Abstract
Fog and dew are often viewed as economic nuisances causing significant financial losses in the transportation industry and agricultural sector. However, they are also critical components of the hydrological cycle, especially in water scarce environments. Water scarcity is one of the major threats to mankind in the 21st century, and this can be due to development pressures, pollution, and/or expanding populations. In water scarce environments, fog and dew represent potentially exploitable ancillary water resources that could ameliorate the water scarce situation, if efficiently harvested. However, two important issues are often overlooked in relation to fog and dew harvesting and potability. First, current fog and dew harvesting technologies are low yielding with great potential for improvements. Second and more importantly, the potability of these water resources is often based on simple analyses that often omit trace metal and biological analyses. The few studies that report trace metal or biological measurements suggest elevated trace metal concentrations or biological contamination that could be of concern to public health. We discuss the potential for fog and dew harvesting technologies and the need for trace metal and biological analyses of these waters before use.
Keywords: fog, dew, nonrainfall water, drylands, ecohydrology
Key Points
Fog and dew are potential ancillary potable resources, but there are potential human health concerns
Current fog and dew harvesting methods could be significantly improved
Fog and dew should be treated before use as potable water
1. Introduction
Perspectives on nonrainfall water (fog and dew) are dependent upon context. For example, in aviation and agriculture, nonrainfall water is regarded as a nuisance causing significant financial losses (Gultepe et al., 2007; Luo & Goudriaan, 2000). In arid and semiarid environments, nonrainfall water is a critical component of the hydrological cycle (Kaseke et al., 2017) that can exceed annual rainfall (Agam & Berliner, 2006) and sustain these ecosystems (Hill et al., 2015; Seely et al., 2005; Wang et al., 2017). Apart from this ecological role, nonrainfall water can also be an exploitable ancillary potable water resource (Klemm et al., 2012; Tomaszkiewicz et al., 2015).
Water scarcity could come in different forms and could occur anywhere. It is estimated that 90% of the world's dryland population reside in developing nations with population growth rates above the global average, exerting more pressure on existing water resources (Wang et al., 2012). Such high population growth rates and development pressures coupled with the potential impacts of global climate change (decrease in rainfall) are projected to increase water stress to more than 66% of the global population by 2025 (Beysens, 2018). On the other hand, regions that are not affected by a decrease in rainfall will be faced with challenges in providing potable water because of increased pollution and incidence of waterborne illnesses (economic water scarcity; Bonell et al., 2005; Gleick, 1998). Given that water scarcity, both physical and economic, is the largest global risk in terms of potential impacts to mankind in the next decade (Forum, 2015), fog and dew could be exploited as ancillary potable water resources.
However, despite this potability potential, we should be cognizant of the potential problems associated with nonrainfall water harvesting. For example, it is unclear how global climate change will affect nonrainfall water in the future, with some fog studies suggesting a significant decrease in fog frequency in the Pacific (Johnstone & Dawson, 2010), while others suggest an increase in the Atlantic (Haensler et al., 2011). Similarly, projected climate change impacts on dew yield during the critical summer months in the Mediterranean are variable and dependent on the model, with some suggesting a 27% drop in dew yield by 2080 (Tomaszkiewicz et al., 2016). Such decreases in fog frequency and dew yield will undoubtedly negatively affect nonrainfall water harvesting in these environments. Therefore, it is paramount that nonrainfall harvesters adopt designs and materials, for example, nanotechnology and biomimicry that will result in higher water yields per nonrainfall water event (Beysens et al., 2013; Park et al., 2013; Sharan et al., 2017), ensuring viability even under the threat of global climate change. At the same time, a few studies have indicated chemical and biological characteristics of nonrainfall water that may be of concern to public health. Therefore, in the interests of public health, there is a need for more potability studies and caution before presenting fog and dew water as an ancillary potable resource to often the most vulnerable members of the global community.
2. Harvesting of Fog and Dew Waters
2.1. Definitions and Harvesting
Although often considered as the same input (Brown et al., 2008), fog and dew are different meteorological phenomena, controlled by different formation processes (Agam & Berliner, 2006; Kaseke et al., 2017; Wang et al., 2017). Fog is the suspended water droplets in the atmosphere at or near the Earth's surface reducing horizontal visibility to less than 1 km (WMO, 1992), while dew is the formation of water droplets on a sufficiently cooled substrate surface below air dew point temperature but above freezing point (Beysens, 1995). Interest in fog as a potable water resource eclipses dew (Figure 1), and this can be attributed to differences in the potential water yield per event; for example, fog water yields can be as high as 50 L m−2 d−1 (Abdul‐Wahab et al., 2007), while dew has a theoretical maximum water yield of 0.8 L m−2 d−1 (Monteith & Unsworth, 2013). However, dew is a more common occurrence globally (Jacobs et al., 2002; Vuollekoski et al., 2015) and in some places such as Mirleft (Morocco), dew may present a better harvesting potential: annual frequency of dew (48.8%) and yield (20 L m−2 yr−1) versus annual fog frequency (5.5%) and yield (1.4 L m−2 yr−1; Lekouch et al., 2012). At the same time, regions such as the Central Namib Desert have an abundance of both fog and dew throughout the year (Henschel & Seely, 2008) and this could augment brackish groundwater during dry periods (Shanyengana et al., 2002). In addition, dew harvesting can be integrated into or conducted from existing infrastructure like roofs or combined with rain harvesting and result in significant financial savings and more total water harvested (Figure 2; Beysens et al., 2013; Clus et al., 2013; Sharan et al., 2007).
Figure 1.
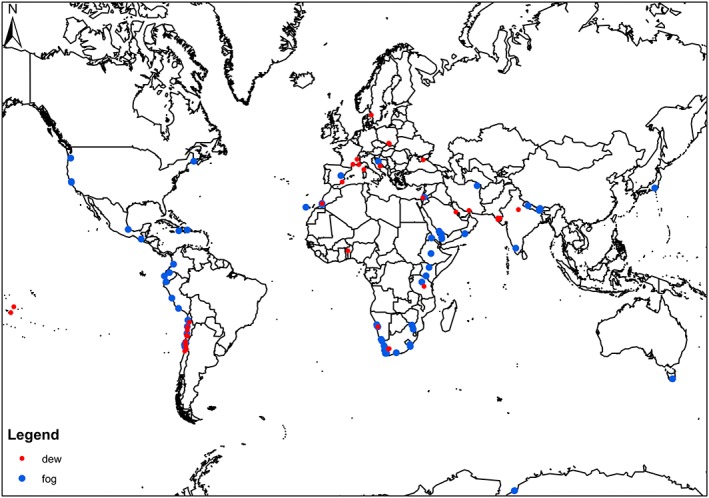
Global distribution of fog and dew collection and/or evaluation projects, both operational and nonoperational.
Figure 2.

Dew collection project in Manquehua Chile, (a) rooftop dew collection and (b) pipe and storage system. The same system is used for rainfall harvesting. CREDIT: Carvajal D./Universidad de La Serena, Chile (with permission).
2.2. Potential Harvesting Technique Improvements
Fog and dew yield are functions of the prevailing meteorological conditions, as well as the efficiencies of the fog collecting mesh (de Dios Rivera, 2011) and receiving substrate surface characteristics of the dew collector (Beysens, 1995). However, the de facto fog harvesting material, Raschel mesh, was not developed specifically for this purpose and the properties of the material in relation to fog harvesting require further investigation (Briassoulis et al., 2007). Its adoption and that of local variants are thus more of convenience and local availability than suitability. Therefore, fog harvesting technologies could benefit from the use of more efficient materials specifically designed for this purpose and this could increase yields by as much as fivefold (Park et al., 2013). Similarly, dew could also benefit from advancements in material science and collector designs developed specifically for passive dew collection (Beysens, 2018; Sharan et al., 2017). For example, origami‐shaped roofs could increase dew yield efficiency by as much as 400% compared to planar surfaces for low yields (<0.02 L m−2 d−1; Beysens et al., 2013). Assuming ceteris paribus, Tables 1 and 2 demonstrate potential fog and dew yields, as well as populations that could be potentially supported during peak fog and dew seasons, calculated from past projects by substituting for more efficient materials and designs. These calculations are important and relevant in that nonrainfall water in most of these areas is meant to supplement and not replace existing conventional water resources. For example, during the dry monsoons in the Kutch region of Western India, the frequent dew events could be a critical potable water resource (Sharan et al., 2007, 2011). While in Asir (Kingdom of Saudi Arabia), peak fog season coincides with peak tourism and water scarcity in this region, fog represents a potentially potable water resource during this period (Gandhidasan & Abualhamayel, 2012). Both examples demonstrate that nonrainfall water potential as a potable resource is critical during particular periods of the year hence the use of seasonal averages (Tables 1 and 2). The potential fog yields may appear high (Table 1); however, a recent mesh developed specifically for fog harvesting collected up to 66 L m−2 d−1 (http://p.dw.com/p/2S5Uz). This demonstrates that such yields are achievable. However, it is important to acknowledge that fog yields will eventually be limited by the liquid water content and duration of the fog event.
Table 1.
Summary and Reevaluation of Fog Water Harvesting Potential for Selected Locations and Potential Number of People Supported by a Single 40‐m2 Fog Collector During the Fog Season, Based on Minimum Water Requirements (7.5 L person−1 d−1; Gleick, 1996)
| Site | Yield L m−2 d−1 | Estimated population, supported by an LFC | |||
|---|---|---|---|---|---|
| SFC | SFCM | SFC | SFCM | Source | |
| Cape Verde | 12 | 60 | 64 | 320 | (Sabino, 2007) |
| South Africa, Lepelfontein | 4.5 | 22.5 | 24 | 120 | (Olivier, 2004) |
| Nepal, Pathivara | 3.6 | 18 | 19 | 96 | (MacQuarrie et al., 2001) |
| Namibia | 2.4 | 12 | 13 | 64 | (Shanyengana et al., 2002) |
| India, Coimbatore | 7.7 | 38.5 | 36 | 205 | (Abhiram et al., 2015) |
| Saudi Arabia | 4 | 20 | 21 | 107 | (Gandhidasan & Abualhamayel, 2012) |
Note. SFC is standard fog collector, SFCM is a standard fog collector modified with a mesh that increases collection efficiency fivefold (Park et al., 2013), and LFC is a large fog collector (40 m2).
Table 2.
Average Dew Yields From Planar Radiative Condensers From Different Field Studies Versus Projected Yields Using a Hollow Funnel Condenser and Origami (40% and 200% Increase in Efficiency, Respectively)
| Site | Average dew yield [L m−2 d−1] | Source | ||
|---|---|---|---|---|
| Planar | Cone | Origami | ||
| Kungsbacka, Sweden | 0.15 | 0.21 | 0.30 | (Nilsson, 1996) |
| Dodoma, Tanzania | 0.06 | 0.08 | 0.12 | (Nilsson, 1996) |
| Ajaccio, France | 0.12 | 0.17 | 0.24 | (Muselli et al., 2002) |
| Jerusalem, Israel | 0.2 | 0.28 | 0.4 | (Berkowicz et al., 2007) |
| Kothara, India | 0.46 | 0.64 | * | (Sharan et al., 2017) |
Values not calculated because efficiency calculation is not valid for reference (planar) values >0.2 L/m2/d (Beysens et al., 2013).
3. Water Quality Concerns
Most fog and dew research suggests that this type of water meets World Health Organization (WHO) standards (Beysens et al., 2006; Klemm et al., 2012; Tomaszkiewicz et al., 2015), mainly based on major cation and anion analyses. However, it must be noted that fog and dew act as atmospheric scrubbers; thus, their chemistry is a function of the air quality and gas‐liquid‐solid heterogeneous interactions in the region (Herckes et al., 2015; Lekouch et al., 2011; Nath & Yadav, 2017). This can result in fog with acidic pH and high ion concentrations due to either oxidation of dimethyl sulfides from oceanic emissions to sulfates, especially along coastal sites (Schemenauer & Cereceda, 1992) or anthropogenic emissions due to industrial activities (Herckes et al., 2015; Sträter et al., 2010). These gas‐liquid‐phase interactions in the atmosphere contribute more to fog because it remains suspended, while in dew, are restricted to the surface of the condensate (Lekouch et al., 2011; Nath & Yadav, 2017). Whereas acidity on its own is not a major human health concern, Sträter et al. (2010) note that acidic pH may result in extraction of heavy metals from aerosol particulates incorporated into fog and result in trace metal concentrations that may exceed local and/or WHO drinking water guidelines. Unfortunately, few studies report trace metal concentrations in fog and dew. However, the existing data do pose serious concerns in this regard. For example, acidic pH, high levels of selenium, arsenic, and nitrates that exceed Chilean guidelines for potable water have been reported in fog water in Alto Patache (Northern Chile; Sträter et al., 2010). Elevated concentrations of aluminum and iron in excess of European Union drinking standards and lead concentrations on the WHO threshold have also been reported in water harvested from dew in Ajaccio (France; Muselli et al., 2006). Therefore, though limited, the data suggest a legitimate potential public health concern related to heavy metals in fog and dew. However, it is impossible to say how prevalent it is due to limited analyses and reporting from past studies.
Similarly, biological aspects of fog and dew water are also often ignored, but the few studies that have investigated them suggest susceptibility to biological contamination including pathogenic forms, for example, coliform and enterococcus (Beysens et al., 2006; Evans et al., 2018; Fuzzi et al., 1997; Muselli et al., 2006). These studies also suggest that biological contamination in fog and dew water can routinely exceed local, regional, and WHO drinking guidelines but can be rectified by disinfection, for example, chlorination (Beysens, 2018; Beysens et al., 2006; Muselli et al., 2006). Given that the most vulnerable populations to water scarcity are dryland populations, whose majority live in developing nations (Wang et al., 2012), the most exposed populations to any public health issues resulting from nonrainfall water use would be these populations since most projects are located or advocated for in these areas (Figure 1). Therefore, we urge cautionary practices, for example, increasing pH, use of filters and noncorrosive pipes and storage facilities, as well as disinfection, to make nonrainfall water potable (Beysens et al., 2006; Muselli et al., 2006; Sharan et al., 2011). We acknowledge that these measures will undoubtedly rise the cost of nonrainfall water per liter. However, according to Sharan et al. (2011), the cost of dew water per liter that had undergone treatment (0.07 US$/L) was still a third of the cost of market price bottled water (0.22US/L) in the Kutch region of Western India, and when rain was factored in because of the dual use of the equipment, the cost dropped to 0.006–0.0075 US$/L (Sharan et al., 2011; Sharan et al., 2017). Meanwhile, in Chile, fog provided better quality potable water at 0.0011–0.0017 US$/L to villages (e.g., Chungungo) compared to the highly government subsidized 0.0016 US$/L of trucked water (Schemenauer et al., 1988). Therefore, although treatment of nonrainfall water increases the cost per unit amount, it is nonetheless cost effective compared to conventional water sources in certain regions of countries such as Chile, Morocco, and India and is also a matter of public health.
4. Summary and Recommendations
It is unlikely that fog and dew harvesting will replace conventional water resources and should thus be viewed as supplementary resources, especially during the driest periods of the year (Gandhidasan & Abualhamayel, 2012; Olivier, 2004; Sharan et al., 2017). At the same time, although nonrainfall water is mainly supplementary, harvesting efficiency can be significantly improved by adoption of materials and designs developed specifically for this purpose (Beysens et al., 2013; Park et al., 2013; Sharan et al., 2017). Mass adoption of these materials will in theory make them more affordable. Based on existing data (Beysens et al., 2006; Klemm et al., 2012; Muselli et al., 2006; Sharan et al., 2011), although we believe fog and dew to be potable after some treatment, we encourage trace metal analyses especially in samples collected from areas influenced by industrial activities or areas with fog and/or dew that exhibits very low pH, since acidic pH of these waters could extract and elevate trace metal concentrations to levels harmful to human health. Interestingly, fog and dew harvesting projects are rarely implemented concurrently and yet both aim to ameliorate water scarcity. It is thus no surprise that despite the many fog and dew reviews (Fessehaye et al., 2014; Khalil et al., 2015; Klemm et al., 2012; Tomaszkiewicz et al., 2015), there has not been a single review that addresses the potability of fog and dew in the same paper. Assuming physical water scarcity and fog harvesting being a viable option, rain harvesting would be a logical extension (Wang & D'Odorico, 2008). And because rain and dew harvesting use primarily the same equipment, modifications to increase dew yields such as insulation (polystyrene foam), special foils, or paint will be minimal (Lekouch et al., 2011). The equipment's multipurpose and potential higher water yield should theoretically reduce the cost of nonrainfall water per liter.
Finally, there does not seem to be much coordination on fog and/or dew harvesting projects worldwide such that it is difficult to analyze or access information about these projects. As an example, the authors of this article reached out to 21 fog and dew researchers enquiring additional information on the status of different projects with the intention of generating a more detailed map for Figure 1 but only received 4 responses or 14% of the requests. This was also partly attributable to the fact that some listed contact information was not current, and despite our best efforts to locate these, emails went unanswered or we could not find alternative contact information. Therefore, it would be beneficial to have a coordinated and updated database where such information is readily available and should include chemical and biological characteristics (parameters) reported in a standardized manner. Such a database could help answer important questions on the prevalence of trace metal toxicity and biological contamination in nonrainfall water and their potential public health impact globally, as well as monitor air quality.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Acknowledgments
The authors declare no financial conflicts or interests. Funding for this work was made available from the U.S. National Science Foundation (IIA‐1427642 and EAR‐1554894). The data used are listed in Tables 1 and 2.
Kaseke, K. F. , & Wang, L. (2018). Fog and dew as potable water resources: Maximizing harvesting potential and water quality concerns. GeoHealth, 2, 327–332. 10.1029/2018GH000171
This article was corrected on 15 JUL 2019. The online version of this article has been modified to include a Conflict of Interest statement.
References
- Abdul‐Wahab, S. A. , Al‐Hinai, H. , Al‐Najar, K. A. , & Al‐Kalbani, M. S. (2007). Fog water harvesting: Quality of fog water collected for domestic and agricultural use. Environmental Engineering Science, 24(4), 446–456. 10.1089/ees.2006.06-0066 [DOI] [Google Scholar]
- Abhiram, M. , Priya, N. D. , & Geetha, P. (2015). Fog harvesting—A wind flow perspective in Western Ghats, Coimbatore, Tamil Nadu. Indian Journal of Science and Technology, 8(28). 10.17485/ijst/2015/v8i28/87123 [DOI] [Google Scholar]
- Agam, N. , & Berliner, P. (2006). Dew formation and water vapor adsorption in semi‐arid environments—A review. Journal of Arid Environments, 65(4), 572–590. 10.1016/j.jaridenv.2005.09.004 [DOI] [Google Scholar]
- Berkowicz, S. , Beysens, D. , Milimouk, I. , Heusinkveld, B. , Muselli, M. , Jacobs, A. , et al. (2007). Urban dew collection in Jerusalem: A three‐year analysis. Paper presented at 4th Conference on Fog, Fog Collection and Dew.
- Beysens, D. (1995). The formation of dew. Atmospheric Research, 39(1‐3), 215–237. 10.1016/0169-8095(95)00015-J [DOI] [Google Scholar]
- Beysens, D. (2018). Dew Water. Gistrup: River Publishers. [Google Scholar]
- Beysens, D. , Brogginib, F. , Milimouk‐Melnytchoukc, I. , Ouazzanid, J. , & Tixiere, N. (2013). New architectural forms to enhance dew collection. Chemical Engineer, 34, 79–84. [Google Scholar]
- Beysens, D. , Ohayon, C. , Muselli, M. , & Clus, O. (2006). Chemical and biological characteristics of dew and rain water in an urban coastal area (Bordeaux, France). Atmospheric Environment, 40(20), 3710–3723. 10.1016/j.atmosenv.2006.03.007 [DOI] [Google Scholar]
- Bonell, M. , Hufschmidt, M. M. , & Gladwell, J. S. (2005). Hydrology and water management in the humid tropics: Hydrological research issues and strategies for water management. Cambridge, UK, and New York: Cambridge Univ. Press. [Google Scholar]
- Briassoulis, D. , Mistriotis, A. , & Eleftherakis, D. (2007). Mechanical behaviour and properties of agricultural nets. Part II: Analysis of the performance of the main categories of agricultural nets. Polymer Testing, 26(8), 970–984. 10.1016/j.polymertesting.2007.06.010 [DOI] [Google Scholar]
- Brown, R. , Mills, A. J. , & Jack, C. (2008). Non‐rainfall moisture inputs in the Knersvlakte: Methodology and preliminary findings: Short communication. Water SA, 34(2), 275–278. [Google Scholar]
- Clus, O. , Lekouch, I. , Muselli, M. , Milimouk‐Melnytchouk, I. , & Beysens, D. (2013). Dew, fog and rain water collectors in a village of S‐Morocco (Idouasskssou). Desalination and Water Treatment, 51(19–21), 4235–4238. 10.1080/19443994.2013.768323 [DOI] [Google Scholar]
- de Dios Rivera, J. (2011). Aerodynamic collection efficiency of fog water collectors. Atmospheric Research, 102(3), 335–342. 10.1016/j.atmosres.2011.08.005 [DOI] [Google Scholar]
- Evans, S. E. , Dueker, M. E. , Logan, J. R. , & Weathers, K. C. (2018). The microbial aerosol composition of iconic fog systems in Coastal Maine and the Namib Desert. Science of the Total Environment. [DOI] [PubMed] [Google Scholar]
- Fessehaye, M. , Abdul‐Wahab, S. A. , Savage, M. J. , Kohler, T. , Gherezghiher, T. , & Hurni, H. (2014). Fog‐water collection for community use. Renewable and Sustainable Energy Reviews, 29, 52–62. 10.1016/j.rser.2013.08.063 [DOI] [Google Scholar]
- Forum, W. E. (2015). Global risks 2015 (pp. 1–69) World Economic Forum, Geneva, Switzerland. [Google Scholar]
- Fuzzi, S. , Mandrioli, P. , & Perfetto, A. (1997). Fog droplets—An atmospheric source of secondary biological aerosol particles. Atmospheric Environment, 31(2), 287–290. 10.1016/1352-2310(96)00160-4 [DOI] [Google Scholar]
- Gandhidasan, P. , & Abualhamayel, H. (2012). Exploring fog water harvesting potential and quality in the Asir Region, Kingdom of Saudi Arabia. Pure and Applied Geophysics, 169(5–6), 1019–1036. 10.1007/s00024-011-0341-z [DOI] [Google Scholar]
- Gleick, P. H. (1996). Basic water requirements for human activities: Meeting basic needs. Water International, 21(2), 83–92. 10.1080/02508069608686494 [DOI] [Google Scholar]
- Gleick, P. H. (1998). The world's water: The biennial report on freshwater resources (1998–99). Washington, DC: Island Press. [Google Scholar]
- Gultepe, I. , Tardif, R. , Michaelides, S. , Cermak, J. , Bott, A. , Bendix, J. , Müller, M. , Pagowski, M. , Hansen, B. , & Ellrod, G. (2007). Fog research: A review of past achievements and future perspectives. Pure and Applied Geophysics, 164(6–7), 1121–1159. 10.1007/s00024-007-0211-x [DOI] [Google Scholar]
- Haensler, A. , Cermak, J. , Hagemann, S. , & Jacob, D. (2011). Will the southern African west coast fog be affected by future climate change? Results of an initial fog projection using a regional climate model. Erdkunde, 65(3), 261–275. 10.3112/erdkunde.2011.03.04 [DOI] [Google Scholar]
- Henschel, J. R. , & Seely, M. K. (2008). Ecophysiology of atmospheric moisture in the Namib Desert. Atmospheric Research, 87(3–4), 362–368. 10.1016/j.atmosres.2007.11.015 [DOI] [Google Scholar]
- Herckes, P. , Marcotte, A. , Wang, Y. , & Collett, J. (2015). Fog composition in the Central Valley of California over three decades. Atmospheric Research, 151, 20–30. 10.1016/j.atmosres.2014.01.025 [DOI] [Google Scholar]
- Hill, A. J. , Dawson, T. E. , Shelef, O. , & Rachmilevitch, S. (2015). The role of dew in Negev Desert plants. Oecologia, 1–11. [DOI] [PubMed] [Google Scholar]
- Jacobs, A. F. , Heusinkveld, B. G. , & Berkowicz, S. M. (2002). A simple model for potential dewfall in an arid region. Atmospheric Research, 64(1–4), 285–295. 10.1016/S0169-8095(02)00099-6 [DOI] [Google Scholar]
- Johnstone, J. A. , & Dawson, T. E. (2010). Climatic context and ecological implications of summer fog decline in the coast redwood region. Proceedings of the National Academy of Sciences, 107(10), 4533–4538. 10.1073/pnas.0915062107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaseke, K. F. , Wang, L. , & Seely, M. K. (2017). Nonrainfall water origins and formation mechanisms. Science Advances, 3(3), e1603131 10.1126/sciadv.1603131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil, B. , Adamowski, J. , Shabbir, A. , Jang, C. , Rojas, M. , Reilly, K. , & Ozga‐Zielinski, B. (2015). A review: Dew water collection from radiative passive collectors to recent developments of active collectors. Sustainable Water Resources Management, 1(4), 1–16. [Google Scholar]
- Klemm, O. , Schemenauer, R. S. , Lummerich, A. , Cereceda, P. , Marzol, V. , Corell, D. , van Heerden, J. , Reinhard, D. , Gherezghiher, T. , & Olivier, J. (2012). Fog as a fresh‐water resource: Overview and perspectives. Ambio, 41(3), 221–234. 10.1007/s13280-012-0247-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekouch, I. , Lekouch, K. , Muselli, M. , Mongruel, A. , Kabbachi, B. , & Beysens, D. (2012). Rooftop dew, fog and rain collection in southwest Morocco and predictive dew modeling using neural networks. Journal of Hydrology, 448, 60–72. [Google Scholar]
- Lekouch, I. , Muselli, M. , Kabbachi, B. , Ouazzani, J. , Melnytchouk‐Milimouk, I. , & Beysens, D. (2011). Dew, fog, and rain as supplementary sources of water in south‐western Morocco. Energy, 36(4), 2257–2265. 10.1016/j.energy.2010.03.017 [DOI] [Google Scholar]
- Luo, W. , & Goudriaan, J. (2000). Dew formation on rice under varying durations of nocturnal radiative loss. Agricultural and Forest Meteorology, 104(4), 303–313. 10.1016/S0168-1923(00)00168-4 [DOI] [Google Scholar]
- MacQuarrie, K. , Pokhrel, A. , Shrestha, Y. , Osses, P. , Schemenauer, R. , Vitez, F. , Kowalchuk, K. , and Taylor, R. (2001). Results from a high elevation fog water supply project in Nepal. Paper presented at Proceedings of the 2nd International Conference on Fog and Fog Collection St. John's, Canada, 15‐20 July 2001.
- Monteith, J. L. , & Unsworth, M. H. (2013). Principles of environmental physics. New York: Routledge Chapman & Hall Inc. [Google Scholar]
- Muselli, M. , Beysens, D. , Marcillat, J. , Milimouk, I. , Nilsson, T. , & Louche, A. (2002). Dew water collector for potable water in Ajaccio (Corsica Island, France). Atmospheric Research, 64(1–4), 297–312. 10.1016/S0169-8095(02)00100-X [DOI] [Google Scholar]
- Muselli, M. , Beysens, D. , Soyeux, E. , & Clus, O. (2006). Is dew water potable? Chemical and biological analyses of dew water in Ajaccio (Corsica Island, France). Journal of Environmental Quality, 35(5), 1812–1817. 10.2134/jeq2005.0357 [DOI] [PubMed] [Google Scholar]
- Nath, S. , & Yadav, S. (2017). A comparative study on fog and dew water chemistry at New Delhi, India. Aerosol and Air Quality Research, 18(1), 26–36. 10.4209/aaqr.2017.01.0033 [DOI] [Google Scholar]
- Nilsson, T. (1996). Initial experiments on dew collection in Sweden and Tanzania. Solar Energy Materials and Solar Cells, 40(1), 23–32. 10.1016/0927-0248(95)00076-3 [DOI] [Google Scholar]
- Olivier, J. (2004). Fog harvesting: An alternative source of water supply on the West Coast of South Africa. GeoJournal, 61(2), 203–214. 10.1007/s10708-004-2889-y [DOI] [Google Scholar]
- Park, K.‐C. , Chhatre, S. S. , Srinivasan, S. , Cohen, R. E. , & McKinley, G. H. (2013). Optimal design of permeable fiber network structures for fog harvesting. Langmuir: the ACS Journal of Surfaces and Colloids, 29(43), 13,269–13,277. 10.1021/la402409f [DOI] [PubMed] [Google Scholar]
- Sabino, A. (2007). Fog collection in the natural park of Serra Malagueta. An alternative source of water for the communities. Paper presented at Proceedings of the 4th International Conference on Fog, Fog Collection and Dew.
- Schemenauer, R. S. , & Cereceda, P. (1992). The quality of fog water collected for domestic and agricultural use in Chile. Journal of Applied Meteorology, 31(3), 275–290. [DOI] [Google Scholar]
- Schemenauer, R. S. , Fuenzalida, H. , & Cereceda, P. (1988). A neglected water resource: The Camanchaca of South America. Bulletin of the American Meteorological Society, 69(2), 138–147. [DOI] [Google Scholar]
- Seely, M. , Henschel, J. R. , & Hamilton, W. J. III (2005). Long‐term data show behavioural fog collection adaptations determine Namib Desert beetle abundance: Research letter. South African Journal of Science, 101(11 & 12), 570–572. [Google Scholar]
- Shanyengana, E. , Henschel, J. , Seely, M. , & Sanderson, R. (2002). Exploring fog as a supplementary water source in Namibia. Atmospheric Research, 64(1‐4), 251–259. 10.1016/S0169-8095(02)00096-0 [DOI] [Google Scholar]
- Sharan, G. , Beysens, D. , & Milimouk‐Melnytchouk, I. (2007). A study of dew water yields on galvanized iron roofs in Kothara (north‐west India). Journal of Arid Environments, 69(2), 259–269. 10.1016/j.jaridenv.2006.09.004 [DOI] [Google Scholar]
- Sharan, G. , Clus, O. , Singh, S. , Muselli, M. , & Beysens, D. (2011). A very large dew and rain ridge collector in the Kutch area (Gujarat, India). Journal of Hydrology, 405(1‐2), 171–181. 10.1016/j.jhydrol.2011.05.019 [DOI] [Google Scholar]
- Sharan, G. , Roy, A. K. , Royon, L. , Mongruel, A. , & Beysens, D. (2017). Dew plant for bottling water. Journal of Cleaner Production, 155, 83–92. 10.1016/j.jclepro.2016.07.079 [DOI] [Google Scholar]
- Sträter, E. , Westbeld, A. , & Klemm, O. (2010). Pollution in coastal fog at Alto Patache, northern Chile. Environmental Science and Pollution Research, 17(9), 1563–1573. 10.1007/s11356-010-0343-x [DOI] [PubMed] [Google Scholar]
- Tomaszkiewicz, M. , Abou Najm, M. , Beysens, D. , Alameddine, I. , & El‐Fadel, M. (2015). Dew as a sustainable non‐conventional water resource: A critical review. Environmental Reviews, 23(999), 1–18. [Google Scholar]
- Tomaszkiewicz, M. , Najm, M. A. , Beysens, D. , Alameddine, I. , Zeid, E. B. , & El‐Fadel, M. (2016). Projected climate change impacts upon dew yield in the Mediterranean basin. Science of the Total Environment, 566, 1339–1348. [DOI] [PubMed] [Google Scholar]
- Vuollekoski, H. , Vogt, M. , Sinclair, V. A. , Duplissy, J. , Järvinen, H. , Kyrö, E.‐M. , Makkonen, R. , Petäjä, T. , Prisle, N. L. , & Räisänen, P. (2015). Estimates of global dew collection potential on artificial surfaces. Hydrology and Earth System Sciences, 19(1), 601–613. 10.5194/hess-19-601-2015 [DOI] [Google Scholar]
- Wang, L. , & D'Odorico, P. (2008). The limits of water pumps. Science, 321(5885), 36–37. 10.1126/science.321.5885.36c [DOI] [PubMed] [Google Scholar]
- Wang, L. , D'Odorico, P. , Evans, J. , Eldridge, D. , McCabe, M. , Caylor, K. , & King, E. (2012). Dryland ecohydrology and climate change: Critical issues and technical advances. Hydrology and Earth System Sciences, 16(8), 2585–2603. 10.5194/hess-16-2585-2012 [DOI] [Google Scholar]
- Wang, L. , Kaseke, K. F. , & Seely, M. K. (2017). Effects of non‐rainfall water inputs on ecosystem functions. Wiley Interdisciplinary Reviews, 4(1). 10.1002/wat1002.1179 [DOI] [Google Scholar]
- WMO (1992). International meteorological vocabulary (2nd ed.). Geneva: World Meteorological Organization. [Google Scholar]


