Abstract
Phthalates are chemical additives to common consumer goods including cleaning products, cosmetics, personal care products, and plastic. Because they are not chemically bound to these products and are widely used, the potential for environmental contamination is significant. Phthalates and their metabolites have been associated with endocrine disruption and reproductive impairment, among other adverse health effects, in laboratory animals and human epidemiologic studies. Common bottlenose dolphins (Tursiops truncatus) are vulnerable to environmental pollutants due to their apex position in the food chain, long life spans, and habitat overlap with developed coastal areas. The objective of this study was to quantify phthalate metabolite concentrations in urine collected from bottlenose dolphins in Sarasota Bay, Florida, during May 2016 (n = 7) and May 2017 (n = 10). Screening of nine phthalate monoester metabolites in bottlenose dolphin urine was performed by liquid chromatography tandem mass spectrometry using methods adapted from those used for analyzing human samples. At least one phthalate metabolite was detected in 71% of the dolphins sampled across both years, with the highest concentrations detected for monoethyl phthalate (MEP; GM = 5.4 ng/ml; 95%CI: 1.3–22.0 ng/ml) and mono‐(2‐ethylhexyl) phthalate (MEHP; GM = 1.9 ng/ml; 95%CI: 1.1–3.2 ng/ml). These data demonstrate exposure to two of the most commonly used phthalates in commercial manufacturing, diethyl phthalate (DEP) and di‐2‐ethylhexyl phthalate (DEHP). This study establishes methods for urinary detection of phthalate metabolites in marine mammals and provides baseline data to address a significant and growing, yet poorly understood, health threat to marine wildlife.
Keywords: marine mammal, contaminant, health, cetacean, plastic
Key Points
Phthalate metabolites were found in 71% of bottlenose dolphins sampled in Sarasota Bay, FL
Monoethyl phthalate (MEP) and mono‐(2‐ethylhexyl) phthalate (MEHP) were the most common metabolites detected in bottlenose dolphin urine
Bottlenose dolphins in Sarasota Bay, FL, are susceptible to phthalate exposure
1. Introduction
1.1. Introduction to Phthalates and Potential Health Concerns
Dialkyl phthalate esters, commonly known as phthalates, are high production volume (HPV) chemicals manufactured for use in a variety of commercial goods, including personal care products, pesticide formulations, medical devices, building materials, electronics, and plastics. As a plasticizer, phthalates functionally increase the flexibility and other desirable properties of plastic products. These chemicals also act as an emulsion agent; phthalates can be added to enhance solubility of mixture components, such as fragrances in personal care products (Agency for Toxic Substances and Disease Registry (ATSDR), 1995, 2002). Certain phthalates have been identified as reproductive and developmental toxicants and endocrine disruptors. Mammalian toxicological studies have suggested that some phthalate compounds can disrupt the secretion of thyroid and growth hormones (Mathieu‐Denoncourt et al., 2015), alter steroidogenesis and lower circulating concentrations of testosterone (Bell, 1982; Meeker & Ferguson, 2014; Sathyanarayana et al., 2017), and impact the production and metabolism of estrogen (Sathyanarayana et al., 2017). The consequences of these hormonal changes may result in reduced fertility, abnormal reproductive organ development, and impacts on pregnancy outcomes (Bell, 1982; Latini et al., 2004). Adverse effects of phthalate exposure, such as altered hormone synthesis, reduced locomotor activity, and impacts on metabolism have also been reported for aquatic organisms, including crustaceans and fish (Oehlmann et al., 2009). To date there are no published studies assessing health outcomes of phthalate exposure to marine mammals. Contaminant exposures, such as polychlorinated biphenyls and other endocrine active compounds, are linked to impaired reproduction and reduced growth rates in cetacean populations and are correlated with altered hormone status (Schwacke et al., 2002, 2011; Wells et al., 2005). Concern over the widespread occurrence of phthalates and potential for detrimental health effects thus extends also to concern over discharge into the marine environment and impacts on coastal ecosystem health. For example, the Southern California Coastal Water Research Project, through a tiered assessment of geographic regions susceptible to wastewater treatment plant effluent and storm water discharge, has identified phthalates as a “chemical of emerging concern” (CEC; Southern California Coastal Water Research Program, 2012).
1.2. Environmental Exposure to Phthalates
With a wide range of applications, phthalates have many entry points into the aquatic environment, including via nonpoint source runoff, point source discharges, such as industrial or municipal wastewater effluents, leaching from plastic products, and atmospheric deposition (ATSDR, 2002; D.‐W. Gao & Wen, 2016). Phthalates are not considered persistent chemicals, but ongoing release of phthalates into the environment lead to pseudo persistence and may result in chronic and ubiquitous environmental exposure risks to wildlife. Marine and aquatic organisms may be exposed through inhalation or ingestion of phthalate‐contaminated air, water, sediment, and prey, and both direct and indirect or accidental ingestion of plastic (Cole et al., 2011; Fossi et al., 2012, 2014; Hu et al., 2016). Plastic additives and environmental contaminants sorbed to plastics are known to be bioavailable to organisms upon ingestion although the significance compared to other pathways is debated (Beckingham & Ghosh, 2017; Koelmans et al., 2016), and the full accounting of the risks of plastics in the environment is an ongoing research and monitoring need (Jahnke et al., 2017). What is clear, however, is that the largest production volume phthalate chemicals are most often utilized in plastics‐related industries, and increasing manufacture of plastics is a driver for continued environmental release of plastic additive chemicals such as phthalates (Rodgers et al., 2014). Use of phthalates has been shifting, however, following regulatory and consumer pressures to eliminate, limit, or replace phthalates in certain products (Erickson, 2017; Zota et al., 2014).
1.3. Phthalate Metabolism and Sampling Considerations
In humans and other mammals, phthalate metabolism occurs quickly through the hydrolysis and conjugation of diester phthalate parent compounds, thereby resulting in detectable monoester metabolites or conjugated compounds in feces and urine (Frederiksen et al., 2007; Wittassek & Angerer, 2008). Phthalate metabolites in urine, rather than the parent diester, have been deemed the most reliable indicator of phthalate exposure in human populations due to the rapid metabolism of diester phthalates in the body, as well as the potential for sample contamination with diester parent compounds from equipment during analysis (Blount et al., 2000; Calafat & McKee, 2006; Frederiksen et al., 2007; Högberg et al., 2008). Phthlates are also biotransformed in the aquatic food web, although metabolic transformation efficiencies tend to increase with trophic level (Hu et al., 2016).
Phthalate metabolites have been previously studied in a variety of marine and aquatic organisms including plankton, molluscs, and crustaceans (Blair et al., 2009), multiple fish species (Fourgous et al., 2016; Ros et al., 2015, 2016; Valton et al., 2014), alligators (Brock et al., 2016), a basking shark (Cetorhinus maximus, Fossi et al., 2014), and several marine mammal species inhabiting the Mediterranean Sea (Fossi et al., 2012, 2014; Baini et al., 2017). In the bulk of these investigations, blubber, organ, and muscle tissues were used to quantify concentrations in organisms, while bile has been analyzed in fish species (Ros et al., 2015; Valton et al., 2014). Only in the American alligator (Alligator mississippiensis) has urinalysis for phthalate metabolites been performed in wildlife species to date (Brock et al., 2016). Given that phthalate diester compounds are readily transformed to the monoester, conjugated and excreted in urine, metabolite measurement in urine provides a noninvasive method for assessing phthalate exposure.
1.4. Study Objectives
Our objective was to quantify urinary phthalate metabolites in bottlenose dolphins sampled in an urban estuary. As top‐level predators and marine mammals with a long lifespan, resident stocks of bottlenose dolphins serve as sensitive gauges to detect disturbances in their local environment (Schwacke et al., 2009, 2011, 2013; Smith et al., 2017; Wells et al., 2004). Urine, although a preferred matrix for biotransformed contaminants, is not a common matrix for wildlife studies. Urine has been used previously to assess bottlenose dolphin exposure to other contaminants, such as perfluorochemicals (Houde, Balmer, et al., 2006) and algal toxins (Twiner et al., 2011), but to our knowledge, this is the first study to quantify phthalate metabolites in bottlenose dolphin urine. Similarly, this is the first study to examine marine mammalian phthalate exposure among a population inhabiting coastal waters in the Gulf of Mexico. This study provides methods and baseline data critical for the development and use of urinary biomonitoring as an indicator for environmental phthalate contamination and helps to develop hypotheses for future studies regarding potential associations between phthalate exposure and health outcomes.
2. Materials and Methods
2.1. Sarasota Bay Dolphins
Bottlenose dolphins in Sarasota Bay, Florida, USA, have been part of a long‐term study to examine their biology, life history, movement patterns, reproduction, health, and behavior since 1970. Resident dolphins in this area belong to a population of approximately 160 individuals, and study methods have included telemetry, underwater acoustic recording, photoidentification (photo‐ID), focal animal behavioral follows, and capture‐release health assessments. Photo‐ID surveys were conducted seasonally until 1992 and subsequently have been conducted over 10 boat‐days each month to monitor the resident population (Wells, 2009). The Sarasota Bay bottlenose dolphin population includes the oldest known female (67 years in 2017), as well as up to five concurrent generations of related individuals (Scott et al., 1990; Wells, 2009, 2014; Wells et al., 1987; Wells et al., 2004). The long‐term study of dolphins in this region has provided the opportunity to conduct both cross‐sectional and longitudinal epidemiologic investigations of health outcomes that may result from environmental exposures and stressors (Hart et al., 2012; Schwacke et al., 2013; Twiner et al., 2011; Wells et al., 2004, 2005), including extensive studies on perfluoroalkyl compounds (Houde et al., 2005; Houde, Balmer, et al., 2006; Houde, Bujas, et al., 2006) and legacy persistent organic pollutants (POPs; Kucklick et al., 2011; Wells et al., 2005; Yordy et al., 2010a, 2010b). Sarasota Bay dolphins are multidecadal, multigenerational, year‐round residents to Sarasota Bay and vicinity. Some individuals have been observed for more than 40 years, through more than 1,500 sightings.
2.2. Sample Collection
Capture‐release health assessment methods have been previously described elsewhere (Wells, 2009). Briefly, small groups of free‐swimming bottlenose dolphins ranging in age between 2 and 50 years old are encircled in shallow water using a seine net measuring 500 m × 4 m and gently restrained by trained personnel. Blood is immediately collected from a ventral fluke vessel, and females are examined by ultrasound to determine pregnancy status. Dolphins that are not in their second or third trimester of pregnancy are then lifted via sling onto a specially designed, padded, and shaded sampling platform at which point measurements of mass, length, and girth are collected, prior to biological sampling. Once onboard, dolphins are gently rolled onto their right side to facilitate urine collection.
Urine was collected via aseptic catheterization (sterile Covidien‐brand Kendall™ or Kangaroo™ catheters) and initially collected into 15‐ or 50‐ml clear polypropylene centrifuge tubes (Corning), depending on volume. In some cases, a sterile 10‐ml Luer Lock disposable syringe (Excelint International Co.) was used to help create a vacuum during catheterization. Aliquots for phthalate metabolite analysis, ranging in volume between 2 and 10 ml, were subsampled from the collection vials into sterile, 10‐ml cryogenic copolymer vials (Denville Scientific) and kept cold on ice in the field. Upon return to land, samples were frozen with liquid nitrogen for transport in vapor shippers and stored below −20 °C until analysis. Urine sample blanks were prepared according to the National Institute of Standards and Technology's (NIST) marine mammal health assessment sample collection protocols similarly to collection of blood (Kucklick et al., 2010). Specifically, deionized water (DI) was pushed through each type of catheter as described above, collected into the same 15‐ or 50‐ml clear polypropylene centrifuge collection tubes as used for urine collection, then dispensed into the same type of cryogenic copolymer vials, kept cold on ice in the field, and frozen with liquid nitrogen for transport. The 2016 and 2017 bottlenose dolphin capture‐release health assessments in Sarasota Bay were conducted under National Marine Fisheries Service Scientific Research Permit No. 15543, as well as IACUC approvals renewed annually by Mote Marine Laboratory.
2.3. Sample Processing and Analysis
Each urine sample was screened for nine phthalate monoester metabolites including: monomethyl phthalate (MMP), monoethyl phthalate (MEP), monoisobutyl phthalate (MiBP), monobutyl phthalate (MBP), monobenzyl phthalate (MBzP), mono‐(2‐ethylhexyl) phthalate (MEHP), mono‐(2‐ethyl‐5‐oxohexyl) phthalate (MEOHP), mono‐(2‐ethyl‐5‐hydroxyhexyl) phthalate (MEHHP), and mono‐isononyl phthalate (MNP). Methods for analyzing phthalate metabolite concentrations in bottlenose dolphin urine were based on protocols established by the Centers for Disease Control and Prevention for human biomonitoring (Center for Disease Control and Prevention (CDC), 2012) and Wenzel et al. (2018). Processing of thawed, room temperature urine samples (1 ml) involved addition of 13C‐labeled phthalate metabolite internal standards (13C‐MEP, 13C‐4‐methyl umbelliferone, 13C‐MeHHP, 13C‐MEHP, 13C‐MBzP; Cambridge Isotope Laboratories) and 4‐methyl umbelliferone glucuronide (Sigma Aldrich), then enzymatic de‐glucuronidation with E. coli β‐glucuronidase (Roche Biomedical, 80 units/mg protein) in a 1 M ammonium acetate buffer, followed by isolation of phthalate metabolites by solid phase extraction (SPE). The enzymatic reaction was assessed by monitoring the conversion of 4‐methyl umbelliferone glucuronide to 4‐methyl umbelliferone. The enzyme used for de‐glucuronidation of conjugated phthalate metabolites does not convert diester phthalate parent compounds to monoesters (Blount et al., 2000). In brief, SPE cartridges (Agilent Bond Elute Nexus, 60 mg, 3 ml) were preconditioned with acetonitrile (1 ml) followed by phosphate buffer (1 ml, 0.14 M NaH2PO4 in 0.85% H3PO4:H2O). Samples were transferred onto cartridges, and cartridges were rinsed with 0.1 M formic acid (4 ml) followed by deionized water (2 ml), and then dried under vacuum. Phthalate metabolites were collected by eluting cartridges with acetonitrile (1 ml) and ethyl acetate (1 ml). The SPE method previously developed for human biomonitoring (CDC, 2012; Wenzel et al., 2018) was modified by increasing the wash step due to the relatively high specific gravity of the dolphin urine samples. Sample extracts were evaporated to dryness in a water bath (55 °C) under a gentle stream of nitrogen gas, then reconstituted in 200 μL of DI water. All reagents used were high purity and solvents were HPLC‐grade. Urine specific gravity was measured in the field with a hand‐held refractometer.
The separation, detection, and quantification of phthalate metabolites in urine extracts was performed by high‐performance liquid chromatography (Agilent 1100 series) with electrospray ionization tandem mass spectroscopy (Applied Biosystems 4000; HPLC‐ESI‐MS/MS). Separation was achieved over 15 min on a C18‐stationary phase analytical column (Waters XBridge 50 × 2.1 mm, 2.5 μm particle size) at a flow rate of 200–300 μL/min with a gradient mobile phase of acetonitrile with 0.1% acetic acid (A) and HPLC‐grade water with 0.1% acetic acid (B). The mobile phase gradient was 2.5 min at 20%A:80%B, followed by a linear ramp to 80%A:20%B by 15 min, then 4 min post‐run at 100%A and column reequilibration at 20%A:80%B for 15 min. Parameters for the mass spectrometer source include the following: entrance potential, −10 V; collision exit potential, −15 V; ion spray voltage, −4,200 V; temperature 450 °C. Compounds and mass transitions monitored are listed in Table S1 (supporting information) along with retention times. An additional confirmation ion was monitored for MEP and MEHP. Quantitation was accomplished using a 4–5 point calibration curve prepared in Type I DI with isotopically labeled phthalate monoester compounds, 4‐methyl umbelliferone (Sigma Aldrich), and target phthalate metabolites obtained as neat compounds from CanSyn (Toronto, CA). Calibration standards were processed through the method along with urine samples (CDC, 2012). Limit of detection (LOD) was estimated as the highest measured concentration in deionized water method blanks (USEPA, 2016) or the lowest standard in the calibration curve. The higher LOD was applied for a conservative approach (Table S1; Wenzel et al., 2018).
Quality assurance/quality control samples processed along with urine samples and calibration standards included DI blanks, DI spike, field blanks of urine collection equipment (catheters and syringes), urine sample duplicates, and urine matrix spikes. Phthalate metabolite concentrations in dolphin urine were corrected for field blanks (see supporting information). Urine concentrations were not adjusted for specific gravity.
2.4. Statistical Analysis
Statistical analyses used Statistica (Version 13, Dell Inc., Round Rock, TX) and R (Version 3.2, R Foundation for Statistical Computing, Vienna, Austria) software packages. Descriptive statistics were calculated for detectable phthalate concentrations, and normality was tested using a Shapiro‐Wilk test. Urinary phthalate metabolite concentrations for two commonly reported metabolites in the literature (MEP and MEHP) and the sum of all nine phthalate metabolites (Σpht) were compared between sampling years, sexes, and age classes (calves and subadults vs. adults) using a Mann‐Whitney U test. For these comparisons, concentrations below the limit of detection were included in the analyses as one half the concentration value of the lowest calibrant (0.5 ng/ml for MEP and 0.05 ng/ml for all other analytes). Dolphins were considered to be calves if captured with their mother, and dolphins 10 years of age or older were deemed adult (Read et al., 1993). Age was determined by analysis of dentinal growth layer groups (Hohn et al., 1989) or known year of birth via field observations. Statistical significance for the year, age, and sex comparisons within the Sarasota Bay data set was evaluated using α = 0.05. For dolphins sampled more than once in a single year, phthalate metabolite concentrations for the first sampling were used for year, sex, and age class comparisons.
3. Data
The data are provided in Figure 1, Table 2, and supporting information.
Figure 1.
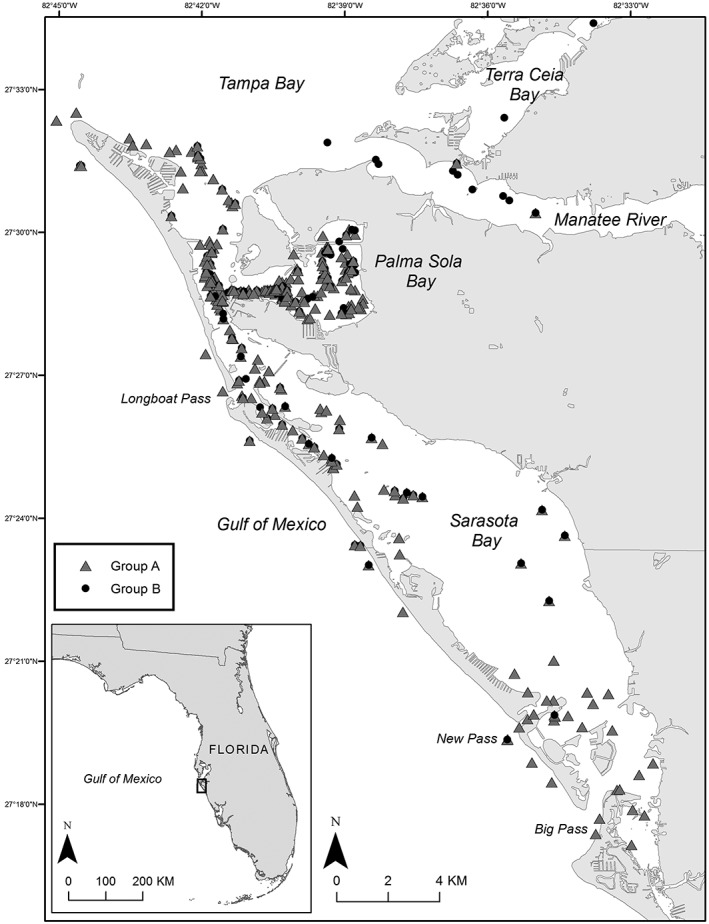
Sighting histories of Sarasota Bay bottlenose dolphins with detected levels of any phthalate metabolite (group A) and no detected levels of any phthalate metabolite (group B). Note: These sightings are for the calendar year prior to capture date.
Table 2.
Urinary Phthalate Metabolite Concentrations (ng/mL, Detected > LODa) for Bottlenose Dolphins Sampled From Sarasota Bay, FL in 2016 and 2017
| Dolphin | Specific gravity | MEPb , c | MEHPb , d | MEOHPe | Total |
|---|---|---|---|---|---|
| 2016 | |||||
| F188 | 1.043 | <LOD | |||
| F223 | 1.035 | <LOD | |||
| F259 | 1.026 | 0.2 | 0.2 | ||
| F292 | 1.040 | 33.4 | 2.0 | 0.1 | 35.5 |
| FB33 | 1.041 | 21.2 | 1.5 | 22.7 | |
| F178 | 1.030 | 1.3 | 1.3 | ||
| F209 | 1.034 | 5.3 | 5.3 | ||
| F209f | ND | 1.0 | 3.4 | 4.4 | |
| 2017 | |||||
| F242 | 1.040 | 3.1 | 1.7 | 0.4 | 5.2 |
| F173 | 1.037 | 1.0 | 1.0 | ||
| F271 | 1.042 | <LOD | |||
| F306 | 1.050 | 1.6 | 1.6 | ||
| F267 | 1.038 | <LOD | |||
| F263 | 1.039 | 5.9 | 0.3 | 6.2 | |
| F151 | 1.035 | 2.1 | 2.1 | ||
| FB07 | 1.045 | 0.9 | 0.9 | ||
| F296 | 1.041 | 3.1 | 3.1 | ||
| F164 | 1.035 | <LOD | |||
| No. at or above LODb | 7 | 9 | 4 | 13 | |
| Mediang | 4.2 | 1.9 | 0.3 | 2.6 | |
| IQRg | 1.6–21.2 | 1.3–2.6 | 0.2–0.4 | 1.2–5.8 | |
| Rangeg | 1.0–33.4 | 0.9–5.9 | 0.1–0.4 | 0.2–35.5 | |
| Meang | 11.0 | 2.3 | 0.3 | 7.1 | |
| S.D.g | 13.3 | 1.6 | 0.1 | 10.8 | |
|
Geometric Mean (95% CI) |
5.4 (1.3–22.0) | 1.9 (1.1–3.2) | 0.2 (0.1–0.6) | 2.9(1.2–7.2) | |
Limit of detection (LOD) is the higher value of either the method detection limit based on analysis of deionized water blanks (USEPA, 2016) or the lowest standard in the calibration curve (see Table S1).
MEP and MEHP concentrations have been corrected for catheter and syringe blanks (see Table S2). All other compounds were <LOD in field blanks.
MEP = monoethyl phthalate.
MEHP = mono‐(2‐ethylhexyl) phthalate.
MEOHP = mono‐(2‐ethyl‐5‐oxohexyl) phthalate.
Recapture sample for F209.
Reported for concentrations at or above the limit of detection. Calculations exclude recapture sample for F209.
4. Results
4.1. Sampled Dolphin Demographics
Urine for phthalate metabolite analysis was opportunistically collected from seven (four female, three male) dolphins in 2016 and 10 (five female, five male) dolphins in 2017. In both years, mother‐calf pairs (2016: n = 1; 2017: n = 2) were sampled for phthalate metabolites (Table 1). F292 and F296 were additional calves sampled in 2016 and 2017, respectively, whose mothers were not sampled for urinary phthalate metabolites. Likewise, F209 was a mother sampled in 2016 with a calf whose urine was not collected. Sampled dolphins were 2 to 34 years of age (Table 1).
Table 1.
Demographics of Bottlenose Dolphins (Tursiops truncatus) Sampled in Sarasota Bay, Florida, 2016–2017
| Dolphin ID | Year | Sex | Age | Age class | Calf of |
|---|---|---|---|---|---|
| F188 | 2016 | M | 20 | Adult | |
| F223 | 2016 | F | 15 | Adult | |
| F259 | 2016 | F | 3 | Calf | FB33 |
| F292 | 2016 | M | 4 | Calf | |
| FB33 | 2016 | F | 34 | Adult | |
| F178 | 2016 | M | 21 | Adult | |
| F209 | 2016 | F | 12 | Adult | |
| F209 (recapture) | 2016 | F | 12 | Adult | |
| F242 | 2017 | M | 27 | Adult | |
| F173 | 2017 | M | 15 | Adult | |
| F271 | 2017 | F | 23 | Adult | |
| F306 | 2017 | M | 4 | Calf | |
| F267 | 2017 | F | 2 | Calf | F151 |
| F263 | 2017 | F | 2 | Calf | FB07 |
| F151 | 2017 | F | 17 | Adult | |
| FB07 | 2017 | F | 33 | Adult | |
| F296 | 2017 | M | 4 | Calf | |
| F164 | 2017 | M | 28 | Adult |
4.2. Phthalate Concentrations
Phthalate metabolites were detected in 71% (N = 12) of the 17 individuals sampled. Method recoveries of MMP, MBP, and MNP assessed by matrix spikes performed with five different dolphin samples split between years was relatively low and variable (25% ± 32%, 68% ± 20% and 64% ± 16%, respectively). Method recovery of MMP and MBP from a spike into deionized water was higher (85% and 99%, respectively), and matrix spike recovery was lower at higher urine specific gravity (Figure S1), indicating that the analytical response of these compounds may be reduced by ion suppression. Because of the poor recovery and potential for matrix effects, MMP was excluded from analyses of phthalate exposure. Matrix spike recovery of all other phthalate metabolites measured was satisfactory (81% to 110%). MBP, MNP, MiBP, MEHHP, and MBzP were not detected in any dolphin samples. Method recovery may contribute to nondetection of MBP and MNP, although environmental prevalence is also expected to be lower for these parent phthalate compounds (Blair et al., 2009; D.‐W. Gao & Wen, 2016). The most commonly detected metabolites were MEP (seven of 18 samples), MEHP (nine of 18 samples), and MEOHP (four of 18 samples; Table 2). The highest concentration detected was for the metabolite MEP (33.4 ng/ml), while MEOHP occurred at the lowest concentrations (0.1 ng/ml). Concentrations for MEP ranged from 1.0 ng/ml to 33.4 ng/ml, MEHP ranged from 0.9 ng/ml to 5.9 ng/ml, and MEOHP ranged from 0.1 ng/ml to 0.4 ng/ml (Table 2). Measures of central tendency for concentrations of each detectable metabolite are reported in Table 2. Urine was collected from one dolphin (F209) during subsequent sampling events 4 days apart, where MEP was detected at different concentrations in each sample, and MEHP was detected only in the recapture sample (Table 2). Concentrations of MEHP and MEP were detected above LOD in some catheter and syringe field blanks used for sample collection (Table S2), and all urine results reported have been corrected for field blank concentrations.
Median and geometric mean concentrations of MEP, MEHP, and Σpht for both sampling years, sexes, and age classes are presented in Table 3. No significant differences in concentrations of MEP, MEHP, or Σpht were observed between years, sexes, or age classes (p > 0.05; Table 3). The geometric mean for total phthalate metabolite (Σpht) concentration for calves was 3.4 ng/ml, while adults measured 2.2 ng/ml (p = 0.39, Table 3). The geometric mean concentrations for Σpht and MEP in 2016 were 3.4 ng/ml and 2.5 ng/ml, respectively. In 2017, Σpht and MEP geometric means were 2.1 ng/ml and 0.7 ng/ml, which were not significantly different than the previous year (p = 0.81, Table 3).
Table 3.
Comparison of Bottlenose Dolphin Phthalate Metabolite Concentrations (ng/mL)a Between Sampling Years, Sexes, and Age Classes (Sarasota Bay, Florida, 2016–2017)
| Category | Level | N |
MEP |
Mediana , cMEP | MEP p | Mediana , c MEHP | MEHP p | GMa , b Σpht | Mediana , c Σpht | Σpht p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2016 | 7 | 2.5 (0.5–13.6) | 1.3 (0.5–21.2) | — | 0.1 (0.02–0.7) | 0.05 (0.05–1.5) | — | 3.4 (0.8–15.0) | 1.7 (0.9–23.0) | — |
| 2017 | 10 | 0.7 (0.4–1.1) | 0.5 (0.5–0.5) | 0.16 | 0.5 (0.1–1.9) | 1.0 (0.05–2.1) | 0.26 | 2.1 (1.2–3.6) | 1.9 (0.9–3.9) | 0.81 | |
| Sex | Male | 8 | 1.4 (0.4–4.7) | 0.9 (0.5–2.4) | — | 0.3 (0.1–1.5) | 0.5 (0.05–1.9) | — | 2.7 (1.0–7.6) | 1.9 (1.3–4.7) | — |
| Female | 9 | 1.0 (0.3–2.9) | 0.5 (0.5–0.5) | 0.47 | 0.3 (0.1–1.2) | 0.05 (0.05–1.5) | 0.89 | 2.4 (1.0–6.0) | 1.7 (0.9–5.7) | 0.89 | |
| Age Class | Calf/ Subadult | 6 | 1.2 (0.2–7.2) | 0.5 (0.5–1.6) | — | 0.4 (0.04–4.7) | 1.0 (0.05–3.1) | — | 3.4 (0.8–14.9) | 2.9 (1.0–7.0) | — |
| Adult | 11 | 1.1 (0.5–2.7) | 0.5 (0.5–3.1) | 1.00 | 0.2 (0.1–0.7) | 0.05 (0.05–1.5) | 0.45 | 2.2 (1.1–4.4) | 1.7 (0.9–5.5) | 0.39 |
Concentrations below the limit of detection were included in analysis as one‐half the lowest calibration standard concentration (0.5 ng/ml for MEP and 0.05 ng/ml for all other compounds). Calculations exclude recapture sample for F209.
Geometric mean (GM) and (95% confidence interval).
Median and (interquartile range).
4.3. Comparison of Sighting Histories
Individual dolphin photo‐ID sighting histories were analyzed as an indication of geographic exposure, as geographic differences in phthalate metabolite concentrations among cetaceans has been previously demonstrated (Fossi et al., 2012). The sightings of each dolphin sampled for phthalate metabolites were examined for the calendar year prior to their health assessment date and plotted on a map according to whether phthalate metabolites were detected (Group A) or not detected (Group B, Figure 1). Teitelbaum et al. (2008) suggested that single spot urine samples in human populations could reliably estimate phthalate exposure within the previous 6 months, while Hauser et al. (2004) found that single spot urinary concentrations of certain phthalate metabolites could signal exposure within the past 3 months. One calendar year was selected as the time frame, rather than 3 or 6 months, in order to increase the number of sightings per dolphin and show a more representative view of their occupation of the survey area. There was considerable geographic overlap in the sightings between both groups, although there were two regions where sightings of Group A appeared to dominate: southeastern Sarasota Bay and northwestern Sarasota Bay, extending into the mouth of Tampa Bay. However, the small sample size for each group prevents definitive conclusions on geographic differences in exposure. Sampling was not targeted specifically for any region of Sarasota Bay; samples in the present study were taken from dolphins opportunistically encountered at the time of sampling in Palma Sola Bay or northwestern Sarasota Bay. Interestingly, dolphin F209 was captured at two different locations within the northwest region of Sarasota Bay 4 days apart, and urine samples differed in levels of MEP and MEHP (Table 2).
5. Discussion
5.1. Phthalate Metabolites in Sarasota Bay Dolphins
This is the first study to report urinary concentrations of phthalate metabolites in bottlenose dolphins, and to our knowledge, any marine mammal. In humans, urine is considered the most reliable matrix for phthalate metabolite detection due primarily to the rapid metabolism of parent compounds and the potential for contamination of other matrices in the analysis of phthalate parent compounds (Blount et al., 2000; Calafat & McKee, 2006; Frederiksen et al., 2007; Högberg et al., 2008). We found that field blanks were a highly beneficial component of the present biomonitoring survey in order to differentiate levels in urine for these ubiquitous compounds, as has been discussed by others (Calafat & Needham, 2009). Thus, we have applied approaches used successfully for human biomonitoring to the study of an important environmental sentinel species in Sarasota Bay, Florida, USA. MEP and MEHP were the most commonly detected metabolites among Sarasota Bay bottlenose dolphins and were also detected at the highest concentrations compared to the other metabolites screened. MEHP, along with MEOHP and MEHHP, is a metabolite of the parent compound DEHP, which is a commonly used phthalate in commercially produced plastic items (ATSDR, 2002). MEP is a metabolite of DEP, a phthalate often added to cosmetics and personal care products (ATSDR, 1995).
Previous studies have demonstrated that despite the potential for daily fluctuations in humans, a single urine sample can predict the average exposure within the previous 3–6 months for some phthalates (Hauser et al., 2004; Teitelbaum et al., 2008); however, it is not known how exposure varies temporally in dolphins and therefore the representation of spot samples. Our findings further support the transient nature of phthalate exposure, based on urinalysis results for a dolphin (F209) that was sampled twice with only 4 days separating sampling events. Similar to most dolphins in the study, MEP and MEHP were the detectable metabolites for F209; however, MEP was higher during her first sampling event (5.3 ng/ml vs. 1.0 ng/ml), while MEHP was only detected during her second sampling event (3.4 ng/ml). These differences could represent geographic influence on exposure or reflect normal variation for an individual (Hauser et al., 2004). The significance of the findings for F209 remains to be understood until additional repeated samples can be collected from bottlenose dolphins.
5.2. Phthalate Metabolite Comparisons to Other Species
It is not particularly surprising to find MEP and MEHP in dolphins, since both of these compounds have been detected in other aquatic wildlife, such as blue mussel tissue (Mytilus edulis, Blair et al., 2009), muscle of Dungeness crab (Metacarcinus magister, Blair et al., 2009), and muscle, brain, liver, and bile from multiple species of fish (Fourgous et al., 2016; Ros et al., 2015, 2016; Valton et al., 2014). MEHP has been detected in blubber of fin whales (Balaenoptera physalus, Fossi et al., 2012, 2014), muscle of basking sharks (Cetorhinus maximus, Fossi et al., 2014), blubber and skin from a bottlenose dolphin (Baini et al., 2017), blubber and skin from a Risso's dolphin (Grampus griseus, Baini et al., 2017), blubber and skin from striped dolphins (Stenella coeruleoalba, Baini et al., 2017), and urine from American alligators (Alligator mississippiensis, Brock et al., 2016). Sarasota Bay dolphins revealed individual variability in the type and amount of phthalate exposure. This has been seen in these other studies as well (Brock et al., 2016; Fossi et al., 2012, 2014; Ros et al., 2015, 2016), where individual and geographic differences were observed.
MEHP and MEP are two of the most commonly reported phthalate metabolites detected in other marine species; however, most studies have reported organ or tissue concentrations (e.g., blubber, skin, muscle, and liver; Baini et al., 2017; Blair et al., 2009; Fossi et al., 2012, 2014; Hu et al., 2016; Ros et al., 2016), which are not directly comparable to urinary concentrations. MEHP has been examined in urine from Florida American alligators, where concentrations (mean = 56.4–4,540 ng/ml; Brock et al., 2016) were much higher than MEHP in Sarasota Bay dolphins (mean = 2.3 ng/ml; Table 4). Bile samples have been examined for certain species of fish where the concentrations of MEHP were also considerably higher (mean roach = 15.5 ng/ml, Valton et al., 2014; mean thicklip gray mullet = 2,530 ng/ml, Ros et al., 2015) than bottlenose dolphin urinary concentrations detected in the present study (Table 4). Bottlenose dolphin urine geometric mean concentrations of MEHP (1.9 ng/ml; 95:CI: 1.1 ng/ml to 3.2 ng/ml, n = 8) and MEP (5.4 ng/ml; 95% CI: 1.3 ng/ml to 22.0 ng/ml, n = 6) were compared to human urine geometric mean concentrations from the National Health and Nutrition Examination Survey (NHANES) for years 2011 to 2012 (MEHP: 1.36 ng/ml, 95% CI: 1.25 ng/ml to 1.49 ng/ml; MEP: 37.9 ng/ml, 95% CI: 33.0 ng/ml to 43.5 ng/ml) and 2009–2010 (MEHP: 1.59 ng/ml, 95% CI: 1.41 ng/ml to 1.79 ng/ml; MEP: 64.4 ng/ml, 95% CI: 58.3 ng/ml to 71.2 ng/ml; CDC, 2017). MEHP and MEP were not detected in all bottlenose dolphin urine samples; however, among those with detectable concentrations, MEP was significantly lower in dolphins than reported human concentrations (p < 0.05, Figure 2a). The MEHP geometric mean concentration for bottlenose dolphins was greater than both NHANES years; however, likely due to the small sample of dolphins, these differences were not statistically significant (p ≥ 0.05, Figure 2b). Additional dolphin sampling may confirm preliminary differences observed in this study.
Table 4.
Comparison of Bottlenose Dolphin MEHP and MEP Urinary Concentrations to Reported Concentrations in Bile and Urine for Other Marine and Aquatic Species
| Metabolite | Species | N | Min | Max | Mean | SD | Units | Matrix | Location | Paper |
|---|---|---|---|---|---|---|---|---|---|---|
| MEHP | Bottlenose Dolphina | 17 | <LOD | 5.9 | 2.4 | 1.6 | ng/ml | Urine | Sarasota Bay, Florida, USA | This study |
| Alligator | 9 | <1.20 | 35,700 | 4,540 | 11,800 | ng/ml | Urine | Everglades, Florida, USA | Brock et al. (2016) | |
| Alligator | 10 | <1.20 | 11,500 | 1,490 | 1,290 | ng/ml | Urine | Okeechobee – Belle Glade, Florida, USA | Brock et al. (2016) | |
| Alligator | 10 | <1.20 | 11,100 | 1,290 | 3,470 | ng/ml | Urine | Okeechobee – Moonshine Bay, Florida, USA | Brock et al. (2016) | |
| Alligator | 9 | <1.20 | 506 | 56.4 | ‐ | ng/ml | Urine | Woodruff, Florida, USA | Brock et al. (2016) | |
| Roach | 1 | ‐ | ‐ | 15.5 | ‐ | ng/ml | Bile | Orge River, France | Valton et al. (2014) | |
| Thicklip Gray Mullet | 29 | <LOD | 20,581 | 2,532.8 | 4,203.6 | ng/ml | Bile | South East Bay of Biscay | Ros et al. (2015) | |
| MEP | Bottlenose Dolphina | 17 | <LOD | 33.4 | 9.6 | 12.7 | ng/ml | Urine | Sarasota Bay, Florida, USA | This study |
| Roach | 4 | ‐ | ‐ | 53 | 15.3 | ng/ml | Bile | Orge River, France | Valton et al. (2014) |
Mean and S.D. calculated for concentrations at or above the LOD (MEP n = 7; MEHP n = 9).
Figure 2.
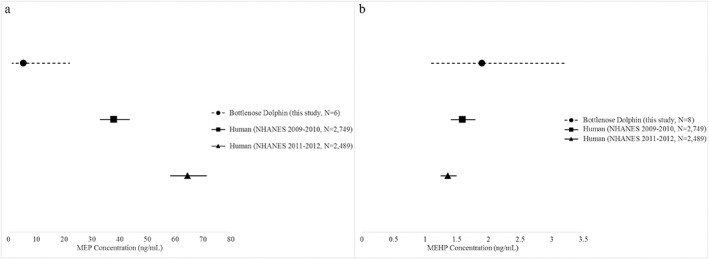
Comparisons of urinary MEP (a) and MEHP (b) concentrations between live bottlenose dolphins sampled from Sarasota Bay, FL in 2016 and 2017 and human concentrations from the National Health and Nutrition Examination Survey (NHANES; CDC, 2017). Human concentrations reported as geometric mean and 95% confidence interval. Bottlenose dolphin concentration reported as geometric mean and 95% confidence interval for animals with detectable concentrations.
5.3. What Do these Data Suggest Regarding Exposure?
Although the sample size was small, this study provides some evidence that urinary levels of MEHP are relatively high in Sarasota Bay bottlenose dolphins based on comparisons to human urine biomonitoring data, warranting more detailed study of exposure routes in the coastal marine environment. Fair et al. (2009) have also reported comparable biomonitoring data between bottlenose dolphins and humans. In their study, triclosan, a common antimicrobial agent and potential endocrine‐disrupting chemical recently restricted by the U.S. Food and Drug Administration, was detected in dolphin plasma from other coastal environments in FL and SC at levels comparable to those in human blood plasma (Fair et al., 2009). The only other study to our knowledge measuring urinary phthalate levels of MEHP in wildlife was conducted by Brock et al. (2016), who found extremely high levels in American alligators. Although the comparison is complicated by interspecies differences in exposure and biotransformation, the levels of MEHP were higher than in human populations and dolphin urine in the current study. These American alligators were sampled from select lakes and wetlands in Florida and may have received exposure from phthalate diesters added to herbicide formulations used for aquatic weed control (Brock et al., 2016). Whether this is a source of exposure for bottlenose dolphins is unknown.
MEP concentrations in bottlenose dolphins were lower than human studies. This result should be expected since human exposure to DEP is primarily through the direct application of cosmetics and personal care products (ATSDR, 1995). Still, MEP was found at the highest concentrations among phthalate metabolites detected in this study, and MEP was not detected in alligators by Brock et al. (2016). While many of the wastewater utilities in the Sarasota area have eliminated direct surface water discharges of treated effluents in recent years and instead have diverted reclaimed wastewater to groundwater recharge or agricultural reuse (Sarasota Bay Estuary Program (SBEP), 2014), these results indicate that other sources of DEP/MEP remain and surface runoff and volatile emissions from agricultural and urban environments are potential concerns.
Two regions of potential interest identified by overlaying dolphin sighting data with phthalate metabolite detections are southeastern and northwestern Sarasota Bay. Southeastern Sarasota Bay drains the City of Sarasota proper and also coincides with an area of Sarasota County that is currently undergoing massive investments in water utility infrastructure in order to replace over 14,000 septic systems that present surface water quality issues (SBEP, 2014). The northwestern region of Sarasota Bay including Palma Sola Bay is within close proximity to Tampa Bay and the outlet of the Manatee River. Tampa Bay is a highly urbanized estuary, and the Manatee River watershed and surrounding Manatee County is agricultural but also experiencing rapid development (Tampa Bay Estuary Program, 2011). The area has a high rate of utilization of reclaimed, treated wastewater for irrigation (SBEP, 2014), and water quality in the basin is potentially impacted by agricultural runoff from widespread use of organic pesticides (Southwest Florida Water Management District, 2001; MacDonald et al., 2004). The Sarasota Bay region, anchored by the city of Sarasota in the south and the city of Bradenton near Palma Sola Bay in the north, was recently ranked the tenth fastest growing metropolitan region in the United States (U.S. Census Bureau, 2017). Proximity to urban environments also has been associated with higher air concentrations of phthalate chemicals (Rudel & Perovich, 2009). Additional sampling of individuals that frequent different regions of the study area and whose locations are known more specifically to the previous 24–48 hr proximate to sampling would help identify the presence of any geographic trends in exposure, including potential sources.
While no significant differences in MEP, MEHP, and Σpht were observed between years, sexes, or age classes, geometric mean and median concentrations of MEHP and Σpht in calves were slightly higher than adults. In our study, the urine samples from three mother‐calf pairs were analyzed for phthalate metabolites, yielding mixed results. F151 had detectable concentrations of MEHP, but her calf (F267) did not have detectable concentrations of any of the phthalate metabolites screened. FB33, the mother of F259, had detectable concentrations of MEP and MEHP, while F259 only had a detectable concentration of MEOHP (near the LOD). Finally, MEHP was the only detectable metabolite in the urine from FB07 and found at lower levels than her calf (F263), who also had a detectable concentration of MEOHP. Phthalates may be transferred to offspring postpartum during lactation, based on the detection in human breast milk of several phthalate diesters, especially the more lipophilic diesters and metabolites such as DEHP/MEHP (Fromme et al., 2011; Main et al., 2006). However, it is uncertain whether the mother‐calf pairs encountered in the present study were nursing or if lactation is a relevant route of phthalate exposure for bottlenose dolphin calves. One possible explanation for the differences observed in mother‐calf exposures could be that the calves, at 2 and 3 years of age, were starting to forage on their own. Weaning of calves among Sarasota Bay dolphins is gradual, with small prey supplementing milk during the first years of life (Wells et al., 2005). Also, calves may metabolize phthalates differently than adult dolphins, evidenced by the detection of MEOHP in calf F259 and higher levels of MEHP than her mother (FB07), suggesting differences in metabolism of DEHP. There are also differences in physiology and activity level (e.g., inhalation rates) between adults and calves that would influence exposure and urinalysis results. Unfortunately, results from this study do not enhance our understanding of maternal phthalate transfer in dolphins because of the few mother‐calf pairs sampled in these years. Maternal transfer of biotransformed compounds is known to be complex, and potentially a compound‐selective process (Yordy et al., 2010a).
5.4. Next Steps
Bottlenose dolphin exposure to DEHP and DEP is concerning due to laboratory and human studies linking these chemicals to adverse health effects including altered hormone synthesis and transport (ATSDR, 2002; Meeker & Ferguson, 2014; Sathyanarayana et al., 2017], male genital developmental abnormalities (ATSDR, 2002; Swan, 2008), reproductive impairment (e.g., lower birth weight, Messerlian et al., 2017; preterm birth, Meeker et al., 2009; delayed time to pregnancy, Thomsen et al., 2017; pregnancy loss, H. Gao et al., 2017), and liver toxicoses including liver cancer (ATSDR, 2002). In fact, MEHP concentrations measured in the dolphins in this study (GM = 1.9 ng/ml; Range = 0.9–5.9 ng/ml) were at comparable levels reported to be associated with a reduced probability of reproductive success in humans (Hauser et al., 2016). More specifically among women undergoing IVF treatment, as urinary concentrations increased, Hauser et al. (2016) observed a decreased probability of intrauterine pregnancies and successful live births, as well as reduced oocyte counts. Although bottlenose dolphin health impacts from phthalate exposure are currently unknown, continued study is warranted especially as production and use of these chemicals persist. Results from this study demonstrate that Sarasota Bay dolphins are exposed to and metabolize these chemicals, thus providing a foundation for future studies investigating the correlation between phthalate metabolite concentrations and other biomarkers of health.
Previous studies of phthalate metabolites in the blubber of marine mammals inhabiting different regions of the Mediterranean Sea have provided evidence that phthalate metabolites, specifically MEHP, may be indicative of environmental microplastic contamination (Baini et al., 2017; Fossi et al., 2012, 2014). If true, this suggests that Sarasota Bay and surrounding areas could be polluted with plastic due to contaminated runoff or effluents; however, to our knowledge, systematic studies of microplastic contamination have not been reported for Sarasota Bay. The detection of MEP in these dolphins suggests exposure also to nonplastic sources, as DEP is commonly incorporated in personal care products, cosmetics, and pesticide/herbicide products. Ultimately, we hope to establish the capability to use urinary phthalate metabolites as a biomarker of environmental contamination and help to identify the source (s) of exposure for Sarasota Bay bottlenose dolphins.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Supporting information
Supporting Information S1
Acknowledgments
Funding for this work was provided by the College of Charleston's Faculty Research and Development grant, the Department of Health and Human Performance's Research and Development grant, and the Department of Geology and Environmental Geosciences. We also thank Abby Wenzel and Wayne McFee for helpful discussions about this project, Krystan Wilkinson for GIS assistance, as well as Eric Zolman, Jared Ragland, Peter Key, and journal peer‐reviewers for their insightful comments to improve this manuscript. Dolphin samples were obtained through health assessments supported primarily by Dolphin Quest, Inc. We are grateful to the staff, collaborators, and volunteers of the Sarasota Dolphin Research Program for ensuring the safe capture, sampling, and release of the dolphins. The data used for these analyses can be found in the text, tables, figures, and supporting information. Supporting information can be found on the web at http://agupubs.onlinelibrary.wiley.com. Disclaimer: The scientific results and conclusions, as well as any opinions expressed herein, are those of the author(s) and do not necessarily reflect the views of the National Oceanic and Atmospheric Administration (NOAA), the National Institute of Standards and Technology (NIST), or the Department of Commerce. Certain commercial equipment, instruments, or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by NIST or NOAA, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Hart, L. B. , Beckingham, B. , Wells, R. S. , Alten Flagg, M. , Wischusen, K. , Moors, A. , et al. (2018). Urinary phthalate metabolites in common bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, FL, USA. GeoHealth, 2, 313–326. 10.1029/2018GH000146
This article was corrected on 15 JUL 2019. The online version of this article has been modified to include a Conflict of Interest statement.
Contributor Information
Leslie B. Hart, Email: hartlb@cofc.edu.
Barbara Beckingham, Email: beckinghamba@cofc.edu.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) (1995). Toxicological profile for diethyl Phthalate. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) (2002). Toxicological profile for di(2‐ethylhexyl) phthalate (DEHP). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [PubMed] [Google Scholar]
- Baini, M. , Martellini, T. , Cincinelli, A. , Campani, T. , Minutoli, R. , Panti, C. , et al. (2017). First detection of seven phthalate esters (PAEs) as plastic tracers in superficial neustonic/planktonic samples and cetacean blubber. Analytical Methods, 9(9), 1512–1520. [Google Scholar]
- Beckingham, B. , & Ghosh, U. (2017). Differential bioavailability of polychlorinated biphenyls associated with environmental particles: Microplastic in comparison to wood, coal and biochar. Environmental Pollution, 220, 150–158. [DOI] [PubMed] [Google Scholar]
- Bell, F. P. (1982). Effects of phthalate esters on lipid metabolism in various tissues, cells and organelles in mammals. Environmental Health Perspectives, 45, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair, J. D. , Ikonomou, M. G. , Kelly, B. C. , Surridge, B. , & Gobas, F. A. (2009). Ultra‐trace determination of phthalate ester metabolites in seawater, sediments, and biota from an urbanized marine inlet by LC/ESI‐MS/MS. Environmental Science & Technology, 43(16), 6262–6268. [DOI] [PubMed] [Google Scholar]
- Blount, B. C. , Silva, M. J. , Caudill, S. P. , Needham, L. L. , Pirkle, J. L. , Sampson, E. J. , Lucier, G. W. (2000). Levels of seven urinary phthalate metabolites in a human reference population. Environmental Health Perspectives, 108, 979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock, J. W. , Bell, J. M. , & Guillette, L. J. (2016). Urinary phthalate metabolites in American alligators (Alligator mississippiensis) . Archives of Environmental Contamination and Toxicology, 71(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Calafat, A. M. , & McKee, R. H. (2006). Integrating biomonitoring exposure data into the risk assessment process: Phthalates [diethyl phthalate and di (2‐ethylhexyl) phthalate] as a case study. Environmental Health Perspectives, 114(11), 1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat, A. M. , & Needham, L. L. (2009). What additional factors beyond state‐of‐the‐art analytical methods are needed for optimal generation and interpretation of biomonitoring data? Environmental Health Perspectives, 117, 1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (CDC) (2017). Fourth report on human exposure to environmental chemicals, updated tables (January 2017). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Retrieved from https://www.cdc.gov/exposurereport/ [Google Scholar]
- Center for Disease Control and Prevention (CDC) (2012). Laboratory procedure manual for phthalate metabolites in urine by HPLC/ESI‐MS/MS. method no. 6303.03. Revised 26 March, 2012.
- Cole, M. , Lindeque, P. , Halsband, C. , & Galloway, T. S. (2011). Microplastics as contaminants in the marine environment: A review. Marine Pollution Bulletin, 62(12), 2588–2597. [DOI] [PubMed] [Google Scholar]
- Erickson, B. E. (2017). Regulators and retailers raise pressure on phthalates. Chemical & Engineering News, 93(25), 11–15. [Google Scholar]
- Fair, P. A. , Mitchum, G. , Hulsey, T. C. , Adams, J. , Zolman, E. , McFee, W. , Wirth, E. , & Bossart, G. D. (2009). Occurrence of triclosan in plasma of wild Atlantic bottlenose dolphins (Tursiops truncatus) and in their environment. Environmental Pollution, 157, 2248–2254. [DOI] [PubMed] [Google Scholar]
- Fossi, M. C. , Coppola, D. , Baini, M. , Giannetti, M. , Guerranti, C. , Marsili, L. , et al. (2014). Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: The case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus). Marine Environmental Research, 100, 17–24. [DOI] [PubMed] [Google Scholar]
- Fossi, M. C. , Panti, C. , Guerranti, C. , Coppola, D. , Giannetti, M. , Marsili, L. , Minutoli, R. (2012). Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Marine Pollution Bulletin, 64(11), 2374–2379. [DOI] [PubMed] [Google Scholar]
- Fourgous, C. , Chevreuil, M. , Alliot, F. , Amilhat, E. , Faliex, E. , Paris‐Palacious, S. , Teil, M. J. , & Goutte, A. (2016). Phthalate metabolites in the European eel (Anguilla anguilla) from Mediterranean coastal lagoons. Science of the Total Environment, 569‐570, 1053–1059. [DOI] [PubMed] [Google Scholar]
- Frederiksen, H. , Skakkebaek, N. E. , & Andersson, A. M. (2007). Metabolism of phthalates in humans. Molecular Nutrition & Food Research, 51(7), 899–911. [DOI] [PubMed] [Google Scholar]
- Fromme, H. , Gruber, L. , Seckin, E. , Raab, U. , Zimmermann, S. , Kiranoglu, M. , et al. (2011). Phthalates and their metabolites in breast Milk—Results from the Bavarian monitoring of breast milk (BAMBI). Environment International, 37(4), 715–722. [DOI] [PubMed] [Google Scholar]
- Gao, D.‐W. , & Wen, Z.‐D. (2016). Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Science of the Total Environment, 541, 986–1001. [DOI] [PubMed] [Google Scholar]
- Gao, H. , Zhang, Y. W. , Huang, K. , Yan, S. Q. , Mao, L. J. , Ge, X. , et al. (2017). Urinary concentrations of phthalate metabolites in early pregnancy associated with clinical pregnancy loss in Chinese women. Scientific Reports, 7(1), 6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, L. B. , Rotstein, D. S. , Wells, R. S. , Allen, J. , Barleycorn, A. , Balmer, B. C. , et al. (2012). Skin lesions on common bottlenose dolphins (Tursiops truncatus) from three sites in the Northwest Atlantic, USA. PLoS One, 7(3), e33081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, R. , Gaskins, A. J. , Souter, I. , Smith, K. W. , Dodge, L. E. , Ehrlich, S. , Meeker, J. D. , Calafat, A. M. , & Williams, P. L. (2016). Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: Results from the EARTH study. Environmental Health Perspectives, 124(6), 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, R. , Meeker, J. D. , Park, S. , Silva, M. J. , & Calafat, A. M. (2004). Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environmental Health Perspectives, 112, 1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högberg, J. , Hanberg, A. , Berglund, M. , Skerfving, S. , Remberger, M. , Calafat, A. M. , et al. (2008). Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environmental Health Perspectives, 116(3), 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn, A. A. , Scott, M. D. , Wells, R. S. , Sweeney, J. C. , & Irvine, A. B. (1989). Growth layers in teeth from known‐age free‐ranging bottlenose dolphins. Marine Mammal Science, 5(4), 315–342. [Google Scholar]
- Houde, M. , Balmer, B. C. , Brandsma, S. , Wells, R. S. , Rowles, T. K. , Solomon, K. R. , Muir, D. C. G. (2006). Perfluoroalkyl compounds in relation to life‐history and reproductive parameters in bottlenose dolphins (Tursiops trunacatus) from Sarasota Bay, Florida, USA. Environmental Toxicology & Chemistry, 25(9), 2405–2412. [DOI] [PubMed] [Google Scholar]
- Houde, M. , Bujas, T. A. , Small, J. , Wells, R. S. , Fair, P. A. , Bossart, G. D. , et al. (2006). Biomagnification of perfluoroalkyl compounds in the bottlenose dolphin (Tursiops truncatus) food web. Environmental Science & Technology, 40(13), 4138–4144. [DOI] [PubMed] [Google Scholar]
- Houde, M. , Wells, R. S. , Fair, P. A. , Bossart, G. D. , Hohn, A. A. , Rowles, T. K. , et al. (2005). Polyfluoroalkyl compounds in free‐ranging bottlenose dolphins (Tursiops truncatus) from the Gulf of Mexico and the Atlantic Ocean. Environmental Science & Technology, 39(17), 6591–6598. [DOI] [PubMed] [Google Scholar]
- Hu, X. , Gu, Y. , Huang, W. , & Yin, D. (2016). Phthalate monoesters as markers of phthalate contamination in wild marine organisms. Environmental Pollution, 218, 410–418. [DOI] [PubMed] [Google Scholar]
- Jahnke, A. , Arp, H. P. H. , Escher, B. I. , Gewert, B. , Gorokhova, E. , Kuhnel, D. , Ogonowski, M. , Potthoff, A. , Rummel, C. , Schmitt‐Jansen, M. , Toorman, E. , & MacLeod, M. (2017). Reducing uncertainty and confronting ignorance about the possible impacts of weathering plastic in the marine environment. Environmental Science & Technology Letters, 4, 85–90. [Google Scholar]
- Koelmans, A. A. , Bakir, A. , Burton, G. A. , & Janssen, C. R. (2016). Microplastic as a vector for chemicals in the aquatic environment: Critical review and model‐supported reinterpretation of empirical studies. Environmental Science & Technology, 50(7), 3315–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucklick, J. , Pugh, R. , Becker, P. , Keller, J. , Day, R. , Yordy, J. , et al. (2010). Specimen banking for marine animal health assessment In Isobe T., Nomiyama K., Subramanian A., & Tanabe S. (Eds.), Interdisciplinary Studies on Environmental Chemistry ‐ Environmental Specimen Bank (pp. 15–23). Terrapub: Ehime, Japan. [Google Scholar]
- Kucklick, J. , Schwacke, L. , Wells, R. , Hohn, A. , Guichard, A. , Yordy, J. , et al. (2011). Bottlenose dolphins as indicators of persistent organic pollutants in the western North Atlantic Ocean and northern Gulf of Mexico. Environmental Science & Technology, 45(10), 4270–4277. [DOI] [PubMed] [Google Scholar]
- Latini, G. , Verrotti, A. , & De Felice, C. (2004). Di‐2‐ethylhexyl phthalate and endocrine disruption: A review. Current Drug Targets. Immune, Endocrine and Metabolic Disorders, 4(1), 37–40. [DOI] [PubMed] [Google Scholar]
- MacDonald, D. D. , Carr, R. S. , Eckenrod, D. , Greening, H. , Grabe, S. , Ingersoll, C. G. , et al. (2004). Development, evaluation, and application of sediment quality targets for assessing and managing contaminated sediments in Tampa Bay, Florida. Archives of Environment Contamination and Toxicology, 46, 147–161. [DOI] [PubMed] [Google Scholar]
- Main, K. M. , Mortensen, G. K. , Kaleva, M. M. , Boisen, K. A. , Damgaard, I. N. , Chellakooty, M. , et al. (2006). Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environmental Health Perspectives, 114(2), 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu‐Denoncourt, J. , Wallace, S. J. , de Solla, S. R. , & Langlois, V. S. (2015). Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non‐mammalian aquatic species. General and Comparative Endocrinology, 219, 74–88. [DOI] [PubMed] [Google Scholar]
- Meeker, J. D. , & Ferguson, K. K. (2014). Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. The Journal of Clinical Endocrinology & Metabolism, 99(11), 4346–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker, J. D. , Hu, H. , Cantonwine, D. E. , Lamadrid‐Figueroa, H. , Calafat, A. M. , Ettinger, A. S. , et al. (2009). Urinary phthalate metabolites in relation to preterm birth in Mexico City. Environmental Health Perspectives, 117(10), 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian, C. , Braun, J. M. , Mínguez‐Alarcón, L. , Williams, P. L. , Ford, J. B. , Mustieles, V. , et al. (2017). Paternal and maternal urinary phthalate metabolite concentrations and birth weight of singletons conceived by subfertile couples. Environment International, 107, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlmann, J. , Schulte‐Oehlmann, U. , Kloas, W. , Jagnytsch, O. , Lutz, I. , Kusk, K. O. , Wollenberger, L. , Santos, E. M. , Paull, G. C. , Van Look, K. J. W. , & Tyler, C. R. (2009). A critical analysis of the biological impacts of plasticizers on wildlife. Philosophical Transactions of the Royal Society B, 394, 2047–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, A. J. , Wells, R. S. , Hohn, A. A. , & Scott, M. D. (1993). Patterns of growth in wild bottlenose dolphins, Tursiops truncatus . Journal of Zoology, 231(1), 107–123. [Google Scholar]
- Rodgers, K. M. , Rudel, R. A. , & Just, A. C. (2014). Phthalates in food packaging, consumer products, and indoor environments In Snedeker S. M. (Ed.), Toxicants in Food Packaging and Household Plastics (pp. 31–59). London: Springer‐Verlag. [Google Scholar]
- Ros, O. , Izaguirre, J. K. , Olivares, M. , Bizarro, C. , Ortiz‐Zarragoitia, M. , Cajaraville, M. P. , et al. (2015). Determination of endocrine disrupting compounds and their metabolites in fish bile. Science of the Total Environment, 536, 261–267. [DOI] [PubMed] [Google Scholar]
- Ros, O. , Vallejo, A. , Olivares, M. , Etxebarria, N. , & Prieto, A. (2016). Determination of endocrine disrupting compounds in fish liver, brain, and muscle using focused ultrasound solid–liquid extraction and dispersive solid phase extraction as clean‐up strategy. Analytical and Bioanalytical Chemistry, 408(21), 5689–5700. [DOI] [PubMed] [Google Scholar]
- Rudel, R. A. , & Perovich, L. J. (2009). Endocrine disrupting chemicals in indoor and outdoor air. Atmospheric Environment, 43, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasota Bay Estuary Program (SBEP) (2014). Sarasota Bay comprehensive conservation and management plan update and state of the bay report. Retrieved from https://www.epa.gov/sites/production/files/2015-09/documents/ccmp.stateofthebay-for-website-august2014.pdf
- Sathyanarayana, S. , Butts, S. , Wang, C. , Barrett, E. , Nguyen, R. , Schwartz, S. M. , et al. (2017). Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. The Journal of Clinical Endocrinology & Metabolism, 102(6), 1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacke, L. H. , Hall, A. J. , Townsend, F. I. , Wells, R. S. , Hansen, L. J. , Hohn, A. A. , et al. (2009). Hematologic and serum biochemical reference intervals for free‐ranging common bottlenose dolphins (Tursiops truncatus) and variation in the distributions of clinicopathologic values related to geographic sampling site. American Journal of Veterinary Research, 70(8), 973–985. [DOI] [PubMed] [Google Scholar]
- Schwacke, L. H. , Smith, C. R. , Townsend, F. I. , Wells, R. S. , Hart, L. B. , Balmer, B. C. , et al. (2013). Health of common bottlenose dolphins (Tursiops truncatus) in Barataria Bay, Louisiana, following the Deepwater Horizon oil spill. Environmental Science & Technology, 48(1), 93–103. [DOI] [PubMed] [Google Scholar]
- Schwacke, L. H. , Voit, E. O. , Hansen, L. J. , Wells, R. S. , Mitchum, G. B. , Hohn, A. A. , Fair, P. A. (2002). Probabilistic risk assessment of reproductive effects of polychlorinated biphenyls on bottlenose dolphins (Tursiops truncatus) from the Southeast United States coast. Environmental Toxicology & Chemistry, 21(12), 2752–2764. [PubMed] [Google Scholar]
- Schwacke, L. H. , Zolman, E. S. , Balmer, B. C. , De Guise, S. , George, R. C. , Hoguet, J. , et al. (2011). Anaemia, hypothyroidism and immune suppression associated with polychlorinated biphenyl exposure in bottlenose dolphins (Tursiops truncatus). Proceedings of the Royal Society of London B: Biological Sciences, 279(1726), 48–57. 10.1098/rspb.2011.0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, M. D. , Wells, R. S. , & Irvine, A. B. (1990). A long‐term study of bottlenose dolphins on the west coast of Florida In Leatherwood S. & Reeves R. R. (Eds.), The Bottlenose Dolphin (pp. 235–244). New York: Academic Press. [Google Scholar]
- Smith, C. R. , Rowles, T. K. , Hart, L. B. , Townsend, F. I. , Wells, R. S. , Zolman, E. S. , et al. (2017). Slow recovery of Barataria Bay dolphin health following the Deepwater Horizon oil spill (2013–2014), with evidence of persistent lung disease and impaired stress response. Endangered Species Research, 33, 127–142. [Google Scholar]
- Southern California Coastal Water Research Program (SCCWRP) (2012). Monitoring strategies for chemicals of emerging concern (CECs) in California's aquatic ecosystems . SCCWRP Technical Report No. 692. Costa Mesa, CA.
- Southwest Florida Water Management District (SFWMD) (2001). Manatee River: Comprehensive watershed management plan. 26 April 2001. Retrieved from http://www.swfwmd.state.fl.us/documents/plans/cwm/cwm-manateeriver.pdf
- Swan, S. H. (2008). Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environmental Research, 108(2), 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampa Bay Estuary Program (TBEP) (2011). Development of numeric nutrient criteria for Boca Ciega Bay, Terra Ceia Bay, and Manatee River, Florida. September 2011. Retrieved from https://www.tbeptech.org/attachments/article/97/TBEP_BCB_TCB_MR_NNC_Full_Technical_Document_Final.pdf
- Teitelbaum, S. L. , Britton, J. A. , Calafat, A. M. , Ye, X. , Silva, M. J. , Reidy, J. A. , et al. (2008). Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environmental Research, 106(2), 257–269. [DOI] [PubMed] [Google Scholar]
- Thomsen, A. M. L. , Riis, A. H. , Olsen, J. , Jönsson, B. A. , Lindh, C. H. , Hjollund, N. H. , et al. (2017). Female exposure to phthalates and time to pregnancy: A first pregnancy planner study. Human Reproduction, 32(1), 232–238. [DOI] [PubMed] [Google Scholar]
- Twiner, M. J. , Fire, S. , Schwacke, L. , Davidson, L. , Wang, Z. , Morton, S. , et al. (2011). Concurrent exposure of bottlenose dolphins (Tursiops truncatus) to multiple algal toxins in Sarasota Bay, Florida, USA. PLoS One, 6(3), e17394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Census Bureau (2017). Press kit: County and metro area population estimates. Table 4: Top 25 Fastest‐growing metro areas (percent change): July 1, 2015 to July 1, 2016. Published Online 23 March 2017. Retrieved from https://www.census.gov/content/dam/Census/newsroom/press-kits/2017/Top%2025%20Fastest%20Metros.pdf
- USEPA (2016). Definition and procedure for the determination of the method detection limit, Revision 2. United States Environmental Protection Agency, Office of Water. Doc. No. EPA 821‐R‐16‐006. December 2016. Retrieved from https://www.epa.gov/sites/production/files/2016-12/documents/mdl-procedure_rev2_12-13-2016.pdf
- Valton, A. S. , Serre‐Dargnat, C. , Blanchard, M. , Alliot, F. , Chevreuill, M. , & Jeanne Teil, M. (2014). Determination of phthalates and their by‐products in tissues of roach (Rutilus rutilus) from the Orge River (France). Environmental Science and Pollution Research, 21, 12,723–12,730. [DOI] [PubMed] [Google Scholar]
- Wells, R. S. (2009). Learning from nature: Bottlenose dolphin care and husbandry. Zoo Biology, 28(6), 635–651. [DOI] [PubMed] [Google Scholar]
- Wells, R. S. (2014). Social structure and life history of bottlenose dolphins near Sarasota Bay, Florida: Insights from four decades and five generations In Yamagiwa J. & Karczmarski L. (Eds.), Primates and Cetaceans (pp. 149–172). Tokyo, Japan: Springer. [Google Scholar]
- Wells, R. S. , Rhinehart, H. L. , Hansen, L. J. , Sweeney, J. C. , Townsend, F. I. , Stone, R. , et al. (2004). Bottlenose dolphins as marine ecosystem sentinels: Developing a health monitoring system. EcoHealth, 1(3), 246–254. [Google Scholar]
- Wells, R. S. , Scott, M. D. , & Irvine, A. B. (1987). The social structure of free‐ranging bottlenose dolphins In Genoways H. (Ed.), Current Mammalogy (pp. 247–305). Boston, MA: Springer. [Google Scholar]
- Wells, R. S. , Tornero, V. , Borrell, A. , Aguilar, A. , Rowles, T. K. , Rhinehart, H. L. , et al. (2005). Integrating life‐history and reproductive success data to examine potential relationships with organochlorine compounds for bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Science of the Total Environment, 349(1), 106–119. [DOI] [PubMed] [Google Scholar]
- Wenzel, A. G. , Brock, J. W. , Cruze, L. , Newman, R. B. , Unal, E. R. , Wolf, B. J. , Somerville, S. E. , & Kucklick, J. R. (2018). Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere, 193, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek, M. , & Angerer, J. (2008). Phthalates: Metabolism and exposure. International Journal of Andrology, 31(2), 131–138. [DOI] [PubMed] [Google Scholar]
- Yordy, J. E. , Wells, R. S. , Balmer, B. C. , Schwacke, L. H. , Rowles, T. K. , & Kucklick, J. R. (2010a). Life history as a source of variation for persistent organic pollutant (POP) patterns in a community of common bottlenose dolphins (Tursiops truncatus) resident to Sarasota Bay, FL. Science of the Total Environment, 408(9), 2163–2172. [DOI] [PubMed] [Google Scholar]
- Yordy, J. E. , Wells, R. S. , Balmer, B. C. , Schwacke, L. H. , Rowles, T. K. , & Kucklick, J. R. (2010b). Partitioning of persistent organic pollutants between blubber and blood of wild bottlenose dolphins: Implications for biomonitoring and health. Environmental Science & Technology, 44(12), 4789–4795. [DOI] [PubMed] [Google Scholar]
- Zota, A. R. , Calafat, A. M. , & Woodruff, T. J. (2014). Temporal trends in phthalate exposures: Findings from the National Health and nutrition examination survey, 2001–2010. Environmental Health Perspectives, 122(3), 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1


