Abstract
Background
Delayed graft function (DGF) is associated with inferior posttransplant outcomes in kidney transplantation. Given these adverse outcomes, we sought to determine the incidence, unique risk factors, and posttransplant outcomes for simultaneous liver kidney (SLK) transplant recipients developing DGF.
Methods
We studied 6214 adult SLK recipients from March 2002 to February 2017 using the Scientific Registry of Transplant Recipients. We determined associations between risk factors and DGF using Poisson multivariate regression and between DGF and graft failure and mortality using Cox proportional hazard analysis.
Results
The overall rate of DGF was 21.8%. Risk factors for DGF in the HCV negative recipient population included pretransplant dialysis (aIRR 3.26, p=0.004), donor BMI (aIRR 1.25 per 5 kg/m2, p = 0.01) and transplantation with a DCD (aIRR 5.38, p=0.001) or imported donor organ (regional share aIRR 1.69, p=0.03; national share aIRR 4.82, p<0.001). DGF was associated with a 2.6-fold increase in kidney graft failure (aHR 2.63, p<0.001), 1.6-fold increase in liver graft failure (aHR 1.62, p<0.001), and 1.6-fold increase in mortality (aHR 1.62, p<0.001).
Conclusions
In HCV negative SLK recipients, recipient pretransplant dialysis and components of kidney graft quality comprise significant risk factors for DGF. Regardless of HCV status, DGF is associated with inferior posttransplant outcomes. Understanding these risk factors during clinical decision making may improve prevention of DGF and may represent an opportunity to improve posttransplant outcomes.
INTRODUCTION
Simultaneous liver kidney (SLK) transplantation in the United States has increased substantially, from 210 cases in 2002 to 730 in 2016.1 This increase is likely due in part to introduction of the Model for End-Stage Liver Disease (MELD) donor allocation system in 2002, which prioritizes liver transplant candidates with associated renal dysfunction. For patients with end-stage liver disease who are on dialysis, SLK offers superior posttransplant patient and liver graft survival compared to liver transplant alone.2,3 Additionally, recipient and graft survival have been shown to be higher in SLK recipients compared to patients undergoing kidney after liver transplant and to patients undergoing liver transplant alone.4–7
Despite the advantages of SLK transplantation, delayed graft function (DGF) is a common and challenging postoperative complication, with up to a 40% reported incidence in single-center studies.8,9 In kidney-alone transplant recipients, DGF is associated with a 20% increase in risk of graft failure after one year and 1.5-fold increased risk of mortality.10–12 Similar findings of inferior posttransplant outcomes in SLK recipients experiencing DGF have been reported; however, these studies were either limited to a single center or did not examine recipient and graft risk factors associated with development of DGF.13,14 Previous work has shown that low quality renal grafts as measured by Kidney Donor Profile Index (KDPI) have been associated with development of DGF in SLK recipients.15 However, it is not clear what component parts of KDPI drive the risk for DGF, if there are risk factors inherent to the graft that are not captured by KDPI, or how recipient factors contribute to DGF risk. Given the adverse consequences of DGF, identifying patient and graft risk factors is important to help guide recipient and donor selection and treatment.
Understanding the risk factors and possible consequences of DGF in SLK recipients may help guide clinical decisions for this increasing solid-organ transplant operation. We sought to determine the donor and recipient factors contributing to DGF risk, as well as the association of DGF with posttransplant outcomes. Elucidating these associations may help inform graft selection and posttransplant management of SLK recipients.
METHODS
Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.16 The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Study population
We studied adult SLK recipients transplanted from March 1, 2002 to February 28, 2017 using SRTR data. We analyzed recipients who were positive for hepatitis C virus (HCV) separately since our time period spanned the introduction and FDA approval of direct acting antiviral (DAA) drugs, confounding outcomes for this patient population. We excluded patients who did not have at least one week of follow-up posttransplant, as our primary outcome was DGF. Our resulting population consisted of 6214 SLK recipients. We used Fisher’s exact tests for categorical variables and Wilcoxon rank-sum tests for continuous variables to compare recipient and donor characteristics between SLK recipients who did and did not develop DGF. We also reported cause of death in both HCV positive and negative populations. Given the high rate of missingness for cause of death and nonspecific etiologies (e.g. cardiac arrest) in the registry, we excluded these variables from multivariate analysis.
Delayed Graft Function (DGF)
Our primary outcome was the development of DGF, defined as need for dialysis in the first 7 days after SLK transplantation. We determined the association between donor and recipient risk factors and DGF using univariate regression models and multivariate Poisson regression with a robust error variance. Sensitivity analysis was performed using multivariate logistic regression. Inferences were not different and therefore, only results from multivariate Poisson regression are shown. Recipient risk factors analyzed included age at transplant, gender, race, HCV status, MELD at transplant, pretransplant hospitalization, pretransplant dialysis, and history of previous liver or kidney transplantation. Pretransplant time on dialysis was analyzed both as a continuous variable as well as a categorical variable of none, less than six weeks, and six weeks or more. We chose six weeks as a clinically relevant time point given its inclusion in UNOS medical criteria for SLK allocation as the definition of sustained acute kidney injury.17
Transplant factors analyzed included year of transplant, kidney and liver cold ischemia time (CIT), and use of induction agents. Donor factors analyzed included age at procurement, gender, race, BMI, terminal creatinine, history of hypertension or diabetes, cause of death, number of HLA mismatches, donation after circulatory death (DCD), donor share type (local, regional or national) and use of machine perfusion for the kidney graft. We defined an imported donor as a regionally or nationally shared organ. We also adjusted for region given previous evidence of regional variability in SLK listing practices.18 This adjustment did not change inferences; therefore results from the more parsimonious model without regional adjustment are shown. In multivariate models, we included risk factors chosen a priori based on clinical significance as well as statistically significant risk factors from univariate analysis.
Effect of Direct-Acting Antivirals (DAA) among HCV positive recipients
Given the introduction of DAAs during the study period, we performed a sensitivity analysis in which we examined changes in risk factors over multiple time periods, defined by DAA use. We found no differences in inferences in the HCV negative population in terms of risk factors between the pre-DAA era (2002–2012), transition era (2013), and post-DAA era (2014–2017). There were no differences in risk factors identified between the overall HCV positive cohort and the pre-DAA era. The post-DAA era for the HCV positive cohort represents a small population of patients (N=669, 27% of HCV positive cohort) and did not achieve convergence in multivariate analysis. We further adjusted our multivariate model for DGF by DAA era and found no changes in inferences, nor was DAA era found to be a significant risk factor for DGF development. Given these findings, we only report results for over the entire study period.
Posttransplant outcomes
We determined the association between DGF and mortality and graft failure using Cox proportional hazard models. Death censored kidney graft failure was defined as kidney retransplant or return to chronic dialysis. Liver graft failure was defined as liver retransplant or death. Risk factors analyzed were the same as for models for the development of DGF. For multivariate analysis, we again included risk factors chosen a priori based on clinical significance as well as statistically significant risk factors from univariate analysis. We used shared frailties to account for underlying variation across regions.
We further investigated the association between DGF and primary nonfunction (PNF) of the renal graft and renal allograft futility (RAF) using multivariate Poisson regression with a robust error variance, including use of interaction analysis. We defined RAF as PNF of the transplanted renal graft or death within three months.9 Finally, we repeated the posttransplant survival analysis excluding recipients whose posttransplant course was complicated by PNF or RAF, as this potentially represents a unique patient population. Our inferences were similar, therefore we only reported our primary analysis including these recipients. For posttransplant outcomes, we performed sensitivity analysis in which subgroups were analyzed separately by HCV status. There were no differences in inferences, therefore only the outcome analyses for the cohort as a whole are presented.
Statistical analysis
Confidence intervals are reported as per the method of Louis and Zeger.19 A two-sided p-value<0.05 was considered significant. All analyses were performed using Stata IC 14.1 (StataCorp, College Station, Texas). To include all patients in our analyses, we performed multiple imputation iterative chained equations to account for missing values for kidney (N=693) and liver (N=261) CIT, using recipient age, gender, race, MELD score, transplant year, organ share type, and donor gender, age, BMI, DCD, liver CIT and cause of death. Missing values were imputed five times to create five complete data sets, which were each independently analyzed. Analyses were then pooled to give final estimates. Inferences from sensitivity analysis without inclusion of imputed values were the same; therefore, only analyses with imputation are presented.
RESULTS
Study Population
HCV negative recipients
Among the 3786 adult HCV negative SLK recipients during the study period, 851 (22.4%) developed DGF. Recipients who developed DGF were slightly younger (56 vs. 57 years old, p=0.003), less likely to be white (65.6% vs. 70.3%, p=0.04), and more likely to be hospitalized at the time of transplant (50.7% vs. 39.5%, p<0.001) in comparison to SLK recipients without DGF (Table 1). SLK recipients with DGF were also more likely to have higher MELD scores at transplant (median 33 vs. 30, p<0.001) and were more likely to be on pretransplant dialysis (83.3% vs. 60.6%, p<0.001).
Table 1.
Characteristics of SLK Recipients.
| HCV negative recipients (N = 3786) | HCV positive recipients (N = 2428) | |||||
|---|---|---|---|---|---|---|
| No DGF | DGF | p-value | No DGF | DGF | p-value | |
| N = 2935 | N = 851 | N = 1926 | N = 502 | |||
| Recipient characteristics | ||||||
| Age at transplant, median (IQR) | 57 (48, 63) | 56 (46, 62) | 0.003 | 56 (52, 61) | 58 (52, 61) | 0.01 |
| Female | 1150 (39.2%) | 359 (42.2%) | 0.1 | 493 (25.6%) | 140 (27.9%) | 0.3 |
| Race | 0.04 | 0.01 | ||||
| White | 2062 (70.3%) | 558 (65.6%) | 1053 (54.7%) | 248 (49.4%) | ||
| Black | 296 (10.1%) | 91 (10.7%) | 473 (24.6%) | 115 (22.9%) | ||
| Other | 577 (19.7%) | 202 (23.7%) | 400 (20.8%) | 139 (27.7%) | ||
| Hospitalization pretransplant | 885 (39.5%) | 299 (50.7%) | <0.001 | 578 (35.9%) | 205 (53.4%) | <0.001 |
| MELD at transplant, median (IQR) | 30 (24, 36) | 33 (26, 39) | <0.001 | 28 (23, 35) | 33 (25, 39) | <0.001 |
| Dialysis pretransplant | <0.001 | <0.001 | ||||
| Any | 1778 (60.6%) | 709 (83.3%) | 1161 (60.3%) | 414 (82.5%) | ||
| < 6 weeks dialysis | 602 (20.5%) | 245 (28.8%) | 339 (17.6%) | 132 (26.3%) | ||
| ≥ 6 weeks dialysis | 1176 (40.1%) | 464 (54.5%) | 822 (42.7%) | 282 (56.2%) | ||
| Years on dialysis, median (IQR) | 0.1 (0.0, 0.4) | 0.1 (0.03, 0.8) | <0.001 | 0.1 (0.0, 0.8) | 0.2 (0.03, 1.5) | <0.001 |
| Previous kidney transplantation | 130 (4.4%) | 42 (4.9%) | 0.5 | 113 (5.9%) | 31 (6.2%) | 0.8 |
| Previous liver transplantation | 325 (11.1%) | 87 (10.2%) | 0.5 | 258 (13.4%) | 70 (13.9%) | 0.8 |
| Donor characteristics | ||||||
| Age at procurement, median (IQR) | 33 (22, 47) | 40 (27, 50) | <0.001 | 33 (23, 46) | 40 (26, 50) | <0.001 |
| Female | 1140 (38.8%) | 357 (42.0%) | 0.1 | 727 (37.7%) | 200 (39.8%) | 0.4 |
| Race | 0.1 | 0.01 | ||||
| White | 1938 (66.0%) | 560 (65.8%) | 1284 (66.7%) | 301 (60.0%) | ||
| Black | 491 (16.7%) | 123 (14.5%) | 307 (15.9%) | 80 (15.9%) | ||
| Other | 506 (17.3%) | 168 (19.7%) | 335 (17.4%) | 121 (24.1) | ||
| BMI, median (IQR) | 25 (22, 29) | 27 (23, 31) | <0.001 | 25 (23, 29) | 26 (23, 30) | 0.004 |
| Creatinine, median (IQR) | 0.9 (0.7, 1.2) | 1.0 (0.7, 1.3) | 0.01 | 0.9 (0.7, 1.2) | 1.0 (0.7, 1.3) | 0.02 |
| History of hypertension | 534 (18.3%) | 243 (29.0%) | <0.001 | 372 (19.4%) | 133 (26.9%) | <0.001 |
| History of diabetes mellitus | 108 (3.7%) | 58 (6.9%) | <0.001 | 65 (3.4%) | 32 (6.5%) | 0.003 |
| HLA mismatches, median (IQR) | 5 (4, 5) | 5 (4, 5) | 0.95 | 5 (4, 6) | 5 (4, 5) | 0.03 |
| Organ share type | <0.001 | 0.03 | ||||
| Local | 2423 (82.6%) | 606 (71.2%) | 1560 (81.0%) | 383 (76.3%) | ||
| Regional | 487 (16.6%) | 233 (27.4%) | 319 (16.6%) | 98 (19.5%) | ||
| National | 25 (0.9%) | 12 (1.4%) | 47 (2.4%) | 21 (4.2%) | ||
| DCD donor | 98 (3.3%) | 62 (7.3%) | <0.001 | 59 (3.1%) | 26 (5.2%) | 0.03 |
| KDPI, median (IQR) | 29 (12, 52) | 41 (19, 63) | <0.001 | 33 (14, 54) | 43 (24, 65) | <0.001 |
| DRI, median (IQR) | 1.2 (1.1, 1.4) | 1.3 (1.1, 1.6) | <0.001 | 1.2 (1.1, 1.) | 1.3 (1.1, 1.6) | <0.001 |
| Kidney on machine perfusion | 617 (21.0%) | 147 (17.3%) | 0.02 | 333 (17.3%) | 72 (14.3%) | 0.1 |
| Transplant characteristics | ||||||
| Kidney CIT, hours, median (IQR) | 10 (8, 13) | 11 (9, 15) | <0.001 | 10 (8, 13) | 11 (9, 15) | <0.001 |
| Liver CIT, hours, median (IQR) | 6 (5, 8) | 7 (5, 8) | <0.001 | 6 (5, 8) | 7 (5, 8) | <0.001 |
| Use of induction immunosuppression | 2345 (79.9%) | 722 (84.8%) | 0.001 | 1543 (80.1%) | 412 (82.1%) | 0.3 |
| Transplant year, median (IQR) | 2011 (2007, 2014) | 2012 (2008, 2015) | <0.001 | 2010 (2006, 2014) | 2012 (2008, 2014) | <0.001 |
Further, HCV negative SLK recipients with DGF were more likely to receive grafts from older donors (median 40 vs. 33 years old, p<0.001) and donors with higher BMI (27 vs. 25, p<0.001) and terminal creatinine (1.0 vs. 0.9, p=0.01). Donors of SLK recipients that developed DGF were more likely to have a history of hypertension (29.0% vs. 18.3%, p<0.001) or diabetes (6.9% vs. 3.7%, p<0.001) and were more likely to be imported (regional share 27.4% vs. 16.6%; national share 1.4% vs. 0.9%, p<0.001) or DCD donors (7.3% vs. 3.3%, p<0.001). These grafts had higher median KDPI (41 vs 29, p<0.001) and DRI (1.3 vs 1.2, p<0.001), and were less likely to be on machine perfusion (17.3% vs. 21.0%, p=0.02).
Finally, HCV negative SLK recipients who developed DGF received grafts with longer median CIT (kidney: 11 hours vs. 10 hours, p<0.001; liver: 7 hours vs 6 hours, p<0.001). Recipients with DGF were more likely to have received induction immunosuppression at transplant (84.8% vs. 79.9%, p=0.001). Transplants for those developing DGF were more likely to occur later in the study period (median 2012 vs 2011, p<0.001).
HCV positive recipients
Among the 2428 adult HCV positive SLK recipients during the study period, 502 (20.7%) developed DGF. Recipients who developed DGF were slightly older (58 vs. 56 years old, p=0.01), less likely to be white (49.4% vs. 54.7%, p=0.01), and more likely to be hospitalized at the time of transplant (53.4% vs. 35.9%, p<0.001) in comparison to SLK recipients without DGF (Table 1). SLK recipients with DGF were also more likely to have higher MELD scores at transplant (median 33 vs. 28, p<0.001) and were more likely to be on pretransplant dialysis (82.5% vs. 60.3%, p<0.001).
Donors for HCV positive SLK recipients with DGF were more likely to older (median 40 vs. 33 years old, p<0.001) and less likely to be white (60.0% vs 66.7%, p=0.01). Donors for recipients who developed DGF had higher BMI (26 vs. 25, p=0.004) and terminal creatinine (1.0 vs. 0.9, p=0.02). These donors were also more likely to have a history of hypertension (26.9% vs. 19.4%, p<0.001) or diabetes (6.5% vs. 3.4%, p=0.003). The donor grafts were also more likely to be imported (regional share 19.5% vs. 16.6%; national share 4.2% vs. 2.4%, p=0.03) or from DCD donors (5.2% vs. 3.1%, p=0.03). Donor grafts for those HCV positive recipients who developed DGF also had a higher median KDPI (43 vs 33, p<0.001) and DRI (1.3 vs 1.2, p<0.001).
In HCV positive SLK recipients, those who developed DGF received grafts with longer median CIT (kidney: 11 hours vs. 10 hours, p<0.001; liver: 7 hours vs 6 hours, p<0.001) and were performed in later years than those without DGF (median year of transplant 2012 vs. 2010, p<0.001).
Cause of death for both HCV negative and positive populations are shown in Table 2. Infection was a common cause of death in both populations, followed by cardiovascular events and malignancies.
Table 2.
Cause of death of SLK Recipients.
| HCV negative recipients (N = 921) | HCV positive recipients (N = 865) | |||
|---|---|---|---|---|
| No DGF | DGF | No DGF | DGF | |
| N = 660 | N = 261 | N = 670 | N = 195 | |
| Unknown | 185 (28.0%) | 71 (27.2%) | 194 (29.0%) | 33 (16.9%) |
| Infection | 127 (19.2%) | 68 (26.1%) | 131 (19.6%) | 54 (27.7%) |
| Cardiovascular | 102 (15.5%) | 43 (16.5%) | 74 (11.0%) | 29 (14.9%) |
| Malignancy | 73 (11.1%) | 10 (3.8%) | 73 (10.9%) | 13 (6.7%) |
| Respiratory failure | 36 (5.5%) | 19 (7.3%) | 18 (2.7%) | 7 (3.6%) |
| Liver graft failure | 31 (4.7%) | 11 (4.2%) | 61 (9.1%) | 19 (9.7%) |
| Kidney graft failure | 18 (2.7%) | 2 (0.8%) | 26 (3.9%) | 4 (2.1%) |
| Hemorrhage | 13 (2.0%) | 7 (2.7%) | 16 (2.4%) | 3 (1.5%) |
| Trauma | 4 (0.6%) | 2 (0.8%) | 6 (0.9%) | 0 (0.0%) |
| Other | 71 (10.8%) | 28 (10.7%) | 71 (10.6%) | 33 (16.9%) |
Delayed Graft Function
HCV negative recipients
In the HCV negative SLK recipient population, significant risk factors for DGF included pretransplant dialysis (aIRR 1.473.267.22, p=0.004), donor BMI (per 5 kg/m2, aIRR 1.061.251.47, p=0.01) and transplantation with a DCD (aIRR 1.935.3815.01, p=0.001) or imported donor (regional share aIRR 1.061.692.69, p=0.03; national share donor aIRR 2.054.8211.30, p<0.001) (Table 3a). The association of pretransplant dialysis with incidence of DGF was more pronounced for recipients with at least 6 weeks dialysis (aIRR 1.583.527.88, p=0.002) and attenuated for recipients with less than 6 weeks dialysis (aIRR 1.042.686.89, p=0.04).
Table 3a.
Risk factors for delayed graft function in HCV negative SLK recipients.
| Adjusted incidence-rate ratios | 95% confidence interval | p-value | |
|---|---|---|---|
| Recipient pretransplant dialysis: none | Reference | -- | -- |
| Any | 3.26 | 1.47, 7.22 | 0.004 |
| < 6 weeks dialysis | 2.68 | 1.04, 6.89 | 0.04 |
| ≥ 6 weeks dialysis | 3.52 | 1.58, 7.88 | 0.002 |
| Donor BMI, per 5 kg/m2 | 1.25 | 1.06, 1.47 | 0.01 |
| DCD | 5.38 | 1.93, 15.01 | 0.001 |
| Donor share type – Local | Reference | -- | -- |
| Regional | 1.69 | 1.06, 2.69 | 0.03 |
| National | 4.82 | 2.05, 11.30 | <0.001 |
| Recipient age, per year | 1.03 | 0.9999, 1.05 | 0.051 |
| Recipient female | 1.04 | 0.64, 1.69 | 0.9 |
| MELD | 1.004 | 0.95, 1.06 | 0.9 |
| Recipient race – white | Reference | ||
| Black | 1.32 | 0.40, 4.35 | 0.8 |
| Hispanic | 0.93 | 0.50, 1.74 | 0.8 |
| Recipient status: not hospitalized | Reference | -- | -- |
| Hospitalized, in ICU | 1.02 | 0.47, 2.23 | 0.96 |
| Hospitalized, not in ICU | 0.62 | 0.25, 1.54 | 0.3 |
| Donor age, per year | 0.997 | 0.98, 1.02 | 0.8 |
| Donor race – white | Reference | ||
| Black | 1.11 | 0.48, 2.54 | 0.8 |
| Hispanic | 1.06 | 0.48, 2.33 | 0.9 |
| Donor creatinine, per mg/dL | 0.97 | 0.61, 1.55 | 0.9 |
| Donor warm ischemia time, min | 0.98 | 0.96, 1.01 | 0.2 |
| Donor cold ischemia time, liver, hours | 1.01 | 0.98, 1.04 | 0.6 |
| Kidney on machine perfusion | 0.74 | 0.43, 1.28 | 0.3 |
| Transplant year | 1.01 | 0.94, 1.09 | 0.8 |
| Use of induction immunosuppression | 1.20 | 0.61, 2.33 | 0.6 |
HCV positive recipients
In multivariable analysis, HCV was associated with a significantly lower incidence of DGF (aIRR 0.330.540.89, p = 0.02). In the HCV positive SLK recipient population, there were no identified additional risk factors for DGF (Table 3b).
Table 3b.
Risk factors for delayed graft function in HCV positive SLK recipients.
| Adjusted incidence-rate ratios | 95% confidence interval | p-value | |
|---|---|---|---|
| Recipient pretransplant dialysis: none | Reference | -- | -- |
| Any | 3.00 | 0.64, 14.01 | 0.2 |
| < 6 weeks dialysis | 4.90 | 0.70, 34.3 | 0.1 |
| ≥ 6 weeks dialysis | 2.80 | 0.56, 13.90 | 0.2 |
| Donor BMI, per 5 kg/m2 | 0.77 | 0.50, 1.19 | 0.2 |
| DCD | 0.48 | 0.06, 3.92 | 0.5 |
| Donor share type – Local | Reference | -- | -- |
| Regional | 0.50 | 0.16, 1.54 | 0.2 |
| National | 1.54 | 0.43, 5.46 | 0.5 |
| Recipient age, per year | 1.03 | 0.96, 1.10 | 0.4 |
| Recipient female | 0.66 | 0.15, 3.00 | 0.6 |
| MELD | 1.03 | 0.91, 1.16 | 0.7 |
| Recipient race – white | Reference | -- | -- |
| Black | 1.95 | 0.66, 5.74 | 0.2 |
| Hispanic | 1.92 | 0.69, 5.31 | 0.2 |
| Recipient status: not hospitalized | Reference | -- | -- |
| Hospitalized, in ICU | 0.27 | 0.05, 1.37 | 0.1 |
| Hospitalized, not in ICU | 0.32 | 0.05, 2.10 | 0.2 |
| Donor age, per year | 1.03 | 1.00, 1.06 | 0.1 |
| Donor race – white | Reference | -- | -- |
| Black | 1.93 | 0.31, 12.13 | 0.5 |
| Hispanic | 1.10 | 0.29, 4.20 | 0.9 |
| Donor creatinine, per mg/dL | 0.52 | 0.17, 1.63 | 0.3 |
| Donor warm ischemia time, min | 1.02 | 0.97, 1.08 | 0.4 |
| Donor cold ischemia time, liver, hours | 0.94 | 0.82, 1.07 | 0.4 |
| Kidney on machine perfusion | 0.80 | 0.27, 2.39 | 0.7 |
| Transplant year | 1.11 | 0.90, 1.37 | 0.3 |
| Use of induction immunosuppression | 4.58 | 1.00, 21.40 | 0.1 |
Posttransplant outcomes
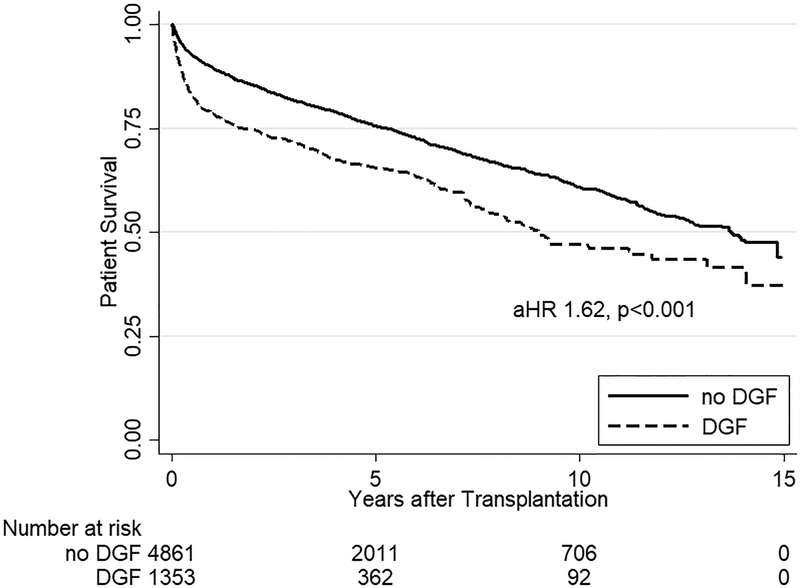
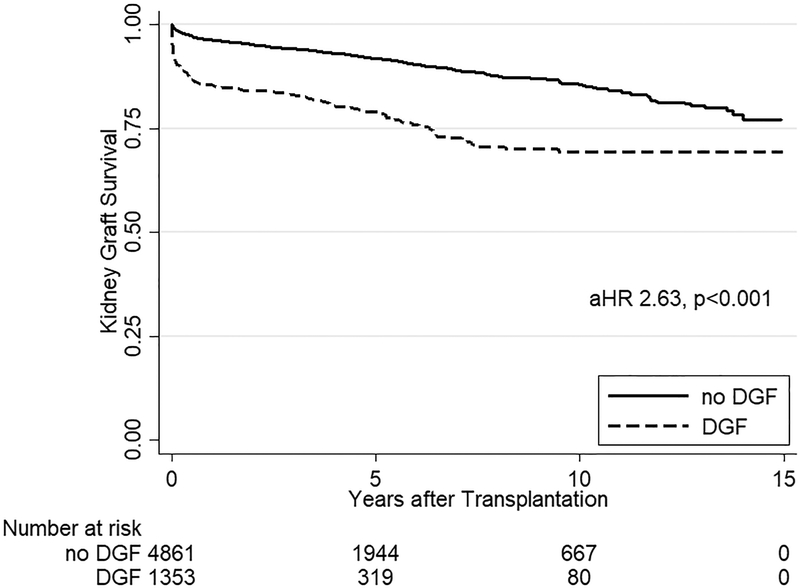
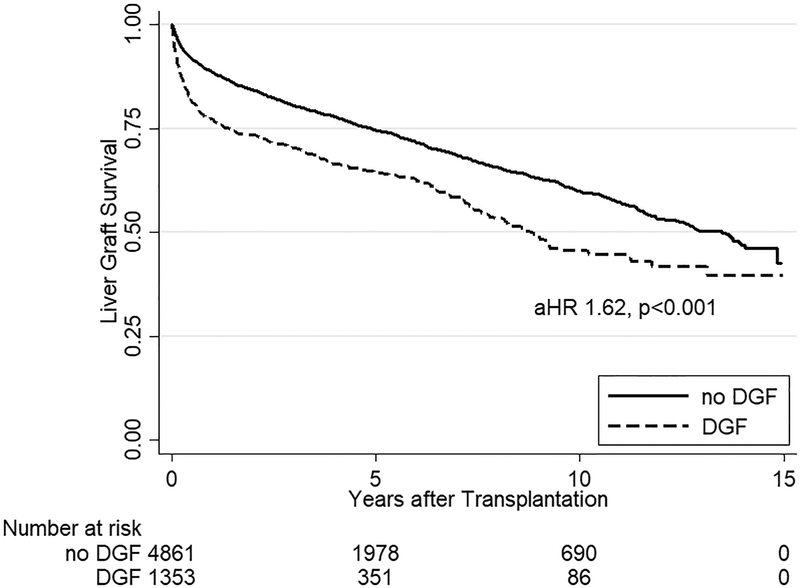
One-year patient survival was 87.2%, one-year death-censored kidney survival was 94.0%, and one-year liver graft survival was 86.0% (Figure 1). Among the 1849 documented liver graft failures, 1683 (91.0%) were due to death. In multivariate analysis for all SLK recipients, DGF was the strongest risk factor for mortality (aHR 1.431.621.82, p<0.001), liver graft failure (aHR 1.441.621.82, p<0.001), and death-censored kidney graft failure (aHR 2.202.633.15, p<0.001) (Table 4).
Figure 1.
Kaplan Meier estimates of patient, kidney graft, and liver graft survival in SLK recipients. SLK recipients with DGF had significantly worse (a) mortality (aHR 1.431.621.82, p<0.001) as well as (b) kidney (aHR 2.202.633.15, p<0.001) and (c) liver graft failure (aHR 1.441.621.82, p<0.001) compared to SLK recipients who did not experience DGF.
Table 4.
Risk Factors for mortality and graft failure in SLK recipients.
| Adjusted Hazard Ratio (95% CI, p-value) | |||
|---|---|---|---|
| Mortality | Kidney Graft Failure | Liver Graft Failure | |
| DGF | 1.62 (1.43–1.82, <0.001) | 2.63 (2.20–3.15, <0.001) | 1.62 (1.44–1.82, <0.001) |
| Previous liver transplant | 1.47 (1.29–1.68, <0.001) | 1.27 (1.01–1.60, 0.04) | 1.47 (1.29–1.67, <0.001) |
| Recipient HCV | 1.45 (1.32–1.61, <0.001) | 1.49 (1.26–1.76, <0.001) | 1.42 (1.29–1.57, <0.001) |
| Previous kidney transplant | 1.38 (1.13–1.69, <0.001) | 1.46 (1.07–1.98, 0.02) | 1.34 (1.10–1.62, 0.004) |
| Hospitalized pretransplant | 1.32 (1.18–1.49, <0.001) | 1.18 (0.96–1.43, 0.1) | 1.33 (1.19–1.49, <0.001) |
| DCD | 1.31 (1.01–1.71, 0.04) | 1.49 (0.98–2.25, 0.1) | 1.40 (1.08–1.80, 0.01) |
| Donor age, per 10 years | 1.17 (1.13–1.22, <0.001) | 1.31 (1.23–1.39, <0.001) | 1.17 (1.13–1.22, <0.001) |
| Recipient age, per 10 years | 1.15 (1.09–1.21, <0.001) | 0.93 (0.85–1.01, 0.1) | 1.12 (1.06–1.18, <0.001) |
| Transplant year | 0.95 (0.93–0.96, <0.001) | 0.95 (0.92–0.97, <0.001) | 0.94 (0.93–0.96, <0.001) |
| Pretransplant dialysis | 1.08 (0.97–1.21, 0.2) | 1.25 (1.03–1.51, 0.02) | 1.06 (0.96–1.19, 0.3) |
| Donor creatinine, per mg/dL | 1.04 (0.99–1.09, 0.2) | 1.10 (1.04–1.16, 0.001) | 1.03 (0.98–1.09, 0.3) |
| Donor female | 1.01 (0.91–1.12, 0.9) | 1.07 (0.91–1.27, 0.4) | 1.02 (0.92–1.13, 0.7) |
| Use of induction immunosuppression | 1.01 (0.90–1.15, 0.8) | 0.94 (0.77–1.15, 0.6) | 1.01 (0.90–1.14, 0.8) |
| Donor cold ischemia time, liver, hours | 1.001 (0.99–1.09, 0.2) | 1.01 (0.99–1.03, 0.3) | 1.01 (0.99–1.02, 0.5) |
| MELD | 0.99 (0.99–1.002, 0.1) | 1.00 (0.97–1.00, 0.05) | 0.99 (0.98–1.001, 0.1) |
| Donor BMI, per 5 kg/m2 | 0.96 (0.92–1.01, 0.1) | 0.97 (0.90–1.04, 0.4) | 0.96 (0.92–1.001, 0.1) |
| Recipient female | 0.96 (0.86–1.07, 0.4) | 1.03 (0.87–1.23, 0.7) | 0.94 (0.85–1.05, 0.3) |
| Donor share type – Local | Reference | Reference | Reference |
| Regional | 0.95 (0.81–1.10, 0.5) | 1.22 (0.98–1.53, 0.1) | 0.90 (0.78–1.05, 0.2) |
| National | 1.12 (0.80–1.58, 0.5) | 1.15 (0.68–1.95, 0.6) | 1.05 (0.74–1.48, 0.8) |
| Recipient race – white | Reference | Reference | Reference |
| Black | 1.14 (0.995–1.30, 0.1) | 1.36 (1.11–1.67, 0.004) | 1.12 (0.98–1.27, 0.1) |
| Other | 0.85 (0.67–1.08, 0.2) | 0.94 (0.74–1.19, 0.6) | 0.87 (0.69–1.10, 0.3) |
| Donor race – White | Reference | Reference | Reference |
| Black | 1.04 (0.91–1.19, 0.6) | 1.24 (1.003–1.54, 0.05) | 1.09 (0.96–1.25, 0.2) |
| Other | 1.02 (0.78–1.34, 0.9) | 1.17 (0.76–1.79, 0.5) | 1.00 (0.76–1.31, 1.0) |
A total of 236 patients had primary nonfunction (PNF) of the renal graft (4.1%). Patients with DGF were more likely to progress to PNF (11.8% vs 2.1%, p<0.001). In multivariate analysis, DGF was the strongest risk factor for PNF (aIRR 3.835.126.84, p<0.001). Other significant risk factors were history of previous liver transplant (aIRR 1.181.652.30, p=0.003), history of previous kidney transplant (aIRR 1.502.193.21, p<0.001), and year of transplant (per year, aIRR 0.900.930.97, p<0.001).
A total of 524 patients had PNF of the transplanted renal graft or death within three months, giving a renal allograft futility (RAF) rate of 8.4%. In multivariate analysis, DGF was the strongest risk factor for RAF (aIRR 2.242.693.24, p<0.001). Other significant risk factors included pretransplant hospitalization (aIRR 1.221.511.85, p<0.001), previous liver (aIRR 1.621.972.40, p<0.001) or kidney (aIRR 1.261.662.19, p<0.001) transplant, year of transplant (per year, aIRR 0.920.940.96, p<0.001), pretransplant dialysis (aIRR 1.051.301.60, p=0.02) and use of a nationally shared (aIRR 1.041.662.66, p=0.03) or DCD organ (aIRR 1.011.482.20, p=0.049). Recipients who received induction immunosuppression were less likely to develop RAF (aIRR 0.650.790.96, p=0.02).
DISCUSSION
In this national study of 6214 SLK recipients in the MELD area, 21.8% developed DGF. HCV status was associated with a decreased incidence of DGF (aIRR 0.54, p = 0.02). Risk factors for the HCV negative recipient population included recipient dialysis prior to SLK (aIRR 1.473.267.22, p=0.004), donor BMI (aIRR 1.061.251.47 per 5 kg/m2), and receipt of an imported (aIRR 1.061.692.69 for regional share; aIRR 2.054.8211.30 for national share) or DCD (aIRR 1.935.3815.01) donor.
Regardless of HCV status, SLK recipients with DGF experienced a 2.6-fold increase in kidney graft failure, 1.6-fold increase in liver failure, 1.6-fold increase in mortality, a 5.1-fold increase in PNF and 2.7-fold increase in RAF. The early complication of DGF is thus associated with inferior long-term outcomes.
Our finding of a 21.8% DGF rate in SLK recipients is similar to a report from Jay et al15 (19% in recipients receiving grafts with KDPI 0–85%), which stands in contrast to a single-center report raising concern for increased DGF rate of 39% in SLK recipients.9 Importantly, we identified specific recipient and donor risk factors for DGF in SLK recipients (recipient pretransplant recipient dialysis, increased donor BMI, imported donors, and DCD donors). While previous studies have demonstrated increased risk of DGF and associated poor outcomes in recipients receiving inferior quality kidney grafts, we identify specific components of the composite quality scores to identify unique risk factors driving this DGF risk in the SLK population. Jay et al, for example, had described increased risk for DGF in SLK recipients receiving high KDPI kidneys, as did Ekser et al in combined liver kidney transplantation.20 Similarly, Levitksy et al previously showed transplant with an expanded criteria donor (ECD) kidney was associated with significant worse one-year patient and kidney graft survival as well as higher rates of DGF.21 Finally, Sharma et al showed the survival advantage conferred by SLK compared to liver alone transplantation diminished with receipt of increasing KDPI grafts.22 In the current study we analyzed component parts of KDPI to further elucidate these previous findings; we demonstrated transplant of DCD organs and increased donor BMI to be the components of KDPI which drives this association in the national cohort. We also found that recipient factors contributed significantly to risk of DGF development.
The differences within our cohort of SLK recipients by HCV status was notable. In adjusted analysis, recipients who were HCV positive had a lower risk of developing DGF and risk factors seen in HCV negative recipients were not associated with DGF development. These results are at odds with the literature reporting higher rates of DGF in the kidney alone transplant population.23 Sensitivity analysis attempting to explore possible mechanisms or risk factors was unrevealing in our current dataset and these differences thus likely represent unmeasured residual confounding unique to the HCV positive population that we are unable to account for in our multivariate model. This area warrants further exploration with more granular data on HCV status of the recipient as well as donor quality.
Our study demonstrates that DGF is associated with inferior posttransplant outcomes in SLK recipients regardless of HCV status. Further, in adjusted analysis, we found DGF to be the strongest risk factor for mortality as well as kidney and liver graft failure. These findings should be considered by the recipient team when evaluated a potential SLK donor, as they are linked to both short and long term posttransplant outcomes. This work also explores the national landscape of SLK transplantation, in contrast to single-center studies that have shown similar associations in their local populations. For example, Hibi et al reported a single-center study in which DGF was the strongest predictor of death-censored kidney graft failure as well as a predictor of mortality.13 Ekser et al also observed this relationship in at their center and showed that DGF and ECD kidneys were significant independent risk factors for patient survival.24 In a national cohort study using SRTR data, Alhamad et al reported high rates of DGF in DCD patients (32.7%) and an association of DGF development with increased mortality and graft failure.14 While the mechanisms of these inferior outcomes are unclear from the current analysis, further work with data on recipient cause of death and postoperative complications may further inform our understanding. This open question is particularly relevant in that we found over 90% of liver graft failures to result from death, which may be due to failure of the liver graft or morbidity unrelated to graft function such as infection or de novo or recurrent neoplasms. The data available for cause of death in our study is not granular enough to explore this question fully, but should form the basis of further investigations.
Lunsford et al quantified futility of renal grafts in SLK transplantation in a study of a single-center experience.9 We extended these findings to a nationwide cohort and, compared to their study, saw lower rates of RAF (8.4% vs. 20.7%). However, the previous study represented a more acutely ill patient population than the current national landscape of SLK recipients. Our current study had lower rates of pretransplant hospitalization (41.9% vs 69.0%) and pretransplant ICU hospitalization (18.3% vs 37.4%), as well as lower median MELD scores at transplant (30 vs. 35). These differences in baseline characteristics may explain differences in futile outcomes, particularly since we found recipient pretransplant hospitalization to be a significant risk factor for PNF and RAF. Similar to Lunsford et al, we found history of previous transplant to be a significant risk factor for RAF. Importantly, we demonstrated the strong association of DGF with negative early posttransplant outcomes of PNF and RAF (5.1-fold and 2.7-fold increased risk, respectively), highlighting the importance of preventing this early complication. Importantly, it has been suggested that the association of DGF with inferior posttransplant outcome is mitigated if PNF is excluded.25 Sensitivity analysis in which we excluded recipients with RAF (PNF or death within 90 days posttransplant) offers opposing evidence to this view, as we found no changes in inferences drawn from the complete study population.
A limitation of our study is the potential for selection bias given the nonuniform listing practices between transplant centers prior to OPTN policy change establishing medical eligibility criteria in 2016, which has previously been cited as a potential bias when analyzing posttransplant outcomes.17,26–28 As has been previously shown, differences in listing affects posttransplant functional recovery in native kidneys, which may confound results from posttransplant outcomes studies.21 An additional limitation is the inability to account for differences in management practices across transplant centers which may affect posttransplant outcomes. We attempted to account for these limitations inherent to our data source by inclusion of multivariate models and sensitivity analysis of subgroups. Additionally, the SRTR dataset contains minimal information on intraoperative and postoperative complications, which may also have an effect on development of DGF and long-term outcomes. To the extent possible, we attempted to mitigate this limitation by including in our analysis models both preoperative surrogate markers for disease severity, including preoperative hospitalization and ICU status, MELD, and pretransplant dialysis as well as postoperative indicators such as early graft rejection of either the kidney or liver. Finally, we cannot determine causality from the observed associations, a limitation of retrospective registry analyses. While DGF may be on the causal pathway to inferior posttransplant outcomes, the observed association may be due to a number of mechanisms or suffer from unmeasured residual confounding. Consequently, further study with even more granular information on perioperative complications as well as cause of death and long-term complications is needed to fully explain the associations that we observe in our study analysis.
Areas for future study should investigate mitigation of DGF risk and associated morbidity and mortality as well as better understanding the causes of mortality in this population, particularly as the association between DGF and inferior outcomes is strong, but the causal mechanisms remain unclear. Several groups have proposed and reported excellent results with two important approaches targeting the transplanted kidney, namely machine perfusion of the graft and delayed transplantation, which can mitigate the risks for development of DGF and resulting morbidity and mortality. For example, Ekser et al demonstrated delayed kidney transplantation of at least two days in combination with continuous hypothermic pulsatile perfusion decreased rates of DGF from 7.3% to 0% compared to simultaneous liver kidney transplantation.24 They further showed this decrease was associated with improved kidney function as well as one and five year patient survival. The same group reported the combination of delayed kidney transplantation and using low KDPI kidneys resulted in excellent three years results.20 Preliminary results from other groups have similarly demonstrated delayed kidney transplantation in high acuity liver recipients permits hemodynamic stabilization and decreases futility.29 Finally, early reports from single-center studies of hypothermic pulsatile machine perfusion have shown promising results in significantly decreasing development of DGF among SLK recipients.30 Future investigation of machine perfusion and delayed transplantation of kidney grafts may contribute to reduction in DGF and its associated morbidity and mortality.
In conclusion, in this national study of SLK recipients, we found DGF to be strongly associated with recipient pretransplant dialysis as well as donor organs recovered from DCD donors and imported from regional or national sources. Subsequently, DGF was associated with inferior posttransplant outcomes, including decreased graft and patient survival in SLK recipients. Incorporating knowledge of risk factors into clinical decision making may improve prevention of DGF and may represent an opportunity to improve posttransplant outcomes.
ACKNOWLEDGMENTS
Funding for this study was provided by the National Institute of Diabetes and Digestive and Kidney Disease and the National Institute on Aging: grant numbers K24DK101828 (PI: Dorry Segev), K23DK115908 (PI: Jacqueline Garonzik Wang), and F32AG053025 (PI: Christine Haugen).
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Abbreviations:
- aHR
adjusted hazard ratio
- aIRR
adjusted incident rate ratio
- BMI
body mass index
- CIT
cold ischemia time
- DAA
direct-acting antiviral
- DCD
donation after circulatory death
- DGF
delayed graft function
- DRI
Donor Risk Index (for liver transplantation)
- HCV
Hepatitis C virus
- HLA
human leukocyte antigen
- HRSA
Health Resources and Services Administration
- KDPI
Kidney Donor Profile Index
- MELD
Model for End-Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- PNF
primary nonfunction
- PRA
Panel Reactive Antibody
- RAF
renal allograft futility
- SLK
simultaneous liver kidney
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose.
REFERENCES
- 1.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant. 2018;18(S1):172–253. doi: 10.1111/ajt.14559 [DOI] [PubMed] [Google Scholar]
- 2.Eason JD, Gonwa TA, Davis CL, et al. Proceedings of consensus conference on simultaneous liver kidney transplantation (SLK). Am J Transplant. 2008;8(11):2243–2251. doi: 10.1111/j.1600-6143.2008.02416.x [DOI] [PubMed] [Google Scholar]
- 3.Locke JE, Warren DS, Singer AL, et al. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation. 2008;85(7):935–942. doi: 10.1097/TP.0b013e318168476d [DOI] [PubMed] [Google Scholar]
- 4.Simpson N, Cho YW, Cicciarelli JC, et al. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: Analysis of UNOS Database. Transplantation. 2006;82(10):1298–1303. doi: 10.1097/01.tp.0000241104.58576.e6 [DOI] [PubMed] [Google Scholar]
- 5.Hmoud B, Kuo YF, Wiesner RH, et al. Outcomes of liver transplantation alone after listing for simultaneous kidney: Comparison to simultaneous liver kidney transplantation. Transplantation. 2015;99(4):823–828. doi: 10.1097/TP.0000000000000438 [DOI] [PubMed] [Google Scholar]
- 6.Martin EF, Huang J, Xiang Q, et al. Recipient survival and graft survival are not diminished by simultaneous liver-kidney transplantation: An analysis of the united network for organ sharing database. Liver Transpl. 2012;18(8):914–929. doi: 10.1002/lt.23440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong TL, Khemichian S, Shah T, et al. Combined Liver-Kidney Transplantation Is Preferable to Liver Transplant Alone for Cirrhotic Patients With Renal Failure. Transplantation. 2012;94(4):411–416. doi: 10.1097/TP.0b013e3182590d6b [DOI] [PubMed] [Google Scholar]
- 8.Wadei HM, Bulatao IG, Gonwa TA, et al. Inferior long-term outcomes of liver-kidney transplantation using donation after cardiac death donors: Single-center and organ procurement and transplantation network analyses. Liver Transpl. 2014;20(6):728–735. doi: 10.1002/lt.23871 [DOI] [PubMed] [Google Scholar]
- 9.Lunsford KE, Bodzin AS, Markovic D, et al. Avoiding Futility in Simultaneous Liver-kidney Transplantation. Ann Surg. 2017;265(5):1016–1024. doi: 10.1097/SLA.0000000000001801 [DOI] [PubMed] [Google Scholar]
- 10.Siddiqi N, McBride MA, Hariharan S. Similar risk profiles for post-transplant renal dysfunction and long-term graft failure: UNOS/OPTN database analysis. Kidney Int. 2004;65(5):1906–1913. doi: 10.1111/j.1523-1755.2004.00589.x [DOI] [PubMed] [Google Scholar]
- 11.Patel SJ, Duhart BT Jr, Krauss AG, et al. Risk factors and consequences of delayed graft function in deceased donor renal transplant patients receiving antithymocyte globulin induction. Transplantation. 2008;86(2):313–320. doi: 10.1097/TP.0b013e31817ef190 [DOI] [PubMed] [Google Scholar]
- 12.Tapiawala SN, Tinckam KJ, Cardella CJ, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21(1):153–161. doi: 10.1681/ASN.2009040412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hibi T, Sageshima J, Molina E, et al. Predisposing Factors of Diminished Survival in Simultaneous Liver/Kidney Transplantation. Am J Transplant. 2012;12(11):2966–2973. doi: 10.1111/j.1600-6143.2012.04121.x [DOI] [PubMed] [Google Scholar]
- 14.Alhamad T, Spatz C, Uemura T, et al. The Outcomes of Simultaneous Liver and Kidney Transplantation Using Donation After Cardiac Death Organs. Transplantation. 2014;98(11):1190–1198. doi: 10.1097/TP.0000000000000199 [DOI] [PubMed] [Google Scholar]
- 15.Jay C, Pugh J, Halff G, et al. Graft quality matters: Survival after simultaneous liver-kidney transplant according to KDPI. Clin Transplant. 2017;31(5):e12933. doi: 10.1111/ctr.12933 [DOI] [PubMed] [Google Scholar]
- 16.Massie AB, Kuricka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8):1723–1730. doi: 10.1111/ajt.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Organ Procurement and Transplantation Network/United Network for Organ Sharing. Simultaneous Liver Kidney (SLK) Allocation Summary of Changes. Available at http://optn.transplant.hrsa.gov/media/1888/kidney_policynotice_slk_201606.pdf.Published 2016. Accessed March 14, 2018.
- 18.Nadim MK, Davis CL, Sung R, et al. Simultaneous liver-kidney transplantation: A survey of US transplant centers. Am J Transplant. 2012;12(11):3119–3127. doi: 10.1111/j.1600-6143.2012.04176.x [DOI] [PubMed] [Google Scholar]
- 19.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekser B, Mangus RS, Kubal CA, et al. Excellent outcomes in combined liver-kidney transplantation: Impact of kidney donor profile index and delayed kidney transplantation. Liver Transpl. 2018;24(2):222–232. doi: 10.1002/lt.24946 [DOI] [PubMed] [Google Scholar]
- 21.Levitsky J, Baker T, Ahya SN, et al. Outcomes and native renal recovery following simultaneous liver-kidney transplantation. Am J Transplant. 2012;12(11):2949–2957. doi: 10.1111/j.1600-6143.2012.04182.x [DOI] [PubMed] [Google Scholar]
- 22.Sharma P, Shu X, Schaubel DE, et al. Propensity score-based survival benefit of simultaneous liver-kidney transplant over liver transplant alone for recipients with pretransplant renal dysfunction. Liver Transpl. 2016;22(1):71–79. doi: 10.1002/lt.24189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forman JP, Tolkoff-Rubin N, Pascual M, et al. Hepatitis C, acute humoral rejection, and renal allograft survival. J Am Soc Nephrol. 2004;15(12):3249–3255. doi: 10.1097/01.ASN.0000145896.16153.43 [DOI] [PubMed] [Google Scholar]
- 24.Ekser B, Mangus RS, Fridell JA, et al. A Novel Approach in Combined Liver and Kidney Transplantation With Long-term Outcomes. Ann Surg. 2017;265(5):1000–1008. doi: 10.1097/SLA.0000000000001752 [DOI] [PubMed] [Google Scholar]
- 25.Kayler LK, Srinivas TR, Schold JD. Influence of CIT-induced DGF on kidney transplant outcomes. Am J Transplant. 2011;11(12):2657–2664. doi: 10.1111/j.1600-6143.2011.03817.x [DOI] [PubMed] [Google Scholar]
- 26.Nadim MK, Sung RS, Davis CL, et al. Simultaneous liver-kidney transplantation summit: Current state and future directions. Am J Transplant. 2012;12(11):2901–2908. doi: 10.1111/j.1600-6143.2012.04190.x [DOI] [PubMed] [Google Scholar]
- 27.Feng S, Trotter JF. Can we stop waiting for godot? Establishing selection criteria for simultaneous liver-kidney transplantation. Am J Transplant. 2012;12(11):2869–2870. doi: 10.1111/j.1600-6143.2012.04295.x [DOI] [PubMed] [Google Scholar]
- 28.Luo X, Massie AB, Haugen CE, et al. Baseline and Center-Level Variation in Simultaneous Liver-Kidney Listing in the United States. Transplantation. 2018;102(4):609–615. doi: 10.1097/TP.0000000000001984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lunsford K, Agopian V, Saharia A, et al. Delayed Renal Implantation Improves Survival Following Simultaneous Liver-Kidney Transplantation in High Acuity Recipients [Abstract]. Am J Transplant. 2017;17(suppl 3). [Google Scholar]
- 30.Korayem I, Gritsch H, Veale J, et al. Effect of Hypothermic Pulsatile Machine Perfusion on Kidney Delayed Graft Function in Simultaneous Liver-Kidney Transplantation: A Single Center Study [Abstract]. Am J Transplant. 2017;17(suppl 3). [Google Scholar]