Abstract
PURPOSE
Proton radiotherapy (PRT) may lessen the neuropsychological risk traditionally associated with cranial radiotherapy for the treatment of pediatric brain tumors by reducing the dose to normal tissue compared with that of photon radiotherapy (XRT). We examined the change in intellectual scores over time in patients with pediatric medulloblastoma treated with craniospinal PRT versus XRT.
METHODS
Intelligence test scores were obtained for a sample of pediatric patients treated between 2007 and 2018 on the same medulloblastoma protocols that differed only in radiotherapy modality (PRT v XRT). Growth curve analyses compared change in scores over time since diagnosis between groups.
RESULTS
Longitudinal intelligence data from 79 patients (37 PRT, 42 XRT) were examined. Groups were similar on most demographic/clinical variables, including sex (67.1% male), age at diagnosis (mean, 8.6 years), craniospinal irradiation dose (median, 23.4 Gy), length of follow-up (mean, 4.3 years), and parental education (mean, 14.3 years). Boost dose (P < .001) and boost margin (P = .001) differed between groups. Adjusting for covariates, the PRT group exhibited superior long-term outcomes in global intelligence quotient (IQ), perceptual reasoning, and working memory compared with the XRT group (all P < .05). The XRT group exhibited a significant decline in global IQ, working memory, and processing speed (all P < .05). The PRT group exhibited stable scores over time in all domains with the exception of processing speed (P = .003).
CONCLUSION
To our knowledge, this is the first study to compare intellectual trajectories between pediatric patients treated for medulloblastoma with PRT versus those treated with XRT on comparable, contemporary protocols. PRT was associated with more favorable intellectual outcomes in most domains compared with XRT, although processing speed emerged as a vulnerable domain for both groups. This study provides the strongest evidence to date of an intellectual sparing advantage with PRT in the treatment of pediatric medulloblastoma.
INTRODUCTION
Craniospinal irradiation (CSI) is associated with significant cognitive risk for patients treated for pediatric medulloblastoma.1-3 Attempts to reduce the whole brain dose and boost volume have yielded evidence of improved intellectual outcomes.3 To achieve the same target clinical dose, proton radiotherapy (PRT) deposits a smaller dose to healthy tissue compared with photon (or x-ray) radiotherapy (XRT).4 In the context of CSI, PRT reduces the dose to healthy brain tissue beyond boost margins, which may result in intellectual sparing relative to XRT.5,6 To date, however, intellectual outcomes have not been compared specifically between pediatric patients treated for medulloblastoma with PRT versus those treated with XRT on comparable treatment protocols.
It remains unclear whether intellectual outcomes after PRT will be superior to modern intensity-modulated XRT protocols. In the only published study to date comparing change in intelligence scores over time between patients with pediatric brain tumor treated with PRT versus XRT, intelligence quotient (IQ) was significantly and persistently lower among patients treated with craniospinal XRT compared with those treated with craniospinal PRT, even though IQ change over time did not differ significantly between groups.5 A recent cross-sectional comparison study found higher IQ and processing speed scores in survivors treated with PRT versus XRT.7 Overall, PRT is not associated with profound cognitive impairment.5,6,8-10 Still, CSI5,6,8-10 and younger age at radiotherapy (RT)8,9 have been associated with increased intellectual risk in PRT samples.
To our knowledge, this is the first longitudinal study comparing change in intelligence indices over time between pediatric patients treated for medulloblastoma with PRT versus a matched cohort of patients treated with XRT. This study is unique in that patients were treated contemporaneously on comparable treatment protocols that differed in RT modality (PRT or XRT). We hypothesized that patients treated with XRT would show larger intellectual declines. Still, we expected to observe significant declines in domains traditionally associated with RT-related risk (ie, processing speed and working memory) in all patients, regardless of RT modality, because of whole-brain RT.
METHODS
Patients
Eligible patients were identified from institutional databases at The Hospital for Sick Children and Texas Children’s Hospital after permission from the respective institutional review boards. Eligibility criteria included (1) histologic confirmation of medulloblastoma, (2) patient age ≤ 18 years at time of RT, (3) treatment with XRT or PRT according to SJMB03 (ClinicalTrials.gov identifier: NCT00085202) or SJMB12 (ClinicalTrials.gov identifier: NCT01878617) protocols between 2007 and 2018, and (4) that patients were proficient in English.
Patients at The Hospital for Sick Children (Canada) received XRT, as standard of care. Canada did not have a proton center during the timeframe of this study. Patients at Texas Children’s Hospital (United States) received PRT, which has been standard of care since 2007, at the MD Anderson Proton Therapy Center.
All patients underwent resection via craniotomy and received RT and chemotherapy according to the SJMB03 or SJMB12 protocol. Patients received standard-dose (ie, 30.6-39.6 Gy) or reduced-dose (ie, 15.0-23.4 Gy) RT to the whole brain and spine and a protocol-determined conformal tumor bed (TB) boost with either 1.0 cm (SJMB03) or 0.5 cm (SJMB12) clinical target volume margin. No patients received boost to the full posterior fossa. Total dose to TB plus margin (ie, boost dose plus CSI dose) ranged from 51.0 to 59.4 Gy. Most patients were standard risk (72.2%) and without recurrence within the timeframe of our observation (86.1%).
Measures
Intelligence scores were abstracted for all eligible patients. Prospective neuropsychological surveillance is standard of care for pediatric patients with medulloblastoma at both institutions. In addition, most patients (91.9%) in the PRT subsample received annual evaluations on a prospective, longitudinal study of intellectual outcomes. At each evaluation, patients were administered the age-appropriate version of one of the following tests: Wechsler Scales of Intelligence11-16 (63.2%), Woodcock-Johnson Tests of Cognitive Abilities, Third Edition17 (36.1%), or the Stanford-Binet Intelligence Test, Fifth Edition18 (0.7%). Baseline and follow-up tests were the same for 88.3% of patients. Table A1 (online only) presents details regarding the test versions administered. We examined scores that represented global IQ (ie, Wechsler/Stanford-Binet Full Scale IQ score; Woodcock-Johnson General Intellectual Ability score), as well as verbal reasoning, perceptual reasoning, working memory, and processing speed. Perceptual reasoning scores were from Wechsler scales only, because a comparable index score was not available on the other measures. Of note, the Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V) does not generate a perceptual reasoning index (PRI) score. NCS Pearson (WISC-V publisher) generated norms for calculating PRI scores from WISC-V subtests, per our request, to enable comparison of scores across versions. WISC-V PRI reliabilities ranged from 0.93 to 0.95 across ages 6 to 16 years.28
The measures have good convergent validity, with correlations for composite scores ranging from 0.80 to 0.92 across measures and test versions administered in this study.19-27 All intellectual indices are reported as standard scores (mean = 100, SD = 15), with lower scores indicating worse functioning.
Statistical Analyses
Summary statistics were stratified by RT group (PRT or XRT) and compared using t tests, χ2 tests, and Mann-Whitney U tests. Growth curve analyses and model fitting were used to determine if the stability/change in intelligence scores over time since diagnosis differed as a function of RT modality. Demographic and clinical characteristics that differed significantly (P < .05) between groups were included in the models as covariates. Covariates remained in the models even if they did not remain statistically significant. Models included participant as a random effect on all time terms. Single time point data were included because these scores contribute to overall group means and add stability to the overall model but do not contribute to or influence slope.
For each intelligence domain (global IQ, verbal reasoning, perceptual reasoning, working memory, and processing speed), we compared growth curve models in which terms were added sequentially to test for differences in scores as a function of RT modality. In model 1, we examined the impact of time since diagnosis across the entire cohort while adjusting for covariates (Intelligence score = time since diagnosis + covariates + [1| participant ID] + ε). To test whether baseline scores differed as a function of RT, we included the fixed effects of RT modality (PRT v XRT) on the intercept in model 2 (Intelligence score = time since diagnosis + RT modality + covariates + [1| participant ID] + ε). In model 3, we added the fixed effects on the slope to test whether slope differed as a function of RT modality (Intelligence score = time since diagnosis × RT modality + covariates + [1| participant ID] + ε). Change in goodness of fit was compared between the models using log likelihood. If model 2 or 3 better fit the data than did model 1, intellectual outcome differed as a function of RT modality. For all analyses, significance was P < .05.
RESULTS
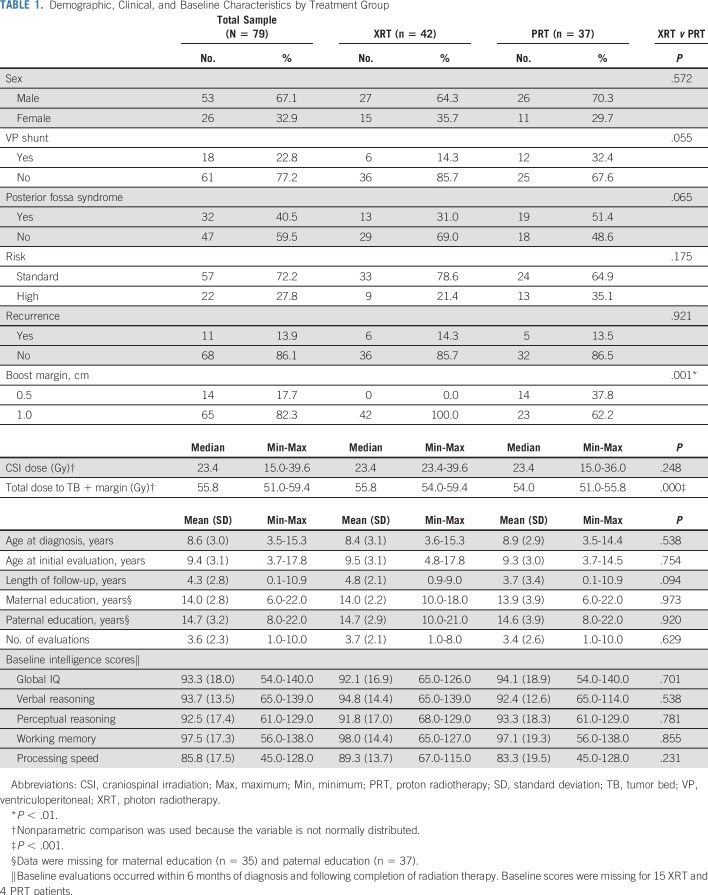
Patient characteristics by RT group are compared in Table 1. As seen in Table 1, the PRT and XRT groups were similar on all clinical and demographic variables, with two exceptions. Median total dose to TB plus margin was higher in the XRT group (55.8 Gy) than in the PRT group (54.0 Gy; U = 345.0; P < .001). In addition, all XRT patients (100.0%) received a TB plus 1.0 cm margin boost; in the PRT group, 62.2% of patients received a TB plus 1.0 cm margin boost, whereas the remaining 37.8% received a TB plus 0.5 cm boost (χ2(1) = 19.3, P = .001). Although statistically significant, the mean differences between groups in boost dose and margin were small (1.3 Gy and 0.2 cm, respectively). The RT groups did not differ on unadjusted intellectual scores at baseline. Although broadly within normal limits, group means fell at the lower end of that range across most domains at baseline.
TABLE 1.
Demographic, Clinical, and Baseline Characteristics by Treatment Group
For each intelligence domain, we examined three models to compare intellectual change over time by RT group. Clinical variables that differed between groups at baseline (ie, boost dose, margin size; Table 1) were included as covariates. Although it was not statistically significant between groups, we included a history of posterior fossa syndrome (PFS) as a covariate because of the known contribution of this postoperative complication to intellectual trajectories and because the proportion of patients with PFS was marginally different between RT groups (P = .065). Test measure (Woodcock-Johnson v Wechsler) was also included as a covariate.
Parameters for the best-fitting models are presented in Table 2 as a function of each intelligence score. (Parameters for all models are presented in Appendix Table A2, online only). Figures 1A to 1E illustrate change in intelligence scores over time since diagnosis by RT group.
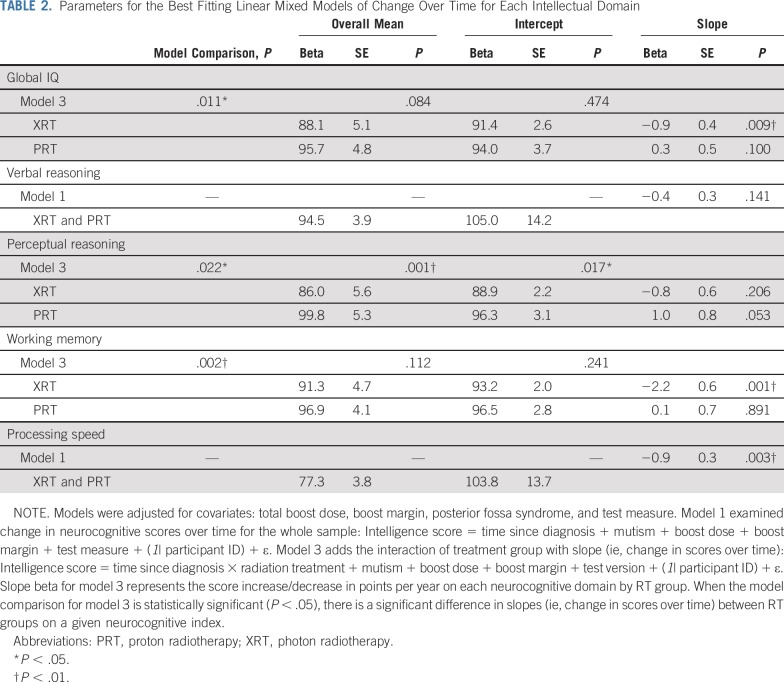
TABLE 2.
Parameters for the Best Fitting Linear Mixed Models of Change Over Time for Each Intellectual Domain
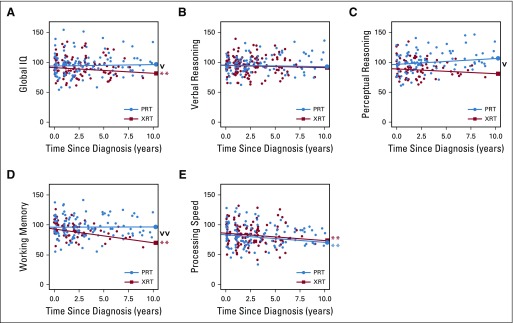
FIG 1.
Change in outcomes scores over time since diagnosis by proton radiotherapy (PRT) and photon radiotherapy (XRT). (A) Global intelligence quotient (IQ). (B) Verbal reasoning. (C) Perceptual reasoning. (D) Working memory. (E) Processing speed. Bold lines are adjusted slopes showing change in neurocognitive scores over time since diagnosis as a function of treatment group. (v) Difference in slopes between PRT and XRT (P < .05). (**) Global IQ, working memory, and processing speed decline (P < .01). (vv) Difference in slopes between PRT and XRT (P < .01).
PRT is associated with stable global IQ scores over time, whereas XRT is associated with significant global IQ decline (Table 2, Fig 1A). For global IQ, the model examining the interaction of treatment with slope (model 3) significantly improved the goodness of fit versus the other models (χ2(1) = 6.9, P = .011). Mean global IQ scores across the entire model were not significantly different between groups (t(73) = −1.7, P = .084), nor was baseline (intercept) performance (t(81) = 2.7, P = .474). Notably, change in global IQ over time (ie, slope) differed between RT groups (t(170) = 2.6, P = .011). Patients treated with PRT showed stable global IQ over time since diagnosis. In contrast, patients treated with XRT lost a statistically significant 0.9 global IQ points per year on average (P = .009).
The PRT and XRT groups did not differ on verbal reasoning scores (Table 2, Fig 1B). For verbal reasoning, neither the model that included the impact of treatment on the intercept (model 2) nor the model that included the interaction of treatment with slope (model 3) improved the goodness of fit versus the base model (model 1; χ2s(1) > 3.8, all p > .05), indicating that there were no significant differences in overall mean scores, baseline performance, or slope between the two groups. It is notable that the model that included the impact of treatment on the intercept approached significance (P = .052) in terms of better fit than did the base model, suggesting possible differences in baseline performance for verbal reasoning scores.
PRT is associated with overall higher and marginally improved perceptual reasoning scores over time compared with XRT (Table 2, Fig 1C). For perceptual reasoning, the model examining the interaction of treatment with slope (model 3) significantly improved the goodness of fit versus the other models (χ2(1) = 5.0, P = .022). Mean perceptual reasoning scores across the entire model were higher for patients in the PRT group compared with those in the XRT group (t(73) = −3.57, P = .001). Adjusted for covariates and model predictors, baseline (intercept) performance for perceptual reasoning also differed between the groups (t(71) = 2.42, P = .017). Change in perceptual reasoning scores over time (ie, slope) differed significantly between RT groups (t(139)=2.31, P = .022). Perceptual reasoning scores increased (improved) by 1.0 points per year on average among patients in the PRT group, although this change was only marginally significant (P = .053). The 0.8 points per year decline observed in the XRT group did not reach statistical significance (P = .206).
PRT is associated with stable working memory, whereas XRT is associated with decline in working memory over time (Table 2, Fig 1D). For working memory, the model examining the interaction of treatment with slope (model 3) significantly improved the goodness of fit versus the other models (χ2(1) = 9.8, P = .002). Mean working memory scores across the entire model did not differ between RT groups (t(74) = −1.59, P = .112), nor did baseline (intercept) performance (t(70) = 1.18, P = .241). Change in working memory scores over time (ie, slope) differed significantly between the two RT groups (t(162) = 3.2, P = .002). Patients treated with PRT exhibited stable working memory scores over time since diagnosis (P = .891). In contrast, working memory scores declined by 2.2 points per year on average among patients in the XRT group (P = .001).
Both PRT and XRT are associated with declines in processing speed over time (Table 2, Fig 1E). For processing speed, neither the model that included the impact of treatment on the intercept (model 2) nor the model that included the interaction of treatment with slope (model 3) improved the goodness of fit versus the base model (model 1; χ2s(1) > 0.58, all p < .44), indicating no significant differences in overall mean scores, baseline performance, or slope between the two groups. Notably, a significant decline in processing speed scores of 0.90 points per year on average was observed in both RT groups (t(200) = 12.96, P = .003).
Neither boost dose nor margin size contributed significantly to any models (all P > .10). All models were reassessed including only SJMB03 patients (n = 65), ensuring comparable chemotherapy, boost dose, and boost margin between groups. Consistent with findings in the combined sample, results revealed the same pattern of better working memory and perceptual reasoning outcomes after PRT versus XRT (all p < .04), as well as no difference between groups in processing speed (P = .50). In contrast to the combined sample, verbal reasoning scores were significantly higher in the PRT group compared with the XRT group (P < .05). A statistically significant difference in global IQ was no longer present (P = .40), although group effects were in the same direction as those seen in the combined sample.
Test measure was a significant covariate in models that included both Wechsler and Woodcock-Johnson scores (all P < .05). Notably, all models tested above were adjusted for test measure.
PFS is a clinically impactful complication, independent of RT modality, as evidenced across most models. PFS was associated with lower scores at baseline (intercept) for global IQ (9.3 points, t(76) = −2.7, P = .008), perceptual reasoning (12.2 points, t(68) = −3.7, P = .001), working memory (13.6 points, t(67) = −4.54, P = 0.001), and processing speed (17.0 points, t(73) = −5.4, P = .001). Notably, baseline verbal reasoning scores were minimally influenced by PFS in this cohort (5.2 points, P > .05).
DISCUSSION
In what we believe to be the first longitudinal study comparing intellectual outcomes between patients treated with PRT versus XRT for pediatric medulloblastoma, PRT was associated with a sparing of function not observed with XRT. Change in global IQ, perceptual reasoning, and working memory scores differed significantly between the PRT and XRT groups. At 4 years after RT, on average, patients treated with PRT exhibited stable performance over time in all domains with the exception of processing speed. In contrast, patients treated with XRT exhibited a significant decline in global IQ, working memory, and processing speed scores.
There have been decades of interest in the potential neuropsychological sparing benefits of PRT, and our findings offer the most conclusive empirical support to date of such an advantage. Previously, our group compared neurocognitive trajectories in prior XRT and PRT cohorts treated with CSI.5 Because those cohorts included patients with varied histologies, who were treated on varied protocols (eg, full posterior fossa boost, TB plus margin boost), and with different lengths of follow-up between groups, conclusions were not definitive regarding a craniospinal PRT benefit. In contrast, the homogeneity of our current sample in terms of histology, disease and treatment factors, timing of treatment, and socioeconomic status (SES) is a notable strength of the current study that affords more confidence that group differences are attributable to RT modality rather than to other sources of systematic variation.
Despite indications of superior intellectual outcomes with PRT, it is important to note that outcomes identified in our XRT cohort are favorable compared with historical reports (eg, 2-4 IQ points decline per year after XRT).29,30 Modern XRT techniques (ie, intensity-modulated RT, reduced boost volume protocols) seem to have yielded intellectual benefits. However, these benefits still do not seem to be as great as those of PRT.
Importantly, processing speed showed significant vulnerability to CSI regardless of RT modality, consistent with findings in other PRT and XRT samples.3,6,31,32 Processing speed tasks reflect both cognitive efficiency and fine motor functioning. Certainly, cerebellar involvement in medulloblastoma places these patients at risk of motor impairment. Furthermore, diffuse irradiation of healthy brain, which is inherent with CSI, is certain to affect white matter whether treatment involves protons or photons. Although sluggish processing remains a risk, the relative sparing observed in other intellectual domains in the current study suggests the normal brain tissue sparing beyond the TB and margin achieved with a PRT boost still results in measurable intellectual benefit.
PRT is still costly, not always covered by insurance, and not widely available; thus, many patients who receive PRT are of higher SES. This is a particular challenge to comparative research because SES is an established contributor to intellectual functioning, development, and recovery.33,34 Again, the design of the current study is advantageous because our RT groups were defined by the standard of care for medulloblastoma at the two involved institutions during the defined timeframe of our investigation. Importantly, because there were no differences between RT groups in terms of parental education, which is an established indicator of SES,35 we were able to avoid this SES confound that has limited PRT-XRT comparison studies attempted in the United States.
Beyond RT modality, PFS emerged as a significant contributor to intellectual function. Interestingly, verbal reasoning was the intellectual domain least affected by PFS, surprising given that postoperative mutism is a hallmark symptom of this syndrome. Although acute symptoms (such as mutism) may abate relatively quickly for some patients, it is increasingly clear that the experience of PFS carries significant, lasting, and broad intellectual risk.6,36 A clinically critical line of research lies in better understanding the factors that lead to the development of PFS and in the discovery of strategies to prevent and reverse the symptoms of and the impairment related to PFS. Of note, verbal reasoning is conceptualized as a crystallized ability, which is generally less susceptible to injury37 than fluid intelligence, possibly explaining the observed relative sparing in this domain.
Although this study heralds notable methodologic advances over previous PRT-XRT comparisons, study limitations must be considered. Random assignment to RT modality may be both impractical and unethical given the popular awareness of PRT in the United States and the strong preliminary support for the superiority of PRT in terms of healthy tissue sparing and the reduction of secondary malignancies and other late effects.38 Even so, because our RT groups were defined by standard of care at the two participating institutions, we were able to achieve comparison groups treated on comparable protocols. The use of different tests across evaluations introduced variance. Although we adjusted for test measure as a covariate in our models, future comparative research should use uniform measurement and a longer follow-up. There were small but statistically significant differences between groups on boost dose and margin. Still, the observed PRT benefit does not seem to be a result of these differences (ie, boost dose/margin did not account for significant variance in our models; most model results were similar when tested on the combined sample or the SJMB03-only subsample).
To our knowledge, this study provides the strongest evidence to date demonstrating an intellectual advantage with PRT in the treatment of pediatric medulloblastoma. Even in the context of CSI, patients treated with PRT exhibited stable intellectual outcomes in most domains and experienced significantly better long-term outcomes in global IQ, perceptual reasoning, and working memory compared with patients treated with XRT. Even so, CSI remains a consistent risk factor for processing speed decline, regardless of RT modality. Neurocognitive sparing did not come at the expense of disease control, with both groups experiencing comparable rates of relapse. Given growing awareness of the sparing potential of PRT among physicians, patients, and families, additional comparison research with larger samples and longer follow-up is needed to guide treatment decisions and cost-benefit decision making. Neuropsychological surveillance, as recommended in the Children’s Oncology Group survivorship guidelines,39 remains essential for pediatric patients with brain tumor treated with RT, regardless of modality. Still, these findings provide compelling evidence that pediatric medulloblastoma should be treated with PRT when available.
APPENDIX
TABLE A1.
Test Type/Version Across Evaluations by Treatment Group
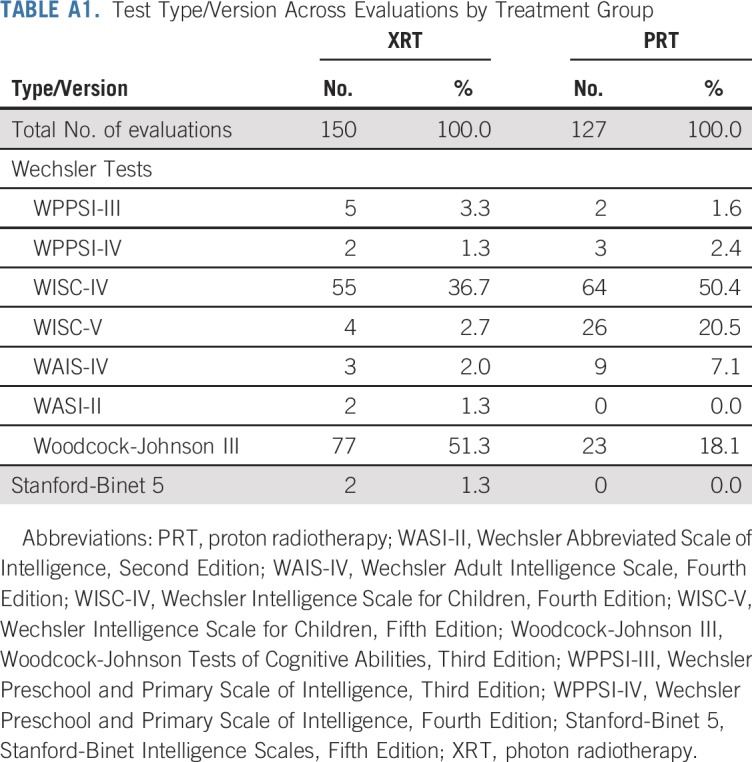
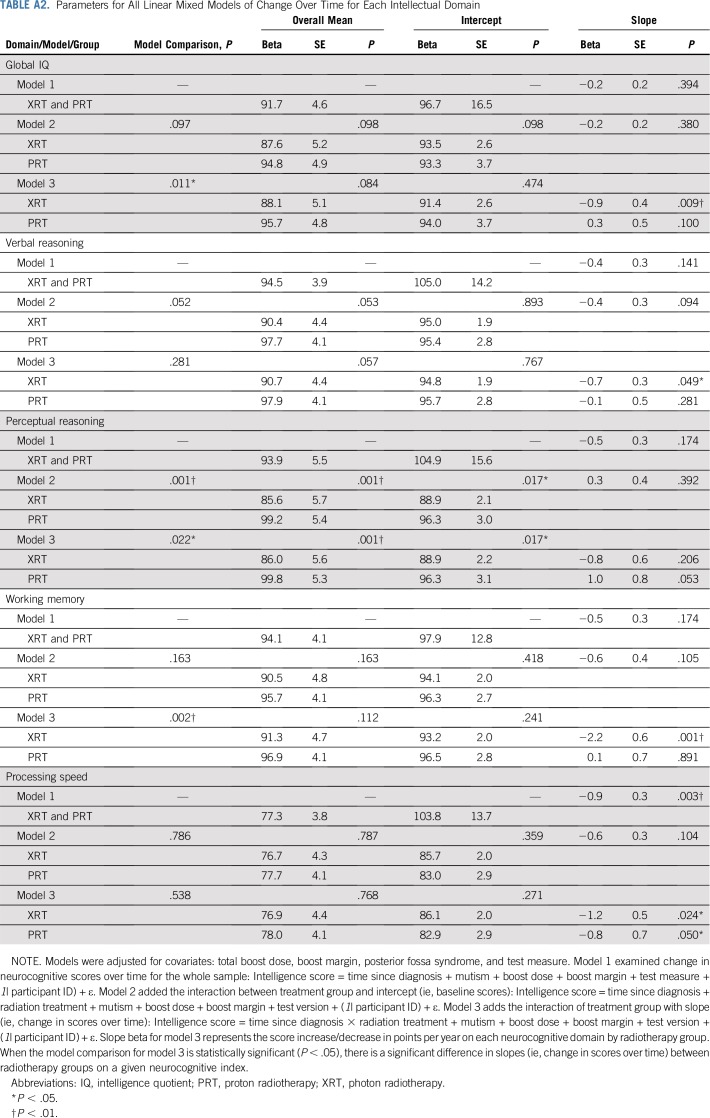
TABLE A2.
Parameters for All Linear Mixed Models of Change Over Time for Each Intellectual Domain
Footnotes
Supported by the National Institutes of Health/National Cancer Institute (R01CA187202 and K07CA157923 to L.S.K.) and the Canadian Institute of Health Research (MOP-123537 to D.M.).
AUTHOR CONTRIBUTIONS
Conception and design: Lisa S. Kahalley, Rachel Peterson, M. Douglas Ris, Anita Mahajan, William E. Whitehead, Murali Chintagumpala, Eric Bouffet, Donald Mabbott
Provision of study material or patients: Lisa S. Kahalley, M. Douglas Ris, Laura Janzen, M. Fatih Okcu, David R. Grosshans, Vijay Ramaswany, Arnold C. Paulino, David Hodgson, Derek S. Tsang, Normand Laperriere, Michael D. Taylor, Murali Chintagumpala, Eric Bouffet, Donald Mabbott
Collection and assembly of data: Lisa S. Kahalley, Rachel Peterson, M. Douglas Ris, Laura Janzen, M. Fatih Okcu, David R. Grosshans, Murali Chintagumpala, Donald Mabbott
Data analysis and interpretation: Lisa S. Kahalley, Rachel Peterson, M. Douglas Ris, M. Fatih Okcu, David R. Grosshans, Anita Mahajan, William E. Whitehead, Robert C. Dauser, Heather M. Conklin, Murali Chintagumpala, Eric Bouffet, Donald Mabbott
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Superior Intellectual Outcomes After Proton Radiotherapy Compared With Photon Radiotherapy for Pediatric Medulloblastoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Arnold C. Paulino
Employment: MD Anderson Cancer Center
Patents, Royalties, Other Intellectual Property: Royalty from Elsevier for book on positron emission tomography/computed tomography in radiotherapy treatment planning
Travel, Accommodations, Expenses: Henry Ford Hospital, University of Southern California
Derek S. Tsang
Other Relationship: Varian Medical Systems (Inst), Mevion Medical Systems (Inst), Hitachi (Inst), RaySearch Laboratories (Inst), IBA (Inst), ProTom (Inst)
Normand Laperriere
Honoraria: Merck/Schering Plough
Consulting or Advisory Role: AbbVie
Eric Bouffet
Research Funding: Roche (Inst), Bristol-Myers Squibb (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 3.Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32:1760–1768. doi: 10.1200/JCO.2013.52.3290. [DOI] [PubMed] [Google Scholar]
- 4.Yock TI, Tarbell NJ. Technology insight: Proton beam radiotherapy for treatment in pediatric brain tumors. Nat Clin Pract Oncol. 2004;1:97–103. doi: 10.1038/ncponc0090. [DOI] [PubMed] [Google Scholar]
- 5.Kahalley LS, Ris MD, Grosshans DR, et al. Comparing Intelligence Quotient Change After Treatment With Proton Versus Photon Radiation Therapy for Pediatric Brain Tumors. J Clin Oncol. 2016;34:1043–9. doi: 10.1200/JCO.2015.62.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahalley LS, Douglas Ris M, Mahajan A, et al. Prospective, longitudinal comparison of neurocognitive change in pediatric brain tumor patients treated with proton radiotherapy versus surgery only. Neuro Oncol. 2019;21:809–818. doi: 10.1093/neuonc/noz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross JP, Powell S, Zelko F, et al. Improved neuropsychological outcomes following proton therapy relative to x-ray therapy for pediatric brain tumor patients. Neuro Oncol. 2019 doi: 10.1093/neuonc/noz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. The Lancet Oncology. 2016;17:287–298. doi: 10.1016/S1470-2045(15)00167-9. [DOI] [PubMed] [Google Scholar]
- 9.Pulsifer MB, Sethi RV, Kuhlthau KA, et al. Early Cognitive Outcomes Following Proton Radiation in Pediatric Patients With Brain and Central Nervous System Tumors. Int J Radiat Oncol Biol Phys. 2015;93:400–7. doi: 10.1016/j.ijrobp.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonini TN, Ris MD, Grosshans DR, et al. Attention, processing speed, and executive functioning in pediatric brain tumor survivors treated with proton beam radiation therapy. Radiother Oncol. 2017;124:89–97. doi: 10.1016/j.radonc.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wechsler D. Wechsler Adult Intelligence Scale. Fourth Edition. San Antonio, TX: NCS Pearson, Inc; 2008. [Google Scholar]
- 12.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. Fourth Edition. San Antonio, TX: NCS Pearson, Inc.; 2012. [Google Scholar]
- 13.Wechsler D. Wechsler Intelligence Scale for Children. Fifth Edition. Bloomington, MD: NCS Pearson, Inc.; 2014. [Google Scholar]
- 14.Wechsler D. Wechsler Intelligence Scale for Children. Fourth Edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 15.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Second Edition. San Antonio, TX: NCS Pearson; 2011. (WASI-II) [Google Scholar]
- 16.Wechsler D. The Wechsler Preschool and Primary Scale of Intelligence. Third Edition. San Antonio, TX: The Psychological Corporation; 2002. (WPPSI-III) [Google Scholar]
- 17.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Cognitive Abilities. Itasca, IL: The Riverside Publishing Company; 2001. [Google Scholar]
- 18.Roid GH. Stanford-Binet Intelligence Scales. Fifth Edition. Itasca, IL: Riverside Publishing; 2003. [Google Scholar]
- 19.Wechsler D. Wechsler Intelligence Scale for Children - Fourth Edition Technical and Interpretive Manual. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 20.Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition Technical and Interpretive Manual. San Antonio, TX: NCS Pearson, Inc; 2008. [Google Scholar]
- 21.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Third Edition Technical Manual. San Antonio: Pearson; 2002. [Google Scholar]
- 22.Wechsler D. Wechsler Intelligence Scale for Children- Fifth Edition Technical and Interpretive Manual. Bloomington, MN: NCS Pearson, Inc.; 2014. [Google Scholar]
- 23.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence - Fourth Edition Technical and Interpretive Manual. San Antonio, TX: NCS Pearson, Inc.; 2012. [Google Scholar]
- 24.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Manual. Second Edition. San Antonio, TX: Psychological Corporation; 2011. [Google Scholar]
- 25.McGrew KS, LaForte EM, Schrank FA.Technical Manual. Woodcock-Johnson IV Rolling Meadows IL; Riverside2014 [Google Scholar]
- 26.McGrew KS, Woodcock RW. Technical Manual Woodcock-Johnson III. Itasca. IL: Riverside Publishing; 2001. [Google Scholar]
- 27.Phelps L, McGrew KS, Knopik SN, et al. The general (g), broad, and narrow CHC stratum characteristics of the WJ III and the WISC III tests: A confirmatory cross battery investigation. School Psychology Quarterly. 2005;20:66–88. [Google Scholar]
- 28.Standardization data from the Wechsler Intelligence Scale for Children. Fifth Edition (WISC-V), Copyright © 2014 NCS Pearson, Inc. Used with permission. All rights reserved.
- 29.Palmer SL, Gajjar A, Reddick WE, et al. Predicting intellectual outcome among children treated with 35-40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17:548–555. doi: 10.1037/0894-4105.17.4.548. [DOI] [PubMed] [Google Scholar]
- 30.Ris MD, Packer R, Goldwein J, et al. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 2001;19:3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 31.Kahalley LS, Conklin HM, Tyc VL, et al. Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psychooncology. 2013;22:1979–1986. doi: 10.1002/pon.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31:3494–500. doi: 10.1200/JCO.2012.47.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoff E. The specificity of environmental influence: socioeconomic status affects early vocabulary development via maternal speech. Child Dev. 2003;74:1368–78. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- 34.Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10:464–80. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 35.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294:2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 36.Schreiber JE, Palmer SL, Conklin HM, et al. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional, prospective study. Neuro Oncol. 2017;19:1673–1682. doi: 10.1093/neuonc/nox135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ris MD, Grosch M, Fletcher JM, et al. Measurement of neurodevelopmental changes in children treated with radiation for brain tumors: what is a true ‘baseline?’. Clin Neuropsychol. 2017;31:307–328. doi: 10.1080/13854046.2016.1216070. [DOI] [PubMed] [Google Scholar]
- 38.Mohan R, Grosshans D. Proton therapy - Present and future. Adv Drug Deliv Rev. 2017;109:26–44. doi: 10.1016/j.addr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Children’s Oncology Group Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancer: Version 5.0, 2018. http://www.survivorshipguidelines.org/