Abstract
Neural regeneration devices interface with the nervous system and can provide flexibility in material choice, implantation without the need for additional surgeries, and the ability to serve as guides augmented with physical, biological (e.g., cellular), and biochemical functionalities. Given the complexity and challenges associated with neural regeneration, a 3D printing approach to the design and manufacturing of neural devices could provide next-generation opportunities for advanced neural regeneration via the production of anatomically accurate geometries, spatial distributions of cellular components, and incorporation of therapeutic biomolecules. A 3D printing-based approach offers compatibility with 3D scanning, computer modeling, choice of input material, and increasing control over hierarchical integration. Therefore, a 3D printed implantable platform could ultimately be used to prepare novel biomimetic scaffolds and model complex tissue architectures for clinical implants in order to treat neurological diseases and injuries. Further, the flexibility and specificity offered by 3D printed in vitro platforms have the potential to be a significant foundational breakthrough with broad research implications in cell signaling and drug screening for personalized healthcare. This progress report examines recent advances in 3D printing strategies for neural regeneration as well as insight into how these approaches can be improved in future studies.
Keywords: 3D bioprinting, spinal cord, nervous system, neural regeneration, tissue engineering
Graphical Abstract
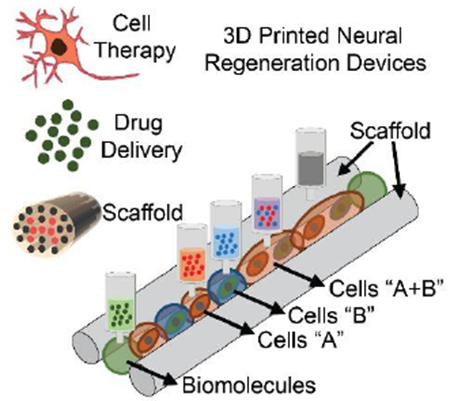
3D printed platforms which combine cells, biomolecules, and scaffolds are growing in promise for combinatorial strategies in neural regeneration. This progress report focuses on the application of various 3D printing approaches to neuronal regeneration devices and mimicking the nervous system on a chip. Current challenges and future opportunities are highlighted to develop clinical implants to treat neurological diseases and injuries.
1. Introduction
The nervous system is structurally separated into two systems: the central nervous system (CNS), comprised of the brain and spinal cord, and the peripheral nervous system (PNS), composed of the cranial and spinal nerves along with their associated ganglia that connect the CNS to the body. These systems are interconnected via an extensive network of nerves and neural cells (i.e., neurons and supporting glial cells) to facilitate communication and relay information (sensorimotor signals) to and from all parts of the body. Typically, neurons receive electrical signals via dendrites or specialized nerve endings and transmit the electrical signals through axons (nerve fibers) to the cell body. Supporting glial cells in the CNS (astrocytes and oligodendrocytes) serve various functions such as insulating the axons and forming the blood-brain barrier. When the nervous system is impacted by an injury or a disease, there is resultant neural cell death, distributed neural networks become disconnected, and the relay of information is disrupted. This loss can lead to many neurological disorders including neurodegenerative diseases (e.g., Alzheimer’s and Parkinson’s disease), stroke, traumatic brain injury (TBI), spinal cord injury (SCI), and peripheral nerve injury (PNI).[1–4]
Nervous system regeneration refers to the re-establishment and repair of functional neural connections, nervous tissue, and cells. Methods to accomplish this through neural tissue engineering involve providing direct replacement of neural cells and/or repair of circuitry by utilizing cell transplantation, bio/chemical-molecular signaling, and a directed guidance “bridge” scaffolding (Figure 1a–c).[5–11] Direct injection of cell-based and/or biomolecular therapies (scaffold-free) for restoring function following nervous system injury has yielded promising outcomes in animal models. However, translating these approaches into practical, clinically available treatments has been limited due to difficulties in selecting optimal cell types for transplantation, cell placement, and cell survival at the injury site due to lack of supporting structures and systems.[12, 13] It has been proposed that using scaffolds to support the cells may be an effective strategy, opening opportunities to test new therapeutic options.[12, 13] In principle, the ideal scaffold should mimic the native tissues as closely as possible, mechanically, organizationally, and biologically. Unfortunately, accurate mimicry of the complex nervous system is challenging via conventional molding techniques, especially when it requires materials and mechanical flexibility, control over cell placement, and precision in anatomical design.[14]
Figure 1.
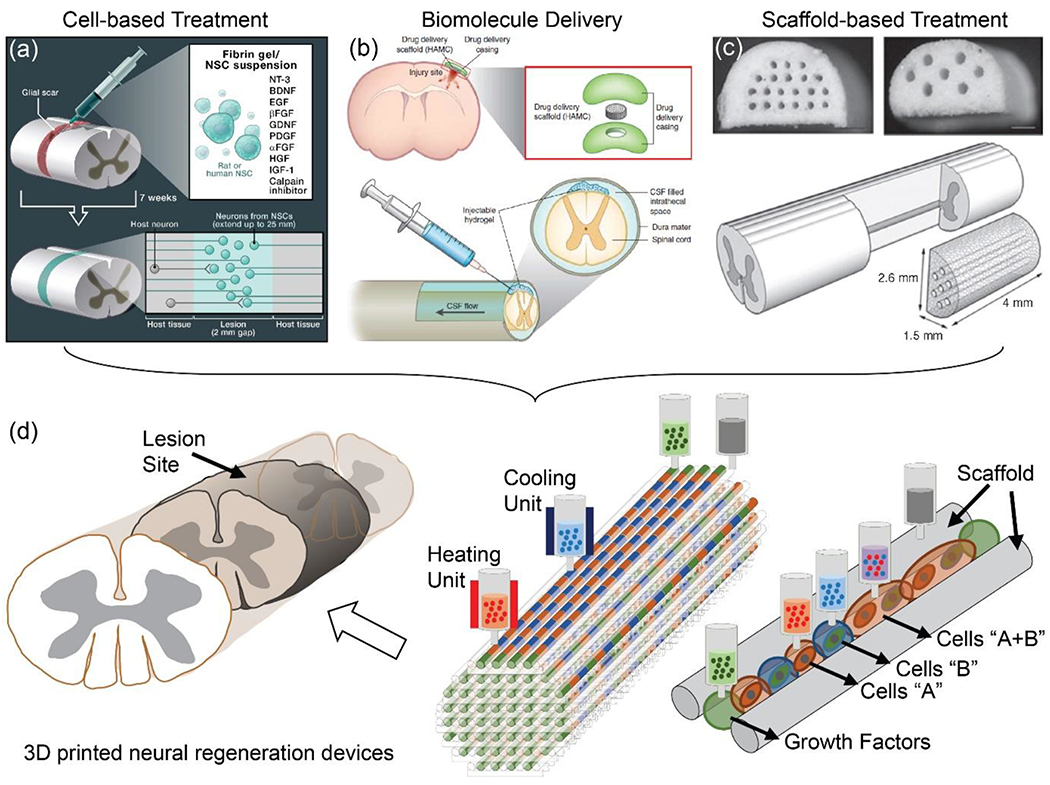
Strategic design of a 3D printed nervous system scaffold to promote neural regeneration. Neural regeneration refers to the re-establishment and repair of functional neural connections, nervous tissue, and cells by (a) controlling the position, growth, and differentiation of transplanted cells [Reproduced with permission.[8] Copyright 2012, Elsevier.], and (b) promoting neurite networks (e.g., sensory and motor) via the inclusion of biomolecules such as neurotrophic factors [Reproduced with permission.[9] Copyright 2014, Nature Publishing Group], (c) within desired channels in the scaffolds [Reproduced with permission.[10] Copyright 2009, Elsevier.]. (d) 3D printing offers promising combinatorial strategies for neural regeneration, by using a common platform to print scaffolds, cells, and biomolecules. Reproduced with permission.[15] Copyright 2018, Wiley-VCH.
3D printing is an additive manufacturing process which is capable of building 3D structures from a computer-aided design (CAD) model in a layer-by-layer fashion to create unique 3D architectures. It potentially enables personalized biomedical device applications which incorporate combinatorial strategies to overcome some of the challenges of neural regeneration (Figure 1d). 3D printing provides four vital features: (1) coupling with 3D imaging technologies to achieve anatomical accuracy; (2) robotics-based biomanufacturing for precision; (3) compatibility with multiple material sets for flexible functionality; and (4) rapid prototyping for combinatorial sampling. Together, these features offer powerful advantages over other methodologies in recreating complex structures such as components of the nervous system. Notably, due to the different environments of the CNS and PNS, as well as neuronal and glial responses to injury, 3D printing also could allow different regeneration strategies for the CNS and PNS.
First, 3D printing allows us to tailor the shape of the scaffold to individual nervous systems and injuries. The combination with 3D imaging enables the acquisition of 3D topological data to design scaffolds that geometrically match the 3D microenvironment of each injury with high anatomical fidelity. Since cell-to-cell contact is critical for outgrowth and ingrowth of axons from grafted cells, 3D printing can play a crucial role in supporting the regenerative ability of the cells when transplanted by ensuring that this cell-to-cell contact is present. Furthermore, 3D printing can provide directionality for regenerating axons, thereby encouraging neural network formation.[16]
Second, in contrast to methodologies which involve printing bare scaffolds and then seeding them with cells or biomolecules post-fabrication, a direct 3D printing approach allows us to print the correct cell types or biomolecules directly onto the desired scaffold for optimal and efficient localization, which can be beneficial in recapitulating complex cyto-architectures such as within the nervous system. For complex nervous system replication, incorporation of different types of cells or molecules into regionally-specific channels could allow for orthotopic reconstruction and optimal regeneration. Hence, 3D printing could permit multicellular neural tissue engineering which is: (i) able to control the position, growth, and differentiation of transplanted cells via direct printing of cells; and (ii) able to form specific neurite networks (e.g., sensory and motor) via printing particular biomolecules (neurotrophic factors, growth factors) within channels.[15, 17]
Third, 3D printing allows for the integration of different classes of multiple materials in a single printer including cells, biomaterials, fibers, polymers, nanomaterials, ceramics, and metals. This flexibility to choose different materials should allow closer mimicry of native tissue-like structures, which is essential for biomedical devices.[18–23] It will enable tuning of mechanical properties of 3D printed devices to achieve enhanced performance for neuroregeneration.[17, 24] It also minimizes mechanical mismatches with surrounding tissues, which is a cause of tissue damage or delamination when the scaffolds are transplanted.[25]
Lastly, 3D printing provides rapid prototyping of organ-level in vitro nervous system platforms.[26] In particular, the platforms offer opportunities for the development of model systems for complex neurological phenomena and targeted treatment of neurological disorders via the alignment of axonal networks and spatial organization of cellular components at the microchip scale. Combinatorial strategies may also solidify the statistical significance of the regeneration.
In this progress report, we discuss the application of 3D printing to neuronal regeneration devices and how this approach benefits nervous system tissue engineering. We also elaborate on the prototyping of customized nervous-system-on-a-chip technologies. First, we discuss general methods and strategies for 3D printing, including selection of biomaterials. Second, we introduce the cellular components and organizational hierarchy of the nervous system. Third, we present recent studies on implantable 3D printed scaffolds for PNS and CNS injuries. Fourth, we describe a 3D printed in vitro nervous-system-on-a-chip with broad research implications in regenerative medicine (e.g., cell signaling and drug screening for personalized healthcare). Finally, we close with a discussion on the current limitations of these strategies and future studies.
2. Design principle for developing 3D printed neural regeneration devices
One of the most important purposes of printing neural regeneration devices (scaffolds) is to create a “living” platform which is transplantable and mimics functional tissues. To generate a living platform, the printed scaffold, cells, and/or biomolecules need to be designed to provide a suitable environment for neural tissue regeneration. Key considerations for the design of the devices include: the printing technology and process methodology; biocompatible materials for cell-laden bioinks and scaffolds; and replication of target structure, injury, or cavity (e.g., via 3D imaging). These characteristics are dependent on the application to the specific injury, as shown in Figure 2.
Figure 2.

Overview of current 3D printed neural regeneration devices and applications.
2.1. 3D printing and bioprinting methodologies
In 3D printed tissue engineering for neural regeneration, bioprinting refers to 3D printing of either biologically inert materials or biomaterials that incorporate cells and other biologics.[27–29] Due to the high water content, a low-viscosity hydrogel is a common biomaterial for bioprinting. Of course, other biomaterials may be considered. For implantable scaffold construction, the viscosity of biocompatible inks needs to be sufficient to maintain the precise architecture of the injured nervous tissues, which is a challenge when these inks are also biodegradable. Depending on the specific regenerative strategy, 3D printed neural regeneration devices are classified into (i) scaffold-only (3D printing), (ii) cell-laden scaffold (bioprinting), and (iii) living scaffold (combining 3D printing and bioprinting, ideally on a single platform).
Extrusion, fused-deposition modeling (FDM), laser-assisted, and stereolithography (SLA) are the most common 3D printing methods to mimic nervous system tissue.[30–32] The purpose of the 3D printed neural tissue model or scaffold is the construction of well-defined neuronal network architectures and neural circuits, which are critical for neural regeneration or nerve injury repairs. Thus, 3D printed scaffolds should provide the mechanical support to maintain a transplanted scaffold within native tissues. Particularly important are channels to allow infiltration of cells and axons or axon propagation.[15, 24, 29, 33, 34]
Architectures of scaffold channels surrounding cells have significant effects on cellular morphology and function, and could change cell migration, attachment, and orientation within the channel of the scaffold.[24, 29] A study of autologous nerve-graft implantation reported that precision matching of internal microstructures of nerve fascicles exhibited effective and rapid functional recovery for 15 mm-long sciatic nerve defects, which shows the importance of multi-microchannel scaffolds.[35] An in vitro study involving seeding rat dorsal root ganglia and Schwann cells performed by Pawar et al. suggested that a wider channel diameter (~80 μm) enhanced the length of axon outgrowth, axon density, and Schwann cell migration compared to channels with smaller diameters (~15 μm).[36] However, the longitudinal alignment of both axons and Schwann cell migration diminished in the wider scaffold channels. In this study, the medium channel diameter (~20-35 μm) represented an ideal compromise between quantity (axon length and density) and quality (axon orientation) of axon growth. Another study involving multiple-channel scaffolds seeded with bone marrow stromal cells showed that an increase in channel diameter from 41 to 64 μm did not reveal a significant difference in the number of axons in an injured rat spinal cord.[37] However, the axon orientation in the smaller diameter channel exhibited a closer resemblance to native spinal cord white matter. On the other hand, blood vessels (~10-15 μm in diameter) entered the scaffold channels, which inhibited the regenerating axons and infiltrated the space for supporting cells. Therefore, the ideal scaffold channel sizes should be large enough to ensure ample support for cells and blood supplies.
In different studies, multichannel peripheral nerve scaffolds with 200–300 μm diameter channels still allowed effective linear alignment of axons as well as vascular and glial cells in 1 cm-long sciatic nerve injuries.[38, 39] Similar effective linear guidance of axons involving scaffold-based spinal cord axon regeneration has also been observed in 150–300 μm diameter microchannel scaffolds both in vitro and in vivo.[15, 34] On the other hand, Krych et al. observed that a channel diameter larger than ~450 μm led to poor nerve regeneration in a rat spinal cord.[40] They observed a reduced number of regenerative axons in a large diameter channel two months post-implantation, presumably due to the inflammatory response, the inability to reach functional synaptic connections between neurons, and the growth of a fibrous scar into the scaffold.[40] In the case of astrocytes in vitro, a smaller diameter scaffold channel (~180 μm) enhanced the extent of astrocyte alignment via the alteration of the astrocyte proliferative morphology.[41] More comparative experiments on the influence of channel diameter on regenerating axons are needed.
In addition, surface structure (i.e., porosity, fill ratio, and pore size) and properties (i.e., roughness, hydrophilicity/hydrophobicity of the 3D printed scaffold) have a significant impact on cell activities such as seeding efficiency and proliferation.[42, 43] A common issue in 3D scaffolds can be irregular cell attachments and proliferation on the inner and outer sides of the scaffolds. For instance, pores that are too small could limit feeding the inner side of a 3D scaffold, whereas large pores could affect the mechanical stability and present suturing issues when the scaffolds are implanted, limiting the linear alignment of neurite growth. For peripheral nerve repair, a pore size of ~10-40 μm and porosity of ~80% led to the most efficient axonal regeneration.[44] However, the influence of these factors on spinal cord injury has not been studied extensively to date.
Surface roughness is another factor which affects neural cell behavior. Smoother surfaces (surface roughness of ~6-50 nm) supported longer axons and more neurite outgrowth/branches in comparison to lager surface roughness (~85-200 nm).[45] Interestingly, in the case of human endothelial cells, a higher surface roughness (~35 nm) of biomaterials enhanced cell adhesion and growth compared to a roughness of ~20 nm.[46] Further, a more hydrophilic surface exhibited a higher rate of cell adhesion and tended to absorb more proteins. Indeed, higher rates of neuronal spreading and neurite outgrowth have been observed as the hydrophobicity of the surface was reduced.[47, 48] Therefore, carefully engineering the local microenvironment is critical for neural tissue engineering scaffolds.
Laser-assisted, inkjet, and extrusion-based 3D printing methods are typically used for bioprinting live cells in hydrogel suspensions (often called cell-laden bioinks) and biomolecules in hydrogel suspensions.[30, 49] In 1999, Odde et al. were the first team responsible for directly bioprinting spinal cord cells via laser-guided direct writing.[50] A near-infrared diode laser light was used to guide arrays of cells in designed 2D patterns with micrometer-scale precision. The printed cells remained viable after exposure to the laser light and exhibited neurite outgrowth. Moreover, since the technique is nozzle-free, it is not affected by clogging or shear stress. Typical viscosity values of printing inks for this laser-assisted bioprinting were ca. 1-300 mPa·s.[30] However, side effects from laser exposure on the target cells need to be carefully considered.
Typical viscosity values of the inkjet printing droplets are limited to ca. 2-20 mPa·s.[51, 52] This range is acceptable for printing cells in media or a hydrogel suspension.[53] However, it is challenging to print a concentrated (dense) polymer solution without clogging. Hence, the method is not suitable for creating a high-resolution 3D structure and multiple-bioink printing processes. On the other hand, extrusion-based 3D printing is capable of incorporating a wide range of materials with viscosities up to 106 mPa·s and with disparate properties.[52] Therefore, although laser-assisted and inkjet printing have been used in bioink printing, extrusion-based printing allows for printing bioinks with higher cell density as well as acellular inks. Also, it can be readily expanded to incorporate multiple inks (materials) including cells, biomolecules, and hydrogels, in which the materials are extruded through their own independent nozzle within a printing system.[30, 54] Alternatively, the extrusion-based printing process can be performed in aqueous environments via freeform reversible embedding of suspended hydrogel (FRESH).[55] Here, the bioinks are printed in a hydrogel bath which prevents dehydration and supports low modulus inks, which, in turn, can enhance cell viability during a lengthy printing process. However, constructing multicellular architectures remains a significant challenge. Recently, commercially available extrusion-based lab-on-a-printer systems have been utilized for tissue engineering studies.[56–59] These printers consist of a variable system which can control the desired temperature inside the syringe, a printing stage which is tunable in the range of 4 °C to 37 °C depending on the specific requirement of the cell-hydrogel suspension for maintain cell viability, and a built-in ultraviolet (UV) light system for sterility and crosslinking. Moreover, depending on the specific requirement for the printing process, the printer can be customized. For instance, to reduce shear stress on cells during extrusion, Willerth et al. have integrated microfluidic channels into the printhead, which allowed a separate flow of cells in bioinks and the associated crosslinker.[56, 60] This enabled low viscous flow, resulting in successful neural differentiation from printed human induced pluripotent stem cells (iPSCs).
Living scaffold printing involves a “one-pot” combination of 3D printing and bioprinting, which requires multiple biomaterials. Extrusion-based 3D printing is versatile for implementing this 3D biomanufacturing process. The printing process, time, pressure, and toxicity of scaffold materials are critical variables influencing cell viability. These devices also span multiple length scales. For instance, for nerve guidance channels, the sizes of the channels typically are on the order of a few tens to hundreds of micrometers in diameter. The lengths of the printed channel structures are in millimeter to centimeter scales.[15, 34] The cell density in the hydrogel matrix is ca. millions of cells per milliliter. Therefore, the volume of the printed cell-laden structure is in the range of ñanoliters within a channel.[15] A lengthy printing process can lead to drying of the cell suspension in the hydrogel (bioink), which results in cell death due to dehydration of hydrogel in air. Our own studies have determined that the printing process of the scaffolds containing cells for replacement of the spinal cord needs to be completed within 30 minutes to prevent this.[15] Additionally, printing pressures of 0.3 to 3 psi are used to maintain viabilities of neural progenitor cells and avoid excessive shear forces. We have also discovered that during this one-pot printing process, low cell viability and lack of long-term functionality can occur.[15] This observation could be due to the use of a toxic solvent and the photopolymerization process.[61–63] Since the maturation time of specific neurons or glial cells may be different, the degradation properties of both bioinks and biomaterials need to be considered and accommodated.[15]
2.2. Biomaterials
3D printed tissue engineering of functional neural systems incorporates multiple biomaterials, biomolecules and/or cells. Biomaterials interact with biological systems and are used to replace and mimic the natural functions of tissues or organs. Biomaterials can be derived from nature or synthesized using polymers, ceramics, metallic or composite materials. For neural regeneration engineering, most of the biomaterials are polymers. In addition, since the elastic moduli of the human brain, spinal cord, and peripheral nerve tissues are ~ 1 kPa, 10 kPa, and 100-500 kPa, respectively,[29, 64–66] soft polymer-based scaffolds have the potential to seamlessly mimic and integrate into the nerve injury. Biomaterial inks for neural regeneration devices can be divided into bioinks and acellular inks.
Cell- and biomolecule-laden inks (bioinks) should be processed under mild conditions close to cell growth environments. On the other hand, acellular inks can be printed in harsh environments such as in high temperatures, intense UV light, or organic solvents. Many different types of biomaterials have been explored for tissue-engineered scaffolds using traditional manufacturing methods.[67] However, only a handful of materials have been successfully 3D printed for living neural regeneration scaffolds (Table 1).[17, 67–69] This is because neural cells are very sensitive and require hybrid materials (cells, biomolecules, culture media, etc.) to promote neural cell regeneration. For successful regeneration of neural networks, long-term viability and functionality of printed cells need to be demonstrated. To this end, optimization of biomaterials incorporated with neurotrophic factors, cells, or extracellular matrix (ECM)-based proteins and structural designs have been developed to improve the properties and performance of the printed constructs for neural regeneration device engineering.
Table 1.
Overview of 3D printed neural regeneration devices
| Application | Materials | Cell Type | Printing Method | Printing Resolution | Printed Structures | Dimension | Solidification | Mechanical Properties | Cell Density | Cell Viability | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioprinted cells in 3D printed scaffolds | |||||||||||
| Cell-laden scaffold for CNS | PCL-PU hydrogel | NSC | Extrusion | ~300-350 μm | Two layers lattice (grid) structure | 20×20×10 mm3 (scaffolds) | Thermal responsive | Modulus 6-8 kPa | 2×106 cells/ml | 78% | [92] |
| Cell-laden scaffold | Alginate, chitosan, agarose | iPSCsa) | Extrusion | ~200-300 μm | Multi-layers lattice | 5×5 mm2 (scaffolds) | CaCl2 crosslinking | Indentation modulus < 5 kPa | 4×107 cells/ml | Day 1:18% Day 9: 103% |
[68] |
| Cell-laden scaffold | Alginate, chitosan, agarose | NSC | Extrusion | ~200-300 μm | Multi-layers lattice | 10×10×10 mm3 (scaffolds) | CaCl2 crosslinking | Compression modulus 7.5 kPa | 5×106 cells/ml | Day 1:75% Day 6 :92% |
[110] |
| Brain-like structure | Gellan gum-RGD | Primary cortical neurons | Manual extrusion (Hand-held printing) | Poor Resolution | Cylindrical consisted with six layers | ~20 mm height and ~10 mm diameter (scaffolds) | CaCl2 crosslinking | - | 1×106 cells/ml | 78-80% | [111] |
| Artificial neural tissue | Cells in Collagen /VEGF in fibrin | Murine NSC (C17.2)+VEGF | Direct inkjet printing | Poor Resolution | Double layer with cells and VEGF | 3×2 mm2 (scaffolds) | pH for collagen, thrombin for fibrin | - | 1×106 cells/ml | ~ 93% | [93] |
| 3D scaffold for CNS | Cells in Matrigel printed on silicone scaffold | iPSC-derived progenitor cells | Extrusion | ~150 μm | Microchannels | ~150 μm width and 5 mm long (channels) | Temperature for Matrigel, moisture for silicone | Modulus ~ 10 MPa | 1×107 cells/ml | > 75% | [15] |
| 3D scaffold for CNS | Cells in Matrigel printed on alginate scaffold | iPSC-derived progenitor cells | Extrusion | ~150 μm | Micro-channels | ~150 μm width and 5 mm long (channels) | Temperature for Matrigel, CaCl2 crosslinking for alginate | Modulus 70-100 kPa | 1×107 cells/ml | > 75% | [15] |
| 3D scaffold for PNS | Fibrinogen, HA, PVA | Schwann cells | Extrusion | ~200 μm | Micro-channels | ~200 μm width and ~14 mm long (channels) | Thrombin | - | 2×105 cells/ml | ~ 98% | [112] |
| 3D scaffold for PNS | Growth factors in GelMa on silicone scaffold | No cells, NGF for sensory path, GDNF for motor path | Extrusion | ~250 μm | bifurcating nerves shape | ~1 mm diameter and ~12 mm length (scaffolds) | UV crosslinking for GelMa, moisture for silicone | Young’s modulus 0.44 MPa | - | - | [17] |
| Seeded cells in 3D printed scaffolds | |||||||||||
| Lattice structure scaffold for CNS | GelMa-DAb) | NSCs | SLA | ~200 μm | Multi-layers lattice | ~10×10×0.8 mm3 (scaffolds) | UV crosslinking | 3×104 cells/ scaffold | [113] | ||
| Lattice structure scaffold for CNS | GelMa/PEGDA | NSCs | SLA | ~200 μm | Multi-layers lattice | ~10×10×0.5 mm3 (scaffolds) | UV crosslinking | Compression modulus ~ 0.45 MPa | 5×103 cells/scaffold | 44.4% | [89] |
| Lattice structure scaffold with electrical stimulator for CNS | PEGDA for scaffold, MWCNT for stimulation | NSCs | SLA | ~200 μm | Multi-layers lattice | ~10×10×0.8 mm3 (scaffolds) | UV crosslinking | Young’s modulus 1.0 MPa | 3×104 cells/scaffold | [106] | |
| 3D scaffold for CNS | GelMa/PEGDA | Neural progenitor cells | SLA | ~200 μm | Micro-channels | ~200 μm width and 2 mm long (channels) | UV crosslinking | Elastic modulus: 260-300 kPa | - | - | [34] |
| Tubular, multi-layer scaffold with electrical stimulator for PNS | PCL/graphene/RGD | Rat Schwann cell | 3D printing and layer-by-layer casting | ~50 μm | Multi-layer Tubular/microchannels | ~15 mm long (scaffolds) | Solvent evaporation | Elastic modulus ~58.63-68.74 MPa | - | > 90% | [69] |
| 3D scaffold for PNS | Collagen | Mesenchymal stem cells | Needle-array assembling (Kenzan Method) | ~500 μm | For implantation, nerve protector (3-mm diameter) was used | ~2 mm diameter and ~3.2 mm length (scaffolds) | Thermal responsive | - | 6×105 cells/ml | - | [114] |
| 3D scaffold for PNS | Silicone | Dorsal root ganglia /Schwann cells | Extrusion | ~250 μm | Micro-channel | ~1 mm diameter and ~12 mm length (scaffolds) | Moisture | Young’s modulus 0.44 MPa | 6×104 cells/ml | - | [17] |
Induced Pluripotent Stem Cells (iPSCs);
DA (Dopamine).
Cell concentrations should be optimized when preparing cell-laden inks. If the concentration is too low, it will be challenging to achieve biologically relevant function. When the concentration is too high, the inks could easily clog the nozzle during printing and will require high shear stresses to be printed, impacting cell viability.[30] As seen in Table 1, the concentrations of the cells in the cell-laden inks are between 106 to 107 cells/ml. For the acellular inks, the printed scaffold must possess strong bioactivity to support cell growth. Diverse methods have been used to modify the bioink materials by mixing with bioactive factors and incorporating enzymatic recognition sites and adhesion factors.[70, 71]
2.2.1. Bioinks (cell or biomolecule encapsulating hydrogels)
Certain criteria need to be met for bioink printing, especially cell-laden printing. First, attention should be given to the required printing pressure and associated shear forces to process the high-viscosity inks to avoid negatively impacting cell viability. Second, the rigidity of the printed structures should be sufficient to retain the desired 3D shape, and this is dependent on the solidification mechanism of various 3D printing technologies, such as UV polymerization, physical/covalent crosslinking, temperature-triggered phase transition, or solvent evaporation.[72–78] Third, the printed 3D constructs should be compatible with neural cells. Since neural stem cells (NSCs) and their progenitors are generally more sensitive and delicate, the mechanical properties of the printed constructs should be carefully considered when preparing the inks and designing the constructs. For example, to promote NSC migration and differentiation into neurons, the elastic moduli of the cell-laden inks was limited to below 1 kPa.[79, 80] Otherwise, the NSCs are more likely to differentiate into glial cells.
In the past few years, tissue engineering has been evolving from 2D to 3D cell culture, which can better mimic the microenvironment of native tissue. To this end, different kinds of naturally derived hydrogels have been used to support 3D cell culture, such as gelatin, collagen, fibrin, hyaluronic acid (HA), and derivatives of natural materials such as alginate, Matrigel, and decellularized extracellular matrix (dECM).[75, 81, 82] These hydrogels can be loaded with various cells, printed into 3D constructs, and gelated (solidified) in different ways. Most natural hydrogels are solidified via physical crosslinking, which can be controlled by temperature, enzymes, or ions. For example, gelatin, collagen, and fibrin are hydrogels derived from natural proteins containing cell-binding domains such as fibronectin, vimentin, vitronectin, and arginylglycylaspartic acid (RGD) peptide motifs, which can promote cell adhesion, differentiation, and growth. Gelatin and collagen hydrogels can be readily crosslinked via thermosensitive gelation.[75] Natural ECM-based hydrogels such as Matrigel and dECM are also thermosensitive hydrogels and provide sufficient cell-binding domains for cell attachment, thereby promoting cellular function.[30] In addition, combining fibrinogen and the enzyme thrombin results in converting fibrinogen to fibrin, which rapidly assembles into a fibrin gel. Properties of these hydrogels are similar to physiological tissues because they are components of the ECM in vivo.[30] However, these hydrogels commonly exhibit too low mechanical strengths to maintain hi-resolution 3D printed structures and low reproducibility in batch-to-batch production. Nevertheless, due to their excellent biocompatibility, these physically crosslinked hydrogels can be directly mixed with living cells or biomolecules and inserted into 3D scaffolds.
Alginate is a derivative of natural materials and an anionic polysaccharide which can be crosslinked using multivalent cations.[76, 83] For example, adding sodium alginate matrix into calcium chloride solution results in the formation of a gel when the sodium ions (Na+) are replaced by calcium ions (Ca2+), and the alginate becomes crosslinked. The viscosity of the hydrogel can be controlled, and the integrity of printed 3D structures maintained. However, unlike other natural hydrogels, alginate does not have sufficient cell-binding domains. To overcome this limitation, RGD peptides have been covalently bonded with alginate to improve cell-adhesion when alginate is used as a cell-laden hydrogel.[84] Similarly, to ensure neuronal differentiation and neurite extension, alginate has been admixed with fibrinogen, which was then cross-linked by a mixture of cross-linking reagents (i.e., chitosan, calcium chloride, thrombin, and genipin).[60]
Synthetic materials are often easier to print into complex 3D constructs with high mechanical performance and structural fidelity. Synthetic hydrogels functionalized with photo-cross-linkable moieties have been actively used as cell- or biomolecule-laden hydrogels for neural regeneration.[72, 73, 85–87] These are covalently crosslinked upon UV irradiation. For instance, gelatin-methacryloyl (GelMa) is a semi-synthetic hydrogel, which consists of gelatin modified with methacrylamide and methacrylate groups. When the GelMa is mixed with a photoinitiator and then exposed to UV light, it changes into a covalently crosslinked hydrogel via photoinitiated radical polymerization.[88, 89] Similarly, poly(ethylene glycol) diacrylate (PEGDA) also has been developed as a crosslinked hydrogel. The covalent network inside these synthetic hydrogels can be designed to enhance the mechanical stability, strength, biocompatibility, absorbability, and usability of scaffolding structures. However, when bioprinting, the toxicity of the free radicals from the photochemical reaction of the photoinitiating system upon absorption of the UV light by the photoinitiator should be considered, since the process can induce the potential for oxidative damage to the printed cells.[63, 90, 91] In addition, the viscosity of the hydrogel (both synthetic and natural) can be tuned by varying the ratio of chemical or biological additives (e.g., thrombin, photoinitiator, and calcium ions, etc.). In some applications, to enhance mechanical properties and cell adhesion, hybrid hydrogel has been incorporated with neural stem cells. For example, biodegradable polyurethane (PU) modified poly(ε-caprolactone) (PCL) hydrogel mixed with soy protein was used as a hybrid bioink.[92] The hydrogels were solidified via thermal-responsive properties at temperatures over 37 ºC.
The hydrogels for the cell-laden inks can be tailored to approach the mechanical properties of the native neural tissues.[75] However, the printed 3D constructs (with cell-laden hydrogel only) lack sufficient strength and stability for long-term cell culture or clinical implantation. To resolve this dichotomy, multi-material hybrid 3D printing of cell-laden and acellular inks has been used to allow the fabrication of 3D scaffolds with anisotropic and gradient mechanical properties.[15]
Apart from cell-encapsulation, neurotrophic factors such as nerve growth factor (NGF), glial cell line‐derived neurotrophic factor (GDNF), neurotrophic factor-3 (NT-3), vascular endothelial growth factor (VEGF), and brain-derived neurotrophic factor (BDNF), encapsulated with hydrogel (i.e., GelMa, polyurethane, and fibrin) have been printed or incorporated to promote the development of neurons and maintain the survival of mature neurons and neurite networks both in vivo and in vitro (also discussed in Section 3).[17, 93, 94]
2.2.2. Acellular inks (scaffold materials)
The goal of the printed scaffold is to recapitulate lost neural circuitry after damage. Hence, the mechanical properties, stability, and immunogenic and inflammatory responses should be considered to meet the requirements of clinical applications.[71] Particularly, for an implantable scaffold construction, a combination of 3D biomedical imaging technology with 3D printing/bioprinting should allow for the reproduction of the precise architecture of injured nervous tissues. To accomplish such a scaffold, the acellular ink materials should possess the proper viscosities to facilitate a high-resolution printing process.
Acellular inks such as thermosetting polymers, which do not need to be compatible with neural cells during 3D printing, can be printed under harsh environments such as high temperatures, toxic solvents, or intense UV light.[73, 77, 95] The benefits of the printed constructs from these inks are that the mechanical performance, thermal properties, hydrophilicity, and degradation rate could be optimized for the various application conditions. In such cases, the designed 3D scaffold structures are usually printed first, and the neural cells are seeded onto the scaffold afterward. Thermoplastic materials such as PCL, poly(lactic acid) (PLA), or poly(lactic-co-glycolic acid) (PLGA) are established synthetic biocompatible and biodegradable polymers used in Food and Drug Administration (FDA) approved devices such as medical implants, drug delivery devices, and tissue constructs.[96] These materials can be printed using FDM, based on the temperature-triggered phase transition.[77, 97, 98] Alternatively, these thermoplastic polymers could also be dissolved in organic solvents and printed using solvent-cast 3D printing technology, whereby fast solvent evaporation induces rapid rigidity, increasing the probability of retaining the designed 3D shapes.[77, 78, 99] In addition, curable thermosetting polymers such as silicone rubber and epoxy have been used for neural regeneration scaffolds.[15, 17] The most common solidification method of such polymers is covalent crosslinking, which can be induced by UV light, temperature, moisture, or catalyst.[72, 73, 86] To enhance cell attachment, these scaffolds could chemically conjugate adhesion RGD peptides to printed scaffolds using standard peptide coupling chemistries.[100] Such modifications allow better control over the cell attachment in the printed scaffolds that more closely mimics nervous tissues.
Both natural and synthetic hydrogels could also comprise acellular inks because covalent networks of these hydrogel structures could enhance mechanical integrity or degradability. Synthetic hydrogels can be printed in complex 3D constructs with high mechanical performance and structural fidelity. For example, synthetic PEGDA and its hybrid exhibited good mechanical properties close to the native spinal cord (~40 kPa) and can be utilized in both SLA and extrusion-based 3D printing approaches.[15, 34] The printed PEGDA generated the precise architecture of injured nervous tissues – a multiple-microchannel scaffold with channel diameter ~200 μm.[15, 34] In some cases, glycerol and methylcellulose were employed into the hydrogels to enhance the printing resolution as needed.[15] To enhance neurite extension, cell adhesion ligands such as arginine–glycine–aspartic acid–serine (RGDS) were covalently attached to PEG hydrogels.[101]
Indeed, cured/crosslinked multi-microchannel scaffolds seeded with cells of a single type have been used for neural regeneration devices.[34] These 3D scaffolds can be readily tuned with the proper size and orientation of the pores, which could promote the neural cell association with the surface and guide cell growth along with the aligned structures. However, if these above-mentioned acellular inks need to be printed during, or immediately after the cell-laden inks are printed, the high temperature, solvent, toxic photoinitiator, or long exposure and intense UV light could affect the viability, proliferation, and/or differentiation of the printed cells. In our own studies, we observed that the free radicals produced by the photoinitiators used to crosslink the PEGDA hydrogels when the scaffold was printed caused human neuronal progenitor cell death, and thus contributed to the low viability observed. We have evaluated three UV sensitive photoinitiators: lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP), 2-hydroxy-1-[4-(2-hydroxyethoxy) phenyl]-2-methyl-1-propanone (Irgacure 2959), and 2,2-Azobis[2-methyl-N- (2-hydroxyethyl) propionamide] (VA-086).[15] It has been demonstrated that low dose near-UV light (365 nm) has not been shown to cause deleterious effects on human mesenchymal stem cell activities,[102] and different cell types react differently to the same photoinitiator.[63] Thus, photochemical effects should not to be equated with free radical effects on cell viability even under low dose UV light. Prior to printing, testing the toxicity of photoinitiators needs to be considered.
The mechanical properties of natural hydrogels (e.g., gelatin, collagen, and fibrin, etc.) are usually poor, and it is difficult to maintain structural integrity long enough for tissue regeneration. However, the alginate-based structure could enhance mechanical properties.[15, 37, 103–105] For example, alginate mixed with methylcellulose (MC) can be printed for 3D scaffolds containing different types of neural cells within 150 μm channels.[15] Indeed, this is the only material that we found would allow printing multiple neural progenitor cell types within a 3D printing assembly. However, controlling the degradation time of these materials needs to be improved for long-term in vivo characterization.
For clinical applications, the development of functional biomaterials in tissue engineering could be critical in preventing a mechanical mismatch with the surrounding tissues, which could further cause tissue damage when the scaffolds are transplanted. The development of new types of soft biodegradable materials has been conducted to mimic nerve tissue (i.e., brain, spinal cord and peripheral nerve) mechanics and tune the biodegradation of the scaffolds, which will, in turn, minimize detrimental effects on cells and axons after implantation into an in vivo model. In addition, to demonstrate the construction of tissue structures, the sizes and shapes of the printed biomaterials should be similar to what would be needed for implantation following a nervous system injury.
Apart from the scaffold materials, conductive inks using diverse materials such as hydrogels, polymers, metal nano- and micro-particles, carbon-based materials (i.e., multiwalled carbon nanotubes [MWCNTs] and graphene, etc.) and liquid metals are being developed and printed as electrodes for bio-electrical neural stimulation and biological neural signal recording.[69, 106–109] These 3D printed electrodes can offer advantages over conventional microfabrication-based devices in several key areas, including: 1) direct fabrication of flexible and transparent sensing and stimulation arrays on biological surfaces; 2) rapid fabrication according to patient-customizable geometries, including 3D and freeform surfaces; and 3) 3D printing of biocompatible and implantable electrodes without any post-annealing process to avoid thermal damage to the target surface.
2.3. 3D imaging
The development of personalized tissue engineering and regenerative strategies aims to create new opportunities to test therapeutic options via reproducing the detailed structural features and functions of native tissues (possibly contributing to solutions for the organ donor shortage).[15, 17, 30, 34, 81, 115–119] A requirement for replicating the cytoarchitecture of functional tissues is a clear understanding of the arrangement and spatial distribution of cellular components. One major advantage of the 3D printing technique is the ability to custom print any desired 3D shape, with or without the addition of precisely positioned cells. The co-development of 3D imaging technologies such as magnetic resonance imaging (MRI), computed tomography (CT), and/or 3D virtual visualization has enabled the acquisition of 3D topological data which allows for the precise reproduction of a 3D object that matches the injury microenvironment of the patient. The accurate reproduction provides a stable and aligned contact between the implanted scaffold and the native nervous system. Therefore, the capacity to fabricate the required geometry and size, with precise internal architecture, renders this approach promising for patient-tailored neural regeneration implants. Hence, 3D printing offers personalized treatments which address specific neurological disease and injury profiles.[17, 34]
The 3D printed patient-specific nervous system scaffolds start by acquiring the anatomical information of the specific nerve injury via 3D imaging (Figure 3a,b).[17, 34, 120] The images are utilized to create a 3D stereolithographic model. From the stereolithographic data, the internal architecture of the 3D model can be further refined using CAD software packages. Then the modified stereolithographic files are sliced into horizontal layers (Slic3r) to create G-code which commands printing pathways. For the living scaffold printing process, multiple dispensing apparati can be controlled by the G-code commands. This 3D printing process allows users to readily custom manufacture any size, shape, and length of an injured nerve and nervous system injury (Figure 3c). Some studies have further quantified the anatomical fidelity by comparing stereolithographic files resulting from taking a scan of the 3D printed models and comparing those to the scans of the original tissue.[120]
Figure 3.
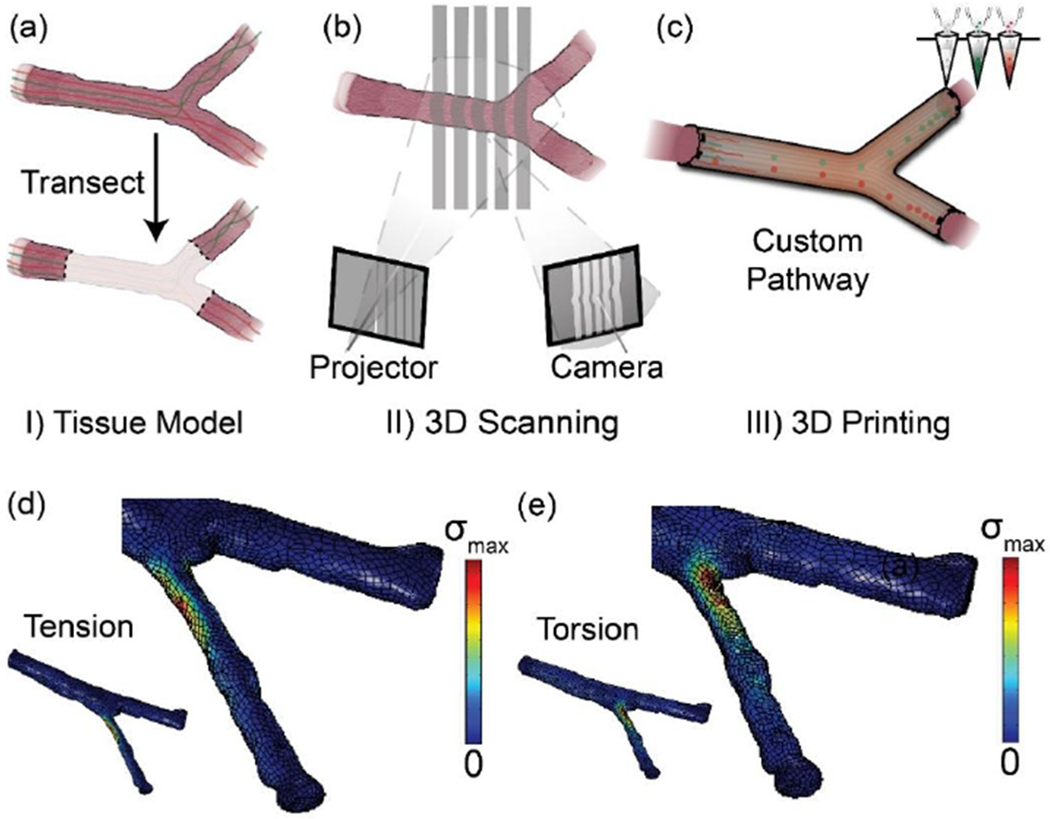
(a)-(c) Personalized nerve guidance pathways via a combination of 3D scanning and 3D printing. The three critical steps are: (a) transection of the nerve tissue model, (b) imaging transected tissue, and (c) 3D printing scaffolds containing path-specific biochemical gradients. (d-e) Computational analysis of the nerve pathways via FEA simulation. Visualizing von Mises stress (σ) distribution in the nerve pathway under both (d) tensile and (e) torsional loading conditions applied to the distal ends of the nerve can be useful in providing an insight to the outcomes from in vivo studies and identifying areas requiring reinforcement. Reproduced with permission.[17] Copyright 2015, Wiley-VCH.
From the stereolithographic files, the mechanical properties of 3D printed scaffolds can be simulated before printing. For example, mechanical simulation tools such as finite element analysis (FEA) have been used for nerve pathway mechanics under both tensile and torsional loading conditions (Figure 3d,e). Such conditions typically occur during surgery and the subsequent regeneration phase.[17] The simulation results can be utilized in identifying areas requiring reinforcement. Hence, the simulation can be helpful not only for determining whether the complex loading conditions of scaffolds could lead to failure in scaffolds but also for determining regions where mechanical deformations are likely. The results could allow the 3D printed model to be optimized to one which provides both an anatomical match as well as enduring strength to survive the harsh conditions of the lengthy regeneration period. Hence, it allows the user to redesign the structure of the printed scaffold, relaxing some of the requirements on the properties of the inks. This is analogous to the incorporation of serpentine and buckled features in electronic skins.[121]
3. Neural cells in the nervous system
The human nervous system is composed of a highly organized and complex cyto-architecture with unique neural populations positioned in specific locations which confer functionality. Despite its complexity, there are only 3 major cell types that arise in the CNS, and all neural cell lineages can be derived from neuronal and glial progenitor cells. The three cell types are neurons, astrocytes, and oligodendrocytes. Neurons integrate and relay information to one another through alterations in their electrical state, whereby their resting electrical potential is perturbed, and they undergo an electrical spike termed an action potential. The electrical charge generated from the action potential travels through long thin extensions called axons and are transmitted to other neurons via branching tree-like structures called axon terminals. Chemical signals in the form of neurotransmitters are released from axon terminals after a neuron has been electrically excited and transmit the chemical signals to the dendrites of other neurons to which they are functionally connected. These functional connections are called synapses and they form the foundation of neuronal networks.
Glial cells refer to astrocytes, oligodendrocytes or microglia and were once thought to play a supportive role to neurons. However, recent evidence suggests that glial cells inhabit dynamic roles in neuronal signal propagation.[122–124] For example, oligodendrocytes wrap neurons in a fatty substance called myelin which increases the conduction velocity of the electrical potential through axons. Alterations in temporal dynamics in neuronal signal transduction from demyelination can inhibit the formation of new neural circuitry, or what is referred to as neuronal plasticity.[125, 126] Furthermore, oligodendrocytes and astrocytes produce growth factors associated with the differentiation, maturation, homeostasis and survival of neurons such as BDNF, NT-3, fibroblast growth factor-2 (FGF-2), GDNF and ciliary neurotrophic factor (CNTF).[126] Astrocytes also play a critical role in the formation and function of synapses directly by regulating neurotransmitter reuptake in the synapse.[123]
The PNS is formed primarily by neural crest stem cells during embryonic development, and a subset of these cells remains in the ganglion post-development that converts from a quiescent state to a more active state after an injury in order to regenerate the damaged tissue.[127] The PNS is also composed of Schwann cells, the peripheral analog of the oligodendrocyte, which are specialized cells that insulate nerve conduction and express genes related to regeneration following injury. These cells begin to proliferate and migrate to the injury site almost immediately after nerve damage.[127] Following migration, Schwann cells align themselves in longitudinal columns called bands of Büngner distal to the basal lamina of the injury, where they produce guides for growing axons and secrete basal lamina components such as laminin, type IV collagen and growth factors.[128] In the PNS, Schwann cells and satellite cells perform analogous roles to oligodendrocytes and astrocytes. Satellite cells support neurons in the ganglia, in a similar fashion to astrocytes in the CNS. These peripheral glial cells also produce many of the same neurotrophic factors that oligodendrocytes and astrocytes produce in the CNS, which are associated with differentiation, maturation, homeostasis and survival in the peripheral nervous system.[125]
After a traumatic injury, the specialized arrangements in the CNS and PNS are perturbed, triggering an inflammatory response which clears away damaged and dead cells. In the PNS, provided the gap for axonal growth is less than 3 cm, it is likely that a successful link from the proximal to distal portion of the injury can be regenerated within a 1-2 week time window without intervention from endogenous pools of neural crest stem cells.[129] However, the adult CNS exhibits little to no regeneration. This is likely due to alterations in the tissue microenvironment, inhibitory factors within the spinal cord including the extracellular matrix and lack of growth factors, limited pools of endogenous stem cell populations and a complex organization of adult structure/function relationships.[130] Therefore, future nervous system regeneration strategies will likely need to be personalized to the lesion of each patient and recapitulate the unique structure and cell identities that were lost.
4. Peripheral nervous system regeneration
The most common surgical repair methodology is either nerve reconstruction by end to end anastomosis or by insertion of nerve grafts.[14, 131] Many of these conventional techniques rely on harvested autologous PNS tissue from the patient or decellularized PNS tissue from allograft sources. However, this standard of treatment can often result in suboptimal outcomes, with patients experiencing extreme dysfunction including the need for additional harvesting surgery, chronic pain and morbidity at the donor site, limitations on graft size and geometry, and potential immune response.[128, 130] Notably, many of the limitations are related to harvesting techniques, customization of the graft tissue or complications from rejection.[5, 132–134] These limitations have inspired alternative graft production methodologies. One is the use of biocompatible conduits or scaffolds as therapeutic options.[134] Previous studies have shown significant advances in the integration of tissue-engineered scaffold conduits to bridge nervous system defects.[14, 135] Indeed, the results from clinical studies, particularly with NeuraGen® (type I collagen scaffold), NeuroMatrixNeuroflex® (type I collagen), NEUROLAC® (poly-DL-lactide-co-caprolactone, PLCL), and NeuroTube® (polyglycolic acid, PGA), are often comparable to autografts in the treatment of lesions.[24, 136] Unfortunately, most of the listed clinical studies found that commercial nerve conduits are not effective for extensive lesions or nerves greater than 3 cm. This limitation is primarily because there is a significant challenge to manufacture patient-specific constructs with clinically relevant size, shape, internal architecture and structural integrity.[137]
To improve the repair of critical gap defects over 3 cm in which successful surgical repair is limited, it has been suggested that intraluminal guidance structures, a bundle of small tubes in a scaffold, might be an option to transplant directly into the lesion cavity.[136, 138–141] An internal lumen architecture guides and promotes specific subtype axon regeneration corresponding to the sensory- and motor-fascicle.[29, 142, 143] The level of complexity can be employed via 3D printing technologies to create defect site-specific molds with internal lumen architectures (i.e., microscale multichannels). Furthermore, the choice of input materials and control over material integration can be tuned to form appropriate scaffolds for peripheral nerve regeneration.
3D printed neural devices should allow the recapitulation of novel complex nerve injuries which are precisely engineered to specific patient anatomies in terms of geometry, mechanics, and biology. In addition, since the elastic modulus of the peripheral nerve tissue is ~100-500 kPa,[66] soft polymer-based scaffolds have the potential to seamlessly biointerface with the endogenous nerve structures. Natural hydrogels such as HA, chitosan, collagen, gelatin, and silk fibroin, have been used for 3D printing peripheral nerve guide scaffolds.[93, 144–147] There is no clear evidence that an implantable scaffold must be biodegradable. However, if it is biodegradable, degradability of scaffolds should complement the rate of nerve regeneration across the nerve gap and then degrade gradually. Too rapid degradation of the scaffold might lead to a detrimental inflammatory reaction, while degradation that is too slow could lead to compression as the tissue expands, or the inability for intercellular communication.[148] Commercial nerve conduits exhibit degradation rates on the order of 3 months to 4 years.[24] For a 10 mm PNS injury, unmyelinated axons cross the gap around the third week. By week 4, myelinated axons are in the middle of the scaffold.[149] Hence, the ideal biodegradation rate of this nerve gap is 3-4 weeks post-implantation. Additionally, the nerve guide scaffold may require nutrient diffusion and inhibit scar tissue infiltration. However, a lack of mechanical support and rapid degradation in vivo limit the use of the natural hydrogel as an implantable scaffold. Similarly, the scaffold-free cell-laden structure could be too weak for nerve implantation; however, it may help in understanding cell-cell or cell-ECM interactions in vitro.[150]
For synthetic hydrogel scaffolds (scaffold-only printed nerve guides), photocurable PEG-based scaffolds have been largely used due to its ready tunability of biochemical, biodegradability, and mechanical properties.[151–154] Evangelista et al. printed both a single channel and multichannel PEGDA scaffold using stereolithography (325 nm wavelength) (Figure 4a,b).[152] To enhance the cell adhesion, PEGDA was conjugated with RGDS before printing. For multichannel scaffolds, the dimensions of ~500 μm channel diameter, ~10 mm length, and ~4 mm outer diameter were used, while for the single channel device the diameter and outer wall thickness were ~1.36 mm and ~2.6 mm, respectively (Figure 4a). Compared to an uninjured nerve (6,080 fibers/mm2), a single lumen (channel) has shown 70% peripheral nerve regeneration (4,492 fibers/mm2) after harvesting at 5 weeks in a rat model of sciatic nerve injury. However, the multichannel scaffold did not show quantifiable axon counts (Figure 4b).
Figure 4.
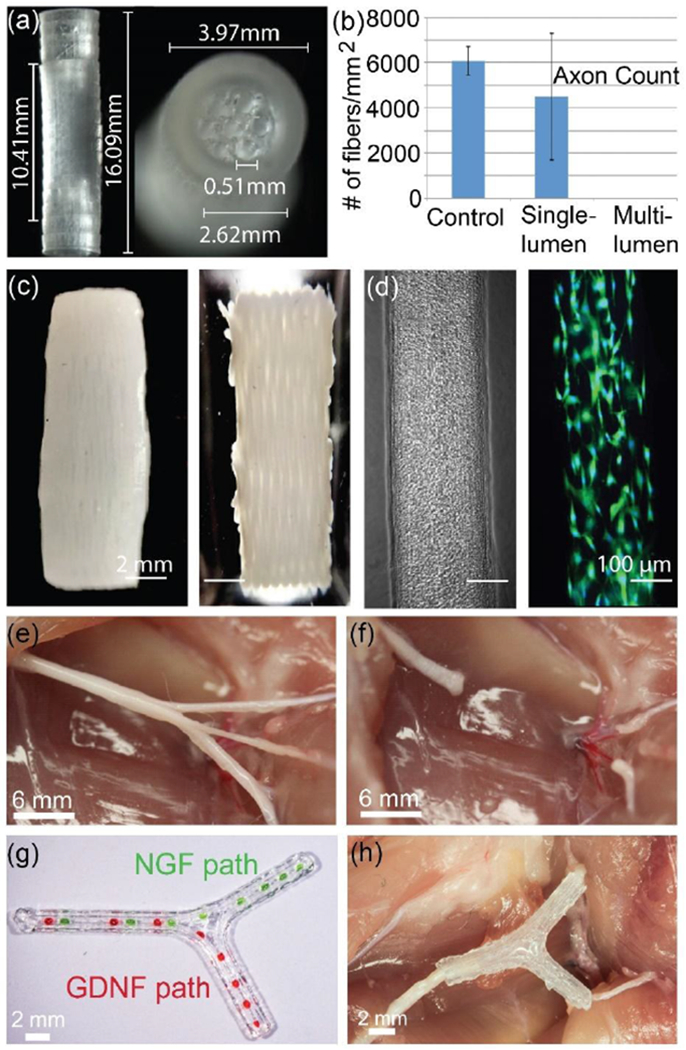
(a) SLA 3D printed PEGDA hydrogel multiple channel nerve guides with cuff for implantation, and (b) their regenerated axon count against a control (uninjured) tissue. Reproduced with permission.[152] Copyright 2015, Thieme Medical Publishers. (c) Fibrin/HA scaffold containing encapsulated Schwann cells via a one-pot printing process. (d) Phase contrast and fluorescence images of printed Schwann cells in a 200 μm width channel. Reproduced with permission.[112] Copyright 2017, Elsevier. (e-h) Extrusion-based 3D printing of a bifurcated silicone nerve guide functionalized with physical cues and path-specific biochemical gradients. (e) Bifurcation pathways in the sciatic nerve which contain branches of sensory and motor nerves, (f) transected nerve pathway, (g) printed NGF gradient for sensory path cues and GDNF gradient for motor path cues in the scaffold, and (h) an implanted 3D printed nerve scaffold. Reproduced with permission.[17] Copyright 2015, Wiley-VCH.
Pateman et al. developed a laser-based micro-stereolithography setup (405 nm laser source) to fabricate a single lumen (channel) PEGDA scaffold.[153] An implantable nerve guide with the dimensions of 1 mm internal diameter, 5 mm length, and a wall thickness of 250 μm was printed. After three weeks, tissue was harvested, and regeneration of axons across a 3 mm injury gap was observed, which was comparable with an autograft control. Interestingly, the approach allows for the fabrication of scaffolds with 50 μm wall thicknesses;[153] however, these scaffold sizes were not suitable for in vivo study due to their delicate nature. In contrast, Zhang et al. have developed a 3D bioprinted scaffold-free nerve construct from human gingiva-derived mesenchymal stem cells (GMSC)-containing collagen that was able to achieve functional recovery in a peripheral nerve injury model when transplanted in vivo.[114] To develop a scaffold-free graft, GMSC spheroid cells were cultured in a 3 mm-diameter Axoguard® nerve protector for 14 days in a facial palsy model before they were transplanted. After 12 weeks, the 3D bioprinted scaffold-free spheroid performed comparably to an autograft in repairing a 5 mm defect in the buccal branch of a rat facial nerve. However, in long nerve gaps, 3D bioprinted scaffolds tend to not perform as well in vivo when compared to their autograft counterparts. For use in longer nerve gaps, mechanical properties of the scaffold should be such as to avoid causing additional compression to the surrounding tissue, nor strain to the regenerating axons. Hence, handling, suturability, stiffness, stability, flexibility, and compressive strength must all be considered for material preparation. Apart from implantation stability, mechanical properties of the PEGDA scaffold influence neurite extension, with a decrease in neurite extension as the PEGDA concentration increases.[101] Therefore, finding optimal conditions for sufficient printing materials is important for PNS regeneration. In addition, at the anatomical level the size of the fascicle varies depending on the type of nerve. The space between fascicles and the outer layer of a single peripheral nerve is called the epineurium and varies between 1 and 100 μm.[155] Thus, printing resolution under 50 μm is of particular interest for neurite guidance and Schwann cell migration, which is strictly linear and guides axons across the injury site in linear arrays with respect to fascicular architecture.[153, 154]
Recent developments in nerve guide scaffolding have changed the concept that scaffolds are merely passive cylindrical structural support devices to those that actively promoted neural outgrowth and axonal regeneration.[156–166] This is enabled via a combination of cell transplants (PNS neurons or Schwann cells), physical guides (scaffold), and biological cues (growth factors). In this regard, 3D printing has been used to fabricate a patient-specific 3D scaffold where biomolecules or cells are embedded in precise positions within the designed matrix to recover sensory and motor functions of nerves.
England et al. extrusion-printed fibrin-based scaffolds containing encapsulated Schwann cells with an initial cell viability of ~ 98% (Figure 4c).[112] To enhance mechanical strength, the fibrinogen-factor XIII ink was reinforced by HA and polyvinyl alcohol (PVA). After seven days in vitro, the printed Schwann cells in hydrogel migrated and formed aligned structures similar to bands of Büngner (Figure 4d).[167] Moreover, after seeding dorsal root ganglia (DRG) neurons, the scaffold sustained cellular growth and provided physical guidance over the alignment of the DRGs (~200 μm width and 14 mm long).[112]
Owens et al. created a multi-lumen cellular scaffold via a multi-material printing approach.[168] Using layers of sacrificial agarose (thermal-sensitive hydrogel) molds and rods, cylindrical nerve conduits of Schwann cell tubes were surrounded by mouse bone marrow stem cells (BMSCs) and multiple lumen channels were formed (~1 cm length, ~2 mm outer diameter, ~500 μm internal lumen diameter). The use of BMSCs allowed the enhancement of Schwann cell adhesions to form isolated Schwann cell rods. For implantation, the graft was surrounded by a collagen layer for reinforcement. After implanting the scaffold into a rat sciatic nerve model for 40 weeks, electrophysiological testing showed recovery of both motor and sensory functions. The regenerative capacity of the scaffold is comparable to that of autologous grafts and commercially available hollow collagen grafts.
Apart from the development of a perfectly cylindrical shape, nerves could have branches, or bifurcations, and tapering. 3D printing can produce such customized complex nerve guides or scaffolds that replicate inherent tissue anatomy. We demonstrated through the combination of 3D scanning and extrusion-based 3D printing, that it was possible to make personalized scaffolds with spatially controlled biochemical growth factors for the regeneration of geometrically and compositionally complex PNS nerve bifurcation pathways in the sciatic nerve (Figure 4e–h).[17] For anatomical accuracy of the nerve, 3D scanning was employed to generate a custom 3D CAD model from a specific nerve injury. Afterward, a silicone-based 3D bifurcating pathway was printed with high fidelity to the tissue model and providing physical cues. 5-7 days post injection of superior cervical ganglion (SCG) neurons into the scaffolds in vitro, the physical cues promoted linear alignment of axons, providing directional neurite outgrowth along the scaffold channels.[17, 150] Schwann cells also aligned along the 3D printed scaffold. Interestingly, the directional neurite outgrowth preferentially followed the printing pathways. This observation suggests that for an implantable scaffold application, a multichannel pathway is better with linear (one direction) printing. Further, path-specific biochemical gradient cues encapsulated with GelMa were placed inside the bifurcating nerve pathway: NGF for sensory path cues and GDNF for motor path cues via the same printing process. In this example, the NGF gradient is intended to attract sensory axons, and the GDNF gradient increases Schwann cell migration within a specific pathway. Since both Schwann cells and DRG cells preferentially follow the RGD peptide and laminin pathways, coating these additives on the surface of a silicone scaffold could be useful to enhance and direct axonal growth.[150, 169, 170] Nevertheless, transplantation of these scaffolds into a 10 mm complex nerve injury in rats demonstrated successfully guided nerve regeneration after 4-6 weeks in vivo, resulting in enhanced functional recovery of the regenerated nerve.[17] Thus, the study suggests that 3D printing may provide a means of regenerating complex nerve injuries, paving the way for the personalized treatment of a wide variety of nerve injuries. Indeed, this concept of combining scanning and printing a 3D subject specific biomimetic nerve guide conduit was recently patented.[171]
Although significant improvements have been made in tissue engineering approaches to address complications of PNS damage, much work remains. For example, there are still no viable approaches to addressing nerve damage in excess of 3 cm. This is due, in part, to the regenerative niche that is responsible for axonal outgrowth in injuries beyond 3 cm.[130] However, drugs have been discovered that slow the closure of the neuronal growth cone, and thus, if these drugs were printed within the nerve conduit, it could be possible to print an enclosed environment that was friendlier to the developmental processes necessary for axonal extension.[39] Such a strategy could even be compatible with a 4D printing approach, where drugs could be temporally released within a nerve conduit to favor axonal extension for a defined period to a distant target.[81, 172] In addition, it is suggested that the ideal scaffold should provide support for the regeneration of various axon subtypes at specific sensory- and motor-specific fascicles.[13, 29] Such phenotype-specific axon regeneration, regionally specific placement, and population specific transplantation is necessary.[142] Thus, peripheral nerve regeneration could involve an all-in-one printing strategy, whereby a scaffold containing various cells and biomolecules with 50 μm thick walls which are strictly linear will guide axons across the injury site in linear arrays that respect fascicular architecture.
5. Central nervous system regeneration
The human CNS is composed of a highly complex cyto-architecture with equally complex functional paradigms. Therefore, improvements in the ability to model aspects of CNS tissue or discrete systems within the CNS have the potential to be of critical importance in a variety of medical conditions. Although animal models and 2D cell culture methodologies with human iPSC-derived neural cells have provided significant insights into developmental biology, disease progression and functional dynamics of CNS networks, much of the inherent complexity in the human CNS is not recapitulated.[173] This is highlighted by the failure rate of pharmaceutical drugs when translated from 2D cell culture models or animal models to human patients.[174] Therefore, it is imperative that future iterations of human CNS models more closely replicate their endogenous counterparts.
5.1. Brain
Advancements in cell technology, such as the homogeneity and specificity of iPSC-derived neural cell types, 3D brain organoids and bioprinting of neural progenitor cells, offer unparalleled platforms to investigate healthy neural dynamics and alterations that lead to disease both in vitro and in vivo. Indeed, Mansour et al. have demonstrated the ability to produce human iPSC-derived brain organoids in vitro that contain multiple cell types and display neuronal functionality more comparable to in vivo physiology as compared to iPSC-derived neuronal cells grown in standard 2D culture conditions. Furthermore, they have transplanted their human brain organoids into the mouse cortex and shown that the graft not only survived, but extended axons to distant targets, exhibited progressive neuronal temporal differentiation patterns, functionally integrated into the neural circuitry of the host and was vascularized by the endogenous tissue.[173] However, the degree of biomimicry of functional 3D brain-like cortical tissue, including the cerebral organoids and physiological functions, remains limited due to the brain’s structural complexity.[43, 175]
Lozano et al. 3D printed (via manual extrusion) brain-like structures as found in the cerebral cortex, with multiple layers of cortical neurons encapsulated with RGD modified gellan gum hydrogel.[111] The encapsulated neurons exhibited a viability of ~80% and could differentiate into neurons and glia. To examine the neurite outgrowth from cortical neurons between adjacent hydrogels, they printed a three-layered structure composed of a middle layer with no cells, sandwiched between bottom and top layers with neurons (Figure 5a,b). After 5 days of culture in vitro, the printed neurons extended axons, and the axons penetrated up to 100 μm into the acellular middle layer (Figure 5c). Although the structure lacked a defined architecture, the ability to control cell and ECM organization could be facilitated to replicate multi-layered brain-like neural circuits and to provide a tool for understanding traumatic brain injuries and neurodegenerative diseases.[111] To construct complex external surface human brain structures, Hinton et al., printed an alginate-based brain model via a combination of the FRESH printing method and MRI images (Figure 5d,e).[55] Complex features of the brain anatomy including the cortex and cerebellum were realized with a resolution of 200 μm. Although the internal structure of the model was not constructed, this study illustrated the potential for building brain tissue with anatomical architecture. In future iterations, it may be necessary to print brain tissue cell subtypes, signaling factor gradients, and vascularization for advanced applications of these more sophisticated brain models.
Figure 5.
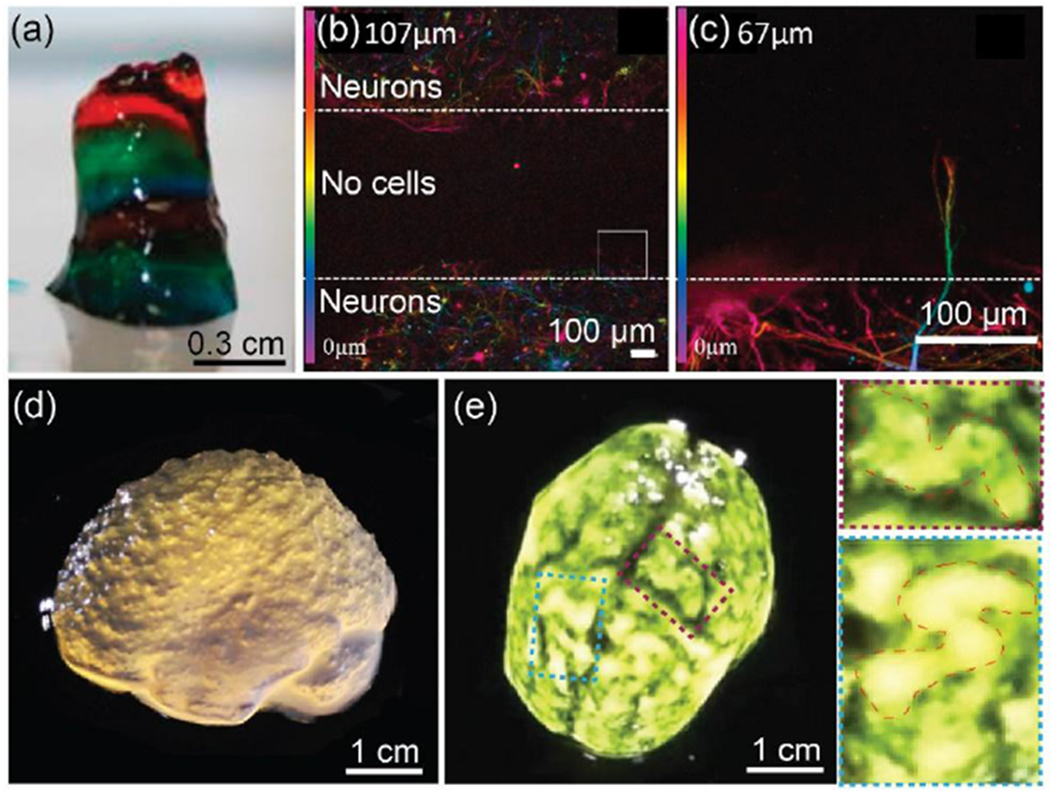
(a-c) 3D printing of brain-like layer structures. (a) Printed brain-like layer structure, each color represents a layer. (b) Confocal microscope image after 5 days of culture. Color bar indicates the depth of the cells along the Z-axis (0 to 107 μm). (c) Close-up images of the square from (b). Color bar indicates the depth of the cells along the Z-axis (0 to 67 μm) of the square. Neurons in hydrogel formed a neuronal network, and the extended axons penetrated into the cell-free middle layer. Reproduced with permission.[111] Copyright 2015, Elsevier. (d-e) 3D printed alginate-based human brain model. (d) Lateral view of the brain model showing microscale anatomical features of the cortex and cerebellum. (e) Top view of the brain model with black dye to enhance visualization of surface folded structures. Two higher resolution regions associated with the folds of the cerebral cortex were outlined. Reproduced with permission.[55] Copyright 2015, American Association for the Advancement of Science.
5.2. Spinal Cord
Following chronic contusion SCI, cell death occurs, resulting in the formation of an astrocytic glial scar around the cavity of the injured tissue which is devoid of function.[176–179] The lesion is caused by direct trauma at the time of injury, leading to a secondary cascade of edema, hemorrhage, inflammation, and events such as lipid peroxidation.[177] SCI lesions exhibit three compartments: the non-neural (stromal) lesion core, astrocyte scar borders and spared neural tissue that is reactive.[176–179] In the stromal lesion core, cellular debris post-injury results in potentially cytotoxic byproducts. An inflammatory response by stromal cells, mesenchymal cells, macrophages and microglial cells clears the cellular debris, and the lesion core becomes comprised of primarily non-neural cells and matrix molecules such as laminins, proteoglycans, collagens and fibronectins. Outside of the lesion core, reactive astrocytes form a several-cell-thick scar border that limits the non-neural lesion core and the adjacent viable neural tissue. The astrocyte scar is relatively continuous with spared but reactive viable neural tissue including microglia, neurons, axons, dendrites, synapses, and oligodendrocytes. The aforementioned reactive astrocyte scar and extracellular matrix proteins in the non-neural lesion core permanently limit cell regeneration, axon extension and neural circuitry reorganization after injury.[179] Notably, several extracellular matrix proteins within the reactive astrocytic glial scar and non-neural stromal core have been implicated in limiting axonal extension and reorganization of spared circuitry. In particular, when matrix proteins such as chondroitin sulfate proteoglycans (CSPGs) have been degraded enzymatically or limited by reducing reactive astrocyte activity within the glial scar in chronic injuries, functional recovery has been observed.[180] Furthermore, regeneration in the CNS is limited by oligodendrocyte and central myelin cell surface inhibitory proteins NI-35/250. Fields et al. have shown that these proteins severely limit transplanted and endogenous axonal outgrowth in both in vitro and in vivo models and also found that these products are produced in the lesion area after damage as myelin breakdown products.[181] The team observed that when oligodendrocyte differentiation, myelin formation or neutralization of NI-35/250 occurred, there was successful regeneration of transected axons over long distances. While there are several potential approaches to limit this secondary damage and improve functional recovery, restoring function after chronic SCI will require strategies to promote targeted axon regeneration, neuronal relay formation, and myelin regeneration.[182–184] Previous attempts have shown significant advances in the integration of tissue-engineered conduits to bridge SCI defects by including (i) cell transplants, (ii) biological cues or glial scar degradation products, and (iii) physical guides (Figure 1).[9, 185–189] Structurally, spinal cord tissue is not homogeneous but contains different neural cell types, arranged with complex spatial distributions.[176, 190–192] Importantly, the regionally specific neuronal subtypes strongly influence axonal growth.[193] Therefore, effectively manufacturing/recreating patient-specific constructs with clinically relevant size, shape, and structural integrity have been advanced by combining neural stem and progenitor cells with 3D printing biocompatible scaffolds to test new therapeutic options for spinal cord injuries.[9, 185–189]
There are two distinct areas where 3D printing is applied to spinal cord scaffolds: (i) cell seeding on printed scaffolds, and (ii) co-printing of cells and scaffolds. Koffler et al. developed a 3D printed hydrogel spinal cord scaffold to support regeneration after SCI via the former approach of cell seeding on a printed scaffold approach.[34] A microscale continuous projection printing technique (μCPP), which is a modified form of stereolithography printing, has been used to build biomimetic hydrogel-based spinal cord scaffolds, made of PEGDA–GelMa (Figure 6a). A single type of neural progenitor cell (NPC) was seeded on to the 2-mm-long scaffold containing 200 μm–diameter multichannels. Previous studies involving scaffolds and nerve regeneration demonstrated that ~200-300 μm diameter microchannel scaffolds were effective in linearly guiding axons.[39, 163] As mentioned earlier, channels larger than ~450 μm in diameter resulted in decreases in nerve regeneration.[40] At 4 weeks post-implantation in rats, the seeded NPCs survived, differentiated, and extended axons throughout the scaffold channels. Importantly, the implanted NPC-derived axons in the scaffold extended into the host spinal cord below the injury site (Figure 6b). The growth of regenerating host axons exhibited a linear pattern, guided by the microchannel architecture of the scaffold. In contrast, empty scaffolds (with no cells) showed only limited host axon growth into scaffolds, and grafting of NPCs (with no scaffolds) extended axons in random orientations. For CNS, a few weeks would potentially allow native OPCs to move in and myelinate the axons. The biodegradability of the synthetic PEGDA-based scaffold allowed observing host axon regeneration and remyelination in an animal at 4 weeks post-implantation. At 6 months post-implantation, the hydrogel scaffolds showed a slow degradation rate (the thickness of the scaffold was reduced by 49%), and channels were still structurally intact and completely filled with NPCs. To determine the functional recovery, locomotor activity was evaluated using the Basso, Beattie, and Bresnahan (BBB) locomotor scale over a 5-month period. Animals implanted with NPC-containing scaffolds showed a compelling functional recovery compared to cell-free scaffolds. At 5 months post-injury, rats with 3D-printed, NPC-filled scaffolds observed recovery of motor evoked potential (MEP) responses, whereas rats with empty scaffolds exhibited baseline noise level, indicating the formation of new ‘neural relays’ across sites of complete spinal cord injury. This 3D printed biomimetic platform could be customized and provided patient-specific spinal cord size and lesion geometry with high anatomical fidelity (when combined with MRI).
Figure 6.
(a) PEGDA/GelMa hydrogel-based spinal cord scaffolds containing 200 μm–diameter multichannels, providing linear alignment of white matter with host axonal tracts in the spinal cord. Grey matter area was printed as a solid. (b) A schematic illustration of the axonal guidance using a 3D printed scaffold. Channels in the scaffold provide linear guidance of rostral-caudal planes, so that grafted cells and host cells can be aligned. Later, host axons in cortico-, rubro-, or reticulo-spinal tracts regenerate into the scaffold and form synaptic connections with grafted neurons inside the channels. The grafted neurons then extend axons, generating the functional connection across the lesion site. Reproduced with permission.[34] Copyright 2019, Nature Publishing Group. (c-e) 3D bioprinted sNPCS and OPCs in a 3D printed scaffold with ~150 μm-diameter channels. (c) Precise spatial distribution of neural cell types in specific channels after 1 day of culture: sNPCs (left), OPCs (middle), and sNPCs and OPCs (right). This allows for the recapitulation of spinal cord architecture with multiple cell types. (d) sNPCs printed in a scaffold after 4 days of culture. (e) Higher resolution image of 3D printed sNPCs in a channel. The sNPCs rapidly differentiated into neurons and extended axons propagating in the designed 3D space. (f) Neurocompatible 3D printed alginate-based scaffolds. The scaffold contained 3 × 3 channels, ~150 μm-diameter channels, and ~1.5 mm × 5 mm sized scaffold. Fluorescence images indicated that bioprinted sNPCs in a 3D printed scaffold were alive 3 days after printing in all three layers. Reproduced with permission.[15] Copyright 2018, Wiley-VCH.
It has recently been demonstrated that the homology of the neural cell types transplanted to the host tissue and their spatial placement is critical to the effective regeneration of specific tracts of the spinal cord.[193] This observation suggests that the generation of specific cell types in specific orthotopic locations may be necessary for optimal regeneration of SCI. In this scenario, loading of cells onto a scaffold (printing cell‐free scaffolds and then seeding them with cells after fabrication) is limited when placing specific neuron subtypes in desired areas, especially onto a microscale multichannel scaffold. To this end, a combination of 3D printing and bioprinting has been proposed to fabricate patient-specific scaffolds where cells and biomolecules are embedded in precise positions within a designed matrix during assembly. This method has the advantage of defining areas where specific neural subtypes should be placed for optimal axonal innervation and connectivity for orthotopic reconstruction of the injured spinal cord.
Using an extrusion process, we have printed a neuro-compatible multichannel scaffold, in which different types of stem-cell derived neural progenitor cells (specifically, spinal neuronal and oligodendrocyte progenitor cells - sNPCs and OPCs) were precisely placed in designated locations (“living” spinal cord model).[15] In this framework, sNPCs are expected to differentiate into regionally specific spinal neurons that subsequently project axons, and OPCs are expected to differentiate into oligodendrocytes that myelinate the axons throughout the scaffold channels, thereby providing a neural relay system across the site of injury. For this design, both sNPCs and OPCs in Matrigel suspensions were bioprinted directly onto biocompatible silicone scaffolds with 150 μm–diameter channels. The precise spatial distribution of cell types in specific channels was demonstrated by separately dispensing sNPCs and OPCs to recapitulate the grey and white matter of the spinal cord (Figure 6c). Both progenitor cells differentiated rapidly and the sNPCs generated axons in the 3D channel space over a period of 4 days (Figure 6d,e). Furthermore, this approach enabled multiple neural cell types to be co-printed in a specific channel - clusters of sNPCs and OPCs were placed with a spatial distribution of ~200 μm within a channel. The printed sNPCs were shown to generate the functional activity of neuronal networks, which is a critical foundation for this therapy. To confirm the functionality of the living scaffold, calcium imaging was performed on sNPCs bioprinted in a silicone scaffold with long axon projections 14 days after printing. In this in vitro test, the neuronal networks responded to high potassium and glutamate, which is indirect evidence of printed sNPCs differentiating into functionally mature neurons.
To mimic the 3D nature of the native spinal cord tissue (architecture and elastic modulus), biodegradable alginate blended with MC has been used for printing multi-layered scaffolds with cells dispersed in channels during manufacture (Figure 6f).[15] Alginate is a polysaccharide-based hydrogel that might lack the protein components needed for cellular adhesion. Enhancing cell attachment with short peptide motifs such as RGD, YIGSR, IKVAV, RNIAEIIKDI, and RYVVLPR could be a useful approach for long term survival. DNA crosslinked hydrogels with stiffnesses ranging from 100 Pa to 30 kPa have also been explored, and it was shown that spinal cord neurons extended more primary dendrites whose lengths were not affected by stiffness, unlike the axons which tended to shorten with increasing stiffness.[194] We have also used PEGDA, a well-researched 3D bioprinting hydrogel with optimal rheological properties, but we found that the photoinitiator used to catalyze the polymerization reaction was detrimental to printed neural cell types.[195] The development of bioinks in the 3D bioprinting field continues to remain a crux, so finding suitable bioinks is an opportunity to significantly advance the field.
In CNS injury repair, many issues remain unresolved regarding cell survival and transplantation techniques. Successful printing of different types of neural progenitor cells and signaling molecules directly onto a specific scaffold channel will enable a multicellular neural tissue engineering approach, where the ability to control the position, growth, and differentiation of transplanted cells will be beneficial in rebuilding damaged tissue. This advancement not only opens the door to new possibilities in investigating the importance of multiple cell identity interactions in vitro in order to model proper arrangement of cell grafts, but even allows for the possibility of generating high-order spatially distributed, organotypically organized cell transplants. When considering that 3D printed scaffolds are inherently 3D structures, it could even be possible to 3D bioprint user-defined structures and culture the structure as an organoid to capture more of the complex cell identities and interactions formed during development.
6. Nervous system on a chip
In vivo studies have been valuable in understanding tissue-level nervous system development and exploring repair strategies.[196] There is also increasing demand to fabricate personalized 3D micro-physiological systems for understanding the development and function of neuronal and glial tissues as well as fundamental and higher order physiological and pathophysiological processes associated with human nerve regeneration and neurological diseases, disorders, and injuries. Therefore, the development of 3D in vitro platforms capable of recapitulating the specificity, complexity, and function of living tissues, specific tissue-like structures, and high order neurophysiological processes could enhance the translatability of neural regeneration treatments and therapeutics, or be utilized for drug testing.[197] Furthermore, 3D in vitro platforms, such as nervous systems-on-a-chip, can have an impact on the study of neurological diseases for which it is difficult to obtain accurate animal models. This could result in broad research implications in fundamental research, drug discovery, and personalized healthcare. Particularly, 3D in vitro platforms have been used to replicate the physiological cell-cell and cell-ECM interactions that could accelerate the development of new therapies.[26, 81, 198–200]
3D printing can offer a novel design and replication of complex tissue-like structures in vitro, at the microscale to full-chip scale, in which multiple specific cells are integrated within a designed matrix.[26, 30, 199, 201–203] An in vitro platform could replicate neurological phenomena, such as cell signaling, cell-cell communication, infection and disease, and regeneration. Hence, such platforms could prove to be valuable and customizable biomanufacturing approaches for realizing complex and specific biomimetic microenvironments for neuroscience studies.
To this end, we previously introduced a customizable 3D printed nervous system-on-a-chip to capture functional dynamics of neural network formation of Schwann cell-axonal interaction and viral uptake using a compartmentalized in vitro model.[26] The approach involved printing microchannels to guide cell interactions and tri-chamber structures for plating distinct cell types: CNS neurons, PNS neurons, Schwann cells, and epithelial cells (Figure 7a). The 3D printed tri-chamber consisted of three layers: (1) microchannels to provide axonal guidance, (2) a sealant layer to protect fluid exchange between chambers, and (3) a top tri-chamber to provide isolation of different cell types and associated media (Figure 7b). Following the chamber printing, cell suspensions were seeded via bioprinting into the individual chamber wells, which were 150 μm in height and 350 μm wide. The models demonstrated that it was possible to direct the growth and assembly of microscale neural features and promote spatial organization of cellular components.
Figure 7.
(a) Schematic of the nervous system showing four primary components: CNS neurons, PNS neurons, Schwann cells, and epithelial cells. (b) 3D printing of tri-chamber consisting of three steps: (I) parallel 350 μm wide channels – providing axonal guidance, (II) a sealant layer – preventing fluidic culture media exchange between the chambers, and (III) a top tri-chamber – providing isolation and organization of specific cell types. (c-e) Biomimetic maturation of 3D printed nervous system on a chip: alignment of axonal networks and spatial organization of cellular components. (c) PNS neurons and axons in chamber 1, (d) Schwann cells in chamber 2, and (e) epithelial cells in chamber 3. (f-h) In vitro model for nervous system viral infection assays: Schwann cells and CNS neurons are resistant to virus infection transmitted from axons (PNS neurons). (f) Infected CNS neurons in chamber 1, (g) infected PNS neurons in chamber 2, and (h) infected Schwann cells in chamber 3 after 10-14 days of culture. Reproduced with permission.[26] Copyright 2016, Royal Society of Chemistry.
As shown in Figure 7c, in order to construct a biomimetic PNS chip model, superior cervical ganglion (SCG) cells were printed in chamber 1. The neurons were cultured for 10-14 days to establish axonal networks throughout each channel. Thus, inter-connection between chambers were only through axonal pathways generated from printed neurons. To provide the inter-connections between axons and cells, Schwann cells and epithelial cells were cultured in chamber 2 and chamber 3, respectively. By characterizing phase contrast micrographs, it was discovered that Schwann cells spontaneously localized to the axon in chamber 2. However, with the absence of axons in the channel, the Schwann cells were randomly distributed (Figure 7d). This observation indicated that self-assembled networks of axon-associated Schwann cells were produced from the model. On the other hand, in chamber 3, axon termini-epithelial cell junctions were formed (Figure 7e).
The capability of integrating these components into lab-on-a-chip style devices could mimic native neural cell interactions. In order to recapitulate the functional interface between axons (CNS neurons) and cells (PNS neurons and Schwann cells), a viral infection assay was applied to the 3D CNS chip model. In the CNS chip model, the CNS system was produced by culturing hippocampal neurons (CNS neurons) in chamber 1 (Figure 7f). The axonal network produced by the CNS neurons penetrated throughout each chamber. Subsequently, in chambers 2 and 3, the PNS system was integrated by culturing PNS neurons cells and Schwann cells, respectively (Figure 7g,h). The chambers were connected via the interpenetrating axonal network that extended from chamber 1, but otherwise isolated. By injecting a virus into chamber 2, the PNS neurons were infected by the virus, and the viral particles spread to both CNS neurons and Schwann cells via axonal transport. The results also showed the alignment of axonal networks and spatial organization of Schwann cells adjacent to the axonal network in chamber 2. The observation of the spread of infection to the CNS neurons and Schwann cells indicated the existence of a potential bottleneck to virus transmission from PNS axons to these cell types. In addition, based on the observation of viral particle transmission through the axon networks, a virus axonal transport rate of 2.0 μm/s was recorded. Indeed, studying viral infection and transport in both PNS and CNS systems is useful for future research in developing treatments for neurological diseases and disorders associated with infectious diseases.[204, 205] Thus, our study suggested that 3D printing can be utilized for the prototyping of microphysiological neural systems to study neurological diseases and disorders and provide emerging therapeutics.
Another application of 3D printing would be to engineer vascular networks and neural networks to form a neurovascular system. Recently, Grasman et al. reported the development of a 3D in vitro vascularized neural tissue model for neurogenesis and neural repair.[198] The model contained an endothelialized microchannel lined by human umbilical vein endothelial cells (HUVECs), acting as a vascular conduit within a 3D collagen hydrogel which served the role of the ECM matrix. In this model, endothelialized microchannels stimulated axonal growth from the DRG and guided the axons toward the channel. Similarly, directed axonal growth was observed when BDNF was localized within the microchannel without HUVECs. Indeed, the model could replicate the complex physiological environment occurring in peripheral nerves after injury, with neural repair observed within 2 weeks. This study shows the potential to recapitulate neural growth and repair via a combinational molding approach.
The complexity of the neural networks could be improved by utilizing 3D printing. We recently demonstrated guided cancer cell HUVECs migration within 3D fibrin matrix via 3D bioprinted cells and 3D printed programmable release capsules.[81] These capsules are comprised of growth factors contained within a GelMa matrix and surrounded by a PLGA shell containing gold nanorods (AuNRs). When a resonant laser wavelength irradiates the printed structure, the photothermal response of AuNRs creates localized heating and leads to rupture of the capsule shell, releasing the contents of the core.[81, 172] This model enables the spatiotemporal control over the generation of signaling growth factor gradients and physical translocation of the cells, subsequently leading to the dynamic mimicking of cellular behaviors in vitro. In addition, the development of more sophisticated vascular networks with neural networks could be made via 3D printing a sacrificial hydrogel ink.[201, 206, 207] Thus, 3D printing has the potential to create a neurovascular system incorporated with specific cells and growth factors, and vascular systems can be 3D printed within the desired 3D matrix. 3D printing techniques could therefore provide flexibility in the design of device architectures to create microenvironmental platforms for in vitro studies of nervous system function, disease modeling, and pharmacology from fundamental science to translational drug discovery.
7. Summary, technical challenges, and outlook
Despite the prevalence of axonal regeneration treatments after neuronal damage, new therapeutic options are needed for both CNS and PNS. As discussed, CNS does not exhibit spontaneous regeneration. Although the PNS can grow axons after injury, when the axonal pathway degenerates, it too has a limited capacity for regrowth. Providing phenotype-specific axon regeneration is a potential therapeutic option for restoring function to damaged axonal tracts after nerve injury for both CNS and PNS. 3D printing has the ability to print specific neural cell subtypes and growth factors in a regionally specific arrangement to mimic the native cytoarchitecture, which can provide a precisely orchestrated re-establishment of neural networks and connections.[12, 193] Hence, 3D printing could provide a means of regenerating complex nerve injuries, paving the way for personalized treatment of a wide variety of nerve injuries. Further, prior to implanting a living scaffold, 3D printed in vitro platforms can be used to test therapeutic options: network formation of specific neural cells under certain conditions, cell-cell and cell-matrix interactions, drug screening, and an understanding of the mechanisms of neuromodulation.
Although significant advances in 3D printed neural regeneration devices and their corresponding applications have been achieved, many obstacles need to be overcome. To date, only a few selected neural cells have been studied. As mentioned, it will likely be necessary to transplant the specific cell types that were lost to confer functional benefit. For example, when cortical neural progenitor cells were transplanted into a spinal cord injury, they survived but failed to successfully integrate. However, when spinal neural progenitor cells were transplanted into a spinal cord injury, they not only survived but matured, extended axons over long distances both rostrally and caudally, and may even have formed functional relays through the lesion area.[208] Further, vascular networks need to be incorporated into the scaffold to provide perfusion of nutrients and diffusible elements. Lastly, finding the optimal combination of biomaterials and cells remains elusive. Careful consideration should be made for the choice and design of biomaterials, based on the following criteria: (i) mechanical properties of biomaterials that attempt to mimic neural tissues; (ii) printability - printing resolutions of less than 50 μm are desired to match with native tissue networks structurally; (iii) integrity, low toxicity, and suitability for multi-layered channel construction; (iv) biodegradation properties; and (v) cell compatibility or containing biological components necessary for neural cell proliferation and adhesion.
Furthermore, many animal models of disease show physiological, biochemical or developmental variations compared to their human equivalents, which impacts the efficacy and safety of treatments developed in these models, sometimes to devastating effect.[209] Protocols to reprogram adult human cells into iPSCs and to directly differentiate iPSCs into distinct neural subtypes offer a virtually unlimited supply of human neural cells which can be used as a platform for testing various cell interactions or treatments both in normal cells and in cells with genetic variants that result in disease.[210–213] It is even possible to derive patient-specific iPSCs for personalized screening of treatments within a discrete cellular system or against the unique genetic constellation of the patient.
As mentioned earlier, regeneration of the CNS is a significant challenge. The development of regionally specific neuronal subtypes could be important components to successful transplantation or disease modeling.[193, 208, 214] The importance of regional specificity is echoed in many endogenous neural structures, but the development of protocols to produce regionally specific cells has been lacking. Therefore, organoid methodologies could be advantageous when modeling disease systems where regional specificity is important. Recent advances in tissue culture techniques have allowed for 3D cell culture methods in which small “organ-like” systems develop in vitro.[43, 173, 175] These 3D methodologies are compatible with human iPSCs and allow investigators to model cell development, disease progression, effects of drug treatments and cell interactions in a system of tissue-specific cells. These 3D cell cultures are even compatible with neural systems and will develop complex networks that more closely replicate human in vivo dynamics. Indeed, these human neural organoids (organoid grafts) can also be printed and transplanted into animal models where they survive, functionally integrate into the endogenous tissue and are vascularized, offering an unprecedented ability to investigate human neurological diseases. Hence, creating 3D bioprinted CNS organoid grafts with a functional vasculature system, which more appropriately mimics complex neural circuitry ,would benefit both in vivo and in vitro applications. Similar attempts have been made to produce in vitro kidney organoids.[215] Alternatively, examining the natural development of defined orthotopic layers and cell populations after bioprinting in the scaffolds, and graft-to-host functional synaptic connectivity within an in vivo physiological tissue environment, may provide a more optimal platform to regenerate lost circuitry.
3D printed neural regeneration devices could also be improved by incorporation with bioelectronics and robotics, which could provide new types of treatment paradigms.[216, 217] Bioelectronics could be used for neural prostheses that allow for the restoration of damaged motor abilities. Despite decades of research, neuromodulatory devices have suffered from signal degradation due to the challenges in determining the optimal geometry to guarantee a stable electrical contact between the electrodes and the 3D conformation of the implantation site. Further, there are often issues with mechanical mismatches between the stimulator and the surrounding tissue.[218, 219] 3D printing technologies could enhance the functionality of the scaffolds by embedding electrodes.
Furthermore, 3D printing can be used to fabricate a conducting composite nerve conduit, in which the whole scaffold is conductive, and incorporate a specific conducting layer or channels within the scaffolds. Conductive biomimetic scaffolds could be designed by modification with conducting particles, polymers, carbon nanotubes, or graphene, which have been used for applications in neural electrodes and interfaces.[220] By incorporating such materials with soft, biocompatible hydrogel matrices in the same 3D printing platform, they can form soft implantable neuroprosthetic scaffolds with an electrically active, conductive surface, that could prevent neural tissue damage. Interfacing conductive scaffolds with transplanted neural cells could be advantageous when investigating optimal stimulation for plasticity or recording neural population dynamics and circuitry. Similarly, the printing of scaffolds with embedded light-emitting diodes could be used for optogenetic stimulation of genetically modified cells for targeted stimulation strategies. However, more information is needed about the toxicity of the conductive inks used in the neural regeneration system, including threshold limits, safe doses, and biocompatibility. For instance, although nanoscale silver particles are now in widespread use in a variety of commercial products, these particles can cause neurotoxicity, reduced neurite outgrowth, inflammation and oxidative stress.[221, 222] Alternative approaches using non-contact stimuli such as light, magnetic fields, or ultrasound could be promising alternatives for neural stimulation devices.[223]
Apart from 3D printed devices which are first printed and then subsequently implanted, a transformative concept would be the direct repair of a nervous system injury via 3D printing on the injured area.[29] Here, the printer is serving as an all-in-one scanner, printer, and robotic surgeon. The disappointing negative results recorded in healing some patients can be partly attributed to the delayed timing of the surgical intervention, especially battlefield and traffic accident sites. Imagine instead if the printer were brought to the site of the accident to repair the injury quickly. This development of in-situ 3D printing is a promising future direction for advanced medical treatments. As a proof of concept, we recently developed a closed-loop feedback system that combines real-time feedback control and direct ink writing of electronic materials and cells using a combination of a delta robot and a computer vision system.[217] This framework allows us to print electrical circuits or living cells directly on moving, freeform, or highly irregular surfaces, including human hands and rat wound beds. With these iterative functions, a 3D printer – possibly combining robotic features and artificial intelligence - could fabricate implantable biomedical devices with the patient’s own cells directly onto the injured area in the nervous system.
Although the development of 3D printed neural regeneration strategies is emerging as a new therapeutic approach for neural diseases and injuries, there is a need for improved understanding of the mechanisms of action and interactions between biomaterials, scaffolds, and neural cells. To this end, more interdisciplinary research from areas of diverse expertise is encouraged to promote optimal repair and functional recovery, which can be translated into effective clinical treatments for patients who sustain neurological diseases and injuries.
Acknowledgements
A.M.P. and M.C.M. acknowledge the Minnesota Spinal Cord Injury and Traumatic Brain Injury Research Grant Program, and the Morton Cure Paralysis Fund. M.C.M. acknowledges the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (Award No. 1DP2EB020537). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also acknowledge Dr. Blake N. Johnson from the Grado Department of Industrial & Systems Engineering at Virginia Tech for his valuable comments and suggestions.
Biographies

Daeha Joung is an Assistant Professor of Physics at Virginia Commonwealth University. Previously, he conducted postdoctoral research in the laboratories of Jeong-Hyun Cho and Michael C. McAlpine at the University of Minnesota (2014-2019). He received a B.S. (2004) in Physics from Soonchunhyang University, an M.S. (2008) in Physics from Eastern Michigan University, and a Ph.D. (2012) in Physics from the University of Central Florida. His primary research interests involve interdisciplinary strategies for forming 3D, heterogeneously integrated, functional biomedical devices.

Ann M. Parr is a Board Certified Neurosurgeon and Associate Professor in the Department of Neurosurgery at the University of Minnesota. Her research centers on transplanting autologous neural stem cells into the injured spinal cord. She has an active translational research laboratory at the UMN Stem Cell Institute, and is interested in mechanisms of recovery using techniques such as histology and immunohistochemistry, optogenetics and animal modeling. Her B.Sc. and M.D. are from Queen’s University, and her Ph.D. and residency were completed at the University of Toronto.

Michael C. McAlpine is the Kuhrmeyer Family Chair Professor of Mechanical Engineering at the University of Minnesota, where he has been since 2015. He received a B.S. in Chemistry with honors from Brown University (2000), and a Ph.D. (2006) in Chemistry from Harvard University. His current research is focused on 3D printing functional materials and devices for biomedical applications, with recent breakthroughs in 3D printed spinal cord implants and 3D printed bionic eyes. He has received several awards for this work, including the Presidential Early Career Award for Scientists and Engineers (PECASE), and the National Institutes of Health Director’s New Innovator Award.
Footnotes
Conflict of Interest
The corresponding author (M. C. McAlpine) holds a patent on this technology: M. C. McAlpine, B. N. Johnson. “Method of Producing a 3D Subject Specific Biomimetic Nerve Conduit.” U.S. Patent 10,405,963 issued September 10, 2019.
Contributor Information
Daeha Joung, Department of Mechanical Engineering, University of Minnesota, Minneapolis, MN 55455, USA; Department of Physics, Virginia Commonwealth University, Richmond, VA 23284, USA.
Nicolas S. Lavoie, Department of Neurosurgery, Stem Cell Institute, University of Minnesota, Minneapolis, MN 55455, USA
Shuang-Zhuang Guo, Department of Mechanical Engineering, University of Minnesota, Minneapolis, MN 55455, USA; School of Materials Science and Engineering, Sun Yat-sen University, Guangzhou 510275, China.
Sung Hyun Park, Department of Mechanical Engineering, University of Minnesota, Minneapolis, MN 55455, USA.
Ann M. Parr, Department of Neurosurgery, Stem Cell Institute, University of Minnesota, Minneapolis, MN 55455, USA
Michael C. McAlpine, Department of Mechanical Engineering, University of Minnesota, Minneapolis, MN 55455, USA
References
- [1].Bruns J Jr, Hauser WA, Epilepsia 2003, 44, 2. [DOI] [PubMed] [Google Scholar]
- [2].Grinsell D, Keating CP, BioMed Res. Int. 2014, 2014, 698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Citron M, Nat. Rev. Drug Discov. 2010, 9, 387. [DOI] [PubMed] [Google Scholar]
- [4].Davie CA, Br. Med. Bull. 2008, 86, 109. [DOI] [PubMed] [Google Scholar]
- [5].Schlosshauer B, Dreesmann L, Schaller H-E, Sinis N, Neurosurgery 2006, 59, 740. [DOI] [PubMed] [Google Scholar]
- [6].Guenard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P, J. Neurosci. 1992, 12, 3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fine EG, Decosterd I, Papaloïzos M, Zurn AD, Aebischer P, Eur. J. Neurosci. 2002, 15, 589. [DOI] [PubMed] [Google Scholar]
- [8].Popovich PG, Cell 2012, 150, 1105. [DOI] [PubMed] [Google Scholar]
- [9].Tam RY, Fuehrmann T, Mitrousis N, Shoichet MS, Neuropsychopharmacology 2014, 39, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].De Laporte L, Yang Y, Zelivyanskaya ML, Cummings BJ, Anderson AJ, Shea LD, Mol. Ther. 2009, 17, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Carballo-Molina OA, Velasco I, Front. Cell. Neurosci. 2015, 9, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Struzyna LA, Harris JP, Katiyar KS, Chen HI, Cullen DK, Neural Regen. Res. 2015, 10, 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Struzyna LA, Katiyar K, Cullen DK, Curr. Opin. Solid State Mater. Sci. 2014, 18, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Faroni A, Mobasseri SA, Kingham PJ, Reid AJ, Adv. Drug Deliv. Rev. 2015, 82, 160. [DOI] [PubMed] [Google Scholar]
- [15].Joung D, Truong V, Neitzke CC, Guo S-Z, Walsh PJ, Monat JR, Meng F, Park SH, Dutton JR, Parr AM, McAlpine MC, Adv. Funct. Mater. 2018, 28, 1801850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kadoya K, Lu P, Nguyen K, Lee-Kubli C, Kumamaru H, Yao L, Knackert J, Poplawski G, Dulin JN, Strobl H, Takashima Y, Biane J, Conner J, Zhang SC, Tuszynski MH, Nat. Med. 2016, 22, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Johnson BN, Lancaster KZ, Zhen G, He J, Gupta MK, Kong YL, Engel EA, Krick KD, Ju A, Meng F, Enquist LW, Jia X, McAlpine MC, Adv. Funct. Mater. 2015, 25, 6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ladd C, So J-H, Muth J, Dickey MD, Adv. Mater. 2013, 25, 5081. [DOI] [PubMed] [Google Scholar]
- [19].Tabatabai A, Fassler A, Usiak C, Majidi C, Langmuir 2013, 29, 6194. [DOI] [PubMed] [Google Scholar]
- [20].Cao T, Ho K-H, Teoh S-H, Tissue Eng. 2003, 9, 103. [DOI] [PubMed] [Google Scholar]
- [21].Hollister SJ, Nat. Mater. 2005, 4, 518. [DOI] [PubMed] [Google Scholar]
- [22].Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJA, Tissue Eng. 2007, 13, 1905. [DOI] [PubMed] [Google Scholar]
- [23].Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR, Trends Biotechnol. 2003, 21, 157. [DOI] [PubMed] [Google Scholar]
- [24].Nectow AR, Marra KG, Kaplan DL, Tissue Eng. Part B Rev. 2012, 18, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJA, Groll J, Hutmacher DW, Adv. Mater. 2013, 25, 5011. [DOI] [PubMed] [Google Scholar]
- [26].Johnson BN, Lancaster KZ, Hogue IB, Meng F, Kong YL, Enquist LW, McAlpine MC, Lab Chip 2016, 16, 1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moroni L, Boland T, Burdick JA, De Maria C, Derby B, Forgacs G, Groll J, Li Q, Malda J, Mironov VA, Mota C, Nakamura M, Shu W, Takeuchi S, Woodfield TBF, Xu T, Yoo JJ, Vozzi G, Trends Biotechnol. 2018, 36, 384. [DOI] [PubMed] [Google Scholar]
- [28].Ozbolat IT, Peng W, Ozbolat V, Drug Discov. Today 2016, 21, 1257. [DOI] [PubMed] [Google Scholar]
- [29].Dixon AR, Jariwala SH, Bilis Z, Loverde JR, Pasquina PF, Alvarez LM, Biomaterials 2018, 186, 44. [DOI] [PubMed] [Google Scholar]
- [30].Murphy SV, Atala A, Nat. Biotechnol. 2014, 32, 773. [DOI] [PubMed] [Google Scholar]
- [31].Pearce JM, Science 2012, 337, 1303. [DOI] [PubMed] [Google Scholar]
- [32].Klein GT, Lu Y, Wang MY, World Neurosurg. 2013, 80, 233. [DOI] [PubMed] [Google Scholar]
- [33].Yoshii S, Oka M, Shima M, Taniguchi A, Akagi M, J. Biomed. Mater. Res. A 2003, 67, 467. [DOI] [PubMed] [Google Scholar]
- [34].Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, Graham L, Lu P, Sakamoto J, Marsala M, Chen S, Tuszynski MH, Nat. Med. 2019, 25, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yan L, Yao Z, Lin T, Zhu Q, Qi J, Gu L, Fang J, Zhou X, Liu X, Neuroreport 2017, 28, 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pawar K, Mueller R, Caioni M, Prang P, Bogdahn U, Kunz W, Weidner N, Acta Biomater. 2011, 7, 2826. [DOI] [PubMed] [Google Scholar]
- [37].Günther MI, Weidner N, Müller R, Blesch A, Acta Biomater. 2015, 27, 140. [DOI] [PubMed] [Google Scholar]
- [38].Stokols S, Sakamoto J, Breckon C, Holt T, Weiss J, Tuszynski MH, Tissue Eng. 2006, 12, 2777. [DOI] [PubMed] [Google Scholar]
- [39].Pawelec KM, Koffler J, Shahriari D, Galvan A, Tuszynski MH, Sakamoto J, Biomed. Mater. 2018, 13, 044104. [DOI] [PubMed] [Google Scholar]
- [40].Krych AJ, Rooney GE, Chen B, Schermerhorn TC, Ameenuddin S, Gross L, Moore MJ, Currier BL, Spinner RJ, Friedman JA, Yaszemski MJ, Windebank AJ, Acta Biomater. 2009, 5, 2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Winter CC, Katiyar KS, Hernandez NS, Song YJ, Struzyna LA, Harris JP, Cullen DK, Acta Biomater. 2016, 38, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Domingos M, Intranuovo F, Russo T, De Santis R, Gloria A, Ambrosio L, Ciurana J, Bartolo P, Biofabrication 2013, 5, 045004. [DOI] [PubMed] [Google Scholar]
- [43].Tang-Schomer MD, White JD, Tien LW, Schmitt LI, Valentin TM, Graziano DJ, Hopkins AM, Omenetto FG, Haydon PG, Kaplan DL, Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kokai LE, Lin YC, Oyster NM, Marra KG, Acta Biomater. 2009, 5, 2540. [DOI] [PubMed] [Google Scholar]
- [45].De Bartolo L, Rende M, Morelli S, Giusi G, Salerno S, Piscioneri A, Gordano A, Di Vito A, Canonaco M, Drioli E, J. Membr. Sci. 2008, 325, 139. [Google Scholar]
- [46].Chung TW, Liu DZ, Wang SY, Wang SS, Biomaterials 2003, 24, 4655. [DOI] [PubMed] [Google Scholar]
- [47].Khorasani MT, Mirzadeh H, Irani S, Radiat. Phys. Chem. 2008, 77, 280. [Google Scholar]
- [48].Lee SJ, Khang G, Lee YM, Lee HB, J. Colloid Interface Sci. 2003, 259, 228. [DOI] [PubMed] [Google Scholar]
- [49].Park JA, Yoon S, Kwon J, Kim YK, Now H, Kim YK, Kim W-J, Yoo J-Y, Jung S, Sci. Rep. 2017, 7, 14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Odde DJ, Renn MJ, Biotechnol. Bioeng. 2000, 67, 312. [DOI] [PubMed] [Google Scholar]
- [51].Calvert P, Chem. Mater. 2001, 13, 3299. [Google Scholar]
- [52].Raney JR, Lewis JA, MRS Bull. 2015, 40, 943. [Google Scholar]
- [53].Xu T, Zhao W, Zhu J-M, Albanna MZ, Yoo JJ, Atala A, Biomaterials 2013, 34, 130. [DOI] [PubMed] [Google Scholar]
- [54].Lewis JA, Adv. Funct. Mater. 2006, 16, 2193. [Google Scholar]
- [55].Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR, Feinberg AW, Sci. Adv. 2015, 1, e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].De la Vega L, Rosas Gómez DA, Abelseth E, Abelseth L, Allisson da Silva V, Willerth SM, Appl. Sci. 2018, 8, 2414. [Google Scholar]
- [57].Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G, Biofabrication 2010, 2, 022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Marga F, Jakab K, Khatiwala C, Shepherd B, Dorfman S, Hubbard B, Colbert S, Forgacs G, Biofabrication 2012, 4, 022001. [DOI] [PubMed] [Google Scholar]
- [59].Norotte C, Marga FS, Niklason LE, Forgacs G, Biomaterials 2009, 30, 5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Abelseth E, Abelseth L, De la Vega L, Beyer ST, Wadsworth SJ, Willerth SM, ACS Biomater. Sci. Eng. 2019, 5, 234. [DOI] [PubMed] [Google Scholar]
- [61].Wang M, Zhai P, Chen X, Schreyer DJ, Sun X, Cui F, Tissue Eng. Part B Rev. 2011, 17, 177. [DOI] [PubMed] [Google Scholar]
- [62].Thomas M, Willerth SM, Front. Bioeng. Biotechnol. 2017, 5, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH, Biomaterials 2005, 26, 1211. [DOI] [PubMed] [Google Scholar]
- [64].Hopkins AM, DeSimone E, Chwalek K, Kaplan DL, Prog. Neurobiol. 2015, 125, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Levental I, Georges PC, Janmey PA, Soft Matter 2007, 3, 299. [DOI] [PubMed] [Google Scholar]
- [66].Borschel GH, Kia KF, Kuzon WM Jr, Dennis RG, J. Surg. Res. 2003, 114, 133. [DOI] [PubMed] [Google Scholar]
- [67].Zhuang P, Sun AX, An J, Chua CK, Chew SY, Biomaterials 2018, 154, 113. [DOI] [PubMed] [Google Scholar]
- [68].Gu Q, Tomaskovic-Crook E, Wallace GG, Crook JM, Adv. Healthcare Mater. 2017, 6, 1700175. [DOI] [PubMed] [Google Scholar]
- [69].Qian Y, Zhao X, Han Q, Chen W, Li H, Yuan W, Nat. Commun. 2018, 9, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mitchell AC, Briquez PS, Hubbell JA, Cochran JR, Acta Biomater. 2016, 30, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ahadian S, Sadeghian RB, Salehi S, Ostrovidov S, Bae H, Ramalingam M, Khademhosseini A, Bioconjugate Chem. 2015, 26, 1984. [DOI] [PubMed] [Google Scholar]
- [72].Hegde M, Meenakshisundaram V, Chartrain N, Sekhar S, Tafti D, Williams CB, Long TE, Adv. Mater. 2017, 29, 1701240. [DOI] [PubMed] [Google Scholar]
- [73].Miao S, Cui H, Nowicki M, Xia L, Zhou X, Lee S-J, Zhu W, Sarkar K, Zhang Z, Zhang LG, Adv. Biosys. 2018, 2, 1800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA, Sci. Rep. 2016, 6, 34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Cui H, Nowicki M, Fisher JP, Zhang LG, Adv. Healthcare Mater. 2017, 6, 1601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pi Q, Maharjan S, Yan X, Liu X, Singh B, van Genderen AM, Robledo-Padilla F, Parra-Saldivar R, Hu N, Jia W, Xu C, Kang J, Hassan S, Cheng H, Hou X, Khademhosseini A, Zhang YS, Adv. Mater. 2018, 30, 1706913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Guo S-Z, Gosselin F, Guerin N, Lanouette AM, Heuzey MC, Therriault D, Small 2013, 9, 4118. [DOI] [PubMed] [Google Scholar]
- [78].Guo S-Z, Heuzey MC, Therriault D, Langmuir 2014, 30, 1142. [DOI] [PubMed] [Google Scholar]
- [79].Kraehenbuehl TP, Langer R, Ferreira LS, Nat. Methods 2011, 8, 731. [DOI] [PubMed] [Google Scholar]
- [80].Her GJ, Wu H-C, Chen M-H, Chen M-Y, Chang S-C, Wang T-W, Acta Biomater. 2013, 9, 5170. [DOI] [PubMed] [Google Scholar]
- [81].Meng F, Meyer CM, Joung D, Vallera DA, McAlpine MC, Panoskaltsis-Mortari A, Adv. Mater. 2019, 31, 1806899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Haring AP, Thompson EG, Tong Y, Laheri S, Cesewski E, Sontheimer H, Johnson BN, Biofabrication 2019, 11, 025009. [DOI] [PubMed] [Google Scholar]
- [83].Hong S, Sycks D, Chan HF, Lin S, Lopez GP, Guilak F, Leong KW, Zhao X, Adv. Mater. 2015, 27, 4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rowley JA, Madlambayan G, Mooney DJ, Biomaterials 1999, 20, 45. [DOI] [PubMed] [Google Scholar]
- [85].Bertassoni LE, Cardoso JC, Manoharan V, Cristino AL, Bhise NS, Araujo WA, Zorlutuna P, Vrana NE, Ghaemmaghami AM, Dokmeci MR, Khademhosseini A, Biofabrication 2014, 6, 024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, Greenfield PT, Calafat NJ, Gounley JP, Ta AH, Johansson F, Randles A, Rosenkrantz JE, Louis-Rosenberg JD, Galie PA, Stevens KR, Miller JS, Science 2019, 364, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hribar KC, Soman P, Warner J, Chung P, Chen S, Lab Chip 2014, 14, 268. [DOI] [PubMed] [Google Scholar]
- [88].Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA, Adv. Mater. 2014, 26, 3124. [DOI] [PubMed] [Google Scholar]
- [89].Zhu W, George JK, Sorger VJ, Zhang LG, Biofabrication 2017, 9, 025002. [DOI] [PubMed] [Google Scholar]
- [90].Lampe KJ, Namba RM, Silverman TR, Bjugstad KB, Mahoney MJ, Biotechnol. Bioeng. 2009, 103, 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yao H, Wang J, Mi S, Polymers 2018, 10, 11. [Google Scholar]
- [92].Lin H-H, Hsieh F-Y, Tseng C-S, Hsu S.-h., J. Mater. Chem. B 2016, 4, 6694. [DOI] [PubMed] [Google Scholar]
- [93].Lee YB, Polio S, Lee W, Dai G, Menon L, Carroll RS, Yoo SS, Exp. Neurol. 2010, 223, 645. [DOI] [PubMed] [Google Scholar]
- [94].Hsieh F-Y, Lin H-H, Hsu S.-h., Biomaterials 2015, 71, 48. [DOI] [PubMed] [Google Scholar]
- [95].Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A, Nat. Biotechnol. 2016, 34, 312. [DOI] [PubMed] [Google Scholar]
- [96].Murphy SV, Skardal A, Atala A, J. Biomed. Mater. Res. A 2013, 101, 272. [DOI] [PubMed] [Google Scholar]
- [97].He Y, Wildman RD, Tuck CJ, Christie SDR, Edmondson S, Sci. Rep. 2016, 6, 20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hutmacher DW, Schantz T, Zein I, Ng KW, Teoh SH, Tan KC, J. Biomed. Mater. Res. 2001, 55, 203. [DOI] [PubMed] [Google Scholar]
- [99].Farahani RD, Dubé M, Adv. Eng. Mater. 2018, 20, 1700539. [Google Scholar]
- [100].Zheng W, Wang Z, Song L, Zhao Q, Zhang J, Li D, Wang S, Han J, Zheng X-L, Yang Z, Kong D, Biomaterials 2012, 33, 2880. [DOI] [PubMed] [Google Scholar]
- [101].Gunn JW, Turner SD, Mann BK, J. Biomed. Mater. Res. A 2005, 72, 91. [DOI] [PubMed] [Google Scholar]
- [102].Ruskowitz ER, DeForest CA, ACS Biomater. Sci. Eng. 2019, 5, 2111. [DOI] [PubMed] [Google Scholar]
- [103].Schütz K, Placht AM, Paul B, Brüggemeier S, Gelinsky M, Lode A, J. Tissue Eng. Regen. Med. 2017, 11, 1574. [DOI] [PubMed] [Google Scholar]
- [104].Atala A, Yoo JJ, Essentials of 3D biofabrication and translation, Elsevier, Amsterdam, Netherlands, 2015. [Google Scholar]
- [105].Li H, Tan YJ, Leong KF, Li L, ACS Appl. Mater. Interfaces 2017, 9, 20086. [DOI] [PubMed] [Google Scholar]
- [106].Lee SJ, Zhu W, Nowicki M, Lee G, Heo DN, Kim J, Zuo YY, Zhang LG, J. Neural Eng. 2018, 15, 016018. [DOI] [PubMed] [Google Scholar]
- [107].Yang C, Cao Q, Puthongkham P, Lee ST, Ganesana M, Lavrik NV, Venton BJ, Angew. Chem. Int. Ed. 2018, 57, 14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Velcescu A, Lindley A, Cursio C, Krachunov S, Beach C, Brown CA, Jones AKP, Casson AJ, Sensors 2019, 19, 1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Salvo P, Raedt R, Carrette E, Schaubroeck D, Vanfleteren J, Cardon L, Sens. Actuators A Phys. 2012, 174, 96. [Google Scholar]
- [110].Gu Q, Tomaskovic-Crook E, Lozano R, Chen Y, Kapsa RM, Zhou Q, Wallace GG, Crook JM, Adv. Healthcare Mater. 2016, 5, 1429. [DOI] [PubMed] [Google Scholar]
- [111].Lozano R, Stevens L, Thompson BC, Gilmore KJ, Gorkin III R, Stewart EM, in het Panhuis M, Romero-Ortega M, Wallace GG, Biomaterials 2015, 67, 264. [DOI] [PubMed] [Google Scholar]
- [112].England S, Rajaram A, Schreyer DJ, Chen X, Bioprinting 2017, 5, 1. [Google Scholar]
- [113].Zhou X, Cui H, Nowicki M, Miao S, Lee SJ, Masood F, Harris BT, Zhang LG, ACS Appl. Mater. Interfaces 2018, 10, 8993. [DOI] [PubMed] [Google Scholar]
- [114].Zhang Q, Nguyen PD, Shi S, Burrell JC, Cullen DK, Le AD, Sci Rep 2018, 8, 6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Clayton TA, Lindon JC, Cloarec O, Antti H, Charuel C, Hanton G, Provost J-P, Le Net J-L, Baker D, Walley RJ, Everett JR, Nicholson JK, Nature 2006, 440, 1073. [DOI] [PubMed] [Google Scholar]
- [116].Rengier F, Mehndiratta A, Von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor H-U, Giesel FL, Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335. [DOI] [PubMed] [Google Scholar]
- [117].Koh A, Kang D, Xue Y, Lee S, Pielak RM, Kim J, Hwang T, Min S, Banks A, Bastien P, Manco MC, Wang L, Ammann KR, Jang K-I, Won P, Han S, Ghaffari R, Paik U, Slepian MJ, Balooch G, Huang Y, Rogers JA, Sci. Transl. Med. 2016, 8, 366ra165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kong YL, Gupta MK, Johnson BN, McAlpine MC, Nano Today 2016, 11, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Johnson BN, McAlpine MC, Biochemist. 2016, 38, 28. [Google Scholar]
- [120].Qiu K, Zhao Z, Haghiashtiani G, Guo S-Z, He M, Su R, Zhu Z, Bhuiyan DB, Murugan P, Meng F, Park SH, Chu CC, Ogle BM, Saltzman DA, Konety BR, Sweet RM, McAlpine MC, Adv. Mater. Technol. 2018, 3, 1700235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kim D-H, Lu N, Ma R, Kim Y-S, Kim R-H, Wang S, Wu J, Won SM, Tao H, Islam A, Yu KJ, Kim T.-i., Chowdhury R, Ying M, Xu L, Li M, Chung H-J, Keum H, McCormick M, Liu P, Zhang Y-W, Omenetto FG, Huang Y, Coleman T, Rogers JA, Science 2011, 333, 838. [DOI] [PubMed] [Google Scholar]
- [122].Perea G, Araque A, Cell Calcium 2005, 38, 375. [DOI] [PubMed] [Google Scholar]
- [123].Perea G, Navarrete M, Araque A, Trends Neurosci. 2009, 32, 421. [DOI] [PubMed] [Google Scholar]
- [124].Santello M, Calì C, Bezzi P, Adv. Exp. Med. Biol. 2012, 970, 307. [DOI] [PubMed] [Google Scholar]
- [125].Monje M, Annu. Rev. Neurosci. 2018, 41, 61. [DOI] [PubMed] [Google Scholar]
- [126].Du Y, Dreyfus CF, J. Neurosci. Res. 2002, 68, 647. [DOI] [PubMed] [Google Scholar]
- [127].Chen Z-L, Yu W-M, Strickland S, Annu. Rev. Neurosci. 2007, 30, 209. [DOI] [PubMed] [Google Scholar]
- [128].Huebner EA, Strittmatter SM, Results Probl. Cell Differ. 2009, 48, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Stemple DL, Anderson DJ, Cell 1992, 71, 973. [DOI] [PubMed] [Google Scholar]
- [130].Wang EW, Zhang J, Huang JH, Neural Regen. Res. 2015, 10, 1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Millesi H, Acta Neurochir. Suppl. 2007, 100, 37. [DOI] [PubMed] [Google Scholar]
- [132].Evans GRD, Anat. Rec. 2001, 263, 396. [DOI] [PubMed] [Google Scholar]
- [133].Ray WZ, Mackinnon SE, Exp. Neurol. 2010, 223, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kehoe S, Zhang XF, Boyd D, Injury 2012, 43, 553. [DOI] [PubMed] [Google Scholar]
- [135].Wang P-H, Tseng I-L, Hsu S.-h., J. Med. Biol. Eng. 2011, 31, 151. [Google Scholar]
- [136].Petcu EB, Midha R, McColl E, Popa-Wagner A, Chirila TV, Dalton PD, Biofabrication 2018, 10, 032001. [DOI] [PubMed] [Google Scholar]
- [137].Pabari A, Lloyd-Hughes H, Seifalian AM, Mosahebi A, Plast. Reconstr. Surg. 2014, 133, 1420. [DOI] [PubMed] [Google Scholar]
- [138].Li J, Rickett TA, Shi R, Langmuir 2008, 25, 1813. [DOI] [PubMed] [Google Scholar]
- [139].Toba T, Nakamura T, Lynn AK, Matsumoto K, Fukuda S, Yoshitani M, Hori Y, Shimizu Y, Int. J. Artif. Organs 2002, 25, 230. [DOI] [PubMed] [Google Scholar]
- [140].Matsumoto K, Ohnishi K, Kiyotani T, Sekine T, Ueda H, Nakamura T, Endo K, Shimizu Y, Brain Res. 2000, 868, 315. [DOI] [PubMed] [Google Scholar]
- [141].Koh HS, Yong T, Teo WE, Chan CK, Puhaindran ME, Tan TC, Lim A, Lim BH, Ramakrishna S, J. Neural Eng. 2010, 7, 046003. [DOI] [PubMed] [Google Scholar]
- [142].Höke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM, J. Neurosci. 2006, 26, 9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, Wright M, Vyas A, Höke A, Exp. Neurol. 2013, 247, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Jansen K, Van Der Werff JFA, Van Wachem PB, Nicolai JPA, De Leij LFMH, Van Luyn MJA, Biomaterials 2004, 25, 483. [DOI] [PubMed] [Google Scholar]
- [145].Alluin O, Wittmann C, Marqueste T, Chabas J-F, Garcia S, Lavaut M-N, Guinard D, Feron F, Decherchi P, Biomaterials 2009, 30, 363. [DOI] [PubMed] [Google Scholar]
- [146].Chen Y-S, Chang J-Y, Cheng C-Y, Tsai F-J, Yao C-H, Liu B-S, Biomaterials 2005, 26, 3911. [DOI] [PubMed] [Google Scholar]
- [147].Yang Y, Ding F, Wu J, Hu W, Liu W, Liu J, Gu X, Biomaterials 2007, 28, 5526. [DOI] [PubMed] [Google Scholar]
- [148].Mahoney MJ, Anseth KS, Biomaterials 2006, 27, 2265. [DOI] [PubMed] [Google Scholar]
- [149].Belkas JS, Shoichet MS, Midha R, Neurol. Res. 2004, 26, 151. [DOI] [PubMed] [Google Scholar]
- [150].Ning L, Sun H, Lelong T, Guilloteau R, Zhu N, Schreyer DJ, Chen X, Biofabrication 2018, 10, 035014. [DOI] [PubMed] [Google Scholar]
- [151].Arcaute K, Mann BK, Wicker RB, Tissue Eng. Part C Methods 2011, 17, 27. [DOI] [PubMed] [Google Scholar]
- [152].Evangelista MS, Perez M, Salibian AA, Hassan JM, Darcy S, Paydar KZ, Wicker RB, Arcaute K, Mann BK, Evans GRD, J. Reconstr. Microsurg. 2015, 31, 327. [DOI] [PubMed] [Google Scholar]
- [153].Pateman CJ, Harding AJ, Glen A, Taylor CS, Christmas CR, Robinson PP, Rimmer S, Boissonade FM, Claeyssens F, Haycock JW, Biomaterials 2015, 49, 77. [DOI] [PubMed] [Google Scholar]
- [154].Mitchel JA, Hoffman-Kim D, PLoS One 2011, 6, e24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Reina MA, Sala-Blanch X, Fabiola M, Arriazu R, Prats-Galino A, Connective tissues of peripheral nerves, McGraw-Hill Medical, New York, NY, USA, 2015. [Google Scholar]
- [156].Kim Y-T, Romero-Ortega MI, MRS Bull. 2012, 37, 573. [Google Scholar]
- [157].Madduri S, Gander B, J. Control. Release 2012, 161, 274. [DOI] [PubMed] [Google Scholar]
- [158].Kapur TA, Shoichet MS, J. Biomed. Mater. Res. A 2004, 68, 235. [DOI] [PubMed] [Google Scholar]
- [159].Miller C, Jeftinija S, Mallapragada S, Tissue Eng. 2002, 8, 367. [DOI] [PubMed] [Google Scholar]
- [160].Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP, Tissue Eng. 2004, 6, 119. [DOI] [PubMed] [Google Scholar]
- [161].Corey JM, Lin DY, Mycek KB, Chen Q, Samuel S, Feldman EL, Martin DC, J. Biomed. Mater. Res. A 2007, 83, 636. [DOI] [PubMed] [Google Scholar]
- [162].Stokols S, Tuszynski MH, Biomaterials 2006, 27, 443. [DOI] [PubMed] [Google Scholar]
- [163].Gros T, Sakamoto JS, Blesch A, Havton LA, Tuszynski MH, Biomaterials 2010, 31, 6719. [DOI] [PubMed] [Google Scholar]
- [164].Gao M, Lu P, Bednark B, Lynam D, Conner JM, Sakamoto J, Tuszynski MH, Biomaterials 2013, 34, 1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Shahriari D, Koffler J, Lynam DA, Tuszynski MH, Sakamoto JS, J. Biomed. Mater. Res. A 2016, 104, 611. [DOI] [PubMed] [Google Scholar]
- [166].Gao M, Lu P, Lynam D, Bednark B, Campana WM, Sakamoto J, Tuszynski M, J. Neural Eng. 2016, 13, 066011. [DOI] [PubMed] [Google Scholar]
- [167].Miller C, Jeftinija S, Mallapragada S, Tissue Eng. 2001, 7, 705. [DOI] [PubMed] [Google Scholar]
- [168].Owens CM, Marga F, Forgacs G, Heesch CM, Biofabrication 2013, 5, 045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Zhu N, Li MG, Guan YJ, Schreyer DJ, Chen XB, Biofabrication 2010, 2, 045002. [DOI] [PubMed] [Google Scholar]
- [170].Sarker MD, Naghieh S, McInnes AD, Ning L, Schreyer D, Chen X, Bioprinting 2019, e00045. [Google Scholar]
- [171].McAlpine MC, Johnson BN (Princeton University; ), U.S. 10,405,963, 2019.
- [172].Gupta MK, Meng F, Johnson BN, Kong YL, Tian L, Yeh Y-W, Masters N, Singamaneni S, McAlpine MC, Nano Lett. 2015, 15, 5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH, Nat. Biotechnol. 2018, 36, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kelava I, Lancaster MA, Dev. Biol. 2016, 420, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Shuler ML, Hickman JJ, Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Bradbury EJ, McMahon SB, Nat. Rev. Neurosci. 2006, 7, 644. [DOI] [PubMed] [Google Scholar]
- [177].Norenberg MD, Smith J, Marcillo A, J. Neurotrauma 2004, 21, 429. [DOI] [PubMed] [Google Scholar]
- [178].Giger RJ, Hollis II ER, Tuszynski MH, Cold Spring Harb. Perspect. Biol. 2010, 2, a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Tsintou M, Dalamagkas K, Seifalian AM, Neural Regen. Res. 2015, 10, 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Cafferty WB, Yang SH, Duffy PJ, Li S, Strittmatter SM, J. Neurosci. 2007, 27, 2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Fields RD, Schwab ME, Silver J, Neuroscientist 1999, 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Ahuja CS, Fehlings M, Stem Cells Transl. Med. 2016, 5, 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Shrestha B, Coykendall K, Li Y, Moon A, Priyadarshani P, Yao L, Stem Cell Res. Ther. 2014, 5, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Milhorat TH, Capocelli AL Jr, Anzil AP, Kotzen RM, Milhorat RH, J. Neurosurg. 1995, 82, 802. [DOI] [PubMed] [Google Scholar]
- [185].Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY, Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Hakim JS, Esmaeili Rad M, Grahn PJ, Chen BK, Knight AM, Schmeichel AM, Isaq NA, Dadsetan M, Yaszemski MJ, Windebank AJ, Tissue Eng. Part A 2015, 21, 2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Madigan NN, Chen BK, Knight AM, Rooney GE, Sweeney E, Kinnavane L, Yaszemski MJ, Dockery P, O’Brien T, McMahon SS, Windebank AJ, Tissue Eng. Part A 2014, 20, 2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Wong DY, Leveque J-C, Brumblay H, Krebsbach PH, Hollister SJ, LaMarca F, J. Neurotrauma 2008, 25, 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Pritchard CD, Slotkin JR, Yu D, Dai H, Lawrence MS, Bronson RT, Reynolds FM, Teng YD, Woodard EJ, Langer RS, J. Neurosci. Methods 2010, 188, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Fry EJ, Clin. Exp. Pharmacol. Physiol. 2001, 28, 253.11251636 [Google Scholar]
- [191].Führmann T, Anandakumaran PN, Shoichet MS, Adv. Healthcare Mater. 2017, 6, 1601130. [DOI] [PubMed] [Google Scholar]
- [192].Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W, Nat. Neurosci. 2017, 20, 637. [DOI] [PubMed] [Google Scholar]
- [193].Dulin JN, Adler AF, Kumamaru H, Poplawski GHD, Lee-Kubli C, Strobl H, Gibbs D, Kadoya K, Fawcett JW, Lu P, Tuszynski MH, Nat. Commun. 2018, 9, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Jiang FX, Yurke B, Firestein BL, Langrana NA, Ann. Biomed. Eng. 2008, 36, 1565. [DOI] [PubMed] [Google Scholar]
- [195].Khandaker M, Orock A, Tarantini S, White J, Yasar O, Int. J. Biomater. 2016, 2016, 3208312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Belanger K, Dinis TM, Taourirt S, Vidal G, Kaplan DL, Egles C, Macromol. Biosci. 2016, 16, 472. [DOI] [PubMed] [Google Scholar]
- [197].Geuna S, Raimondo S, Fregnan F, Haastert-Talini K, Grothe C, Eur. J. Neurosci. 2016, 43, 287. [DOI] [PubMed] [Google Scholar]
- [198].Grasman JM, Ferreira JA, Kaplan DL, Adv. Funct. Mater. 2018, 28, 1803822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Villar G, Graham AD, Bayley H, Science 2013, 340, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Taylor MP, Kobiler O, Enquist LW, Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 17046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA, Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS, Nat. Mater. 2012, 11, 768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Miri AK, Nieto D, Iglesias L, Goodarzi Hosseinabadi H, Maharjan S, Ruiz‐Esparza GU, Khoshakhlagh P, Manbachi A, Dokmeci MR, Chen S, Shin SR, Zhang YS, Khademhosseini A, Adv. Mater. 2018, 30, 1800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Crawford JR, Curr. Neurol. Neurosci. Rep. 2010, 10, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Lunney JK, Int. J. Biol. Sci. 2007, 3, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Lin NYC, Homan KA, Robinson SS, Kolesky DB, Duarte N, Moisan A, Lewis JA, Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Liu X, Yuk H, Lin S, Parada GA, Tang TC, Tham E, de la Fuente-Nunez C, Lu TK, Zhao X, Adv. Mater. 2018, 30, 1704821. [DOI] [PubMed] [Google Scholar]
- [208].Dell’Anno MT, Wang X, Onorati M, Li M, Talpo F, Sekine Y, Ma S, Liu F, Cafferty WBJ, Sestan N, Strittmatter SM, Nat. Commun. 2018, 9, 3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Shanks N, Greek R, Greek J, Philos. Ethics Humanit. Med. 2009, 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S, Cell 2007, 131, 861. [DOI] [PubMed] [Google Scholar]
- [211].Park I-H, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ, Cell 2008, 134, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA, Science 2007, 318, 1917. [DOI] [PubMed] [Google Scholar]
- [213].Forsberg M, Hovatta O, Front. Physiol. 2012, 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Rosenzweig ES, H Brock J, Lu P, Kumamaru H, Salegio EA, Kadoya K, Weber JL, Liang JJ, Moseanko R, Hawbecker S, Huie JR, Havton LA, Nout-Lomas YS, Ferguson AR, Beattie MS, Bresnahan JC, Tuszynski MH, Nat. Med. 2018, 24, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R, Nat. Methods 2019, 16, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Park SH, Su R, Jeong J, Guo S-Z, Qiu K, Joung D, Meng F, McAlpine MC, Adv. Mater. 2018, 30, 1803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Zhu Z, Guo S-Z, Hirdler T, Eide C, Fan X, Tolar J, McAlpine MC, Adv. Mater. 2018, 30, 1707495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Patil AC, Thakor NV, Med. Biol. Eng. Comput. 2016, 54, 23. [DOI] [PubMed] [Google Scholar]
- [219].Lacour SP, Courtine G, Guck J, Nat. Rev. Mater. 2016, 1, 16063. [Google Scholar]
- [220].Fattahi P, Yang G, Kim G, Abidian MR, Adv. Mater. 2014, 26, 1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Rezvani E, Raffertya A, McGuinness C, Kennedy J, Acta biomater. 2019, 94, 145. [DOI] [PubMed] [Google Scholar]
- [222].Repar N, Li H, Aguilar JS, Li QQ, Drobne D, Hong Y, Nanotoxicology 2018, 12, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Wang Y, Guo L, Front. Neurosci. 2016, 10, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]