Abstract
Exercise holds potential to reduce substance use, but engaging patients in exercise regimens is challenging. Contingency management (CM) interventions can directly address adherence by targeting initiation and maintenance of exercise behavior. This study evaluated the efficacy of a CM-reinforced exercise intervention as an adjunct to standard outpatient substance use disorder treatment. Participants were 120 patients with substance use disorders who were randomly assigned to standard care with CM for completing exercise goals or CM for completing general non-exercise goals weekly for a four-month treatment period. Urine samples were tested for evidence of illicit substance use up to twice a week during treatment, and at follow-ups through 12 months. Results found that the CM-Exercise condition demonstrated during-treatment improvements on several physical activity and relevant psychosocial functioning indices (e.g., self-efficacy for exercise). CM-Exercise had no advantage relative to the CM-General condition in decreasing substance use. Overall, this study adds to a small body of well powered trials assessing effects of exercise interventions as adjunct treatment for substance use disorders and finds no benefit over an alternate CM approach in terms of drug abstinence.
Keywords: physical activity, adjuvant substance use disorder treatment, incentives
Regular physical activity (any bodily movement produced by the contraction of skeletal muscles that results in an increase in energy expenditure above resting energy expenditure) and exercise (a type of physical activity consisting of planned, structured, and repetitive bodily movement done to improve and/or maintain one or more components of physical fitness) have numerous health benefits, including improved executive function, self-perceived quality of life, physical functioning that enables completing daily living activities, and sleep quality, and reduced depressive symptoms, anxiety symptoms, and risk of many diseases and conditions (Pescatello, Riebe, & Thompson, 2013; Physical Activity Guidelines Advisory Committee, 2018; Powell et al., 2019). There are also biological, psychological, social and physiological reasons to expect direct and indirect benefits of physical activity and exercise on substance use (Bock et al., 2012; Brellenthin & Koltyn, 2016; Linke & Ussher, 2015; Lynch, Peterson, Sanchez, Abel, & Smith, 2013; Read & Brown, 2003; Smith & Lynch, 2013; Zschucke, Heinz, & Ströhle, 2012). For example, exercise interventions reduce negative emotional states common in patients with substance use disorders and that might precipitate relapse (Cooney et al., 2013; Wipfli, Rethorst, & Landers, 2008). Correlational and cross-sectional evidence concludes that increased physical activity is associated with reduced substance use problems (Korhonen, Kujala, Rose, & Kaprio, 2009; Ströhle et al., 2007).
Reviews generally find benefits of physical activity and exercise programs among people with licit and illicit substance use disorders. Common conclusions are that such programs are feasible and safe, and reduce anxiety and depressive symptoms (Giesen, Deimel, & Bloch, 2015; Hallgren, Vancampfort, Giesen, Lundin, & Stubbs, 2017). Empirical studies of laboratory or structured exercise interventions in adults with licit and illicit substance use disorders find reductions in cravings (Buchowski et al., 2011; Daniel, Cropley, Ussher, & West, 2004; Ussher, Sampuran, Doshi, West, & Drummond, 2004), withdrawal symptoms (Bock, Marcus, King, Borrelli, & Roberts, 1999; Taylor, Ussher, & Faulkner, 2007), and stress (Cutter et al., 2014).
Exercise interventions may also impact substance use behavior directly, but reviews are cautious. One review concluded that evidence supports increased abstinence and reduced craving or negative mood with adjuvant exercise in the treatment of illicit drug and alcohol use disorder and cigarette smoking (Wang, Wang, Wang, Li, & Zhou, 2014). Other reviews found benefits of exercise on smoking cessation (Ussher et al., 2014; Zschucke et al., 2012), but that evidence of effects on other substance use was preliminary (Zschucke et al., 2012). Reviews of studies among persons with alcohol use disorder found evidence of positive effects of exercise on fitness and other health outcomes but not on abstinence (Giesen et al., 2015; Hallgren et al., 2017). There is also general consensus that a lack of rigorous and non-preliminary studies complicates drawing conclusions (Daniel et al., 2004; Giesen et al., 2015; Hallgren et al., 2017; Ussher et al., 2014; Wang et al., 2014; Zschucke et al., 2012). Two rigorous trials in residential stimulant use disorder patients randomized to a supervised exercise program or a health education control condition found reduced relapse rates following discharge from treatment in subgroup analyses, with one finding effects on relapse only among those with low severity (≤ 18 days) stimulant use in the month prior to treatment (Rawson et al., 2015), and the other finding greater reductions in relapse rates among those with high versus low adherence to the exercise program (Trivedi et al., 2017). Adherence has also been noted as likely contributing to effects of exercise on abstinence in non-preliminary trials among alcohol use disorder outpatients (Jensen, Nielsen, & Ekstrøm, 2018; Roessler et al., 2017) and in smoking cessation studies (Marcus et al., 1999, 2005; Prapavessis et al., 2016; Ussher et al., 2015).
Low adherence to exercise regimens is a challenge, with both supervised (Ussher, Lewis, Aveyard, Manyonda, West, Lewis, Marcus, Riaz, Taylor, Barton, et al., 2015) and home-based (Ussher, Taylor, & Faulkner, 2014) programs, and even in residential settings (Trivedi et al., 2017). In fact, adherence has widespread effects on exercise and other complex behavioral health outcomes (Martin, Williams, Haskard, & Dimatteo, 2005), including in patients with stimulant use disorder, alcohol use disorder, and other chronic illnesses (Brown et al., 2014; Church et al., 2010; Dunn, Trivedi, Kampert, Clark, & Chambliss, 2005; Trivedi et al., 2017).
Contingency management (CM) is useful for increasing engagement in and outcomes of substance use treatment (Ainscough, McNeill, Strang, Calder, & Brose, 2017; Benishek et al., 2014; Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Prendergast, Podus, Finney, Greenwell, & Roll, 2006). In these interventions, patients receive tangible incentives for adherence behaviors such as submitting drug negative urine samples. Reinforcement principles can also be applied toward engaging in physical activity and exercise (DeVahl, King, & Williamson, 2005; Epstein, Paluch, Kilanowski, & Raynor, 2004; Faith et al., 2001; Jeffery, Wing, Thorson, & Burton, 1998), and a meta-analysis finds reinforcement is effective for initiating and maintaining exercise (Mitchell et al., 2013). As an example, Petry et al. (2013) randomized 45 sedentary adults to a CM intervention in which they received incentives for walking 10,000 steps per day per pedometers or a control condition in which they were encouraged to meet the same step goal consistent with guidelines but without reinforcement. Participants in the CM condition met walking goals on significantly more days (82.5% versus 55.3%) than those in the control condition. However, that study did not enroll persons with substance use problems.
Two randomized studies applied CM to exercise adherence and evaluated preliminary efficacy in reducing drinking. In a non-treatment sample, Weinstock, Petry, Pescatello, & Henderson (2016) randomized 70 heavy drinking sedentary college students to 8-week interventions, one of which reinforced them for meeting objectively-verified weekly exercise goals, with the goal of increasing and then maintaining exercise over time at a level of ≥ 150 minutes per week of moderate intensity or ≥ 75 minutes per week of vigorous intensity exercise (Pescatello et al., 2013). Students in the CM condition self-reported greater exercise frequency, but changes in exercising were unrelated to drinking. In a clinical sample, Brown et al., (2014) randomized 48 alcohol-dependent sedentary outpatients to brief advice about exercising or a CM intervention, in which they received incentives for attending moderate intensity (exercise at a rate that achieved 55–69% of age-predicted maximal heart rate; Franklin, Whaley, Howley, & Balady, 2000) group aerobic exercise sessions, and 62% came to at least 8 of 12 sessions. Those in the CM intervention reduced some indices of drinking more than those receiving brief advice, but effects were not maintained at follow-up and self-reports of time spent in moderate intensity exercise and estimated maximal oxygen consumption (VO2 max, higher values indicate greater cardiovascular health) obtained during a cardiovascular fitness test did not differ between conditions. Patients who attended aerobic exercise sessions did have improved maximal oxygen consumption, an objective measure of physical fitness that correlates with physical activity levels, and in this subgroup, correlations were significant between adherence to exercising and drinking outcomes.
Thus, exercise may hold potential as a supplemental substance use treatment intervention, but it is difficult to ascertain its impact when adherence is poor, and rigorous studies are needed. This study was a pragmatic trial to assess effects of reinforcing adherence with physical activity and exercise activities performed on a weekly basis among outpatient substance use disorder treatment patients. Exercise activities were tailored to patient preferences and abilities but aimed to increase over time and then maintain at a level of ≥ 150 minutes of moderate intensity exercise per week, per expert guidelines (Pescatello et al., 2013). The active control condition reinforced completion of non-exercise behaviors related to general treatment goals (e.g., attending a medical appointment, job skills training), to control for time, attention, and contingent reinforcement. We hypothesized that patients in the CM-Exercise condition would improve dimensions of physical activity and fitness related outcomes above and beyond their counterparts in the CM condition reinforcing completion of non-exercise goals. We evaluated relations between adherence and health outcomes. In addition, self-efficacy, motivation, and social support are associated with sustaining an exercise regimen (Buckworth, Lee, Regan, Schneider, & DiClemente, 2007; Cress et al., 2005), engaging in exercise is associated with improved quality of life in substance use disorder treatment patients (Muller & Clausen, 2015), and sleep quality improves with exercise (Kelley & Kelley, 2017). Thus, these factors were assessed. Final analyses examined study intervention effects on reducing substance use.
Methods
Participants
Participants were 120 patients enrolled in a community outpatient substance abuse treatment clinic. Inclusion criteria were age 18–65 years, English speaking, DSM-IV diagnosis of cocaine, opioid or marijuana use disorder, and written permission from a health care professional to participate in an exercise program. Exclusion criteria were: serious uncontrolled psychiatric conditions (e.g., actively suicidal, psychotic); past 6-month history of myocardial infarction, stroke, unstable angina, coronary surgery or angioplasty, uncontrolled arrhythmia, or hyperthyroidism; blood pressure >165 mmHg systolic or >95 mmHg diastolic; body mass index (BMI) >45 kg/m2; grossly abnormal physical functioning; pregnant; or in recovery for gambling disorder. Figure 1 shows numbers screened and enrolled. The study was powered at 0.80 and alpha =.05 to detect a medium effect size between conditions of about d = 0.50 and enrolled ~60 patients per condition (Cohen, 1988).
Figure.
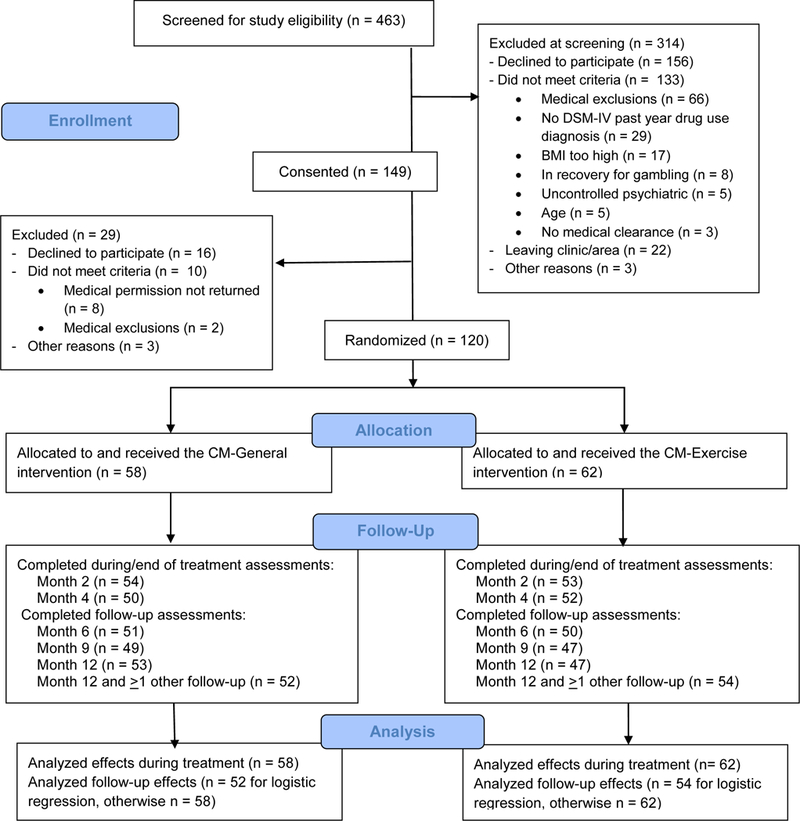
Participant flow through the study.
Assessments
Participants provided informed consent, as approved by the University Institutional Review Board. Research staff administered baseline assessments. Participants completed checklists derived from the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) to assess substance use diagnoses (American Psychiatric Association, 2000), and the Addiction Severity Index (McLellan & Hunkeler, 1998; McLellan et al., 1992) evaluated functioning across medical, employment, alcohol, drug, legal, family/social, and psychiatric domains; scores range 0 (lowest severity of problems in the domain) to 1 (highest severity of problems in the domain). An Alcosensor IV Alcometer (Intoximeters, St. Louis, MO) tested for recent drinking, and OnTrak TestSticks (Varian, Inc., Walnut Creek, CA) screened urine samples for stimulants, opioids, and marijuana. A urine pregnancy test (EarlyPregnancyTests.com; Bellingham, WA) was administered at intake to women of childbearing potential to assess eligibility criteria.
Participants completed the International Physical Activity Questionnaire, Short Form (IPAQ) (Craig et al., 2003) which is a reliable and valid assessment of total and moderate and vigorous intensity physical activity volume in MET·mins/wk, walking for at least 10 mins in MET·mins/wk, and sitting in mins/day.
Health-related fitness and medical outcomes included the following: Handgrip, an indicator of overall body strength, is assessed in the dominant hand by a Jamar dynamometer (Lafayette Instrument Co., Lafayette, IN) and involves 2 trials to obtain average kg (Pescatello et al., 2013). The Floor Transfer Test assessed lower body strength, balance, and functional ability; it involves moving from a standing position to the floor with buttocks to the ground and returning to standing, with the quicker of two trials recorded in seconds (Murphy, Olson, Protas, & Overby, 2003). The Sit-and-Reach Test assessed low back and hip-joint flexibility (Jackson et al., 1998). Individuals sit with soles 10–12 in apart and flat against a box (32×50×45 cm) with a measuring stick placed on top of the box and extending over it towards patient’s legs. The patient is instructed to slowly reach forward as far as possible toward the stick and box with both hands, keeping hands parallel and holding the furthest position for 2 sec. The score is the most distant point (in cm) reached with fingertips toward the box, with best of 3 trials recorded (ACSM, 2006; Lemmink et al., 2003). The Push-Up Test, a measure of muscle strength and endurance, recorded consecutive push-ups (Pescatello et al., 2013). A modified version of the Harvard Step Test (Keen & Sloan, 1958) assessed cardiovascular fitness. Following 5 minutes of quiet rest, individuals step onto and down from a step 30 cm (11.8 inches) in height for 3 minutes at a rate of 30 steps per minute. Heart rate is measured for 30 seconds at 1, 2 and 3 minutes after completion of stepping. A Physical Fitness Score is [(duration of exercise in seconds × 100) / (sum of the three heart rates)]. This step test has demonstrated reliability and sensitivity for assessing cardiovascular fitness (Jinzhou, Fu, Zhang, Li, & Shan, 2008). To assess blood pressure (BP) after 5 minutes of seated rest, a semiautomatic digital device (Omron, Lake Forest, IL) took at least two readings, 3 minutes apart, until consecutive readings were within 5 mm Hg and with the last two readings averaged (Pickering et al., 2005). Body mass index (BMI) was calculated from weight in kg/height in m2.
Assessments of psychosocial functioning were: The Self-Efficacy for Exercise Scale (Resnick & Jenkins, 2000) assessed confidence about exercising regularly across various scenarios (e.g., when tired, when under pressure from work, during bad weather), with items rated 0 (“Cannot do at all”) to 10 (“Highly certain can do”), and the average taken as the scale score (range 0 to 180, least to greatest self-efficacy). The scale has good internal consistency and predictive validity and correlates with other measures of exercising (Shaughnessy, Resnick, & Macko, 2004). The Intrinsic Motivation Inventory (Buckworth et al., 2007) evaluated motivation to engage in exercise, and is reliable and valid (Lee & DiClemente, 2001; Vallerand & Fortier, 1998). Items (e.g., “I enjoy participating in physical activity very much”) are rated 1 (“Not true at all’) to 7 (“Very true”), with negative items (e.g., “I participate in physical activity because I have no other choice”) reverse coded, and all item ratings summed to produce a scale score (range 21 to 147, least to greatest intrinsic motivation). Physical Activity Social Support scale has established psychometrics in evaluating support from friends and family related to physical activity (Eyler et al., 1999; Sallis, Grossman, Pinski, Patterson, & Nader, 1987; Sallis, Hovell, & Richard Hofstetter, 1992). Items ask about how often family and friends provide support in the past 2 months (e.g., “How often did my family give me encouragement to stick with me exercise program?”), with items rated 1 (“Never”) to 5 (“Very often”) (or not applicable), and the applicable items averaged to produce a Family score and a Friends score (range 13 to 65, less to more social support). The Quality of Life Inventory (Frisch, Cornell, Villanueva, & Retzlaff, 1992) assesses satisfaction in life areas. It has test-retest reliability of .80–.91, correlates with other measures of well-being (Frisch et al., 1992), and is positively impacted by CM treatments (Petry, Alessi, & Hanson, 2007). The total score (range −51 to 102) reflects subjective well-being in life domains considered to be of at least some importance to the individual, with greater scores reflecting greater well-being. The Pittsburgh Sleep Quality Index (Buysse, Reynolds III, Monk, Berman, & Kupfer, 1989) is a widely used, reliable and valid measure of sleep, with items referring to sleep quality in the past month; lower scale scores indicate better sleep quality.
Participants received $25 for completing the baseline psychosocial assessment, $25 for returning the medical form, and $25 for the baseline physical assessment. They received $50 for evaluations at Months 2, 4, 6, and 9, and $75 at Month 12. Figure 1 shows completion rates, which did not differ by conditions, p > .05, except for higher Month 12 completion rates in CM-Exercise, x2 (1) values = 5.23, p = .02.
Procedures
All participants received standard intensive outpatient services as delivered by the outpatient clinic, which consisted of group therapy focusing on 12-steps, relapse prevention, drug refusal skills, and daily planning. Treatment involves 4 hours of care 3–4 days per week for weeks, followed by gradual reductions. By week 9, most come to the clinic for only one aftercare group session per week.
Participants submitted urine and breath samples up to twice weekly (Mon-Thur or Tues-Fri) in weeks 1–8 and once weekly in weeks 9–16. Samples were tested for marijuana, cocaine, methamphetamine, amphetamine, opioids, and alcohol as outlined above. Compensation for each sample submitted was $2, regardless of results. The research staff congratulated participants for each substance for which they tested negative and encouraged them to discuss any use in group. In addition to standard care and sample monitoring, individuals received one of two weekly CM interventions described below. The first CM session was 30–45 minutes, including explanations of how to earn incentives, and subsequent sessions were about 20 minutes.
A computerized program (Charpentier, 2003) randomized participants. It stratified assignment on Baseline sample result (positive for any drug vs none) and exercise level (< or ≥ 30 min weekly).
Treatments
CM for General Non-Exercise Behaviors (CM-General).
Persons in this condition completed a Personal Needs Assessment evaluating: employment, education, family, housing, medical/ psychiatric, legal, sobriety, social/recreational, personal improvement, and transportation concerns. They identified two to four areas, and each week, they completed Behavioral Contracts specifying activities related to those areas. For example, if the goal was education, individuals could enroll in a GED course, and if the goal was medical, they could attend a doctor’s appointment. Contracts listed required objective verifications such as receipts or brochures (Petry, Martin, & Finocche, 2001). To ensure separation between conditions, exercise and physical activities (e.g., bowling, swimming, walking) were not reinforced in this condition.
Participants earned one chance to draw a slip of paper and win a prize for each contracted goal (e.g., go to an NA meeting, complete a job application) completed and verified (up to 3/week). If they completed three goals within the week, they earned at least four bonus draws; bonus draws escalated such that if they completed three goals 2 weeks in a row, they received five bonus draws, and three goals 3 weeks in a row resulted in six bonus draws, and so on, up to a maximum of 10 bonus draws/week. In total, up to 187 draws over 16 weeks was possible.
If a patient failed to complete (or verify) three goals in a given week, draws for any completed goals were provided, but no bonus draws occurred that week and bonus draws reset to four for the next week in which at least three goals were completed. Absences required preapproval; unexcused absences reset bonus draws. Once a patient completed three goals 2 weeks in a row, bonus draws returned to the highest number previously achieved.
The prize bowl contained 500 slips. Half (250) stated “Good job!” but were not associated with a prize. The other half were winning: 204 stated “Small”; 45 stated “Large,” and one “Jumbo.” When individuals drew a winning slip, they chose among prizes in that category during the CM session.
Small prizes were items such as bus tokens, socks, toiletries, and food items, and large prizes were $20 gift cards, watches, coffee makers, etc. Jumbo prizes included stereos, TVs and $100 gift cards. Slips were returned to the bowl after each draw. To introduce the system, individuals could “draw until they win” (small, large or jumbo, whichever came first) in the initial CM session after determining the first behavioral contract.
CM for Exercise (CM-Exercise).
The first session explicated rationales for increasing exercise. After assessing participants most physically active period and current level with the PPAQ, staff described the American College of Sports Medicine (ACSM)) recommendations for developing and maintaining overall health and fitness (modified as needed by physician’s advice) of exercising 3–5 times per week, for ≥30 min each time, including moderate intensity aerobic, resistance, and flexibility exercises, to total ≥ 150 minutes per week (Garber et al., 2011; Pescatello et al., 2013). They also encouraged lifestyle physical activity of household, occupational, and leisure activities (Garber et al., 2011; Pescatello et al., 2013; Physical Activity Guidelines Advisory Committee, 2018) (Blair et al, 1992; Dunn et al., 1998), and recommended planned and short bouts (10 min) of exercising to cope with cravings. The goal was to move individuals to an equivalent of walking a 15–20 min mile pace for 2–3 miles, 3–4 days or more per week (Garber et al., 2011; Pescatello et al., 2013). Exercise programs that allow for various types of physical activities (e.g., aerobic, resistance) are recommended; and, goal setting, behavioral contracts, positive reinforcement, and having physical activity options are important for adherence to exercise programs (Cress et al., 2005).
The staff introduced the Behavioral Contract, collaboratively deciding on three physical activities each week. One initial activity included going to the YMCA, fees for which were paid by the research grant. Especially for sedentary participants, initial behavior contracts might have focused on increasing engagement in low intensity, daily physical activities, and then increased (e.g., walk 10,000 steps per day on four days this week). Documentation included workout output on the YMCA equipment, signed forms, receipts, pedometer readings, or cell phone recorded videos.
Draws earned per activity, bonus draws, and the prize bowl were identical to that above. These individuals also “drew until winning” in the first session for completing an initial contract.
Therapy adherence.
Two master’s level staff provided both CM conditions. They received didactic training and completed written tests and role plays of CM, for both conditions, before study initiation. Ongoing supervision consisted of review of behavioral contracts and audiotapes. Two raters, blinded to conditions and achieving inter-rater reliability of 0.83, coded tapes from 20% of participants (selected randomly but ensuring roughly equal ratings of conditions and staff) on the Contingency Management Competence Scale for Activities (Petry et al., 2010). They rated items such as “To what extent did the therapist state how many draws would be earned at the next session if the client completed activities?” on a Likert scale (1 = not at all/ poor, 3 = somewhat/ adequate, 7 = extensive/ excellent). In CM-General sessions, means (standard deviations) were 4.9 (0.3), reflecting “good,” and they were similar in CM-Exercise sessions at 5.1 (0.6), indicating competent delivery of CM in both conditions, p > .20. A review of all goals revealed no exercise behaviors were assigned in the CM-General condition and no non-exercise related goals were assigned in the CM-Exercise condition.
Data analysis
Chi-square and t-test tests compared conditions on demographics and Baseline variables.
The primary aim was to assess effects on physical activity, and primary outcomes were IPAQ physical activity scores. Secondary aims assessed effects on health-related fitness and psychosocial functioning measures. For changes in outcomes over time, hierarchical mixed models (HLM; Hedeker, 1993) examined effects of study condition, time, and condition by time interactions. Separate analyses examined effects during treatment (Baseline, Month 2, Month 4) and through follow-up (Baseline, Months 2, 4, 6, 9, 12). Analyses assessed linear changes over time, with time at Baseline coded zero and follow-ups coded as days from Baseline to each follow-up. For all outcomes, initial models examined effects of Time and specified random intercepts and slopes; three models (IPAQ Vigorous activity days, Vigorous activity minutes, and Vigorous activity MET mins per week) converged only with the slope variance component fixed. After convergence, condition was added, and main (study condition, time) and interaction (condition by time) effects examined.
Exploratory aims assessed weeks in treatment and longest consecutive weeks of abstinence from alcohol, stimulants, marijuana, and opioids concurrently as measured by breath and urine samples. We also report the number of samples submitted and proportion of negative samples for all drugs concurrently. Proportions were calculated both including only submitted samples in the denominator (i.e., no assumptions about whether missing samples reflected use) and using 24 in the denominator (total expected samples, assuming missing samples were positive). Independent t-tests compared conditions on these indices, with data available from all randomized participants. Analyses other than hierarchical mixed models were conducted with IBM® SPSS® Statistics version 26.
Results
Baseline Characteristics
Table 1 shows descriptive statistics and statistical tests for variables assessed only at baseline (demographics, substance use disorder status, baseline self-reported days of substance use in the past 90 days and urine drug toxicology test results, ASI); Descriptive statistics for variables assessed repeatedly are in Table 2 (physical activity outcomes), and Supplemental Table 1 (health-related fitness, medical, and psychosocial functioning outcomes). Groups were generally similar at baseline, including no differences on most physical activity, fitness, and psychosocial outcomes. The two exceptions were Systolic BP and Diastolic BP, which were higher in the CM-Exercise condition compared to CM-General condition.
Table 1.
Demographic and baseline characteristics
| Variable | CM-General | CM-Exercise | Statistic test, p |
|---|---|---|---|
| N | 58 | 62 | |
| Age | 39.3 (10.7) | 36.7 (10.0) | t(118)=1.33, .19 |
| Male, % (n) | 74.1 (43) | 77.4 (48) | X2 (1) = 0.18, .68 |
| Hispanic ethnicity, % (n) | 12.1 (7) | 12.9 (8) | X2 (1) = 0.02, .89 |
| Race, % (n) | X2 (2) = 1.51, .47 | ||
| Black or African American | 41.4 (24) | 37.1 (23) | |
| White | 53.4 (31) | 51.6 (32) | |
| Other | 5.2 (3) | 11.3 (7) | |
| Marital Status. % (n) | X2 (2) = 0.22, .90 | ||
| Never married | 63.8 (37) | 59.7 (37) | |
| Married/Cohabitating | 15.5 (9) | 17.7 (11) | |
| Divorced/Separated/Widowed | 20.7 (12) | 22.6 (14) | |
| Years education | 12.2 (1.8) | 12.6 (2.0) | t(118)= −1.33, .19 |
| Any income, past 30 days, % (n) | 15.5% (9) | 16.1% (10) | X2 (1) = 0.01, .93 |
| Income if any, past 30 days | $700 ($648) | $723 ($1,093) | t(118)= −0.10, .92 |
| Access to a car for own use, % (n) | 17.2% (10) | 21% (13) | X2 (1) = 0.27, .60 |
| DSM-IV diagnosis, % (n) | |||
| Alcohol | 60.3 (35) | 56.5 (35) | X2 (1) = 0.19, .67 |
| Marijuana | 37.9 (22) | 37.1 (23) | X2 (1) = 0.01, .93 |
| Opioid | 51.7 (30) | 48.4 (30) | X2 (1) = 0.13, .72 |
| Stimulant | 62.1 (36) | 53.2 (33) | X2 (1) = 0.96, .33 |
| Baseline sample positive, % (n) | |||
| Any substance | 12.1 (7) | 9.7 (6) | X2 (1) = 0.18, .67 |
| Alcohol | 0.0 (0.0) | 0.0 (0.0) | -- |
| Cocaine | 3.4 (2) | 0.0 (0.0) | X2 (1) = 2.17, .14 |
| Marijuana | 10.3 (6) | 8.1 (5) | X2 (1) = 0.19, .67 |
| Methamphetamine | 0.0 (0.0) | 0.0 (0.0) | -- |
| Opioids | 0.0 (0.0) | 1.6 (1) | X2 (1) = 0.94, .33 |
| Days of use in the past 90 | |||
| Any substance | 23.7 (22.4) | 26.0 (21.2) | t(118)=−0.59, .56 |
| Alcohol | 12.2 (19.7) | 13.1 (20.1) | t(118)=−0.27, .79 |
| Cocaine | 6.9 (13.6) | 8.2 (14.9) | t(118)=−0.48, .63 |
| Marijuana | 7.4 (15.6) | 8.1 (16.1) | t(118)=−0.25, .80 |
| Methamphetamine | 1.0 (7.9) | 0.2 (1.9) | t(118)=0.77, .44 |
| Opioids | 9.9 (18.5) | 11.2 (17.6) | t(118)=−0.42, .68 |
| Addiction Severity Index | |||
| Alcohol | 0.08 (0.16) | 0.08 (0.16) | t(118)=0.02, .98 |
| Drug | 0.08 (0.09) | 0.07 (0.09) | t(118)=0.26, .80 |
| Employment | 0.83 (0.24) | 0.77 (0.23) | t(118)=1.29, .20 |
| Family/social | 0.14 (0.19) | 0.19 (0.25) | t(118)=−1.37, .17 |
| Legal | 0.19 (0.24) | 0.17 (0.24) | t(118)=0.49, .63 |
| Medical | 0.14 (0.26) | 0.15 (0.27) | t(118)=−0.37, .71 |
| Psychiatric | 0.25 (0.20) | 0.22 (0.21) | t(118)=1.02, .31 |
Notes. Values are means (with standard deviations in parentheses) unless noted. CM = contingency management. Addiction Severity Index scores range 0 (lowest severity of problems in the domain) to 1 (highest severity of problems in the domain). Self-efficacy for Exercise Scale scores range 0 to 180, least to greatest self-efficacy, Intrinsic Exercise Motivation Inventory scores range 21 to 147, least to greatest intrinsic motivation. On the Pittsburg Sleep Quality Index, lower scale scores indicate better sleep quality. Physical Activity Social Support scores range 13 to 65, less to more social support. Quality of Life scores range −51 to 102, least to greatest well-being.
Table 2.
Raw means and standard deviations for physical activity outcomes at baseline through each follow-up.
| Raw Scores | ||||||
|---|---|---|---|---|---|---|
| Outcome | Baseline | Month 2 | Month 4 | Month 6 | Month 9 | Month 12 |
| Vigorous MET·mins/wk | ||||||
| CM-General | 724.8 (1,237.6) | 1,022.6 (1,662.7) | 1,947.8 (3,3069.0) | 1,777.0 (2,628.2) | 1,760.0 (2,956.4) | 1,405.8 (2,245.8) |
| CM-Exercise | 834.2 (1,623.5) | 2,615.7 (2,684.4) | 1,913.6 (1,884.3) | 2,551.3 (2,588.9) | 2909.3 (3,452.7) | 2,728.0 (3,141.0) |
| Moderate ME·mins/wk |
||||||
| CM-General | 1,156.6 (1,479.2) | 1,574.7 (1,584.4) | 1,626.1 (1,733.8) | 2,122.1 (1,825.1) | 2,257.1 (1,799.8) | 2,049.6 (1,881.0) |
| CM-Exercise | 1,360.0 (1,819.5) | 1,973.7 (1,764.5) | 2,338.4 (1,736.4) | 2,624.3 (1,860.9) | 2,881.5 (1,897.2) | 2,610.0 (2,134.4) |
| Walking MET·mins/wk | ||||||
| CM-General | 2,235.5 (1,562.7) | 2,662.7 (1,517.6) | 2,459.5 (1,525.3) | 2,405.1 (1,602.1) | 2,085.7 (1,614.2) | 2,474.3 (1,664.7) |
| CM-Exercise | 2,536.5 (1,359.6) | 3,429.1 (1,069.6) | 3,216.8 (1,247.8) | 3,366.0 (959.4) | 3,083.1 (1,331.6) | 3,044.1 (1,440.2) |
| Total MET MET·mins/wk | ||||||
| CM-General | 4,116.9 (2,976.8) | 5,260.1 (3,397.1) | 6,033.4 (4,651.8) | 6,304.3 (4,576.1) | 6,102.8 (4,635.8) | 5,929.8 (3,999.6) |
| CM-Exercise | 4,730.4 (3,350.5) | 8,018.5 (3,959.7) | 7,468.8 (3,361.9) | 8,541.7 (3,971.9) | 8,873.8 (5,066.7) | 8,392.1 (5,577.2) |
| Vigorous activity days/wk | ||||||
| CM-General | 1.5 (2.2) | 1.8 (2.0) | 1.8 (2.3) | 2.0 (2.4) | 1.6 (2.4) | 1.6 (2.3) |
| CM-Exercise | 1.7 (2.3) | 3.3 (2.0) | 2.6 (1.9) | 2.9 (2.4) | 2.8 (2.7) | 2.7 (2.6) |
| Moderate activity days/wk | ||||||
| CM-General | 3.6 (2.9) | 3.6 (2.5) | 2.9 (2.7) | 4.0 (2.6) | 4.0 (2.6) | 3.9 (3.0) |
| CM-Exercise | 2.8 (2.8) | 4.1 (2.5) | 4.3 (2.3) | 4.3 (2.4) | 4.8 (2.2) | 4.4 (2.9) |
| Walking activity mins/day | ||||||
| CM-General | 176.2 (124.0) | 184.3 (122.6) | 170.3 (136.6) | 206.4 (171.0) | 155.4 (159.7) | 187.7 (146.2) |
| CM-Exercise | 161.9 (144.0) | 255.0 (162.4) | 240.5 (162.0) | 244.6 (130.8) | 220.4 (147.0) | 241.3 (148.6) |
| Sitting mins/day | ||||||
| CM-General | 362.3 (216.8) | 277.9 (169.0) | 246.0 (170.1) | 254.0 (140.0) | 270.5 (187.1) | 285.0 (211.2) |
| CM-Exercise | 307.3 (187.6) | 229.4 (127.1) | 227.0 (142.2) | 251.1 (123.5) | 243.7 (191.2) | 256.3 (171.0) |
Note: Walking activity days/wk captures days walked for at least 10 minutes.
Intervention phase outcomes
Table 2 shows descriptive statistics for physical activity outcomes at each time point, and Table 3 shows associated results of hierarchical mixed model analyses. CM-Exercise participants had higher overall levels of physical activity, within-group improvements over time, and improvements over time compared to the CM-General condition on several outcomes. Specifically, Vigorous MET*mins/wk and Total MET*mins/wk were higher overall in CM-Exercise compared to CM-General. Within the CM-Exercise condition, improvements over time occurred on Moderate MET*mins/wk, Walking MET*mins/wk, and Vigorous activity days/wk; these variables did not change over time in CM-General. Condition by time interactions also occurred. Moderate activity days/wk increased over time in CM-Exercise and decreased over time in CM-General. Walking activity minutes/day increased over time in CM-Exercise and did not change over time in CM-General. Other outcomes changed similarly or did not change over time in both conditions.
Table 3.
Physical activity: Hierarchical mixed model results.
| Baseline to Month 4 | Baseline to Month 12 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Time β1 |
SE | T ratioa | P | Condition P |
Condition ×Time p |
Time β1 |
SE | T ratioa | P | Condition P |
Condition ×Time p |
| Vigorous | ||||||||||||
| MET·mins/wk | ||||||||||||
| CM-General | 10.74 | 3.79 | 2.83 | .01 | .02 | .73 | 2.55 | 1.00 | 2.56 | .01 | .09 | .41 |
| CM-Exercise | 9.11 | 2.83 | 3.22 | <.01 | 4.01 | 1.45 | 2.77 | <.01 | ||||
| Moderate | ||||||||||||
| MET·mins/wk | ||||||||||||
| CM-General | 3.85 | 2.68 | 1.44 | .15 | .53 | .22 | 2.88 | 0.80 | 3.58 | <.001 | .17 | .45 |
| CM-Exercise | 8.66 | 2.77 | 3.13 | <.01 | 4.14 | 1.01 | 4.10 | <.001 | ||||
| Walking MET·mins/wk | ||||||||||||
| CM-General | 1.67 | 2.64 | 0.63 | .53 | .14 | .21 | −0.11 | 0.78 | −0.15 | .88 | <.01 | .38 |
| CM-Exercise | 5.82 | 1.91 | 3.05 | <.01 | 0.81 | 0.70 | 1.16 | .25 | ||||
| Total MET·mins/wk | ||||||||||||
| CM-General | 16.66 | 8.39 | 2.45 | .02 | .04 | .42 | 5.60 | 1.79 | 3.13 | <.01 | .02 | .29 |
| CM-Exercise | 23.47 | 4.92 | 4.78 | <.001 | 8.87 | 2.47 | 3.59 | <.001 | ||||
| Vigorous activity days/wk | ||||||||||||
| CM-General | <0.01 | <0.01 | 0.78 | .44 | .20 | .23 | <0.01 | <0.01 | 0.63 | .53 | .06 | .66 |
| CM-Exercise | 0.01 | <0.01 | 2.46 | .02 | <0.01 | <0.01 | 1.11 | .27 | ||||
| Moderate activity days/wk | ||||||||||||
| CM-General | −0.01 | <0.01 | −1.97 | .05 | .11 | < .001 | <0.01 | <0.01 | 1.35 | .18 | .98 | .10 |
| CM-Exercise | 0.01 | <0.01 | 3.49 | <.001 | 0.01 | <0.01 | 3.43 | <.001 | ||||
| Walking activity mins/day | ||||||||||||
| CM-General | −0.04 | 0.23 | −0.18 | .86 | .91 | .03 | 0.01 | 0.07 | 0.15 | .88 | <.01 | .18 |
| CM-Exercise | 0.73 | 0.25 | 2.87 | .01 | 0.15 | 0.08 | 1.96 | .05 | ||||
| Sitting mins/day | ||||||||||||
| CM-General | −0.65 | 0.27 | −2.39 | 0.02 | .76 | .29 | −0.06 | 0.10 | −0.61 | .54 | .97 | .43 |
| CM-Exercise | −1.06 | 0.27 | −3.86 | <.001 | −0.17 | 0.09 | −1.93 | .06 | ||||
Approximate degrees of freedom associated with 7-ratios vary with each analysis depending on number of observed vs missing data points (available from authors on request).
Note: SE = standard err. ß1 coefficient = the rate of change per unit time for the CM-Exercise condition when coded 0 (and CM-General coded 1); ß1 coefficient the rate of change per unit time for CM-General condition when coded 0 (and CM-Exercise coded 1). Walking activity days/wk captures days walked for at least 10 minutes.
For health-related fitness and medical outcomes, descriptive statistics are in Supplemental Table 1 and results of hierarchical mixed model analyses are in Supplemental Table 2. Fitness and medical outcomes were unimproved in the CM-Exercise condition compared to CM-General condition, with one exception: Floor transfer was faster (better) overall in CM-Exercise compared to CM-General. Diastolic BP and Systolic BP were higher overall in CM-Exercise compared to CM-General, but also increased over time among CM-General participants.
For psychosocial functioning outcomes, descriptive statistics are in Supplemental Table 1 and results of analyses are in Table 4. The CM-Exercise condition conferred more benefits and protective effects on psychosocial functioning outcomes than on fitness and medical outcomes. Intrinsic motivation to exercise was higher overall in CM-Exercise compared to CM-General, and there was a significant condition by time interaction, with decreased motivation over time in the CM-General compared to CM-Exercise condition. There was also a significant condition by time interaction on Self-efficacy for exercise, which decreased over time in CM-General compared to CM-Exercise. Among CM-Exercise participants, Quality of life increased over time. These results occurred in the context of declines over time in Physical activity social support from family and Physical activity social support from friends in the CM-Exercise condition. Among CM-General participants, sleep quality decreased (improved) with time.
Table 4.
Psychosocial outcomes: Hierarchical mixed model results.
| Baseline to Month 4 |
Baseline to Month 12 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Time β1 |
SE | T ratioa | P | Condition P |
Condition × Time p |
Time β1 |
SE | T ratioa | P | Condition P |
Condition × Time p |
| Self-efficacy for exercise | ||||||||||||
| CM-General | −0.01 | <0.01 | −3.01 | <.01 | .06 | .01 | <0.01 | <0.01 | 0.82 | .41 | <.01 | .33 |
| CM-Exercise | 0.00 | <0.01 | 0.50 | .62 | <−0.01 | <0.01 | −0.55 | .58 | ||||
| Intrinsic motivation to exercise | ||||||||||||
| CM-General | −0.05 | 0.02 | −2.02 | .05 | .05 | .03 | −0.11 | 0.08 | −1.41 | .16 | .03 | .43 |
| CM-Exercise | 0.02 | 0.02 | .97 | .33 | −0.04 | 0.02 | −1.87 | .06 | ||||
| Quality of life | ||||||||||||
| CM-General | 0.05 | 0.04 | 1.28 | .20 | .72 | .20 | 0.02 | 0.02 | 1.23 | .22 | .28 | .21 |
| CM-Exercise | 0.02 | 0.03 | 3.36 | <.001 | −0.01 | 0.01 | −0.49 | .63 | ||||
| Physical activity family support | ||||||||||||
| CM-General | −0.02 | 0.01 | −0.89 | .38 | .43 | .61 | −0.01 | 0.00 | −3.73 | <.01 | .56 | .45 |
| CM-Exercise | −0.02 | 0.01 | −2.12 | .04 | <−0.01 | 0.00 | −1.61 | .11 | ||||
| Physical activity friends support | ||||||||||||
| CM-General | −0.01 | 0.01 | −0.47 | .64 | .22 | .16 | −0.01 | <0.01 | −2.88 | <.01 | .44 | .74 |
| CM-Exercise | −0.03 | 0.01 | −2.86 | <.01 | −0.01 | <0.01 | −2.59 | .01 | ||||
| Sleep quality | ||||||||||||
| CM-General | −0.01 | 0.01 | −2.41 | .02 | .67 | .62 | −0.07 | 0.07 | −0.97 | .33 | .65 | .29 |
| CM-Exercise | −0.01 | 0.01 | −1.78 | .08 | 0.01 | 0.01 | 0.82 | .41 | ||||
Approximate degrees of freedom associated with 7-ratios vary with each analysis depending on the effect considered and number of observed data points (available from authors on request).
Note: SE = standard err. β1 coefficient = the rate of change per unit time for the CM-Exercise condition when coded 0 (and CM-General coded 1); β1 coefficient the rate of change per unit time for CM-General condition when coded 0 (and CM-Exercise coded 1).
Most remaining intervention phase outcomes, including those related to substance use, did not differ between conditions (see Table 5). Exceptions were adherence with completing contracted goals and prize earnings, both of which were higher in CM-General compared to CM-Exercise.
Table 5.
Retention, adherence, prize earnings, and abstinence outcomes.a
| CM-General | CM-Exercise | t (118) | p value | |
|---|---|---|---|---|
| Number of weeks in treatment | 8.7 (7.6) | 9.3 (7.3) | −0.46 | .65 |
| Number of activities completed | 32.3 (16.1) | 25.7 (15.7) | 2.29 | .02 |
| Prize earnings ($) | $248.60 ($164.40) | $179.50 ($149.90) | 2.37 | .02 |
| Number of samples submitted | 15.2 (6.5) | 13.1 (7.0) | 1.63 | .11 |
| Longest duration of abstinence weeks | 6.2 (5.1) | 4.9 (4.5) | 1.40 | .16 |
| % of samples negative for all substances (of submitted samples) | 81.6 (33.1) | 82.4 (30.4) | −0.14 | .89 |
| % of samples negative for all substances (of 24 samples scheduled) | 54.7 (32.7) | 48.7 (31.4) | 1.03 | .31 |
Values are mean (SD) unless otherwise noted.
Outcomes assessed baseline through Month 12 follow-up
CM-Exercise condition participants had higher overall levels of physical activity and improvements over time compared to the CM-General condition on some outcomes when assessed baseline through Month 12 (Tables 2 and 3). Walking MET*mins/week, Total MET*mins/wk, and Walking activity mins/day were higher overall in CM-Exercise compared to CM-General. Among CM-Exercise participants, Walking activity mins/day also increased over time.
Regarding health-related fitness, medical and psychosocial outcomes baseline through Month 12 (Supplemental Tables 1 and 2, and Table 4), Floor transfer was faster overall, and Diastolic BP and Systolic BP were higher overall, in CM-Exercise compared to CM-General. Meanwhile, Self-efficacy for exercise and Motivation to exercise were higher overall in CM-Exercise versus CM-General. Physical activity social support from family also decreased over time among CM-General participants. Remaining fitness, medical, and psychosocial measures changed similarly or did not change over time in both conditions.
There were no study-related serious adverse effects, but two CM-Exercise participants sustained mild injuries while exercising. Both declined medical treatment and resumed exercising after 1–4 days of rest.
Discussion
This randomized controlled trial demonstrated that reinforcing exercise in outpatient substance abuse treatment patients was efficacious in improving several physical activity indices (total physical activity and vigorous activity expenditure, volume of moderate activity and walking) and one fitness measure (push-ups) over the short term. The CM-Exercise condition also conferred protective effects against decrements in self-efficacy for exercise and motivation to exercise observed in the CM-General condition, and increased quality of life. These effects occurred in the context of declines over time in the CM-Exercise but not CM-General condition in levels of social support for engaging in physical activity. Reinforcing exercise provided no unique or additional effect on reducing drug use relative to reinforcing other goal behaviors. The comparator condition was an enhanced intervention provided adjunct to usual care, but one that likely did not obscure effects of reinforcing exercise on abstinence. A prior study in cocaine and heroin use disorder patients assessed effects of a similar CM-General condition and found that abstinence did not differ from that observed in a standard care condition (Petry et al., 2006). This study did not compare CM for exercise to standard care alone or exercise without CM, and thus did not assess the efficacy of CM per se. This study assessed the relative efficacy of different behavioral targets of reinforcement, and the results do not indicate an advantage of reinforcing exercise over other goal behaviors of the type examined here in improving substance use outcomes. Reinforcing exercise did result in improvements in several physical activity and psychosocial functioning outcomes, consistent with findings in nonclinical samples of positive impacts of physical activity on over 35 chronic diseases and health conditions (2018 Physical Activity Guidelines Advisory Committee, 2018).
This study is the first well-powered study to assess effects of CM for exercise tailored to individual preferences and based on promising findings in prior CM studies (Brown et al., 2014; Weinstock et al., 2016). Personal preferences were accommodated in both conditions, and all participants were encouraged to select meaningful and desired activities. CM-Exercise condition participants were encouraged to select types of physical activities they most enjoyed, such as walking, bowling, attending fitness classes, or using specific exercise equipment. Likewise, individuals in the CM-General condition elected to work on individualized steps related to, for example, finding a job, improving relationships, or addressing legal problems. Adherence was higher with these non-exercise behaviors than with those involving exercise. Exercise adherence in this study compares favorably with that observed in two preliminary trials on CM for exercise among substance use treatment patients (Brown et al., 2014; Weinstock et al., 2016). Still, some patients may benefit from interventions that directly address motivation to exercise (Weinstock et al., 2016). On the other hand, rates of physical activity in this study were fairly high, even at baseline. Participants in this study sat on average five to six hours per day, and did about 3 hours of walking per day, perhaps related to the high rates of unemployment and low rates of car ownership in this sample.
Low exercise adherence is often cited as contributing to a lack of observed effects of exercise on substance use outcomes. This study aimed to address this issue by reinforcing exercise activities, and did not find differences between conditions on substance use outcomes. Focusing on studies not limited by weaknesses discussed above, two trials compared supervised group-based moderate intensity exercise to a health education control condition in residential stimulant use disorder treatment patients, and did not find overall differences between conditions on abstinence following treatment discharge (Rawson et al., 2015; Trivedi et al., 2017). However, post hoc analyses found increased abstinence among patients with high versus low exercise adherence (Trivedi et al., 2017), and in those with low severity (< 18 days) stimulant use prior to treatment (Rawson et al., 2015). Two studies in alcohol use disorder outpatients found no differences between supervised group-based vigorous exercise or individual vigorous exercise (Jensen et al., 2018), or between supervised group or individual exercise (intensity not prescribed (Roessler et al., 2017) compared to usual alone. One of those studies noted that diminished exercise adherence over time likely contributed to results (Jensen et al., 2018). The other found that in the overall sample, moderate compared to low levels of exercise was associated with lower rates of post-treatment excessive drinking; adherence was not assessed (Roessler et al., 2017). Among sedentary women smokers, supervised vigorous exercise (Marcus et al., 2005), but not supervised moderate exercise (Marcus et al., 1999), improved smoking outcomes relative to psychosocial treatment without exercise. In the former, those with high versus low adherence had improved outcomes (Marcus et al., 2005). However, a trial in pregnant smokers did not find that vigorous exercise improved smoking outcomes compared to behavioral support only; a median 29% of sessions were attended (Ussher et al., 2015). Others assessed effects of exercise for maintenance of smoking cessation in nonpregnant women, and found no benefits of exercise relative to a contact control condition; on average, 53.8% of exercise sessions were attended (Prapavessis et al., 2016). The current study is the first assessment of exercise for treating substance use disorder that did not restrict participation to individuals with a specific substance use disorder, and results resembled those among persons selected for specific substance use disorders, discussed above.
The comparator condition in this study reinforced completion of general goal-related activities excluding exercise. This comparator controlled for differences between groups in attention, a known problem in this literature (Giesen et al., 2015). A similar condition also did not improve abstinence relative to usual care in prior research (Petry et al., 2006). Nonetheless, reinforcing exercise compared to treatment as usual might be most likely to show positive effects of the intervention on abstinence. A yoked design might also be considered, but yoked conditions have never shown benefits in CM studies (Lussier et al., 2006; Prendergast et al., 2006) and require some violations of random assignment. This study also did not test effects of reinforcing exercise on exercise adherence. Substantial evidence demonstrates that reinforcement increases adherence with target behaviors, but effects of unobserved factors cannot be ruled out.
Many individuals elected not to participate in this study (Figure 1), primarily because they did not want to exercise (i.e., self-selection), and some participants in the CM-Exercise condition failed to complete even one week of physical activities. Substance use disorder outpatients (Abrantes et al., 2011) and residential treatment patients (Stoutenberg, Warne, Vidot, Jimenez, & Read, 2015) express positive attitudes toward exercise incorporated into treatment, as do current and ex-heroin users about exercise in general (not in the context of treatment) (Neale, Nettleton, & Pickering, 2012). In this study, an exercise intervention may have had better effects in individuals with inherent interest or proclivity toward exercising, though the need for and benefit of CM in these circumstances is questionable. Another consideration is that relatively high intensity exercise (i.e., 150 minutes of moderate activity or 75 minutes of vigorous activity per week) and high intensity of support/supervision may be needed for reductions in substance use. However, no exercise or supervision intensity has proven clearly efficacious to date. Further, recruitment data and perhaps exercise adherence results here guard against adoption of reinforcing exercise as a widely applicable strategy for addressing drug use in outpatient substance use treatment samples. In addition, no exercise or supervision intensity has proven clearly efficacious to date.
There are study limitations to consider. Participants were drawn from a single clinic, and there was high treatment drop out (on average mid-way through the intervention period), as is typical in outpatient settings (Capoccia et al., 2011; Hoffman et al., 2011; Wickizer et al., 1994). The study was designed to be a test of CM for a heterogeneous set of substance use disorders in a real-world treatment program, where the patients served are not limited to a specific type of substance use disorder. This approach increases generalization and is consistent with compelling biopsychosocial reasons to expect the benefits of exercise to apply widely to clinical and nonclinical populations, given appropriate tailoring for personal circumstances. Findings here are consistent with studies restricted to patients with specific substance use disorders, but potential differences between substance use disorders in neurobiological contributions related to exercise and substance use (Robertson et al., 2016) or other factors cannot be ruled out. The possibility that low rates of substance use prior to and during treatment in the current study (Table 3; see also Petry et al., 2005, 2012), while not uncommon (Petry, Alessi, Marx, Austin, & Tardif, 2005; Petry, Barry, Alessi, Rounsaville, & Carroll, 2012), may have rendered reductions in drug use difficult to detect.
There are also strengths of this study. It was conducted in a community outpatient substance use treatment clinic, employed minimal inclusion and exclusion criteria, and enrolled a diverse sample, increasing generalization of the findings. The two conditions applied reinforcement similar in all respects other than the target behavior so effects of reinforcing exercise explicitly could be isolated while controlling for time and attention effects. The CM-Exercise intervention provided a free gym membership to reduce costs and allow flexibility in times, types and locations for exercising, and it engendered fairly good adherence to exercise by reinforcing it. Rigor methods were employed, such as randomization to study conditions, standard objective indices, and an intent-to-treat approach to analyses.
The results of this study, in the context of the existing literature, suggest potential future directions for this area of research. In this study, reinforcing exercise aimed to address issues related to exercise adherence often cited in the literature as limiting conclusions. This study did not assess effects of reinforcing versus not reinforcing exercise adherence. There might also be important exercise dose-related relations to consider, with critical links to adherence. To address these questions, future research might experimentally vary adherence and experimentally vary exercise intensity, while attending to adherence. There is also potential in mobile and sensing technologies (wearables, and those that attach to exercise equipment). For example, such devices might improve the process of monitoring adherence, monitoring quality of exercise, and providing feedback on criterion levels of exercise.
Conclusions
This study adds to the small but growing body of non-preliminary trials on exercise interventions among substance use treatment patients. Consistent with most such studies in this literature, this study did not find benefits of exercise on substance use outcomes. There also appears to be no substance use disorder for which exercise interventions are clearly efficacious in reducing substance use. A consistent finding relates to observed or suspected effects of adherence on outcomes. There remains a small number of trials not limited by common methodological weaknesses and few in any one patient population in this literature, and potential experimental manipulations and devices that may advance this field of research.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants T32 AA007290, R01 AA021446, R01 AA023502, and R01 DA027615. Funders did not have any further role in study design; in the collection, analysis and interpretation of the data; in the writing of the report; or, in the decision to submit the paper for publication.
This research was presented in part at the American Psychological Association annual conference.
Contributor Information
Sheila M. Alessi, Department of Medicine and the Calhoun Cardiology Center – Behavioral Health Division, University of Connecticut School of Medicine
Carla J. Rash, Department of Medicine and the Calhoun Cardiology Center – Behavioral Health Division, University of Connecticut School of Medicine
Linda S. Pescatello, Department of Psychology, University of Connecticut.
References
- 2018 Physical Activity Guidelines Advisory Committee. (2018). 2018 Physical Activity Guidelines Advisory Committee Scientific Report. To the Secretary of Health and Human Service; Washington, D.C: Retrieved from https://health.gov/paguidelines/secondedition/report/pdf/PAG_Advisory_Committee_Report.pdf [Google Scholar]
- Abrantes AM, Battle CL, Strong DR, Ing E, Dubreuil ME, Gordon A, & Brown RA (2011). Exercise preferences of patients in substance abuse treatment. Mental Health and Physical Activity, 4(2), 79–87. 10.1016/j.mhpa.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainscough TS, McNeill A, Strang J, Calder R, & Brose LS (2017). Contingency Management interventions for non-prescribed drug use during treatment for opiate addiction: A systematic review and meta-analysis. Drug and Alcohol Dependence, 178(November 2016), 318–339. 10.1016/j.drugalcdep.2017.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Task Force on DSM-IV: Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Association. [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, & Festinger DS (2014). Prize-based contingency management for the treatment of substance abusers: a meta-analysis. Addiction, 109(9), 1426–1436. 10.1111/add.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock BC, Fava JL, Gaskins R, Morrow KM, Williams DM, Jennings E, … Marcus BH (2012). Yoga as a complementary treatment for smoking cessation in women. Journal of Women’s Health, 21(2), 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, & Roberts MR (1999). EXERCISE EFFECTS ON WITHDRAWAL AND MOOD AMONG WOMEN ATTEMPTING SMOKING CESSATION, 24(3), 399–410. [DOI] [PubMed] [Google Scholar]
- Brellenthin AG, & Koltyn KF (2016). Exercise as an adjunctive treatment for cannabis use disorder. The American Journal of Drug and Alcohol Abuse, 42(5), 481–489. 10.1080/00952990.2016.1185434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Minami H, Read JP, Marcus BH, Jakicic JM, … Stuart GL (2014). A preliminary, randomized trial of aerobic exercise for alcohol dependence. Journal of Substance Abuse Treatment, 47(1), 1–9. 10.1016/jjsat.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, & Martin PR (2011). Aerobic Exercise Training Reduces Cannabis Craving and Use in Non-Treatment Seeking Cannabis-Dependent Adults. PLoS ONE, 6(3), e17465. 10.1371/journal.pone.0017465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckworth J, Lee RE, Regan G, Schneider LK, & DiClemente CC (2007). Decomposing intrinsic and extrinsic motivation for exercise: Application to stages of motivational readiness. Psychology of Sport and Exercise, 8(4), 441–461. https://doi.org/10.1016Zj.psychsport.2006.06.007 [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburg Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. 10.1152/ajpheart.00471.2010 [DOI] [PubMed] [Google Scholar]
- Charpentier PA (2003). gRAND urn randomization program, version 1.10 [computer software]. Yale University, Connectitut. [Google Scholar]
- Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, … Earnest CP (2010). Effects of Aerobic and Resistance Training on Hemoglobin A 1c Levels in Patients With Type 2 Diabetes. JAMA, 304(20), 2253 10.1001/jama.2010.1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analyses for the social sciences. Hillsdale, NJ, Lawrence Erlbauni Associates. [Google Scholar]
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, … Mead GE (2013). Exercise for depression. Cochrane Database of Systematic Reviews, 311(9), 2432–2433. 10.1002/14651858.CD004366.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, … Oja P (2003). International Physical Activity Questionnaire: 12-Country Reliability and Validity. Medicine & Science in Sports & Exercise, 35(8), 1381–1395. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- Cress ME, Buchner DM, Prohaska T, Rimmer J, Brown M, Macera C, … Chodzko-Zajko W (2005). Best practices for physical activity programs and behavior counseling in older adult populations. Journal of Aging and Physical Activity, 13(1), 61–74. [DOI] [PubMed] [Google Scholar]
- Cutter CJ, Schottenfeld RS, Moore BA, Ball SA, Beitel M, Savant JD, … Barry DT (2014). A pilot trial of a videogame-based exercise program for methadone maintained patients. Journal of Substance Abuse Treatment, 47(4), 299–305. 10.1016/jjsat.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J, Cropley M, Ussher MH, & West R (2004). Acute effects of a short bout of moderate versus light intensity exercise versus inactivity on tobacco withdrawal symptoms in sedentary smokers. Psychopharmacology, 174(3), 320–326. 10.1007/s00213-003-1762-x [DOI] [PubMed] [Google Scholar]
- DeVahl J, King R, & Williamson JW (2005). Academic Incentives for Students Can Increase Participation in and Effectiveness of a Physical Activity Program. Journal of American College Health, 53(6), 295–298. 10.3200/JACH.53.6.295-298 [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, & Chambliss HO (2005). Exercise treatment for depression. American Journal of Preventive Medicine, 28(1), 1–8. https://doi.org/10.1016Zj.amepre.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Epstein LH, Paluch RA, Kilanowski CK, & Raynor HA (2004). The Effect of Reinforcement or Stimulus Control to Reduce Sedentary Behavior in the Treatment of Pediatric Obesity. Health Psychology, 23(4), 371–380. 10.1037/0278-6133.23.4.371 [DOI] [PubMed] [Google Scholar]
- Eyler AA, Brownson RC, Donatelle RJ, King AC, Brown D, & Sallis JF (1999). Physical activity social support and middle-and older-aged minority women: results from a US survey. Soc Sci Med, 49(6), 781–789. 10.1016/s0277-9536(99)00137-9 [DOI] [PubMed] [Google Scholar]
- Faith MS, Berman N, Heo M, Pietrobelli A, Gallagher D, Epstein LH, … Allison DB (2001). Effects of Contingent Television on Physical Activity and Television Viewing in Obese Children, 107(5), 1043–1048. [DOI] [PubMed] [Google Scholar]
- Franklin BA, Whaley MH, Howley ET, & Balady GJ (2000). American College of Sports Medicine: ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins Philadelphia. [Google Scholar]
- Frisch MB, Cornell J, Villanueva M, & Retzlaff PJ (1992). Clinical validation of the Quality of Life Inventory. A measure of life satisfaction for use in treatment planning and outcome assessment. Psychological Assessment, 4(1), 92–101. 10.1037/1040-3590.4.1.92 [DOI] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M, … Swain DP (2011). Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. [DOI] [PubMed] [Google Scholar]
- Giesen ES, Deimel H, & Bloch W (2015). Clinical Exercise Interventions in Alcohol Use Disorders: A Systematic Review. Journal of Substance Abuse Treatment, 52, 1–9. 10.1016/j.jsat.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Hallgren M, Vancampfort D, Giesen ES, Lundin A, & Stubbs B (2017). Exercise as treatment for alcohol use disorders: systematic review and meta-analysis. British Journal of Sports Medicine, 51(14), 1058–1064. 10.1136/bjsports-2016-096814 [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Wing RR, Thorson C, & Burton LR (1998). Use of personal trainers and financial incentives to increase exercise in a behavioral weight-loss program. Journal of Consulting and Clinical Psychology, 66(5), 777. [DOI] [PubMed] [Google Scholar]
- Jensen K, Nielsen Ch., & Ekstrøm CT (2018). Physical exercise in the treatment of alcohol use disorder (AUD) patients affects their drinking habits: A randomized controlled trial. Scandinavian Journal of Public Health, 47(4), 462–468. 10.1177/1403494818759842 [DOI] [PubMed] [Google Scholar]
- Jinzhou Y, Fu Y, Zhang R, Li X, & Shan G (2008). The reliability and sensitivity of indices related to cardiovascular fitness evaluation. Kinesiology: International Journal of Fundamental and Applied Kinesiology, 40(2), 139–146. [Google Scholar]
- Keen EN, & Sloan AW (1958). Observations on the Harvard Step Test. Journal of Applied Physiology, 13(2), 241–243. 10.1152/jappl.1958.13.2.241 [DOI] [PubMed] [Google Scholar]
- Kelley GA, & Kelley KS (2017). Exercise and sleep: a systematic review of previous metaanalyses. Journal of Evidence-Based Medicine, 10(1), 26–36. 10.1111/jebm.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T, Kujala UM, Rose RJ, & Kaprio J (2009). Physical Activity in Adolescence as a Predictor of Alcohol and Illicit Drug Use in Early Adulthood: A Longitudinal Population-Based Twin Study. Twin Research and Human Genetics, 12(03), 261–268. 10.1375/twin.12.3.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RE, & DiClemente CC (2001). Extrinsic and intrinsic motivation: Which is important for exercise? Medicine & Science in Sports & Exercise, 33(5), S112. [Google Scholar]
- Linke SE, & Ussher MH (2015). Exercise-based treatments for substance use disorders: evidence, theory, and practicality. The American Journal of Drug and Alcohol Abuse, 41(1), 7–15. 10.3109/00952990.2014.976708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, & Higgins ST (2006). A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction, 101(2), 192–203. 10.1111/j.1360-0443.2006.01311.x [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, & Smith MA (2013). Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neuroscience & Biobehavioral Reviews, 37(8), 1622–1644. https://doi.org/10.1016Zj.neubiorev.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, King TK, Parisi AF, Pinto BM, Roberts M, … Abrams DB (1999). The Efficacy of Exercise as an Aid for Smoking Cessation in Women. Archives of Internal Medicine, 159(11), 1229 10.1001/archinte.159.11.1229 [DOI] [PubMed] [Google Scholar]
- Marcus BH, Lewis B, Hogan J, King T, Albrecht A, Bock B, … Abrams D (2005). The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: A randomized controlled trial. Nicotine & Tobacco Research, 7(6), 871–880. 10.1080/14622200500266056 [DOI] [PubMed] [Google Scholar]
- McLellan AT, & Hunkeler E (1998). Alcohol & Drug Abuse: Patient Satisfaction and Outcomes in Alcohol and Drug Abuse Treatment. Psychiatric Services, 49(5), 573–575. 10.1176/ps.49.5.573 [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, … Argeriou M (1992). The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment, 9(3), 199–213. [DOI] [PubMed] [Google Scholar]
- Mitchell MS, Goodman JM, Alter DA, John LK, Oh PI, Pakosh MT, & Faulkner GE (2013). Financial Incentives for Exercise Adherence in Adults. American Journal of Preventive Medicine, 45(5), 658–667. 10.1016/j.amepre.2013.06.017 [DOI] [PubMed] [Google Scholar]
- Muller AE, & Clausen T (2015). Group exercise to improve quality of life among substance use disorder patients. Scandinavian Journal of Public Health, 43(2), 146–152. 10.1177/1403494814561819 [DOI] [PubMed] [Google Scholar]
- Murphy MA, Olson SL, Protas EJ, & Overby AR (2003). Screening for Falls in Community-Dwelling Elderly. Journal of Aging and Physical Activity, 11(1), 66–80. 10.1123/japa.11.166 [DOI] [Google Scholar]
- Neale J, Nettleton S, & Pickering L (2012). Heroin users’ views and experiences of physical activity, sport and exercise. International Journal of Drug Policy, 23(2), 120–127. 10.1016/j.drugpo.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Riebe D, & Thompson PD (2013). ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Carroll KM, Hanson T, MacKinnon S, Rounsaville B, & Sierra S (2006). Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. Journal of Consulting and Clinical Psychology, 74(3), 592–601. 10.1037/0022-006X.74.3.592 [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, & Hanson T (2007). Contingency management improves abstinence and quality of life in cocaine abusers. Journal of Consulting and Clinical Psychology, 75(2), 307–315. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Marx J, Austin M, & Tardif M (2005). Vouchers Versus Prizes: Contingency Management Treatment of Substance Abusers in Community Settings. Journal of Consulting and Clinical Psychology, 73(6), 1005–1014. 10.1037/0022-006X.73.6.1005 [DOI] [PubMed] [Google Scholar]
- Petry NM, Andrade LF, Barry D, & Byrne S (2013). A randomized study of reinforcing ambulatory exercise in older adults. Psychology and Aging, 28(4), 1164–1173. 10.1037/a0032563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, & Carroll KM (2012). A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. Journal of Consulting and Clinical Psychology, 80(2), 276–285. 10.1037/a0026883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Martin B, & Finocche C (2001). Contingency management in group treatment: a demonstration project in an HIV drop-in center. Journal of Substance Abuse Treatment, 21(2), 89–96. 10.1016/S0740-5472(01)00184-2 [DOI] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. (2018). Physical activity guidelines advisory committee scientific report. Washington, D.C. [Google Scholar]
- Powell KE, King AC, Buchner DM, Campbell WW, DiPietro L, Erickson KI, … Katzmarzyk PT (2019). The scientific foundation for the Physical Activity Guidelines for Americans. Journal of Physical Activity and Health, 16(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Prapavessis H, De Jesus S, Fitzgeorge L, Faulkner G, Maddison R, & Batten S (2016). Exercise to Enhance Smoking Cessation: the Getting Physical on Cigarette Randomized Control Trial. Annals of Behavioral Medicine, 50(3), 358–369. 10.1007/s12160-015-9761-9 [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, & Roll J (2006). Contingency management for treatment of substance use disorders: A meta-analysis. Addiction, 101(11), 1546–1560. 10.1111/j.1360-0443.2006.01581.x [DOI] [PubMed] [Google Scholar]
- Rawson RA, Chudzynski J, Mooney L, Gonzales R, Ang A, Dickerson D, … Cooper CB (2015). Impact of an exercise intervention on methamphetamine use outcomes post-residential treatment care. Drug and Alcohol Dependence, 156, 21–28. 10.1016/j.drugalcdep.2015.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read JP, & Brown RA (2003). The role of physical exercise in alcoholism treatment and recovery. Professional Psychology: Research and Practice, 34(1), 49–56. 10.1037/0735-7028.34.1.49 [DOI] [Google Scholar]
- Resnick B, & Jenkins LS (2000). Testing the Reliability and Validity of the Self-Efficacy for Exercise Scale, 49(3), 154–159. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Chudzynski J, Mooney LJ, Rawson RA, Dolezal BA, … London ED (2016). Effect of Exercise Training on Striatal Dopamine D2/D3 Receptors in Methamphetamine Users during Behavioral Treatment. Neuropsychopharmacology, 41(6), 1629–1636. 10.1038/npp.2015.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler KK, Bilberg R, Søgaard Nielsen A, Jensen K, Ekstrøm CT, & Sari S (2017). Exercise as adjunctive treatment for alcohol use disorder: A randomized controlled trial. PLoS ONE, 12(10), 1–14. 10.1371/journal.pone.0186076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis JF, Grossman RM, Pinski RB, Patterson TL, & Nader PR (1987). The Development of Scales to Measure Social Support and Exercise Behaviors ‘ for Diet, 16, 825–836. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Hovell MF, & Richard Hofstetter C (1992). Predictors of adoption and maintenance of vigorous physical activity in men and women. Preventive Medicine, 21(2), 237–251. 10.1016/0091-7435(92)90022-A [DOI] [PubMed] [Google Scholar]
- Shaughnessy M, Resnick BM, & Macko RF (2004). Reliability and validity testing of the short self-efficacy and outcome expectation for exercise scales in stroke survivors. Journal of Stroke and Cerebrovascular Diseases, 13(5), 214–219. 10.1016/jjstrokecerebrovasdis.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Smith MA, & Lynch WJ (2013). The Neurobiology of Exercise and Drug-Seeking Behavior In Routledge Handbook of Physical Activity and Mental Health (pp. 500–512). Routledge. [Google Scholar]
- Stoutenberg M, Warne J, Vidot D, Jimenez E, & Read JP (2015). Attitudes and Preferences Towards Exercise Training in Individuals with Alcohol Use Disorders in a Residential Treatment Setting. Journal of Substance Abuse Treatment, 49, 43–49. 10.1016/jjsat.2014.08.008 [DOI] [PubMed] [Google Scholar]
- Ströhle A, Höfler M, Pfister H, Müller A-G, Hoyer J, Wittchen H-U, & Lieb R (2007). Physical activity and prevalence and incidence of mental disorders in adolescents and young adults. Psychological Medicine, 37(11), 1657–1666. 10.1017/S003329170700089X [DOI] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, & Faulkner G (2007). The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review, (January 2006), 534–543. 10.1111/j.1360-0443.2006.01739.x [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Rethorst CD, Carmody T, Grannemann BD, Walker R, … Nunes EV (2017). Randomized Controlled Trial Comparing Exercise to Health Education for Stimulant Use Disorder. The Journal of Clinical Psychiatry, 78(8), 1075–1082. 10.4088/JCP.15m10591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher MH, Sampuran AK, Doshi R, West R, & Drummond DC (2004). Acute effect of a brief bout of exercise on alcohol urges. Addiction, 99(12), 1542–1547. 10.1111/j.1360-0443.2004.00919.x [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor AH, & Faulkner GEJ (2014). Exercise interventions for smoking cessation. Cochrane Database of Systematic Reviews, (8). 10.1002/14651858.CD002295.pub5 [DOI] [PubMed] [Google Scholar]
- Ussher M, Lewis S, Aveyard P, Manyonda I, West R, Lewis B, … Coleman T (2015). The London Exercise And Pregnant smokers (LEAP) trial: a randomised controlled trial of physical activity for smoking cessation in pregnancy with an economic evaluation. Health Technology Assessment, 19(84), 1–136. 10.3310/hta19840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallerand RJ, & Fortier MS (1998). Measures of intrinsic and extrinsic motivation in sport and physical activity: A review and critique. Advances in Sport and Exercise Psychology Measurement, 81–101. [Google Scholar]
- Walters-Salas E by T. (2012). The Challenge of Patient Adherence. Bariatric Nursing and Surgical Patient Care, 7(4), 186–186. 10.1089/bar.2012.9960 [DOI] [Google Scholar]
- Wang D, Wang Y, Wang Y, Li R, & Zhou C (2014). Impact of Physical Exercise on Substance Use Disorders: A Meta-Analysis. PLoS ONE, 9(10), e110728. 10.1371/journal.pone.0110728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J, Petry NM, Pescatello LS, & Henderson CE (2016). Sedentary college student drinkers can start exercising and reduce drinking after intervention. Psychology of Addictive Behaviors, 30(8), 791–801. 10.1037/adb0000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipfli BM, Rethorst CD, & Landers DM (2008). The anxiolytic effects of exercise: a meta-analysis of randomized trials and dose-response analysis. Journal of Sport and Exercise Psychology, 30(4), 392–410. [DOI] [PubMed] [Google Scholar]
- Zschucke E, Heinz A, & Ströhle A (2012). Exercise and Physical Activity in the Therapy of Substance Use Disorders. The Scientific World Journal, 2012, 1–19. 10.1100/2012/901741 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


