Abstract
Background:
Forkhead box protein A1 (FOXA1) promotes luminal differentiation, and hypermethylation of the gene can be a mechanism of developing estrogen receptor-negative (ER−) breast cancer. We examined FOXA1 in breast tumor and adjacent normal tissue in relation to reproductive factors, particularly higher parity and no breastfeeding, that are associated with ER− tumors.
Methods:
We performed immunohistochemistry for FOXA1 in breast tumors (n=1,329) and adjacent-normal tissues (n=298) in the Women’s Circle of Health Study (949 Blacks and 380 Whites). Protein expression levels were summarized by histology (H) scores. Generalized linear models were used to assess FOXA1 protein expression in relation to reproductive factors by ER status.
Results:
ER+ vs. ER− tumors had higher FOXA1 protein expression (P<0.001). FOXA1 expression was higher in tumor versus paired adjacent-normal tissue in women with ER+ or non-triple-negative cancer (both P<0.001), but not in those with ER− or triple-negative cancer. Higher number of births (1, 2, and 3+) was associated with lower FOXA1 protein expression in ER+ tumors (differences in H score, or β= −8.5, 95% CI= −15.1 to −2.0), particularly among parous women who never breastfed (β= −10.4, −19.7 to −1.0), but not among those who breastfed (β = −7.5, 95% CI= −16.9 to 1.8). The associations for ER− tumors were similar, although they were not statistically significant.
Conclusions:
In this tumor-based study, higher parity was associated with lower FOXA1 expression in ER+ tumors, and breastfeeding may ameliorate the influence.
Impact:
These findings contribute to our understanding of FOXA1 methylation and breast cancer etiology.
Keywords: FOXA1, breast cancer, reproductive factors, African Americans/Blacks
Introduction
In the United States, African American/Black women are more likely than White women to be diagnosed with poor prognosis breast cancers, particularly estrogen receptor-negative (ER−) breast cancer.1,2 Until recently, little was known with respect to biological risk factors for ER− breast cancer in Black women. Data from the African American Breast Cancer Epidemiology and Risk (AMBER) Consortium provide convincing evidence that, while having children is associated with reduced risk of ER+ breast cancer, it is linked to increased risk of ER− breast cancer in Black women.3 Notably, breastfeeding may modulate the association, as the increased risk of ER− cancer was not observed among those who breastfed,3 a finding that is consistent with data from predominately White women.4 There also appeared to be differential risk relationships by ER status with age at menarche.5,6 Together, these findings suggest that hormonal exposure in early years could affect later development of ER− breast cancer in Black women, who are more likely to experience menarche at a younger age than White women, have more children, and not breastfeed.7
The mechanisms underlying relationships between reproductive factors and development of ER− breast cancer are largely unknown. It is possible that early reproductive events and hormone perturbation could influence breast cancer subtypes through effects on progenitor cells in the mammary gland.8 One possible mechanism whereby reproductive events could influence whether luminal progenitor cells give rise to ER− versus ER+ breast tumors is through DNA methylation, an epigenetic modification that occurs in specific patterns throughout development. DNA methylation may be affected by the milieu of hormonal changes that occur during puberty, pregnancy, and lactation.9,10 Our group compared methylation patterns between Black and White women and found that one of the top differentially methylated loci in ER− breast tumors was within the Forkhead box A1 (FOXA1) gene.11 As a pioneer transcription factor, FOXA1 promotes the differentiation of luminal progenitors to mature luminal cells while repressing the basal phenotype.12–16 BRCA1-deficient breast tumors, the vast majority of which exhibit a ER− and basal-like phenotype, also have increased DNA methylation and silencing of the FOXA1 gene.17 Our analysis also showed that FOXA1 DNA methylation levels in ER− tumors may differ by number of births and breastfeeding behaviors,11 suggesting that FOXA1 may be an important link between reproductive exposures and the rise of ER− tumors.
To further elucidate the potential mechanism that reproductive factors modulate ER− breast cancer risk through FOXA1, we examined FOXA1 protein expression in breast tumor and adjacent normal tissue from 949 Black women and 380 White women in relation to reproductive factors. We hypothesized that the change of FOXA1 protein expression from adjacent normal to tumor tissue may predict ER status. Also, we predicted that FOXA1 protein expression in tumor tissues would be lower in women with more children and no history of breastfeeding.
Materials and Methods
Patient Samples
Breast tumor tissue samples were from participants in the Women’s Circle of Health Study (WCHS), a case-control study conducted in metropolitan New York City and 10 counties in eastern New Jersey which was designed to investigate risk factors for aggressive breast cancer in Black and White women. Details on study recruitment and participation rates have been described elsewhere.18,19 This study was approved by the Institutional Review Boards of all participating institutions and all study participants provided written informed consent prior to the baseline interview. In-home interviews were conducted to obtain data on known and suspected risk factors for breast cancer. As part of the informed consent, participants were asked to sign a release for pathology reports and archived tumor specimens, with more than 95% of patients agreeing. Formalin-fixed paraffin-embedded tumor and matched adjacent-normal tissue blocks were requested from hospitals where the diagnostic surgical procedure was performed; for a subset of cases (44%) where hospitals would not release blocks, whole sections were requested. Hematoxylin and Eosin stained sections of tissue specimens were reviewed by a study pathologist (T. K.) for annotating tumor-dense regions for the construction of TMAs. Three 0.6 mm cores from a tumor tissue block and if available, two cores from an adjacent-normal tissue block were placed into TMA blocks for analysis. Completed TMAs and whole sections were stored in nitrogen-filled desiccators at room temperature to preserve antigenicity. Clinical and tumor characteristics, including the expression status of hormone receptors (HR, i.e., ER and progesterone receptor [PR]) and human epidermal growth factor receptor 2 (HER2), were based on patients’ pathology reports. The statistical analysis included 1,329 cases with conclusive FOXA1 staining results from either invasive cancer or ductal carcinoma in situ (DCIS), including 298 who also had adjacent normal tissue.
Immunohistochemistry and image analysis
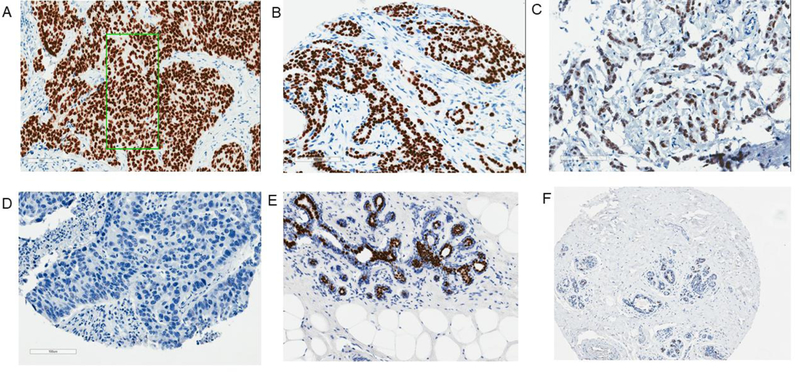
TMAs and whole sections containing breast tumor samples were stained for FOXA1 using the monoclonal primary antibody HNF-3α (Santa Cruz Biotechnology, Catalog No. sc-101058, Dallas, TX), which we had previously optimized.11 Stained slides were digitally imaged at ×20 magnification using the Aperio ScanScope XT (Aperio Technologies, Vista, CA). Automated image analysis of immunohistochemistry staining was performed with Aperio GENIE, a computer-assisted classifier to identify tumor regions.20 Whole section slides were annotated manually to identify tumor epithelial regions for the image analysis. Adjacent-normal tissue sections followed the same manual annotation process as tumor tissue sections to identify regions of lobule and ducts. Tissue cores or whole sections with low cellularity (<25 cells) were excluded. The percent of cells stained was recorded in each intensity category: 0, 1+ (only partial or weak staining), 2+ (moderate and complete staining), and 3+ (intense and complete staining). Tumor cores on TMAs were collapsed into patient-level data using a cellularity-weighted approach, as previously described.21 A histological score (H-score) at the patient level was calculated by the formula: [1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+)] × 100 (Figure 1).22 Tumor and adjacent-normal tissue followed the same scoring protocol. Distributions of FOXA1 protein expression according to specimen and tissue types in ER+ and ER− tumors are presented in Supplemental Table 1.
Figure 1.
FOXA1 IHC in breast tissue. A. whole section tumor tissue (H-score = 275) (x20, the green frame was the annotated area for automatic scoring); B–D. TMA tumor cores (H-score = 203, 74, and 0, respectively); E. whole section adjacent-normal tissue (H-score = 55); F. TMA adjacent-normal core (H-score = 34)
Reproductive characteristics
Data on reproductive history, including age at first menstrual period (age at menarche), number of pregnancies, and the outcome of each pregnancy were collected as a part of a comprehensive in-person interview. For each birth, participants were asked about the status and duration of breastfeeding. Women were defined as postmenopausal if they reported that they had ceased menstruation naturally at least one year before the reference date, or if they had both ovaries removed.
Statistical analysis
We examined the difference in FOXA1 protein expression between the paired tumor and adjacent normal tissue by paired t-tests, overall and within molecular subtypes. The molecular subtype was defined as hormone receptors (ER and PR) positive (HR+)/HER2−, HR+/HER2+, HR−/HER2+, or HR−/HER2−. We derived the difference of H-score between the tumor and adjacent normal tissue and modeled the difference for predicting ER+ (vs. ER−) tumors using logistic regressions, adjusting for age, race, grade, and stage.
FOXA1 H-scores were examined in relation to clinicopathological and reproductive characteristics using analysis of variance (ANOVA). For reproductive characteristics, the associations were examined separately for ER+ and ER− tumors because their influences on breast cancer risk vary by ER status.7,23 Associations of reproductive characteristics with FOXA1 protein expression in tumors were assessed using generalized linear models with the gamma distribution with log link, adjusted for age at diagnosis and race. The model fit was assessed based on the Bayesian information criterion. We performed two sets of sensitivity analyses to evaluate the potential influence of confounding bias. First, because 102 (7.7%) and 17 (1.2%) participants had a missing value on grade and stage, respectively, these variables were not included in the final model. Regression models were fit with additional adjustment for breast cancer stage and tumor grade to evaluate their influence on the associations. Second, we further included specimen type (TMA vs. whole section tissue) in the regression models, as FOXA1 protein expression levels tended to be higher in whole sections than TMAs (Supplemental Table 1). Because the association of parity with ER− tumor risk differed by breastfeeding in Blacks,3 we evaluated the difference in the association of parity with FOXA1 protein expression stratified by ever versus never breastfeeding and then race. Statistical interactions were assessed by examining a product term of parity and breastfeeding among parous women, using Wald tests in regression models. The analyses were performed with SAS v9.4. All tests of statistical significance were two-sided; a P-value of less than 0.05 was considered statistically significant.
Results
The univariate results showed that FOXA1 protein expression was lower in tumors of Black (versus White) women and of more aggressive characteristics, with expression lower in tumors that were higher grade, larger in size, and more advanced stage (Table 1). Invasive ductal carcinoma had lower FOXA1 protein expression than DCIS, but invasive lobular carcinoma showed the highest levels among the histological types. ER− tumors had lower FOXA1 protein expression than ER+ tumors, and triple-negative breast cancer (TNBC), i.e., HR−/HER2− (H-score = 45), had the lowest expression among the molecular subtypes. Comparisons between paired tumor and adjacent normal tissues showed significant upregulation of FOXA1 protein expression in tumor tissues among HR+/HER2+, HR+/HER2−, and HR−/HER2+ subtypes (all P<0.001), but downregulation in tumor tissues among TNBC (P=0.005) (Figure 2). In multivariable models adjusting for age, race, grade, and stage, the increase of FOXA1 protein expression from adjacent normal to tumor tissue significantly predicted ER+ vs. ER− subtypes (odds ratio = 1.13 95% CI= 1.09– 1.18, P<0.001) and non-TNBC vs. TNBC subtypes (odds ratio= 1.22, 95% CI= 1.14–1.31, P<0.001 for each 10 point increase in H-score; Supplemental Table 2).
Table 1.
FOXA1 protein expression according to demographic and tumor characteristics of study participants
| Characteristic | N | H-score, means (SD) | P-value1 |
|---|---|---|---|
| Total | 1329 | 157 (90) | – |
| Race | 0.001 | ||
| Black | 949 | 151 (95) | |
| White | 380 | 170 (74) | |
| Age | 0.74 | ||
| <40 | 153 | 154 (93) | |
| 40–49 | 366 | 154 (93) | |
| 50–59 | 445 | 157 (88) | |
| ≥60 | 365 | 161 (88) | |
| Histology | <0.001 | ||
| Ductal carcinoma in situ | 217 | 171 (80) | |
| Invasive ductal carcinoma | 968 | 151 (92) | |
| Invasive lobular carcinoma | 94 | 191 (63) | |
| Invasive mammary carcinoma and other invasive | 47 | 135 (86) | |
| Tumor grade | |||
| Low | 180 | 175 (65) | <0.001 |
| Intermediate | 480 | 183 (72) | |
| High | 567 | 125 (102) | |
| Tumor size (cm) | 0.005 | ||
| <1.0 | 227 | 163 (78) | |
| 1.0 – 1.9 | 408 | 160 (87) | |
| ≥2.0 | 478 | 143 (100) | |
| AJCC Stage | 0.001 | ||
| 0, I | 731 | 165 (84) | |
| II | 418 | 143 (97) | |
| III, IV | 163 | 151 (93) | |
| Lymph node status | 0.41 | ||
| Negative | 813 | 155 (90) | |
| Positive | 388 | 159 (91) | |
| ER status | <0.001 | ||
| Positive | 960 | 185 (69) | |
| Negative | 348 | 75 (92) | |
| PR status | <0.001 | ||
| Positive | 854 | 182 (72) | |
| Negative | 426 | 102 (100) | |
| HER2 status | 0.001 | ||
| Positive /equivocal | 246 | 172 (85) | |
| Negative | 989 | 149 (92) | |
| Molecular subtype | <0.001 | ||
| HR+2/HER2+ | 156 | 186 (70) | |
| HR+/HER2− | 696 | 179 (74) | |
| HR− /HER2+ | 79 | 136 (105) | |
| HR− /HER2− | 200 | 45 (70) |
ANOVA
Hormone receptor positive included ER+/PR+, ER+/PR−, and ER−/PR+ tumors
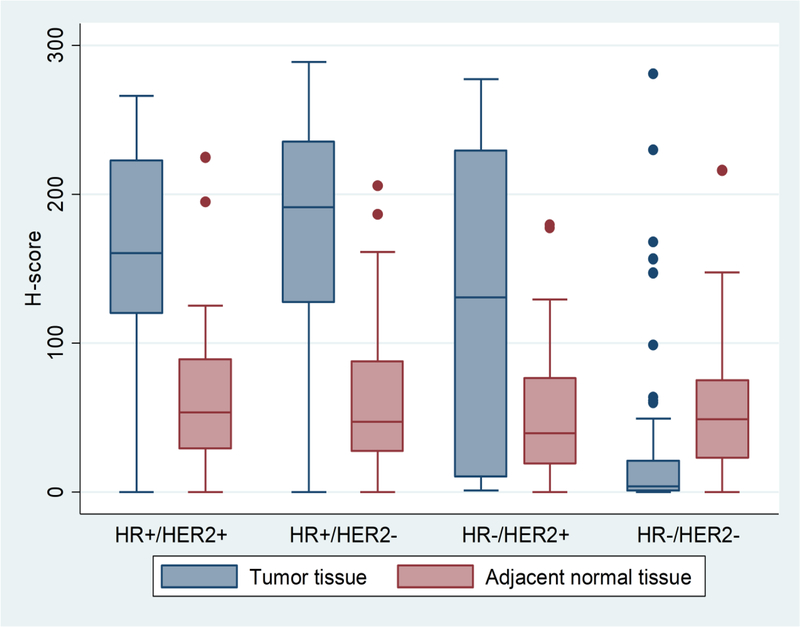
Figure 2.
Box plot of FOXA1 protein expression in tumor and adjacent-normal tissue by molecular subtype of breast cancer.
Note: A total of 298 patients with both tumor and adjacent-normal tissue; among them 254 with subtype information. The H-score of FOXA1 protein expression were different between tumor and adjacent-normal tissue overall (P<0.001) and within each subtype (P=0.005 for HR−/HER2− and P<0.001 for the other three subtypes; paired t-tests). The FOXA1 protein expression in adjacent-normal tissue were similar between the subtypes (P=0.78, ANOVA).
Table 2 shows the univariate results for associations between FOXA1 protein expression and reproductive characteristics in tumors according to ER status, and in adjacent normal tissue. Among parous women, later age at first live birth was associated with higher levels of FOXA1 protein expression in ER− breast cancer (P = 0.019). Having more children was associated with lower FOXA1 H-scores in ER+ tumors (P = 0.046). A similar reduction of FOXA1 protein expression with increased parity was also observed for ER− tumors and adjacent normal tissue, but the differences were not statistically significant. FOXA1 protein expression did not appear to differ by history of breastfeeding among parous women.
Table 2.
Associations of reproductive characteristics with FOXA1 protein expression in ER+ tumors, ER− tumors, and adjacent normal tissue
| ER+ tumors | ER− tumors | Adjacent normal tissue2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reproductive characteristics | N | H-score, mean (SD) | P-value1 | N | H-score, mean (SD) | P-value1 | N | H-score, mean (SD) | P-value1 |
| Age at menarche, years | 0.55 | 0.61 | 0.70 | ||||||
| <11 | 117 | 190 (67) | 38 | 62 (75) | 32 | 56 (42) | |||
| 11–12 | 391 | 183 (69) | 134 | 75 (90) | 125 | 59 (46) | |||
| ≥13 | 449 | 187 (70) | 176 | 79 (97) | 140 | 63 (45) | |||
| Age at first live birth, years | 0.87 | 0.019 | 0.49 | ||||||
| <24 | 442 | 185 (72) | 189 | 63 (88) | 135 | 59 (44) | |||
| 25–29 | 169 | 182 (65) | 53 | 74 (86) | 46 | 65 (52) | |||
| ≥30 | 145 | 185 (69) | 47 | 105 (102) | 45 | 68 (46) | |||
| Parity | 0.42 | 0.13 | 0.20 | ||||||
| Nulliparous | 204 | 189 (65) | 59 | 92 (96) | 72 | 54 (43) | |||
| Parous | 756 | 185 (70) | 298 | 72 (91) | 226 | 62 (46) | |||
| Number of births (among parous women) | 0.046 | 0.28 | 0.50 | ||||||
| 1 | 198 | 193 (68) | 68 | 87 (92) | 61 | 68 (48) | |||
| 2 | 282 | 186 (67) | 111 | 65 (89) | 83 | 62 (47) | |||
| ≥3 | 276 | 177 (74) | 110 | 70 (92) | 82 | 59 (44) | |||
| Breastfeeding (among parous women) | 0.93 | 0.77 | 0.16 | ||||||
| Never | 393 | 185 (71) | 150 | 73 (94) | 120 | 66 (51) | |||
| Ever | 363 | 184 (70) | 139 | 70 (88) | 106 | 58 (40) | |||
| Duration of breastfeeding (among women who breastfed), months | 0.22 | 0.74 | 0.77 | ||||||
| <12 | 213 | 188 (69) | 84 | 68 (83) | 66 | 57 (39) | |||
| ≥12 | 150 | 179 (70) | 55 | 73 (94) | 40 | 59 (41) | |||
| Menopausal status | 0.85 | 0.39 | 0.18 | ||||||
| Premenopause | 438 | 185 (70) | 167 | 71 (90) | 163 | 57 (45) | |||
| Postmenopause | 522 | 186 (69) | 181 | 79 (94) | 135 | 64 (46) | |||
ANOVA
Among participants who had adjacent normal tissue (n=298)
In multivariable analyses (Table 3), there were suggested associations that parous women had lower FOXA1 protein expression in ER+ and ER− tumors compared to nulliparous women, but the estimates (β = −5.3 [P=0.32] and −13.4 [P=0.27], respectively) were not significant. Among parous women with ER+ tumors, a higher number of births was associated with lower FOXA1 protein expression in tumors (β = −8.5, 95% CI= −15.1 to −2.0, P=0.010). When stratified by breastfeeding, the association between number of births and FOXA1 protein expression in ER+ tumors was significant in those who never breastfed (β = −10.4, 95% CI= −19.7 to −1.0, P=0.029), but not among those who reported having breastfed (β = −7.5, 95% CI= −16.9 to 1.8, P=0.11) (P-interaction = 0.57). Additional adjustment for tumor grade, breast cancer stage, and specimen type did not change the associations (Supplemental Table 3). Among women with ER− breast cancer, a higher number of births was also associated with lower FOXA1 protein expression overall (β = −10.3, P=0.13) and in those who never breastfed (β = −13.9, P=0.20). The associations were not statistically significant, likely due to reduced sample size.
Table 3.
FOXA1 protein expression in relation to parity and history of breastfeeding
| ER+ tumors | ER− tumors | |||||
|---|---|---|---|---|---|---|
| N | β (95% CI)1 | P-value | N | β (95% CI)1 | P-value | |
| Parous vs. Nulliparous (ref.) | 960 | −5.3 (−15.6, 5.1) | 0.32 | 348 | −13.4 (−37.4, 10.7) | 0.27 |
| Number of births (1, 2, 3+) | 756 | −8.5 (−15.1, −2.0) | 0.010 | 289 | −10.3 (−23.5, 2.9) | 0.13 |
| Number of birth stratified by history of breastfeeding (BF) | ||||||
| Never BF | 393 | −10.4 (−19.7, −1.0) | 0.029 | 150 | −13.9 (−35.1, 7.3) | 0.20 |
| Ever BF | 363 | −7.5 (−16.9, 1.8) | 0.11 | 139 | −3.6 (−21.9, 14.7) | 0.70 |
| P-interaction2 = 0.57 | P-interaction2 = 0.30 | |||||
Generalized linear models adjusting for age and race.
Wald tests for the interaction between number of births and breastfeeding
When further stratified by race (Table 4), a borderline-significant inverse association between parity and FOXA1 protein expression in ER− tumors was evident in samples from Black women (β = −11.8, P=0.07), but not in samples from White women (β = 5.7, P=0.84). In ER− tumors from Black women, the inverse association was stronger in women who had never breastfed compared to those who had breastfed (β = −16.9 [P=0.11] vs. −3.8 [P=0.38]), although the estimates were not significant. Age at menarche was not associated with FOXA1 protein expression in ER+ or ER− tumors in multivariable models (Supplemental Table 4).
Table 4.
FOXA1 protein expression in relation to parity and history of breastfeeding by race
| ER+ tumors | ER− tumors | ||||||
|---|---|---|---|---|---|---|---|
| Race | N | β (95% CI)1 | P-value | N | β (95% CI)1 | P-value | |
| Parous vs. Nulliparous (ref.) | Black | 661 | −7.1 (−21.5, 7.3) | 0.33 | 281 | −22.0 (−49.7, 5.8) | 0.12 |
| White | 299 | −1.9 (−16.4, 12.5) | 0.79 | 67 | 3.5 (−49.8, 56.7) | 0.90 | |
| Number of births (1, 2, 3+) | Black | 549 | −6.8 (−14.4, 0.8) | 0.08 | 247 | −11.8 (−24.6, 1.0) | 0.07 |
| White | 207 | −14.1 (−26.7, −1.5) | 0.028 | 42 | 5.7 (−50.7, 62.0) | 0.84 | |
| Number of birth stratified by history of breastfeeding (BF) | |||||||
| Never BF | Black | 306 | −8.7 (−19.4, 2.1) | 0.11 | 135 | −16.9 (−37.3, 3.5) | 0.11 |
| Ever BF | 243 | −5.5 (−16.5, 5.5) | 0.33 | 112 | −3.8 (−21.6, 14.1) | 0.68 | |
| P-interaction2 = 0.64 | P-interaction = 0.32 | ||||||
| Never BF | White | 87 | −17.8 (−35.5, −0.2) | 0.047 | 15 | −3 | |
| Ever BF | 120 | −13.0 (−30.8, 4.9) | 0.16 | 27 | −3 | ||
| P-interaction = 0.58 | |||||||
Generalized linear models adjusting for age.
Wald tests for the interaction between number of births and breastfeeding
Estimates not shown because small number of participants in the stratum (N <30).
Discussion
We observed that ER+ vs. ER− tumors had higher FOXA1 protein expression, and the comparisons of FOXA1 expression between tumor and adjacent normal tissue suggest that FOXA1 is upregulated in ER+ and other non-TN breast cancers, but downregulated in TNBC. These findings are consistent with the role of FOXA1 in promoting the luminal differentiation but suppressing the basal differentiation of the progenitor cells during breast tumorigenesis. Also, a higher number of births was associated with lower FOXA1 protein expression in ER+ tumors, and the association was attenuated in women who breastfed. For ER− tumors, consistent associations of parity and breastfeeding with FOXA1 were observed overall and in Black women, although the estimates were not significant possibly due to reduced sample size. Our race-specific findings should be interpreted with caution because of the smaller sample size of White women compared to Black women. We were unable to examine the association between FOXA1 protein expression in ER− tumors and parity by breastfeeding in White women. Another limitation was that we were unable to eliminate potential confounding bias, as a limited number of confounders were adjusted. In addition, the tissue samples were not from a single source, as a large proportion of patients’ tissue samples were only available as whole section slides. However, the difference would not lead to systematic bias because the specimen type (whole section versus TMA) was unlikely related to the reproductive factors and its inclusion in the multivariable models did not change the results.
The biological action of FOXA1 is key to the function of ER in breast cancer.12–15 FOXA1 is a pioneer factor that renders chromatin accessible to transcription factors, including ER, so that ER can promote the transcription of its target genes.24 Most breast tumors, including ER− or basal-like tumors, are thought to arise from luminal progenitor cells.25 Together with ER and GATA-3, another transcription factor, FOXA1 induces luminal cell differentiation and suppress the basal phenotype.16 Studies have shown that FOXA1 and ER are co-expressed in breast tumors and lower FOXA1 expression levels correlate with higher tumor grade,26,27 a feature of ER− tumors and TNBC. From our previous work11 and others,10 we speculate that, in many cases, the reproductive factor-associated changes in FOXA1 protein levels reflect the levels of DNA methylation at the FOXA1 gene. Interestingly, Gong et al. showed that the BRCA1 protein can regulate the expression of FOXA1 by impeding EZH2 methyltransferase activity, and propose that silencing or mutation of the BRCA1 gene abrogates this inhibitory effect ultimately leading DNA methylation at FOXA1. They further suggested that the resultant repression of FOXA1 allows cells to acquire a basal-like phenotype,17 consistent with earlier findings that most BRCA1-deficient breast tumors are basal-like TNBC.28 This suggests that parity-associated methylation of FOXA1 may predispose transformed cells to develop into TNBC.
Our findings on FOXA1 protein expression in ER− breast cancer are consistent with epidemiological and tumor DNA methylation data. Epidemiological evidence of parity in relation to ER− breast cancer in Black women has been confirmed by the AMBER Consortium.3 In the consortium, which consisted of 1,252 cases of ER− tumors and 14,180 controls, Black women who had more children were at higher risk of ER− breast cancer compared to those with fewer children. In addition, the positive association of parity with ER− tumors was attenuated by breastfeeding, that is, among women who breastfed, their risk of ER− tumors did not increase with the higher number of births.3,4,23 A consistent finding was observed in the Breast Cancer Family Registry with predominately White women.4 In our earlier investigation, higher parity was associated with DNA hypermethylation of FOXA1 in ER− tumors, particularly for Black women who never breastfed.11 The evidence is further supported by the current study that FOXA1 protein expression in ER− tumors was lower, which may result from hypermethylation, among Black women with a higher number of births and no breastfeeding. Although the association of parity with FOXA1 protein expression in ER− tumors was not significant, the estimates of beta coefficients were similar or somewhat stronger in ER− compared with ER+ tumors. The findings warrant confirmation with a larger sample of women with ER− tumors.
In AMBER, ever versus never breastfeeding was associated with a decreased risk of ER− breast cancer. However, we did not find an association of breastfeeding itself with FOXA1 protein expression. The reason for this null finding is unclear. Pregnancy can alter DNA methylation in mammary gland epithelial cells and “epigenetic memory” of pregnancy has been observed in animal models.29,30 The pregnancy-related DNA methylation changes may help prime the mammary gland for lactation. During lactation, prolactin and mammary gland epithelial cells enhance milk protein and lipid synthesis, and the process may promote DNA demethylation of lactation-specific genes, including DNA methyltransferases, which is an important driver of DNA methylation.31,32 However, the potential contribution of lactation on DNA methylation in the FOXA1 signaling axis is unclear. Also, there may be mechanisms other than modulation of FOXA1 whereby parity and breastfeeding affect breast cancer risk. It has been hypothesized that postpartum involution promotes remodeling of terminal duct lobular units, with immune and inflammatory reactions, which are hallmarks of ER− tumors,33,34 and breastfeeding can ameliorate these processes.35,36 Studies directly examining FOXA1 and related factors in postpartum breast tissue in women with and without breastfeeding, or animal studies mimicking parity with and without breastfeeding, may further elucidate the mechanisms.
Another risk factor for ER− breast cancer in Black women is early age at menarche.5 However, we did not observe an association of age at menarche with FOXA1 protein expression in breast tumors. Studies have suggested radiation exposure in puberty as a mechanism of developing ER− tumors related to early age at menarche. Early versus late age at menarche often leads to a longer duration between menarche and first live births, a period during which undifferentiated ductal cells may be highly susceptible to DNA damage caused by carcinogens.37,38 The role of FOXA1 in promoting luminal cell differentiation between menarche and a full-term pregnancy is unclear and warrants investigations.
Our study has several strengths. To our knowledge, this study is the first reporting relationships between reproductive characteristics and FOXA1 protein expression in breast tumors and adjacent normal tissue. A subset of our samples provided both tumor and adjacent normal tissue, allowing for modeling to what extent that the change of FOXA1 from normal to tumor tissue was associated with the rise of ER+ tumors and non-TNBC. Because of a relatively small sample size of those with adjacent normal tissue, this sub-analysis should be considered exploratory. Other strengths include that our study population comprised a large number of Black women, a population with higher risk of ER− tumors than Whites. Also, we used automated imaging analysis to derive an objective assessment of FOXA1 protein expression.
In conclusion, this tumor-based study showed that FOXA1 protein expression in ER+ breast tumors was inversely associated with parity, and the association was attenuated in women who breastfed. Results were largely consistent for ER− tumors although a larger sample is required for confirmation. The observation is in line with our previous findings of FOXA1 DNA hypermethylation in breast tumors, and further supports the potential roles of FOXA1 in the mechanism of parity and breastfeeding influencing the risk of ER− tumors.
Supplementary Material
Acknowledgments:
This work was supported by grants from the US Army Medical Research and Material Command (DAMD-17-01-1-0334) (to D.H. Bovbjerg and C.B. Ambrosone), the National Cancer Institute (R01 CA100598 to C.B. Ambrosone; P01 CA151135 to C.B. Ambrosone; R01 CA185623 to E.V. Bandera and C.C. Hong; R01 CA225947 to C.B. Ambrosone and M.J. Higgins; K07 CA201334 to T.Y. Cheng; and CCSGs to Roswell Park Cancer Institute (P30 CA016056) and Rutgers Cancer Institute of NJ (P30 CA072720), the Breast Cancer Research Foundation (to C.B. Ambrosone) and a gift from the Philip L Hubbell family (to C.B. Ambrosone). In addition, the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey Department of Health, is funded by the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute under contract HHSN261201300021I and control No. N01-PC-2013-00021, the National Program of Cancer Registries (NPCR), Centers for Disease Control and Prevention under grant NU5U58DP006279-02-00 as well as the State of New Jersey and the Rutgers Cancer Institute of New Jersey.
Footnotes
Conflict of interest statement: The authors declare no potential conflicts of interest.
References
- 1.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017;67:439–48. [DOI] [PubMed] [Google Scholar]
- 2.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, et al. Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. J Clin Oncol 2015;33:2254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer JR, Viscidi E, Troester MA, Hong CC, Schedin P, Bethea TN, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Work ME, John EM, Andrulis IL, Knight JA, Liao Y, Mulligan AM, et al. Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the Breast Cancer Family Registry. Br J Cancer 2014;110:1367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrosone CB, Zirpoli G, Hong CC, Yao S, Troester MA, Bandera EV, et al. Important Role of Menarche in Development of Estrogen Receptor-Negative Breast Cancer in African American Women. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrosone CB, Zirpoli GR, Bovbjerg DH, Shankar J, Hong CC, McCann SE, et al. Associations between estrogen receptor-negative breast cancer and timing of reproductive events differ between African American and European American women. Cancer Epidemiol Biomarkers Prev 2014;23:1115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrosone CB, Zirpoli G, Ruszczyk M, Shankar J, Hong CC, McIlwain D, et al. Parity and breastfeeding among African-American women: differential effects on breast cancer risk by estrogen receptor status in the Women’s Circle of Health Study. Cancer Causes Control 2014;25:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barcellos-Hoff MH. Does microenvironment contribute to the etiology of estrogen receptor-negative breast cancer? Clin Cancer Res 2013;19:541–8. [DOI] [PubMed] [Google Scholar]
- 9.Huh SJ, Clement K, Jee D, Merlini A, Choudhury S, Maruyama R, et al. Age- and pregnancy-associated DNA methylation changes in mammary epithelial cells. Stem Cell Reports 2015;4:297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh S, Gu F, Wang CM, Lin CL, Liu J, Wang H, et al. Genome-wide DNA methylation profiling reveals parity-associated hypermethylation of FOXA1. Breast Cancer Res Treat 2014;147:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinal AC, Buas MF, Wang D, Cheng DT, Sucheston-Campbell L, Hu Q, et al. FOXA1 hypermethylation: link between parity and ER-negative breast cancer in African American women? Breast Cancer Res Treat 2017;166:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 2011;43:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature 2009;462:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, et al. FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development 2010;137:2045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu X, Jeselsohn R, Pereira R, Hollingsworth EF, Creighton CJ, Li F, et al. FOXA1 overexpression mediates endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer. Proc Natl Acad Sci U S A 2016;113:E6600–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernardo GM, Bebek G, Ginther CL, Sizemore ST, Lozada KL, Miedler JD, et al. FOXA1 represses the molecular phenotype of basal breast cancer cells. Oncogene 2013;32:554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong C, Fujino K, Monteiro LJ, Gomes AR, Drost R, Davidson-Smith H, et al. FOXA1 repression is associated with loss of BRCA1 and increased promoter methylation and chromatin silencing in breast cancer. Oncogene 2015;34:5012–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrosone CB, Ciupak GL, Bandera EV, Jandorf L, Bovbjerg DH, Zirpoli G, et al. Conducting Molecular Epidemiological Research in the Age of HIPAA: A Multi-Institutional Case-Control Study of Breast Cancer in African-American and European-American Women. J Oncol 2009;2009:871250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandera EV, Chandran U, Zirpoli G, McCann SE, Ciupak G, Ambrosone CB. Rethinking sources of representative controls for the conduct of case-control studies in minority populations. BMC Med Res Methodol 2013;13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurinavicius A, Laurinaviciene A, Ostapenko V, Dasevicius D, Jarmalaite S, Lazutka J. Immunohistochemistry profiles of breast ductal carcinoma: factor analysis of digital image analysis data. Diagn Pathol 2012;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allott EH, Cohen SM, Geradts J, Sun X, Khoury T, Bshara W, et al. Performance of Three-Biomarker Immunohistochemistry for Intrinsic Breast Cancer Subtyping in the AMBER Consortium. Cancer Epidemiol Biomarkers Prev 2016;25:470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsch FR, Varella-Garcia M, Bunn PA Jr., Di Maria MV, Veve R, Bremmes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 2003;21:3798–807. [DOI] [PubMed] [Google Scholar]
- 23.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat 2008;109:123–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer KB, Carroll JS. FOXA1 and breast cancer risk. Nat Genet 2012;44:1176–7. [DOI] [PubMed] [Google Scholar]
- 25.Gross K, Wronski A, Skibinski A, Phillips S, Kuperwasser C. Cell Fate Decisions During Breast Cancer Development. Journal of developmental biology 2016;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badve S, Turbin D, Thorat MA, Morimiya A, Nielsen TO, Perou CM, et al. FOXA1 expression in breast cancer--correlation with luminal subtype A and survival. Clin Cancer Res 2007;13:4415–21. [DOI] [PubMed] [Google Scholar]
- 27.Thorat MA, Marchio C, Morimiya A, Savage K, Nakshatri H, Reis-Filho JS, et al. Forkhead box A1 expression in breast cancer is associated with luminal subtype and good prognosis. J Clin Pathol 2008;61:327–32. [DOI] [PubMed] [Google Scholar]
- 28.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 2003;100:8418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz TA, Liao SG, Palmieri VJ, Dearth RK, Pathiraja TN, Huo Z, et al. Targeted DNA Methylation Screen in the Mouse Mammary Genome Reveals a Parity-Induced Hypermethylation of Igf1r That Persists Long after Parturition. Cancer Prev Res (Phila) 2015;8:1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dos Santos CO, Dolzhenko E, Hodges E, Smith AD, Hannon GJ. An epigenetic memory of pregnancy in the mouse mammary gland. Cell Rep 2015;11:1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melnik BC, Schmitz G. Milk’s Role as an Epigenetic Regulator in Health and Disease. Diseases 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Luo J, Zhang C, Ma Y, Sun S, Zhang T, et al. Mechanism of prolactin inhibition of miR-135b via methylation in goat mammary epithelial cells. J Cell Physiol 2018;233:651–62. [DOI] [PubMed] [Google Scholar]
- 33.Safonov A, Jiang T, Bianchini G, Gyorffy B, Karn T, Hatzis C, et al. Immune Gene Expression Is Associated with Genomic Aberrations in Breast Cancer. Cancer Res 2017;77:3317–24. [DOI] [PubMed] [Google Scholar]
- 34.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 2010;12:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schedin P Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer 2006;6:281–91. [DOI] [PubMed] [Google Scholar]
- 36.Faupel-Badger JM, Arcaro KF, Balkam JJ, Eliassen AH, Hassiotou F, Lebrilla CB, et al. Postpartum remodeling, lactation, and breast cancer risk: summary of a National Cancer Institute-sponsored workshop. J Natl Cancer Inst 2013;105:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat 1982;2:5–73. [DOI] [PubMed] [Google Scholar]
- 38.Tang J, Fernandez-Garcia I, Vijayakumar S, Martinez-Ruis H, Illa-Bochaca I, Nguyen DH, et al. Irradiation of juvenile, but not adult, mammary gland increases stem cell self-renewal and estrogen receptor negative tumors. Stem cells 2014;32:649–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.