Abstract

Metal–organic framework nanosheets (MONs) are attracting increasing attention as a diverse class of two-dimensional materials derived from metal–organic frameworks (MOFs). The principles behind the design of layered MOFs that can readily be exfoliated to form nanosheets, however, remain poorly understood. Here we systematically investigate an isoreticular series of layered MOFs functionalized with alkoxy substituents in order to understand the effect of substituent alkyl chain length on the structure and properties of the resulting nanosheets. A series of 2,5-alkoxybenzene-1,4-dicarboxylate ligands (O2CC6H2(OR)2CO2, R = methyl–pentyl, 1–5, respectively) was used to synthesize copper paddle-wheel MOFs. Rietveld and Pawley fitting of powder diffraction patterns for compounds Cu(3–5)(DMF) showed they adopt an isoreticular series with two-dimensional connectivity in which the interlayer distance increases from 8.68 Å (R = propyl) to 10.03 Å (R = pentyl). Adsorption of CO2 by the MOFs was found to increase from 27.2 to 40.2 cm3 g–1 with increasing chain length, which we attribute to the increasing accessible volume associated with increasing unit-cell volume. Ultrasound was used to exfoliate the layered MOFs to form MONs, with shorter alkyl chains resulting in higher concentrations of exfoliated material in suspension. The average height of MONs was investigated by AFM and found to decrease from 35 ± 26 to 20 ± 12 nm with increasing chain length, with the thinnest MONs observed being only 5 nm, corresponding to five framework layers. These results indicate that careful choice of ligand functionalities can be used to tune nanosheet structure and properties, enabling optimization for a variety of applications.
Short abstract
A series of layered copper metal−organic frameworks (MOFs) were synthesized, utilizing benzene-1,4-dicarboxylates difunctionalized in the 2,5-positions with alkoxy pendent chains (methoxy-pentoxy) as the organic ligands. Increasing the chain length from propoxy to pentoxy increased the interlayer distance, resulted in increased CO2 uptake, and aided the ultrasonic exfoliation of these MOFs to form nanosheets, producing MONs down to just 5 nm thick.
1. Introduction
Metal–organic framework nanosheets (MONs) are free-standing, nominally two-dimensional materials formed by the coordination of organic ligands to metal ions or clusters.1−4 MONs share many of the advantages of other metal–organic materials, such as the ease with which their structures can be varied and new properties introduced. However, they also benefit from the high aspect ratio, vast external surface area, and nanoscopic dimensions of other two-dimensional materials such as graphene, boron nitride, and molybdenum disulfide. These properties have already enabled MONs to outperform their bulk counterparts in a variety of applications, including gas separation,5 water purification,6 sensing,7−12 catalysis,13−20 and optoelectronics.21−26
The development of metal–organic frameworks (MOFs) over the past two decades has been focused on understanding how to design and tune the properties of open porous three-dimensional structures, for applications including gas storage,27 catalysis,28 and sensing.29−31 During this time, a large number of layered MOFs have been reported. It is these MOFs that provide a platform for the development of MONs. Two distinct approaches have emerged to convert layered MOFs into MONs: (1) “bottom-up” self-assembly of subcomponents in the presence of a directing interface5,32 or chemical species33,34 and (2) “top-down” exfoliation from layered MOFs. For the latter approach, a wide variety of methods have been developed including freeze–thaw,35 ball-milling,36,37 intercalation,38,39 and ultrasonication.7,36,40−49 Among these, liquid exfoliation using ultrasound is one of the most widely applicable and readily scalable methods, which has been widely applied in the exfoliation of other two-dimensional (2D) materials.50−53 The vast majority of MONs reported to date are based on known MOF structures being repurposed to form nanosheets. Understanding how to optimize the design of layered MOFs to facilitate exfoliation into free-standing nanosheets is therefore an important and necessary new crystal engineering challenge.
Functionalization of 2,5-dihydroxybenzene-1,4-dicarboxylic acid (H2DHBDC) with pendant flexible substituents has proved useful for understanding the structure–property relationships of three-dimensional MOFs, particularly regarding their gas adsorption properties, as well as their framework flexibility, introducing new behaviors and optimizing their design for the adsorption of specific gases.54,55 In most of these systems, the pendant chains sit within the pores of the MOF and do not affect the overall structure, although they can influence the architecture that is formed.54 We have recently developed this approach further in a series of layered MOFs designed to incorporate short alkyl-ether chains between the layers intended to aid exfoliation by weakening interlayer interactions and increasing interactions with solvent molecules.47 In a related study, two layered MOFs functionalized with either −O(CH2)4CH3 or −O(CH2)3OCH3 chains which bestowed hydrophobic or hydrophilic character, respectively, were exfoliated in a wide range of solvents.57 The more hydrophobic system formed thinner, more well-defined nanosheets comprising fewer framework layers. Optimization and control of the exfoliation process by framework design, however, remain key challenges, which we seek to address in the current study.
Here, we report a series of layered MOFs (Figure 1) formed using DHBDC2– ligands functionalized with alkyl chains (methyl to pentyl). We show that increasing chain length improves exfoliation to yield MONs of improved aspect ratio. We suggest that increasing chain length weakens the interlayer interactions and improves interactions of the framework layers with solvent molecules, leading to the enhanced exfoliation. The study is underpinned by a detailed characterization of the MOF series in their solvated and unsolvated forms, enabling the effect of alkyl chain length on their structure to be understood. We also report on the CO2/N2 adsorption behavior of the MOFs, which can be related to the trend in their unit-cell dimensions.
Figure 1.

General reaction scheme for the targeted syntheses of MOFs [Cu(n)(DMF)]n, where n = 1–5. R groups in dicarboxylic acid ligands precursors H21 to H25 are defined, and the terminology used throughout this Article is provided. [*When using H21 and H22, the anticipated MOF structures were not formed (see text)].
2. Results and Discussion
2.1. MOF Syntheses and Characterization
Dicarboxylic acids H21–H2454 and H2557 were synthesized via Williamson etherification of dimethoxy-2,5-dihydroxybenzenedicarboxylate with iodomethane or 1-bromoalkane (where alkane = ethane-pentane) following previously reported protocols. High yields (83–91%) of 2,5-bis(alkoxy)-1,4-benzenedicarboxylic acid (alkoxy = methoxy-pentoxy) were obtained, and compound purity was confirmed through 1H NMR spectroscopy (Figures S1–S5). MOFs were formed by the solvothermal reaction of H21–H25 (10 mol % excess) and copper nitrate in DMF at 110 °C for 36 h. Green microcrystalline powders were obtained following slow cooling of the mixtures to room temperature and were then analyzed by powder X-ray diffraction (PXRD), Fourier-transform infrared (FTIR) spectroscopy, thermogravimetric analysis (TGA), and elemental analysis to understand their structure.
In line with our previous study, H25 was found to give a compound with the formula Cu(5)(DMF). TGA shows a 15.2% mass loss in the region 110–230 °C (theoretical 15.5% mass loss, Figure S7); FTIR shows the C = O stretch of coordinated DMF at 1668 cm–1 (Figure S6), shifted from the expected wavenumber of 1676 cm–1 for free DMF,58 and elemental analysis shows good agreement for the empirical formula CuC21H31NO7 (Table S1).
For the first time, we were also able to determine the structure of Cu(5)(DMF) (Figure 2b) from a high-resolution PXRD pattern collected at Diamond Light Source, beamline I11, and refine the structural model by Rietveld methods (see Figures S15 and S16; CCDC 1910582). Carboxylate groups of four ligands of 5 coordinate to Cu2 dimers in a bidentate bridging manner, forming the paddle-wheel (PW) secondary building unit (SBU) (Figure 1). DMF is coordinated at the axial positions to form a distorted square-pyramidal coordination environment around the Cu2 atoms, which are separated by a distance of 3.16 Å. Ligand 5 connects adjacent PWs, which form a square-net topology within a layer (Figure 2c). The pentoxy chains and DMF molecules extend out of the layer interdigitating with those in adjacent layers, holding them together through van der Waals forces (Figure 2b). This layered structure is isoreticular with that of MOF-259 and our previously reported single-crystal structure for Zn(X)(DMF) where X is 2,5-bis(3-methoxypropoxy)-1,4-benzenedicarboxylate (i.e., R = O(CH2)3OCH3).47
Figure 2.

(a) PXRD patterns of microcrystalline powders resulting from the syntheses of Cu(1–5)(DMF), collected at Diamond Light Source, beamline I11 (λ = 0.826015 Å). (b) Crystal structure of Cu(5)(DMF) as refined through Rietveld methods, viewed down the c-axis. H atoms are omitted for clarity. (c) Square net structure of Cu(5)(DMF), viewed down the Cu-PW Cu–Cu axis. H atoms and coordinated DMF molecules omitted for clarity. (d) Space-filling view of Cu(5)(DMF) showing −O(CH2)4CH3 chain and axial DMF packing. Copper, carbon, hydrogen, nitrogen, and oxygen atoms are depicted in purple, gray, white, blue, and red, respectively.
Dicarboxylic acids H2n (n = 3, 4) form MOFs Cu(n)(DMF), with structures closely related to Cu(5)(DMF). Mass losses corresponding to the loss of coordinated DMF were observed by TGA over the temperature range of 90–240 °C; FTIR showed the C=O stretch around 1665 cm–1 of coordinated DMF; elemental analysis was consistent with the anticipated formula unit (Table S1). Pawley refinements of synchrotron PXRD data indicated unit cell parameters similar to Cu(5)(DMF) (Table 1 and Figures S11–S13), which suggests that they adopt the same square-grid topology. There is an increase in the volume of the unit cell with increasing alkyl chain length (3–5) from 1009 to 1155 Å3. However, it is difficult to attribute this to expansion along a single axis because of the triclinic lattice symmetry. The flexibility of the alkyl chains, potential of the aromatic rings to rotate, and interplay with coordinated solvent molecules and pores mean that the effect of each additional methylene unit is likely to be more subtle than simply moving the layers apart.
Table 1. Unit Cell Parameters Determined through Pawley Refinements of PXRD Data (298 K) for Cu(3–5)(DMF) and Cu(3–5) and Rietveld Refinement of Cu(5)(DMF)a.
| MOF | refinement | space group | a (Å) | b (Å) | c (Å) | α (deg) | β (deg) | γ (deg) | vol (Å3) | Rwpb | Rwp′c |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu(X)(DMF)47 | N/A | P-1 | 10.42 | 10.82 | 10.88 | 85.21 | 74.99 | 67.51 | 1095 | N/A | N/A |
| Cu(3)(DMF) | Pawley | P-1 | 10.60 | 10.62 | 10.81 | 71.12 | 79.91 | 61.22 | 1009 | 0.0224 | 0.0894 |
| Cu(4)(DMF) | Pawley | P-1 | 10.62 | 10.80 | 10.94 | 76.01 | 62.54 | 81.99 | 1080 | 0.0377 | 0.1539 |
| Cu(5)(DMF) | Pawley | P-1 | 10.84 | 10.81 | 10.86 | 83.79 | 79.74 | 67.34 | 1155 | 0.0256 | 0.1129 |
| Cu(5)(DMF) | Rietveld | P-1 | 10.84 | 10.80 | 10.85 | 83.82 | 79.82 | 67.44 | 1154 | 0.0517 | 0.2455 |
| Cu(3) | Pawley | P-1 | 10.81 | 10.69 | 11.00 | 71.51 | 77.35 | 63.35 | 1072 | 0.0655 | 0.1278 |
| Cu(4) | Pawley | P-1 | 10.42 | 10.72 | 11.03 | 77.27 | 64.11 | 82.87 | 1081 | 0.0474 | 0.0942 |
| Cu(5) | Pawley | P-1 | 10.86 | 11.04 | 10.87 | 84.04 | 76.89 | 70.65 | 1197 | 0.0593 | 0.1101 |
All unit cell parameters are rounded to two decimal places; more accurate values and associated errors are provided in the Supporting Information (Table S3). X = 2,5-bis(3-methoxypropoxy)-1,4-benzenedicarboxylate (i.e., R = O(CH2)3OCH3).
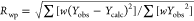 and
and
 , where yobs is the observed intensity, ycalc the
calculated intensity, w the weight, and bkgr the
background.
, where yobs is the observed intensity, ycalc the
calculated intensity, w the weight, and bkgr the
background.
Rietveld refinements were attempted on high-resolution synchrotron PXRD patterns of Cu(n)(DMF) (n = 3, 4) but it was not possible to solve the structures or to obtain models suitable for Rietveld refinement. However, Cu sites could be located from electron density maps. Cu2 units are situated with their centroids at the inversion centers within the structures (Table S5). Locating the Cu atoms allowed calculation of the 2D directional connectivity within layers (based on distances between Cu–Cu centroids and length of a coordinating BDC ligand) and confirmed the anticipated square-grid network structure. This enabled calculation of the interlayer distance, which was found to increase 8.37 < 9.42 < 9.91 Å from Cu(3)(DMF) to Cu(5)(DMF), correlating with increasing alkyl chain length (Figure S14). Particle morphologies of Cu(3–5)(DMF) were visualized using scanning electron microscopy (SEM). Platelets were observed with lateral dimensions in the range of 1–10 μm (Figure S22).
Microcrystalline powders resulting from the reactions using H2n (n = 1, 2) were similarly assessed. Elemental analyses (Table S1) were not consistent with the anticipated Cu(n)(DMF) (n = 1, 2) formula. Indexing and Pawley refinement indicated a mixture of phases present. Guo et al. reported a structurally similar Cu-PW MOF using 1, which has water capping the axial positions of the PW. Although TGA indicated that water may be present (4.5% mass loss between 50 and 110 °C), this phase was not observed in our synthesis (Figure S9).60 There are no examples of layered MOFs using 2 as the linker, to the best of our knowledge. Attempts to grow larger crystals of these materials were unsuccessful. Analogous syntheses using zinc and H21 or H22, attempting to grow single crystals, resulted in cubic crystals of MOFs isoreticular with the 3D MOF-5,61 in which 1,4-benzenedicarboxylate ligands (BDC) were replaced by 1 or 2, respectively. These materials were therefore excluded from further studies. Similar crystallizations using zinc and H23–5 also resulted in the 3D MOF-5 structure.
2.2. MOF Desolvation
We have previously observed that desolvation of this class of layered PW-MOFs can take place during ultrasonic exfoliation in noncoordinating solvents, for instance with acetonitrile (MeCN) for Cu(5)(DMF).57 Desolvation is also anticipated to occur during gas adsorption measurements where frameworks are typically first activated through heating under dynamic vacuum (in our work, 160 °C for 10 h), in order to remove any solvent coordinated or present from the pores. Before moving on to discuss the results of exfoliation or gas sorption studies, we therefore first sought to characterize the structures of the desolvated MOFs. This is particularly important for these systems as the parent, nonfunctionalized Cu(BDC)(DMF) undergoes a structural rearrangement upon desolvation, in which the carboxylate groups coordinate to the vacated axial position of the Cu2 dimer of neighboring layers to form a porous nonlayered 3D MOF, Cu(BDC).62,63
Figure 3 shows PXRD patterns of Cu(3–5)(DMF) after gas adsorption analyses and after sonication in DMF or MeCN, compared with the as-synthesized MOFs. A distinct new PXRD pattern was observed for each of the MOFs following gas adsorption measurements or exfoliation in MeCN. Gas adsorption resulted in peak broadening and poorly defined patterns; however, peak positions broadly match those observed from exfoliating in MeCN, indicating a similar phase has been formed in both cases. Sonication in MeCN gave sharper diffraction patterns. These samples were then digested using DCl/D2O in d6-DMSO for 1H NMR spectroscopy. No DMF or MeCN was observed in any of the digested Cu(3–5) samples (Figures S23–S25), indicating that exfoliation in MeCN results in complete removal of DMF from compounds Cu(3–5)(DMF), and there is no coordination by MeCN.
Figure 3.
PXRD patterns (λ = 1.5406 Å) of (a) Cu(3)(DMF), (b) Cu(4)(DMF), and (c) Cu(5)(DMF) MOFs after gas adsorption analyses and after exfoliation in DMF and MeCN, compared with as-synthesized MOFs.
Pawley fitting of the PXRD patterns after exfoliation in MeCN indicated crystal phases distinct from the solvated MOFs, which were assigned as the desolvated phases Cu(3), Cu(4), and Cu(5) (Table 1). There are slight increases in unit cell volume compared to the solvated structures. Without a full structure solution, it is difficult to explain this. Locating the Cu atoms (Table S5) again allowed calculation of the interlayer distances in the same manner as for Cu(3–5)(DMF). These increased with the trend 8.68 < 9.37 < 10.03 Å from Cu(3) to Cu(5), with the only notable increase upon desolvation being for Cu(3)(DMF) to Cu(3) (8.37–8.68 Å). It is worth noting that these structures do not undergo the equivalent rearrangement upon desolvation of Cu(BDC)(DMF), instead maintaining their layered structure. We suggest this difference is due to the increased interlayer distance of Cu(n)(DMF) compared to Cu(BDC)(DMF) (5.2 Å); sterics of the alkoxy arms may prevent the slipping of the layers and the close proximity needed to establish 3D coordination.
The diffraction patterns for all three frameworks following sonication in DMF were found to match those of the as-synthesized material, indicating no substantial structural changes had occurred. Some broadening of the PXRD peaks was observed, most prominently in the case of Cu(3)(DMF), consistent with decreases in crystallite size during sonication.
2.3. Gas Adsorption
Figure 4 shows the N2 adsorption isotherms (77 K) for Cu(3–5). The BET surface areas were determined as 8.6, 9.0, and 5.4 m2 g–1, respectively. The type III adsorption isotherms,64 observed with negligible N2 uptake below 0.7 P/P0, are consistent with related alkoxy functionalized MOFs, where the pore space is not accessible for nonpolar gases.65 CO2 adsorption (195 K) follows a type Ib isotherm, with a moderate type H1 hysteresis loop. CO2 uptake increases from 27.2, to 32.9, to 40.2 cm3 g–1, from Cu(3) to Cu(5). This relatively large uptake, compared to N2, could be due to increased penetration of the framework layers by CO2, which has a higher binding affinity for the Cu sites. The unit cell volume increases by 11% (1072 to 1197 Å3, Table 1) from Cu(3) to Cu(5), which could account for the increasing uptake of CO2, also bearing in mind that the DMF has been removed, leaving coordinatively unsaturated Cu sites. If the unit cell volume remained constant across the series, it could be anticipated that the CO2 uptake would decrease with an increase in alkyl chain length, because of the increased packing density of the chains between the 2D framework layers, but this is presumably compensated by the observed unit cell expansion.
Figure 4.

(a) CO2 and (b) N2 isotherms of Cu(3–5), recorded at 195 and 77 K, respectively. Gas adsorption is represented by darker shades, and desorption by lighter shades. Isotherms for Cu(3), Cu(4), and Cu(5) are red, blue, and green, respectively.
2.4. Ultrasonic Exfoliation To Form MONs
In order to exfoliate the MOFs, 5 mg of Cu(3–5)(DMF) was suspended in 6 mL of DMF or MeCN and sonicated for 12 h in a water bath. Samples were rotated in the bath to ensure even exposure, and the bath was fitted with a water coil to maintain temperature, which equilibrated at 21 °C.57 The samples were then centrifuged for either 1 h at 1500 rpm or 4.5 h at 4500 rpm to remove the larger unexfoliated MOF crystallites. A calibration curve, obtained by serial dilution of a suspension containing a known mass of Cu(3–5)(DMF), was used to estimate the concentration of the nanosheets in suspension (Table 2 and Figures S26–S35).
Table 2. Concentration (mg mL–1) of MONs Remaining in Suspension Following Different Centrifugation Protocols.
| solvent | ratea | time (h) | Cu(3)(DMF) | Cu(4)(DMF) | Cu(5)(DMF) |
|---|---|---|---|---|---|
| DMF | 1500 | 1 | 0.23 | 0.22 | 0.13 |
| 4500 | 4.5 | 0.13 | 0.07 | 0.05 | |
| MeCN | 1500 | 1 | 0.40 | 0.24 | 0.11 |
| 4500 | 4.5 | 0.11 | 0.06 | 0.04 |
Rate is in revolutions per minute (rpm).
As expected, the concentration of MONs in suspension decreased in all cases as the centrifugation rate increased from 1500 to 4500 rpm (Table 2). No visible sedimentation was observed in any of the samples after a week. Interestingly, shorter alkyl chains resulted in higher concentrations of material in suspension after exfoliation in both DMF and MeCN, from both centrifugation protocols. The concentration of material in suspension for Cu(3–5)(DMF) was higher following exfoliation in DMF than in MeCN following the faster (and longer) centrifugation protocol. From the slower (and shorter) centrifugation protocol, Cu(3-4)(DMF) had a higher concentration in MeCN, but Cu(5)(DMF) had a higher concentration in DMF. These results demonstrate the importance of solvent–surface interactions in optimizing exfoliation and the effect that even small changes in the surface functionalities can have.
To assess the effect that chain length has on MON particle sizes following ultrasonic exfoliation in MeCN, suspensions following centrifugation (1500 rpm, 1 h) were deposited onto freshly cleaved mica and analyzed by AFM. A statistical analysis of the nanosheet dimensions was undertaken from resulting AFM images (Figures 5 and S36–S38). As the alkyl chain length increases from Cu(3) to Cu(5), the average thickness of the observed nanosheets decreased from 35 nm, through 22 nm, to 20 nm. Objects with heights of only 5 nm were observed in all three samples, corresponding to just five layers of framework. There is also an increase in lateral dimensions, from 222 to 348 nm across the series. These averages sit within a broad particle size distribution, with particles ranging between 50 and 900 nm. However, the observed differences in lateral dimensions between Cu(3)–Cu(4) and Cu(4)–Cu(5) are statistically significant (unpaired t test at 99% confidence level), and differences in both lateral dimensions and height are significant between Cu(3) and Cu(5) (unpaired t test, 99% confidence level). Additionally, the general trends are corroborated through dynamic light scattering (DLS) measurements (Figure S42 and Table S6), which confirmed the mean hydrodynamic diameter increased 185 < 213 < 247 nm (Table 3). As noted elsewhere, DLS underestimates nanosheet lateral dimensions with respect to AFM,66,67 in part because of the Stokes–Einstein equation assumption of spherical particles.68
Figure 5.

Topographic AFM images of nanosheets of Cu(3), Cu(4), and Cu(5) (a–c) observed after ultrasonic exfoliation of Cu(3–5)(DMF) in MeCN, centrifugation (1500 rpm for 1 h), and deposition onto mica. Associated scatter plots of MON dimensions (d–f).
Table 3. Summary of Statistical Size Data from Cu(3–5) MONs Observed after Exfoliation of MOFs Cu(3–5)(DMF) in MeCNa.
| Cu(3) | Cu(4) | Cu(5)57 | |
|---|---|---|---|
| n | 129 | 114 | 94 |
| x̅ LD ± SD | 222 ± 95 | 275 ± 148 | 348 ± 202 |
| x̅H ± SD | 35 ± 26 | 22 ± 13 | 20 ± 12 |
| x̅ aspect ratio ± SDb | 10 ± 7 | 15 ± 9 | 19 ± 10 |
| diameterc | 185 | 213 | 247 |
LD = largest lateral dimension (nm). H = height (nm) and SD= standard deviation (nm).
Mean aspect ratio determined for individual particles.
Hydrodynamic diameter (nm) according to number-averaged DLS data.
Overall, it is remarkable that increasing the alkyl chain length by just two methylene units almost doubles the aspect ratio of the nanosheets formed, from 10 to 19. The opposite trend might be expected as each extra methylene unit will contribute additional van der Waals interactions. However, the longer alkyl chains also increase the interlayer distance, reducing other interlayer interactions and potentially making the layers easier to shear apart during sonication. Solvent is also expected to play a key role in offsetting the energy penalty for creating new interfaces during exfoliation.69,70 Contact angle measurements revealed complete surface wetting (0°) upon addition of either DMF or MeCN to thin films of Cu(3–5)(DMF) MONs on mica compared to angles of 11° and 14°, respectively, for uncoated mica surfaces. The decrease in concentration of material in suspension with increasing alkyl chain length indicates less favorable solvent–MON interactions. This could also result in more of the larger particles sedimenting out of suspension during centrifugation, resulting in a lower average particle size distribution for the MONs with longer alkyl chains. However, given the larger lateral dimensions of the nanosheets of Cu(5) compared to Cu(3), we suggest that weaker interlayer interactions are the major driver for the thinner nanosheets observed in this case.
In contrast to exfoliation in MeCN, AFM analyses of the samples resulting from exfoliation in DMF showed only a small number of MONs with heights <50 nm across all samples. Cu(3)(DMF) heights ranged from 50 to 200 nm, Cu(4)(DMF) 40–150 nm and Cu(5)(DMF) 40–350 nm (Figures S39–41) with a broad lateral size distribution comparable to those observed in MeCN. A key difference between exfoliation in DMF compared to MeCN is that DMF remains coordinated to the axial position of the Cu-PW throughout the exfoliation process when carried out in DMF, indicated by no change to the diffraction pattern post sonication in DMF (Figure 3). Considering Cu(5)(DMF), the alkyl chains and DMF from adjacent layers interdigitate, contributing to the interlayer interactions (see Figure 2). If DMF is removed, the interactions between the remaining alkyl chains are likely to be weaker, potentially accounting for the thinner MONs observed following exfoliation in MeCN.
3. Conclusions
We have developed a series of layered MOFs incorporating ligands with alkyl side-chains of different length which are situated between the layers, in order to understand how this affects the nanosheets formed through liquid ultrasonic exfoliation of these MOFs. Ligands 1–5, which are 2,5-bis(alkoxy)benzene-1,4-dicarboxylates with alkoxy chains ranging from methoxy to pentoxy, were used to generate a series of Cu PW-based layered MOFs. Pawley and Rietveld refinements of synchrotron PXRD data revealed that three of the compounds, Cu(3–5)(DMF), formed an isoreticular series of layered PW MOFs with the square-grid topology. The fully desolvated structures Cu(3–5) were also characterized by Pawley refinement, and both series of compounds showed an increase in unit cell volume with increasing alkyl chain length. CO2 uptake in the relatively dense layered frameworks increases by nearly 50% from 27.2 to 40.2 cm3 g–1 with an increase in chain length across the series Cu(3–5), whereas negligible N2 uptake was observed in all cases.
Liquid exfoliation in an ultrasonic bath produced suspensions of MONs in DMF and MeCN with shorter alkyl chains found to result in higher concentrations of material in suspension for both solvents. Although higher concentrations of material were typically observed in DMF, this was found to correspond to thick sheets >50 nm in height. AFM revealed the formation of nanosheets in MeCN with thicknesses as low as 5 nm, corresponding to just five layers of the framework. The difference in behavior between the two solvents was attributed to removal of the apical DMF from the Cu PWs in MeCN, weakening interlayer interactions. We observed a decrease in the mean height of the MONs in MeCN from 35 nm > 22 nm > 20 nm for Cu(3)–Cu(4)–Cu(5), respectively. This trend of thinner nanosheets with increasing chain length was attributed to reducing the interlayer interactions and increasing the interlayer distance.
Together, these results demonstrate that an isoreticular series of MONs enables systematic studies to elucidate the rules governing the exfoliation of layered materials to form nanosheets. These insights will help in the design of new MOFs that are more readily exfoliated to form high concentrations of high-aspect-ratio nanosheets. The ability of MONs to be systematically tuned and optimized in this way provides them an advantage over other two-dimensional materials that are not modular in construction and will enable MONs to make an important contribution to sensing, catalysis, separation, electronics, and composite materials applications.
4. Experimental Section
Materials and reagents were obtained from multiple different commercial suppliers and used without further purification, specifically: dimethoxy-2,5-dihydroxybenzenedicarboxylate (Sigma-Aldrich, 99+%), methyl iodide (Alfa Aesar, 99%, stabilized with copper), ethyl bromide (Sigma-Aldrich, 98%), propyl bromide (Sigma-Aldrich, 99%), butyl bromide (Acros, 99%), pentyl bromide (Alfa Aesar, 99%), copper nitrate trihydrate (SLS, 98%), acetonitrile (Fisher, ≥ 99.9%) and n,n-dimethylformamide (Fisher, ≥99%).
4.1. Ligand Precursor Syntheses
Ligand precursors H21 to H24 were synthesized in close accordance with previously reported methodologies.54 Generally, dimethoxy-2,5-dihydroxybenzenedicarboxylate (1 g, 4.42 mmol) and K2CO3 (1.83 g, 13.28 mmol) were placed in a Schlenk flask and suspended in 30 mL of N,N-dimethylformamide (DMF). The corresponding alkyl halide was added under stirring via syringe (13.26 mmol of methyl iodide, ethyl bromide, propyl bromide, butyl bromide, or pentyl bromide, respectively). The reaction mixture was stirred overnight at 85 °C and transferred into a round-bottom flask, and the DMF was removed under reduced pressure. A NaOH solution (100 mL H2O, 400 mg NaOH) was added, and the product was refluxed overnight. After the mixture was allowed to cool to room temperature, aqueous HCl (10%) was added to precipitate the products as off-white powders. Yields: H21, 91% (0.91 g, 4.02 mmol); H22, 90% (0.91 g, 4.02 mmol); H23, 83% (1.0 g, 3.55 mmol); H24, 86% (1.2 g, 3.88 mmol).
The method was adapted for H25.47 A 2.089 g (9.24 mmol) sample of dimethoxy-2,5-dihydroxybenzenedicarboxylate was used, and other reagents were scaled accordingly. Instead of refluxing in NaOH(aq), the functionalized ester was refluxed in a 1:1 (v:v) mixture of THF and KOH(aq) (5%), before HCl workup in the same way to precipitate a white powder. Yield: H25, 87% (2.714 g, 8.02 mmol).
4.2. MOF Syntheses
Typically, Cu(NO3)2·3H2O (100 mg, 0.414 mmol) and H2(1–5) (0.455 mmol, 10% excess) were dissolved in 10 mL of DMF and sealed into 12 mL reaction vials with Teflon-lined lids. These were heated to 110 °C, held for 36 h, and then cooled at 0.1 °C min–1 to 25 °C. Green solids were isolated through centrifugation and washed with DMF (3 × 5 mL) and diethyl ether (2 × 5 mL). The resulting green microcrystalline powders were air-dried prior to characterization. Yields: Cu(1)(DMF), 82%; Cu(2)(DMF), 97%; Cu(3)(DMF), 93%; Cu(4)(DMF), 82%; Cu(5)(DMF), 89%. All yields are based on the anticipated structural formula [Cu(1–5)(DMF)]n and calculated based on Cu.
4.3. Ultrasonic Exfoliation
Five mg of the respective MOFs were suspended in 6 mL of solvent (DMF or MeCN). Mixtures were vortexed for 30 s before being placed in a Fisher brand Elmasonic P 30H ultrasonic bath (2.75 L, 380/350 W, UNSPSC 42281712) filled with water. Samples were held and rotated around the bath at 40 rpm using an adapted Heidolph RZR 2020 overhead stirrer with a multisample holder. The ultrasonic bath was operated at 100% and 80 kHz, and samples were sonicated for 12 h. A cooling water coil was equipped to prevent bath heating upon prolonged use, with the bath temperature equilibrating at 21 °C.
4.4. Characterization
TGA was performed using a PerkinElmer Pyris 1 TGA from 30 to 600 °C at 10 °C min–1, under a 20 mL min–1 N2 flow. FTIR specta were recorded using a PerkinElmer Spectrum One spectrometer equipped with a diamond ATR accessory. Data were collected from 500 to 4000 cm–1 using a resolution of 1 cm–1 and 16 scans. Elemental analyses were performed using a Elementar vario MICRO cube. Samples for SEM analysis were loaded onto a carbon sticky tab on an aluminum sample stub and coated with approximately 20 nm of gold using an Edwards S150B sputter coater. SEM micrographs were collected using a TESCAN VEGA3 LMU SEM instrument, operating at 15 keV and using the secondary electron detector. N2 and CO2 adsorption isotherms were measured using a Micromeritics 3 Flex volumetric gas adsorption instrument. Prior to dosing, samples were activated on the Micromeritics Smart Vac Prep Sample preparation station at 160 °C and 10–3 mbar for 10 h. Approximately 50 mg of solid, dried sample was used in each case. CO2 and N2 gases used had purity grades of 99.5 and 99.999%, respectively. N2 adsorption isotherms were recorded at 77 K using a liquid nitrogen bath for cooling of the sample cell, and CO2 adsorption isotherms were conducted at 195 K using a dry ice/isopropanol slurry for cooling of the sample cell. The Brunauer–Emmett–Teller equation was used to calculate the specific surface area from adsorption data obtained at 0.05 ≤ P/P0 ≤ 0.3. 1H NMR spectroscopy was performed on a Bruker Avance IIIHD 400 MHz spectrometer. 1H chemical shifts are reported in parts per million on the δ scale and referenced to the residual proton resonance of the solvent. UV–vis spectra were recorded with a Varian Cary 50 UV spectrophotometer using a resolution of 1 nm and scan speed of 600 nm min–1, controlled with PerkinElmer Spectrum One software. Measurements were made using 10 mm path length quartz cuvettes. AFM images were obtained using a Bruker Multimode 5 AFM equipped with a Nokia 10× visualizing lens, operating in soft tapping-mode using Bruker OTESPA-R3 cantilevers. Samples were prepared by dropping 10 μL (sample dependent) of suspension onto a freshly cleaved mica substrate. Images were processed using standard techniques with Gwyddion software.71 DLS data were collected using a Malvern Zetasizer Nano Series particle size analyzer equipped with a He–Ne laser at 633 nm, operating in backscatter mode (173°). Contact angle measurements were made using a Ramé-Hart goniometer.
4.5. Powder X-ray Diffraction Data
PXRD data were recorded at the University of Sheffield on a Bruker D8 ADVANCE powder X-ray diffractometer or using synchrotron radiation at beamline I11 at Diamond Light Source.72−74 Unit cell dimensions for samples Cu(3–5)(DMF) were determined by indexing of synchrotron PXRD patterns in the range 3° ≤ 2θ ≤ 40° and fitting by Pawley refinement,75 using TOPAS software.76,77 A structural model for Cu(5)(DMF), adapted from a previously published single-crystal structure,47 was subsequently fitted to the PXRD pattern by Rietveld refinement78 using TOPAS. Pawley and Rietveld refinement details are provided in the Supporting Information (Tables S3 and S4 and Figures S11–S20).
Acknowledgments
D.J.A. and J.A.F. thank the EPSRC (EP/K503149/1), the Ramsay Memorial Fellowship (JAF), and the University of Sheffield’s Vice Chancellors Fellowship (JAF) for funding. T.M.R. and L.B. acknowledge the Leverhulme Foundation for funding. P.V. and R.A.F. acknowledge financial support from the German Research Foundation within research unit FOR2433. A.S. acknowledges the German Research Foundation for a Postdoctoral Fellowship. The authors thank Diamond Light Source for beam time; Drs. Stephen Thompson and Claire Murray at beamline I11 for assistance with data collection; and Michael Harris, Christopher Hill, and the University of Sheffield BioMedical Sciences EM unit for SEM analysis.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.inorgchem.9b01128.
Material syntheses and characterization, PXRD refinement procedures, gas adsorption isotherms, UV data, AFM data, and statistical analyses (PDF)
Accession Codes
CCDC 1910582 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- Ashworth D. J.; Foster J. A. Metal-Organic Framework Nanosheets (MONs): A New Dimension in Materials Chemistry. J. Mater. Chem. A 2018, 6 (34), 16292–16307. 10.1039/C8TA03159B. [DOI] [Google Scholar]
- Zhao M.; Huang Y.; Peng Y.; Huang Z.; Ma Q.; Zhang H. Two-Dimensional Metal-Organic Framework Nanosheets: Synthesis and Applications. Chem. Soc. Rev. 2018, 47 (16), 6267–6295. 10.1039/C8CS00268A. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Peng J.; Wang W.; Liu S.; Zhao Q.; Huang W. Ultrathin Two-Dimensional Metal-Organic Framework Nanosheets for Functional Electronic Devices. Coord. Chem. Rev. 2018, 377, 44–63. 10.1016/j.ccr.2018.08.023. [DOI] [Google Scholar]
- Xu M.; Yang S. S.; Gu Z.-Y. Two-Dimensional Metal-Organic Framework Nanosheets: A Rapidly Growing Class of Versatile Nanomaterials for Gas Separation, MALDI-TOF Matrix and Biomimetic Applications. Chem. - Eur. J. 2018, 24, 15131–15142. 10.1002/chem.201800556. [DOI] [PubMed] [Google Scholar]
- Rodenas T.; Luz I.; Prieto G.; Seoane B.; Miro H.; Corma A.; Kapteijn F.; Llabrés I Xamena F. X.; Gascon J. Metal-Organic Framework Nanosheets in Polymer Composite Materials for Gas Separation. Nat. Mater. 2015, 14 (1), 48–55. 10.1038/nmat4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang H.; Hong L. Polycationic Polymer-Regulated Assembling of 2D MOF Nanosheets for High-Performance Nanofiltration. ACS Appl. Mater. Interfaces 2017, 9 (33), 28079–28088. 10.1021/acsami.7b08383. [DOI] [PubMed] [Google Scholar]
- Li Z. Q.; Qiu L. G.; Wang W.; Xu T.; Wu Y.; Jiang X. Fabrication of Nanosheets of a Fluorescent Metal-Organic Framework [Zn(BDC)(H2O)]n (BDC = 1,4-Benzenedicarboxylate): Ultrasonic Synthesis and Sensing of Ethylamine. Inorg. Chem. Commun. 2008, 11 (11), 1375–1377. 10.1016/j.inoche.2008.09.010. [DOI] [Google Scholar]
- Xu H.; Gao J.; Qian X.; Wang J.; He H.; Cui Y.; Yang Y.; Wang Z.; Qian G. Metal-Organic Framework Nanosheets for Fast-Response and Highly Sensitive Luminescent Sensing of Fe3+. J. Mater. Chem. A 2016, 4 (28), 10900–10905. 10.1039/C6TA03065C. [DOI] [Google Scholar]
- He L.; Duan F.; Song Y.; Guo C.; Zhao H.; Tian J.-Y.; Zhang Z.; Liu C.-S.; Zhang X.; Wang P.; et al. 2D Zirconium-Based Metal-Organic Framework Nanosheets for Highly Sensitive Detection of Mucin 1: Consistency between Electrochemical and Surface Plasmon Resonance Methods. 2D Mater. 2017, 4 (2), 025098. 10.1088/2053-1583/aa6fc6. [DOI] [Google Scholar]
- Yang Q.; Zhou L.; Wu Y. X.; Zhang K.; Cao Y.; Zhou Y.; Wu D.; Hu F.; Gan N. A Two Dimensional Metal-Organic Framework Nanosheets-Based Fluorescence Resonance Energy Transfer Aptasensor with Circular Strand-Replacement DNA Polymerization Target-Triggered Amplification Strategy for Homogenous Detection of Antibiotics. Anal. Chim. Acta 2018, 1020, 1–8. 10.1016/j.aca.2018.02.058. [DOI] [PubMed] [Google Scholar]
- Chen J.; Shu Y.; Li H.; Xu Q.; Hu X. Nickel Metal-Organic Framework 2D Nanosheets with Enhanced Peroxidase Nanozyme Activity for Colorimetric Detection of H2O2. Talanta 2018, 189 (March), 254–261. 10.1016/j.talanta.2018.06.075. [DOI] [PubMed] [Google Scholar]
- Hu S.; Yan J.; Huang X.; Guo L.; Lin Z.; Luo F.; Qiu B.; Wong K. Y.; Chen G. A Sensing Platform for Hypoxanthine Detection Based on Amino-Functionalized Metal Organic Framework Nanosheet with Peroxidase Mimic and Fluorescence Properties. Sens. Actuators, B 2018, 267, 312–319. 10.1016/j.snb.2018.04.055. [DOI] [Google Scholar]
- Zhan G.; Zeng H. C. Synthesis and Functionalization of Oriented Metal-Organic-Framework Nanosheets: Toward a Series of 2D Catalysts. Adv. Funct. Mater. 2016, 26 (19), 3268–3281. 10.1002/adfm.201505380. [DOI] [Google Scholar]
- Cao L.; Lin Z.; Peng F.; Wang W.; Huang R.; Wang C.; Yan J.; Liang J.; Zhang Z.; Zhang T.; et al. Self-Supporting Metal-Organic Layers as Single-Site Solid Catalysts. Angew. Chem., Int. Ed. 2016, 55 (16), 4962–4966. 10.1002/anie.201512054. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Zhao M.; Han S.; Lai Z.; Yang J.; Tan C.; Ma Q.; Lu Q.; Chen J.; Zhang X.; et al. Growth of Au Nanoparticles on 2D Metalloporphyrinic Metal-Organic Framework Nanosheets Used as Biomimetic Catalysts for Cascade Reactions. Adv. Mater. 2017, 29 (32), 1700102. 10.1002/adma.201700102. [DOI] [PubMed] [Google Scholar]
- Dong R.; Zheng Z.; Tranca D. C.; Zhang J.; Chandrasekhar N.; Liu S.; Zhuang X.; Seifert G.; Feng X. Immobilizing Molecular Metal Dithiolene-Diamine Complexes on 2D Metal-Organic Frameworks for Electrocatalytic H2Production. Chem. - Eur. J. 2017, 23 (10), 2255–2260. 10.1002/chem.201605337. [DOI] [PubMed] [Google Scholar]
- Ling P.; Qian C.; Gao F.; Lei J. Enzyme-Immobilized Metal-Organic Framework Nanosheets as Tandem Catalysts for the Generation of Nitric Oxide. Chem. Commun. 2018, 54 (79), 11176–11179. 10.1039/C8CC05068F. [DOI] [PubMed] [Google Scholar]
- Lan G.; Li Z.; Veroneau S. S.; Zhu Y.-Y.; Xu Z.; Wang C.; Lin W. Photosensitizing Metal-Organic Layers for Efficient Sunlight-Driven Carbon Dioxide Reduction. J. Am. Chem. Soc. 2018, 140 (39), 12369–12373. 10.1021/jacs.8b08357. [DOI] [PubMed] [Google Scholar]
- He T.; Ni B.; Zhang S.; Gong Y.; Wang H.; Gu L.; Zhuang J.; Hu W.; Wang X. Ultrathin 2D Zirconium Metal-Organic Framework Nanosheets: Preparation and Application in Photocatalysis. Small 2018, 14 (16), 1703929. 10.1002/smll.201703929. [DOI] [PubMed] [Google Scholar]
- Huang J.; Li Y.; Huang R. K.; He C. T.; Gong L.; Hu Q.; Wang L.; Xu Y. T.; Tian X. Y.; Liu S. Y.; et al. Electrochemical Exfoliation of Pillared-Layer Metal-Organic Framework to Boost the Oxygen Evolution Reaction. Angew. Chem., Int. Ed. 2018, 57 (17), 4632–4636. 10.1002/anie.201801029. [DOI] [PubMed] [Google Scholar]
- Saines P. J.; Tan J. C.; Yeung H. H. M.; Barton P. T.; Cheetham A. K. Layered Inorganic-Organic Frameworks Based on the 2,2-Dimethylsuccinate Ligand: Structural Diversity and Its Effect on Nanosheet Exfoliation and Magnetic Properties. Dalt. Trans. 2012, 41 (28), 8585–8593. 10.1039/c2dt30648d. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Hui L.; Ran W.; Lu Y.; Jia D. Facile Synthesis of Two-Dimensional (2D) Nanoporous NiO Nanosheets from Metal-Organic Frameworks with Superior Capacitive Properties. New J. Chem. 2016, 40 (2), 1100–1103. 10.1039/C5NJ02261D. [DOI] [Google Scholar]
- Benmansour S.; Abhervé A.; Gómez-Claramunt P.; Vallés-García C.; Gómez-García C. J. Nanosheets of Two-Dimensional Magnetic and Conducting Fe(II)/Fe(III) Mixed-Valence Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9 (31), 26210–26218. 10.1021/acsami.7b08322. [DOI] [PubMed] [Google Scholar]
- Hu X.; Wang Z.; Lin B.; Zhang C.; Cao L.; Wang T.; Zhang J.; Wang C.; Lin W. Two-Dimensional Metal-Organic Layers as a Bright and Processable Phosphor for Fast White-Light Communication. Chem. - Eur. J. 2017, 23 (35), 8390–8394. 10.1002/chem.201702037. [DOI] [PubMed] [Google Scholar]
- Xing W.; Ye P.; Lu J.; Wu X.; Chen Y.; Zhu T.; Peng A.; Huang H. Tellurophene-Based Metal-Organic Framework Nanosheets for High-Performance Organic Solar Cells. J. Power Sources 2018, 401 (August), 13–19. 10.1016/j.jpowsour.2018.08.078. [DOI] [Google Scholar]
- Jayaramulu K.; Masa J.; Morales D. M.; Tomanec O.; Ranc V.; Petr M.; Wilde P.; Chen Y. T.; Zboril R.; Schuhmann W.; et al. Ultrathin 2D Cobalt Zeolite-Imidazole Framework Nanosheets for Electrocatalytic Oxygen Evolution. Adv. Sci. 2018, 5 (11), 1801029. 10.1002/advs.201801029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-R.; Kuppler R. J.; Zhou H.-C. Selective Gas Adsorption and Separation in Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38 (5), 1477. 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]
- Huang Y. B.; Liang J.; Wang X. S.; Cao R. Multifunctional Metal-Organic Framework Catalysts: Synergistic Catalysis and Tandem Reactions. Chem. Soc. Rev. 2017, 46 (1), 126–157. 10.1039/C6CS00250A. [DOI] [PubMed] [Google Scholar]
- Morris R. E.; Brammer L. Coordination Change, Lability and Hemilability in Metal-Organic Frameworks. Chem. Soc. Rev. 2017, 46 (17), 5444–5462. 10.1039/C7CS00187H. [DOI] [PubMed] [Google Scholar]
- Evans J. D.; Garai B.; Reinsch H.; Li W.; Dissegna S.; Bon V.; Senkovska I.; Fischer R. A.; Kaskel S.; Janiak C.; et al. Metal-Organic Frameworks in Germany: From Synthesis to Function. Coord. Chem. Rev. 2019, 380, 378–418. 10.1016/j.ccr.2018.10.002. [DOI] [Google Scholar]
- Grigoropoulos A.; McKay A. I.; Katsoulidis A. P.; Davies R. P.; Haynes A.; Brammer L.; Xiao J.; Weller A. S.; Rosseinsky M. J. Encapsulation of Crabtree’s Catalyst in Sulfonated MIL-101(Cr): Enhancement of Stability and Selectivity between Competing Reaction Pathways by the MOF Chemical Microenvironment. Angew. Chem., Int. Ed. 2018, 57 (17), 4532–4537. 10.1002/anie.201710091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G.; Otsubo K.; Yamada T.; Sakaida S.; Kitagawa H. Superprotonic Conductivity in a Highly Oriented Crystalline Metal- Organic Framework Nanofil. J. Am. Chem. Soc. 2013, 135 (20), 7438–7441. 10.1021/ja402727d. [DOI] [PubMed] [Google Scholar]
- Pustovarenko A.; Goesten M. G.; Sachdeva S.; Shan M.; Amghouz Z.; Belmabkhout Y.; Dikhtiarenko A.; Rodenas T.; Keskin D.; Voets I. K.; et al. Nanosheets of Nonlayered Aluminum Metal-Organic Frameworks through a Surfactant-Assisted Method. Adv. Mater. 2018, 30 (26), 1707234. 10.1002/adma.201707234. [DOI] [PubMed] [Google Scholar]
- Lan G.; Li Z.; Veroneau S. S.; Zhu Y.-Y.; Xu Z.; Wang C.; Lin W. Photosensitizing Metal-Organic Layers for Efficient Sunlight-Driven Carbon Dioxide Reduction. J. Am. Chem. Soc. 2018, 140, 12369. 10.1021/jacs.8b08357. [DOI] [PubMed] [Google Scholar]
- Wang X.; Chi C.; Zhang K.; Qian Y.; Gupta K. M.; Kang Z.; Jiang J.; Zhao D. Reversed Thermo-Switchable Molecular Sieving Membranes Composed of Two-Dimensional Metal-Organic Nanosheets for Gas Separation. Nat. Commun. 2017, 8, 14460. 10.1038/ncomms14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y.; Li Y.; Ban Y.; Yang W. Two-Dimensional Metal-Organic Framework Nanosheets for Membrane-Based Gas Separation. Angew. Chem., Int. Ed. 2017, 56 (33), 9757–9761. 10.1002/anie.201703959. [DOI] [PubMed] [Google Scholar]
- Peng Y.; Li Y.; Ban Y.; Jin H.; Jiao W.; Liu X.; Yang W. Metal-Organic Framework Nanosheets as Building Blocks for Molecular Sieving Membranes. Science (Washington, DC, U. S.) 2014, 346 (6215), 1356. 10.1126/science.1254227. [DOI] [PubMed] [Google Scholar]
- Ding Y.; Chen Y. P.; Zhang X.; Chen L.; Dong Z.; Jiang H. L.; Xu H.; Zhou H. C. Controlled Intercalation and Chemical Exfoliation of Layered Metal-Organic Frameworks Using a Chemically Labile Intercalating Agent. J. Am. Chem. Soc. 2017, 139 (27), 9136–9139. 10.1021/jacs.7b04829. [DOI] [PubMed] [Google Scholar]
- Song W.-J. J. Intracellular DNA and MicroRNA Sensing Based on Metal-Organic Framework Nanosheets with Enzyme-Free Signal Amplification. Talanta 2017, 170 (February), 74–80. 10.1016/j.talanta.2017.02.040. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar P.; Mukhopadhyay A.; Savitha G.; Moorthy J. N. Orthogonal Self-Assembly of a Trigonal Triptycene Triacid: Signaling of Exfoliation of Porous 2D Metal-Organic Layers by Fluorescence and Selective CO2capture by the Hydrogen-Bonded MOF. J. Mater. Chem. A 2017, 5 (11), 5402–5412. 10.1039/C6TA11110F. [DOI] [Google Scholar]
- Ashworth D. J.; Cooper A.; Trueman M.; Al-Saedi R. W. M.; Smith L. D.; Meijer A. J.; Foster J. A. Ultrasonic Exfoliation of Hydrophobic and Hydrophilic Metal-Organic Frameworks to Form Nanosheets. Chem. - Eur. J. 2018, 24 (68), 17986–17996. 10.1002/chem.201803221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.-Z.; Maeda Y.; Xu Q. Top-down Fabrication of Crystalline Metal-Organic Framework Nanosheets. Chem. Commun. 2011, 47 (29), 8436–8438. 10.1039/c1cc12510a. [DOI] [PubMed] [Google Scholar]
- Xu G.; Yamada T.; Otsubo K.; Sakaida S.; Kitagawa H. Facile “Modular Assembly” for Fast Construction of a Highly Oriented Crystalline MOF Nanofilm. J. Am. Chem. Soc. 2012, 134 (40), 16524–16527. 10.1021/ja307953m. [DOI] [PubMed] [Google Scholar]
- Tan J. C.; Saines P. J.; Bithell E. G.; Cheetham A. K. Hybrid Nanosheets of an Inorganic-Organic Framework Material: Facile Synthesis, Structure, and Elastic Properties. ACS Nano 2012, 6 (1), 615–621. 10.1021/nn204054k. [DOI] [PubMed] [Google Scholar]
- Junggeburth S. C.; Diehl L.; Werner S.; Duppel V.; Sigle W.; Lotsch B. V. Ultrathin 2D Coordination Polymer Nanosheets by Surfactant-Mediated Synthesis. J. Am. Chem. Soc. 2013, 135, 6157–6164. 10.1021/ja312567v. [DOI] [PubMed] [Google Scholar]
- Beldon P. J.; Tominaka S.; Singh P.; Saha Dasgupta T.; Bithell E. G.; Cheetham A. K. Layered Structures and Nanosheets of Pyrimidinethiolate Coordination Polymers. Chem. Commun. 2014, 50 (31), 3955–3957. 10.1039/C4CC00771A. [DOI] [PubMed] [Google Scholar]
- Foster J. A.; Henke S.; Schneemann A.; Fischer R. A.; Cheetham A. K. Liquid Exfoliation of Alkyl-Ether Functionalised Layered Metal-Organic Frameworks to Nanosheets. Chem. Commun. 2016, 52 (69), 10474–10477. 10.1039/C6CC05154E. [DOI] [PubMed] [Google Scholar]
- Liu H.-L.; Chang Y.-J.; Fan T.; Gu Z.-Y. Two-Dimensional Metal-Organic Framework Nanosheets as a Matrix for Laser Desorption/Ionization of Small Molecules and Monitoring Enzymatic Reactions at High Salt Concentrations. Chem. Commun. 2016, 52 (88), 12984–12987. 10.1039/C6CC07371A. [DOI] [PubMed] [Google Scholar]
- Cliffe M. J.; Castillo-Martínez E.; Wu Y.; Lee J.; Forse A. C.; Firth F. C. N.; Moghadam P. Z.; Fairen-Jimenez D.; Gaultois M. W.; Hill J. A.; et al. Metal-Organic Nanosheets Formed via Defect-Mediated Transformation of a Hafnium Metal-Organic Framework. J. Am. Chem. Soc. 2017, 139 (15), 5397–5404. 10.1021/jacs.7b00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y.; Huang Y.; Zhu Y.; Chen B.; Wang L.; Lai Z.; Zhang Z.; Zhao M.; Tan C.; Yang N.; et al. Ultrathin Two-Dimensional Covalent Organic Framework Nanosheets: Preparation and Application in Highly Sensitive and Selective DNA Detection. J. Am. Chem. Soc. 2017, 139 (25), 8698–8704. 10.1021/jacs.7b04096. [DOI] [PubMed] [Google Scholar]
- Fan Z.; Nomura K.; Zhu M.; Li X.; Xue J.; Majima T.; Osakada Y. Synthesis and Photocatalytic Activity of Ultrathin Two-Dimensional Porphyrin Nanodisks via Covalent Organic Framework Exfoliation. Commun. Chem. 2019, 2 (1), 1–8. 10.1038/s42004-019-0158-8. [DOI] [Google Scholar]
- Kim J.; Kwon S.; Cho D. H.; Kang B.; Kwon H.; Kim Y.; Park S. O.; Jung G. Y.; Shin E.; Kim W. G.; et al. Direct Exfoliation and Dispersion of Two-Dimensional Materials in Pure Water via Temperature Control. Nat. Commun. 2015, 6, 1–9. 10.1038/ncomms9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari P.; Kaur N.; Sharma V.; Mobin S. M. High-Yield Graphene Produced from the Synergistic Effect of Inflated Temperature and Gelatin Offers High Stability and Cellular Compatibility. Phys. Chem. Chem. Phys. 2018, 20 (30), 20096–20107. 10.1039/C8CP02263A. [DOI] [PubMed] [Google Scholar]
- Henke S.; Schneemann A.; Kapoor S.; Winter R.; Fischer R. A. Zinc-1,4-Benzenedicarboxylate-Bipyridine Frameworks - Linker Functionalization Impacts Network Topology during Solvothermal Synthesis. J. Mater. Chem. 2012, 22 (3), 909–918. 10.1039/C1JM14791A. [DOI] [Google Scholar]
- Henke S.; Schneemann A.; Wütscher A.; Fischer R. A. Directing the Breathing Behavior of Pillared-Layered Metal-Organic Frameworks via a Systematic Library of Functionalized Linkers Bearing Flexible Substituents. J. Am. Chem. Soc. 2012, 134 (22), 9464–9474. 10.1021/ja302991b. [DOI] [PubMed] [Google Scholar]
- Ashworth D. J.; Cooper A.; Trueman M.; Al-Saedi R. W. M.; Smith L. D.; Meijer A. J. H. M.; Foster J. A. Ultrasonic Exfoliation of Hydrophobic and Hydrophilic Metal-Organic Frameworks To Form Nanosheets. Chem. - Eur. J. 2018, 24 (68), 17986–17996. 10.1002/chem.201803221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coblentz Society, Inc. Evaluated Infrared Reference Spectra. In NIST Chemistry Web Book, NIST Standard Reference Database Number 69; Linstrom P.J., Mallard W.G., Eds.; National Institute of Standards and Technology: Gaithersburg MD; 20899. 10.18434/T4D303. [DOI]
- Li H.; Eddaoudi M.; Groy T. L.; Yaghi O. M. Establishing Microporosity in Open Metal-Organic Frameworks: Gas Sorption Isotherms for Zn(BDC) (BDC = 1,4-Benzenedicarboxylate). J. Am. Chem. Soc. 1998, 120 (33), 8571–8572. 10.1021/ja981669x. [DOI] [Google Scholar]
- Guo Z.; Reddy M. V.; Goh B. M.; San A. K. P.; Bao Q.; Loh K. P. Electrochemical Performance of Graphene and Copper Oxide Composites Synthesized from a Metal-Organic Framework (Cu-MOF). RSC Adv. 2013, 3 (41), 19051–19056. 10.1039/c3ra43308k. [DOI] [Google Scholar]
- Eddaoudi M.; Kim J.; Rosi N.; Vodak D.; Wachter J.; O’Keeffe M.; Yaghi O. M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science (Washington, DC, U. S.) 2002, 295 (5554), 469–472. 10.1126/science.1067208. [DOI] [PubMed] [Google Scholar]
- Carson C. G.; Hardcastle K.; Schwartz J.; Liu X.; Hoffmann C.; Gerhardt R. A.; Tannenbaum R. Synthesis and Structure Characterization of Copper Terephthalate Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2009, 2009 (16), 2338–2343. 10.1002/ejic.200801224. [DOI] [Google Scholar]
- Carson C. G.; Brunnello G.; Lee S. G.; Jang S. S.; Gerhardt R. A.; Tannenbaum R. Structure Solution from Powder Diffraction of Copper 1,4- Benzenedicarboxylate. Eur. J. Inorg. Chem. 2014, 2014, 2140–2145. 10.1002/ejic.201301543. [DOI] [Google Scholar]
- Thommes M.; Kaneko K.; Neimark A. V.; Olivier J. P.; Rodriguez-Reinoso F.; Rouquerol J.; Sing K. S. W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87 (9–10), 1051–1069. 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- Schneemann A.; Takahashi Y.; Rudolf R.; Noro S.; Fischer R. A. Influence of Co-Adsorbates on CO 2 Induced Phase Transition in Functionalized Pillared-Layered Metal-Organic Frameworks. J. Mater. Chem. A 2016, 4 (33), 12963–12972. 10.1039/C6TA03266D. [DOI] [Google Scholar]
- Liscio A.; Kouroupis-Agalou K.; Betriu X. D.; Kovtun A.; et al. Evolution of the Size and Shape of 2D Nanosheets during Ultrasonic Fragmentation Evolution of the Size and Shape of 2D Nanosheets during Ultrasonic Fragmentation. 2D Mater. 2017, 4, 025017. 10.1088/2053-1583/aa57ff. [DOI] [Google Scholar]
- Mukhopadhyay A.; Maka V. K.; Savitha G.; Moorthy J. N. Photochromic 2D Metal-Organic Framework Nanosheets (MONs): Design, Synthesis, and Functional MON-Ormosil Composite. Chem. 2018, 4 (5), 1059–1079. 10.1016/j.chempr.2018.03.013. [DOI] [Google Scholar]
- Lotya M.; Rakovich A.; Donegan J. F.; Coleman J. N. Measuring the Lateral Size of Liquid-Exfoliated Nanosheets with Dynamic Light Scattering. Nanotechnology 2013, 24 (26), 265703. 10.1088/0957-4484/24/26/265703. [DOI] [PubMed] [Google Scholar]
- Shen J.; He Y.; Wu J.; Gao C.; Keyshar K.; Zhang X.; Yang Y.; Ye M.; Vajtai R.; Lou J.; et al. Liquid Phase Exfoliation of Two-Dimensional Materials by Directly Probing and Matching Surface Tension Components. Nano Lett. 2015, 15 (8), 5449–5454. 10.1021/acs.nanolett.5b01842. [DOI] [PubMed] [Google Scholar]
- Hernandez Y.; Nicolosi V.; Lotya M.; Blighe F. M.; Sun Z.; De S.; McGovern I. T.; Holland B.; Byrne M.; Gun’ko Y. K.; et al. High-Yield Production of Graphene by Liquid-Phase Exfoliation of Graphite. Nat. Nanotechnol. 2008, 3 (9), 563–568. 10.1038/nnano.2008.215. [DOI] [PubMed] [Google Scholar]
- Nečas D.; Klapetek P. Gwyddion: An Open-Source Software for SPM Data Analysis. Cent. Eur. J. Phys. 2012, 10 (1), 181–188. 10.2478/s11534-011-0096-2. [DOI] [Google Scholar]
- Tartoni N.; Thompson S. P.; Tang C. C.; Willis B. L.; Derbyshire G. E.; Wright A. G.; Jaye S. C.; Homer J. M.; Pizzey J. D.; Bell A. M. T. High-Performance X-Ray Detectors for the New Powder Diffraction Beamline I11 at Diamond. J. Synchrotron Radiat. 2008, 15 (1), 43–49. 10.1107/S0909049507046250. [DOI] [PubMed] [Google Scholar]
- Thompson S. P.; Parker J. E.; Potter J.; Hill T. P.; Birt A.; Cobb T. M.; Yuan F.; Tang C. C. Beamline I11 at Diamond: A New Instrument for High Resolution Powder Diffraction. Rev. Sci. Instrum. 2009, 80 (7), 075107. 10.1063/1.3167217. [DOI] [PubMed] [Google Scholar]
- Thompson S. P.; Parker J. E.; Marchal J.; Potter J.; Birt A.; Yuan F.; Fearn R. D.; Lennie A. R.; Street S. R.; Tang C. C. Fast X-Ray Powder Diffraction on I11 at Diamond. J. Synchrotron Radiat. 2011, 18 (4), 637–648. 10.1107/S0909049511013641. [DOI] [PubMed] [Google Scholar]
- Pawley G. S. Unit-Cell Refinement from Powder Diffraction Scans. J. Appl. Crystallogr. 1981, 14 (6), 357–361. 10.1107/S0021889881009618. [DOI] [Google Scholar]
- Coelho A. A.TOPAS Academic, version 4.1; see http://www.topas-academic.net.
- Coelho A. A.; Evans J.; Evans I.; Kern A.; Parsons S. The TOPAS Symbolic Computation System. Powder Diffr. 2011, 26 (S1), S22–S25. 10.1154/1.3661087. [DOI] [Google Scholar]
- Rietveld H. M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2 (2), 65–71. 10.1107/S0021889869006558. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



