Abstract
Tryptophan hydroxylase (TPH) activity was detected in cultured epidermal melanocytes and dermal fibroblasts with respective Km of 5.08 and 2.83 mM and Vmax of 80.5 and 108.0 µmol/min. Low but detectable TPH activity was also seen in cultured epidermal keratinocytes. Serotonin and/or its metabolite and precursor to melatonin, N-acetylserotonin (NAS), were identified by LC/MS in human epidermis and serum. Endogenous epidermal levels were 113.18 ± 13.34 and 43.41 ± 12.45 ng/mg protein for serotonin (n=8/8) and NAS (n=10/13), respectively. Their production was independent of race, gender and age. NAS was also detected in human serum (n=13/13) at a concentration 2.44 ± 0.45 ng/mL, while corresponding serotonin levels were 295.33 ± 17.17 ng/mL (n=13/13). While there were no differences in serum serotonin levels, serum NAS levels were slightly higher in females. Immunocytochemistry studies showed localization of serotonin to epidermal and follicular keratinocytes, eccrine glands, mast cells and dermal fibrocytes. Endogenous production of serotonin in cultured melanocytes, keratinocytes and dermal fibroblasts was modulated by UVB. In conclusion, serotonin and NAS are produced endogenously in the epidermal, dermal and adnexal compartments of human skin and in cultured skin cells. NAS is also detectable in human serum. Both serotonin and NAS inhibited melanogenesis in human melanotic melanoma at concentrations of 10−4-10−3 M. They also inhibited growth of melanocytes. Melanoma cells were resistant to NAS inhibition, while serotonin inhibited cell growth only at 10−3 M. In summary, we characterized a serotonin-NAS system in human skin that is a part of local neuroendocrine system regulating skin homeostasis.
Keywords: epidermis, serotonin, N-acetylserotonin, skin cells
Introduction
Human skin composed of the epidermis, dermis and hypodermis (subcutaneous fat adjacent to dermis also called as subcutis) is the largest body organ that not only serves as the protective barrier 1,2 but is also empowered with sensory and regulatory functions necessary for organismal survival 3. Thus, it has the capability to sense and adapt to environmental changes and to regulate and protect local and central body homeostasis 3. These functions are coordinated by the cutaneous neuroendocrine system 4–6. The epidermis represents the most-outer layer of the skin that builds a protective barrier against environmental stressors including ultraviolet radiation (UVR)7 through differentiation of keratinocytes and their interaction with melanocytes 1,2,8.
Serotonin (5HTP), a product of sequential metabolic transformation of L-tryptophan (Trp) →hydroxyTrp→5HTP is produced across different species representing an ancient molecule with pluripotent and diverse activities 9–13. The rate-limiting step in serotonin synthesis is tryptophan hydroxylation at position 5 on the indole ring to produce 5-hydroxytryptophan (TrpOH) in a reaction catalyzed by tryptophan hydroxylase and requiring molecular oxygen and the reducing cofactor 6-tetrahydrobiopterin 14–16. There are two isoforms of the enzyme encoded by separate genes. TPH1 expression is the highest in the pineal gland 15, while being detected in different body organs and cells types including skin 16, and TPH2 expressed predominantly in neuronal cells and representing the main isoform in the brain 17. Both isoenzymes are expressed in the human skin with TPH2 being predominantly expressed in normal and malignant melanocytes and retinal pigment epithelium 18–21. TrpOH can also be produced in the skin through H2O2- and ultraviolet A (UVA) irradiation induced a free-radical-mediated oxidation of L-tryptophan 22.
Serotonin is also a precursor to melatonin, a molecule with diverse biological activities 9,23,24, formed through sequential acetylation to N-acetylserotonin (NAS) by either arylalkylamine N-acetyltransferase/serotonin N-acetyltransferase (AANAT/SNAT) 23–25 or arylamine N-acetyltransferase (NAT) with mixed arylamine/arylalkylamine substrate specificity 26–28, and following methylation by hydroxyindole-O-methyltransferase/N-acetylserotonin methyltransferase (HIOMT/ASMT) 23,24,29. All these enzymes are expressed in the human skin 18,30. NAS is also a product of melatonin metabolism by CY1B1 23, an enzyme also expressed in the human skin 31,32. Thus, in the skin serotoninergic and melatoninergic pathways are tightly interconnected with NAS representing a precursor to and a product of melatonin metabolism 18,33.
Serotonin regulates a wide range of physiological and pathological processes in humans predominantly through interaction with cell surface membrane-bound G protein coupled receptors or ligand-gated ion channels classified into seven general families (5HTR1–7R) 10–12,34,35. They can attenuate (5HTR1/5) or stimulate (5HTR4/6/7) adenylate cyclase activity or enhance production of inositol-triphosphate (IP3) and diaglycerol (DAG) (5HTR2AR) or function as an ion channel (5HTR3R) 34,35.
Human skin or skin cells express genes and proteins of 5HTR1A, 1B, 2A, 2B, 2C, 3 and 7 receptors, whose activation would contribute to the pleiotropic effects of serotonin on skin functions including vascular and inflammatory responses, wound healing and potentially contributing to regulation of hair cycling and melanin pigmentation 36–41. However, there is a paucity of information on the biological role of NAS except its contribution to photoprotection against UVB 42. In addition, it is unclear whether NAS is only produced locally or whether it can enter the systemic circulation. Therefore, we decided to characterize 5HT/NAS systems in the human skin.
Materials and Methods
Chemicals
Charcoal stripped fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA, USA). Serotonin and N-acetylserotonin were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade acetonitrile, water and acetic acid (Fisher scientific, Pittsburgh, PA, USA) were used for HPLC. For LC-MS system, acetonitrile, water and formic acid (Sigma-Aldrich, St. Louis, MO, USA) were used. Dulbecco’s modified Eagle’s medium (DMEM) with high glucose (4,500 mg/L), 1% penicillin-streptomycin solution (10,000 units of penicillin and 10 mg of streptomycin in 1 mL 0.9% NaCl), DMSO, ethanol, HEPES (1 M), L-3,4-dihydroxyphenylalanine (L-DOPA), NaOH, non-essential amino acids (NEAA) (100×), RPMI-1640 medium, serotonin, sodium pyruvate (100 mM), Triton® X-100, were purchased from Sigma-Aldrich (St. Louis, MO, USA). FBS, 0.05% trypsin/0.53 mM EDTA solution, 1×PBS (pH 7.4), L-glutamine (200 mM) were supplied by Thermo Fisher Scientific (Waltham, MA, USA). Deuterated standard of N-acetylserotonin [N-acetylserotonin-d7 (NAS-D7)] was received from the National Institute of Mental Health Chemical Synthesis and Drug Supply Program NIMH # A-906, Bethesda, MD, USA.
Human skin and serum
The use of human tissues was approved by both UTHSC and UAB Institutional Review Boards as an exempt protocol #4 and collected as described previously 43,44. The skin specimens (n=13) were obtained from both males and females [30 to 90 years old] of African-American and Caucasian races and the epidermis was peeled and extracted with 75% acetonitrile with homogenization as described previously 45–47. Serum samples were collected from thirteen healthy adult volunteers (12 Caucasians and 1 Hispanic, with an age range of 25 to 61 years including 10 females and 3 males) according to the IRB protocol #7526 (Dr. A. Postlethwaite, P.I.) as described previously 43,44. The samples were extracted using 90% methanol and processed as described in 43–46 and were kept in −80 °C for further experiments.
Cell cultures
For enzymatic assays, passages 4 and 5, respectively, of normal human epidermal keratinocytes and dermal fibroblasts of the primary cultures established from foreskins following the standards protocols used in our laboratory 36 were used. In addition, we have used immortalized line of adult epidermal melanocytes (PIG-1, gift of Dr. LePoole from Loyola University), which was grown in melanocyte growth media (MGM) supplemented with melanocyte growth factors (MGF) (Lonza Walkersville Inc, Walkersville, MD, USA). Immortalized adult human epidermal keratinocytes (HaCaT) were cultured in Dulbecco’s minimal essential media (DMEM) supplemented with 5% charcoal stripped fetal bovine serum (Serum Source International, Inc. Charlotte, NC, USA) and 1% Antibiotic-Antimycotic Solution (Mediatech, Inc. Manassas, VA, USA)48.
For functional assays, performed at the University of Munster, human epidermal HaCaT keratinocyte line was purchased from ATCC (LGC Standards, Middlesex, UK) while MNT-1 cells representing a human melanotic melanoma line was acquired as a gift from Dr. Cédric Delevoye (Institute Curie, Paris, France). HaCaT cells were cultured in RPMI-1640 supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 2 mM L-glutamine, 1% (v/v) streptomycin-penicillin solution 49. Normal neonatal human melanocytes (Cell Applications, Inc., San Diego, CA, USA) were cultured in Melanocytes Medium254 supplemented with human melanocyte growth supplement (HMGS) (Thermo Fisher Scientific, Waltham, MA, USA). MNT-1 cells were maintained in DMEM medium supplemented with 20% (v/v) heat-inactivated fetal bovine serum, 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 1% (v/v) NEAA, 1% (v/v) streptomycin-penicillin solution at 37 °C in a humidified atmosphere of 5% CO2 in air 50. Cells in the logarithmic growth phase were used in all experiments while 80–90% monolayers of confluent MNT-1 cells were harvested with a mixture of 0.05% trypsin-EDTA solution. The media were changed every 48 h.
Cell viability assay
Cells were seeded on 96-well plates at the density of 1.5×104 cells/well in the culture medium and thereafter grown to subconfluence. Incubation with NAS and serotonin was performed in dose- (10–7–10–3 M) and time-dependent manner (24, 48 and 72 h). Cell viability was evaluated by MTT assay. Briefly, MTT (5 mg/mL) was dissolved in 1×PBS, filtered through a 0.22 μM Millipore® filter and stored at 4 °C. After desired incubation time, cells were washed twice with 1×PBS, then 100 μL of MTT in culture medium (final dilution, 1:10) was added to each well, and incubated for 3 h at 37 °C to allow MTT metabolism. The formazan produced was dissolved in 100 μL acidic isopropanol and absorbance was measured at 595 nm using a BioTek ELx808™ microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Measurement of melanin content
Cells were seeded on 6-well plates (Sarstedt, Nümbrecht, Germany) at the density of 3×105 cells/well and were allowed to attach overnight. The cells were then incubated for 72 h in fresh medium containing various concentrations of NAS or serotonin dissolved in ethanol as described above. For determination of melanin content, the MNT-1 cells were harvested, washed with 1×PBS, centrifuged at 1,000×g for 10 min (4 °C), and solubilized in 500 μL of 1 N NaOH for 2 h at 80 °C. The absorbance was measured at 405 nm using a BioTek ELx808™ microplate reader, and results were presented as the percentage of the control sample.
DOPA oxidase activity of tyrosinase
MNT-1 cells were seeded on 6-well plates and incubated with NAS or serotonin for 72 h as described earlier. After that, cells were harvested, washed with 1×PBS, centrifuged at 1,000×g for 10 min (4 °C), lysed 0.5% Triton® X-100 in 1×PBS on ice. The lysates were subsequently centrifuged at 16,000×g for 15 min (4 °C), 300 μL resultant supernatant was added to 300 μL of 5 mM L-DOPA in 1×PBS, and incubated for 1 h at 37 °C. The dopachrome formation was evaluated by measuring absorbance at 475 nm using a BioTek ELx808™ microplate reader, and results were presented as the percentage of the control sample.
Liquid chromatography-mass spectrometry (LC-MS) detection of serotonin and N-acetylserotonin and quantification
In order to detect serotonin and N-acetylserotonin, the dried extracts from epidermal and serum samples were re-dissolved in methanol and analyzed by LC-MS as described previously 43–46. The UPLC (ultra-performance liquid chromatography) separation was performed on a Waters ACQUITY I-Class UPLC system (Waters, Milford, USA) consisting of a binary pump, an autosampler, a column manager, a degasser and a diode-array detector (DAD). An Agilent Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm, Agilent Technologies, Santa Clara, CA, USA) maintained at 35 °C was used with a mobile phase consisting of the following linear gradient of acetonitrile containing 0.1% formic acid: 15% for 1.5 min, 15 – 30% for 0.1 min, 30% for 0.9 min, 30 – 100% for 0.5 min, 100% for 3 min, 100 – 15% for 0.1 min, and 15% for 0.9 min. The flow rate was 0.3 mL/min and the DAD was operated in the range of 200 – 400 nm. The UPLC was connected to a Xevo™ G2-S QTof mass spectrometer (Waters, Milford, USA), a quadrupole (Q) hybrid with orthogonal acceleration time-of-flight (Tof) tandem mass spectrometer (MS) in positive ion mode. The scan range was 50 to 1200 Da in positive mode, and all MS values were collected in centroid mode. The capillary and cone voltages were 3.0 kV and 30 V, respectively. The desolvation gas was maintained at 1000 L/h at a temperature of 500 °C. The cone gas was 100 L/h with a source temperature of 150 °C. The data acquisition rate was 0.3 s, with a 20 second interval. The lockspray frequency was every 20 s using Leucine Enkephalin solution (100 ng/mL) as the lockspray reference compound (m/z 556.2771) with a flow rate of 5 μL/min. The MS data were collected with full scan mode with low (6 V) and high (ramp from 20 V to 40 V) collision energy (CE) data channels to get both the parent ions (MS) and the daughter ions (MS/MS). All data were acquired and processed by Waters MassLynx v4.1 software. The relative concentrations of NAS product were calculated from the UPLC Xevo™ G2-S QTof MS peak areas in relation to standards curves generated using the corresponding ions of standards. The values are presented as means ± SE or as individual values.
The limits of detection, accuracy, linearity and recovery have been investigated as described by Carter et al.51. The samples were dissolved in methanol and applied LC-MS using Xevo G2-XS QTof LC-MS system (Waters, Milford, MA, USA). The mass range of 100 to 1,000 Da in positive mode was scanned using the continuum mode with scan time of 1 second. The capillary and cone voltages were 1.7 kV and 40 V, respectively. The desolvation gas flow rate was set on 800 L/h with source temperature of 120 °C. 200 ng/mL leucine enkephalin (m/z = 556.2771) was used as the lockspray reference compound at the flow rate of 10 uL/min with lockspray interval of 10 seconds and scan time of 1 second. UPLC conditions were the same as above except column temperature (25 °C). The mass chromatograms were processed by Waters MassLynx v4.1 software. EIC (Extracted ion chromatograms) were performed using m/z = 160.076± 0.0004 [M+H-NH2COCH3]+ for NAS and 164.077± 0.0004 for NAS-D7. As an internal standard we have used NAS-D7 during sample preparation that included a standard curve and recovery determination. For preparation of standard curves we used 0.5, 5, 25 and 500 ng/mL NAS and NAS-D7 as an internal standard (n = 2).
Supplemental figure 1 shows NAS/NAS-D7 linearity over a range of 5–500 pg with a slope of 13.75±0.62 and a coefficient of r2 ≥ 0.9998. Table 1 shows the intrarun (within run) and interrun (between run) accuracy and precision for the determination. Percent relative error (%RE) represents (Ce−Ct)/Ct] × 100 where Ce is the experimental concentration determined from the calibration curve slope, and Ct represents theoretical concentration. Percent relative standard deviation (%RSD) represents (SD/Cavg) × 100, being a measure of assay precision, where Cavg is the average concentration calculated, and SD is the standard deviation of Cavg. The ratios of NAS/NAS-D7 were plotted to form calibration curves over concentration rage of 2.28–228 nM of NAS standards (n = 2 for each standard) suspended in fetal bovine serum (FBS). The limits of quantitation (LOQ) were calculated as the ratio of signal to noise and it was 23 for the lowest concentration of NAS, 0.5 ng/mL.
Table 1.
Precision and accuracy of the NAS assays
| ng/mL | Intrarun | Interrun (9 days) | ||||
|---|---|---|---|---|---|---|
| 1-st | 2-nd | |||||
| RE (%)* | RSD (%)** | RE (%)* | RSD (%)** | RE (%)* | RSD (%)** | |
| 0.5 | 28.87 | 2.61 | 12.59 | 129 | 19.54 | 8.22 |
| 5 | −7.78 | 5.01 | 15.24 | 8.00 | 5.36 | 13.25 |
| 50 | −4.94 | 4.82 | 16.78 | 1.35 | 7.54 | 12.15 |
, Relative error: [Ce-Ct)/Ct] X 100 where Ce is the experimental concentration and Ct is theoretical concentration;
, Percent relative standard deviation: (SD/Cavg) X 100 where Cavg is the average concentration and SD is the standard deviation
The extraction efficiency was determined as follows, FBS samples containing 0.5, 5, and 50 ng/mL NAS were processed alongside equivalent concentrations of the standard prepared without serum. The NAS-D7 standard was added to the samples before analysis by LC-MS. The recovery was calculated as the ratio of peak areas, FBS vs. non-FBS. NAS recovery for 0.5, 5 and 50 ng/mL was 157.1, 86.4 and 35.8%, respectively.
Serotonin levels
Serotonin levels in the epidermis and serum were measured using ELISA kit from MyBioSource, Inc. (San Diego, CA, USA) following manufacture’s recommendations. Briefly, 100 μL of samples and standard solutions were added to the wells and incubated for 2 h at 37 °C. Biotin-antibody was added to the wells and incubated for 1 h at 37 °C. The wells were washed with wash buffer followed by adding HRP-avidin to each well and incubated for 1 h at 37 °C. After washing with buffer for 5 times, TBM substrate was added to each well and incubated for 20 min at 37 °C. After adding stop solution, the absorbance was measured at 450 nm.
Tryptophan hydroxylase activity assays
Cells were detached and processed as described previously 36. Cell pellets were suspended in ice cold 5 mM Tris-HCl (pH 7.0) containing 0.2% Triton X-100 and homogenized. Homogenate was further incubated in an ultrasound bath for 1 min and then used for determination of enzymatic activity. The standard reaction conditions contained 0.1 to 2 mM L-tryptophan, 0.3 mM 6-methyltetrahydropterin, 200 mM ammonium sulfate, 7 mM DTT, 25 μg/mL catalase, 25 μM ferrous ammonium sulfate, 50 mM of 2-N-morpholino)ethanesulfonic acid (MES) buffer (pH 7.0), with atmospheric oxygen. The assays was initiated by the addition of 10 μL of a 3 mM stock of 6-MePH4 6-methyltetrahydropterin). The final volume of reaction mixture was 100 μL. The reaction was stopped by addition of 6 M perchloric acid. The production of hydroxytryptophan (TrpOH) was measured using and HPLC with fluorometric detection as described previously 26,30. The relative concentrations of TrpOH product were calculated from the HPLC peak areas in relation to standards curves generated using the corresponding TrpOH standard. The Km and Vmax values were calculated from Michaelis-Menten plots using Prism 4.00 (GraphPad Software, San Diego, CA, USA).
Immunocytochemical detection of serotonin in human skin
Immunocytochemistry was performed as described previously 18,52. Briefly, frozen sections were performed on normal human scalp skins from three different donors, obtained after elective plastic surgery with informed consent. The skin was embedded in OCT medium (RA Lamb, East Sussex, UK). Multiple cryosections of different human skins were air-dried, fixed in ice-cold acetone, hydrated in PBS, and blocked in 10% donkey serum. They were incubated with rabbit anti-serotonin polyclonal antibody (abcam ab8882) diluted 1:100 for 1 h. Then the sections were washed in PBS and incubated using the DAKO LSAB2 HRP/3-amino-9-ethylcarbazole (AEC) system (DAKO Ltd, Cabridgeshire, UK) according to the manufacturer’s instructions, which includes a biotin-labelled anti-rabbit antibody followed by streptavidin. Negative controls were processed as above, except with primary antibody against serotonin replaced with donkey serum.
Detection of dopamine (DA) and 5HT in cultured skin cells treated with UVB
Detailed description of the experimental design is found in the supplemental file. Briefly human fibroblasts (HF), immortalized HaCaT keratinocytes and co-cultured epidermal keratinocytes and melanocytes were cultured as described previously 53,54. UVB treatment protocols followed those described by Skoboviat et al 54. The protocols of cell extraction and electrochemical detection of 5HT after HPLC separation followed the protocols described by Williams et al 55.
Statistics
Student t-test was performed as indicated using Prism 4.00 (GraphPad Software, San Diego, CA, USA) presented as means ± SE or SD as indicated. Statistically significant differences are considered when *, p<0.05; **, p<0.01; ***, p<0.001 (student t-Test) and #, p<0.05; ##, p<0.01; ###, p<0.001 (Mann-Whitney test or One-way ANOVA test).
Results and Discussion
TPH activity in human skin cells
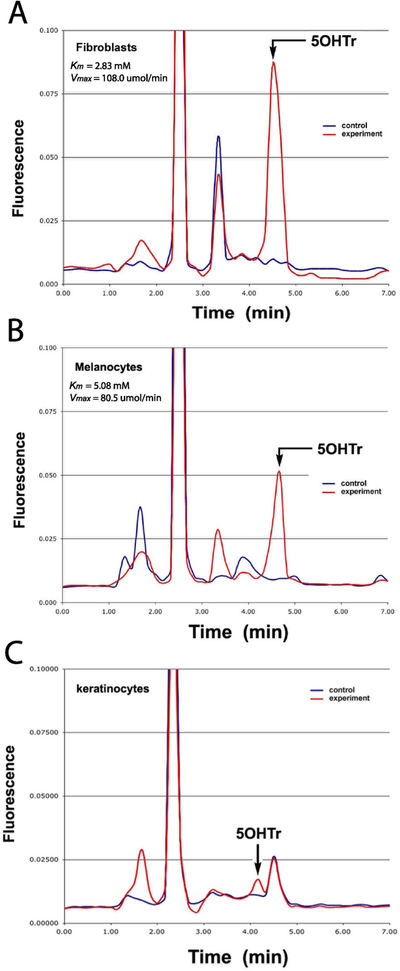
Previously we detected the TPH1 gene and protein expression in the main cellular compartments of the human and rodent skin including epidermis, dermis and adnexal structures 21,26,30, while the TPH2 gene was detected predominantly in melanocytes and retinal pigment epithelium cells 19,20. TPH activity was also detected in rodent melanoma cells using a radiometric method 27, while TrpOH was detected in human melanoma cells by LCMS 56. In the current study we measured TPH activity in extracts of in human epidermal melanocytes, fibroblasts and keratinocytes by measuring TrpOH production using HPLC with fluorometric detection (Fig. 1). The precise retention time of the product was confirmed by spiking the samples with a Trp(OH) standard. The Michaelis-Menten plots of values obtained after plotting TPH activity vs tryptophan concentrations (0.1 to 2 mM) L-tryptophan showed respective values for Km of 5.08 and 2.83 mM and V max 80.5 and 108.0 μmol/min for melanocytes and fibroblasts. The TPH activity in epidermal keratinocytes was too low to obtain Michaelis-Menten plots. Thus, human dermal fibroblasts and epidermal melanocytes express enzymatically active TPH with low activity detected in epidermal keratinocytes, consistent with previous immunocytochemical studies 18,21,22 and western blot assays 3 performed on the skin or skin cells that showed relatively lower expression of TPH in epidermal keratinocytes in comparison to normal and malignant melanocytes. It is important to say that we do not know how different are levels of TPH activity in skin cells in comparison to pineal gland, since human pineal glands have not been available in our laboratory. However, we expect that these levels will be significantly higher in the pineal gland, since it produces melatonin for systemic use. For comparison in other neuroendocrine system we found that murine pituitary POMC gene expression is >1,000 higher in the murine pituitary than murine skin 57 indicating that differences in the expression between skin and central endocrine organs can be substantial.
Figure 1.
Tryptophan hydroxylase (TPH) activity in skin cells. TPA activity was measured as production of 5-hydroxytryptophan (5OHTr) in extracts from dermal fibroblasts (passages 4 and 5) (A),immortalized line of epidermal melanocytes (PIG-1) (B) and keratinocytes (passages 4 and 5)(C) using HPLC with fluorimetric detection. Experimental represents samples incubated with L-tryptophan, while control samples were incubated without the substrate. The assays were repeated 4 times.
Characterization and quantification of serotonin and NAS in the epidermis and serum
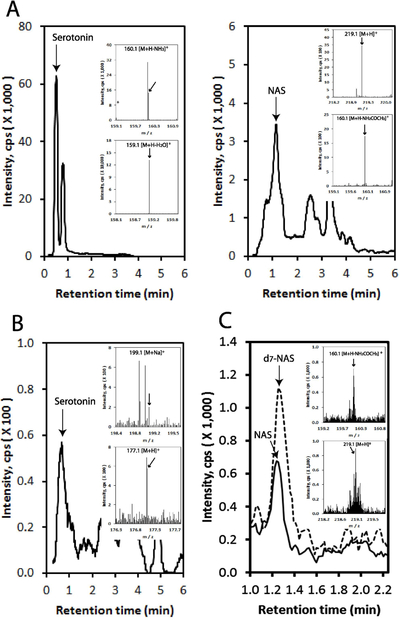
Previously we demonstrated the ability of the mammalian skin to transform serotonin to NAS and further to melatonin, and measured melatonin metabolism in this organ 26–28,30,47,58. In the present study we detected serotonin and NAS in the epidermis and human serum using Xevo™ G2 qTof mass spectrometer. As shown in Fig. 2 serotonin was detected in the epidermis at m/z=160.1 [M+H-NH3]+ and 159.1 [M+H-H2O]+, while NAS at 219.1 [M+H]+ and 160.1 [M+H-NH2COCH3]+ in extracted-ion chromatogram (EIC) with RT corresponding to serotonin and NAS standards, respectively. Thus, similarly to rodent skin 26–28,30,58, human epidermis is able to produce endogenously NAS. In human serum serotonin was detected at m/z=199.1 [M+Na]+ and 177.1 [M+H]+ (Fig. 2B), which was expected. Using the qTOF at extremely precise range 160.076 ± 0.0004 [M+H-NH2COCH3]+ we also detected NAS in the human serum (Fig. 2C).
Figure 2.
Detection of serotonin and NAS in human epidermis and serum. LC-MS was performed as described in Materials and Methods. EICs are shown using m/z = 159.1 [M+H-H2O]+ (serotonin in epidermis), 219.1 [M+H]+ (NAS in epidermis), 199.1 [M+Na]+ (serotonin in serum) and 160.076 [M+H-NH2COCH3]+ (NAS in serum). Pre-purified sample (using a long column (WatersC18 column, 250 × 4.6 mm, 5 μm particle size) with a gradient of acetonitrile in water (40–100%) at a flow rate of 0.5 ml/min (15 min), followed by a wash with 100% acetonitrile for 30 min at a flow rate of 0.5 ml/min and for 20 min at a flow rate of 1.5 ml/min) was used for serotonin in epidermis. All other EICs were performed using crude extracts. A, detection of serotonin and NAS in the epidermis; B, detection of serotonin in the serum. C, detection of NA in the serum using Xevo G2-XS QTof LC-MS system (Waters, Milford, MA, USA). The analysis was described in Materials and Methods. NAS-D7 was used as an internal standard (broken line), and EIC was performed using m/z = 160.076 ± 0.0004 for Abs Window (Da) for NAS and using m/z = 164.077 for NAS-D7. Inserts show mass spectra of RTs corresponding standards. Thirteen independent assays were performed for different samples with two LC/MS repeats for each sample. The epidermal extracts were obtained from intact human skin and serum was collected as described in Materials and Methods.
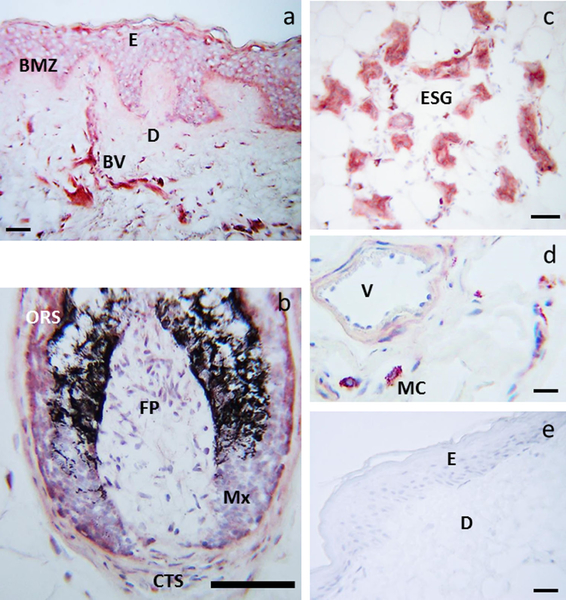
The histologic distribution of the serotonin antigen was then tested in frozen sections of normal human scalp (Fig. 3). Immunoreactivity was detected in keratinocytes of the basal, suprabasal and spinous layers with strong expression in melanocytes and focal expression in dermal blood vessels (Fig. 3a). Serotonin immunoreactivity was also detected in keratinocytes of the outer root sheath and hair bulb and follicular melanocytes of the hair follicle and fibroblasts of dermal papilla (Fig. 3b). In the dermis, serotonin immunoreactivity was also present in eccrine glands (Fig. 3c) and mast cells (Fig. 3d). This localization is similar to the previously reported distribution of SNAT, melatonin and TPH antigens in frozen sections of human skin 18,52,59 with one difference: melatonin was predominantly distributed in the upper layers of the epidermis which was not the pattern seen in TPH and serotonin antigen distribution 18,52 (Fig. 3). Thus, we have provided strong evidence that serotonin and NAS are produced in the human epidermis. No secondary antibody binding was observed as evidenced by absence of staining when the primary antibody was replaced by donkey serum (Fig. 3e).
Figure 3.
Immunolocalization of serotonin in human scalp. Serotonin immunoreactivity was detected cytoplasmically and membranously in keratinocytes and melanocytes of the basal layer, and in keratinocytes of the suprabasal and spinous layers, as well as in dermal blood vessels (BV) (a), ORS and matrix cells of the hair follicle, FP and CTS (b), ESG (c) and mast cells (d). Control: primary antibody replaced with donkey serum and secondary antibody only (e). BMZ: basement membrane zone, CTS: D: dermis, E: epidermis, ESG: eccrine/sweat glands, FP: dermal papilla fibroblasts, Mx: hair follicle matrix, ORS: outer rout sheath, V: lumen of the blood vessel. Scale bar: 20 μm (a), 100 μm (b), 50 μm (c), 13 μm (d), 23 μm (e). The immunostains are from the scalp skin of the 35 years old male donor, a representative of three donors with multiple stains performed for each skin sample
Previously, we have quantified levels of melatonin and its metabolites in the human epidermis 45–47. In the present studies we measured serotonin levels using ELISA assay and NAS by qTof LC-MS using epidermis obtained from 13 subjects (the same extracts as those used for measurement of melatonin and metabolites 45,46) and serum. NAS was detected in 13 of 13 serum samples and in 10 of 13 epidermal samples. Supplemental Figure 1 and Table 1 demonstrate the linearity, accuracy and limits of detection of NAS by by qTLC-MS. The detected average values for serum and epidermal samples (2.44 and 43.41 ng/mL, respectively) were within the detection limits and at the linear range of the assay. Since the recovery of NAS at the lower range (0.5 ng/mL) was higher than expected (157.1%), similar to the reported by Carter et al. 51 (123.4%), we submitted an extracted sample of FBS for qTLC-MS analysis using m/z = 160.076 and found a small but detectable corresponding peak in FBS (not shown). This could explain higher than expected recovery of NAS at low range, see above.
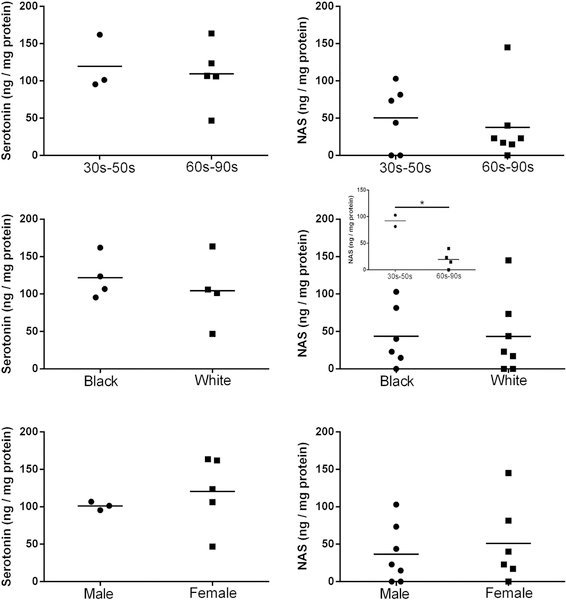
As shown in Table 2 serotonin levels in the epidermis were 2.6 times higher than that of NAS, while 121 times higher than NAS in the serum. Epidermal levels of these compounds were independent of race, gender and age. Although we noted higher NAS levels in African-Americans (30 to 50 yrs) vs older Caucasian males (60 to 90 yrs), this difference would require confirmation on a larger cohort of patients (Fig. 4; supplemental Fig. 2). Interestingly, a similar trend was reported for melatonin content in the epidermis 46. In the human serum there were no differences in serotonin levels depending on age and gender, with slightly higher NAS levels in females than males (supplemental Fig. 2). Backlund et al have recently detected N-acetyltryptamine in the rhesus macaque, rat and human plasma samples 60, while Carter et al detected NAS in the serum of two children 51. The listed by Carter et al 51 values of 15 pg/mL and 24 pg/mL) of plasma levels of NAS in children with attention disorders that were 70 to 100 times lower than reported by us, which requires explanation. These differences can be due to our use of serum samples while the cited authors used plasma or in differences in subjects’ selection. We repeated analysis of 13 human serum samples using m/z = 160.076 on Xevo G2-XS QTof LC-MS system and found the values at similar range (6.13 ± 0.67 ng/mL) as in Table 2. Moreover, our cohort was composed of healthy adult individuals while their cohort consisted of two young children’s with autism. We also acknowledge the limitation of this study represented by limited number of human subjects (n=13). Therefore, future studies using a large cohort of donors are required to define a precise correlation between race age and health status and NAS levels. It is worthy to cite old literature reporting acetylation of serotonin in the rodent 61 and human liver 62, which indicates that this organ can supply systemic circulation with NAS. Interestingly, White et al. 62 reported a wide variability in the 13 livers tested to acetylate serotonin.
Table 2.
Serotonin and NAS content in the human epidermis and serum
| Serotonin | NAS | |
|---|---|---|
| Epidermis (ng/mg protein) | 113.18 ± 13.34 (n=13) | 43.41 ± 12.45 (n=13) |
| Serum (ng/mL serum) | 295.33 ± 17.17 (n=8) | 2.44 ± 0.45 (n=13) |
The data represent averages ±SE from different patients (n) with the assay repeated at least 3 times.
Figure 4.
Levels of serotonin and NAS in the epidermis. Serotonin was quantified using ELISA kit from MyBioSource, Inc. (San Diego, CA) and NAS was quantified with qTOF LC-MS (EIC) using corresponding standard (m/z = 219.1 [M+H]+, *p < 0.05 at Student t-test.
We also tested the effect of UVB on the production of serotonin in melanocytes, keratinocytes and fibroblasts using a chemiluminescent detection system coupled to HPLC separation (supplemental Fig. 3). Briefly, the basic levels of serotonin in sham irradiated fibroblasts, HaCaT keratinocytes and co-cultured melanocytes and keratinocytes (0 mJ/cm2) were 120.4, 24.9 and 7.7 pg/105 cells, respectively. Only in HaCaT and co-cultured cells did UVB treatment enhance serotonin production at 10 mJ/cm2. One hundred mJ/cm2 attenuated this production in all cell types, which may be secondary to the partial toxic effect of this UVB dose 63. On the other hand UVB inhibited dopamine production in a dose-dependent manner in fibroblasts, HaCaT keratinocytes and melanocytes from the respective basal levels of 26.3, 19.2 and 23.8 pg/105 cells (supplemental Fig. 4). Interestingly, catecholamines are produced endogenously by skin cells 64, and dopamine has a potential to inhibit serotonin and melatonin synthesis 24,65–68. Therefore, our preliminary data are encouraging for further studies on UVB regulation of interactions between serotoninergic/melatoninergic and dopaminergic systems.
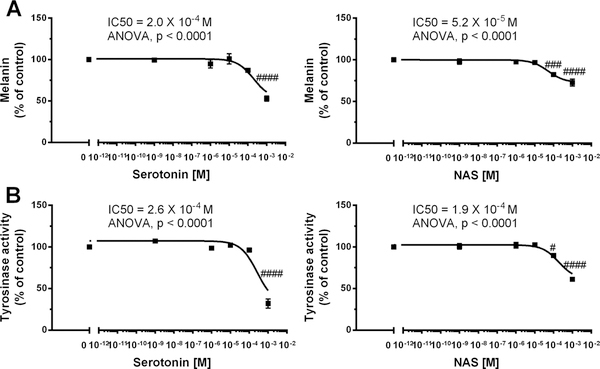
Serotonin’s role in the regulation of pigmentary responses is complex and dependent on ligand activation of a particular receptor 8,41,69,70. In melanophores pigment granule dispersion or aggregation is dependent on activation on 5HT3 or 5HT1/2, respectively 69. In human melanomas serotonin or serotonin uptake inhibitors were reported to inhibit melanogenesis 8,70. However, 5HT1A/2A or 5HT2B can stimulate or inhibit melanogenesis, respectively 40,41. Therefore, we tested the phenotypic effects of serotonin and NAS on melanogenic activity using the pigmented melanoma line MNT-1 (Fig. 5). Thus, melanin synthesis was significantly inhibited only by high doses (10−4 and 10−3 M) of serotonin and NAS with respective IC50 of 2.0 × 10−4 and 5.2 × 10−5 M (Fig. 5A, B). The need for high serotonin concentration suggests that anti-melanogenic effect is independent of membrane bound 5TH receptors. A similar pattern of regulation was observed for tyrosinase activity with corresponding IC50 for serotonin and NAS being 2.6 × 10−4 and 1.9 × 10−4 M (Fig. 5C, D). Although the described effects of serotonin are in agreement with studies on human melanomas 8,70 and human melanocytes and murine immortalized melanocytes 41 we believe that the effect will be dependent on cellular genotype and composition of the corresponding receptors. As it relates to NAS, this is the first demonstration of an inhibitory effect on melanogenesis, since previous studies on inhibition of melanogenesis by 10−4 M NAS showed a lack of effect in rodent melanomas 71 or some inhibition in anagen murine skin but without statistical significance 72. These combined, and presented results and literature reports demonstrate that the regulation of melanin pigmentation by serotonin and NAS is complex, dependent on species, normal and malignant phenotype, and receptor expression and a probability involve metabolic transformation 5HT→NAS→melatonin, since human melanomas can transform tryptophan to melatonin 56 and MNT-1 expresses TPH1 and HIOMT 73.
Figure 5.
Inhibition of melanogenesis by serotonin and NAS in MNT-1 melanoma cells Cells were incubated with graded concentrations of serotonin or NAS for 72 h in a dose-dependent manner and proceeded for melanin assay (A) and tyrosinase activity (B) as described in Materials and Methods. Data were presented as the mean±SE (n=6), while values were normalized and expressed as percentage of the control value i.e. cells incubated in presence of 0.2% ethanol. Statistically significant differences were indicated as #P < 0.05, ###P < 0.001, ####P < 0.0001 by one way ANOVA. The experiments were independently repeated three times with similar results.
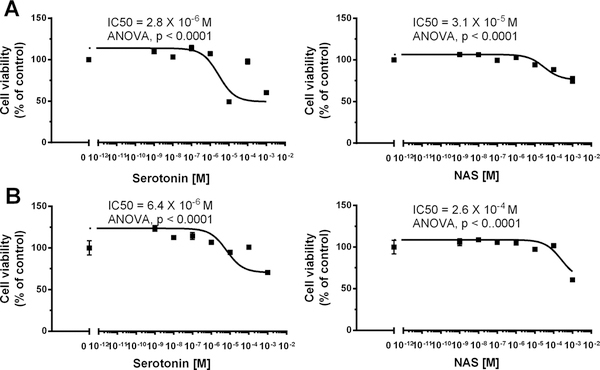
We also tested the effect of serotonin and NAS on growth of MNT-1 melanoma and normal epidermal melanocytes in cell culture (supplemental Fig. 5; Fig. 6). While NAS had no marked effect on melanoma cell growth, serotonin inhibited it only at very high concentration of 10−3 M (supplemental Fig. 5). In the case of normal epidermal melanocytes serotonin and NAS moderately inhibited cell growth with respective IC50 of 2.8–6.4 × 10−6 and 0.3–2.6 × 10−4 M (Fig. 6). The inhibitory effect of serotonin on melanocytes growth is consistent with previous report 26. These results show that while serotonin and to lower degree NAS can inhibit growth of human melanocytes, while melanoma cells are becoming resistant to such regulation, especially in case of NAS. Although the above concentrations of the ligands are unphysiological as relates to the serum levels, they are produced locally and, therefore, are considered as a part of physiological local response system. Regarding NAS, it is noteworthy that it can protect human keratinocytes and melanocytes against UVB induced damage 42,74. It has a similar effect on induction of genes involved in anti-oxidative response, attenuation of ROS and DNA damage and increases nuclear levels of p53 phosphorylated at Serine 15, similarly to the effects excreted melatonin and its metabolites 42,74. The above results suggest that NAS is not a mere product of serotonin or melatonin metabolism but it has its own biological activity that includes regulation of pigment cells and involvement in photoprotective activities. The relatively high concentration of NAS necessary to achieve the phenotypic effects suggests an existence of a nuclear receptor for this ligand.
Figure 6.
Inhibition of normal neonatal epidermal melanocytes growth by serotonin and NAS. Dose dependent curves of MTT viability assay were generated after 48 (A) and 72 (B) h of treatment with serotonin and NAS. Data were presented as the mean±SE (n=6), while values were normalized and expressed as percentage of the control value i.e. cells incubated in presence of 0.2% ethanol. The neonatal keratinocytes were from the commercial sources and were grown as described in the materials and methods. The experiments were independently repeated three times with similar results.
Conclusions
In this report we provide evidence that serotonin and NAS are produced endogenously in the epidermal, dermal and adnexal compartments of human skin and in cultured skin cells that also show detectable tryptophan hydroxylase activity, a rate-limiting enzyme in the serotoninergic pathway. Epidermal serotonin levels were independent of race, gender and age, but its production could be affected by environmental factors such as UVB. NAS epidermal levels were higher in young African-Americans vs old Caucasian males having a similar trend to that reported previously for melatonin. The present and reported previously detection of NAS in human serum indicated its availability to serve as a substrate for enzymatic transformation to melatonin in peripheral organs expressing HIOMT. Therefore, NAS can enter the circulation from different peripheral organs expressing NAT1/2, especially liver and perhaps skin. Nevertheless, its presence in the circulation and peripheral organs could explain why the C57BL6 mouse with inactive AANAT can produce melatonin. Serotonin and NAS can regulate the skin pigmentary activity including an antiproliferative effect in normal melanocytes and an inhibition of melanogenesis in melanoma cells. This suggests that NAS is not merely an intermediate or product of serotonin and melatonin metabolism but that it also has bioregulatory activity in skin cells, in addition to previously reported protection against the UVB induced damage. This property is shared by melatonin and its metabolites. In conclusion, we propose that cutaneous production of serotonin and NAS is a part of a local neuroendocrine system that regulates skin homeostasis.
Supplementary Material
Acknowledgements
The present study was supported by NIH grant 1R01AR056666–01A2 and in part by NIH grants 1R01AR073004–01A1 and R01 AR071189–01A1 and VA merit grant (No. 1I01BX004293–01A1) to ATS, and the German Research Foundation [Deutsche Forschungsgemeinschaft (DFG)] (grant number: KL2900/2–1) to KK. The authors would like to express their gratitude to Dr. Cédric Delevoye (Structure and Membrane Compartments, Institute Curie, Paris Sciences & Lettres Research University, Centre National de la Recherche Scientifique, UMR144, Paris, France) for a kind gift of MNT-1 cells. We thank Dr. David Klein for help in acquiring N-acetylserotonin-d7
Abbreviations:
- NAS
N-acetylserotonin
- yrs
years old
- UVB
ultraviolet B
- FBS
fetal bovine serum
- TCA
trichloroacetic acid
- SDS
sodium dodecyl sulfate
- ESI
electrospray ionization
- MS
mass spectrometry
- LC-MS
liquid chromatography mass spectrometry
- HPLC
high-pressure liquid chromatography
- UPLC
ultra-performance liquid chromatography
- qTOF
quadrupole time-of-flight
- NAS-D7
N-acetylserotonin-d7
References
- 1.Elias PM, Menon G, Wetzel BK, Williams JJ. Barrier requirements as the evolutionary “driver” of epidermal pigmentation in humans. American Journal of Human Biology. 2010;22(4):526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias PM. The skin barrier as an innate immune element. Seminars In Immunopathology. 2007;29(1):3–14. [DOI] [PubMed] [Google Scholar]
- 3.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21(5):457–487. [DOI] [PubMed] [Google Scholar]
- 5.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34(6):827–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015;103:72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology. 2018;159(5):1992–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski A TD, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. [DOI] [PubMed] [Google Scholar]
- 9.Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell Mol Life Sci. 2017;74(21):3863–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin AM, Young RL, Leong L, et al. The Diverse Metabolic Roles of Peripheral Serotonin. Endocrinology. 2017;158(5):1049–1063. [DOI] [PubMed] [Google Scholar]
- 11.Spohn SN, Mawe GM. Non-conventional features of peripheral serotonin signalling - the gut and beyond. Nat Rev Gastroenterol Hepatol. 2017;14(7):412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv J, Liu F. The Role of Serotonin beyond the Central Nervous System during Embryogenesis. Front Cell Neurosci. 2017;11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buznikov GA, Lambert HW, Lauder JM. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305(2):177–186. [DOI] [PubMed] [Google Scholar]
- 14.Kema IP, de Vries EG, Muskiet FA. Clinical chemistry of serotonin and metabolites. J Chromatogr B Biomed Sci Appl. 2000;747(1–2):33–48. [DOI] [PubMed] [Google Scholar]
- 15.Mockus SM, Vrana KE. Advances in the molecular characterization of tryptophan hydroxylase. J Mol Neurosci. 1998;10(3):163–179. [DOI] [PubMed] [Google Scholar]
- 16.Tidemand KD, Peters GH, Harris P, Stensgaard E, Christensen HEM. Isoform-Specific Substrate Inhibition Mechanism of Human Tryptophan Hydroxylase. Biochemistry. 2017;56(46):6155–6164. [DOI] [PubMed] [Google Scholar]
- 17.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299(5603):76. [DOI] [PubMed] [Google Scholar]
- 18.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19(2):176–194. [DOI] [PubMed] [Google Scholar]
- 19.Slominski AT, Kleszczynski K, Semak I, et al. Local melatoninergic system as the protector of skin integrity. Int J Mol Sci. 2014;15(10):17705–17732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zmijewski MA, Sweatman TW, Slominski AT. The melatonin-producing system is fully functional in retinal pigment epithelium (ARPE-19). Mol Cell Endocrinol. 2009;307(1–2):211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slominski A, Pisarchik A, Johansson O, et al. Tryptophan hydroxylase expression in human skin cells. Biochim Biophys Acta. 2003;1639(2):80–86. [DOI] [PubMed] [Google Scholar]
- 22.Schallreuter KU, Salem MA, Gibbons NC, et al. Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 1: Epidermal H2O2/ONOO(−)-mediated stress abrogates tryptophan hydroxylase and dopa decarboxylase activities, leading to low serotonin and melatonin levels. FASEB J. 2012;26(6):2457–2470. [DOI] [PubMed] [Google Scholar]
- 23.Tan D, Reiter RJ. Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019;2(1):44–66. [Google Scholar]
- 24.Cipolla-Neto J, Amaral FGd. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocrine Reviews. 2018;39(6):990–1028. [DOI] [PubMed] [Google Scholar]
- 25.Klein DC, Coon SL, Roseboom PH, et al. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–357; discussion 357–308. [PubMed] [Google Scholar]
- 26.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur J Biochem. 2003;270(16):3335–3344. [DOI] [PubMed] [Google Scholar]
- 27.Slominski A, Pisarchik A, Semak I, Sweatman T, Szczesniewski A, Wortsman J. Serotoninergic system in hamster skin. J Invest Dermatol. 2002;119(4):934–942. [DOI] [PubMed] [Google Scholar]
- 28.Semak I, Korik E, Naumova M, Wortsman J, Slominski A. Serotonin metabolism in rat skin: characterization by liquid chromatography-mass spectrometry. Arch Biochem Biophys. 2004;421(1):61–66. [DOI] [PubMed] [Google Scholar]
- 29.Donohue SJ, Roseboom PH, Illnerova H, Weller JL, Klein DC. Human hydroxyindole-O-methyltransferase: presence of LINE-1 fragment in a cDNA clone and pineal mRNA. DNA Cell Biol. 1993;12(8):715–727. [DOI] [PubMed] [Google Scholar]
- 30.Slominski A, Pisarchik A, Semak I, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002;16(8):896–898. [DOI] [PubMed] [Google Scholar]
- 31.Slominski AT, Kim TK, Janjetovic Z, et al. Differential and Overlapping Effects of 20,23(OH)(2)D3 and 1,25(OH)(2)D3 on Gene Expression in Human Epidermal Keratinocytes: Identification of AhR as an Alternative Receptor for 20,23(OH)(2)D3. Int J Mol Sci. 2018;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SL, Justiniano R, Williams JD, Cabello CM, Qiao S, Wondrak GT. The Tryptophan-Derived Endogenous Aryl Hydrocarbon Receptor Ligand 6-Formylindolo[3,2-b]Carbazole Is a Nanomolar UVA Photosensitizer in Epidermal Keratinocytes. J Invest Dermatol. 2015;135(6):1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slominski AT, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ, Paus R. Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. J Invest Dermatol. 2018;138(3):490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millan MJ, Marin P, Bockaert J, Mannoury la Cour C. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci. 2008;29(9):454–464. [DOI] [PubMed] [Google Scholar]
- 35.Alexander SP, Christopoulos A, Davenport AP, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors. Br J Pharmacol. 2017;174 Suppl 1:S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. Journal of cellular physiology. 2003;196(1):144–153. [DOI] [PubMed] [Google Scholar]
- 37.Nordlind K, Azmitia EC, Slominski A. The skin as a mirror of the soul: exploring the possible roles of serotonin. Exp Dermatol. 2008;17(4):301–311. [DOI] [PubMed] [Google Scholar]
- 38.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. [DOI] [PubMed] [Google Scholar]
- 39.Sadiq A, Shah A, Jeschke MG, et al. The Role of Serotonin during Skin Healing in Post-Thermal Injury. Int J Mol Sci. 2018;19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou L, Cai M, Ren Y, et al. The different roles of 5-HT1A/2A receptors in fluoxetine ameliorated pigmentation of C57BL/6 mouse skin in response to stress. J Dermatol Sci. 2018;92(3):222–229. [DOI] [PubMed] [Google Scholar]
- 41.Oh EJ, Park JI, Lee JE, et al. A novel role of serotonin receptor 2B agonist as an anti-melanogenesis agent. Int J Mol Sci. 2016;17(4):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janjetovic Z, Jarrett SG, Lee EF, Duprey C, Reiter RJ, Slominski AT. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci Rep. 2017;7(1):1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slominski AT, Kim TK, Li W, et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep. 2015;5:14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slominski AT, Kim TK, Hobrath JV, et al. Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols. Sci Rep. 2017;7(1):11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim TK, Lin Z, Li W, Reiter RJ, Slominski AT. N1-Acetyl-5-Methoxykynuramine (AMK) is produced in the human epidermis and shows antiproliferative effects. Endocrinology. 2015;156(5):1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim TK, Lin Z, Tidwell WJ, Li W, Slominski AT. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol Cell Endocrinol. 2015;404:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim TK, Kleszczynski K, Janjetovic Z, et al. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013;27(7):2742–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skobowiat C, Brozyna AA, Janjetovic Z, et al. Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. Journal of pineal research. 2018;65(2):e12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleszczynski K, Tukaj S, Kruse N, Zillikens D, Fischer TW. Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. Journal of pineal research. 2013;54(1):89–99. [DOI] [PubMed] [Google Scholar]
- 50.Kleszczyński K, Bilska B, Stegemann A, et al. Melatonin and its metabolites ameliorate UVR-induced mitochondrial oxidative stress in human MNT-1 melanoma cells. 2018;19(12):3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carter MD, Calcutt MW, Malow BA, Rose KL, Hachey DL. Quantitation of melatonin and n-acetylserotonin in human plasma by nanoflow LC-MS/MS and electrospray LC-MS/MS. J Mass Spectrom. 2012;47(3):277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab. 2008;19(1):17–24. [DOI] [PubMed] [Google Scholar]
- 53.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301(3):E484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol. 2013;168(3):595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams JM, Steketee JD. Time-dependent effects of repeated cocaine administration on dopamine transmission in the medial prefrontal cortex. Neuropharmacology. 2005;48(1):51–61. [DOI] [PubMed] [Google Scholar]
- 56.Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002;511(1–3):102–106. [DOI] [PubMed] [Google Scholar]
- 57.Slominski A, Ermak G, Hwang J, Mazurkiewicz J, Corliss D, Eastman A. The expression of proopiomelanocortin (POMC) and of corticotropin releasing hormone receptor (CRH-R) genes in mouse skin. Biochim Biophys Acta. 1996;1289(2):247–251. [DOI] [PubMed] [Google Scholar]
- 58.Slominski A, Baker J, Rosano TG, et al. Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. The Journal of biological chemistry. 1996;271(21):12281–12286. [DOI] [PubMed] [Google Scholar]
- 59.Fischer TW, Slominski A, Tobin DJ, Paus R. Melatonin and the hair follicle. Journal of pineal research. 2008;44(1):1–15. [DOI] [PubMed] [Google Scholar]
- 60.Backlund PS, Urbanski HF, Doll MA, et al. Daily Rhythm in Plasma N-acetyltryptamine. J Biol Rhythms. 2017;32(3):195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weissbach H, Redfield BG, Axelrod J. The exzymic acetylation of serotonin and other naturally occurring amines. Biochim Biophys Acta. 1961;54:190–192. [DOI] [PubMed] [Google Scholar]
- 62.White TA, Jenne JW, Evans DA. Acetylation of serotonin in vitro by a human N-acetyltransferase. Biochem J. 1969;113(4):721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone-proopiomelanocortin system in human melanocytes. Mol Endocrinol. 2006;20(10):2539–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126(9):1948–1965. [DOI] [PubMed] [Google Scholar]
- 65.Tosini G, Dirden JC. Dopamine inhibits melatonin release in the mammalian retina: in vitro evidence. Neurosci Lett. 2000;286(2):119–122. [DOI] [PubMed] [Google Scholar]
- 66.Nguyen-Legros J, Chanut E, Versaux-Botteri C, Simon A, Trouvin JH. Dopamine inhibits melatonin synthesis in photoreceptor cells through a D2-like receptor subtype in the rat retina: biochemical and histochemical evidence. J Neurochem. 1996;67(6):2514–2520. [DOI] [PubMed] [Google Scholar]
- 67.Naoi M, Maruyama W, Takahashi T, Ota M, Parvez H. Inhibition of tryptophan hydroxylase by dopamine and the precursor amino acids. Biochem Pharmacol. 1994;48(1):207–211. [DOI] [PubMed] [Google Scholar]
- 68.Kuhn DM, Arthur R Jr., Dopamine inactivates tryptophan hydroxylase and forms a redox-cycling quinoprotein: possible endogenous toxin to serotonin neurons. J Neurosci. 1998;18(18):7111–7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ali SA, Salim S, Sahni T, Peter J, Ali AS. 5-HT receptors as novel targets for optimizing pigmentary responses in dorsal skin melanophores of frog, Hoplobatrachus tigerinus. Br J Pharmacol. 2012;165(5):1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McEwan M, Parsons PG. Inhibition of melanization in human melanoma cells by a serotonin uptake inhibitor. J Invest Dermatol. 1987;89(1):82–86. [DOI] [PubMed] [Google Scholar]
- 71.Slominski A, Pruski D. Melatonin inhibits proliferation and melanogenesis in rodent melanoma cells. Exp Cell Res. 1993;206:189–294. [DOI] [PubMed] [Google Scholar]
- 72.Slominski A, Chassalevris N, Mazurkiewicz J, Maurer M, Paus R. Murine skin as a target for melatonin bioregulation. Exp Dermatol. 1994;3(1):45–50. [DOI] [PubMed] [Google Scholar]
- 73.Kleszczynski K, Bilska B, Stegemann A, et al. Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int J Mol Sci. 2018;19(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janjetovic Z, Nahmias ZP, Hanna S, et al. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. Journal of pineal research. 2014;57(1):90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.