Abstract

A dual seven-membered cyclic carbonate/olefin monomer was synthesized from CO2 and cis-1,4-butenediol and polymerized. The properties of the polymer were controlled using divergent catalytic strategies toward the stereochemistry of the olefin. Ring-opening polymerization of the cyclic carbonate using an organocatalytic approach retained the cis-stereoconfiguration of the olefin and yielded a hard semicrystalline polymer (Tm 115 °C). Ring-opening metathesis polymerization using Grubbs’ catalyst proceeded with high trans-stereoregularity (95%) and produced a soft amorphous polymer (Tg −22 °C). Cis to trans isomerization of the polymer was possible using Cu(I) salts under UV light. In all polymers, the C=C double bond remained available for postpolymerization modification and thermoset resins were formed by cross-linking. From this single monomer, cis-trans-cis triblock copolymers, with potential applications as thermoplastic elastomers, were synthesized by combining both strategies using cis-1,4-butenediol as a chain transfer agent.
Controlling polymer stereochemistry is a powerful strategy to manipulate its physical properties.1−8 The influence of the stereochemistry of alkene bonds in polymer chains is particularly remarkable. In nature, poly(cis-isoprene) (natural rubber) has thus vastly superior elastomeric properties compared with poly(trans-isoprene) (gutta percha).9−11 Catalysis has been an efficient way to control synthetic polymers’ stereochemistry by generating chain tacticity, yet examples of its application in cis/trans isomerism remain comparatively rare. Hillmyer and co-workers for example demonstrated the stereoselective ring-opening metathesis polymerization of cis-cyclooctene monomers using Ru catalysts (trans-selective)12 and Mo catalysts (cis-selective).13 Becker and Dove reported an organocatalytic stereocontrolled synthesis of unsaturated polyesters, resulting in a tunable cis/trans ratio, crystallinity, and mechanical properties.14 Postpolymerization catalysis was also exploited to isomerize poly(propylene maleate) into poly(propylene fumarate) toward 3D printing and medical applications.15−17
The ability to drastically change polymer properties through cis/trans isomerization, e.g. from hard to soft materials, is particularly appealing in the context of synthetic thermoplastics elastomers (TPEs), which have a wide range of applications including 3D printing, elastomers (e.g., in footwear), pressure-sensitives adhesives, and coatings.18,19 TPEs have similar properties to chemically cross-linked rubbers,20 but they can be melt-(re)processed like thermoplastics. TPEs are usually ABA triblock copolymers in which hard/crystalline end-blocks (A) can microphase-separate from the soft/amorphous midblock (B) and act as physical cross-links to strengthen the elastic matrix. To the best of our knowledge, these TPEs are always made from two different monomers and may require purification of the middle block. Motivated by the desire to prepare hard or soft materials, as well as triblock TPEs, from one single monomer and in one pot, we anticipated that cis/trans stereoselective polymerization of a selected monomer would provide the desired platform. Here we describe such a monomer, which is a dual cyclic carbonate/olefin. We also demonstrate that these polymers are amenable to cross-linking and thermosets formation.
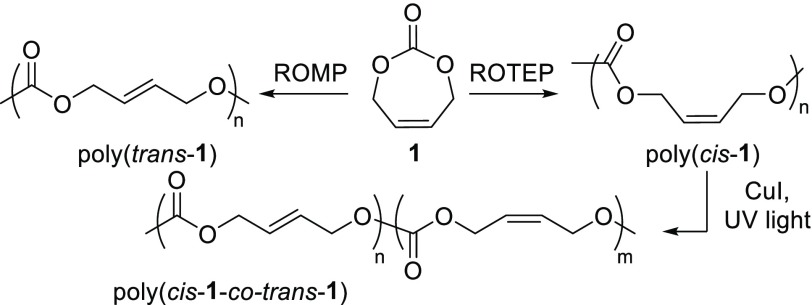
cis-1,4-Butenediol is a 1,4-diol which may be derived from erythritol,21 a fermentation product of glucose (Figure 1). The corresponding cyclic carbonate, 4,7-dihydro-1,3-dioxepin-2-one (1), was readily prepared in one step from CO2, in 51% crystalline yield, following a procedure reported by our group (Figure 1).22 CO2 insertion was achieved using 2 equiv of 2,2,6,6-tetramethylpiperidine (TMP) with cyclization facilitated by 1 equiv of para-toluenesulfonate chloride (TsCl). 1 is a seven-membered cyclic compound with both a carbonate and an alkene functionality, providing opportunities for ring-opening polymerization using metathesis (ROMP) or transcarbonation (referred to here as ROP) methodologies. We reasoned that ROMP and ROP would both produce a polycarbonate, but could show opposite stereoselectivity toward the configuration of the polymer alkene bonds.
Figure 1.
Retrosynthetic approach to 1,4-butenediol from erythritol; synthesis of 4,7-dihydro-1,3-dioxepin-2-one (1); ORTEP23 view of the crystal structure of 1 (displacement ellipsoids at 50% probability level).
First, various organo- and metal-based catalysts were screened for the ROP of 1 in solution (Table S1). When using 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) as the catalyst (Table 1), ROP proceeded rapidly at room temperature with 100% conversion after 10 min, at a monomer/catalyst/initiator ([1]0:[cat.]0:[I]0) feed ratio of 100:1:1 (Table 1, entry 2). The quantitative conversion of the monomer to polymer is consistent with the high ring strain of 1 calculated by DFT (Figure S56). For each of the ROP catalysts tested, the cis configuration of the monomer’s alkene was maintained in the polymer as evidenced by 1H NMR spectroscopy (Figure 2). Using TBD led to a good correlation between theoretical and experimental Mn values measured by Size-Exclusion Chromatography (SEC), with dispersities (ĐM) of 1.3–1.8 (Tables 1 and S1). ROP could also occur at 60 °C in molten monomer using Sn(Oct)2 or TBD as the catalyst (Table 1 entry 4 and Table S1 entry 5, respectively). MALDI-ToF spectrometry and 1H NMR spectroscopy confirmed the presence of 4-methyl benzyl alcohol and OH chain ends (Figure S27). Initial rate kinetic studies carried out with TBD indicated first-order kinetics (kobs = 0.117 s–1) with respect to monomer concentration (Figures S30–S31). Mn was found to increase linearly with conversion (Figures S32–S33) with an Mn,SEC of 22 100 g mol–1 achievable (degree of polymerization (DP) ≈ 200, Figure S18).
Table 1. Polymerization of 1 Using ROTEP and ROMP Catalysis with Divergent Cis/Trans Stereoselectivitya.
| entry | [cat.] | [1]0:[cat.]0:[I]0b | temp (°C) | time (min)c | conv (%)d | cis/transe (m:n) | Mn,theo (g mol–1)f | Mn,NMR (g mol–1)g | Mn,SEC [ĐM] (g mol–1)h |
|---|---|---|---|---|---|---|---|---|---|
| 1 | TBD | 50:1:1 | 22 | 1 | 100 | 100:0 | 5820 | 6500 | 6400 [1.65] |
| 2 | TBD | 100:1:1 | 22 | 10 | 100 | 100:0 | 11 520 | 10 800 | 11 000 [1.62] |
| 3 | TBD | 150:1:1 | 22 | 40 | 100 | 100:0 | 17 220 | 17 100 | 18 100 [1.53] |
| 4 | Sn(Oct)2i | 100:0.5:1 | 60 | 10 | 70j | 100:0 | 8070 | 9400 | 8600 [1.16] |
| 5 | Sn(Oct)2i | 200:0.5:1 | 60 | 18 | 41j | 100:0 | 9400 | 7300 | 8900 [1.27] |
| 7 | GII | 150:1:0 | 22 | 30 | 100 | 15:85 | 17 190 | – | 22 600 [2.02] |
| 8 | GIIk | 150:1:0 | 22 | 30 | 100 | 5:95 | 17 190 | – | 22 500 [1.58] |
| 9 | GII | 300:1:0 | 22 | 50 | 100 | 10:90 | 34 290 | – | 34 300 [1.80] |
| 10 | GIIi | 150:1:0 | 120 | 30 | 100 | 20:80 | 17 190 | – | 23 200 [2.69] |
In CH2Cl2 with [1]0 = 1 mol L–1 unless stated otherwise.
I is 4-MeBnOH.
Reaction times not optimized.
Calculated by relative integration of the alkene protons in 1 (δ = 5.82 ppm, t (J = 1.8 Hz)) and poly(1) (cis δ = 5.80 ppm, t (J = 4.1 Hz), trans δ = 5.90 ppm, t (J = 3.0 Hz)) in the 1H NMR spectrum in CDCl3.
Determined by relative integration of alkene protons in the trans and cis polymer.
Calculated as Mr(I)+ (Mr(1) × [1]0/[I]0 × conv/100%); for ROMP: Mr(I) = Mr(CHPh) and [I]0 = [cat.]0.
For ROTEP: calculated by the relative integration of the methylene protons in I (δ = 5.12 ppm, s) and in poly(1); undeterminable for ROMP.
Calculated by SEC relative to polystyrene standards in CHCl3 or THF eluent, ĐM = Mw/Mn.
No solvent.
Reaction quenched as stirring stopped.
Reaction carried out under UV light (λ = 365 nm).
Figure 2.
Alkene and methylene regions of the 1H NMR spectra of poly(1), demonstrating the divergence of the catalytic strategies used, and the partial isomerization from poly(cis-1) (top) to poly(trans-1) (bottom), using CuI under UV light (middle).
Next, the ROMP of 1 was carried out (Scheme 1), catalyzed by Grubbs second generation catalyst (GII). Quantitative conversion was achieved at 22 °C in solution after 30 min at the [1]0:[GII]0 150:1 ratio. Good control over Mn and ĐM was observed across the range of loadings tested (Table 1, entries 7–10 and Table S2). An Mn,SEC of up to 34 300 g mol–1 (DP ≈ 300) was achieved after 50 min (Table 1, entry 9). Satisfyingly, GII favored the formation of the trans isomer with stereoregularities of up to 95% obtainable in 30 min under UV, as observed by 1H NMR spectroscopy (Figure 2 and Table 1, entry 8). The trans selectivity of the reaction was found to be sensitive to time, monomer concentration, and temperature (Table S2). Longer reaction times and high dilution favored formation of the trans isomer, with a maximum selectivity of 95% observed after 92 h (Table S2, entry 9). At 120 °C, trans selectivity decreased to 88% and 80% when performing reactions in 1,2-tetrachloroethane or neat monomer, respectively (Table S2, entry 6, Table 1, entry 10).
Scheme 1. Divergent Stereoselective Strategies Used for the ROP of 1.
Postpolymerization isomerization was also explored as DFT indicated the trans isomer was thermodynamically more stable (Figure S57). Isomerization of the double bond was possible under UV light at room temperature, using 1 alkene equivalent of CuI (52% conversion of poly(cis-1) to trans isomer, 68 h) (Figure S10). No degradation of the polymer was observed by SEC (Figure S13) or NMR spectroscopy (Figure S12).
The appearance and solubility of poly(trans-1) are significantly different from those of poly(cis-1). At room temperature poly(trans-1) is a viscoelastic solid, soluble in THF, whereas poly(cis-1) is a powder, insoluble in THF but soluble in CHCl3 (Figure S8). Thermogravimetric analysis (TGA) indicated that the onset of thermal degradation (Td,onset) for both polymers occurred at 152 °C (Table S4, entries 1 and 2). Differential scanning calorimetry (DSC) revealed for the cis polymer the presence of a minor glass transition at −24 °C (Tg) and a melting transition at 115 °C (Tm) (Figure S46). For the 5:95 (cis/trans) polymer, a major single Tg at −22 °C was observed, attributed to the cis regime (Figure 3 and Figure S47). A Tm was not observed. The significant difference in crystallinity of the trans and cis polymers highlights the impact of the double-bond isomerism on material properties.
Figure 3.

DSC thermograms of poly(cis-1) (top) and poly(trans-1) (bottom).
Cross-linking experiments were conducted to explore the potential of the polymers for thermoset formation and coating applications.24−26 Trimethylolpropane tris(3-mercaptopropionate) was reacted with poly(cis-1) and a cis/trans (12:88) polymer in the presence of photoinitiator Irgacure 819 at three alkene/thiol [C=C]0:[SH]0 loadings (1:1, 2:1, 5:1). DSC analysis confirmed the formation of highly cross-linked networks with no Tm or Tg being observed up to 140 °C at ratios of 1:1 and 2:1 (Figures S51–S53). Crystallinity was still observed for a weakly cross-linked 5:1 sample (Figure S50). TGA revealed increasing thermal stability with increasing cross-linking (Table S6, maximum Td,onset = 193 °C).
The synthesis of a cis-trans-cis triblock was then attempted, utilizing a sequential ROMP/ROTEP concept originally demonstrated by Hillmyer and co-workers,27−29 albeit this time with one single monomer, 1 (Scheme 2). Cis-butene-1,4-diol was used as a chain transfer agent (CTA) for the ROMP of 1 in a [1]0:[GII]0:[CTA]0 ratio of 20:0.005:1 (Table 2, entry 1). THF was used as the solvent to decrease deactivation of the CTA by GII,30 and a [CTA]0:[GII]0 ratio of 200:1 was chosen to ensure high incorporation of CTA in the polymer.31 After 45 h, an aliquot indicated 100% conversion of 1 to poly(1) with a cis/trans ratio of 7:93 and an Mn,SEC (ĐM) of 2100 (2.02) g mol–1 (Table 2 entry 1). MALDI-ToF spectrometry revealed the presence of OH chain ends (Figure S31), and 1H NMR spectroscopy confirmed the incorporation of the CTA into the polymer’s backbone with the expected 1:20 ratio (Figure S21). TBD and more monomer were then added in a 2:20 ratio with respect to [CTA]0. 1H NMR spectroscopy indicated 100% conversion of monomer after 3 min giving a polymer (ABA-1) with a cis/trans content of 54:46 and an Mn,SEC (ĐM) of 4600 (2.06) g mol–1. 1H DOSY NMR spectroscopy confirmed the presence of only one polymer species, with both cis and trans regimes, and the sequential increase of molar mass (decrease in diffusion coefficient from 2.06 × 10–8 to 1.48 × 10–8 s–1, Figures S22 and S24). The experiment was repeated at a [1]0:[GII]0:[CTA]0 feed ratio of 40:0.005:1, with additional monomer and TBD (40:1 with respect to [CTA]0). A cis-trans-cis ABA triblock (ABA-2) of higher Mn (Mn,SEC = 8900 g mol–1, Đ = 1.60, cis/trans 56:44) was obtained (Table 2, entry 2). 13C{1H} NMR spectroscopy gave some insight into the copolymer chain microstructure, revealing limited transcarbonation reactions and well-defined blocks (Figure S25). This was supported by DSC analysis, which showed phase separation and retention of the crystallinity of the cis end-blocks (Figure S55).18
Scheme 2. Sequential ROMP/ROTEP Strategy for the Formation of Cis-Trans-Cis Triblock Copolymers.
Table 2. Synthesis of Poly(cis-1-b-trans-1-b-cis-1).
| entry | n:CTA | cis/trans | Mtheo (g mol–1)b | Mn,NMR (g mol–1)c | Mn, SECd (g mol–1)d | ĐMd |
|---|---|---|---|---|---|---|
| 1 | 20:1 | 54:46 (3:97) | 4650 (2370) | 3700 (2200) | 4600 (2100) | 2.06 (2.02) |
| 2 | 40:1 | 54:46 (3:97) | 9210 (4650) | 10 000 (5000) | 8900 (4700) | 1.60 (1.67) |
Sequential procedure outlined in Scheme 2, with [1]0 = 1 mol L–1. Monomer conversion quantitative for each step. Values in brackets were obtained after the ROMP step.
Calculated as Mr(CTA) + (2 × n × Mr(1)).
Calculated by the relative integration of the methylene protons in the CTA (δ = 4.19–4.27 ppm) and the polymer (δ = 4.60 – 4.80 ppm).
Calculated by SEC relative to polystyrene standards in THF eluent.
In conclusion, we have demonstrated that from novel dual monomer 1, divergent stereoselective polymerization catalytic strategies can direct polymer properties between a soft amorphous material (Tg −22 °C) and a hard semicrystalline material (Tm 115 °C). These thermoplastics can further be transformed into thermosets via cross-linking of the alkene polymer backbone. A CTA has also been employed to synthesize triblock ABA polymers from this single monomer, with potential applications as thermoplastic elastomers. Our ongoing efforts are now aimed at investigating the effect of composition and molar mass of these triblock copolymers on nanoscales morphologies and mechanical properties. Finally, the carbonate function of these polymers introduces some opportunities for chemical and biological degradation. We anticipate that understanding the degradability of these materials will facilitate their adoption as sustainable alternatives in various commodity and specialty applications.
Acknowledgments
Analytical facilities were provided through the Material and Chemical Characterisation Facility (MC2) at the University of Bath. We thank the University of Bath HPC for computing resources, the UK EPSRC, and the University of Bath (studentship for TMG), as well as the Royal Society (RG/150538, UF/160021 fellowship to AB) for research funding.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b06259.
Experimental and computational procedures, NMR spectra of monomer and polymers, Plot of Mn and ĐM vs. conversion, polymerization kinetic data, images of SEC traces, MALDI-ToF MS, TGA-MS, DSC traces and DMA data, DFT calculations data and associated digital repository (PDF)
Single-crystal X-ray diffraction data for 1 (CIF)
The authors declare no competing financial interest.
Supplementary Material
References
- Worch J. C.; Prydderch H.; Jimaja S.; Bexis P.; Becker M. L.; Dove A. P.. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 2019, 10.1038/s41570-019-0117-z. [DOI] [Google Scholar]
- Domski G. J.; Rose J. M.; Coates G. W.; Bolig A. D.; Brookhart M. Living alkene polymerization: New methods for the precision synthesis of polyolefins. Prog. Polym. Sci. 2007, 32 (1), 30–92. 10.1016/j.progpolymsci.2006.11.001. [DOI] [Google Scholar]
- Lu X. B.; Ren W. M.; Wu G. P. CO2 Copolymers from Epoxides: Catalyst Activity, Product Selectivity, and Stereochemistry Control. Acc. Chem. Res. 2012, 45 (10), 1721–1735. 10.1021/ar300035z. [DOI] [PubMed] [Google Scholar]
- Dijkstra P. J.; Du H. Z.; Feijen J. Single site catalysts for stereoselective ring-opening polymerization of lactides. Polym. Chem. 2011, 2 (3), 520–527. 10.1039/C0PY00204F. [DOI] [Google Scholar]
- Carpentier J.-F. Discrete Metal Catalysts for Stereoselective Ring-Opening Polymerization of Chiral Racemic β-Lactones. Macromol. Rapid Commun. 2010, 31 (19), 1696–1705. 10.1002/marc.201000114. [DOI] [PubMed] [Google Scholar]
- Miyake G. M.; Chen E. Y. X. Synthesis of highly syndiotactic polymers by discrete catalysts or initiators. Polym. Chem. 2011, 2 (11), 2462–2480. 10.1039/c1py00245g. [DOI] [Google Scholar]
- Stanford M. J.; Dove A. P. Stereocontrolled ring-opening polymerisation of lactide. Chem. Soc. Rev. 2010, 39 (2), 486–494. 10.1039/B815104K. [DOI] [PubMed] [Google Scholar]
- Thomas C. M. Stereocontrolled ring-opening polymerization of cyclic esters: synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39 (1), 165–173. 10.1039/B810065A. [DOI] [PubMed] [Google Scholar]
- Bunn C. W.; B W. H. Molecular structure and rubber-like elasticity I. The crystal structures of β gutta-percha, rubber and polychloroprene. Proc. R. Soc. London: Ser. A 1942, 180 (980), 40–66. 10.1098/rspa.1942.0024. [DOI] [Google Scholar]
- Goodman A.; Schilder H.; Aldrich W. The thermomechanical properties of gutta-percha: II. The history and molecular chemistry of gutta-percha. Oral Surg., Oral Med., Oral Pathol. 1974, 37 (6), 954–961. 10.1016/0030-4220(74)90448-4. [DOI] [PubMed] [Google Scholar]
- Bateman L.The Chemistry and Physics of Rubber-Like Substances; Wiley: New York, 1964. [Google Scholar]
- Kobayashi S.; Pitet L. M.; Hillmyer M. A. Regio- and Stereoselective Ring-Opening Metathesis Polymerization of 3-Substituted Cyclooctenes. J. Am. Chem. Soc. 2011, 133 (15), 5794–5797. 10.1021/ja201644v. [DOI] [PubMed] [Google Scholar]
- Jeong H.; Kozera D. J.; Schrock R. R.; Smith S. J.; Zhang J.; Ren N.; Hillmyer M. A. Z-Selective Ring-Opening Metathesis Polymerization of 3-Substituted Cyclooctenes by Monoaryloxide Pyrrolide Imido Alkylidene (MAP) Catalysts of Molybdenum and Tungsten. Organometallics 2013, 32 (17), 4843–4850. 10.1021/om400583t. [DOI] [Google Scholar]
- Bell C. A.; Yu J. Y.; Barker I. A.; Truong V. X.; Cao Z.; Dobrinyin A. V.; Becker M. L.; Dove A. P. Independent Control of Elastomer Properties through Stereocontrolled Synthesis. Angew. Chem., Int. Ed. 2016, 55 (42), 13076–13080. 10.1002/anie.201606750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.; Dolder C. K.; Walker J. M.; Mishra R.; Dean D.; Becker M. L. Synthesis and Biological Evaluation of Well-Defined Poly(propylene fumarate) Oligomers and Their Use in 3D Printed Scaffolds. Biomacromolecules 2016, 17 (2), 690–697. 10.1021/acs.biomac.6b00014. [DOI] [PubMed] [Google Scholar]
- Wilson J. A.; Luong D.; Kleinfehn A. P.; Sallam S.; Wesdemiotis C.; Becker M. L. Magnesium Catalyzed Polymerization of End Functionalized Poly(propylene maleate) and Poly(propylene fumarate) for 3D Printing of Bioactive Scaffolds. J. Am. Chem. Soc. 2018, 140 (1), 277–284. 10.1021/jacs.7b09978. [DOI] [PubMed] [Google Scholar]
- DiCiccio A. M.; Coates G. W. Ring-Opening Copolymerization of Maleic Anhydride with Epoxides: A Chain-Growth Approach to Unsaturated Polyesters. J. Am. Chem. Soc. 2011, 133 (28), 10724–10727. 10.1021/ja203520p. [DOI] [PubMed] [Google Scholar]
- Wang W.; Lu W.; Goodwin A.; Wang H.; Yin P.; Kang N.-G.; Hong K.; Mays J. W. Recent advances in thermoplastic elastomers from living polymerizations: Macromolecular architectures and supramolecular chemistry. Prog. Polym. Sci. 2019, 95, 1–31. 10.1016/j.progpolymsci.2019.04.002. [DOI] [Google Scholar]
- Drobny J. G.Handbook of Thermoplastic Elastomers, 2nd ed.; William Andrew Publishing: Oxford, 2014. [Google Scholar]
- Bhowmick A. K.; Stephens H.. Handbook of Elastomers, 2nd ed.; Taylor & Francis: Boca Raton, FL, 2000. [Google Scholar]
- Raju S.; Jastrzebski J.; Lutz M.; Gebbink R. Catalytic Deoxydehydration of Diols to Olefins by using a Bulky Cyclopentadiene-based Trioxorhenium Catalyst. ChemSusChem 2013, 6 (9), 1673–1680. 10.1002/cssc.201300364. [DOI] [PubMed] [Google Scholar]
- McGuire T. M.; Lopez-Vidal E. M.; Gregory G. L.; Buchard A. Synthesis of 5-to 8-membered cyclic carbonates from diols and CO2: A one-step, atmospheric pressure and ambient temperature procedure. J. CO2 Util. 2018, 27, 283–288. 10.1016/j.jcou.2018.08.009. [DOI] [Google Scholar]
- Farrugia L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854. 10.1107/S0021889812029111. [DOI] [Google Scholar]
- Stößer T.; Li C.; Unruangsri J.; Saini P. K.; Sablong R. J.; Meier M. A. R.; Williams C. K.; Koning C. Bio-derived polymers for coating applications: comparing poly(limonene carbonate) and poly(cyclohexadiene carbonate). Polym. Chem. 2017, 8 (39), 6099–6105. 10.1039/C7PY01223C. [DOI] [Google Scholar]
- Koning C. E.; Sablong R. J.; Nejad E. H.; Duchateau R.; Buijsen P. Novel coating resins based on polycarbonates and poly(ester-co-carbonate)s made by catalytic chain growth polymerization of epoxides with CO2 and with anhydride/CO2. Prog. Org. Coat. 2013, 76 (12), 1704–1711. 10.1016/j.porgcoat.2013.05.004. [DOI] [Google Scholar]
- Sainz M. F.; Souto J. A.; Regentova D.; Johansson M. K. G.; Timhagen S. T.; Irvine D. J.; Buijsen P.; Koning C. E.; Stockman R. A.; Howdle S. M. A facile and green route to terpene derived acrylate and methacrylate monomers and simple free radical polymerisation to yield new renewable polymers and coatings. Polym. Chem. 2016, 7 (16), 2882–2887. 10.1039/C6PY00357E. [DOI] [Google Scholar]
- Pitet L. M.; Hillmyer M. A. Combining Ring-Opening Metathesis Polymerization and Cyclic Ester Ring-Opening Polymerization To Form ABA Triblock Copolymers from 1,5-Cyclooctadiene and d,l-Lactide. Macromolecules 2009, 42 (11), 3674–3680. 10.1021/ma900368a. [DOI] [Google Scholar]
- Arrington K. J.; Waugh J. B.; Radzinski S. C.; Matson J. B. Photo- and Biodegradable Thermoplastic Elastomers: Combining Ketone-Containing Polybutadiene with Polylactide Using Ring-Opening Polymerization and Ring-Opening Metathesis Polymerization. Macromolecules 2017, 50 (11), 4180–4187. 10.1021/acs.macromol.7b00479. [DOI] [Google Scholar]
- Xiang S.; Zhang Q.; Zhang G.; Jiang W.; Wang Y.; Zhou H.; Li Q.; Tang J. Facile Synthesis of Block Copolymers by Tandem ROMP and eROP from Esters Precursors. Biomacromolecules 2014, 15 (8), 3112–3118. 10.1021/bm500723k. [DOI] [PubMed] [Google Scholar]
- Bielawski C. W.; Scherman O. A.; Grubbs R. H. Highly efficient syntheses of acetoxy- and hydroxy-terminated telechelic poly(butadiene)s using ruthenium catalysts containing N-heterocyclic ligands. Polymer 2001, 42 (11), 4939–4945. 10.1016/S0032-3861(00)00504-8. [DOI] [Google Scholar]
- Hillmyer M. A.The Preparation of Functionalized Polymers by Ring-Opening Metathesis; California Institute of Technology: 1995.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.