Abstract
PURPOSE
Approximately 15% to 43% of esophageal adenocarcinomas (EACs) are human epidermal growth factor receptor 2 (HER2) positive. Because dual-agent HER2 blockade demonstrated a survival benefit in breast cancer, we conducted a phase II feasibility study of trastuzumab and pertuzumab added to neoadjuvant chemoradiotherapy (nCRT) in patients with EAC.
PATIENTS AND METHODS
Patients with resectable HER2-positive EAC received standard nCRT with carboplatin and paclitaxel and 41.4 Gy of radiotherapy, with 4 mg/kg of trastuzumab on day 1, 2 mg/kg per week during weeks 2 to 6, and 6 mg/kg per week during weeks 7, 10, and 13 and 840 mg of pertuzumab every 3 weeks. The primary end point was feasibility, defined as ≥ 80% completion of treatment with both trastuzumab and pertuzumab. An exploratory comparison of survival with a propensity score–matched cohort receiving standard nCRT was performed, as were exploratory pharmacokinetic and biomarker analyses.
RESULTS
Of the 40 enrolled patients (78% men; median age, 63 years), 33 (83%) completed treatment with trastuzumab and pertuzumab. No unexpected safety events were observed. R0 resection was achieved in all patients undergoing surgery, with pathologic complete response in 13 patients (34%). Three-year progression-free and overall survival (OS) were 57% and 71%, respectively (median follow-up, 32.1 months). Compared with the propensity score–matched cohort, a significantly longer OS was observed with HER2 blockade (hazard ratio, 0.58; 95% CI, 0.34 to 0.97). Results of pharmacokinetic analysis and activity on [18F]fluorodeoxyglucose positron emission tomography scans did not correlate with survival or pathologic response. Patients with HER2 3+ overexpression or growth factor receptor–bound protein 7 (Grb7) –positive tumors at baseline demonstrated significantly better survival (P = .007) or treatment response (P = .016), respectively.
CONCLUSION
Addition of trastuzumab and pertuzumab to nCRT in patients with HER2-positive EAC is feasible and demonstrates potentially promising activity compared with historical controls. HER2 3+ overexpression and Grb7 positivity are potentially predictive for survival and treatment response, respectively.
INTRODUCTION
In resectable esophageal cancer (EC), a significant median gain in survival of 25 months can be achieved with neoadjuvant chemoradiotherapy (nCRT) administered according to the CROSS regimen compared with surgery alone.1,2 Nevertheless, survival remains poor; EC accounts for 508,600 deaths per year worldwide.3
In esophageal adenocarcinoma (EAC), 15% to 43% of tumors demonstrate human epidermal growth factor receptor 2 (HER2; ErbB-2) positivity,4-9 and inconsistent conclusions regarding the relationship between HER2 overexpression and clinical outcome have been published.8,10-12 In the phase III ToGA trial,13 the HER2-targeting monoclonal antibody trastuzumab significantly improved survival in patients with HER2-positive advanced gastric cancer. After positive results in a phase I/II study, the randomized RTOG 1010 trial (ClinicalTrials.gov identifier: NCT01196390) is currently investigating the addition of trastuzumab to carboplatin and paclitaxel and 50.4 Gy of radiotherapy in EAC.7 In patients with early breast cancer, the combination of trastuzumab and pertuzumab, a monoclonal antibody inhibiting ligand-dependent HER2 heterodimerization, has demonstrated higher pathologic complete response (pCR) compared with chemotherapy and trastuzumab alone.14 Preclinical studies15,16 in gastric cancer have demonstrated a synergistic effect on tumor inhibition with dual-agent HER2 blockade. In advanced HER2-positive gastric or gastroesophageal junction cancer, the JACOB study showed that the addition of pertuzumab to trastuzumab and chemotherapy resulted in a nonsignificant median survival benefit of 3.3 months, along with a manageable increase in grade ≥ 3 toxicity.17 Given the potential of dual-agent HER2 targeting, we hypothesized that addition of trastuzumab and pertuzumab to nCRT in resectable HER2-positive EAC could potentially improve survival. Because no data are available on the safety of this combination before major surgery, we first conducted a feasibility study.
Pharmacokinetic analyses in gastroesophageal cancer have suggested that exposure to low trastuzumab serum concentrations correlate with worse prognosis18 and that lower pertuzumab concentrations are reached in gastric19 compared with breast cancer.20 Therefore, we additionally assessed the pharmacokinetics of trastuzumab and pertuzumab. Furthermore, because definite predictors for response are lacking in EAC, we performed exploratory biomarker analyses to identify relevant biomarkers for HER2-targeted therapy, including HER2/HER3 and growth factor-binding protein 7 (Grb7) expression8,21-27, HER2 extracellular domain (sHER2) level, and activity on [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT).
PATIENTS AND METHODS
Study Design and Patient Eligibility Criteria
We performed a multicenter phase II study with trastuzumab and pertuzumab added to nCRT followed by esophagectomy in patients with HER2-positive EAC. Eligible patients had surgically resectable, histologically proven HER2-positive adenocarcinoma of the esophagus or gastroesophageal junction, without evidence of metastatic spread. Patients with celiac nodes and paraesophageal lymph nodes, including subcarinal and paratracheal lymph nodes, were considered eligible; those with other mediastinal nodes and nodes distal from the celiac trunk were considered ineligible. Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1 and adequate hematologic, renal, and hepatic function were required. Patients who had received prior treatment with chemotherapy, radiotherapy, or HER2-targeted therapy or had severely impaired lung function or clinically significant cardiovascular disease, including a left ventricular ejection fraction (LVEF) < 55% (Data Supplement) were excluded.
The trial was approved by the medical ethical review committee of the Amsterdam University Medical Center and conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written, informed voluntary consent.
Treatment Plan
Patients received paclitaxel (50 mg/m2), carboplatin (area under the curve [AUC], 2), and radiotherapy (23 × 1.8 Gy over 5 weeks).1 Trastuzumab was administered at a loading dose of 4 mg/kg on day 1, 2 mg/kg once per week during weeks 2 to 6, and 6 mg/kg once every 3 weeks during weeks 7 to 13. Pertuzumab was administered at a dose of 840 mg once every 3 weeks (Data Supplement). Surgery was performed in week 14, with a transthoracic or transhiatal approach and a two-field lymph node dissection.
Dose Modifications
Dose reductions were not allowed for trastuzumab; pertuzumab dose was reduced if grade 3 to 4 diarrhea or rash occurred, without dose re-escalation thereafter. Dose delays for trastuzumab and pertuzumab were permitted for treatment-related adverse events (AEs).
Study End Points and Statistical Analyses
The primary objective was to assess the feasibility of neoadjuvant treatment, in terms of completion of trastuzumab and pertuzumab, using a one-sample test for a binomial proportion with normal approximation and a one-sided P value of < .05. Treatment would be regarded feasible if ≥ 80% of patients completed treatment; a rate of ≤ 62% would be considered unfeasible. On the basis of a single-stage Fleming design, 40 patients were required to achieve an α of 0.05 and a power of 0.80.
Secondary end points were safety, response, and survival. Exploratory end points were pharmacokinetics in relation to safety, response, and survival and biomarker analyses in relation to response and survival. All tests were two sided, with P values < .05 considered statistically significant. SPSS Statistics for Windows (version 25.0; IBM, Armonk, NY) was used for statistical analyses.
Safety.
The safety population included all patients who received at least one dose of trastuzumab and pertuzumab. AEs were graded using the National Cancer Institute Common Toxicity Criteria for Adverse Events (version 4.03) and the Radiation Oncology Group criteria; postoperative complications were graded according to Clavien-Dindo. Left ventricular systolic dysfunction (LVSD) was defined as ≥ 10% absolute decrease from baseline to an absolute value of < 50% with (symptomatic) or without (asymptomatic) cardiac failure symptoms.28,29
Response and survival.
Pathologic response was assessed according to the criteria of Mandard et al.30 Patients were clinically assessed through physical examination, laboratory tests, and multigated acquisition and CT scans before treatment. During treatment, weekly screening was performed using laboratory tests and physical examination.
Survival analyses included all patients who received at least one dose of trastuzumab and pertuzumab. Progression-free (PFS) and overall survival (OS) were estimated using Kaplan-Meier analyses, measured from treatment initiation to documented disease progression or death, respectively.
To compare the observed survival with that of patients receiving conventional nCRT according to CROSS, we performed propensity score matching with data from the Netherlands Cancer Registry (NCR). Using a logistic regression model, we matched on sex, performance status, number of comorbidities, age, hospital volume, tumor stage, and tumor length. Because determination of HER2 status is not standard clinical practice for patients without metastases, it could not be included as a matching variable. Matching was performed at a one-to-four ratio with a maximum allowable absolute difference in propensity score of 0.1, optimized to match patients with the fewest number of matches first. Survival was analyzed using a Cox proportional hazards model, after confirmation of the proportional hazards assumption.
Pharmacokinetic and biomarker analyses.
For pharmacokinetic assessments, predose serum samples were obtained on days 1, 8, 15, 22, 29, 43, 64, and 85 to measure trough concentrations (Cmin), and postdose serum samples were obtained on days 1, 22, 43, 64, and 85 to measure peak concentrations (Cmax). Trastuzumab and pertuzumab were isolated from serum and analyzed using liquid chromatography with mass spectrometry. In serum samples collected on days 1, 22, 43, 64, and 85, sHER2 was measured using a fully automated, two-site sandwich immunoassay (ADVIA Centaur XP, Siemens, Munich, Germany;31 Data Supplement).
Tissue tumor samples for biomarker analyses obtained at baseline and post-treatment were stained for HER2/HER3 and Grb7 using anti-HER2/neu, HER3, and anti-Grb7 rabbit monoclonal antibodies. Slides were scored for HER2 using the Hoffman scoring system, with HER2 positivity defined as immunohistochemistry (IHC) 3+ or IHC2+ with amplification of HER2, as assessed with silver in situ hybridization.32,33 Membranous HER3 expression was scored using the HER2 IHC scoring guidelines,32 and IHC2+ and IHC3+ scores were defined as positive. For Grb7, the percentage of positive membranous stained cells and the maximal intensity of staining were assessed. Tumors were Grb7 positive when scoring at least IHC2+ in 10% of tumor cells (Data Supplement). Survival for HER2 IHC3+ and IHC2+ with amplification was assessed using a Gehan-Breslow-Wilcoxon survival curve.34
Associations between the difference in FDG uptake on pre- and post-treatment PET/CT scans and pathologic response and survival were analyzed. Scans were performed according to the standard clinical protocols at each institution and reviewed using an Hermes workstation (Hermes Medical Solutions, Stockholm, Sweden). Volumes of interest were drawn over the primary tumor, from which maximum standardized uptake values (SUVmax) were calculated. The associations of secondary outcomes with the degree of pathologic response and survival were assessed using nonparametric tests. Additional methods are described in the Data Supplement.
RESULTS
Patient Characteristics
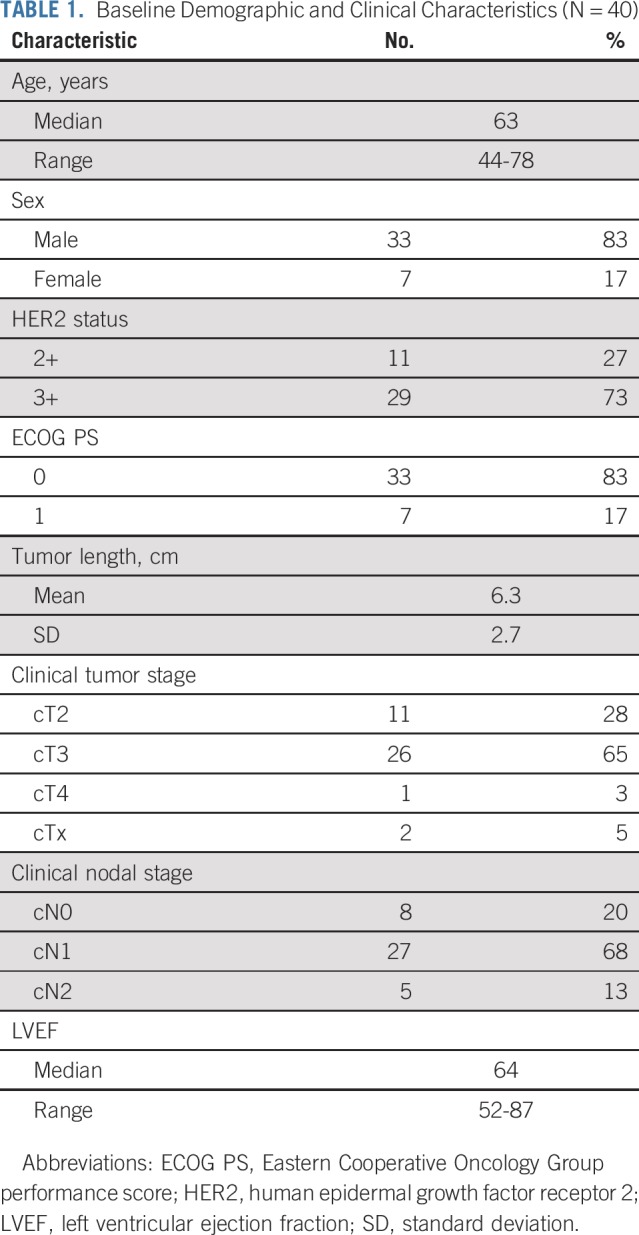
Forty patients were enrolled between April 2014 and September 2016 and were observed through February 2019. A majority of included patients were men (n = 33; 83%) and had an ECOG performance score of 0 (n = 33; 83%). Median age was 63 years (interquartile range [IQR], 57-69). Most tumors were cT3 (65%) and cN1 (58%; Table 1).
TABLE 1.
Baseline Demographic and Clinical Characteristics (N = 40)
Adherence to Treatment
Thirty-three of 40 patients completed treatment with trastuzumab and pertuzumab without dose modifications (completion rate, 83%). One or more doses of trastuzumab and/or pertuzumab were omitted in seven patients because of LVEF decrease (n = 3), trial discontinuation at the patient’s request (n = 3), or death (n = 1). Three (8%) of 40 patients missed one dose of chemotherapy because of LVEF decrease (n = 2) or hospitalization (n = 1).
Safety
Overall toxicity.
The most common AEs of any grade were fatigue (76%), diarrhea (73%), and nausea (70%; Table 2). One patient (3%) died as a result of pulmonary fibrosis during the trial. Of the grade ≥ 3 AEs (n = 19; 48%), diarrhea (20%) and dysphagia (18%) were most common.
TABLE 2.
AEs in Enrolled Patients (N = 40)
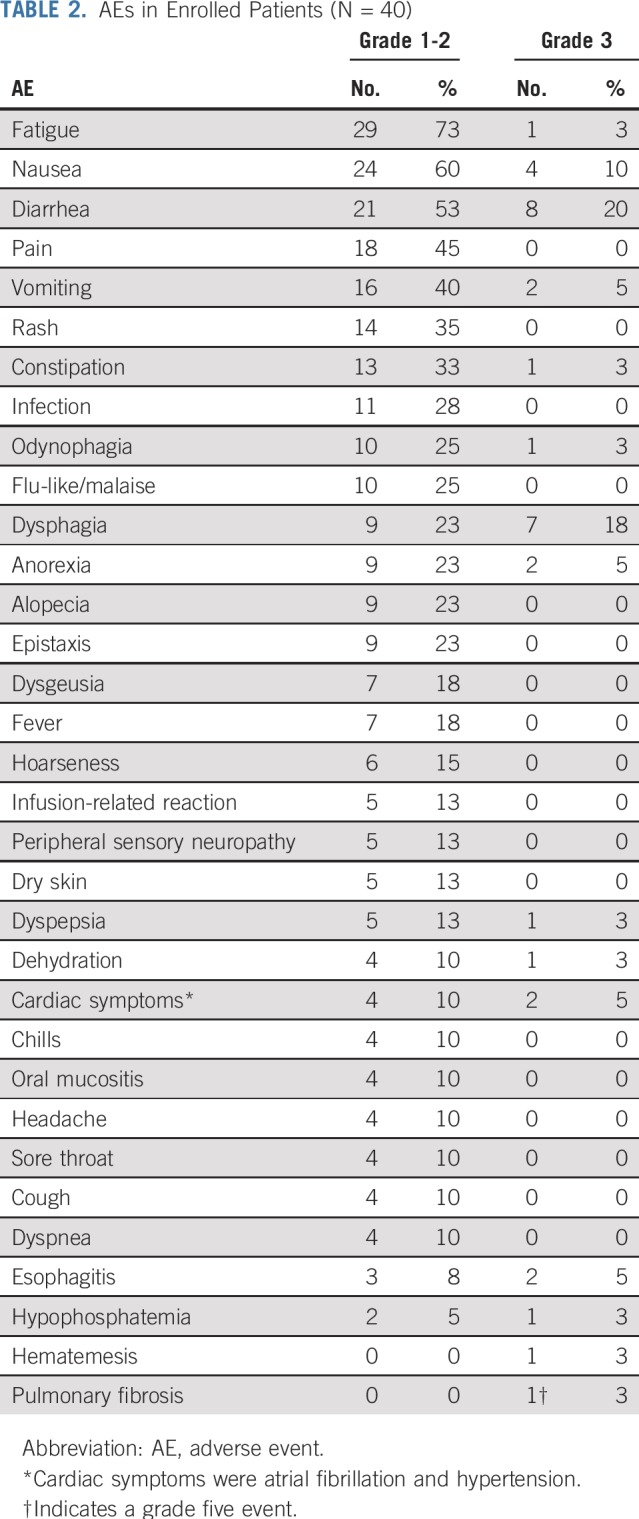
Eleven patients (28%) had one or more serious AE (SAE), requiring (prolongation of) hospitalization; vomiting (n = 4), nausea (n = 3), and GI hemorrhage (n = 2) were the most prevalent causes (Data Supplement). All but one patient recovered from the SAEs; aggravation of preexisting pulmonary fibrosis led to a fatal outcome.
Cardiotoxicity.
Thirty-seven patients were included in the cardiac safety analysis. Because of early non–cardiac-related discontinuation (n = 2) or death (n = 1), post-treatment measurements were not performed in three patients. The mean (± standard deviation [SD]) pretreatment LVEF was 64% (± 7.8%); it was 59% (± 8.4%) post-treatment. Five patients (14%) developed LVSD, of whom one patient had symptomatic LVSD and one patient missed a dose of trastuzumab and pertuzumab because of LVEF decrease. However, cardiotoxicity did not result in delay of surgery in any of these patients.
Postoperative complications.
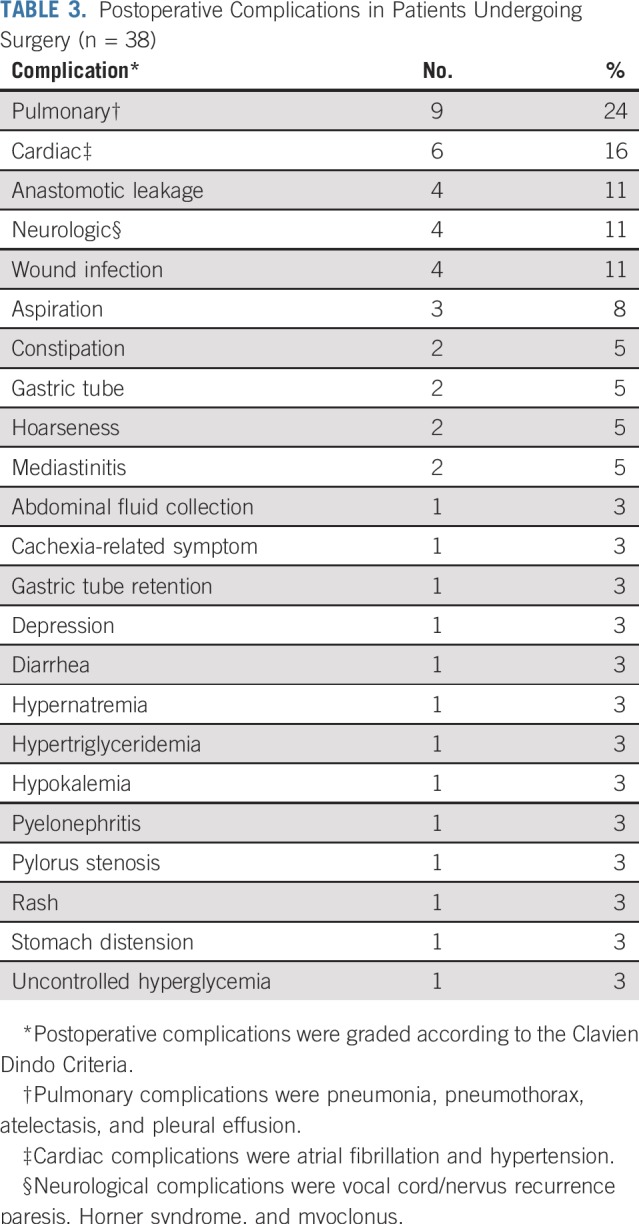
Two patients did not undergo surgery (5%); one patient died before surgery as a result of pulmonary fibrosis, and one patient developed interval metastases. No patient died within 30 and 90 days after surgery, and no unforeseen complications were reported. A majority of postoperative complications were pulmonary (24%) or cardiac complications (16%; Table 3). Four patients (11%) experienced anastomotic leakage.
TABLE 3.
Postoperative Complications in Patients Undergoing Surgery (n = 38)
Response and Survival
R0 resection was achieved in all 38 patients undergoing surgery (100%), of whom 13 (34%) had a pCR (Mandard 1). Mandard 2, 3, 4, and 5 responses were observed in 10 (26%), 10 (26%), three (8%), and two patients (5%), respectively (Data Supplement).
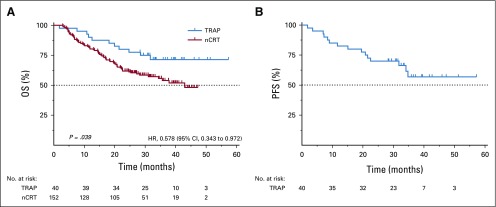
Median follow-up was 32.1 months (IQR, 28.3-39.4). Median PFS and OS were not achieved during follow-up. One- and 3-year PFS rates were 82.5% and 56.8%, respectively. One- and 3-year OS rates were 90.0% and 71.3%, respectively (Fig 1).
FIG 1.
(A) Overall survival (OS) and (B) progression-free survival (PFS) in patients receiving neoadjuvant chemoradiotherapy (nCRT) alone or nCRT plus trastuzumab and pertuzumab (TRAP). HR, hazard ratio.
Results were compared with the propensity score–matched cohort from the NCR receiving nCRT (Data Supplement). The assumption of proportional hazards was met (P = .671). Patients receiving nCRT, trastuzumab, and pertuzumab were at significantly lower hazards of death compared with patients receiving nCRT only (hazard ratio, 0.58; 95% CI, 0.34 to 0.97; Fig 1).
Pharmacokinetic Analysis
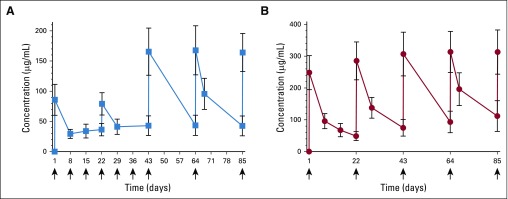
Pharmacokinetic data were evaluable for 37 patients. The mean (± SD) observed Cmin and Cmax for trastuzumab were 38.4 (± 10.8) and 128.9 µg/mL (± 23.2), respectively (Fig 2A); for pertuzumab, they were 89.5 (± 24.8) and 292.3 µg/mL (± 52.5), respectively (Fig 2B). All patients had a pertuzumab Cmin > 20 µg/mL. The AUC concentration of trastuzumab and pertuzumab did not correlate with cardiac toxicity (P = .652 and P = .728, respectively). Between patients with and without pCR or disease progression, Cmin concentrations of trastuzumab and pertuzumab were not significantly different (all P > .05).
FIG 2.
Mean trough and peak concentrations of (A) trastuzumab and (B) pertuzumab in serum. Horizontal bars indicate standard deviations.
Biomarker Analyses
Serum samples from 40 patients were available. Median baseline sHER2 was 11.9 µg/mL (IQR, 10.7-14.6; Data Supplement). sHER2 baseline levels were not significantly different in patients with and without disease progression (P = .890), nor were they different in patients with pCR or residual disease (P = .158).
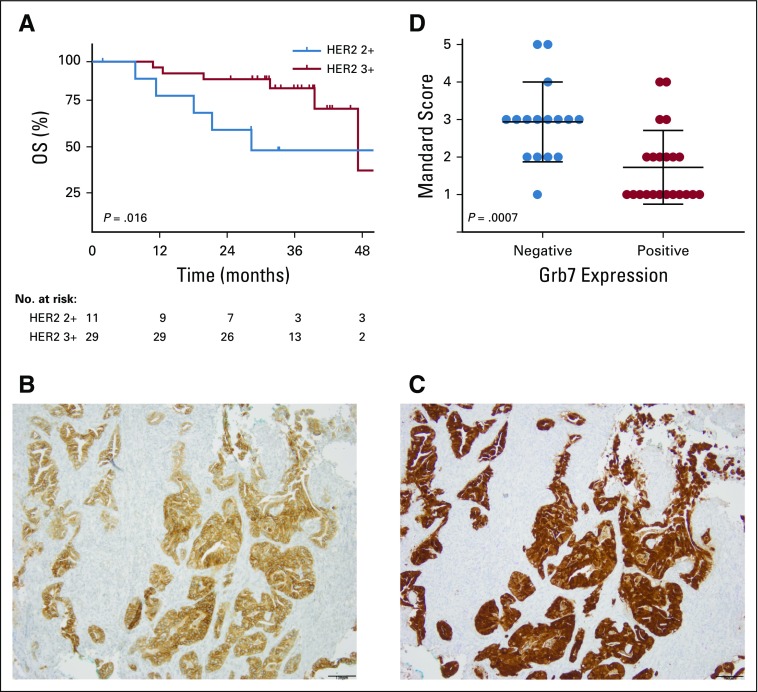
Patients with HER2 IHC3+ tumors at baseline (n = 29) demonstrated a significantly better OS compared with HER2 IHC2+ patients with gene amplification (n = 11; P = .016; Fig 3A). HER3 expression and Grb7 expression were positive in 58% (n = 23) and 55% (n = 22) of patients, respectively (Figs 3B and 3C; Data Supplement). Treatment response was significantly better for patients with Grb7-positive tumors at baseline (P = .001; Fig 3D). No correlation between pretreatment HER2 expression and Mandard score was observed, nor did pre- or post-treatment HER3 expression demonstrate a correlation with treatment response or survival.
FIG 3.
(A) Overall survival (OS) of patients with human epidermal growth factor receptor 2 (HER2) immunohistochemistry (IHC) 2+ and IHC3+ tumors. (B) HER3 and (C) growth factor–binding protein 7 (Grb7) expression at 10× magnification. (D) Treatment response by Mandard score in patients with Grb7-positive and -negative tumors.
Baseline PET/CT scans were available from 38 patients. Scans from two patients could not be analyzed because of logistic reasons. Six patients did not have post-treatment scans because of death (n = 1) or discretion of the local investigator (n = 5). Post-treatment scans were performed at a median time of 8.1 weeks (IQR, 7.0-8.3) after completing nCRT, with five scans prematurely performed within 6 weeks after nCRT completion. Median SUVmax at baseline was 12.1 (IQR, 8.7-15.9); it was 4.5 (IQR, 3.5-5.5) after treatment. No significant difference in ∆SUVmax was identified in patients with v without disease progression (P = .417), nor in patients with high v low Mandard scores (P = .349). After exclusion of prematurely performed scans, still no relationship between ∆SUVmax and Mandard score or progression was observed (all P > .05). Baseline SUVmax did not predict progression or death (P = .846).
DISCUSSION
This is the first study to our knowledge demonstrating the feasibility of the addition of both trastuzumab and pertuzumab to standard nCRT with carboplatin and paclitaxel in patients with resectable HER2-positive EAC, with 83% of patients receiving the complete treatment schedule.
Toxicity was similar to that with standard nCRT (CROSS), with the exception of increased incidences of rash and grade 3 diarrhea compared with the CROSS trial1 (35% v 0% and 20% v 1%, respectively), most likely because of pertuzumab-induced inhibition of the epidermal growth factor receptor.29,35,36 AEs were manageable and did not result in treatment discontinuation of trastuzumab or pertuzumab or in delay of surgery. In the JACOB trial,17 cardiac toxicity was similar in patients with and without pertuzumab. Incidence of LVSD was higher in our study compared with the JACOB trial. It should, however, be noted that LVEF changes cannot directly be attributed to trastuzumab and pertuzumab alone, because CRT may also influence cardiac function.37,38 Nevertheless, because no cardiac toxicity was observed in a phase I/II study7 investigating trastuzumab with chemotherapy and 50.4 Gy of radiotherapy, the extent to which radiotherapy influences cardiac toxicity remains unclear. Postoperative complication rates were lower in the TRAP study compared with the CROSS trial,1 with anastomotic leakage reported in 10% versus 22% and pulmonary complications in 24% versus 46%, respectively.
Compared with the CROSS trial,1 higher R0 resection and pCR rates were observed (100% v 92% and 34% v 23% for EAC, respectively). Direct comparison with a propensity score–matched cohort treated with the CROSS regimen may also suggest that addition of trastuzumab and pertuzumab to nCRT is more effective than nCRT alone. Unfortunately, a major limitation is that HER2 status could not be retrieved for the propensity score–matched cohort. Nonetheless, in previous retrospective cohort studies of patients with EAC treated with nCRT,8,12 no significant association between baseline HER2 status and survival was observed, and therefore, the influence of HER2 status on prognosis is probably limited.
In contrast to the ToGA trial,13 we identified no correlation between the pharmacokinetics of trastuzumab and pathologic response or survival. Furthermore, compared with a phase IIIb study39 in advanced gastric cancer, our patients achieved higher trastuzumab Cmin values. However, as this trial also demonstrated,39 we observed no association with increased efficacy. All patients reached the pertuzumab target concentration of > 20 µg/mL, and our results on pertuzumab trough values were comparable to those of previous studies in breast20 and gastroesophageal cancers.19 Similar to previous studies on gastroesophageal cancer in the metastatic setting,17,19 we chose to double the pertuzumab dose compared with that used in breast cancer studies.40 This suggests that higher doses are needed in upper GI compared with breast cancer to reach similar pharmacokinetic concentrations, although the mechanism underlying this difference remains unknown.19
Exploratory biomarker analysis were performed to identify subgroups that benefit most from HER2 targeting.41 Our data support previous reports21,22,24 showing that patients with high HER2 expression potentially benefit most from HER2-targeting agents. However, available studies on the relationship between HER2 status and survival of patients treated with nCRT8,12 have not investigated the difference in survival between HER2 IHC3+ and HER2 IHC2+ with amplification. Therefore, it is still an open question whether high HER2 expression is a true predictive, and potentially prognostic, biomarker in this population. We also identified Grb7 as a potential treatment response biomarker. This is in line with previous observations of Grb7 as a therapeutic target in esophageal27 and breast cancers,42 because it strongly binds HER2 and could play an essential role in HER2 signaling. Therefore, patients with Grb7-overexpressing tumors may be more sensitive to HER2-targeting treatment.26,27,42 Alternatively, this could be a result of coamplification of Grb7 with HER2, located on chromosome 17, which has been reported in breast cancer.42,43 Nevertheless, here we report Grb7 negativity in 45% of HER2-positive patients. Therefore, Grb7 holds potential value as a predictive biomarker, and further investigation is warranted. The previous finding of sHER2 as a prognostic and predictive marker22,44,45 could not be confirmed in this trial, as also has been reported by others.24
PET/CT scans performed in our study were executed at a prolonged interval after nCRT (8 rather than 4-6 weeks), considering previous studies demonstrating the substantial influence of radiation-induced inflammation on pathologic response assessment by PET/CT.46-48 Nevertheless, we did not observe a correlation between decreased FDG uptake and pathologic response.49,50 Exclusion of prematurely performed scans did not influence outcome. Although FDG PET/CT scanning is valuable to determine the presence of metastases,49 its value as a predictive tool for treatment response seems questionable.51
In conclusion, the addition of trastuzumab and pertuzumab to nCRT is safe and tolerable for patients with HER2-positive EAC, and preliminary efficacy results seem promising. Because data on an HER2-positive control group are lacking, a randomized phase III study is warranted to demonstrate the superiority of the addition of trastuzumab and pertuzumab. High HER2 and Grb7 may serve as biomarkers to predict survival and treatment response, respectively.
ACKNOWLEDGMENT
We thank all patients and investigators participating in this trial, as well as the nursing staff of the Amsterdam University Medical Center. We also thank Héctor van den Boorn for statistical support.
Footnotes
Presented as a poster at the 2016 ASCO Annual Meeting, Chicago, IL, June 3-7, 2016; the 2018 ASCO Annual Meeting, Chicago, IL, June 1-5, 2018; and at the 2019 ESMO Annual Meeting, Barcelona, Spain, September 26-October 1, 2019.
Supported by the Academic Medical Center, Amsterdam, the Netherlands and by an unrestricted research grant from Hoffmann-La Roche, Basel, Switzerland.
AUTHOR CONTRIBUTIONS
Conception and design: Maarten C.C.M. Hulshof, Ron A.A. Mathôt, Cornelis J.A. Punt, Maurice J.C. van der Sangen, Mark I. van Berge Henegouwen, Hanneke W.M. van Laarhoven
Administrative support: Ron A.A. Mathôt, Hanneke W.M. van Laarhoven
Provision of study material or patients: Kausilia K. Krishnadath, Rob H.A. Verhoeven, Laurens V. Beerepoot, Maartje Los, Hanneke W.M. van Laarhoven
Collection and assembly of data: Charlotte I. Stroes, Sandor Schokker, Aafke Creemers, Stephanie O. van der Woude, Roel J. Bennink, Kausilia K. Krishnadath, Rob H.A. Verhoeven, Geert-Jan Creemers, Grard A.P. Nieuwenhuijzen, Laurens V. Beerepoot, Joos Heisterkamp, Maartje Los, Annemieke Cats, Geke A.P. Hospers, Mark I. van Berge Henegouwen, Hanneke W.M. van Laarhoven
Data analysis and interpretation: Charlotte I. Stroes, Sandor Schokker, Aafke Creemers, Remco J. Molenaar, Roel J. Bennink, Ron A.A. Mathôt, Cornelis J.A. Punt, Rob H.A. Verhoeven, Martijn G.H. van Oijen, Geert-Jan Creemers, Maurice J.C. van der Sangen, Laurens V. Beerepoot, Joos Heisterkamp, Marije Slingerland, Annemieke Cats, Geke A.P. Hospers, Maarten F. Bijlsma, Mark I. van Berge Henegouwen, Sybren L. Meijer, Hanneke W.M. van Laarhoven
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Feasibility and Biomarker Study of Neoadjuvant Trastuzumab and Pertuzumab With Chemoradiotherapy for Resectable Human Epidermal Growth Factor Receptor 2–Positive Esophageal Adenocarcinoma: TRAP Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sandor Schokker
Travel, Accommodations, Expenses: Roche
Ron A.A. Mathôt
Consulting or Advisory Role: Merck Sharp & Dohme, Bayer, CSL Behring, Zeria Pharmaceutical
Research Funding: Bayer, CSL Behring, Shire
Cornelis J.A. Punt
Consulting or Advisory Role: Bayer (Inst)
Travel, Accommodations, Expenses: Nordic Pharma
Rob H.A. Verhoeven
Research Funding: Bristol-Myers Squibb (Inst)
Martijn G.H. van Oijen
Research Funding: Roche (Inst), Nordic Group (Inst), Servier (Inst), Amgen (Inst), Merck (Inst)
Grard A.P. Nieuwenhuijzen
Consulting or Advisory Role: Medtronic (Inst)
Research Funding: Medtronic (Inst)
Laurens V. Beerepoot
Consulting or Advisory Role: Servier
Geke A.P. Hospers
Consulting or Advisory Role: Roche (Inst), Merck Sharp & Dohme (Inst), Amgen (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst)
Research Funding: Bristol-Myers Squibb (Inst)
Maarten F. Bijlsma
Consulting or Advisory Role: Servier
Research Funding: Celegene
Travel, Accommodations, Expenses: Servier
Mark I. van Berge Henegouwen
Consulting or Advisory Role: Medtronic (Inst), Johnson & Johnson (Inst), Mylan (Inst)
Research Funding: Olympus (Inst), Stryker (Inst)
Travel, Accommodations, Expenses: Johnson & Johnson
Hanneke W.M. van Laarhoven
Honoraria: Lilly/ImClone
Consulting or Advisory Role: Lilly/ImClone, Nordic Group, Bristol-Myers Squibb, Servier
Research Funding: Bristol-Myers Squibb (Inst), Bayer Schering Pharma (Inst), Celgene (Inst), Janssen-Cilag (Inst), Lilly (Inst), Nordic Group (Inst), Philips Healthcare (Inst), Roche (Inst), Merck Sharp & Dohme (Inst), Servier (Inst)
Travel, Accommodations, Expenses: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Hardwick RH, Barham CP, Ozua P, et al. Immunohistochemical detection of p53 and c-erbB-2 in oesophageal carcinoma; no correlation with prognosis. Eur J Surg Oncol. 1997;23:30–35. doi: 10.1016/s0748-7983(97)80139-4. [DOI] [PubMed] [Google Scholar]
- 5.Polkowski W, van Sandick JW, Offerhaus GJA, et al. Prognostic value of Laurén classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol. 1999;6:290–297. doi: 10.1007/s10434-999-0290-2. [DOI] [PubMed] [Google Scholar]
- 6.Reichelt U, Duesedau P, Tsourlakis MC, et al. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol. 2007;20:120–129. doi: 10.1038/modpathol.3800712. [DOI] [PubMed] [Google Scholar]
- 7.Safran H, Dipetrillo T, Akerman P, et al. Phase I/II study of trastuzumab, paclitaxel, cisplatin and radiation for locally advanced, HER2 overexpressing, esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2007;67:405–409. doi: 10.1016/j.ijrobp.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 8.Plum PS, Gebauer F, Krämer M, et al. HER2/neu (ERBB2) expression and gene amplification correlates with better survival in esophageal adenocarcinoma. BMC Cancer. 2019;19:38. doi: 10.1186/s12885-018-5242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duhaylongsod FG, Gottfried MR, Iglehart JD, et al. The significance of c-erb B-2 and p53 immunoreactivity in patients with adenocarcinoma of the esophagus. Ann Surg. 1995;221:677–683, discussion 683-684. doi: 10.1097/00000658-199506000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slamon DJ, Clark GM, Wong SG, et al: Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177-182, 1987. [DOI] [PubMed]
- 11.Ross JS, Fletcher JA. The HER-2/neu oncogene: Prognostic factor, predictive factor and target for therapy. Semin Cancer Biol. 1999;9:125–138. doi: 10.1006/scbi.1998.0083. [DOI] [PubMed] [Google Scholar]
- 12.Creemers A, Ebbing EA, Hooijer GKJ, et al. The dynamics of HER2 status in esophageal adenocarcinoma. Oncotarget. 2018;9:26787–26799. doi: 10.18632/oncotarget.25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 14.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 15.Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita-Kashima Y, Iijima S, Yorozu K, et al. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res. 2011;17:5060–5070. doi: 10.1158/1078-0432.CCR-10-2927. [DOI] [PubMed] [Google Scholar]
- 17.Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372–1384. doi: 10.1016/S1470-2045(18)30481-9. [DOI] [PubMed] [Google Scholar]
- 18.Cosson VF, Ng VW, Lehle M, et al. Population pharmacokinetics and exposure-response analyses of trastuzumab in patients with advanced gastric or gastroesophageal junction cancer. Cancer Chemother Pharmacol. 2014;73:737–747. doi: 10.1007/s00280-014-2400-5. [DOI] [PubMed] [Google Scholar]
- 19.Kang YK, Rha SY, Tassone P, et al. A phase IIa dose-finding and safety study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2-positive advanced gastric cancer. Br J Cancer. 2014;111:660–666. doi: 10.1038/bjc.2014.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortés J, Swain SM, Kudaba I, et al. Absence of pharmacokinetic drug-drug interaction of pertuzumab with trastuzumab and docetaxel. Anticancer Drugs. 2013;24:1084–1092. doi: 10.1097/CAD.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 21.Shah MA, Kang YK, Thuss-Patience PC, et al. Biomarker analysis of the GATSBY study of trastuzumab emtansine versus a taxane in previously treated HER2-positive advanced gastric/gastroesophageal junction cancer. Gastric Cancer. 2019;22:803–816. doi: 10.1007/s10120-018-00923-7. [DOI] [PubMed] [Google Scholar]
- 22.Baselga J, Cortés J, Im SA, et al. Biomarker analyses in CLEOPATRA: A phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2–positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32:3753–3761. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 23.Baselga J, Lewis Phillips GD, Verma S, et al. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin Cancer Res. 2016;22:3755–3763. doi: 10.1158/1078-0432.CCR-15-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bianchini G, Kiermaier A, Bianchi GV, et al. Biomarker analysis of the NeoSphere study: Pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Res. 2017;19:16. doi: 10.1186/s13058-017-0806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nencioni A, Cea M, Garuti A, et al. Grb7 upregulation is a molecular adaptation to HER2 signaling inhibition due to removal of Akt-mediated gene repression. PLoS One. 2010;5:e9024. doi: 10.1371/journal.pone.0009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pogue-Geile KL, Kim C, Jeong JH, et al. Predicting degree of benefit from adjuvant trastuzumab in NSABP trial B-31. J Natl Cancer Inst. 2013;105:1782–1788. doi: 10.1093/jnci/djt321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawada G, Niida A, Hirata H, et al. An integrative analysis to identify driver genes in esophageal squamous cell carcinoma. PLoS One. 2015;10:e0139808. doi: 10.1371/journal.pone.0139808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Ramshorst MS, van Werkhoven E, Honkoop AH, et al. Toxicity of dual HER2-blockade with pertuzumab added to anthracycline versus non-anthracycline containing chemotherapy as neoadjuvant treatment in HER2-positive breast cancer: The TRAIN-2 study. Breast. 2016;29:153–159. doi: 10.1016/j.breast.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 29.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma: Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 31.Esteva FJ, Cheli CD, Fritsche H, et al. Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res. 2005;7:R436–R443. doi: 10.1186/bcr1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 33.Bartley AN, Washington MK, Ventura CB, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: Guideline from the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Am J Clin Pathol. 2016;146:647–669. doi: 10.1093/ajcp/aqw206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez RLMC, Naranjo JD. A pretest for choosing between logrank and Wilcoxon tests in the two-sample problem. Metron. 2010;68:111–125. [Google Scholar]
- 35.Swain SM, Schneeweiss A, Gianni L, et al. Incidence and management of diarrhea in patients with HER2-positive breast cancer treated with pertuzumab. Ann Oncol. 2017;28:761–768. doi: 10.1093/annonc/mdw695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul T, Schumann C, Rüdiger S, et al. Cytokine regulation by epidermal growth factor receptor inhibitors and epidermal growth factor receptor inhibitor associated skin toxicity in cancer patients. Eur J Cancer. 2014;50:1855–1863. doi: 10.1016/j.ejca.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Haj Mohammad N, Kamphuis M, Hulshof MCCM, et al. Reduction of heart volume during neoadjuvant chemoradiation in patients with resectable esophageal cancer. Radiother Oncol. 2015;114:91–95. doi: 10.1016/j.radonc.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Hatakenaka M, Yonezawa M, Nonoshita T, et al. Acute cardiac impairment associated with concurrent chemoradiotherapy for esophageal cancer: Magnetic resonance evaluation. Int J Radiat Oncol Biol Phys. 2012;83:e67–e73. doi: 10.1016/j.ijrobp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 39. doi: 10.1200/JCO.2016.71.6852. Shah MA, Xu RH, Bang YJ, et al: HELOISE: Phase IIIb randomized multicenter study comparing standard-of-care and higher-dose trastuzumab regimens combined with chemotherapy as first-line therapy in patients with human epidermal growth factor receptor 2–positive metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 35:2558-2567, 2017. [DOI] [PubMed] [Google Scholar]
- 40.Swain SM, Baselga J, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandrekar SJ, Sargent DJ. Clinical trial designs for predictive biomarker validation: Theoretical considerations and practical challenges. J Clin Oncol. 2009;27:4027–4034. doi: 10.1200/JCO.2009.22.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadler Y, González AM, Camp RL, et al. Growth factor receptor-bound protein-7 (Grb7) as a prognostic marker and therapeutic target in breast cancer. Ann Oncol. 2010;21:466–473. doi: 10.1093/annonc/mdp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luoh SW, Ramsey B, Hanlon Newell A, et al. HER-2 gene amplification in human breast cancer without concurrent HER-2 over-expression. Springerplus. 2013;2:386. doi: 10.1186/2193-1801-2-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. doi: 10.1007/s00535-015-1046-3. Zhou J, Peng Z, Liu Y, et al: Predictive value of serum HER2 ECD in patients with HER2-positive advanced gastric cancer treated with trastuzumab plus chemotherapy. J Gastroenterol 50:955-961, 2015 [Erratum: J Gastroenterol 51:509-510, 2016] [DOI] [PubMed] [Google Scholar]
- 45.Shi HZ, Wang YN, Huang XH, et al. Serum HER2 as a predictive biomarker for tissue HER2 status and prognosis in patients with gastric cancer. World J Gastroenterol. 2017;23:1836–1842. doi: 10.3748/wjg.v23.i10.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stiekema J, Vermeulen D, Vegt E, et al. Detecting interval metastases and response assessment using 18F-FDG PET/CT after neoadjuvant chemoradiotherapy for esophageal cancer. Clin Nucl Med. 2014;39:862–867. doi: 10.1097/RLU.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 47.Arnett ALH, Merrell KW, Macintosh EM, et al. Utility of 18F-FDG PET for predicting histopathologic response in esophageal carcinoma following chemoradiation. J Thorac Oncol. 2017;12:121–128. doi: 10.1016/j.jtho.2016.08.136. [DOI] [PubMed] [Google Scholar]
- 48. Valkema MJ, Noordman BJ, Wijnhoven BPL, et al: Accuracy of 18F-FDG PET/CT in predicting residual disease after neoadjuvant chemoradiotherapy for esophageal cancer. J Nucl Med 60:1553-1559, 2019. [DOI] [PubMed]
- 49.Kim MK, Ryu JS, Kim SB, et al. Value of complete metabolic response by (18)F-fluorodeoxyglucose-positron emission tomography in oesophageal cancer for prediction of pathologic response and survival after preoperative chemoradiotherapy. Eur J Cancer. 2007;43:1385–1391. doi: 10.1016/j.ejca.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 50.McLoughlin JM, Melis M, Siegel EM, et al. Are patients with esophageal cancer who become PET negative after neoadjuvant chemoradiation free of cancer? J Am Coll Surg. 2008;206:879–886, discussion 886-887. doi: 10.1016/j.jamcollsurg.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 51.Noordman BJ, Spaander MCW, Valkema R, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): A prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018;19:965–974. doi: 10.1016/S1470-2045(18)30201-8. [DOI] [PubMed] [Google Scholar]