Abstract
Background:
We have recently demonstrated that human-CD47 (hCD47) expressed on endothelial cells of porcine lung xenografts extended median graft survival from 3.5 days to 8.7 days in baboons. Intra-bone bone marrow transplantation (IBBMTx) in a pig-to-baboon model was previously shown to markedly prolong the duration of macrochimerism up to 21 days from 1–4 days by intravenous BMTx. We now examined whether the use of hCD47 transgenic (Tg) BM further prolonged the duration of chimerism following IBBMTx. We then tested if lung xenograft survival was prolonged following IBBMTx.
Methods:
Baboons received GalTKO-hCD47/hCD55Tg (n=5) or -hCD55Tg (n=1) or -hCD46/HLA-E Tg (n=1) pig IBBMTx. Macrochimerism, anti-pig T cells and antibody responses were assessed. Animals received lung xenografts from either hCD47+ or hCD47-porcine lungs 1–3 months later.
Results:
All baboons that received hCD47Tg porcine IBBM maintained durable macrochimerism >30 days, and two maintained chimerism for >8 weeks. Notably, anti-pig antibody levels decreased over time and anti-pig cellular unresponsiveness developed following IBBMTx. Lungs from hCD47Tg IBBMTx matched pigs were transplanted at day 33 or day 49 after IBBMTx. These animals showed extended survival up to 13 and 14 days, while animals that received lungs from hCD47 negative pigs displayed no prolonged survival (1–4 days).
Conclusion:
This is the first report demonstrating durable macrochimerism beyond 8 weeks, as well as evidence for B cell tolerance in large animal xenotransplantation. Using hCD47Tg pigs as both IBBMTx and lung donors prolongs lung xenograft survival. However, additional strategies are required to control the acute loss of lung xenografts.
Introduction
Studies involving the kidney and heart transplant of life-supporting solid organ xenografts in pig-to-baboon models have shown graft survival >6 months1–3 and heterotopic hearts for over 2 years 4. Despite this progress, the success of porcine lung grafts in baboons remains frustratingly limited, with an average survival of 3 days, even with the use of multi-transgenic donors 5. Using porcine lungs from donors in which human CD47 (hCD47) is expressed on endothelial cells (ECs), we have recently reported consistent prolonged survival to >7 days. This result is in contrast to results with porcine lung xenografts lacking hCD47 expression, which were uniformly rejected within 7 days of transplant 6. Expression of hCD47 appears to protect ECs from acute vascular rejection during the period immediately following transplant. However, as the grafts ultimately went on to be rejected between 7–10 days post-transplant, it is clear that additional strategies are required for the long-term survival of lung xenografts.
Previous data has shown that durable mixed xenogeneic chimerism following bone marrow transplantation (BMTx) leads to tolerance of T and B cells via suppression of the production of xenoantibodies, including anti-non-Gal natural antibody (nAb) in mouse models 7–12. We hypothesized that BMTx prior to lung xenograft transplant in our model would lead to mixed chimerism, which would facilitate prolonged lung xenograft survival. However, previous attempts of BMTx using traditional methods of intravenous (IV) administration were not successful in maintaining durable macrochimerism. Previous studies have shown that following α−1,3-galactocyltransferase knockout (GalTKO) pig BMTx, chimerism was typically lost within 48 hours 13, except in one case where it persisted for 4 days 14. In order to improve engraftment with persistent chimerism, we have recently developed a novel technique of intra-bone BM transplantation (IBBMTx) in a pig-to-baboon model, in which the cells are infused directly into the long bones of the recipient. By utilizing this strategy, we have reported peripheral blood macrochimerism for up to 21 days with evidence of BM engraftment, which is proven by colony forming unit assays 15. In addition, these baboons that received IBBMTx showed prolonged xeno kidney graft survival 15. Therefore, in this study we applied the IBBMTx strategy in our xeno lung Tx experiment.
Recent studies have demonstrated that interspecies incompatibility between CD47 and its ligand, signal regulatory protein alpha causes direct phagocytosis of porcine cells by human macrophages 16. We have successfully produced hCD47 Tg GalTKO miniature swine that express hCD47 in all blood cell lineages 17. Using endothelial cells isolated from these hCD47 pigs markedly reduced phagocytosis by both human and baboon macrophages (Yamada K et al. manuscript in preparation). We hypothesized that IBBMTx from hCD47 Tg pig donors would further prolong peripheral macrochimerism, which might facilitate extended lung xenograft survival in our pig-to-baboon model.
In this study, we first examined whether the use of BM cells from hCD47 Tg pigs along with our IBBMTX procedure 15 would extend the duration of macrochimerism and reduce anti-pig preformed antibody levels, as well as T cell unresponsiveness following IBBMTx in baboons. We next tested whether baboon recipients of IBBMTx would have prolonged lung xenograft survival, and if hCD47 Tg GalTKO pigs and/or syngeneic donors (produced from the same cell line to BM donor, which means SLA was also matched to the BM donor) are required as donors of lung xenografts.
MATERIALS AND METHODS
Animals
All animal work was conducted in accordance with NIH and USDA guidelines and with approval from the Columbia University Institutional Animal Care and Use Committee.
Recipients:
Seven baboons (Papio hamadryas) purchased from Mannheimer Foundation, Homestead, FL or gifted from The University of Oklahoma Health Sciences Center (Oklahoma City, OK) were used as recipients. The baboons with anti-non Gal cytotoxicity <40% were selected from screening cytotoxicity assay, as previously described 6 (Table 1).
Table 1.
Recipients, donors (IBBM and lungs) and numbers & sites of BM administration
| Baboon ID | 15P17 | 14P3 | 14P84 | 14P54 | 2715 | 14P55 | 17214 |
|---|---|---|---|---|---|---|---|
| Number of BM cells intraosseously infused (109/kg) | 3.7 | 0.38 | 0.96 | 0.2 | 0.3 | 0.8 | 0.02 |
| Body weight of baboon (kg) | 7.5 | 8.6 | 7.5 | 8.4 | 11 | 7.7 | 10 |
| Cytotoxicity (%) | 29 | 29 | 36 | 21 | 12.8 | 14.5 | 21.9 |
| Tg of GalTKO BM donor | hCD47 hCD55 CRI |
hCD47 hCD55 CRI |
hCD47 hCD55 CRI |
hCD47 hCD55 CR) |
hCD47 hCD55 CRI |
hCD55 | hCD46 HLA-E Rev |
| Body weight of BM donor (kg) | 12.7 | 15 | 13.8 | 11.8 | 13 | 46.7 | > 50kg (defrosted BM cells) |
| Infused sites | Both tibia | Both tibia | Both tibia | Both tibia | Both tibia | Both tibia | Both tibia |
| Tg of GalTKO Lung donor | hCD47 hCD55 (CRI) |
hCD47 hCD46 TFPI SLA-II low (Rev) |
GalTKO alone (SLA-DH) |
hCD47 hCD46 TFPI (Rev) |
hCD47 hCD55 (CRI) |
hCD47 hCD55 (CRI) |
hCD46 HLA-E B4KO (Rev) |
| SLA matching between BM and lung donor | Matched syngeneic |
Mismatched | Class I mismatched | Mismatched | Matched syngeneic | Matched | Matched syngeneic |
| Body weight of Lung donor (kg) | 11.8 Lower lobe graft |
9 | 9.6 | 10.7 | 17 Lower lobe graft |
10 | 14.5 |
BM: bone marrow, CRI: SLA-HH hCD47/hCD55 Tg miniature swine, Rev: pig donor provided by Revivicor Inc., SLA: swine leukocyte antigen, TFPI: tissue factor pathway inhibitor, Tg: transgene
Donors:
Two different lines of GalT-KO pigs were used as donors. One was the MHC-inbred Columbia/Sachs GalT-KO miniature swine with or without the addition of transgenic hCD47 and/or hCD55 17. They were swine leukocyte antigen (SLA)-HH or DH type miniature swine. Especially, the SLA-HH hCD47/hCD55 Tg pig was called CRI (Table 1). The others were hCD46/hCD47/tissue factor pathway inhibitor (TFPI), hCD46/hCD47/TFPI/SLA-II low Tg GalT-KO, hCD46/human leukocyte antigen (HLA)-E and β4GalNT2-KO (B4KO) hCD46/HLA-E produced and provided by Revivicor Inc., Blacksburg, as previously described 18. (Table 1, 2).
Table 2.
Results of delayed lung transplantation in recipients of IBBMTx
| Baboon ID | PBMC and BM hCD47 expression of BM donor |
Timing of lung Tx |
Chimerism at the time of lung Tx |
Reduction of anti-pig nAb after IBBMTx |
IFN gamma ELSIPOT |
Graft survival (day) |
Lung findings |
SLA matching between BM and Lung donors |
hCD47 expression on graft lung ECs |
|---|---|---|---|---|---|---|---|---|---|
| 15P17 | hCD47+ | 49 | Yes | Yes | Pan pig unresponsiveness | 13 | Graft loss but partially aerated | Syngeneic cell line | Positive |
| 2715 | hCD47+ | 33 | Yes | Yes | Pan pig unresponsiveness | 14 | Graft loss but partially aerated | ||
| 17214 | Without hCD47 | 28 | NO Lost chimera on day 25 |
Yes | Pan pig unresponsiveness | 1 | Almost hemorrhagic /congestion | Negative | |
| 14P3 | hCD47+ | 91 | No Lost chimera on day 70 |
Yes | Pan pig unresponsiveness | 4 | Almost hemorrhagic /congestion | Not syngeneic cell
line, SLA mismatched |
|
| 14P84 | hCD47+ | 69 | No Lost chimera on day 70 |
Yes | Pan pig unresponsiveness | 2 | Totally hemorrhagic | ||
| 14P54 | hCD47+ | 96 | No Lost chimera on day 70 |
Yes | Unresponsive to BM donor but small response to Rev | 26 hours | Almost hemorrhagic /congestion |
IBBMTx: intra-bone bone marrow transplantation, IFN: interferon, EC: endothelium, nAb: natural antibody
Surgical procedure
Intra-bone Bone Marrow Tx (IBBMTx)
The baboons underwent splenectomy as well as central venous line insertion on day −14 to −7 prior to IBBMTx. BM harvest was performed as previously described 15. Collagen gel matrix, Cellmatrix (WAKO Inc, Osaka, JAPAN) was used for the IBBMTx to hold donor BM cells in the recipient BM cavity, as previously described 15. In a similar manner to our previous studies, approximately half of the processed pig BM cells were mixed with the collagen gel matrix and infused into the bone cavities of the tibiae of the recipients. The rest of the pig BM cells were intravenously infused on the same day.
Delayed lung Tx
Recipients of pig BM received a lung from another donor pig 28 to 90 days after IBBMTx. This wide range was due to limited availability of lung donors of the required size. BM recipient baboons received a left lung graft from either SLA-matched or mismatched GalTKO pig with or without a hCD47Tg. The lung graft was harvested from donor pigs and immediately perfused with Perfadex (XVIVO Perfusion AB, Göteborg, Sweden) and then transplanted orthotopically by left thoracotomy in a non-life-supporting condition as previously described 6, 19,6,18 (Table1, 2). The total ischemic time was within 1 hour in all the cases. All baboons were sedated for 16 hours after the lung Tx under ventilation to rule out technical failure with observation by thoracotomy, as previously reported 6.
Immunosuppression/conditioning regimen
All animals received a modified non-myeloablative conditioning regimen as previously reported (Supplemental Fig 1A) 15, consisting of 100 cGy of total body irradiation on day −7 and 700 cGy of thymic irradiation on day −2, Rituximab (Genentech, San Francisco, CA, USA) at 20 mg/kg on day −14 to deplete B cells and anti-thymocyte globulin (ATG, Thymoglobulin, Genzyme, Lyon, France) at 10 mg/kg on day −3 and −2 to deplete T cells. Additionally, tacrolimus (Prograf, Astellas Pharma Inc., Tokyo, Japan, 0.05 to 0.15 mg/kg/day, to achieve a target blood level of 5 to 15 ng/ml, discontinued before the lung Tx) and mycophenolate mofetil (MMF, Genentech, San Francisco, CA, USA, at 70 mg/kg/day) were administered intravenously over 24 hours daily from day −6. Anti-CD154 mAb (ABI793, HuMAb-Mouse, Medarex Inc., Annandale, NJ, 20 mg/kg/day IV) was administered three times a week beginning on day 2 and discontinued before the lung Tx. Cobra Venom Factor (CVF, Advanced Research Technologies, San Diego, CA, USA) was administered daily for complement inhibition from day −1 until the end of the study. For anti-coagulation, heparin (10 U/kg/h IV) was administered prior to the start of IBBMTx for a target activated clotting time of 130 to 150 seconds, and prostacyclin (FLOLAN, 30 ng/kg/min, GlaxoSmithKline, NC, USA) was administered daily from day 0 for 14 days 15 to prevent thrombotic microangiopathy 20. Recombinant porcine cytokines, porcine stem cell factor (pSCF. Gift from BioTransplant, Inc., Charlestown, MA, USA) and porcine interleukin-3 (pIL-3, BioTransplant, Inc.) were given intravenously over 24 hrs daily at 30 ug/kg/day from day 0 to 28 after IBBMTx. Ganciclovir at 10 mg/kg/day was administered intravenously once a day throughout experimental period from day 0 to prevent baboon cytomegalovirus disease after xeno IBBMTx transplant. Additionally, all baboons received methylprednisolone at 5 mg/kg, given intravenously soon after revascularization of the lung grafts to reduce pulmonary edema associated with ischemia-reperfusion injury 21. Our protocol for xeno lung Tx in this study as well as in our previous study of primary xeno lung Tx includes anti-IL6 receptor Ab (Tocilizumab, ACTEMRA, San Francisco, CA, USA) at 10 mg/kg once a week from the day of the lung Tx, based on our previous data showing that IL-6 in sera increased following xeno lung Tx19. Since thrombosis is a well-known side effect of anti CD154 mAb22, we switched from anti-CD154mAb to anti-CD40mAb after the lung Tx. Anti-CD40 mAb was administered three times a week from 2 days after the lung Tx (Supplemental Fig 1B).
Flow cytometry (FCM) to detect pig BM cell chimerism and Tg expression in donor peripheral blood mononuclear cells (PBMC) and BM
For baboon cell populations, antibodies against CD3 (BD Pharmingen: clone SP34–2), CD4 (BD Horizon: clone L200) and CD8 (BD Horizon RPA –T8) for T cells, CD20 (BD Horizon: clone 2H7) and CD22 (Life technology, MHCD 2201) for B cells, NHP CD45 (BD biosciences: D058–1283) for all baboon leukocytes were used. In order to detect pig cells specifically (macrochimerism), we used biotinylated 1030H1–19 (mouse anti-pan-pig tissue, IgM) and allophycocyanine (APC)-Streptavidin (BD Biosciences). After incubation, cells were washed twice and lysed with FACS lysing solution (BD Biosciences, San Jose, CA), and then washed twice again. Cells were read on FACS Canto II (Becton Dickinson, Mountain View, CA, USA) for detection of cell-bound antibodies, and data was analyzed using FlowJo (V.10. 1, Tree Star, Ashland, OR, USA). The percentage of pig cells (macrochimerism) staining with anti-pan-pig antibody in gating for whole leukocytes was determined by comparing to PBMC staining from the naïve pig cells as positive control, and naïve baboon cells as negative control. To confirm Tg expression in donor pigs’ PBMC and BM, anti-human hCD47Ab (BD Pharmingen, clone: B6H12), hCD55 (Biolegend, clone: JS11), hCD46 (BD Pharmingen, clone: E4. 3) and HLA-E (Biolegend, clone: 3D12) were used.
ELISPOT assay and Mixed Lymphocyte Reaction (MLR)
Both interferon gamma (IFNγ) ELISPOT and MLR assays were performed using methods previously reported 1, 6, 15. Polyvinylidene difluoride 96-well plates (Millipore) were coated with anti-IFNγ Ab. Then, baboon PBMC and irradiated cells (self, donor, third party, human and media) were incubated for 48 hours. After incubation, biotinylated anti-IFNγ Ab was used to detect IFNγ secreted from baboon PBMC. For MLR, baboon PBMCs were stained with eFluor, and incubated with irradiated stimulator cells (self, donor, third party, human and media) for 72 hours. After incubation, cells were evaluated on a FACS Canto II (Becton-Dickinson) with eFluor detection (647 nm excitation) via an APC filter. Dye expression was dampened by even distribution into daughter cells. Stimulation index was calculated based on anti-self-proliferation.
Antibody-FCM
Preformed nAb as well as elicited anti-donor antibodies were assessed using PBMC from donor pigs incubated with decomplemented recipient sera detected with anti-human IgG conjugated to fluorescein isothiocyanate (FITC). Briefly, each 1×105/tube of donor PBMC was incubated with each recipient baboon sera by the time series for 30 minutes. The cells were washed and then incubated with IgG-FITC as a secondary Ab. The cells were acquired on FACS Canto II, as previously described 23. Results were analyzed using FlowJo (V.10. 1, Tree Star, Ashland, OR, USA). The level of nAb was calculated as 100%. For the sera of 15P17, 14P55, 2715 and 17214, BM donor pig cells were used for the assays, since the baboons received the pig lungs from the donors that were either syngeneic or SLA matched to the BM donors. For 14P3, 14P54 and 14P84, lung donor PBMC were used because the lung donors were SLA-mismatched to the BM donors.
Histology
-
a)
Formalin-embedded specimens: Lung specimens were fixed in 10% formaldehyde and embedded in paraffin. Tissues were sectioned, stained using hematoxylin and eosin (H&E), and examined by an experienced pathologist.
-
b)
Frozen tissue for immunofluorescence analysis: Frozen samples were used for immunofluorescence (IF). Anti-human IgM, IgG, C3, C5b and C4d conjugated to FITC (all from DAKO, Carpentaria, CA), were used for IF to assess antibody binding and complement activation in the graft 6. To confirm actual Tg expression in the donor lungs, the right lung was used and stained with anti-human CD46 (BD Biosciences, San Jose), hCD55 (JS11; BioLegend, San Diego, CA), and hCD47 (Abcam, Cambridge, MA). Endothelial cells were stained with anti-CD31 antibody (ab28364, Abcam, Cambridge, MA).
Results
Tg expression of hCD47 and hCD55 in donors
We examined Tg expression of pig donors’ PBMCs and BM. Expression of hCD47 and hCD55 was confirmed on PBMCs and BM cells isolated from hCD47/hCD55 Tg pigs (Fig 1C and D) and their absence was confirmed on PBMCs and BM cells from non-hCD47/CD55 Tg pigs (Fig 1A, B). We also assessed hCD46 and HLA-E expression on PBMCs and BM of an hCD46/HLA-E Tg pig. hCD46 was positive on both PBMC and BM cells (Fig 1F and G) while HLA-E expression on BM was weak (Fig 1F and G).
Figure 1:

Tg expression assessed by FCM. (A-D) X-axis demonstrates hCD55 and Y–axis demonstrates hCD47. (E, F) X-axis demonstrates HLA-E and Y-axis demonstrates hCD46. A: PBMC of GalTKO. B: PBMC of hCD55 Tg Sachs GalTKO pig, C: PBMC of hCD47/hCD55 Tg pig, D: BM of hCD47/hCD55 Tg Sachs GalTKO pig. In hCD47/hCD55 Tg pig PBMC and BM, both Tg were well expressed, whereas hCD47 expression was absent in Sachs GalTKO and hCD55 GalTKO pigs. E: PBMC of GalTKO pig, F: PBMC of hCD46/HLA-E Tg Revivicor pig, G: BM of hCD46/HLA-E Tg Revivicor pig. The expression of HLA-E was weak in BM cells. BM: bone marrow, FCM: flow cytometry, h: human, HLA: human leukocyte antigen, PBMC: peripheral blood mononuclear cell, Tg: transgene.
Clinical outcomes following IBBMTX
None of the animals developed lethal clinical complications such as severe anemia or thrombocytopenia (Fig. 2A and B) and no baboon developed leukopenia following IBBMTx except for several days following the administration of ATG (Fig 2 C). All of the cases remained clinically stable.
Figure 2:
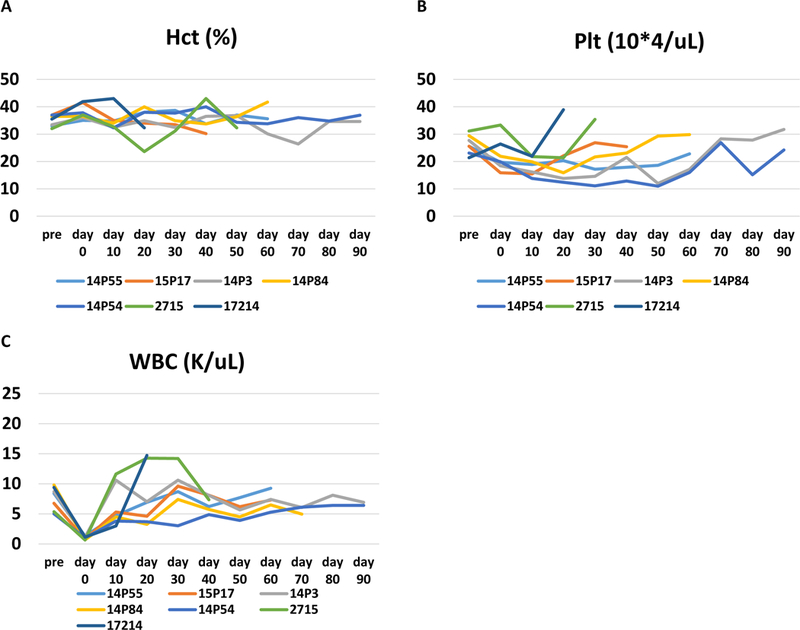
Hematocrit (A, %), Platelet count (B, 10*4/uL) and white blood cell count (C, K/uL) of each baboon. None of the animals required a blood transfusion to treat anemia or thrombocytopenia following IBBMTx. Hct: Hematocrit, Plt: platelet, WBC: white blood cells.
Durable macrochimerism was maintained longer than 8 weeks following IBBMTx using hCD47 Tg pigs as donors
Table 1 summarizes the numbers of BM cells in IBBMTx, Tg information of donors and sites of infusion. Fig 3A shows % peripheral macrochimerism in baboons that received hCD47+/hCD55+ GalTKO Sachs pig IBBMTx (n=5. red lines), hCD55+ GalTKO Sachs pig IBBMTx (blue), hCD46/HLA-E/GalTKO Revivicor pig (green) and GalTKO Sachs pig alone (historic control 15, n=6, black) assessed by FCM. Macrochimerism was uniformly detectable beyond 4 weeks in baboon recipients of hCD47+/hCD55+ GalTKO IBBMTx (red lines). Four of 5 baboons maintained macrochimerism beyond 60 days. These animals received delayed lung grafts at days 33, 49, 69, 91 and 96, respectively. Two animals received a delayed lung graft while chimerism was still present (arrows in Fig 3B). One baboon (2715) received a delayed lung graft from a syngeneic hCD47 Tg pig on day 33 after IBBMTX and maintained chimerism for 7 days after delayed lung Tx (day 40 after IBBMTX). The other baboon (15P17) received a delayed lung graft at day 49. Although macrochimerism decreased at PODs 2 and 7, it reappeared and remained detectable at 13 days after delayed lung Tx (Dashed line with red square symbols in Fig 3B). In contrast, neither of the baboons that received lung grafts from hCD55+ GalTKO or hCD46/HLA-E GalTKO pigs maintained macrochimerism beyond 4 weeks. The level of chimerism in these two baboons (Fig 3A blue and green) was similar to historic controls of GalTKO IBBMTx 15 (Fig 3A black lines).
Figure 3:



A: The percentage of chimerism in each case following IBBMTx. 15P17 and 2715 received a lung Tx while they had peripheral chimerism (15P17 on day 49 and 2715 on day 33 following IBBMTx, respectively). Arrows indicate the day of lung Tx. B: FCM profiles of macrochimerism in blood and BM of 15P17. C: The average percentage with standard deviation of macrochimerism following IBBMTx before organ Tx. Black lines: GalTKO alone porcine BM recipients (n=6). Red lines: recipients of hCD47/hCD55/GalTKO (n=5). Blue line: recipient of hCD55/GalTKO pig (n=1). Yellow line: recipients of hCD46/HLA-E/GalTKO (n=1). IBBMTx: intra-bone bone marrow transplantation.
When we calculated the average percent of chimerism following IBBMTx until day of delayed lung Tx in baboons that received a hCD47+/hCD55+ GalTKO IBBMTx (n=4. red line), hCD55+ GalTKO IBBMTx (light blue), hCD46/HLA-E GalTKO (yellow) and GalTKO alone (historic control 15, n=6, black), the beneficial effects of hCD47 expression on BM cells were clearly demonstrated (Fig 3C). % of chimerism at day 30 following IBBMTx in the baboons that received hCD47+/hCD55 GalTKO IBBMTx was significantly higher than that of GalTKO alone (t-test p = 0.0073).
The marked decline of anti-donor IgG antibodies following IBBMTx
We assessed the level of anti-non Gal nAb pre and post IBBMTx. No elicited anti-non Gal nAb was observed following IBBMTx in any of the recipients. Four baboons, including one with hCD55/GalTKO without hCD47Tg, were followed for >60 days. Notably, all three baboons that received hCD47+Tg IBBMTx had a reduction of anti-pig nAb more than 30% compared to pre IBBMTx levels (31%, 40% and 53% reduction. Red lines in Fig 4A), while one animal that received hCD55+ BM without hCD47 had only a 20% reduction at day 60 (blue line in Fig 4) after IBBMTx. Furthermore, the levels of anti-non Gal nAb levels among three recipients (14P3, 14P84 and 14P54), which were followed up beyond 60 days after hCD47+/hCD55+ porcine IBBMTx, declined markedly (Fig 4). Two baboons (15P17 and 2715) that received hCD47+ GalTKO lung grafts syngeneic to BM donors at Day 49 or Day 33 had slightly decreased anti-pig nAb after lung Tx, suggesting that residual anti-pig nAb was absorbed in the lung grafts.
Figure 4:

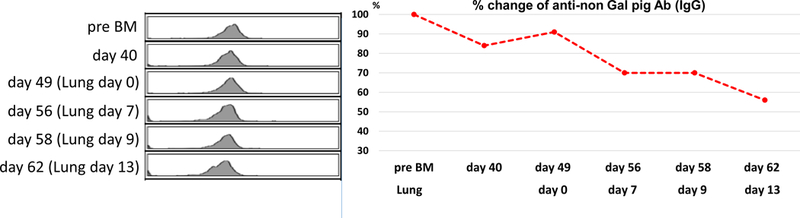

A: % reduction of anti-pig IgG binding assessed by FCM following IBBMTx. The value of each baboon’s preformed nAb was considered as 100 %. >30% reduction pf IgG levels was observed 60 days following hCD47+ IBBMTx (14P3, 14P84 and 14P84). B, C: FCM data and % of reduction of antibody throughout the experiment (B: #15P17, C: #2715). The value of Pre BM was evaluated as 100 %. Further decline was observed following lung Tx. Ig: immunoglobin, nAb: natural antibody.
Absence of T cell sensitization measured by IFN gamma ELISPOT and MLR assay following IBBMTx
The IFN gamma ELISPOT assay was used to assess T cell memory responses as well as NK responses, and MLR assays were used to assess proliferative responses just prior to lung transplantation. Fig 5A shows the number of spots of IFN-gamma seen on ELISPOT assay at pre IBBMTx without any conditioning and the time of delayed lung Tx from all seven baboons that received IBBMTx. Anti-donor pig unresponsiveness was observed, while the anti-human response was uniformly preserved in all the recipients prior to the lung Tx. Assays of baboons 14P3, 14P54 and 14P55 that were set up beyond 60 days still showed donor pig specific unresponsiveness. Weak responses against third-party pig stimulators were detected. We also performed cellular assays at the time of graft loss for baboons 15P17, 14P3, 2715 and 14P55. Unfortunately, we could not perform cellular assays at the time of graft loss in the remaining two baboon recipients due to insufficient blood samples. The results at the time of graft loss demonstrated that no anti-lung donor cellular response against donors was observed even though the lung grafts were being lost. Fig 5B shows stimulation indexes of MLR assays of four recipients at the time of pre BM and prior to delayed lung Tx. We unfortunately did not have enough cells to set up for the remaining three recipients. 14P55 and 17214 had anti-pig specific unresponsiveness with strong anti-human responses. Notably, 2715 showed donor-pig specific unresponsiveness (Fig 5A–5, 5B–2 and Supplemental Fig 2A and 2B).
Figure 5:
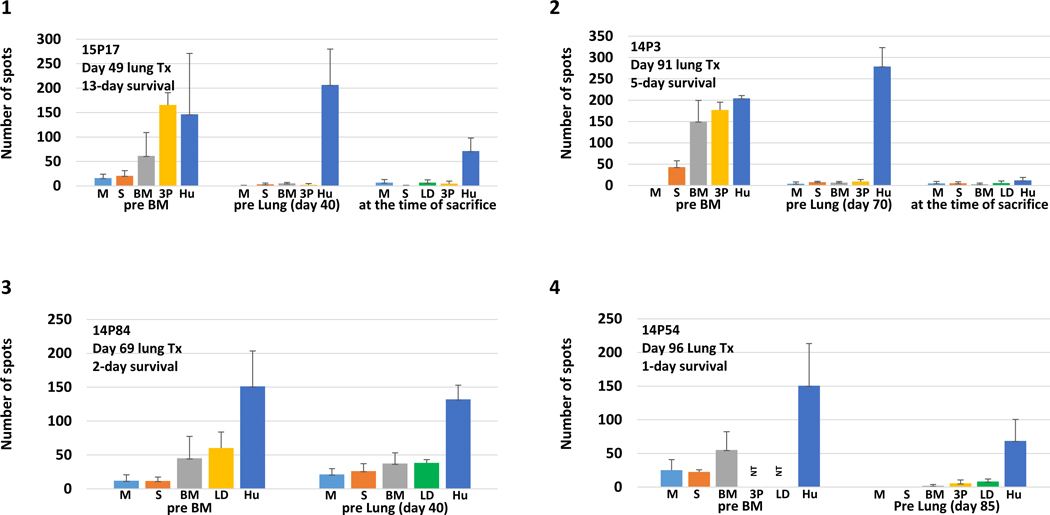

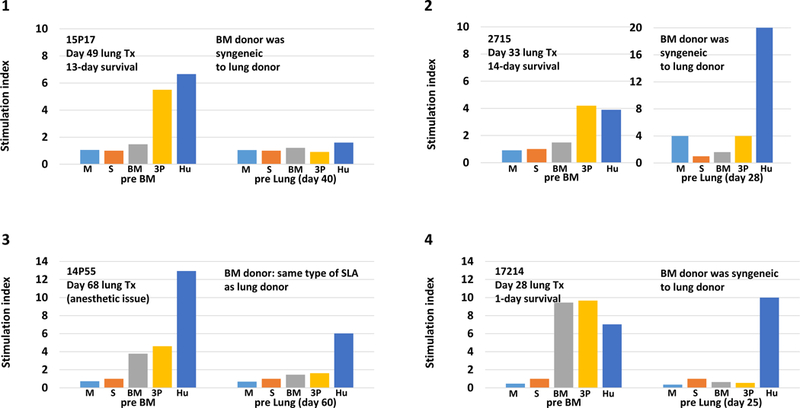
A: Number of spots of IFN gamma ELISPOT assays pre BM, at time of delayed lung Tx, and at the time of sacrifice from IBBMTx recipients. (1) 15P17, pre BM, 40 days following IBBMTx, at the time of sacrifice. (2) 14P3, pre BM, 70 days and at time of sacrifice, (3) 14P84, pre BM and 40 days, (4) 14P54 pre BM and 85 days, (5) 2715, pre BM, 28 days and at the time of sacrifice, (6) 14P55, pre BM and 60 days and at the time of sacrifice, and (7) 17214, pre BM, 25 days. Anti-BM and lung donor pig unresponsiveness was observed while anti-human response was uniformly preserved in all recipients prior to lung Tx in comparison with the results of pre BM without any conditioning. At the time of sacrifice, anti-lung donor unresponsiveness was observed in 15P17, 14P3,2715 and 14P55. Assays of baboons 14P3, 14P54 and 14P55 that were set up beyond 60 days still showed donor pig-specific unresponsiveness. Small responses against the third-party pig stimulator were seen. B: Stimulation indexes of MLR assays, pre BM and at the time of delayed lung Tx (4 baboons recipients of IBBMTx). (1) #15P17, pre BM and 40 days following IBBMTx, (2) 2715 pre BM and 28 days, (3) 14P55 pre BM and 60 days and (4)17214 pre BM and 25 days. Although 15P17 had general unresponsiveness, the other three demonstrated anti-human responses at the pre lung period. 14P55 and 17214 had anti-pig-specific unresponsiveness while 2715 showed donor pig-specific unresponsiveness in comparison with the results of pre BM.
Target cells: medium (M), self (S), bone marrow donor (BM), lung donor (LD), third party pig (3P) and human (H). NT: not tested.
Outcome of delayed lung transplant following IBBMTX, suggesting that hCD47 on vascular endothelium of lung graft as well as syngeneic combination of IBBMTx donors and lung donor are required
We addressed whether baboon recipients of IBBMTx had prolonged lung xenograft survival, and if hCD47 Tg GalTKO pigs and/or syngeneic swine are required as donors of lung xenografts. All baboons were anti-pig unresponsive which was assessed by ELISPOT assays at the time of delayed lung Tx, and they received a delayed lung Tx using the left lung from GalTKO swine with various Tgs 28 to 96 days following IBBMTx (Table 2). All seven received a delayed lung Tx. One baboon, 14P55, had a failed delayed lung Tx procedure due to anesthetic issues during surgery (14P55 with hCD55+/GalTKO IBBMTx).
As shown in Table 2, two of the other six baboons maintained their delayed transplanted lung xenografts for 13 days and 14 days respectively. These baboons received hCD47+/hCD55+/GalTKO IBBMTx and lung grafts using syngeneic donors to BM donors. Both were stable until the morning of POD 13 (15P17) or POD 14 (2715). However, since they developed fits of coughing, we performed thoracotomy to check the lung grafts. Thoracotomy of 15P17 at POD 13 after delayed lung Tx showed graft loss but the graft was partially aerated. Histology confirmed that areas of aeration remained although hemorrhagic changes were observed (Fig 6–1). Immunofluorescence staining showed only minimal IgM and IgG deposition, and no C3, C5b or C4d was detectable in the viable area of the excised lung graft (Fig 6 2–6). Non-viable area did not provide a quality assessment due to high background of staining. The minimal IgM or IgG deposits are likely due to residual preformed nAb, not elicited Abs based on FCM data (Fig 4B). The lung graft of baboon 2715 at POD 14 also showed similar findings. In these two cases (15P17 and 2715), we confirmed hCD47 expression on ECs of lung grafts (Fig 7A shows representative findings from the graft of baboon 15P17).
Figure 6:

1) Pathological findings of graft lung of 15P17 at the time of euthanasia on day 13 following lung Tx. There were viable aerated areas in the graft lung although hemorrhagic changes were also seen. 2–6) Immunofluorescence staining of IgM (2), IgG (3), C3 (4), C5b (5) and C4d (6). There was some weak IgM and IgG deposition in the lung graft. However, no deposition of IgG, C3 or C5b was observed.
Figure 7:

hCD47 expression in graft lung of 15P17 (A) and 14P54 (B). Green: hCD47, Red: CD31. A: Orange/yellow color indicates hCD47 expression on ECs. B: hCD47 expression was only positive on alveolar epithelium. No hCD47 expression on ECs of the grafts.
However, in contrast to these 2 cases, all other hCD47 Tg lung donor pigs did not express hCD47 on lung EC. Two animals (14P3, 14P54) received BM from hCD47/hCD55 Columbia/Sachs miniature swine, and then received SLA-mismatched lung grafts from Revivicor multi-Tg, including hCD47, GalTKO pigs. Despite hCD47 Tg, these lung donor lungs had positive expression of hCD47 only on alveolar epithelium and not on ECs, as was also observed in our previous study 6. The lung grafts were rejected in a relatively acute period (Fig 7B shows representative findings from the graft of baboon 14P54). Baboon 14P84 also received BM from a hCD47/hCD55 Tg Columbia/Sachs miniature swine (SLA-HH), and then received the lung graft from SLA-mismatched Columbia/Sachs GalTKO alone without any Tg miniature swine (SLA-DH). Although we found a decline in anti-lung donor Ab level prior to lung Tx, the lung graft was rejected on POD 2. Baboon 17214 received the lung graft from a hCD46/HLA-E/B4KO GalTKO without hCD47Tg pig that was syngeneic to the IBBMTx donor. Although the animal had no elicited Ab and no anti-donor cellular responses, the graft was rejected on POD 1.
Discussion
We have previously shown that survival of lung grafts from hCD47+GalTKO swine extended to a median of 9 days compared to 3 days for hCD47-lungs or lungs lacking expression of hCD47 on lung EC’s in a pig-to-baboon model 6. Our data shows that lung grafts from pig donors are highly susceptible to acute humoral rejection which can be mitigated, but not totally avoided, by the transgenic expression of hCD47 on endothelium of donor lungs.6 By utilizing a mixed chimerism strategy we have achieved global immune tolerance, including at the level of B cells and NK cells in mouse models 24, 25. We have shown that durable mixed xenogeneic chimerism is also possible in mice with human immune systems, which has also resulted in tolerance of human T cells 26, 27.
In this study we have for the first time achieved durable porcine chimerism for over 60 days in a non-human primate. In keeping with the results from rodent studies this chimerism was associated with a marked decline in anti-pig preformed nAb. Durable chimerism was achieved in these baboons by combining our IBBMTx method 15 with the use of hCD47Tg GalTKO pig donors17. Notably, we found that the level of anti-non Gal nAb IgG decreased markedly following IBBMTx by using hCD47 Tg BM. Although this has been previously reported in a GalTKO mouse model 26, 27 our findings are the first demonstration of durable macrochimerism with reduction of anti-non Gal nAb in a pig-to-baboon BMTx model. Even though the baboons received immunosuppression following IBBMTx, reduction of anti-non Gal nAb was not seen in the previous study with the same immunosuppressive regimen that lost macrochimerisms by day 21 following IBBMTx using GalTKO pigs as donors15. The second major achievement is the fact that both baboons that received hCD47+ IBBMTx had 13 days and 14 days survival of syngeneic delayed lung grafts in which ECs express hCD47. Although survival of greater than 2 weeks wasn’t achieved, this reproducible result of 2-week survival of lung xenografts are the first reported cases in a pig-to-baboon Tx model.
We have previously shown the effectiveness of IBBMTx, which allowed persistent chimerism for up to 21 days following transplant of porcine BM from GalTKO alone swine15. This is in contrast to only a couple of days survival after intravenous BM Tx 13, 14. In this study, we have clearly demonstrated prolonged peripheral macrochimerism beyond 60 days by utilizing IBBMTx with hCD47+/hCD55+ GalTKO BM. Although these donors included hCD55Tg, neither hCD55Tg nor hCD46 alone facilitated BM cells to survive beyond 30 days, indicating that hCD47 expression on BM cells is essential for durable chimerism. We also confirmed that the IBBMTx strategy is essential, based on our previous study demonstrating the limited duration of chimerism following hCD47+/hCD55+ GalTKO BM Tx using traditional intravenous infusion 17.
A major advancement in this challenging model is that two consecutive baboons displayed 13 days and 14 days survival of lung xenografts that were transplanted 1 to 2 months following IBBMTx. However, although all baboons receiving IBBMTx, including other 4 baboons, had no anti-donor T cell responses assessed by ELISPOT or MLR at the time of delayed lung Tx, all lung grafts were rejected with hemorrhagic changes. Our data from this study suggest that several key elements must be used to optimize the survival regimen. Despite the fact that anti-pig T cell unresponsiveness was achieved following IBBMTx, 1) lung xenograft EC’s must express hCD47, 2) syngeneic donors must be used for IBBMTx and subsequent delayed lung Tx, and 3) the waiting period for delayed lung Tx needs to be longer than 2 months following IBBMTx in order to allow nAbs to decline further. Our previous studies in allogeneic pig or non-human primate models demonstrated that presence of macrochimerism is important for accepting delayed transplanted kidneys 28, 29. Both of the two longest survivors in our current study had macrochimerism at the time of delayed lung Tx, at day 33 and day 49 respectively. However, the lung grafts were transplanted at early time points after IBBMTx, and these grafts were lost with hemorrhagic changes, suggesting that further reduction of nAb is likely required to improve graft survival. Unfortunately, because of limited availability of syngeneic donors, two baboons (14P3 and 14P54) whose preformed nAb decreased >60% (Fig 4) received SLA-mismatched lung xenografts in which ECs did not express hCD47. Future studies of delayed xeno lung Tx >3 months following IBBMTx utilizing hCD47+ syngeneic pigs will clarify the role of persistence of chimerism in lung xenografts.
Baboons for our experiments are mostly within the 7–14 kg range. Since delayed lung donors must be size matched to recipients, we cannot use siblings of the same litter for IBBMTx donors and delayed lung xenograft donors because of the waiting period between IBBMTx and the delayed lung Tx. Therefore, a specific cloning or breeding plan is required to optimize ideal donor and recipient pairings in our experimental design. In addition, SLA-matched lung donors to BM donors with hCD47 expression might be another option if future experiments confirm that minor antigen disparity does not cause rejection of delayed lungs. These very necessary steps point the way forward to optimizing the regimen for successful lung xenografts.
Finally, although the two consecutive baboons that displayed 13 days and 14 days survival of lung xenografts transplanted 1–2 months following IBBMTx is a major advancement in this challenging model, our success with xeno lung transplantation still lags significantly behind the recent prolonged survival results of cardiac 3, 4 and renal 1, 2 xenografts. Lung xenografts appear to be highly susceptible to antibody-mediated rejection, so preformed nAb levels should be as close to zero as possible, as assessed by FCM binding of porcine cells either in PBMC or ECs. Ab adsorption by donor organs might also be helpful at the time of delayed lung Tx. Additional strategies related to inhibiting the innate immune response within the lung may be needed to target innate host defenses 19, 30–32 due to the fact that the alveolar capillary network is particularly susceptible to endothelial activation or destruction associated with the innate response. Recent genetic modification techniques have allowed for the addition of multiple genes and knockout of B4 or NeuGc non Gal antigens 33–35. However, recent reports also suggest the existence of other as yet undefined antigens associated with additional KO manipulation 34,27. Our previous studies in rodents showed that inducing durable mixed chimerism tolerizes nAb-forming B cells of all specificities, obviating the need to identify each possible target of nAbs 7, 8. The results obtained here suggest that tolerance of nAb-forming B cells is similarly achieved by inducing mixed chimerism. Future studies will build on this work to achieve even more lasting macrochimerism in pig-to-baboon models. Experiments to optimize future experimental designs for successful lung xenografts are currently in progress.
Supplementary Material
Fig. 1A. Immunosuppression regimen and procedures of IBBMTx
Fig. 1B Immunosuppression regimen and procedures of delayed lung Tx
Fig. 2A IFN gamma ELISPOT assays at pre IBBMTx, day 28 after IBBMTx (pre delayed lung Tx), and POD 14 after delayed lung Tx.
Fig. 2B. MLR assays at pre IBBMTx and day 28 after IBBMTx (pre delayed lung Tx) in baboon 2715. Lymphocyte gated cells were analyses.
Acknowledgments
We thank Dr. Tomoshi Tsuchiya and Dr. Takeshi Nagayasu for their critical review of the manuscript. We thank Dr. Robert Hawley for his advice and information regarding transgenic pigs in this study. We also thank Harrison Glor for his technical assistance and Haruna Shimizu for her editorial assistance. We thank Genzyme for their gift of rabbit ATG and Genentech for their gift of Rituximab. We also thank Biotransplant for their gift of pSCF and pIL-3. This research was supported by NIH grant, P01AI45897, and sponsored research funding from Lung Biotechnology PBC. FCM analysis in this article was performed in the CCTI flow cytometry core, supported in part by the Office of the Director, NIH, under awards S10RR027050.
Grant supports: Supported by NIAID 5P01AI045897
Abbreviation
- Ab
antibody
- APC
allophycocyanine
- ATG
anti thymocyte globulin
- BM
bone marrow
- CVF
cobra venom factor
- EC
endothelium
- FCM
flow cytometry
- FITC
fluorescein isothiocyanate
- GalTKO
α-1,3-galactocyltransferase knockout
- H&E
hematoxylin and eosin
- HLA
human leukocyte antigen
- IBBMTX
intra-bone bone marrow transplantation
- IF
immunofluorescence
- IFN
interferon
- Ig
immunoglobulin
- IL
interleukin
- IV
intravenous
- MLR
mixed lymphocyte reaction
- MMF
mycophenolate mofetil
- nAb
natural antibody
- PBMC
peripheral blood mononuclear cell
- POD
post-operative day
- SCF
stem cell factor
- SLA
swine leukocyte antigen
- TFPI
tissue factor pathway inhibitor
- Tg
transgenic
- Tx
transplantation
References
- 1.Rivard CJ, Tanabe T, Lanaspa MA, et al. Upregulation of CD80 on glomerular podocytes plays an important role in development of proteinuria following pig-to-baboon xeno-renal transplantation -an experimental study. Transplant international: official journal of the European Society for Organ Transplantation 2018, 31:1164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation 2017, 24.doi : 10.1111/xen.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018, 564:430–3. [DOI] [PubMed] [Google Scholar]
- 4.Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 2016, 7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdorf L, Laird C, O’Neill N, et al. Xenogeneic Lung Transplantation: Extending Life-Supporting Organ Function Using Multi-Transgenic Donor Pigs and Targeted Drug Treatments. The Journal of Heart and Lung Transplantation 2016, 35:S188. [Google Scholar]
- 6.Watanabe H, Sahara H, Nomura S, et al. GalT-KO pig lungs are highly susceptible to acute vascular rejection in baboons, which may be mitigated by transgenic expression of hCD47 on porcine blood vessels. Xenotransplantation 2018, 25:e12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksentijevich I, Sachs DH, Sykes M: Humoral tolerance in xenogeneic BMT recipients conditioned by a nonmyeloablative regimen. Transplantation 1992, 53:1108–14. [DOI] [PubMed] [Google Scholar]
- 8.Lee LA, Sergio JJ, Sachs DH, Sykes M: Mechanism of tolerance in mixed xenogeneic chimeras prepared with a nonmyeloablative conditioning regimen. Transplant Proc 1994, 26:1197–8. [PubMed] [Google Scholar]
- 9.Ohdan H, Swenson KG, Kitamura H, Yang YG, Sykes M: Tolerization of Gal alpha 1,3Gal-reactive B cells in pre-sensitized alpha 1,3-galactosyltransferase-deficient mice by nonmyeloablative induction of mixed chimerism. Xenotransplantation 2001, 8:227–38. [DOI] [PubMed] [Google Scholar]
- 10.Yang YG, deGoma E, Ohdan H, Bracy JL, Xu Y, Iacomini J, Thall AD, Sykes M: Tolerization of anti-Galalpha1–3Gal natural antibody-forming B cells by induction of mixed chimerism. The Journal of experimental medicine 1998, 187:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohdan H, Yang YG, Shimizu A, Swenson KG, Sykes M: Mixed chimerism induced without lethal conditioning prevents T cell-and anti-Gal alpha 1,3Gal-mediated graft rejection. J Clin Invest 1999, 104:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohdan H, Yang YG, Swenson KG, Kitamura H, Sykes M: T cell and B cell tolerance to GALalpha1,3GAL-expressing heart xenografts is achieved in alpha1,3-galactosyltransferase-deficient mice by nonmyeloablative induction of mixed chimerism. Transplantation 2001, 71:1532–42. [DOI] [PubMed] [Google Scholar]
- 13.Griesemer A, Liang F, Hirakata A, et al. Occurrence of specific humoral non-responsiveness to swine antigens following administration of GalT-KO bone marrow to baboons. Xenotransplantation 2010, 17:300–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng YL, Dor FJ, Kuwaki K, et al. Bone marrow transplantation from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Xenotransplantation 2004, 11:361–70. [DOI] [PubMed] [Google Scholar]
- 15.Tasaki M, Wamala I, Tena A, et al. High incidence of xenogenic bone marrow engraftment in pig-to-baboon intra-bone bone marrow transplantation. Am J Transplant 2015, 15:974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ide K, Wang H, Tahara H, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A 2007, 104:5062–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tena AA, Sachs DH, Mallard C, et al. Prolonged Survival of Pig Skin on Baboons After Administration of Pig Cells Expressing Human CD47. Transplantation 2017, 101:316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper DKC, Hara H, Iwase H, et al. Justification of specific genetic modifications in pigs for clinical organ xenotransplantation. Xenotransplantation. 2019, 15:e12516. doi: 10.1111/xen.12516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahara H, Sekijima M, Ariyoshi Y, et al. Effects of carbon monoxide on early dysfunction and microangiopathy following GalT-KO porcine pulmonary xenotransplantation in cynomolgus monkeys. Xenotransplantation 2018, 25: doi: 10.1111/xen12359. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf MZ, Raslan Z, Atkinson L, et al. Prostacyclin reverses platelet stress fibre formation causing platelet aggregate instability. Sci Rep. 2017, 17;7:5582. doi: 10.1038/s41598-017-05817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003, 167: 490–511. [DOI] [PubMed] [Google Scholar]
- 22.Barrile R, van der Meer AD, Park H, et al. Organ-on-Chip recapitulates thrombosis induced by an anti-CD154 monoclonal antibody: Translational Potential of Advanced Microengineered Systems. Clin Pharmacol Ther. 2018, 104:1240–1248. doi: 10.1002/cpt.1054. [DOI] [PubMed] [Google Scholar]
- 23.Liang F, Wamala I, Scalea J, et al. Increased levels of anti-non-Gal IgG following pig-to-baboon bone marrow transplantation correlate with failure of engraftment. Xenotransplantation. 2013;20:458–68. doi: 10.1111/xen.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sykes M, Sachs DH: Bone marrow transplantation as a means of inducingtolerance. Seminars in immunology 1990, 2:401–17. [PubMed] [Google Scholar]
- 25.Sharabi Y, Sachs DH: Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. The Journal of experimental medicine 1989, 169:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe M, Qi J, Sykes M, Yang YG: Mixed chimerism induces donor-specific T-cell tolerance across a highly disparate xenogeneic barrier. Blood 2002, 99:3823–9. [DOI] [PubMed] [Google Scholar]
- 27.Lan P, Wang L, Diouf B, et al. Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood 2004, 103:3964–9. [DOI] [PubMed] [Google Scholar]
- 28.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation 1995;59:256–62. [PubMed] [Google Scholar]
- 29.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DMJ, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest 2000;105:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreisel D, Goldstein DR: Innate immunity and organ transplantation: focus on lung transplantation. Transplant international : official journal of the European Society for Organ Transplantation 2013, 26:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelmann B, Massberg S: Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013, 13:34–45. [DOI] [PubMed] [Google Scholar]
- 32.Cantu E, Lederer DJ, Meyer K, et al. Gene set enrichment analysis identifies key innate immune pathways in primary graft dysfunction after lung transplantation. Am J Transplant 2013, 13:1898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler JR, Martens GR, Estrada JL, et al. Silencing porcine genes significantly reduces human-anti-pig cytotoxicity profiles: an alternative to direct complement regulation. Transgenic Res 2016, 25:751–9. [DOI] [PubMed] [Google Scholar]
- 34.Martens GR, Reyes LM, Butler JR, et al. Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells From GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs. Transplantation 2017, 101:e86–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee W, Miyagawa Y, Long C, et al. Expression of NeuGc on Pig Corneas and Its Potential Significance in Pig Corneal Xenotransplantation. Cornea 2016, 35:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1A. Immunosuppression regimen and procedures of IBBMTx
Fig. 1B Immunosuppression regimen and procedures of delayed lung Tx
Fig. 2A IFN gamma ELISPOT assays at pre IBBMTx, day 28 after IBBMTx (pre delayed lung Tx), and POD 14 after delayed lung Tx.
Fig. 2B. MLR assays at pre IBBMTx and day 28 after IBBMTx (pre delayed lung Tx) in baboon 2715. Lymphocyte gated cells were analyses.


