Abstract
Climate change presents complex and wide‐reaching threats to human health. A variable and changing climate can amplify and unmask ecological and socio‐political weaknesses and increase the risk of adverse health outcomes in socially vulnerable regions. When natural disasters occur in such areas, underlying climatic conditions may amplify the public health crisis. We describe an emerging epidemic of Zika virus (ZIKV) in Ecuador following the 2016 earthquake, which coincided with an exceptionally strong El Niño event. We hypothesize that the trigger of a natural disaster during anomalous climate conditions and underlying social vulnerabilities were force multipliers contributing to a dramatic increase in ZIKV cases postearthquake.
Keywords: climate change, natural disaster, Zika virus, vulnerable populations
Key Points
When natural disasters occur in areas made vulnerable by climate change, the effects are multiplied
Climate change makes vulnerable regions even more vulnerable and plays a role in the root cause of the eventual impacts of natural disasters
Multisectoral coordination is needed to implement surveillance and response systems to detect and protect vulnerable communities
1. Introduction
Climate change is emerging as one of the greatest modern threats to human health (Wang & Horton, 2015; Watts et al., 2017). Over the past decade, increasingly unambiguous science has linked anthropogenic emissions with unprecedented worldwide changes in the stability of our climate and environment (Pachauri et al., 2014). It is anticipated that as the planet continues to warm we will see novel, complex, and wide‐reaching impacts on human health (Kim, 2016). A variable and changing climate can amplify and unmask underlying ecological and socio‐political weaknesses (Luber & Lemery, 2015) and increase the risk of adverse health outcomes in vulnerable (i.e., low income) as well as socioeconomically stable regions. When natural disasters occur, climate change may amplify the public health crisis. Here we describe an emerging epidemic of Zika virus (ZIKV) in the coastal province of Manabi, Ecuador, following the 2016 earthquake which coincided with an exceptionally strong El Niño event. In coastal Ecuador, El Niño events are associated with heavy rainfall and warmer air temperatures and have been shown to be associated with outbreaks of dengue fever (Stewart‐Ibarra & Lowe, 2013). We hypothesize that the trigger of the earthquake during anomalous climate conditions coupled with underlying social vulnerabilities was the principal force multipliers of this outbreak. Extreme climate events associated with climate change will likely play a larger role in human health outcomes during natural disasters in the future.
2. The Earthquake
On 16 April 2016, a magnitude 7.8 earthquake occurred off the coast of Ecuador, impacting approximately 720,000 people and placing the country's health system on maximum alert. According to the Pan American Health Organization (World Health Organization, 2016a), essential infrastructure, including hospitals and health‐care facilities, were rendered inoperative – both due to unstable structural damage as well as loss of electricity, rupture of water and gas pipes, and other damages. Reports by the United States Agency for International Development (USAID) estimated 660 fatalities, 40 missing persons, 4,605 injuries, 9,750 damaged buildings, over 30,000 people displaced, and 720,000 people requiring humanitarian assistance (United States Agency for International Development, 2016). Many rural communities were isolated, while cities became chaotic with influxes of displaced populations. The national Secretary for Risk Management, municipal governments, public health offices at national and local levels, and international aid bodies (e.g., United Nation UN Office for Disaster Risk Reduction (UNISDR), United Nations International Children's Emergency Fund (UNICEF), Red Cross, Pan American Health Organization/World Health Organization (PAHO/WHO), United States Agency for International Development (USAID), Médecins Sans Frontières, among others) reacted after the event, providing emergency medical teams, mobile water treatment units, and temporary shelter for residents (World Health Organization, 2016a). The earthquake caused tremendous social and environmental disturbance, resulting in population displacement, a breakdown in piped water and sanitation infrastructure, a disruption in health‐care services, and increases in psychological distress. This resulted in a surge in morbidity and mortality from both communicable and noncommunicable diseases, including diseases transmitted by the Aedes aegypti mosquito.
3. The Virus
The first reported cases of Zika virus (ZIKV) infections in Ecuador were documented on 15 January 2016 (World Health Organization, 2016b), just months before the earthquake. ZIKV is an arbovirus transmitted to people by the Aedes aegypti and Aedes albopictus mosquitoes, and also by other transmission routes, including sexual transmission (Petersen et al., 2016). As of August 2017, 6,811 suspected and confirmed cases of ZIKV have been reported from Ecuador, including seven cases of congenital ZIKV syndrome and 660 confirmed cases in pregnant women (Pan American Health Organization/World Health Organization, 2017a, 2017b). In the majority of symptomatic patients, ZIKV infections result in a mild acute febrile illness associated with rash, conjunctivitis, arthralgia, and headache, with the potential for late neurologic complications such Guillain‐Barre Syndrome (Petersen et al., 2016). Despite a relatively mild clinical course in symptomatic healthy adults, the effect on fetal development can be devastating. Fetal death, fetal growth restriction, and a spectrum of central nervous system abnormalities have been documented in more than 40% of cases of maternal infection (Brasil et al., 2016). In a recent study of U.S. women with evidence of ZIKV infection during the first trimester of pregnancy, 11% had an infant with a birth defect (Honein et al., 2017). The same vectors that carry ZIKV also transmits dengue virus (DENV) and chikungunya virus (CHIKV), which cocirculate in the same populations and cause significant morbidity in coastal Ecuador. In 2016, there were 14,150 reported DENV cases and 1,860 cases of CHIKV in the country (Pan American Health Organization, 2017).
Leading up to 16 April 2016, 92 total cases of ZIKV had been documented countywide. In the wake of the earthquake, this number rapidly escalated from 92 to 1,106 total cases in just 3 months (United Nations International Children's Emergency Fund, 2016) (Figure 1). Eighty‐percent of these cases were reported in the province of Manabi, the region most impacted by the earthquake (United Nations International Children's Emergency Fund, 2016; Pan American Health Organization, 2017) (Figure 2). A vector control intervention was instituted by the Ministry of Health (MoH) after the earthquake, which included larvicide (temefos/abate) application, intrahouse fumigation, thermal nebulizers, and bed nets targeted at displaced people and women of fertile age. This emergency intervention lasted until June 2016.
Figure 1.
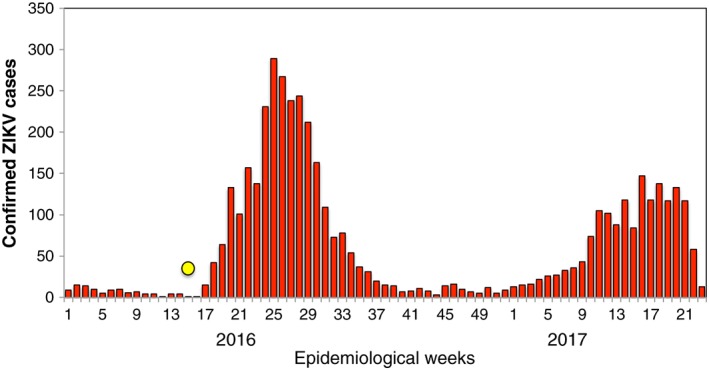
Confirmed ZIKV cases by epidemiological week (EW) in Ecuador. The date of the earthquake is indicated as a yellow circle (EW 15, 2016). Source: Data published by the Ecuadorian Ministry of Public Health.
Figure 2.
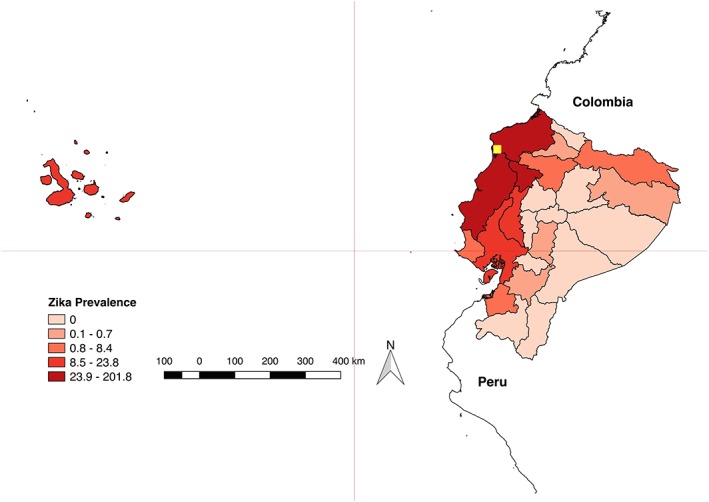
Laboratory‐confirmed Zika cases per 100,000 population, by province. Ecuador. EW 1 of 2016 to EW 23 of 2017. The location of the epicenter of the earthquake is shown in yellow. Source: Data published by the Ecuadorian Ministry of Public Health (Pan American Health Organization, 2017).
4. Social Vulnerability
“Social vulnerability” is an indicator of the sensitivity of a community to disturbances from environmental hazards and natural disasters and a metric of its capacity to respond, adapt, and recover (Cutter et al., 2003). Similarly, the Intergovernmental Panel on Climate Change (Pachauri et al., 2014) views social vulnerability as a function of the degree to which a population will respond to a given change in climate, and its adaptive capacity or the “degree to which adjustments in practices, processes, or structures can moderate or offset the potential for damage.” The ability of a community to demonstrate adaptive capacity or resilience in the face of natural disasters and climate change is dependent upon its technical, institutional, economic, and cultural ability, as well as existing health‐care infrastructure (Beccari, 2016). Many indices attempt to objectively capture “vulnerability” and “resilience,” although diverse demographics and the uniqueness of each natural disaster make these indices incredibly spatiotemporally dependent.
As a developing middle‐income country, many geographic regions and social sectors of Ecuador are underresourced. Our prior studies from coastal Ecuador found that social vulnerabilities (e.g., lack of social cohesion, poor housing conditions, and inadequate access to piped water in the home) interact with local climate conditions to increase the risk of Ae. aegypti transmitted diseases in urban areas (Stewart Ibarra et al., 2013; Stewart‐Ibarra et al., 2014). According to the United Nations Development Programme (Bermudez, 2017), following the earthquake, the “country's emergency response capabilities were overwhelmed, unleashing decades of pent up vulnerability.” Significant efforts are still underway to rebuild essential infrastructure. According to the World Bank, since 2016, urban unemployment rose from 4.5% to 6.5% and urban underemployment increased from 11.7% to 18.8%, an effect that was linked to the earthquake (World Bank, 2017). These indicators suggest that the country was indeed socially vulnerable when the earthquake struck and serves as an exemplar of population vulnerability in the face of climate change.
5. The Climate
The distribution and prevalence of vector‐borne diseases is influenced by fluctuations in temperature and precipitation (Gage et al., 2008), and large‐scale climate phenomena such as the El Niño–Southern Oscillation (Bi et al., 2005; Fisman et al., 2016; Githeko et al., 2000). The conditions that allowed for such a rapid spread of ZIKV in Manabi are likely multifactorial and include the following: historically high densities of Aedes aegypti during the rainy season (February to May), the occurrence of an exceptionally strong El Niño event (increasing the odds of arbovirus outbreaks) (Muñoz et al., 2017), a historically high burden of dengue fever, and underlying social vulnerability associated with poverty. The earthquake compounded and intensified these vulnerabilities in the following ways: displaced populations sleeping outdoors who were at risk for increased exposure to mosquito bites, water accumulation in containers near human settlements due to lack of piped water, movement of people from most affected areas into other areas allowing for spread of ZIKV, lack of access to basic health care, lack of health care for pregnant women potentially affected by ZIKV, low use of contraceptives, and a breakdown in local governance. Although this earthquake occurred in a socioeconomically depressed community, the impacts mentioned above could likewise occur in wealthier communities following a disaster, increasing their vulnerability to similar negative repercussions.
Climate variability affects the spread of vector‐borne diseases both directly, through ecologic changes that alter the abundance of insect and zoonotic vectors, and indirectly through changes in human behavior in response to the climate and environment. Prior studies in southern coastal Ecuador have shown that local weather conditions (rainfall and minimum temperature) are drivers of dengue transmission and Aedes aegypti proliferation (Stewart Ibarra et al., 2013; Stewart‐Ibarra et al., 2014). In terms of direct effects, air temperatures influence vector development rates, reproductive behavior and mortality and warmer temperatures increase rates of viral replication in the mosquito vector up to a certain optimal temperature (Morin et al., 2013). A recent paper by Mordecai et al. (2017) showed the effects of temperature on DEN, ZIKV, and CHIKV transmission risk across Latin American and the Caribbean and found the maximum risk of transmission occurred from 26 to 29°C. Precipitation also influences habitat suitability for mosquito larvae and pupae; however, the effects depend on local social conditions. Increases in rainfall can increase the availability of larval habitat (e.g., rain filled containers located outdoors); decreases in rainfall and water scarcity, as seen during seasonal El Niño climate variations (Gagnon et al., 2001; Poveda et al., 2000) and postearthquake scenarios, can also increase the availability of larval habitat due to impacts on water storage patterns (e.g., tap‐water filled containers) (Ashby et al., 2017; Moreno‐Madriñán & Turell, 2017; Stanforth et al., 2016). Over longer time scales, rainfall, temperature, and humidity influence land cover and land use, which can alter development of vector populations (Fuller et al., 2009).
One of the most important drivers of interannual variability in the climate in coastal Ecuador is the El Niño–Southern Oscillation (ENSO) (Santoso et al., 2013). Prior studies have demonstrated the impact of ENSO on local climate anomalies and, as a result, on dengue outbreaks in coastal Ecuador (Borbor‐Cordova, 2016; Recalde‐Coronel et al., 2014; Rossel & Cadier, 2009; Stewart Ibarra et al., 2013), and similar effects have been documented elsewhere (Colon‐Gonzalez et al., 2011; Hurtado‐Diaz et al., 2007; Johansson et al., 2009). In Ecuador, El Niño events are associated with warmer local air temperatures and increased rainfall, increasing the likelihood of outbreaks of diseases transmitted by Ae. aegypti. Prior studies suggest that the climate variables that predict the intensity and timing of dengue outbreaks include minimum, maximum, and mean SST temperature; minimum land surface temperature; local rainfall; and relative humidity and wind velocity (Morin et al., 2013; Stewart Ibarra et al., 2013). As ZIKV is transmitted by the same mosquito vectors as DENV, it is likely that these same climate variables are important drivers of ZIKV transmission.
As a result of global warming, sea surface temperatures since the beginning of the century have steadily warmed (United States Environmental Protection Agency, 2017), which directly impacts vector abundance and the appearance of mosquito transmitted diseases. Some studies predict that the frequency of severe El Niño events will increase with climate change (Cai et al., 2014; Timmermann et al., 1999). The relationship between climate change and ENSO is complex yet inextricably linked (Santoso et al., 2013). Long‐term climate trends are associated with anthropogenic climate change signals, while year‐to year and decadal variations are driven by other global climate phenomena (Muñoz et al., 2016a, 2016b). When positive short‐term temperature anomalies are superimposed upon long‐term warming, they combine to create anomalous ecologic conditions (Muñoz et al., 2016a, 2016b) conducive to the spread of arboviruses such as ZIKV, DENV, and CHIKV. According to the U.S. National Oceanic and Atmospheric Administration (NOAA), the warming effects of the 2015‐2016 El Niño event contributed to making 2016 the third consecutive warmest year on record (National Oceanic and Atmospheric Administration, 2016). At the time of the earthquake in Ecuador, a strong El Niño event was smoldering in the Pacific Ocean (National Oceanic and Atmospheric Administration, 2015), and ZIKV had just emerged in Ecuador.
The effect of climate variability on the spread of ZIKV in South America has been proposed (Muñoz et al., 2016a, 2016b; Paz & Semenza, 2016) and, based on retrospective climate models, could have been predicted (Muñoz et al., 2017). In Manabi, rising temperatures (Figure 3), increasing sea surface temperatures, an ongoing El Niño event, and high abundance of mosquito vectors, followed by an earthquake in a socially vulnerable region, may have allowed an outbreak of a climate‐sensitive emerging infectious disease.
Figure 3.
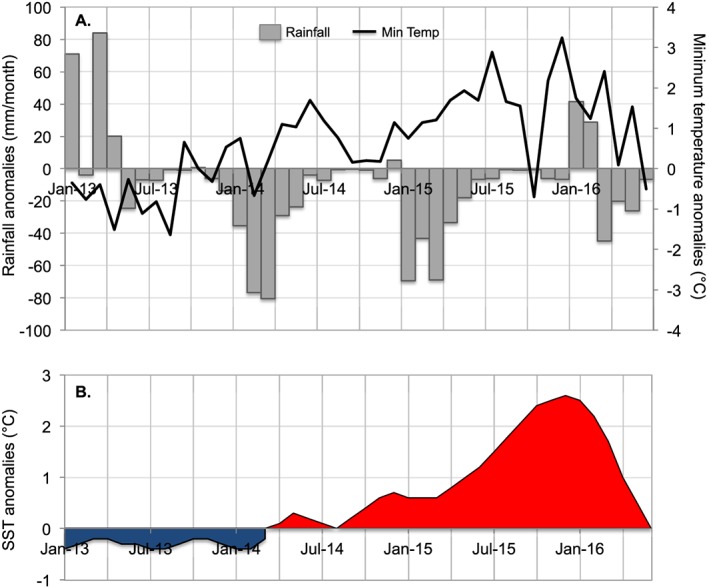
Local climate conditions in the city of Manta, Manabi Province, Ecuador, were warmer and drier than average during the 2015–2016 El Niño event. (a) Monthly anomalies in rainfall and minimum temperature from Manta, Manabi Province, Ecuador, and (b) anomalies in sea surface temperature (SST) in the Niño 3.4 region, also known as Oceanic Niño Index (ONI). Positive SST anomalies (ocean warming) shown in red; negative SST anomalies (ocean cooling) shown in blue. Climate anomalies were calculated using monthly means from a long‐term time series for rainfall (1975–2016) and for minimum temperature (1981–2016). Climate data were provided for the Manta weather station by the National Institute for Meteorology and Hydrology (INAMHI) of Ecuador. ONI data (3 month running mean of ERSST.v5 anomalies in the Niño 3.4 region) were provided by the National Oceanic and Atmospheric Administration (NOAA) Climate Prediction Center (CPC) of NOAA/ National Weather Service (http://origin.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ONI_v5.php).
6. Conclusions
The outbreak of ZIKV in Manabi, Ecuador, following the earthquake of 2016 demonstrates the negative synergistic effects of natural disasters when superimposed upon anomalous climate conditions in socially vulnerable regions. In this case example, an exceptionally strong El Niño event created environmental conditions that favored mosquito vector replication just as ZIKV first appeared in Ecuador. Following the earthquake, the incidence of ZIKV in Manabi surged as social conditions broke down in the aftermath of the earthquake. We hypothesize that the trigger of a natural disaster during anomalous climate conditions and underlying social vulnerabilities was force multipliers contributing to the spread of ZIKV in this region of Ecuador.
As the planet continues to warm in this century, it is likely that we will see an increase in extreme climate events that will compound the impact of acute and protracted humanitarian crisis. To mitigate the impact, multisectoral coordination is needed in order to strengthen ties between the health sector, governmental entities, and research and disaster management communities. Such intersectoral cooperation should aim to (1) have the ability to quickly implement uniform health surveillance in the aftermath of major “natural” disasters, (2) have integrated climate‐health surveillance systems in place to support evaluations of the extent to which disaster‐related health impacts can be attributed to climate, and (3) create models that identify vulnerable communities and geographic regions and thus have longitudinal surveillance and early warning systems available in these places. Another recommendation would be to increase the adaptive capacity of high risk communities in order to reduce their vulnerability.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Acknowledgments
This paper represents a collaboration through the University of Colorado Consortium on Climate Change and Health. Stewart‐Ibarra receives support from the National Science Foundation (DEB EEID 1518681 and DEB RAPID 1641145). Borbor‐Cordova receives support from Centro Internacional del Pacifico para la Reduccion de Riesgos de Desastres at ESPOL. No other authors have financial disclosures or conflicts of interest to declare.
Sorensen, C. J. , Borbor‐Cordova, M. J. , Calvello‐Hynes, E. , Diaz, A. , Lemery, J. , & Stewart‐Ibarra, A. M. (2017). Climate variability, vulnerability and natural disasters: A case study of Zika virus in Manabi, Ecuador following the 2016 earthquake. GeoHealth, 1, 298–304. 10.1002/2017GH000104
This article was corrected on 15 JUL 2019. The online version of this article has been modified to include a Conflict of Interest statement.
References
- Ashby, J. , Moreno‐Madriñán, M. J. , Yiannoutsos, C. T. , & Stanforth, A. (2017). Niche modeling of dengue fever using remotely sensed environmental factors and boosted regression trees. Remote Sensing, 9(4), 328. [Google Scholar]
- Beccari, B. (2016). A comparative analysis of disaster risk, vulnerability and resilience composite indicators. PLoS Currents, 8 10.1371/currents.dis.453df025e34b682e9737f95070f9b970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, P. , Parton, K. A. , & Tong, S. (2005). El Nino‐Southern Oscillation and vector‐borne diseases in Anhui, China. Vector Borne and Zoonotic Diseases, 5(2), 95–100. [DOI] [PubMed] [Google Scholar]
- Borbor‐Cordova, M. J. , Beltran, E. , Cardenas, W. , Endy, T. , Finkelstein, J. L. , King, C. , … Stewart‐Ibarra, A. (2016). Case study 5.C vector‐virus microclimate surveillance system for dengue control in Machala, Ecuador In Climate Services for Health: Improving Public Health Decision‐Making in a New Climate [Internet]. Geneva, Switzerland: World Meteorological Association and World Health Organization; Retrieved from https://public.wmo.int/en/resources/library/climate-services-health-case-studies [Google Scholar]
- Brasil, P. , Pereira, J. P. , Moreira, E. , Ribeiro Nogueira, R. M. , Damasceno, L. , Wakimoto, M. , … Nielsen‐Saines, K. (2016). Zika virus infection in pregnant women in Rio de Janeiro. The New England Journal of Medicine, 375(24), 2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez, N. (2017). A year after the Ecuadorian earthquake, we still have work to do. United Nations Development programme. Retrieved from http://undp.org/content/undp/en/home/blog/2017/4/17/A-un-a-o-del-terremoto-en-Ecuador-a-n-queda-mucho-por-haver.html [Google Scholar]
- Cai, W. , Borlace, S. , Lengaigne, M. , van Rensch, P. , Collins, M. , Vecchi, G. , … Jin, F.‐F. (2014). Increasing frequency of extreme El Niño events due to greenhouse warming. Nature Climate Change, 4(2), 111–116. [Google Scholar]
- Colon‐Gonzalez, F. J. , Lake, I. R. , & Bentham, G. (2011). Climate variability and dengue fever in warm and humid Mexico. The American Journal of Tropical Medicine and Hygiene, 84(5), 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter, S. L. , Boruff, B. J. , & Shirley, W. L. (2003). Social vulnerability to environmental hazards. Social Science Quarterly, 84(2), 242–261. [Google Scholar]
- Fisman, D. N. , Tuite, A. R. , & Brown, K. A. (2016). Impact of El Niño Southern Oscillation on infectious disease hospitalization risk in the United States. Proceedings of the National Academy of Sciences of the United States of America, 114(1), 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, D. O. , Troyo, A. , & Beier, J. C. (2009). El Niño Southern Oscillation and vegetation dynamics as predictors of dengue fever cases in Costa Rica. Environmental Research Letters, 4. 140111‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage, K. L. , Burkot, T. R. , Eisen, R. J. , & Hayes, E. B. (2008). Climate and vector‐borne diseases. American Journal of Preventive Medicine, 35(5), 436–450. [DOI] [PubMed] [Google Scholar]
- Gagnon, A. S. , Bush, A. B. , & Smoyer‐Tomic, K. E. (2001). Dengue epidemics and the El Niño Southern Oscillation. Climate Research, 19(1), 35–43. [Google Scholar]
- Githeko, A. K. , Lindsay, S. W. , Confalonieri, U. E. , & Patz, J. A. (2000). Climate change and vector‐borne diseases: A regional analysis. Bulletin of the World Health Organization, 78(9), 1136–1147. [PMC free article] [PubMed] [Google Scholar]
- Honein, M. A. , Dawson, A. L. , Petersen, E. E. , Jones, A. M. , Lee, E. H. , Yazdy, M. M. , … Ellington, S. R. (2017). Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA, 317(1), 59–68. [DOI] [PubMed] [Google Scholar]
- Hurtado‐Diaz, M. , Riojas‐Rodriguez, H. , Rothenberg, S. J. , Gomez‐Dantés, H. , & Cifuentes, E. (2007). Short communication: Impact of climate variability on the incidence of dengue in Mexico. Tropical Medicine & International Health, 12(11), 1327–1337. [DOI] [PubMed] [Google Scholar]
- Johansson, M. A. , Dominici, F. , & Glass, G. E. (2009). Local and global effects of climate on dengue transmission in Puerto Rico. PLoS Neglected Tropical Diseases, 3(2), e382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E. J. (2016). The Impacts of Climate Change on Human Health In The United States: A Scientific Assessment (pp. 312). Washington, DC: US Global Change Research Program. [Google Scholar]
- Luber G., & Lemery J. (Eds.) (2015). Global Climate Change and Human Health: From Science To Practice. San Francisco, CA: John Wiley. [Google Scholar]
- Mordecai, E. A. , Cohen, J. M. , Evans, M. V. , Gudapati, P. , Johnson, L. R. , Lippi, C. A. , … Weikel, D. P. (2017). Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Neglected Tropical Diseases, 11(4), e0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno‐Madriñán, M. J. , & Turell, M. (2017). Factors of concern regarding Zika and other Aedes aegypti‐transmitted viruses in the United States. Journal of Medical Entomology, 54(2), 251–257. [DOI] [PubMed] [Google Scholar]
- Morin, C. W. , Comrie, A. C. , & Ernst, K. (2013). Climate and dengue transmission: Evidence and implications. Environmental Health Perspectives, 121(11–12), 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz, Á. G. , Thomson, M. C. , Goddard, L. , Aldighieri, S. (2016a). The Latin American and the Caribbean climate landscape for ZIKV transmission (IRI Tech. Rep., 2016–01).
- Muñoz, Á. G. , Thomson, M. C. , Goddard, L. , & Aldighieri, S. (2016b). Analyzing climate variations at multiple timescales can guide Zika virus response measures. GigaScience, 5(1), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz, Á. G. , Thomson, M. C. , Stewart‐Ibarra, A. M. , Vecci, G. , Chourio, X. , Nájera, P. , … Yang, X. (2017). Could the recent Zika epidemic have been predicted? Frontiers in Microbiology. 10.3389/fmicb.2017.01291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration (2015). Strong El Nino Sets the Stage for 2015–2016 Winter Weather (NOAA, active weather alerts, 2015). Retrieved from http://www.noaanews.noaa.gov/stories2015/101515-noaa-strong-el-nino-sets-the-stage-for-2015-2016-winter-weather.html [Google Scholar]
- National Oceanic and Atmospheric Administration (2016). 2016 Marks Three Consecutive Years of Record Warmth for the Globe. (NOAA, News and Features, 2016). Retrieved from http://www.noaa.gov/stories/2016-marks-three-consecutive-years-of-record-warmth-for-globe [Google Scholar]
- Pachauri, R. K. , Allen, M. R. , Barros, V. R. , Broome, J. , Cramer, W. , Christ, R. , … van Ypserle, J. P. (2014). Climate change 2014: Synthesis report In Pachauri R. & Meyer L. (Eds.), Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Intergovernmental Panel on Climate Change (pp. 151). Geneva, Switzerland: IPCC. [Google Scholar]
- Pan American Health Organization (2017). Regional Zika Epidemiological Update (Americas) May 2017. Retrieved from http://www.paho.org/hq/index.php?option=com_content&id=11599&Itemid=41691 [Google Scholar]
- Pan American Health Organization/World Health Organization (2017a). Zika‐Epidemiological Report 28 June 2017. Retrieved from http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=35027&Itemid=270 [Google Scholar]
- Pan American Health Organization/World Health Organization (2017b). Zika cumulative cases. Retrieved from http://www.paho.org/hq/index.php?option=com_content&view=article&id=12390&Itemid=42090&lang=en [Google Scholar]
- Paz, S. , & Semenza, J. C. (2016). El Nino and climate change—Contributing factors in the dispersal of Zika virus in the Americas? Lancet, 387(10020), 745. [DOI] [PubMed] [Google Scholar]
- Petersen, L. R. , Jamieson, D. J. , Powers, A. M. , & Honein, M. A. (2016). Zika virus. The New England Journal of Medicine, 374(16), 1552–1563. [DOI] [PubMed] [Google Scholar]
- Poveda, G. , Graham, N. E. , Epstein, P. R. , Rojas, W. , Quiñones, M. L. , Velez, I. D. , & Martens, W. J. (2000). Climate and ENSO variability associated with vector‐borne diseases in Colombia. Presented at the Proceedings of the 10th Symposium on Global Change Studies 2000 Jan 10 (pp. 183–204), American Meteorological Society, Dallas, TX.
- Recalde‐Coronel, G. C. , Barnston, A. G. , & Muñoz, Á. G. (2014). Predictability of December‐April rainfall in coastal and Andean Ecuador. Journal of Applied Meteorology and Climatology, 53(6), 1471–1493. [Google Scholar]
- Rossel, F. , & Cadier, E. (2009). El Niño and prediction of anomalous monthly rainfalls in Ecuador. Hydrological Processes, 23, 3253–3260. [Google Scholar]
- Santoso, A. , McGregor, S. , Jin, F. , , Cai, W. , England, M. H. , An, S. I. , … Guilyardi, E. (2013). Late‐twentieth‐century emergence of the El Niño propagation asymmetry and future projections. Nature, 504(7478), 126–130. [DOI] [PubMed] [Google Scholar]
- Stanforth, A. , Moreno‐Madriñán, M. J. , Max, J. , & Ashby, J. (2016). Exploratory analysis of dengue fever niche variables within the Río Magdalena watershed. Remote Sensing, 8(9), 770. [Google Scholar]
- Stewart Ibarra, A. M. , Ryan, S. J. , Beltran, E. , Mejía, R. , Silva, M. , & Muñoz, Á. (2013). Dengue vector dynamics (Aedes aegypti) influenced by climate and social factors in Ecuador: Implications for targeted control. PLoS One, 8(11), e78263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart‐Ibarra, A. M. , & Lowe, R. (2013). Climate and non‐climate drivers of dengue epidemics in southern coastal ecuador. The American Journal of Tropical Medicine and Hygiene, 88(5), 971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart‐Ibarra, A. M. , Munoz, A. G. , Ryan, S. J. , Beltrán Ayala, E. , Borbor‐Cordova, M. J. , Finkelstein, J. L. , … Rivero, K. (2014). Spatiotemporal clustering, climate periodicity, and social‐ecological risk factors for dengue during an outbreak in Machala, Ecuador, in 2010. BMC Infectious Diseases, 14, 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann, A. , Oberhuber, J. , Bacher, A. , Esch, M. , Latif, M. , & Roeckner, E. (1999). Increased El Niño frequency in a climate model forced by future greenhouse warming. Nature, 398(6729), 694. [Google Scholar]
- United Nations International Children's Emergency Fund (2016). Twelvefold Increase in Zika Cases Since Ecuador Earthquake (UNICEF Press Release, 18 July 2016). Retrieved from https://www.unicef.org/media/media_91912.html [Google Scholar]
- United States Agency for International Development (2016). Ecuador–Earthquake. Fact Sheet #5, Fiscal Year 2016. Retrieved from https://www.usaid.gov/sites/default/files/documents/1866/ecuador_eq_fs05_05-06-2016.pdf [Google Scholar]
- United States Environmental Protection Agency (2017). Climate change indicators: Sea surface temperature. Retrieved from https://www.epa.gov/climate-indicators/climate-change-indicators-sea-surface-temperature
- Wang, H. , & Horton, R. (2015). Tackling climate change: The greatest opportunity for global health. Lancet, 386(10006), 1798–1799. [DOI] [PubMed] [Google Scholar]
- Watts, N. , Adger, N. , Ayeb‐Karlsson, S. , Bai, Y. , Byass, P. , Campbell‐Lendrum, D. , … Costello, A. (2017). The Lancet countdown: Tracking progress on health and climate change. Lancet, 389(10074), 1151–1164. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2016a). The Earthquake in Ecuador: Significant damage to health facilities; emergency medical teams deployed, World Health Organization/Pan American Health Organization, 121(June 2016), 1–3. [Google Scholar]
- World Health Organization (2016b). Zika Virus Infection--Guyana, Barbados and Ecuador (WHO, Disease Outbreak News. 2016). Retrieved from http://www.who.int/csr/don/20-january-2016-zika-guyana-barbados-ecuador/en/ [Google Scholar]
- World Bank (2017). Overview, the World Bank in Ecuador. Retrieved from http://www.worldbank.org/en/country/ecuador/overview] [Google Scholar]


