Abstract
PURPOSE
Platinum-based therapy is the standard of care in patients who have HER2-negative, advanced esophagogastric cancer (AEGC). Retrospective data suggest that intratumoral ERCC1 levels may determine platinum sensitivity. A randomized, phase II study was performed in patients with AEGC to explore whether the efficacy of a platinum-based therapy with fluorouracil, leucovorin, and oxaliplatin (FOLFOX) versus a non–platinum-containing regimen of irinotecan and docetaxel (IT) differed according to ERCC1 levels.
PATIENTS AND METHODS
Overall, 202 untreated patients with HER2-negative AEGC and a Zubrod performance status of 0-1 were evaluated prospectively for mRNA expression of ERCC1 level and then randomly assigned to FOLFOX or IT, stratified by the intratumoral statuses of ERCC1 low (< 1.7) or high (≥ 1.7). Objectives were to assess progression-free survival (PFS) and overall survival (OS) in all patients treated with FOLFOX compared with IT, stratified by low and high ERCC1 levels, and to assess for interactive effects between ERCC1 expression and treatment arm.
RESULTS
Eighty-six percent of patients had ERCC1 values < 1.7. Thus, evaluation of the ERCC1-high subgroup was limited. Grade ≥ 3 anemia, dehydration, diarrhea, and fatigue were greater in patients with IT. Occurrences of grade ≥ 3 neuropathy and decreased neutrophils were greater in patients with FOLFOX. In all patients, FOLFOX had a statistically superior median PFS compared with IT (5.7 v 2.9 months; hazard ratio, 0.68; P = .02). In patients with ERCC1 levels < 1.7 receiving FOLFOX, PFS and response rate were statistically superior to IT, with no significant difference in OS.
CONCLUSION
The evaluation of ERCC1 in patients with upper GI tumors was thwarted by an overwhelming predominance of low ERCC1 mRNA expression. Nonetheless, distribution of treatment effects on PFS did not vary with expression. For all patients and for those with low ERCC1 expression, FOLFOX was superior in efficacy to IT.
INTRODUCTION
In 2019, there will be more than 43,500 occurrences of esophagogastric cancer diagnosed in the United States and more than 26,650 deaths.1 The majority of patients who present have a high risk of recurrence. Many chemotherapeutic drugs, including fluoropyrimidines, platinums, irinotecan, taxanes, TAS 102, the anti-VEGFR antibody ramucirumab, and immunotherapy, have demonstrated activity in this cancer.2-11 Generally, the combination of fluoropyrimidines and platinums is used as front-line therapy. Although response rates with cytotoxic combinations range from 30%-50%, there can be significant toxicity associated with these regimens, and median overall survival (OS) remains largely between 6 and 9 months, with progression-free survival (PFS) in the 4- to 5-month range.
Doublets of fluorouracil (FU) and leucovorin plus oxaliplatin (FOLFOX) or irinotecan combined with docetaxel (IT) have demonstrated similar efficacy. FOLFOX has a reported response rate of 45%, a time to progression of 6.2 months, and an OS of 8.6 months; IT has reported response rates of 45%, a time to progression of 4.5 months, and an OS of 8.2 months.12,13 These regimens, FOLFOX and IT, have not been compared yet. Generally, most combination chemotherapy in HER2-negative, advanced esophagogastric cancer has demonstrated a PFS of 4-6 months and an OS of 9-12 months.2-4,14,15 Identifying predictive markers of outcome and response to chemotherapy could help tailor treatment to allow patients to have exposure to drugs that may show maximum benefit.
ERCC1
The nucleotide excision repair pathway is a DNA repair pathway involved in repair of bulky, helix-distorting DNA lesions caused by ultraviolet light or chemicals, including platinum compounds. The cytotoxic effect of platinum compounds is based on the formation of these bulky, intrastrand, platinum-DNA adducts, and removal of these adducts from genomic DNA is mediated by the nucleotide excision repair pathway.16 Recognition and repair of the platinum-induced damage results in platinum resistance. Critical in this pathway is the ERCC1 gene (and protein); its low expression has been associated with platinum sensitivity. Several studies across tumor types have evaluated ERCC1 mRNA expression and its potential to predict treatment response to platinum compounds.
ERCC1 and Gastric Cancer
In largely retrospective evaluations, ERCC1 has been shown to predict response to platinum-based therapy. There has been evaluation by immunohistochemistry (IHC), mRNA levels by reverse transcriptase polymerase chain reaction, and single nucleotide polymorphisms. There has been no correlation between the methodologies assessed. Generally, IHC is an accessible technique although often criticized for potential variability in interpretation. Thereby, use of reverse transcriptase polymerase chain reaction allows for semiquantitative assessment of ERCC1, potentially allowing for consistency in evaluation. In gastric cancer, several retrospective evaluations have shown that ERCC1 has a statistically significant relationship to response and survival in the advanced, perioperative and adjuvant setting.17-21 A study of preoperative cisplatin and infusional FU in patients with gastric cancer assessed ERCC1 retrospectively and demonstrated higher response rates and OS in patients with low ERCC1 mRNA levels. Another trial, SWOG S0356, tested preoperative oxaliplatin combined with protracted-infusion FU and 4,500 cGy of external-beam radiation for esophageal cancer. Of the 90 patients evaluable for this trial, 53 (58.8%) had specimens analyzed for ERCC1. ERCC1 mRNA levels within the primary tumor had a statistically significant inverse relationship to 2-year OS (37% v 72%; P = .04) and 2-year PFS (17% v 67%; P < .0004). The cutoff level was 1.66 × 10−3 in relation to expression of the β-actin gene, consistent with the previously reported cutpoint of 1.7 × 10−3.22,23 Further support that ERCC1 expression predicts platinum sensitivity came from an evaluation of patients receiving S1 with cisplatin, in whom low ERCC1 expression was associated with a higher response rate.21 In another trial of patients with gastric cancer treated with FOLFOX, the median survival time was significantly longer for patients with low compared with high ERCC1 expression (15.8 v 6.2 months; P < .0001).20
The relationship between ERCC1 and platinum sensitivity has been demonstrated across tumor types; data in NSCLC, colon cancer, and ovarian cancer corroborate the inverse relationship of ERCC1 and platinum sensitivity. Further, given that these have largely been retrospective evaluations, it has been difficult to discern whether ERCC1 is a predictive or prognostic marker. Prospective treatment assignment based on the presence of ERCC1 by IHC or gene expression has had mixed results.24,25
PATIENTS AND METHODS
Patients
Eligibility criteria included patients with unresectable advanced or metastatic HER2-negative adenocarcinoma of the esophagus, stomach, or gastroesophageal junction who were treatment naive or had completed adjuvant therapy at least 180 days before registration. If HER2 status was not known, tissue specimen submission was required for testing. Tumor tissue submission also was required to assess ERCC1. Patients must have had a Zubrod performance status of 0-1 with adequate organ function, defined as follows: hemoglobin ≥ 9 g/dL; ANC ≥ 1,500/μL; platelets > 100,000/μL, total bilirubin ≤ 1.5 mg/dL (regardless of whether patients had liver involvement secondary to tumor); AST and ALT both ≤ 3 times the institutional upper limit of normal (unless the liver was involved with tumor, in which case both AST and ALT must be ≤ 5 times the institutional upper limit of normal), serum creatinine < 1.5 mg/dL or creatinine clearance > 60 mL/min; and no sensory neuropathy > grade 1. Patients may have had measurable and/or nonmeasurable disease as assessed by computed tomography scans or magnetic resonance imaging 28 days or 42 days before registration, accordingly.
The study was approved by the local institutional review boards, and informed consent was obtained from all participants. All eligible patients were randomly assigned irrespective of ERCC1 status. If ERCC1 expression or HER2 status could not be determined, or if the patient had HER2-positive status, the patient was ineligible.
mRNA Quantification
Samples were to be fixed in formalin for 8-24 hours and then paraffin embedded according to institutional procedures. All gene expression levels were measured at a Clinical Laboratory Improvement Amendments–approved laboratory (Response Genetics, Los Angeles, CA); microdissection was performed on all formalin-fixed, paraffin-embedded tumor samples to ensure that only tumor cells were dissected. Details about the procedure for microdissection, mRNA isolation, and quantification are provided.26 Results were to be provided in 7-10 days.
Treatment
Treatment consisted of random assignment to either a platinum- or a non–platinum-containing arm with stratification for ERCC1 level. The regimens were as follows: Arm 1 was FOLFOX, specifically oxaliplatin 85 mg/m2 intravenously (IV) over 2 hours on day 1, leucovorin 400 mg/m2 IV over 2 hours on day 1, FU 400 mg/m2 IV bolus over 1 hour on day 1, and FU 2,400 mg/m2 IV over 46-48 hours via CADD pump on days 1 and 2. Each cycle was to be administered every 14 days. Arm 2 was IT, specifically irinotecan 65 mg/m2 IV over 90 minutes with docetaxel 30 mg/m2 IV over 30 minutes on days 1 and 8. Each cycle was to be administered every 21 days. Dose adjustments of all agents were made according to toxicity experienced during the preceding cycle, using National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) for toxicity grading and serious adverse event reporting. The maximum dose delay for any reason was 4 weeks; omitted doses were not made up, and patients were taken off protocol treatment for dose delays of more than 4 weeks or dose adjustments of 2 or more levels.
Patients underwent disease assessment (by computed tomography or magnetic resonance imaging) every 6 weeks and were treated until disease progression occurred or until symptomatic deterioration, unacceptable toxicity, or treatment delay for any reason lasted longer than 4 weeks. Patients were observed until death or 3 years after registration, whichever occurred first.
Study Endpoints
The primary endpoint of this phase II study was PFS. Secondary endpoints included OS, toxicities, and overall response rate (ORR; confirmed and unconfirmed complete and partial response).
Statistical Considerations
The primary study hypothesis was that patients with high-ERCC1 status who were treated with IT would have superior PFS compared with those treated with FOLFOX. It was anticipated that roughly 50% of patients would fall into each of the high or low ERCC1 subgroups according to observed data in colorectal and esophageal cancer studies.27 One hundred eligible patients in each ERCC1 subgroup provided at least 80% power to detect hazard ratios (HRs) of 1.55 or greater across a range of potential median PFS values (3.0-5.5 months in the inferior arm and 4.7-9.9 months in the superior arm) according to a 10% one-sided log-rank test, 3 years of accrual, and 2 years of follow-up. The total accrual goal was 200 eligible patients. Patients were randomly assigned to treatment with FOLFOX or IT with stratification by ERCC1 level (high v low, ≥ 1.7 v < 1.7, respectively) and disease site (esophageal v gastric/gastroesophageal junction).
The observed data did not align with our ERCC1 distributional assumption, in that approximately 86% of the patients had ERCC1 values lower than 1.7. Thus, the primary objective of assessing PFS in patients with high ERCC1 by treatment arm was limited by low statistical power, and the interaction analysis of treatment effect by the a priori ERCC1 threshold was not feasible. Thus, the analysis consisted of the following: (1) assessments of PFS, OS, and ORR in all patients treated with FOLFOX compared with those treated with IT, (2) assessments of PFS, OS, and ORR in patients with low-ERCC1 status treated with FOLFOX compared with those treated with IT, (3) an assessment of PFS in patients with high-ERCC1 status treated with FOLFOX compared with those treated with IT, and (4) an investigation into whether differences in PFS between the two treatment arms varied by ERCC1 levels. The analysis plan also included estimation of ERCC1 threshold(s) that defined categories of differential treatment effects on PFS given observed evidence of an interaction between ERCC1 expression and treatment arm.
All eligible patients were included in the analyses by randomized treatment assignment regardless of actual treatments received according to the intent-to-treat principle. Probabilities of OS and PFS were estimated using the Kaplan-Meier method. Statistical differences in event rates between treatment arms were assessed via Cox regression model with stratification for ERCC1 level (high v low) and disease site. Rates of ORR in the subset of patients with measurable disease were compared via the Fisher’s exact test or χ2 test as appropriate. A series of plotted Kaplan-Meier estimates was used to assess variation of treatment arm differences in PFS according to ERCC1 levels (quartiles).
RESULTS
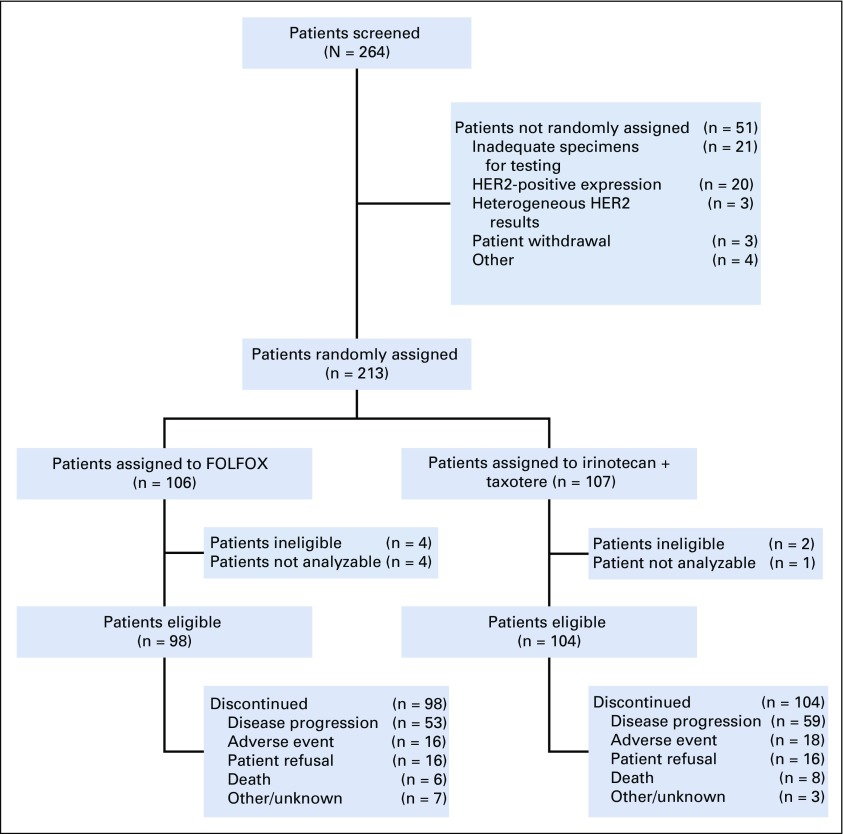
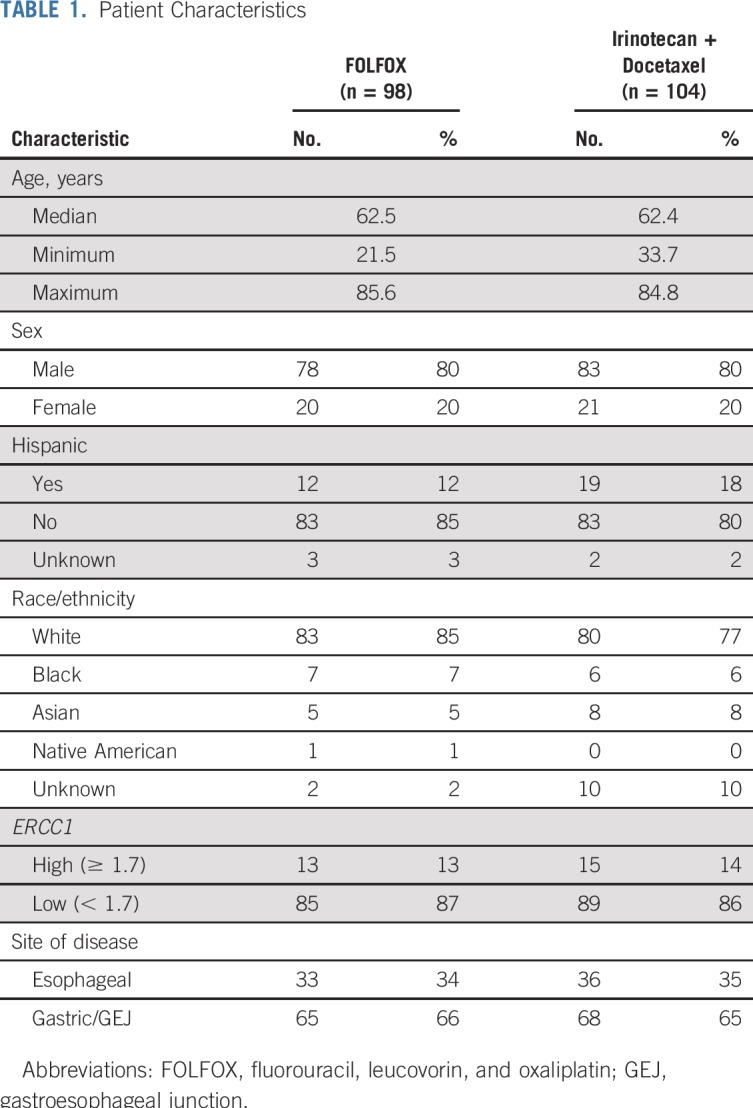
The study was activated on March 1, 2012, and closed to accrual on April 1, 2015, after meeting the accrual goal with 264 patients registered to the initial screening. The median time from screening to random assignment was 8.73 days (range, 3-20 days). Fifty-one patients were not randomly assigned, most commonly because of inadequate specimens for testing or HER2-positive expression. Two-hundred thirteen patients were randomly assigned. Six patients were deemed ineligible, and five additional patients were excluded from analyses because of death or withdrawal before random assignment; thus, 202 patients were included in the primary analysis (Fig 1). Patient characteristics are listed in Table 1.
FIG 1.
CONSORT flow diagram. FOLFOX, fluorouracil, leucovorin, and oxaliplatin.
TABLE 1.
Patient Characteristics
Safety
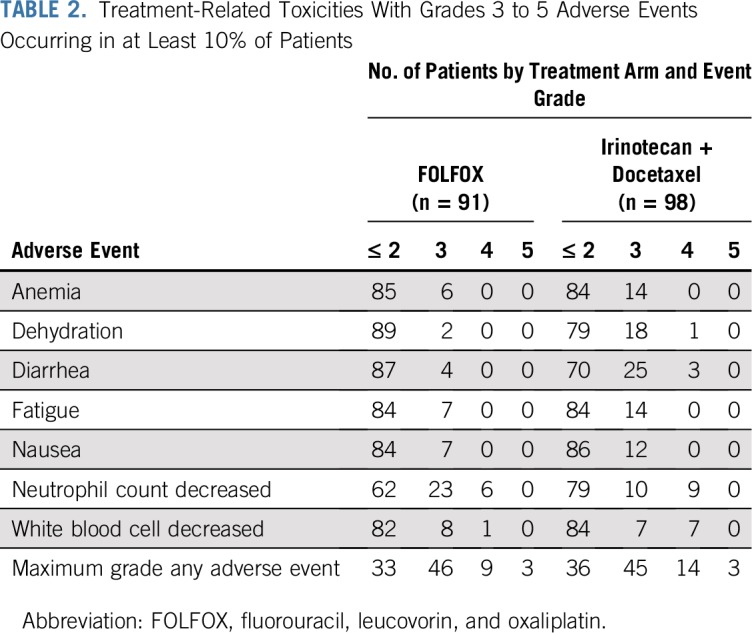
One hundred eighty-nine patients were assessed for adverse events, because 14 patients did not receive protocol therapy. Among the 91 patients assessed for adverse events on the FOLFOX arm, three treatment-related deaths were reported (lung infection and oral mucositis, each n = 1, and a sudden death of unknown cause). Nine additional patients experienced grade 4 adverse events. In 98 patients assessed for adverse events on the IT arm, three treatment-related deaths were reported (multiorgan failure, n = 2; respiratory failure, n = 1). Fourteen additional patients experienced grade 4 adverse events (primarily hematologic events; Table 2).
TABLE 2.
Treatment-Related Toxicities With Grades 3 to 5 Adverse Events Occurring in at Least 10% of Patients
Efficacy
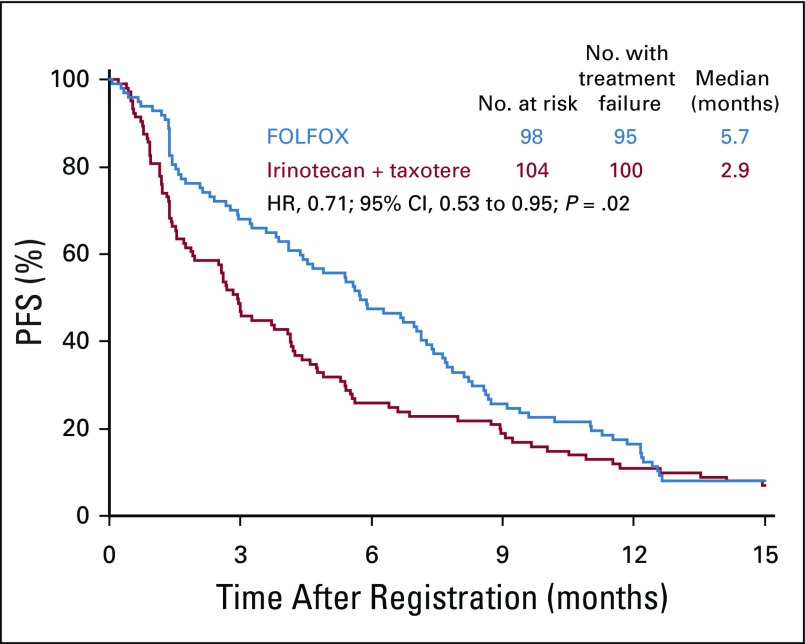
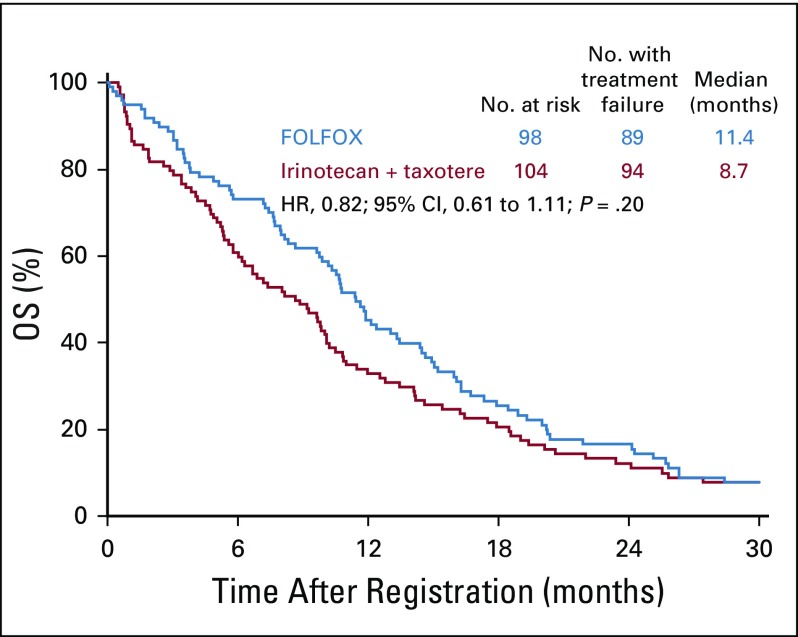
The median PFS was significantly longer in the FOLFOX arm (n = 98) than in the IT arm (n = 104): 5.7 months (95% CI, 4.4 to 7.1 months) versus 2.9 months (95% CI, 1.9-4.1 months). The HR was 0.71 (95% CI, 0.53 to 0.95; P = .02; Fig 2). The median OS was greater with FOLFOX than IT, but this difference did not reach statistical significance: 11.4 months (95% CI, 9.8 to 13.5 months) versus 8.7 months (95% CI, 6.0 to 10.1 months); the HR was 0.82 (95% CI, 0.61 to 1.11; P = .20; Fig 3). A higher ORR was observed in the FOLFOX arm than in the IT arm, although this difference was not statistically significant: 33 of 79 evaluable patients (42%; 95% CI, 31% to 53%) versus 26 of 88 evaluable patients (30%; 95% CI, 20% to 39%; P = .10). Twenty patients (25%) in the FOLFOX arm and 21 patients (24%) in the IT arm had stable disease.
FIG 2.
Progression-free survival (PFS) in all patients. FOLFOX, fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio.
FIG 3.
Overall survival (OS) in all patients. FOLFOX, fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio.
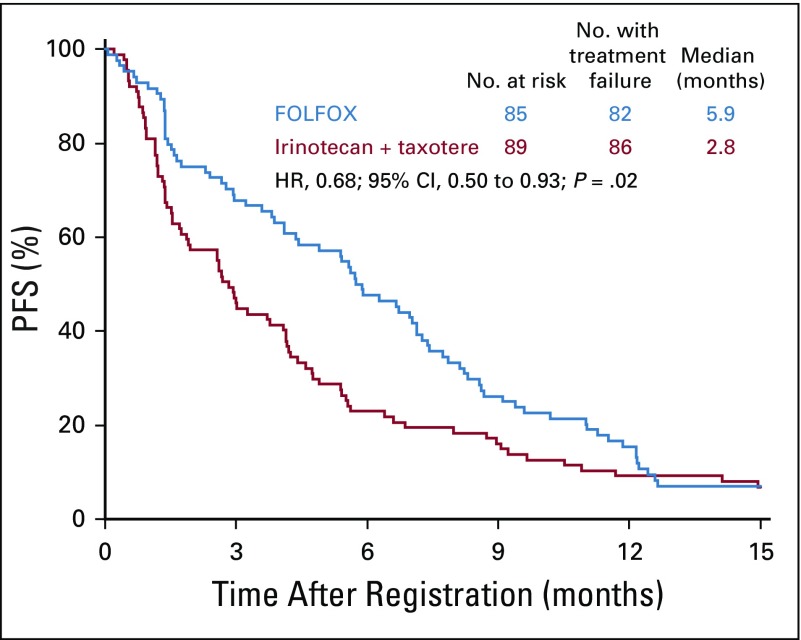
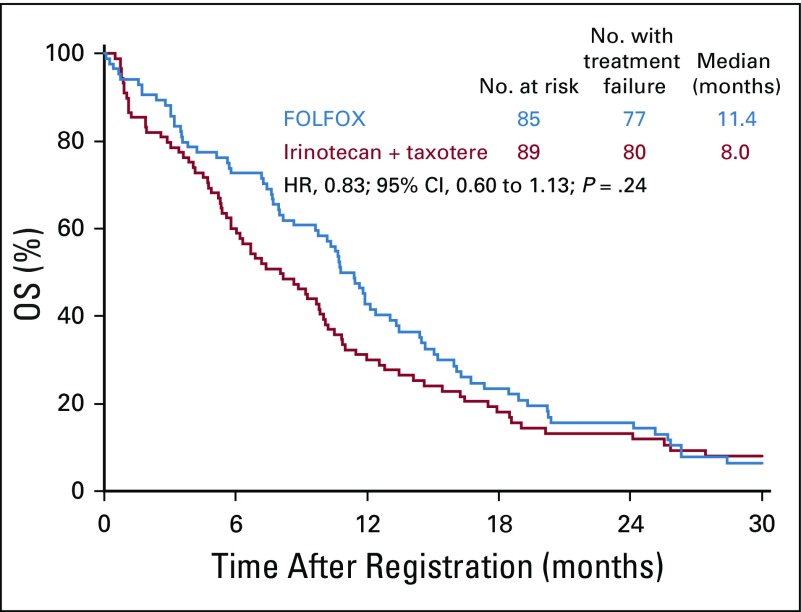
Similar to the total cohort, in the ERCC1-low subgroup, the median PFS was improved in the FOLFOX arm compared with the IT arm: 5.9 months (n = 85; 95% CI, 4.1 to 7.1 months) versus 2.8 months (n = 89; 95% CI, 1.7 to 4.1 months), respectively. The HR was 0.68 (95% CI, 0.50 to 0.93; P = .02; Fig 4). The median OS for the ERCC1-low subgroup was 11.4 months (95% CI, 8.7 to 13.0 months) in the FOLFOX arm and was 8.0 months (95% CI, 5.8 to 10.0 months) in the IT arm; the HR was 0.83 (95% CI, 0.60 to 1.13; P = .24; Fig 5). Of the 70 and 76 evaluable patients with measurable disease in the FOLFOX and IT arms, 31 (44%; 95% CI, 32% to 57%) versus 21 (28%; 95% CI, 18% to 39%) responses were seen (P = .04; Appendix Table A2, online only). In the FOLFOX versus in the IT arm, there were 17 patients (24%) versus 20 patients (26%) with stable disease.
FIG 4.
Progression-free survival (PFS) in patients with ERCC1-low status. FOLFOX, fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio.
FIG 5.
Overall survival (OS) in patients with ERCC1-low status. FOLFOX, fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio.
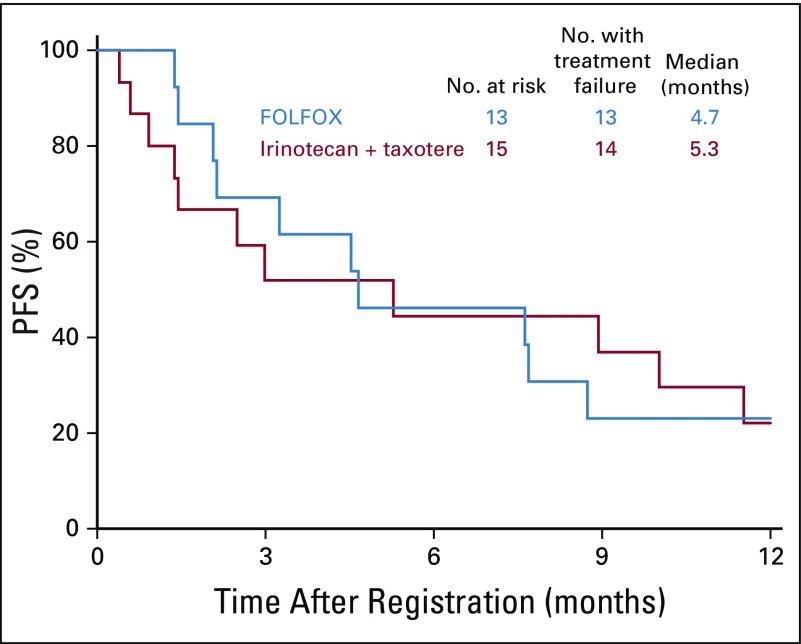
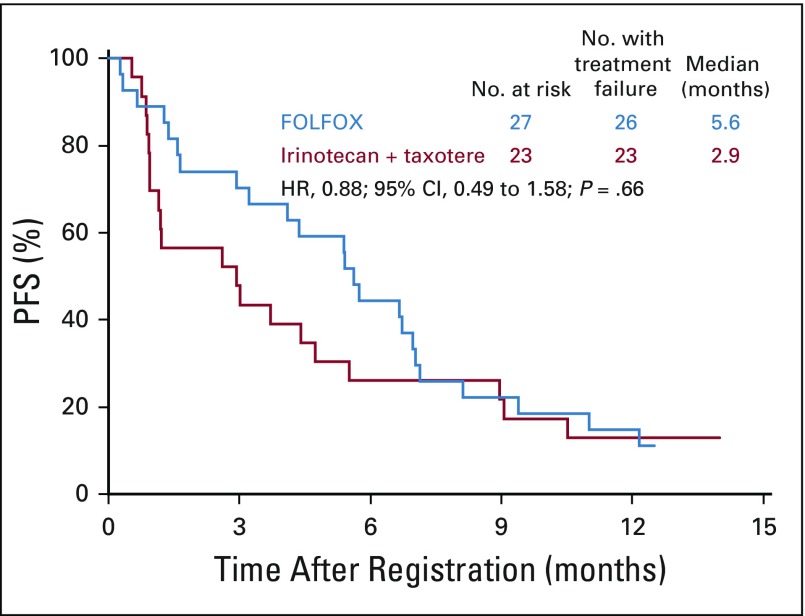
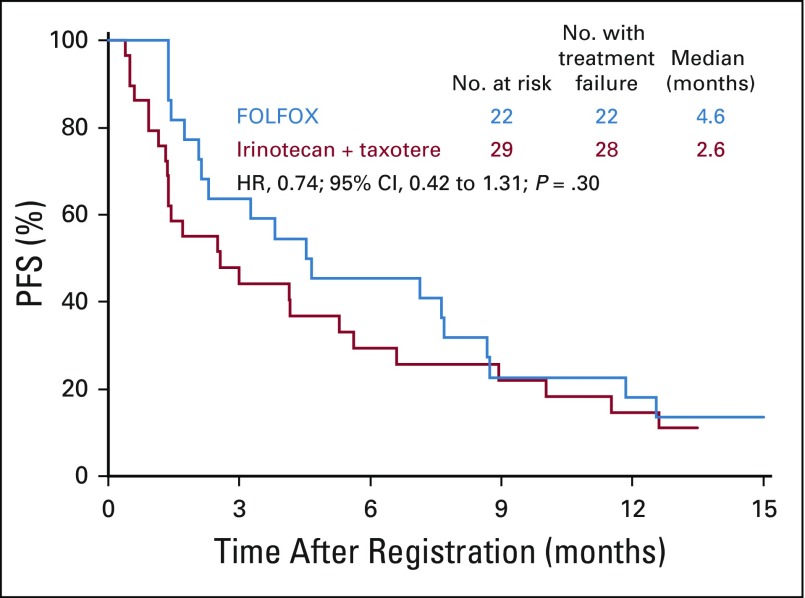
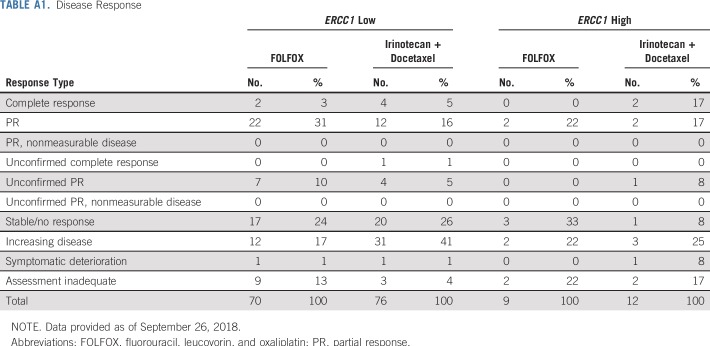
In the ERCC1-high subgroup, the median PFS was similar in the FOLFOX and IT arms: 4.7 months (n = 13; 95% CI, 2.1 to 8.7 months) versus 5.3 months (n = 15; 95% CI, 0.9 to 11.5 months); the HR was 0.91 (95% CI, 0.4 to 2.0; P = .83; Fig 6). Of the nine and 12 evaluable patients with measurable disease in the FOLFOX and IT arms, two (22%; 95% CI, 0% to 60%) versus five (42%; 95% CI, 15% to 72%) responses were seen (P = .64; Appendix Table A1).
FIG 6.
Progression-free survival (PFS) in patients with ERCC1-high status. FOLFOX, fluorouracil, leucovorin, and oxaliplatin.
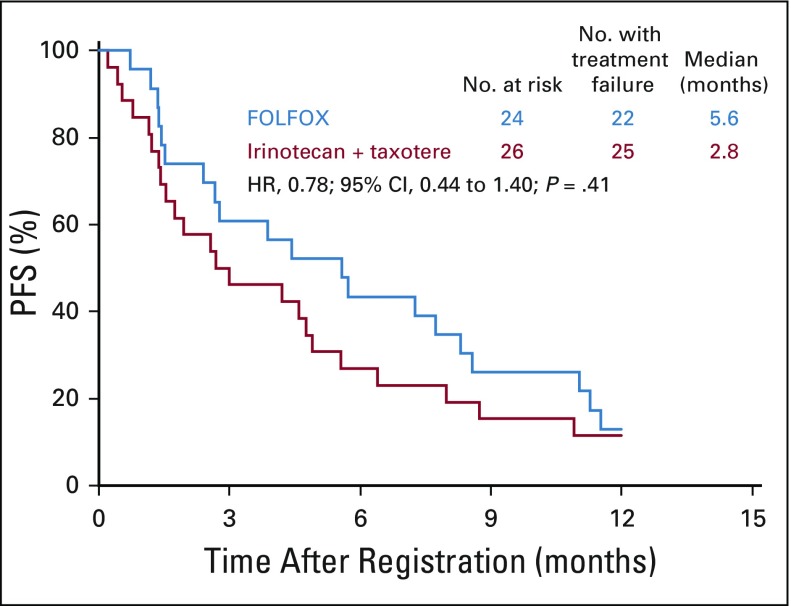
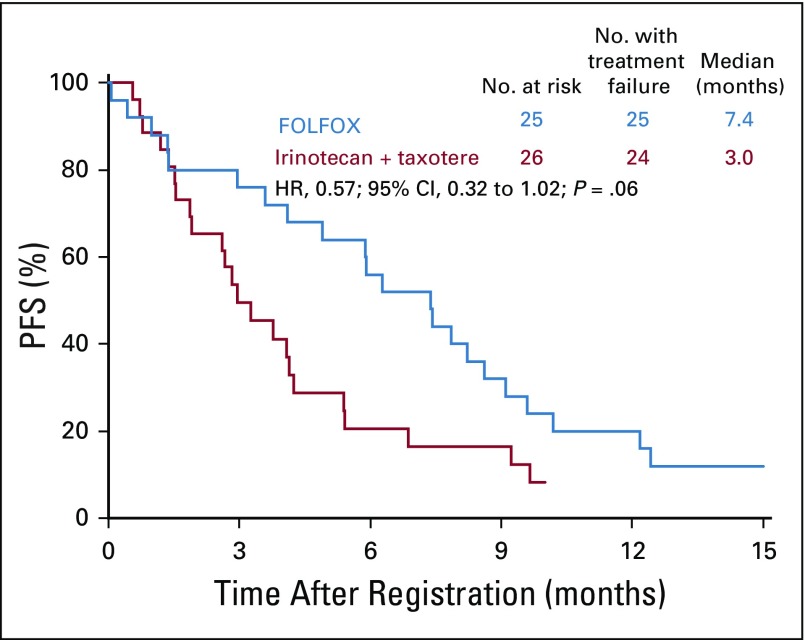
The PFS by treatment arm was plotted within ERCC1 quartiles: 0.20-0.80, ≥ 0.81-0.1.10, ≥ 1.11-1.42, and ≥ 1.43-5.71. These plots showed a consistent pattern of improved PFS in the FOLFOX versus the IT arm and, thus, little evidence of differential treatment effects on PFS across ERCC1 levels in this population (Appendix Figs A1, A2, A3, and A4, online only). Thus, no statistical tests for interactions were performed.
DISCUSSION
To our knowledge, this was the first study to prospectively validate intratumoral gene expression of ERCC1 as a potential marker of response to platinum-based chemotherapy. This randomized, phase II trial in patients with advanced esophagogastric cancer did not demonstrate a differential treatment effect on PFS on the basis of ERCC1 levels. Despite the retrospective data reported across various tumor types, evaluated by IHC or gene expression, which have shown an inverse relationship of ERCC1 and platinum sensitivity, this prospective evaluation did not confirm those results.
Our proposed assumption, based on several retrospective studies, was that the number of patients with ERCC1-high and ERCC1-low statuses would be split 50/50. The interim evaluation revealed that the statistical value of ERCC1-high/-low statuses was approximately 20/80 and not 50/50, as had been hypothesized. As such, we adjusted our objectives, because there was not enough statistical power to assess treatment effects in the group of patients with ERCC1-high status. The variation in the prospective evaluation of the ERCC1-high/-low distribution differed from that reported in prior studies. We have not identified the cause of this difference in distribution of ERCC1 expression. We conducted an investigation and validation of the tissue handling, laboratory techniques, and data. Further evidence that there was not an issue with the procedure of evaluation of ERCC1 derives from a contemporaneous trial evaluating ERCC1 in colorectal cancer, the MAVERICC trial.28 This study was designed to prospectively evaluate ERCC1 in patients with metastatic colorectal cancer randomly assigned to FOLFOX with bevacizumab versus fluorouracil, leucovorin, irinotecan with bevacizumab (ie, platinum- and non–platinum-containing regimens). This study also assumed a 50/50 split for ERCC1-high/-low statuses, and the interim data analysis also found a similar 20/80 distribution, consistent with our observations.
Another consideration is that the cutoff level of 1.7, which had been identified previously in other studies, may not have been accurate. To assess this more, a series of Kaplan-Meier curves were done to show if there was a differential treatment effect on PFS across varying levels (Figs 5 and 6, Appendix Figs A1 through A4), and this effect was not found. The curves demonstrate a similar pattern in each, suggesting that the treatment effects on PFS do not significantly vary by ERCC1 mRNA level.
Other techniques have been used to assess ERCC1, including evaluation by IHC and evaluation of ERCC1 polymorphisms. Validated quantitative immunofluorescence assays have been plagued by variability in antibody performance, and none has been reproducibly used in a study of prospective treatment assignment.29 However, given the well-documented impact of post-translational modification of ERCC1 on DNA repair, one limitation of our study may have been reliance on ERCC1 mRNA levels for patient categorization.30 Unfortunately, there has not been prospective validation of any technique used to evaluate ERCC1.
Overall, there was a statistically significant improvement in PFS for FOLFOX compared with IT, a finding that was consistent across ERCC1 levels. In the ERCC1-low group, there was a statistically significant improvement in PFS and ORR but not OS, although there was a numeric improvement noted. This result may have been due to the smaller numbers in each of the subgroups. To our knowledge, there have not been any prior randomized trials comparing FOLFOX to IT. This significant improvement with FOLFOX may suggest that the addition of a platinum as well as FU is important in the first-line treatment of advanced gastric cancer.
This study was the first, to our knowledge, to prospectively analyze the use of ERCC1 expression as a predictive biomarker with real-time measurement and treatment assignment. Unfortunately, this study did not validate or identify ERCC1 as a predictive marker of platinum sensitivity in upper GI tumors. It did support the use of FOLFOX, a platinum-containing regimen, as a standard and superior front-line regimen compared with the non–platinum-containing IT.
APPENDIX
FIG A1.
Progression-free survival (PFS) across ERCC1 levels: first quartile. FOLFOX, fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio.
FIG A2.
Progression-free survival (PFS) across ERCC1 levels: second quartile. FOLFOX, fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio.
FIG A3.
Progression-free survival (PFS) across ERCC1 levels: third quartile. FOLFOX, fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio.
FIG A4.
Progression-free survival (PFS) across ERCC1 levels: fourth quartile. FOLFOX, fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio.
TABLE A1.
Disease Response
Footnotes
Presented in part at the American Society of Clinical Oncology Annual Meeting, June 2-6, 2017, Chicago, IL.
Supported by the National Cancer Institute of the National Institutes of Health Award Nos. CA180888, CA180819, CA180820, CA180821, CA180830, CA189830, CA189971, CA189953, CA189858, CA180798, CA189808, CA189972, CA180801, CA189957, CA189872, CA189822, CA 189856, CA189952, CA180846, CA189804, CA189860, CA189853, and legacy Nos. CA11083 and CA35119.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: Syma Iqbal, Heinz-Josef Lenz, David Ilson
Collection and assembly of data: Syma Iqbal, Heinz-Josef Lenz, Barbara Burtness, Chaitali S. Nangia, Afsaneh Barzi, Charles J. Schneider, Jane Jijun Liu, Efrat Dotan, Howard S. Hochster
Data analysis and interpretation: Syma Iqbal, Shannon McDonough, Heinz-Josef Lenz, Barbara Burtness, Katherine A. Guthrie, Howard S. Hochster
Provision of study material or patients: Heinz-Josef Lenz, David Ilson, Barbara Burtness, Afsaneh Barzi, Charles J. Schneider, Howard S. Hochster
Administrative support: Heinz-Josef Lenz, Chaitali S. Nangia, Jane Jijun Liu, Howard S. Hochster
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized, Phase II Study Prospectively Evaluating Treatment of Metastatic Esophageal, Gastric, or Gastroesophageal Cancer by Gene Expression of ERCC1: SWOG S1201
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Syma Iqbal
Honoraria: Celgene, Eisai, F Hoffmann La Roche AG
Consulting or Advisory Role: F Hoffmann La Roche AG
Speakers' Bureau: Celgene, Eisai
Research Funding: Bayer, Onyx
Travel, Accommodations, Expenses: Celgene, Eisai
Heinz-Josef Lenz
Honoraria: Merck Serono, Roche, Bayer, Boehringer Ingelheim, Isofol, GSK
Consulting or Advisory Role: Merck Serono, Roche, Bayer, BMS, GSK
Travel, Accommodations, Expenses: Merck Serono, Bayer
David Ilson
Consulting or Advisory Role: Lilly, ImClone, Roche, Genentech, Bristol-Myers Squibb, Pieris Pharmaceuticals, Merck, Bayer, AstraZeneca, Taiho Pharmaceutical, Astellas Pharma
Barbara Burtness
Honoraria: Astra Zeneca
Consulting or Advisory Role: Merck, Debiopharm Group, AstraZeneca, Bristol-Myers Squibb, Alligator Biosciences, Aduro Biotech, GlaxoSmithKline, Celgene, CUE Biopharma, Maverick Therapeutics, Rakuten, Nanobiotix, Macrogenics, ALX Oncology
Research Funding: Merck (Inst), Aduro (Inst), Formation Biologics (Inst), Bristol-Myers Squibb (Inst), Exelixis (Inst)
Travel, Accommodations, Expenses: Merck, Debiopharm Group, Boehringer Ingelheim
Chaitali S. Nangia
Consulting or Advisory Role: Novartis
Speakers' Bureau: Merck, Novartis
Travel, Accommodations, Expenses: Novartis
Afsaneh Barzi
Consulting or Advisory Role: Merrion, bioTheranostics, Bayer Technology System
Research Funding: Bayer (Inst), Merck (Inst)
Jane Jijun Liu
Employment: Illinois CancerCare
Speakers' Bureau: Genentech
Travel, Accommodations, Expenses: Genentech
Efrat Dotan
Honoraria: Pfizer, Boston Medical
Consulting or Advisory Role: Boston Biomedical, ARMO BioSciences
Research Funding: Pfizer (Inst), Bayer (Inst), Incyte (Inst), Boston Biomedical (Inst), Merck (Inst), MedImmune (Inst), GSK (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Howard S. Hochster
Consulting or Advisory Role: Bayer, Genentech, Amgen, Exelixis
No other potential conflicts of interest were reported.
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2018. [Google Scholar]
- 2.Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 4.Enzinger PC, Burtness BA, Niedzwiecki D, et al. CALGB 80403 (Alliance)/E1206: A randomized phase II study of three chemotherapy regimens plus cetuximab in metastatic esophageal and gastroesophageal junction cancers. J Clin Oncol. 2016;34:2736–2742. doi: 10.1200/JCO.2015.65.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 7.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 8.Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1437–1448. doi: 10.1016/S1470-2045(18)30739-3. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 10.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 11.Pozzo C, Barone C, Szanto J, et al. Irinotecan in combination with 5-fluorouracil and folinic acid or with cisplatin in patients with advanced gastric or esophageal-gastric junction adenocarcinoma: Results of a randomized phase II study. Ann Oncol. 2004;15:1773–1781. doi: 10.1093/annonc/mdh473. [DOI] [PubMed] [Google Scholar]
- 12.Louvet C, André T, Tigaud JM, et al. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol. 2002;20:4543–4548. doi: 10.1200/JCO.2002.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Park SR, Chun JH, Yu MS, et al. Phase II study of docetaxel and irinotecan combination chemotherapy in metastatic gastric carcinoma. Br J Cancer. 2006;94:1402–1406. doi: 10.1038/sj.bjc.6603133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: A randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 15.Ajani JA, Fodor MB, Tjulandin SA, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005;23:5660–5667. doi: 10.1200/JCO.2005.17.376. [DOI] [PubMed] [Google Scholar]
- 16.Reardon JT, Sancar A. Nucleotide excision repair. Prog Nucleic Acid Res Mol Biol. 2005;79:183–235. doi: 10.1016/S0079-6603(04)79004-2. [DOI] [PubMed] [Google Scholar]
- 17.Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16:309–316. doi: 10.1200/JCO.1998.16.1.309. [DOI] [PubMed] [Google Scholar]
- 18.Kwon HC, Roh MS, Oh SY, et al. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol. 2007;18:504–509. doi: 10.1093/annonc/mdl430. [DOI] [PubMed] [Google Scholar]
- 19.Napieralski R, Ott K, Kremer M, et al. Combined GADD45A and thymidine phosphorylase expression levels predict response and survival of neoadjuvant-treated gastric cancer patients. Clin Cancer Res. 2005;11:3025–3031. doi: 10.1158/1078-0432.CCR-04-1605. [DOI] [PubMed] [Google Scholar]
- 20.Wei J, Zou Z, Qian X, et al. ERCC1 mRNA levels and survival of advanced gastric cancer patients treated with a modified FOLFOX regimen. Br J Cancer. 2008;98:1398–1402. doi: 10.1038/sj.bjc.6604317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsubara J, Nishina T, Yamada Y, et al. Impacts of excision repair cross-complementing gene 1 (ERCC1), dihydropyrimidine dehydrogenase, and epidermal growth factor receptor on the outcomes of patients with advanced gastric cancer. Br J Cancer. 2008;98:832–839. doi: 10.1038/sj.bjc.6604211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leichman LP, Goldman BH, Bohanes PO, et al. S0356: A phase II clinical and prospective molecular trial with oxaliplatin, fluorouracil, and external-beam radiation therapy before surgery for patients with esophageal adenocarcinoma. J Clin Oncol. 2011;29:4555–4560. doi: 10.1200/JCO.2011.36.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohanes PO, Goldman BH, Benedetti J, et al. Association of excision repair cross-complementation group 1 (ERCC1) gene expression (GE) with outcome in stage II-III esophageal adenocarcinoma (EA) patients treated with preoperative platinum-based chemoradiation (CRT) in a phase II cooperative study (SWOG 0356) J Clin Oncol. 2011;29 (suppl; abstr 4023) [Google Scholar]
- 24.Cobo M, Isla D, Massuti B, et al. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: A phase III trial in non-small-cell lung cancer. J Clin Oncol. 2007;25:2747–2754. doi: 10.1200/JCO.2006.09.7915. [DOI] [PubMed] [Google Scholar]
- 25.Lee SM, Falzon M, Blackhall F, et al. Randomized prospective biomarker trial of ERCC1 for comparing platinum and nonplatinum therapy in advanced non–small-cell lung cancer: ERCC1 trial (ET) J Clin Oncol. 2017;35:402–411. doi: 10.1200/JCO.2016.68.1841. [DOI] [PubMed] [Google Scholar]
- 26.Horikoshi T, Danenberg KD, Stadlbauer TH, et al. Quantitation of thymidylate synthase, dihydrofolate reductase, and DT-diaphorase gene expression in human tumors using the polymerase chain reaction. Cancer Res. 1992;52:108–116. [PubMed] [Google Scholar]
- 27.Leichman L, Goldman BH, Benedetti JK, et al. : Oxaliplatin (OXP) plus protracted infusion 5-fluorouracil (PIFU) and external beam radiation (EBRT) prior to surgery (S) for potentially curable esophageal adenocarcinoma (EA): A Southwest Oncology Group (SWOG) phase II trial with molecular correlates (S0356). J Clin Oncol 27, 2009. (suppl; abstr 4513) [Google Scholar]
- 28.Parikh AR, Lee FC, Yau L, et al. MAVERICC, a randomized, biomarker-stratified, phase II study of mFOLFOX6-bevacizumab versus FOLFIRI-bevacizumab as first-line chemotherapy in metastatic colorectal cancer. Clin Cancer Res. 2019;25:2988–2995. doi: 10.1158/1078-0432.CCR-18-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehra R, Zhu F, Yang DH, et al. Quantification of excision repair cross-complementing group 1 and survival in p16-negative squamous cell head and neck cancers. Clin Cancer Res. 2013;19:6633–6643. doi: 10.1158/1078-0432.CCR-13-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Oliva AB, Lachaud C, Szyniarowski P, et al. USP45 deubiquitylase controls ERCC1-XPF endonuclease-mediated DNA damage responses. EMBO J. 2015;34:326–343. doi: 10.15252/embj.201489184. [DOI] [PMC free article] [PubMed] [Google Scholar]