Abstract
Objective
The neutrophil to lymphocyte ratio (NLR) is a non-specific, easy-to-obtain marker of inflammation associated with morbidity and mortality in systemic, psychiatric, and age-related inflammatory conditions. Given the growing trend of substance use disorder (SUD) in older adults, and the relationship between inflammation and SUD elevated NLR may serve as a useful inflammatory biomarker of the combined burden of aging and SUD. The present study focused on cocaine use disorder (CUD) to examine if cocaine adds further inflammatory burden among older adults, by comparing NLR values between older adults with CUD and a non-cocaine using, aged-matched, nationally representative sample.
Methods
The dataset included 107 (86% male) participants (aged 50–65 years) with cocaine use disorder. NLR was derived from complete blood count tests by dividing the absolute value of peripheral neutrophil concentration by lymphocyte concentration. For comparison, we extracted data from age-matched adults without CUD using the National Health and Nutrition Examination Survey. Individuals with immunocompromising conditions were excluded (e.g., rheumatoid arthritis and sexually transmitted infections such as HIV). A doubly-robust inverse probability-weighted regression adjustment (IPWRA) propensity score method was used to estimate group differences on NLR while controlling for potential confounding variables (age, gender, race, income, nicotine, marijuana and alcohol use).
Results
The IPWRA model revealed that the CUD sample had significantly elevated NLR in comparison to non-cocaine users, with a moderate effect size (β weight = 0.67).
Conclusion
Although non-specific, NLR represents a readily obtainable inflammatory marker for SUD research. CUD may add further inflammatory burden to aging cocaine users.
Keywords: Aging, Cocaine, Inflammation, Lymphocytes, Neutrophils
INTRODUCTION
Cocaine use disorder (CUD) is a persistent condition, often leading to health complications and emergency department visits. Cocaine use in older adults is on the rise [1–3]. Estimates suggest the prevalence of weekly cocaine use and CUD among individuals ≥ 50 increased from 2011–2015 at a rate of 236% and 271%, respectively [4]. Aging is associated with decreased health and a significant changes in immune functioning called ‘immunosenescence’. An important feature of these changes is the low-grade inflammation that can be compounded by substance use and pro-inflammatory conditions such as diabetes and atherosclerosis [5–7]. As cocaine exposure is associated with elevated inflammation and altered immune functioning [8,9], the presence of CUD might exacerbate inflammatory processes in aging adults. Those with CUD are at greater risk for developing HIV and hepatitis C infection [10–12]. Further, the presence of CUD accelerates the progression of inflammatory diseases, such as atherosclerosis [13,14]. Identifying inflammatory biomarkers in aging adults with CUD can help establish important biological mechanisms of action that could lead to better interventions and reduce the inflammatory burden on this sensitive population.
Neutrophils are first responder white blood cells that are mobilized to sites of acute endothelial damage and/or infection [15]. While neutrophils are important in acute inflammation and defense of bacterial and fungal infections, chronically elevated neutrophil activity can lead to tissue damage [16]. Lymphocytes are another type of white blood cell central to the immune response. Lymphocytes are key in the response to infection and are related to the progression of inflammatory and autoimmunity diseases [17,18]. The neutrophil to lymphocyte ratio (NLR) is obtained from complete blood counts (typically 1,000 /μl). Because neutrophil and lymphocyte counts are standard in routine blood tests, the NLR represents a cost-effective and easily available but non-specific marker of inflammation. NLR is associated with incidence, morbidity, and mortality in several systemic diseases, including cardiovascular diseases and malignancies [19–21]. More recently, elevated NLR has been associated with schizophrenia and mood disorders [22–28]. Both neutrophils and lymphocytes have also been implicated in substance use. For instance, neutrophils increase after heavy alcohol consumption [29] and higher NLR is related to risk for developing alcoholic liver disease and other alcohol-related complications [30]. Nicotine is also related to increases in neutrophils and/or lymphocytes and extant evidence suggests that smoking cessation might reverse these changes [31–35]. High NLR was also reported in subjects with heroin dependence [36,37].
No previous study has evaluated NLR in the context of cocaine use. While some inflammatory makers have been shown to decrease after acute administration of cocaine [38], chronic cocaine use generally leads to immune system activation. Chronic cocaine use modulates cytokine levels toward a pro-inflammatory (i.e., decreased interleukin [IL]-10 and increased tumor necrosis factor-α) profile [39–42] and activates glial cells (e.g., astrocytes and microglia) in the brain [8,43]. Cocaine administration also modulates neutrophil levels: intravenous administration of cocaine increases neutrophils within the first seven days of exposure [44,45]. Further, cocaine alters the functioning of neutrophils; both the antibacterial and tumoricidal functions of neutrophils increase after cocaine exposure [46]. Cocaine also has a broad effect on lymphocyte activity [47,48]. Beyond direct effects on immune cells, cocaine use generally enhances hypothalamic-pituitary-adrenal axis activity, which further influences immune functioning (for a more detailed review on the immunology of substance use disorders, please see [49]. Although short-term activation of the immune system can be adaptive, chronic activation due to repeated cocaine use can lead to a neuroinflammatory state that has a negative impact on central nervous system functioning [43].
While cocaine is associated with these marked changes in neutrophils and lymphocytes, NLR has not yet been evaluated as a potential maker of inflammation in aging adults with CUD. Older adults with cocaine addiction present unique issues to treatment, including complicated co-morbidities that add to the already large public heath burden of substance use disorders [50]. Aging alone is associated with low-grade inflammation [5,6]. Further, in non-drug using populations, age is positively associated with NLR [51]. The added burden of cocaine use in older populations might be expected to increase NLR. The current study examined the hypothesis that chronic cocaine use adds exacerbated inflammatory burden among older adults (aged 50–65 years old) by comparing NLR values between an aging CUD group and an age-matched nationally representative sample. If CUD adds further insult to typical immunosenescence observed in aging populations, then aging adults with CUD should have increased NLR compared with age-matched controls.
METHODS
Participants
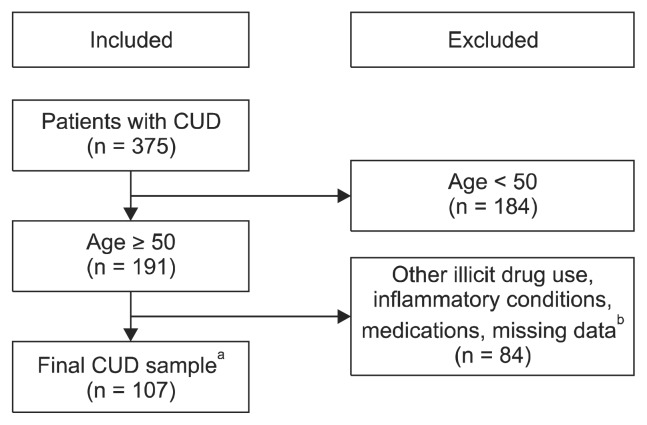
The sample included 107 participants meeting standardized diagnostic criteria for CUD based on the Structured Clinical Interview (SCID) for the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV)/the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) and 1,309 age-matched, non-cocaine using controls. With the transition from DSM-IV to DSM-5, not all participants were evaluated under the same DSM. All participants in the CUD group endorsed 3 or more symptoms on the SCID, which resulted in 83 participants meeting DSM-IV criteria for cocaine dependence and 24 participants meeting DSM-5 criteria for CUD. SCIDs were performed by Master’s level trained clinicians or supervised graduate student trainees. The data from CUD participants were collected at the Center for Neurobehavioral Research on Addiction between 2011 and 2018. Data for non-cocaine using adults were extracted from the National Health and Nutrition Examination Survey (NHANES years 2013–2014 and 2015–2016). For the CUD group, records were included if they met the following requirements: a) CUD diagnosis, b) age 50 to 65 years, c) files were retrievable, and d) LabCorp blood lab panel data was available. Records were excluded if there was evidence of a) illicit drug use other than marijuana or cocaine, b) a current inflammatory condition not associated with natural aging (e.g., rheumatoid arthritis and sexually transmitted inflections – please refer to Fig. 1 for full exclusionary criteria), c) major psychiatric disorder previously associated with inflammation, including mood disorders and psychotic disorders, d) current medication influencing inflammatory processes or central nervous system function, or e) neurological disease. Records were not excluded if the participant had an existing pro-inflammatory condition associated with normal aging (e.g., hypertension or diabetes). The cocaine sample consort is presented in Figure 1. For the aged-matched non-cocaine using sample, records were excluded if they reported: a) age < 50 or > 65 years, b) illicit drug use other than marijuana, c) any inflammatory conditions not associated with normal aging, including sexually transmitted infections (see Fig. 2 for full exclusionary criteria), d) current medication influencing inflammatory processes or central nervous system function, or e) neurological disease. The NHANES consort is presented in Figure 2. A detailed explanation of inclusion/exclusion criteria is provided in the Supplementary Materials (available online). All CUD participants provided informed consent and their anonymity was persevered. All procedures involving the CUD sample were approved by the University of Texas Health Science Center at Houston Committee for the Protection of Human Subjects (no. HSC-MS-05-0322).
Fig. 1.
Cocaine use disorder (CUD) sample consort.
aParticipants with the following existing pro-inflammatory conditions associated with normal aging were included in the sample: hypertension, elevated serum glucose, diabetes, and hyperlipidemia.
bParticipants were excluded if they reported any illicit drug use other than cocaine and marijuana. Participants were excluded if they had irretrievable files or missing LabCorp data (n = 56). Participants were also excluded if they were on anti-inflammatory medications or had inflammatory medical conditions (n = 28) including hepatitis C, HIV, rheumatoid arthritis, syphilis, and trichomoniasis.
Fig. 2.
National Health and Nutrition Examination Survey (NHANES) sample consort.
aExclusionary criteria (other than age) were as follows. Participants were excluded if they reported using the following illicit drugs: cocaine, heroin, methamphetamine, or anabolic steroids. Participants were also excluded if they reported any of the following inflammatory diseases or conditions: rheumatoid arthritis/psoriatic arthritis, gout, liver disease, cancer, preventative aspirin use, or asthma attack within the past year. The following sexually transmitted infections were also exclusionary: hepatitis B, hepatitis C, HIV or chlamydia. Participants were excluded if they had any of the following conditions that may affect inflammation within the past 30 days: flu, pneumonia, ear infection, common cold, or stomach/intestinal illness. Participants reporting medications potentially influencing the central nervous system or inflammatory processes were excluded. For example: anti-fungals, anti-infectives, penicillin, analgesics, hormone modifiers, steroids, immunosuppressive agents, and antipsychotics.
Measures
Neutrophil to lymphocyte ratio
As part of standard medical screening for participation in CUD research studies at the Center for Neurobehavioral Research on Addiction, participants provided a blood sample. Peripheral blood was collected from fasting participants in the morning (between 8 AM–12 PM) by venipuncture into heparin-containing vacutainers. Samples were sent on the same day of blood draw to LabCorp for standard blood laboratory panel analysis, including comprehensive metabolic panel, complete blood count, and thyroid panel. The NHANES data, blood draw, and analysis procedures are described in detail online (https://www.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?BeginYear=2013). For both the CUD and NHANES datasets, NLR was calculated by dividing the absolute value of peripheral neutrophil concentration (1,000 cells /μl) by lymphocyte concentration (1,000 cells /μl) based on standard laboratory panel data.
Variable Selection and Harmonization
Selection of exclusionary criteria and key covariates for inclusion in the propensity scoring model was based on a literature search related to potential sources of inflammation followed by group discussion and consensus among the authors. Based on this selection process, the following variables were included as covariates in the final statistical model (detailed below): age, gender, race, estimated income, nicotine use, marijuana use, and alcohol use (note that current use of other illicit substances was exclusionary in both groups). The final exclusion and matching covariates required harmonization between datasets. While the NLR calculation was necessarily derived from equivalent standardized laboratory methods (neutrophil and lymphocyte counts 1,000 cells /μl), several of the covariates were measured differently between the CUD and NHANES datasets. Specifics of the variable selection and harmonization process are enumerated in the Supplementary Material (available online); to summarize, for the formal data analysis the outcome measure NLR and the covariate age were treated as continuous variables. The covariate income level was treated as an ordinal categorical variable. The covariates race, gender, alcohol, nicotine, and marijuana use were treated as nominal categorical variables.
Statistical Analyses
A doubly robust propensity scoring method, inverse-probability-weighted regression adjustment (IPWRA), was employed via the teffects command in STATA [52,53] to estimate group differences (CUD vs. NHANES) on NLR while controlling for predetermined covariates: age, gender, race, income, nicotine use, marijuana use, and alcohol use. IPWRA combines augmented inverse probability weighting of covariates (treatment model) and regression variable adjustment (outcome model) and concurrently implements both models to estimate the outcome parameters. This combined approach is robust to misspecification, thus optimizing “treatment” effects estimates (i.e., cocaine use) in observational datasets like those used presently. By (1) sampling from a population of diagnostically-defined individuals with CUD and a nationally representative age-matched sample of the population, (2) eliminating as many inflammation-related factors as possible in both datasets and (3) including potential covariates in the model, we aimed to meet the IPWRA assumptions of covariate balance, overlap, and conditional mean independence of outcomes [53].
RESULTS
The CUD sample (n = 107) was 86% male and 14% female. The race distribution was heavily African American (Table 1). This distribution is representative of the sample of metropolitan-area Houston cocaine users typically in enrolled in Center for Neurobehavioral Research on Addiction studies. As commonly observed in CUD, some participants met criteria for other substance use disorders. Fifteen participants met for current alcohol use disorder, while seven participants met for current cannabis use disorder. Cocaine was the primary substance of abuse for all CUD participants. The NHANES sample was 50% male. The NHANES categories were collapsed to African American, White, and Other to match the CUD dataset (Table 1). Standardized measures of substance use disorder were not obtained in the NHANES dataset. Note that the demographic imbalance between the CUD and NHANES samples underscores the utility of the IPWRA methodology.
Table 1.
Sample characteristics
| Variable | CUD (n = 107) | NHANES (n = 1,309) | |
|---|---|---|---|
| Age (yr) | Mean | 54.4 | 57.4 |
| Sex (n) | Male | 92 | 701 |
| Female | 15 | 608 | |
| Race (n) | African American | 90 | 329 |
| White | 9 | 571 | |
| Other | 8 | 510 | |
| Income (USD) | Monthly median | 800 | 1,250 |
| Education (yr) | 12.6 | 12.7 | |
CUD, Cocaine use disorder; NHANES, National Health and Nutrition Examination Survey; USD, United States dollar.
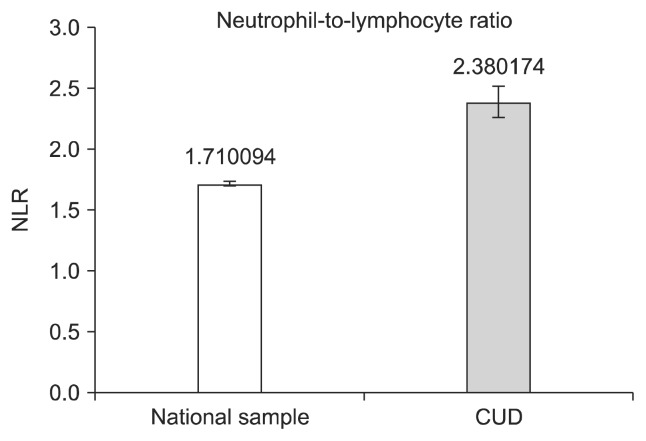
Shown in Figure 3, the NLR means and standard errors for the groups were CUD = 2.38 (± 0.13); controls = 1.71 (± 0.02). The propensity score model revealed a statistically reliable difference in NLR between the groups, β = 0.67, robust standard error = 0.14, p < 0.001. The β weight of 0.67 indicates a moderate effect size, confirming the initial hypothesis of greater inflammation in CUD subjects aged 50–65 versus an age matched nationally representative sample. To examine if the model adequately balanced the covariates, the overidentification test was applied via the STATA tebalance overid command [53]. The overidentification test result was χ2 (10) = 3.09, p = 0.97, thus failing to reject the null hypothesis that the model-adjusted means of the covariates were equivalent across groups.
Fig. 3.
Neutrophil to lymphocyte ratio values from the propensity score matching model. Error bars represent standard deviations.
CUD, cocaine use disorder; NLR, neutrophil to lymphocyte ratio.
DISCUSSION
The current study used NLR—a non-specific biomarker of inflammation—to test if CUD adds further inflammatory burden in aging adults. We used a doubly robust propensity score method to account for potential confounding factors including age, gender, race, income, and other drug use. After harmonization on these important variables, comparison of NLR values between the two groups revealed that NLR was increased in older adults with CUD in comparison to the aged-matched, control group without CUD. To the best of our knowledge, this is the first study that compared NLR values in a CUD sample to a nationally representative dataset, and also the first to focus specifically on cocaine use and aging.
The biochemical pathway that exists between inflammation/NLR, cocaine, and aging has not yet been clearly elucidated. As discussed above, cocaine can affect immune functioning, leading to a state of low-grade chronic inflammation. Aging itself is marked by several changes in the immune system, including the development of a chronic pro-inflammatory state frequently called ‘inflammaging’ [54]. Immunosenescence changes include involution of the thymus [55], decline in T and B cell functioning [56], decrease in IL-2 production [57] and alterations of neutrophil phagocytic capability [58]. Human aging is also associated with elevated activation of the sympathetic nervous system and stress response [59], primarily through decreased norepinephrine reuptake [60,61]. For instance, as a robust modulator of monoamine transmission, cocaine also alters both central and peripheral norepinephrine function [13,62,63]. Cocaine is also a sigma-1 receptor agonist, acting on various leukocyte populations [64]. Exposure to cocaine leads to reduced proliferation of T cells and decreased IL-2 levels [65]. Therefore, both aging and cocaine use might lead to increased NLR possibly through reduced number of lymphocytes due to decreased IL-2 levels among other factors and/or increased number of neutrophils through increased stress response. It is worth mentioning that neither aging nor chronic cocaine use induce leukocyte changes outside the normal range count. Moreover, because of the relatively wide variation of leukocyte count within the normal range, the cutoff for NLR is debated [27,66]. Accordingly, most studies with NLR rely on a reference population, due to challenge translating observed group differences into individual cases.
There are several notable limitations to this study. First, the dataset lacked the size and distributional properties to examine the synergistic effect of cocaine dose-response (i.e., lifetime use) on inflammation and age. As we did not have a comparable younger CUD population, we cannot fully interpret the inflammatory effects related uniquely to cocaine use versus those related directly to age. However, our findings suggest a combined impact of cocaine and aging, such that presence of CUD adds further inflammatory burden to the increase in inflammation associated with aging. Second, the manner in which the CUD dataset and the NHANES dataset were collected did not allow for perfect harmonization, which likely added noise and decreased the precision of the estimated effect. Third, the relationship between peripheral inflammatory markers and neuroinflammation is still being characterized and is in the nascent stages of understanding, but has been the source of contention in the scientific literature [49,67]. To further complicate this limitation, NLR is non-specific inflammatory marker, leaving restricted insight regarding the mechanisms by which cocaine and aging interact to increase inflammation. Subsequent work will require more focused investigation using specific immune markers, neuroimaging, and their relative relationships to NLR.
In conclusion, elevated NLR values were observed in older patients with CUD when compared to age-matched non-CUD controls. These findings support the role of NLR as a readily available blood-based biomarker with potential scientific utility. Given that NLR is readily obtainable from standard laboratory panels, it may be particularly valuable in the examination of large, representative cohort or longitudinal datasets of SUD and other disease processes. Future studies investigating the relation between elevated NLR and clinical outcomes in patients with CUD are needed. If inflammatory mechanisms underlying anhedonia, stress, and impaired cognitive function in CUD are associated with higher NLR values, this would have implications for treatment [41,68]. Currently there is a growing trend toward testing anti-inflammatory agents as pharmacological tools in addiction treatment [41,69,70]. Further investigation of NLR as an indicator of inflammatory response would aid in these medication development efforts. Further, although beyond the scope of the present project, development of a cutoff value for NLR that optimally discriminates between healthy controls and individuals with cocaine use disorder may provide unique value to the literature.
Supplementary Information
Acknowledgments
This study was funded in parts by NIDA P50 DA 009262, NIDA R01 DA044895, and the McGovern Research Scholar Award.
Footnotes
This work was presented as a poster at the College on Problems of Drug Dependence, San Antonio, 2019.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conception and design of the study: Scott D. Lane, Charles E. Green, and Joy M. Schmitz. Acquisition and analysis of data: Amber M. Berumen, Kira E. Gomez, Jessica Vincent, Heather E. Soder, Scott D. Lane, Robert Suchting, Charles E. Green. Original draft and figures: Heather E. Soder. Final editing of manuscript: Heather E. Soder, Scott D. Lane, Charles E. Green, Joy M. Schmitz, Margaret C. Wardle, Amber M. Berumen, Kira E. Gomez, Jessica Vincent, Robert Suchting, Antonio L. Teixeira.
REFERENCES
- 1.Wu LT, Blazer DG. Illicit and nonmedical drug use among older adults: a review. J Aging Health. 2011;23:481–504. doi: 10.1177/0898264310386224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colliver JD, Compton WM, Gfroerer JC, Condon T. Projecting drug use among aging baby boomers in 2020. Ann Epidemiol. 2006;16:257–265. doi: 10.1016/j.annepidem.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann SW, Fingerhood M. Substance-use disorders in later life. N Engl J Med. 2018;379:2351–2360. doi: 10.1056/NEJMra1805981. [DOI] [PubMed] [Google Scholar]
- 4.John WS, Wu LT. Trends and correlates of cocaine use and cocaine use disorder in the United States from 2011 to 2015. Drug Alcohol Depend. 2017;180:376–384. doi: 10.1016/j.drugalcdep.2017.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buford TW. Hypertension and aging. Ageing Res Rev. 2016;26:96–111. doi: 10.1016/j.arr.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assar ME, Angulo J, Rodríguez-Mañas L. Diabetes and ageing-induced vascular inflammation. J Physiol. 2016;594:2125–2146. doi: 10.1113/JP270841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda) 2017;32:9–19. doi: 10.1152/physiol.00012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox Res. 2013;23:174–188. doi: 10.1007/s12640-012-9334-7. [DOI] [PubMed] [Google Scholar]
- 9.Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiasson MA, Stoneburner RL, Hildebrandt DS, Ewing WE, Telzak EE, Jaffe HW. Heterosexual transmission of HIV-1 associated with the use of smokable freebase cocaine (crack) AIDS. 1991;5:1121–1126. doi: 10.1097/00002030-199109000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Parikh N, Nonnemacher MR, Pirrone V, Block T, Mehta A, Wigdahl B. Substance abuse, HIV-1 and hepatitis. Curr HIV Res. 2012;10:557–571. doi: 10.2174/157016212803306023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyndall MW, Currie S, Spittal P, Li K, Wood E, O’Shaughnessy MV, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17:887–893. doi: 10.1097/00002030-200304110-00014. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin GC, Roth MD, Tashkin DP. Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J Neuroimmunol. 1998;83:133–138. doi: 10.1016/S0165-5728(97)00229-4. [DOI] [PubMed] [Google Scholar]
- 14.Bachi K, Mani V, Jeyachandran D, Fayad ZA, Goldstein RZ, Alia-Klein N. Vascular disease in cocaine addiction. Atherosclerosis. 2017;262:154–162. doi: 10.1016/j.atherosclerosis.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilgus TA, Roy S, McDaniel JC. Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care (New Rochelle) 2013;2:379–388. doi: 10.1089/wound.2012.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 17.Brooks SM, McGowan K, Bernstein IL, Altenau P, Peagler J. Relationship between numbers of beta adrenergic receptors in lymphocytes and disease severity in asthma. J Allergy Clin Immunol. 1979;63:401–406. doi: 10.1016/0091-6749(79)90213-6. [DOI] [PubMed] [Google Scholar]
- 18.Buseyne F, Burgard M, Teglas JP, Bui E, Rouzioux C, Mayaux MJ, et al. Early HIV-specific cytotoxic T lymphocytes and disease progression in children born to HIV-infected mothers. AIDS Res Hum Retroviruses. 1998;14:1435–1444. doi: 10.1089/aid.1998.14.1435. [DOI] [PubMed] [Google Scholar]
- 19.Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14:573–577. doi: 10.1586/14779072.2016.1154788. [DOI] [PubMed] [Google Scholar]
- 20.Küçük E, Kocayiğit İ, Günel C, Düzenli H. Neutrophil-to-lymphocyte ratio in occlusive vascular diseases: the literature review of the past 10 years. World J Emerg Med. 2016;7:165–172. doi: 10.5847/wjem.j.1920-8642.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isaac V, Wu CY, Huang CT, Baune BT, Tseng CL, McLachlan CS. Elevated neutrophil to lymphocyte ratio predicts mortality in medical inpatients with multiple chronic conditions. Medicine (Baltimore) 2016;95:e3832. doi: 10.1097/MD.0000000000003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moody G, Miller BJ. Total and differential white blood cell counts and hemodynamic parameters in first-episode psychosis. Psychiatry Res. 2018;260:307–312. doi: 10.1016/j.psychres.2017.11.086. [DOI] [PubMed] [Google Scholar]
- 23.Mayda H, Ahsen A, Bağcioğlu E, Öztürk A, Bahçeci B, Soyuçok E, et al. Effect of increased Neutrophil-to-Lymphocyte Ratio (NLR) and Decreased Mean Platelet Volume (MPV) values on inflammation in acute mania. Noro Psikiyatr Ars. 2016;53:317–320. doi: 10.5152/npa.2016.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivković M, Pantović-Stefanović M, Dunjić-Kostić B, Jurišić V, Lačković M, Totić-Poznanović S, et al. Neutrophil-to-lymphocyte ratio predicting suicide risk in euthymic patients with bipolar disorder: moderatory effect of family history. Compr Psychiatry. 2016;66:87–95. doi: 10.1016/j.comppsych.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Bustan Y, Drapisz A, Ben Dor DH, Avrahami M, Schwartz-Lifshitz M, Weizman A, et al. Elevated neutrophil to lymphocyte ratio in non-affective psychotic adolescent inpatients: evidence for early association between inflammation and psychosis. Psychiatry Res. 2018;262:149–153. doi: 10.1016/j.psychres.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Aydin Sunbul E, Sunbul M, Yanartas O, Cengiz F, Bozbay M, Sari I, et al. Increased neutrophil/lymphocyte ratio in patients with depression is correlated with the severity of depression and cardiovascular risk factors. Psychiatry Investig. 2016;13:121–126. doi: 10.4306/pi.2016.13.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazza MG, Lucchi S, Tringali AGM, Rossetti A, Botti ER, Clerici M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:229–236. doi: 10.1016/j.pnpbp.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Mazza MG, Lucchi S, Rossetti A, Clerici M. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in non-affective psychosis: A meta-analysis and systematic review. World J Biol Psychiatry. 2019 doi: 10.1080/15622975.2019.1583371. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Kanwar KC, Tikoo A. Hematological lesions in rat following heavy alcohol ingestion. J Environ Pathol Toxicol Oncol. 1992;11:241–245. [PubMed] [Google Scholar]
- 30.Nand N, Malhotra P, Dhoot DK. Clinical profile of alcoholic liver disease in a tertiary care centre and its correlation with type, amount and duration of alcohol consumption. J Assoc Physicians India. 2015;63:14–20. [PubMed] [Google Scholar]
- 31.Bridges RB, Wyatt RJ, Rehm SR. Effects of smoking on inflammatory mediators and their relationship to pulmonary dysfunction. Eur J Respir Dis Suppl. 1986;146:145–152. [PubMed] [Google Scholar]
- 32.Phillips B, Esposito M, Verbeeck J, Boué S, Iskandar A, Vuillaume G, et al. Toxicity of aerosols of nicotine and pyruvic acid (separate and combined) in Sprague-Dawley rats in a 28-day OECD 412 inhalation study and assessment of systems toxicology. Inhal Toxicol. 2015;27:405–431. doi: 10.3109/08958378.2015.1046000. [DOI] [PubMed] [Google Scholar]
- 33.Olatunji LA, Michael OS, Adeyanju OA, Areola ED, Soladoye AO. Anti-inflammatory and antithrombotic effects of nicotine exposure in oral contraceptive-induced insulin resistance are glucocorticoid-independent. Pharmacol Rep. 2017;69:512–519. doi: 10.1016/j.pharep.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Jensen EJ, Pedersen B, Frederiksen R, Dahl R. Prospective study on the effect of smoking and nicotine substitution on leucocyte blood counts and relation between blood leucocytes and lung function. Thorax. 1998;53:784–789. doi: 10.1136/thx.53.9.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roethig HJ, Koval T, Muhammad-Kah R, Jin Y, Mendes P, Unverdorben M. Short term effects of reduced exposure to cigarette smoke on white blood cells, platelets and red blood cells in adult cigarette smokers. Regul Toxicol Pharmacol. 2010;57:333–337. doi: 10.1016/j.yrtph.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Cıcek E, Demırel B, Cıcek IE, Kıraç AS, Eren I. Increased neutrophil-lymphocyte and platelet-lymphocyte ratios in male heroin addicts: a prospective controlled study. Clin Psychopharmacol Neurosci. 2018;16:190–196. doi: 10.9758/cpn.2018.16.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzel D, Yazici AB, Yazici E, Erol A. Evaluation of immunomodulatory and hematologic cell outcome in heroin/opioid addicts. J Addict. 2018;2018 doi: 10.1155/2018/2036145. 2036145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta K, Sharma R, Singh V, Masoomi R, Dileepan KN, He J, et al. Intravenous cocaine results in an acute decrease in levels of biomarkers of vascular inflammation in humans. Cardiovasc Toxicol. 2018;18:295–303. doi: 10.1007/s12012-017-9440-0. [DOI] [PubMed] [Google Scholar]
- 39.Pellegrino TC, Dunn KL, Bayer BM. Mechanisms of cocaine-induced decreases in immune cell function. Int Immunopharmacol. 2001;1:665–675. doi: 10.1016/S1567-5769(00)00051-5. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz P, Berho M, Steele BW, Hao L. Peripheral human T lymphocyte maintenance of immune functional capacity and phenotypic characteristics following in vivo cocaine exposure. Clin Immunol Immunopathol. 1998;88:271–276. doi: 10.1006/clin.1998.4579. [DOI] [PubMed] [Google Scholar]
- 41.Xu W, Flick T, Mitchel J, Knowles C, Ault K. Cocaine effects on immunocompetent cells: an observation of in vitro cocaine exposure. Int J Immunopharmacol. 1999;21:463–472. doi: 10.1016/S0192-0561(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 42.Fox HC, D’Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, et al. Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol. 2012;27:156–166. doi: 10.1002/hup.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Periyasamy P, Guo ML, Buch S. Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy. 2016;12:1310–1329. doi: 10.1080/15548627.2016.1183844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jankowski MM, Ignatowska-Jankowska B, Glac W, Swiergiel AH. Cocaine administration increases CD4/CD8 lymphocyte ratio in peripheral blood despite lymphopenia and elevated corticosterone. Int Immunopharmacol. 2010;10:1229–1234. doi: 10.1016/j.intimp.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Di Francesco P, Falchetti R, Gaziano R, Lanzilli G, Belogi L, Ravagnan G, et al. Differential effects of short-term or prolonged cocaine exposure on peripheral blood cells in mice. Life Sci. 1994;54:2015–2020. doi: 10.1016/0024-3205(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 46.Baldwin GC, Buckley DM, Roth MD, Kleerup EC, Tashkin DP. Acute activation of circulating polymorphonuclear neutrophils following in vivo administration of cocaine. A potential etiology for pulmonary injury. Chest. 1997;111:698–705. doi: 10.1378/chest.111.3.698. [DOI] [PubMed] [Google Scholar]
- 47.Bagasra O, Forman L. Functional analysis of lymphocytes subpopulations in experimental cocaine abuse. I. Dose-dependent activation of lymphocyte subsets. Clin Exp Immunol. 1989;77:289–293. [PMC free article] [PubMed] [Google Scholar]
- 48.Ruiz P, Cleary T, Nassiri M, Steele B. Human T lymphocyte subpopulation and NK cell alterations in persons exposed to cocaine. Clin Immunol Immunopathol. 1994;70:245–250. doi: 10.1006/clin.1994.1036. [DOI] [PubMed] [Google Scholar]
- 49.Salem H, Lane SD, Teixeira AL. Immunology of substance use disorders. In: Teixeira AL, Bauer ME, editors. Immunopsychiatry: a clinician’s introduction to the immune basis of mental disorders. New York: Oxford University Press; 2019. pp. 165–178. [Google Scholar]
- 50.Yarnell SC. Cocaine abuse in later life: a case series and review of the literature. Prim Care Companion CNS Disord. 2015;17:PCC.14r01727. doi: 10.4088/PCC.14r01727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Chen Q, Luo X, Hong J, Pan K, Lin X, et al. Neutrophil-to-lymphocyte ratio positively correlates to age in healthy population. J Clin Lab Anal. 2015;29:437–443. doi: 10.1002/jcla.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.StataCorp. Stata Statistical Software: Release 15. College Station TX: StataCorp LLC; 2017. [Google Scholar]
- 53.StataCorp. STATA treatment-effects reference manual: potential outcomes/counterfactual outcomes. Release 13. College Station: Stata Press; 2013. [Google Scholar]
- 54.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 55.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riley RL, Blomberg BB, Frasca D. B cells, E2A, and aging. Immunol Rev. 2005;205:30–47. doi: 10.1111/j.0105-2896.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 57.Wu WT, Pahlavani M, Cheung HT, Richardson A. The effect of aging on the expression of interleukin 2 messenger ribonucleic acid. Cell Immunol. 1986;100:224–231. doi: 10.1016/0008-8749(86)90021-3. [DOI] [PubMed] [Google Scholar]
- 58.Wessels I, Jansen J, Rink L, Uciechowski P. Immunosenescence of polymorphonuclear neutrophils. ScientificWorld-Journal. 2010;10:145–160. doi: 10.1100/tsw.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun AY. Aging and in vivo norepinephrine-uptake in mammalian brain. Exp Aging Res. 1976;2:207–219. doi: 10.1080/03610737608257177. [DOI] [PubMed] [Google Scholar]
- 61.Esler M, Hastings J, Lambert G, Kaye D, Jennings G, Seals DR. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol Regul Integr Comp Physiol. 2002;282:R909–R916. doi: 10.1152/ajpregu.00335.2001. [DOI] [PubMed] [Google Scholar]
- 62.Suhara T, Farde L, Halldin C, Någren K, Karlsson P. Effects of cocaine on [11C]norepinephrine and [11C] beta-CIT uptake in the primate peripheral organs measured by PET. Ann Nucl Med. 1996;10:85–88. doi: 10.1007/BF03165058. [DOI] [PubMed] [Google Scholar]
- 63.Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2009;14:119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marasco CC, Goodwin CR, Winder DG, Schramm-Sapyta NL, McLean JA, Wikswo JP. Systems-level view of cocaine addiction: the interconnection of the immune and nervous systems. Exp Biol Med (Maywood) 2014;239:1433–1442. doi: 10.1177/1535370214537747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klein TW, Matsui K, Newton CA, Young J, Widen RE, Friedman H. Cocaine suppresses proliferation of phytohemagglutinin-activated human peripheral blood T-cells. Int J Immunopharmacol. 1993;15:77–86. doi: 10.1016/0192-0561(93)90033-U. [DOI] [PubMed] [Google Scholar]
- 66.Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10:12. doi: 10.1186/s13104-016-2335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bendorius M, Po C, Muller S, Jeltsch-David H. From systemic inflammation to neuroinflammation: the case of neurolupus. Int J Mol Sci. 2018;19:E3588. doi: 10.3390/ijms19113588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wardle MC, Vincent JN, Suchting R, Green CE, Lane SD, Schmitz JM. Anhedonia is associated with poorer outcomes in contingency management for cocaine use disorder. J Subst Abuse Treat. 2017;72:32–39. doi: 10.1016/j.jsat.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmitz JM, Green CE, Hasan KM, Vincent J, Suchting R, Weaver MF, et al. PPAR-gamma agonist pioglitazone modifies craving intensity and brain white matter integrity in patients with primary cocaine use disorder: a double-blind randomized controlled pilot trial. Addiction. 2017;112:1861–1868. doi: 10.1111/add.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Birath JB, Briones M, Amaya S, Shoptaw S, Swanson AN, Tsuang J, et al. Ibudilast may improve attention during early abstinence from methamphetamine. Drug Alcohol Depend. 2017;178:386–390. doi: 10.1016/j.drugalcdep.2017.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.