Abstract
Objectives
To compare fluorescence in situ hybridization (FISH) and a commercially available sequencing assay for comprehensive genomic profiling (CGP) to determine the best approach to identify gene rearrangements (GRs) in large B-cell lymphomas (LBCLs).
Methods
Comparison of standard-of-care FISH assays (including a two-probe approach for MYC; break-apart and fusion probes) and an integrated genomic DNA/RNA sequencing CGP approach on a set of 69 consecutive LBCL cases.
Results
CGP detected GRs, including those involving MYC (1), BCL-2 (3), and BCL-6 (3), not detected by FISH. FISH detected non–IgH-MYC (4) and BCL-6 (2) GRs that were not detected by CGP. In four instances, standalone CGP or FISH testing would have missed a double-hit lymphoma.
Conclusions
CGP was superior to FISH in the detection of IgH-MYC rearrangements but was inferior for the detection of non–IgH-MYC rearrangements. Our study demonstrates the rationale for development of a customized approach to identify GRs in LBCLs.
Keywords: High-grade B-cell lymphoma, FISH, MYC, Sequencing, Comprehensive genomic profiling, Gene rearrangements, Prognosis
Large B-cell lymphomas (LBCLs) are a biologically complex group of hematolymphoid neoplasms whose molecular profile has prognostic implications. This fact is reflected in the current World Health Organization (WHO) classification scheme, in which LBCLs can be further classified into diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS); high-grade B cell lymphoma (HGBL), NOS; and HGBL with MYC and BCL-2 and/or BCL-6 rearrangements, or so-called double- and triple-hit lymphomas.1 HGBLs are usually more aggressive than their DLBCL, NOS counterparts and are now commonly being treated with more aggressive treatment regimens,2 making the distinction between these categories clinically significant.1,3-5
Fluorescence in situ hybridization (FISH) is the current gold standard for detecting rearrangements in LBCLs.1 There are various options when it comes to which FISH probes to use, and the choice of probe alters the sensitivity for detection depending on the breakpoint in a given case. This difference has previously been discussed with regard to various MYC probes.6,7 In theory, this limitation should be abrogated by the technology used in comprehensive genomic profiling (CGP) studies. Given that CGP studies have recently become accessible for routine clinical use, the use of standalone CGP in the detection of these rearrangements could be a cost-effective alternative to FISH testing while providing additional mutational data that have prognostic and therapeutic significance. However, experimental data comparing the sensitivity of CGP and FISH for detection of clinically important rearrangements in LBCL are lacking. With the emergence of the availability of CGP in routine clinical practice, such data must be compiled to determine the most sensitive and clinically relevant testing algorithm for LBCLs.
This study was performed to produce data to guide testing algorithms for detection of MYC, BCL-2, and BCL-6 rearrangements in LBCLs. We did this by performing a commercially available CGP approach and standard-of-care FISH studies (including a two-probe approach for MYC; break-apart and fusion probes) on a set of consecutive LBCL cases. Our results provide a rationale for development of a customized sequencing approach to detect gene rearrangements in LBCLs.
Materials and Methods
Study Group
Data were collected from 69 consecutive LBCL cases on which FISH and CGP studies were performed during routine clinical care. Cases classified as B‐lymphoblastic leukemia/lymphoma (terminal deoxynucleotidyl transferase [TdT]+) and blastoid variant mantle cell lymphoma (cyclin D1+, t(11;14)(q13;q32)+) were excluded. Clinical data, including age, nodal/extranodal site of involvement, germinal center/non–germinal center immunophenotype as per the Hans algorithm, and double expressor status, were obtained from our electronic medical record.
Immunohistochemical Studies
Immunohistochemical stains had been performed as part of the diagnostic workup in individual cases and were reviewed for this study. Immunohistochemical stains were performed using formalin-fixed, paraffin-embedded tissue sections and an automated immunostainer (DAKO). All tissue sections underwent heat-induced antigen retrieval. Antibodies used were CD20, CD3, CD5, CD10, BCL-6, MUM-1, BCL-2, MYC, Ki-67, CD19, and CD30 (Leica Biosystems). The presence of Epstein-Barr virus was detected using an Epstein-Barr virus-encoded small RNA fluorescein-conjugated oligonucleotide probe supplied in hybridization solution (Leica Biosystems).
FISH
FISH was performed at our institution using formalin‐fixed, paraffin‐embedded tissue sections and the protocols recommended by the manufacturers for each of the following probes: MYC break‐apart probe (Abbott Molecular/Vysis), BCL-6 break-apart probe (Abbott Molecular/Vysis), and dual‐color fusion probes for IGH-MYC and IGH‐BCL2 (Abbott Molecular/Vysis). A total of 200 interphase nuclei were analyzed for each probe in areas of interest and stored using the Applied Imaging/Cytovision system. A neoplasm assessed by using fixed, paraffin‐embedded tissue sections was considered positive if observed signals were clearly more than 10% for IGH-BCL2 and IgH-MYC fusion and more than 15% for BCL6 and MYC break-apart probes, based on validation studies performed in our laboratory. These cutoffs are the result of the cytogenetic laboratory validation of FISH probes in tissue sections for clinical use (because of the known difficulty in providing an exact count in tissue sections in which nuclei are in or out of the plane of section). Importantly, FISH assays were performed on unstained slides collected from the same blocks used for CGP studies.
CGP
Integrated genomic RNA/DNA profiling was performed at Foundation Medicine using the FoundationOne Heme assay (Foundation Medicine). This hybrid capture approach uses massive parallel sequencing on the Illumina HiSeq 2500 according to their previously published method.8 Formalin-fixed, paraffin-embedded tissue sections were sent to Foundation Medicine for analysis. A total of 306 genes were interrogated for genomic alterations by DNA, and 265 genes were interrogated for gene fusions by RNA using a hybrid-capture, next-generation sequencing approach. All tissue submitted for this study contained tumor cells that were 20% or more of the nucleated cells present in the samples. The genomic positions of the MYC breakpoints were mapped using the human genome browser (hg19 assembly) available at https://genome.ucsc.edu/cgi-bin/hgGateway. The specific coordinates of regions baited in the IgH, MYC, BCL-2, and BCL-6 genes with this CGP platform were previously reported.8
Results
The population studied had an average age of 60 years (range, 33-92 years). The sex distribution was 31 (45%) women and 38 (55%) men. Most biopsy specimens (n = 41; 59%) were from extranodal sites, with the remainder (n = 28; 41%) being nodal in location. Sixty-four percent (n = 44) of cases were classified as germinal center immunophenotype and 36% (n = 25) of cases were non–germinal center cell type by Hans algorithm. Forty-five percent (n = 30) of cases were double expressors for MYC and BCL-2, and 55% (n = 39) were not double expressors by immunohistochemistry as defined by BCL-2 expression in at least 50% of all lymphoma cells and MYC expression in at least 40% of all lymphoma cells, as per WHO criteria.1
DNA/RNA profiling studies detected a wide variety of genetic alterations in various genes. The most common mutations detected in this cohort were alterations in BCL-2, BCL-6, MLL2, TP53, and CREBBP. Overall, CGP detected 21 BCL-2 rearrangements, 20 BCL-6 rearrangements, five IgH-MYC rearrangements, and two non–IgH-MYC rearrangements. These non–IgH-MYC rearrangements both were rearrangements involving MYC intron 1. All BCL-2 rearrangements detected involved IgH. Half of the BCL-6 rearrangements (10/20) involved the IgH locus; other translocation partners included IGL, IKZF1, RHOH, SNHG1, IGK, HSP90AA1, and TRA2B.
FISH detected 18 BCL-2 rearrangements, 19 BCL-6 rearrangements, four IgH-MYC rearrangements, and six non–IgH-MYC rearrangements. These data are compared with those of CGP in Table 1. Note that for BCL6, a total of 22 rearrangements were identified using both assays and that neither assay alone could identify all 22 of these.
Table 1.
Overall Sensitivities of FISH and CGP in the Detection of MYC, BCL-2, and BCL-6 Gene Rearrangements
| No. (%) of Positive Cases | ||
|---|---|---|
| Genes Involved in the Rearrangement | FISH | CGP |
| IgH-MYC | 4/5 (80) | 5/5 (100) |
| Non–IgH-MYC | 6/6 (100) | 2/6 (33) |
| IgH-BCL-2 | 18/21 (86) | 21/21 (100) |
| BCL-6 | 19/22 (86) | 20/22 (91) |
CPG, comprehensive genomic profiling; FISH, fluorescence in situ hybridization.
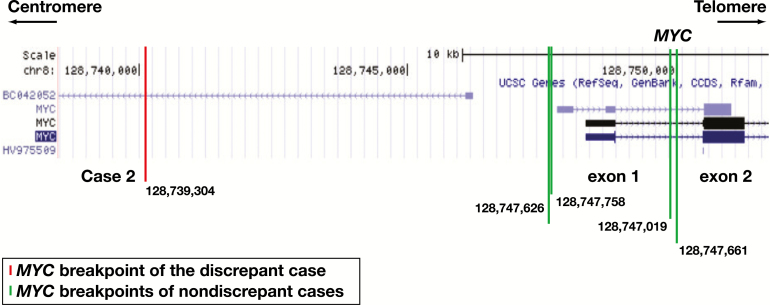
Of the 69 cases studied, 12 (17%) cases had discordant CGP and FISH results; these cases are delineated in Table 2. Note that in three cases (4, 7, and 10), BCL-6 gene rearrangements were detected by CGP but not FISH and in two cases (5 and 11) were detected by FISH but not CGP. Non–IgH-MYC rearrangements were detected in four cases (1, 3, 8 and 11) by FISH but not CGP. Case 2 demonstrates an instance in which CGP detected an IgH-MYC rearrangement that was not detected by FISH. A comparison between the location of the breakpoints within the MYC gene for discordant and concordant cases is show in Figure 1. Cases 6, 9, and 12 harbored IgH-BCL-2 rearrangements that were detected by CGP alone, while FISH did not detect any IgH-BCL-2 rearrangements not found by CGP. Cases that demonstrated discrepant results were reviewed at Foundation One and in-house confirming the reported results in all cases.
Table 2.
Translocations and Rearrangements Detected by FISH and CGP in Cases With Discordant Resultsa
| Case No. | Translocations and Genes Rearrangement (FISH) | Translocations and Genes Rearrangement (CGP) |
|---|---|---|
| 1 | Non–IgH-MYC and IgH-BCL-2 | IgH-BCL-2 |
| 2 | IgH-BCL-2 | IgH-BCL-2 and IgH-MYC |
| 3 | Non–IgH-MYC | None |
| 4 | IgH-BCL-2 | IgH-BCL-2 and BCL-6 |
| 5 | BCL-6 | None |
| 6 | BCL-6 | IgH-BCL-2 and BCL-6 |
| 7 | None | BCL-6 |
| 8 | Non–IgH-MYC | None |
| 9 | IgH-MYC | IgH-MYC and IgH-BCL-2 |
| 10 | None | BCL-6 |
| 11 | Non–IgH-MYC and BCL-6 | None |
| 12 | None | IgH-BCL-2 |
CPG, comprehensive genomic profiling; FISH, fluorescence in situ hybridization.
aThose depicted in bold are cases in which the discrepancy would have led to misclassification of the lymphoma as the diagnosis of high-grade B cell lymphoma with MYC and BCL-2 or BCL-6 gene rearrangements would not be rendered.
Figure 1.
Mapping of the MYC breakpoints at 8q24.21 (MYC locus) detected by the comprehensive genomic profiling (CGP) assay. The breakpoint depicted by the red line represents a rearrangement detected only by CGP, whereas breakpoints depicted in green represent those that were detected by both CGP and fluorescence in situ hybridization (FISH) studies. The breakpoint localization is indicated at the bottom of each line. Note the far centromeric location of the breakpoint not detected by the FISH assay. Only exons 1 and 2 of MYC are shown. The data were generated using UCSC Genome Browser on human (GRCh37/h19) assembly (https://genome.ucsc.edu/).
A comparison of the sensitivities of NGS and FISH for each rearrangement is shown in Table 1. The sensitivities of FISH in detecting IgH-MYC, non–IGH-MYC, IgH-BCL-2, and BCL-6 rearrangements were 80%, 100%, 86%, and 86%, respectively. The sensitivities of NGS in detecting IgH-MYC, non–IGH-MYC, IgH-BCL-2, and BCL-6 rearrangements were 100%, 33%, 100%, and 91%, respectively.
Interestingly, only two (33%) of the six “double-hit” lymphomas in this cohort were also double expressors by immunohistochemistry (IHC), as defined by the WHO criteria delineated above. Conversely, 28 (93%) of the 30 double expressors by IHC were not double-hit lymphomas when analyzed by a combined CGP and FISH approach. Table 3 shows the IHC results for MYC, BCL-2, and BCL-6 in cases with discrepant results by CGP and FISH.
Table 3.
Expression of MYC, BCL-2, and BCL-6 as Detected by Immunohistochemical Studies for Each of the Discrepant Cases
| Case No. | MYC Expression, % | BCL-2 Expression, % | Double Expressor MYC and BCL2 | BCL-6 Expression, % | Double Hit |
|---|---|---|---|---|---|
| 1 | 80 | <10 | No | >30 | Yes |
| 2 | 90 | >50 | Yes | >30 | Yes |
| 3 | 80 | 100 | Yes | >30 | No |
| 4 | 10 | 90 | No | 60 | No |
| 5 | 60 | 90 | Yes | 20 | No |
| 6 | 10 | >50 | No | >30 | No |
| 7 | 60 | 80 | Yes | >30 | No |
| 8 | 80 | 70 | Yes | >30 | No |
| 9 | 60 | >50 | Yes | >30 | Yes |
| 10 | 70 | >50 | Yes | >30 | No |
| 11 | 40 | >50 | Yes | >30 | Yes |
| 12 | >90 | >90 | Yes | <30 | No |
Discussion
The current classification and treatment approach in LBCLs hinges greatly on the ability to detect clinically significant rearrangements involving MYC, BCL-2, and BCL-6. Except for tumors with blastic morphology, the presence of MYC and BCL-2 and/or BCL-6 rearrangements can significantly alter the classification of LBCLs from DLBCL, NOS to HGBL with MYC and BCL-2 and/or BCL-6 rearrangements in the current WHO classification scheme.1 Determining the appropriate classification is important due to the poor prognosis of double- and triple-hit lymphomas and their requirement for escalated chemotherapeutic approaches.1,3-5
To date, FISH has been considered the gold-standard assay for detection of these gene rearrangements.1 However, it has been documented that the use of different FISH probe sets alters the sensitivity of detection for various translocation breakpoints, particularly for breakpoints that are more centromeric in location. This has been best described for MYC FISH probes.6,7 The recent emergence of clinically accessible comprehensive genomic profiling methods has increased the frequency at which CGP is performed in clinical practice. Evaluation of hematopoietic neoplasms, specifically LBCLs, for recurrent mutations is rapidly becoming a necessity for optimal classification, prognostication, and management.9,10 This trend is expected to continue as additional druggable mutations and immunotherapy options are developed. As a result, in our current practice and in accordance with our clinicians’ requests, we currently perform both FISH and CGP on all newly diagnosed LBCLs. We recognize that this approach is likely not routine at most medical centers and that, with the emergence of CGP, guidelines for testing in these cases need to be established. However, this approach has provided an important study set of clinical cases that has allowed us to directly compare current FISH and CGP assays for detection of MYC, BCL-2, and BCL-6 gene rearrangements.
The value and increasing role of NGS assays is apparent in the increasing number of clinically available assays capable of providing individualized data, including point mutations, chimeric transcripts, and gene expression. Such assays that are currently available include the TruSight RNA fusion panel (Illumina) and FusionPlex Pan-Heme Kit (ArcherDx). These molecular assays allow for the detection of fusions, even without prior knowledge of fusion partners or breakpoints, and provide information regarding relative expression of selected genes (eg, for cell of origin) and mutations over key hotspot regions.11 It is expected that assays such as these will have an increasing role in the evaluation of patients with lymphomas.
The current literature comparing FISH and CGP in the detection of recurrent gene rearrangements is lacking, and to our knowledge, this is the first study to date exploring this comparison.
In our cohort, BCL-2, TP53, BCL-6, MLL2, and CREBBP were the most commonly mutated genes as detected by CGP. The particularly high frequencies of BCL-2 and BCL-6 mutations are in keeping with previous data indicating these to be the genes most commonly mutated in DLBCL.9,10 Given the relatively high proportion of germinal center-type cases present in this cohort, the predominance of BCL-2 mutations correlates well with previous literature.1 A complete list of genomic alterations can be seen in Supplemental Table 1, and a comprehensive table including the demographic data and results for each case studied can be seen in Supplemental Table 2 (all supplemental materials can be found at American Journal of Clinical Pathology online).
Regarding the detection of recurrent gene rearrangements, CGP showed higher sensitivity compared with FISH for the detection of IgH-MYC (100% and 80%, respectively), IgH-BCL2 (100% and 86%, respectively), and BCL-6 (91% and 86%, respectively) translocations. The ability to detect non–IgH-MYC rearrangements was superior using the FISH assay, however, with the sensitivity being 100% for FISH and only 33% for CGP.
Importantly, four cases (6% of the cohort) would have been misclassified as DLBCL, NOS rather than HGBL with MYC and BCL-2 and/or BCL-6 rearrangements if standalone FISH or CGP was used. Of these double-hit cases, two (2.9% of the cohort; 33% of all double hits detected in the cohort) demonstrated non–IgH-MYC translocations detected by FISH that were not detected by CGP. The remaining two potentially misclassified double-hit cases (2.9% the cohort; 33% of all double hits detected in the cohort) showed rearrangements by CGP that were not detected by FISH, including an IgH-MYC and IgH-BCL-2 rearrangement.
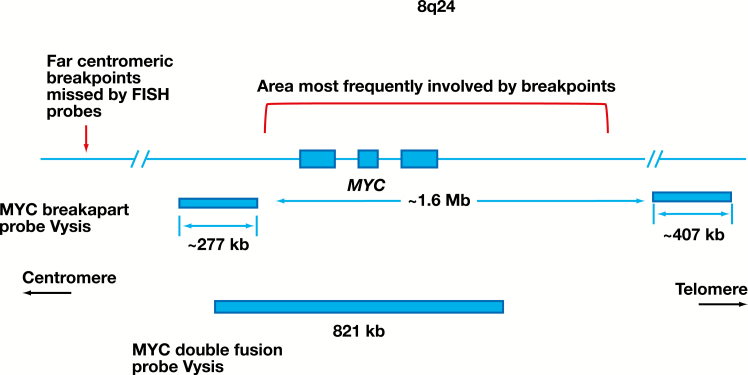
The IgH-MYC rearrangement that was detected by CGP but not by FISH was positioned in a more centromeric location than those that were detected by both CGP and FISH studies, as shown in Figure 1. This finding supports the previously reported notion that far centromeric breakpoints in the MYC gene can be missed by FISH assays using both break-apart and fusion probes.6,7 The distribution of 8q24 breakpoints and the representation of the region covered by the FISH probes are pictographically represented in Figure 2. In addition, both FISH probes would miss cryptic insertions of IgH into the MYC locus and vice versa. Other limitations include cutting artifacts and overlapping of the nuclei in tissue sections that may result in loss and overlap of FISH signals complicating the interpretation of the FISH results. It has been suggested that a dual approach, using a break-apart probe to cover the far telomeric breakpoints together with a double-fusion probe, may maximize detection of MYC gene rearrangements using FISH.6 The coordinates of translocations detected by CGP and missed by the FISH probes used in our workflow are listed in Supplemental Table 2.
Figure 2.
Schematic diagram of the distribution of most frequent breakpoints involving the MYC gene. The covered regions by break-apart and dual-fusion MYC FISH probes are shown. The region where most of the IGH-MYC breakpoints occur is indicated.12-15 Note the lack of coverage for both probe sets at the far centromeric portion of the MYC gene.
While a higher sensitivity of CGP over FISH for detection of most gene rearrangements was seen, a notable exception was the seeming propensity for the integrated DNA/RNA profiling approach used for this study to miss non–IgH-MYC rearrangements, as this approach detected only two (33%) of six of these rearrangements. As recently published,16 some non–IgH-MYC rearrangements are in the large, noncoding region surrounding the MYC gene. Due to the size of this region, it is challenging to capture all possible non–IgH-MYC rearrangements using CGP. Regardless of the breakpoint surrounding MYC, IgH-MYC rearrangements are captured at the IgH locus.
In addition to increased sensitivity for detection of most recurrent translocations in LBCL, another benefit of including CGP studies in the diagnostic algorithm for LBCLs is the additional prognostic and therapeutic information that it might provide in an individual patient. For instance, MYD88 mutations were detected in nine cases in this cohort, which represents patients who might benefit from ibrutinib therapy.17 A PTEN mutation was detected in one case, representing a patient who might benefit from therapy with PI3K inhibitors. EZH2 mutations were detected in 10 cases, which is a population of patients who might benefit from inhibitor therapy.18 In terms of prognostic information, CGP provides data regarding mutations in MYD88, CD79B, BCL-6, NOTCH1, NOTCH2, EZH2, and BCL-2, which have all been shown to correlate with distinct categories of LBCL with prognostic implications.19 The number of each of these prognostically significant mutations detected in this cohort of LBCLs can be found in Supplemental Figure 1.
In summary, either CGP or FISH alone is inadequate to detect all MYC, BCL-2, and BCL-6 gene rearrangements and thus cannot correctly characterize all LBCLs. Currently, a combined approach using CGP, given its superior sensitivity for the detection of most LBCL gene rearrangements (IgH-MYC, BCL-2, and BCL-6) and the additional clinically useful information provided by these studies, and the Vysis MYC break-apart probe, to detect non–IgH-MYC rearrangements missed by CGP, seems to be the most cost-efficient and sensitive method for classifying these neoplasms at this time. Alternatively, to further optimize the cost to clinical impact ratio, an integrated genomic DNA/RNA profiling panel could be developed that is specifically geared toward LBCLs. The development of such a platform seems to be the next logical step to aid in the classification, prognostication, and treatment of these neoplasms.
Theoretically, the ideal platform, in the context of DLBCL, should incorporate a baiting schema that covers all potential breakpoints of the MYC, BCL-2, and BCL-6 genes. After appropriate validation against the current gold standard of FISH testing, this single assay could emerge as more sensitive for detection of clinically significant rearrangements and provide prognostic and targetable mutation information.
Supplementary Material
Dr Vega received funding from the National Cancer Institute, National Institutes of Health (grant R01CA222918).
References
- 1. Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Rev 4th ed. Lyon, France: IARC; 2017. [Google Scholar]
- 2. Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129:280-288. [DOI] [PubMed] [Google Scholar]
- 3. Johnson NA, Savage KJ, Ludkovski O, et al. . Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Gouill S, Talmant P, Touzeau C, et al. . The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica. 2007;92:1335-1342. [DOI] [PubMed] [Google Scholar]
- 5. Li S, Desai P, Lin P, et al. . MYC/BCL6 double-hit lymphoma (DHL): a tumour associated with an aggressive clinical course and poor prognosis. Histopathology. 2016;68:1090-1098. [DOI] [PubMed] [Google Scholar]
- 6. Muñoz-Mármol AM, Sanz C, Tapia G, et al. . MYC status determination in aggressive B-cell lymphoma: the impact of FISH probe selection. Histopathology. 2013;63:418-424. [DOI] [PubMed] [Google Scholar]
- 7. May PC, Foot N, Dunn R, et al. . Detection of cryptic and variant IGH-MYC rearrangements in high-grade non-Hodgkin’s lymphoma by fluorescence in situ hybridization: implications for cytogenetic testing. Cancer Genet Cytogenet. 2010;198:71-75. [DOI] [PubMed] [Google Scholar]
- 8. He J, Abdel-Wahab O, Nahas MK, et al. . Integrated genomic DNA/RNA profiling of hematologic malignancies in the clinical setting. Blood. 2016;127:3004-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmitz R, Wright GW, Huang DW, et al. . Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378:1396-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chapuy B, Stewart C, Dunford AJ, et al. . Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24:679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim B, Lee H, Shin S, et al. . Clinical evaluation of massively parallel RNA sequencing for detecting recurrent gene fusions in hematologic malignancies. J Mol Diagn. 2019;21:163-170. [DOI] [PubMed] [Google Scholar]
- 12. Joos S, Falk MH, Lichter P, et al. . Variable breakpoints in Burkitt lymphoma cells with chromosomal t(8;14) translocation separate c-myc and the IgH locus up to several hundred kb. Hum Mol Genet. 1992;1:625-632. [DOI] [PubMed] [Google Scholar]
- 13. Joos S, Haluska FG, Falk MH, et al. . Mapping chromosomal breakpoints of Burkitt’s t(8;14) translocations far upstream of c-myc. Cancer Res. 1992;52:6547-6552. [PubMed] [Google Scholar]
- 14. Busch K, Keller T, Fuchs U, et al. . Identification of two distinct MYC breakpoint clusters and their association with various IGH breakpoint regions in the t(8;14) translocations in sporadic Burkitt-lymphoma. Leukemia. 2007;21:1739-1751. [DOI] [PubMed] [Google Scholar]
- 15. Einerson RR, Law ME, Blair HE, et al. . Novel FISH probes designed to detect IGK-MYC and IGL-MYC rearrangements in B-cell lineage malignancy identify a new breakpoint cluster region designated BVR2. Leukemia. 2006;20:1790-1799. [DOI] [PubMed] [Google Scholar]
- 16. Chong LC, Ben-Neriah S, Slack GW, et al. . High-resolution architecture and partner genes of MYC rearrangements in lymphoma with DLBCL morphology. Blood Adv. 2018;2:2755-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee JH, Jeong H, Choi JW, et al. . Clinicopathologic significance of MYD88 L265P mutation in diffuse large B-cell lymphoma: a meta-analysis. Sci Rep. 2017;7:1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bohers E, Mareschal S, Bertrand P, et al. . Activating somatic mutations in diffuse large B-cell lymphomas: lessons from next generation sequencing and key elements in the precision medicine era. Leuk Lymphoma. 2015;56:1213-1222. [DOI] [PubMed] [Google Scholar]
- 19. Schmitz R, Wright GW, Huang DW, et al. . Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378:1396-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.