Abstract
Background:
Accurate estimates for the contemporary burden of chronic liver disease (CLD) are vital for setting clinical, research, and policy priorities. We aimed to review the incidence, prevalence, and mortality of CLD and its resulting complications, including cirrhosis and hepatocellular carcinoma (HCC).
Findings:
The epidemiology of CLD is shifting, reflecting implementation of large-scale hepatitis B vaccination and hepatitis C treatment programs, the rising prevalence of the metabolic syndrome, and increasing alcohol misuse. In this setting, data from the Centers for Disease Control and the Global Burden of Disease Study demonstrate the incidence and clinical impact of CLD and its complications continue to rise worldwide. We conclude by discussing limitations of available data and outline steps on improving data infrastructure.
Conclusion:
The global burden of CLD and cirrhosis is substantial. Although vaccination, screening, and anti-viral treatment campaigns for hepatitis B and C have reduced CLD burden in some parts of the world, concomitant increases in injection drug use, alcohol misuse and metabolic syndrome threaten these trends. Ongoing efforts to address CLD-related morbidity and mortality require accurate contemporary estimates of epidemiology and outcomes.
Keywords: Alcoholic liver disease, Hepatitis B, Liver cancer, Hepatitis C, Nonalcoholic fatty liver disease
Introduction
Chronic liver disease (CLD) and cirrhosis account for 44,000 deaths in the United States and 2 million deaths worldwide each year, in addition to a high burden of disability and increased healthcare utilization.1, 2 However, mortality estimates for CLD are likely conservative and underestimate its true burden.3 The most common etiologies of CLD and cirrhosis are chronic hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol-related liver disease (ALD), and non-alcoholic fatty liver disease (NAFLD). Multiple recent developments are reshaping the epidemiology of CLD and cirrhosis including neonatal HBV vaccination campaigns, improved HCV treatment access and effectiveness, the opioid crisis, the obesity epidemic, and increasing rates of alcohol misuse.
Accurate estimates of the contemporary burden of cirrhosis is vital for setting clinical, research, and policy priorities. In light of the ever-changing nature of CLD epidemiology, we aimed to review the global incidence, prevalence, and mortality of CLD and its resulting complications including cirrhosis and hepatocellular carcinoma (HCC).
Incidence, Prevalence and Mortality of CLD
Global Burden of CLD
Globally, 1.5 billion persons had CLD in 2017, most commonly due to NAFLD (60%), HBV (29%), HCV (9%), and ALD (2%).4 In European countries, the median cirrhosis prevalence was 833/100,000 (range 447-1100) but data on cirrhosis prevalence in other areas, particularly resource-limited settings, are sparse.5, 6 Similarly, accurate accounting of cirrhosis and CLD incidence is difficult in most areas due to a paucity of high-quality, prospective data.6 Based on data from the Global Burden of Disease study, the age-standardized incidence rate of cirrhosis and CLD was 20.7/100,000 in 2015, a 13% increase from 2000 (Tables 1-2).7 The estimated incidence of cirrhosis in Europe is 26.0/100,000, and the incidence in Asia ranges from 16.5/100,000 in East Asia to 23.6/100,000 in Southeast Asia.7
Table 1.
Prevalence, Incidence, Mortality and Costs of CLD and Cirrhosis Globally
| Author (year) | Country/Region | Prevalence (per 100,000 pop.)* |
Incidence (per 100,000 person- years)* |
Mortality (per 100,000 person- years)* |
Costs | |
|---|---|---|---|---|---|---|
| Cirrhosis | ||||||
| Global Burden of Disease (2018)4 | All | Cirrhosis and CLD: 1.5 billion (2017)* | Cirrhosis and CLD: 5.2 million (2017)* | - | Cirrhosis and CLD YLDs 1.8 million (2017) | |
| Mokdad (2014)1 | All | - | - | 15.7 (2010) | - | |
| Mokdad (2014)1 | Asia | - | - | 8.2-33.7 (2010) | - | |
| Mokdad (2014)1 | Europe | - | - | 10.2-20.0 (2010) | - | |
| Mokdad (2014)1 | Latin America | - | - | 15.8-27.5 (2010) | - | |
| Mokdad (2014)1 | North Africa/MiddleEast | - | - | 20.2 (2010) | - | |
| Mokdad (2014)1 | Oceania | - | - | 41.5 (2010) | - | |
| Mokdad (2014)1 | Sub-Saharan Africa | - | - | 12.9-24.2 (2010) | ||
| Ratib (2017)156 | United Kingdom | - | 35.9 (2009) | 5.9 (2009) | ||
| HCV | ||||||
| WHO (2017)24 | All | 1,000 (2015) | 23.7 (2015) | 402,000 (2015)* | - | |
| WHO (2017)24 | Africa | 1,000 (2015) | 31.0 (2015) | - | - | |
| WHO (2017)24 | Eastern Mediterranean | 2,300 (2015) | 62.5 (2015) | - | - | |
| WHO (2017)24 | Europe | 1,500 (2015) | 61.8 (2015) | - | - | |
| WHO (2017)24 | Americas | 700 (2015) | 6.4 (2015) | - | - | |
| WHO (2017)24 | South-East Asia | 500 (2015) | 14.8 (2015) | - | - | |
| WHO (2017)24 | Western Pacific | 700 (2015) | 6.0 (2015) | - | - | |
| HBV | ||||||
| WHO (2017)24 | All | 3,500 (2015) | 1,300 (age <5 years) (2015) | 884,000 (2015)* | - | |
| WHO (2017)24 | Africa | 6,100 (2015) | 3,000 (age <5 years)(2015) | - | - | |
| WHO (2017)24 | Eastern Mediterranean | 3,300 (2015) | 1,600 (age <5 years) (2015) | - | - | |
| WHO (2017)24 | Europe | 1,600 (2015) | 400 (age <5 years) (2015) | - | - | |
| WHO (2017)24 | Americas | 700 (2015) | 200 (age <5 years) (2015) | - | - | |
| WHO (2017)24 | South-East Asia | 2,000 (2015) | 700 (age <5 years) (2015) | - | - | |
| WHO (2017)24 | Western Pacific | 6,200 (2015) | 900 (age <5 years) (2015) | - | - | |
| NAFLD | ||||||
| Global Burden of Disease (2018)4 | All | NASH cirrhosis 11,061 (2017) | - | - | - | |
| Younossi (2015)54 | All | 25,000 | - | - | - | |
| Younossi (2015)54 | Africa | 13,500 | - | - | - | |
| Li (2019)55 | Asia | 33,900(2012-2017) | 5,090 (1999-2019) | |||
| Wong (2015)60 | Hong Kong | - | 3,400 (2008-2013) | - | - | |
| Zhou (2011)59 | China | - | 9,100 (2009) | - | - | |
| Chang (2016)62 | South Korea | 2,970 (2002-2013) | ||||
| Younossi (2015)54 | Europe | 23,710 | - | - | - | |
| Younossi (2016)157 | Germany, France, Italy, United Kingdom | €35 billion direct costs (€354-1163 per patient) | ||||
| Kanerva (2014)158 | Finland | 41,150 | - | - | - | |
| Zelber-Sagi (2014)61 | Israel | - | 2,714 (2003-2010) | - | - | |
| Younossi (2015)54 | Middle East | 31,790 | - | - | - | |
| Younossi (2015)54 | South America | 30,450 | - | - | - | |
| Riquelme (2009)159 | Chile | 23,400 | - | - | - | |
| ALD | ||||||
| Global Burden of Disease (2018)4 | All | 26 million (2017)* | 903,700 annually (2017)* | YLDs 400,100 | ||
| Rehm (2013)68 | All | - | - | 7.2 (2010) | - | |
| HCC | ||||||
| Global Burden of Disease (2017/2018)4,103 | All | 803,400 (2017)* | 953,100 annually (2017)* | 12.1 (2015) | YLDs 229,500 (2017) |
YLD: years lived with disability
Where total population unavailable, the total number of cases are presented
Table 2.
Prevalence, Incidence, Mortality and Costs of CLD and Cirrhosis in North America
| Author (year) | Data Source | Prevalence (per 100,000 pop.) |
Incidence (per 100,000 person-years) |
Mortality (per 100,000 person-years) |
Costs | |
|---|---|---|---|---|---|---|
| Cirrhosis | ||||||
| Beste (2015)14 | US VA system | 1,058 (2013) | 167 (2012) | 126 (2013) | - | |
| Flemming (2018)13 | Routinely collected health data from Ontario, Canada | 840 (2016) | 90 (2016) | - | - | |
| Scaglione (2015)15 | NHANES | 302 (2010) | - | 2-year mean proportion of deaths 26.4% (1999-2006) | - | |
| Mellinger (2018)71 | Truven Marketscan Commercial Claims and Encounters Database | 270 (2015) | - | - | - | |
| Tapper (2018)2 | CDC | - | - | 12.2 (1999-2016) | - | |
| HCV | ||||||
| CDC (2018)27 | National notifiable diseases surveillance system | - | 1.0 (2016) | 4.5 (2016) | - | |
| Hofmeister (2019)21 | NHANES | 900 (2013-2016) | ||||
| Beste (2015)14 | US VA system | HCV cirrhosis:503 (2013) | HCV cirrhosis: 77.9 (2012) | - | - | |
| HBV | ||||||
| CDC (2018)27 | National notifiable diseases surveillance system | - | 1.0 (2016) | 0.5 (2016) | ||
| Roberts (2016)160 | NHANES | 300 (2012) | - | - | - | |
| Beste (2015)14 | US VA system | HBV cirrhosis:503 (2013) | HBV cirrhosis: 3.3 (2012) | |||
| NAFLD | ||||||
| Browning (2004)57 | Dallas Heart Study | 34,000 | ||||
| Kanwal (2016)16 | US VA system | 17,610 (2011) | 2,500 (2011) | - | - | |
| Allen (2018)52 | Olmsted County, Minnesota | - | 329 (2014) | - | - | |
| Younossi (2016)157 | Markov model | - | - | - | $103 billion direct costs ($1613 per patient) | |
| Beste (2015)14 | US VA system | NASH cirrhosis: 161 (2013) | NASH cirrhosis: 30.3 (2012) | - | - | |
| Kabbany (2017)51 | NHANES | NASH cirrhosis: 178 (2009-2012) | ||||
| ALD | ||||||
| Mellinger (2018)71 | Truven Marketscan Commercial Claims and Encounters Database | Alcoholic cirrhosis: 100 (2015) | - | - | $44,835 per-person (alcoholic cirrhosis) vs. $23,319 per-person (non-alcoholic cirrhosis) | |
| Beste (2015)14 | US VA system | Alcoholic cirrhosis: 327 (2013) | Alcoholic cirrhosis: 47.8 (2012) | - | - | |
| HCC | ||||||
| White (2017)106 | US Cancer Statistics registry | - | 6.7 (2012) | - | - | |
| Altekruse (2014)105 | SEER/CDC | - | 5.9 (2006-2010) | 4.3 (2006-2010) | - | |
| Tapper (2018)2 | CDC | - | - | 3.6 (1999-2016) | - |
There have been small increases in cirrhosis incidence in Europe, high-income Asia-Pacific, East Asia, Southeast Asia, and South Asia from 2000-2015.7 Although HBV vaccination and viral hepatitis treatment programs have curbed the incidence of cirrhosis in many countries, including Japan and Taiwan, the rising burden of obesity, metabolic syndrome, and alcohol misuse threatens these trends.7-9
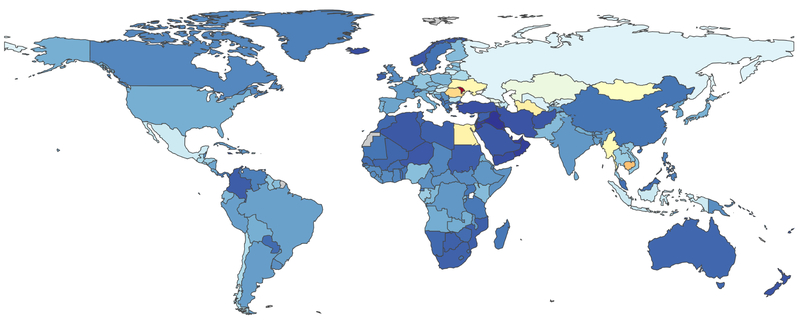
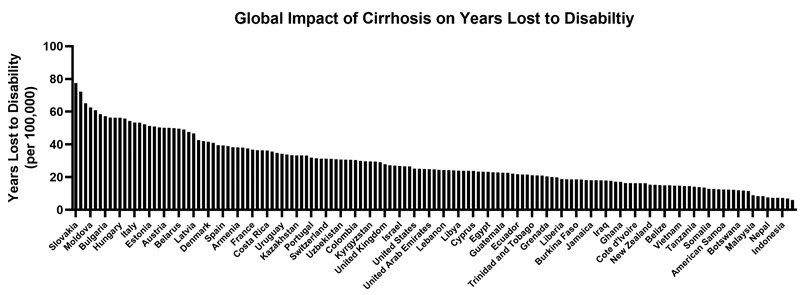
The major complications of CLD – cirrhosis (1.2 million deaths) and liver cancer (790,000 deaths) – account for 3.5% of all deaths worldwide.10 In Figure 1 we detail the global age-adjusted mortality per 100,000 persons across regions. Cirrhosis-related mortality decreased from 20.0/100,000 person-years in 1980 to 15.8/100,000 person-years in 2010.1 Although decrease mortality rates were pronounced in East Asia, North Africa/Middle East, and high-income Asia Pacific, increases in mortality were observed in many other parts of the world including South Asia, Central Asia, and Eastern Europe. These disparate trends are likely related to differences in the distribution of underlying CLD etiologies and strategies to curb CLD burden. Deaths from cirrhosis increased two-fold in sub-Saharan Africa between 1980 and 2010, primarily due to viral hepatitis and ALD.1 Although viral hepatitis remains the driving force behind cirrhosis-related mortality in Asia, deaths are declining due to increased HBV vaccination and treatment of viral hepatitis.1, 11, 12 ALD is currently responsible for most cirrhosis-related deaths in Europe but trends vary between countries with mortality decreasing in some (Austria, Denmark, France, Germany, Hungry) and increasing in others (Finland, Ireland, UK).9 Beyond mortality, disability is another metric of morbidity. We therefore detail in Figure 2 the years lost to disability attributed to cirrhosis.
Figure 1: Global Burden of Cirrhosis Mortality.
In this figure, abstracted from the Global Burden of Disease Study (https://vizhub.healthdata.org/gbd-compare/. accessed 4/2/19), we detail the age-adjusted risk of mortality (per 100,000 persons) attributed to cirrhosis. Legend (mortality per 100,000 persons): Dark to light blue (0-40). Yellow (40-50), Yellow-Orange (50-60), Orange-Red (60-70), Red (>70)
Figure 2: Global Burden of Years Lost to Disability Due to Cirrhosis.
In this this figure, we detail the years lost to disability (YLD) due to cirrhosis. YLD is a function of the disease incidence, the disability weight (on functioning) and the average duration of illness. Data abstracted from the Global Burden of Disease Study (https://vizhub.healthdata.org/gbd-compare/, accessed 4/2/19).
Burden of CLD in North America
Estimates detailing the epidemiology of CLD in North America are also limited by the paucity of longitudinal, population-based data. Baby boomers (born 1945-1965) comprise half of cirrhosis cases in North America with a relatively higher prevalence among blacks, Hispanics, and those with lower levels of education.13-15 The estimated prevalence of cirrhosis ranges from 300-1,000/100,000 conditional on the variable risk of CLD across populations (Table 2).13-15 For example, the estimated incidence of cirrhosis is 167/100,000 person-years within the US Veterans Affairs (VA) system compared to 90/100,000 person-years in a population from Ontario, Canada.13, 14 These discrepant estimates may be explained by ascertainment bias or differences in the patient populations including the VA’s predominantly male population with a higher burden of viral hepatitis, alcohol misuse, and metabolic syndrome.16, 17
The prevalence of cirrhosis has increased 1.5-2 fold over the past two decades.13-15 While most prevalent cirrhosis cases are in the baby boomer cohort, incident cirrhosis diagnoses are highest and rising disproportionately in younger Americans.13, 14 As reflected in a Canadian population-based cohort where risk-factors such as metabolic syndrome and alcohol misuse are rising among young people, age-specific cirrhosis incidence increased 22% from 1997-2016.13
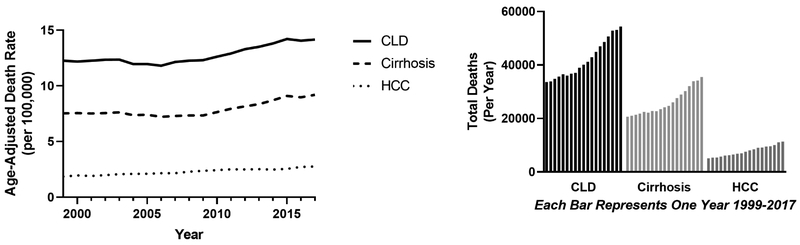
After several decades of stability from the 1970-2008, CLD-related mortality has since steadily risen with disproportionate relative increases among young people, women, non-Hispanics, whites, and Native Americans.2, 3 Following a National Center for Health Statistics (NCHS) study that demonstrated a 65% increase in cirrhosis mortality from 2009-2016, we present updated statistics through 2017 (Table 3).2 Age-adjusted mortality rates from CLD and cirrhosis continued to rise, with estimates of 14.2 (95% CI 14.0-14.3) and 9.2 (95% CI 9.1-9.3) per 100,000, respectively in 2017 (Figure 3).
Table 3.
Mortality from CLD, cirrhosis and HCC in the US, overall and among various subgroups from 1999-2017
| Combined liver- related mortality rate/100,00 (95% CI) |
CLD mortality rate/100,000 (95% CI) |
Cirrhosis mortality rate/100,000 (95% CI) |
HCC mortality rate/100,000 (95% CI) |
||
|---|---|---|---|---|---|
| Overall | 15.20 (15.16-15.23) | 12.86 (12.84-12.89) | 7.96 (7.94-7.98) | 2.34 (2.33-2.35) | |
| Sex | Female | 10.04 (10.00-10.07) | 9.06 (9.03-9.09) | 5.27 (5.25-5.30) | 0.97 (0.96-0.98) |
| Male | 21.04 (20.99-21.09) | 17.10 (17.05-17.15) | 10.96 (10.93-11.00) | 3.92 (3.90-3.95) | |
| Age | 25-34 | 1.81 (1.78-1.84) | 1.71 (1.69-1.74) | 0.73 (0.71-0.75) | 0.10 (0.09-0.11) |
| 35-44 | 8.53 (8.47-8.60) | 8.18 (8.11-8.24) | 4.62 (4.57-4.67) | 0.36 (0.35-0.37) | |
| 45-54 | 26.15 (26.04-26.27) | 23.56 (23.46-23.67) | 14.68 (14.59-14.76) | 2.59 (2.56-2.63) | |
| 55-64 | 42.33 (42.17-42.49) | 34.95 (34.81-35.10) | 22.28 (22.16-22.39) | 7.37 (7.30-7.44) | |
| 65-74 | 47.73 (47.52-47.94) | 38.53 (38.34-38.72) | 24.79 (24.64-24.94) | 9.21 (9.11-9.30) | |
| 75-84 | 53.68 (53.39-53.96) | 42.15 (41.89-42.40) | 27.16 (26.96-27.37) | 11.53 (11.40-11.66) | |
| 85+ | 46.30 (45.88-46.72) | 37.32 (36.94-37.70) | 21.17 (20.88-21.45) | 8.98 (8.80-9.17) | |
| Race | Native American | 33.00 (32.51-33.49) | 29.89 (29.42-30.35) | 16.93 (16.59-17.28) | 3.12 (2.96-3.29) |
| Asian/Pacific Islander | 9.70 (9.57-9.82) | 4.96 (4.87-5.05) | 2.90 (2.83-2.97) | 4.76 (4.67-4.84) | |
| Black | 14.40 (14.31-14.49) | 10.99 (10.91-11.07) | 6.29 (6.23-6.35) | 3.43 (3.38-3.47) | |
| White | 15.20 (15.16-15.23) | 13.31 (13.28-13.34) | 8.33 (8.31-8.36) | 2.06 (2.05-2.07) | |
| Ethnicity | Hispanic | 22.08 (21.95-22.21) | 18.33 (18.21-18.45) | 12.49 (12.39-12.59) | 3.74 (3.69-3.80) |
| Non-Hispanic | 14.56 (14.53-14.59) | 12.33 (12.30-12.36) | 7.52 (7.50-7.54) | 2.22 (2.20-2.23) | |
| Geographic region | Northeast | 12.97 (12.91-13.04) | 10.71 (10.65-10.77) | 6.38 (6.33-6.43) | 2.28 (2.25-2.30) |
| Midwest | 13.71 (13.65-13.77) | 11.69 (11.63-11.75) | 7.08 (7.03-7.12) | 2.00 (1.98-2.03) | |
| South | 16.16 (16.11-16.21) | 13.82 (13.78-13.87) | 8.85 (8.81-8.89) | 2.31 (2.29-2.33) | |
| West | 15.20 (15.16-15.23) | 14.24 (14.18-14.30) | 8.71 (8.66-8.76) | 2.79 (2.76-2.82) | |
Data source: Centers for Disease Control and Prevention National Center for Health Statistics
Age-adjusted rate listed for all categories with exception of age strata
Combined liver-related definition: C22.0, K70, K71, K72, K73, K74, K75, K76
Cirrhosis mortality definition: K74.6, K74.5, K70.3
CLD mortality definition: K70, K71, K72, K73, K74, K75, K76
HCC mortality definition: C22.0
Figure 3: Mortality Due to Chronic Liver Disease (CLD), Cirrhosis and Hepatocellular Carcinoma (HCC) in the US (1999-2017).
In the left panel we show how the age-adjusted mortality for CLD, cirrhosis, and HCC has changed over time. While HCC-related mortality has slowly climbed from 1999, mortality due to CLD and cirrhosis began rising after 2008, continuously through 2017
In the right panel we show the raw numbers of deaths attributed primarily to CLD, cirrhosis, and HCC for each year from 1999-2017
Specific Chronic Liver Diseases
The relative contribution of viral hepatitis, NAFLD, and ALD to the global burden of CLD is rapidly shifting.4, 7, 8, 18, 19 Globally, HBV incidence and its complications have been reduced by widespread vaccination and anti-viral treatment programs. In contrast, although many patients with chronic HCV infection are being successfully treated with direct acting antiviral (DAA) therapy, reducing future risk of developing cirrhosis or HCC20, the opioid epidemic and intravenous drug use patterns have resulted in an increased number of acute HCV infections.21 In parallel, NAFLD is becoming an increasingly important cause of CLD worldwide concurrent with a rising burden of obesity and metabolic syndrome. Similarly, alcohol consumption is responsible for an estimated 27% of liver-related deaths worldwide, highest in Europe, and has been increasing in many countries globally.22
HCV Infection
Approximately 71 million people worldwide (1.0%) have chronic HCV with a prevalence of 1.0% in the US, 1.5-1.8% in Europe, 1.0% in Africa, and 0.5-0.7% in Asia.23-26 Most HCV infections are caused by genotypes 1 and 3, which are estimated to account for 44% and 25% of infections, respectively.26 There are an estimated 1.8 million new HCV infections per year (incidence rate 23.7/100,000) with the highest rates in the Eastern Mediterranean (62.5 per 100,000) and Europe (61.8/100,000).24 In the US, an estimated 41,200 new HCV infections occurred in 2016 (incidence rate 13.9/100,000).27 In contrast to other infectious diseases, including human immunodeficiency virus, malaria and tuberculosis, deaths from viral hepatitis have been increasing over the last 15 years.28 HCV has been a leading cause of cirrhosis and hepatocellular carcinoma (HCC), accounting for an estimated 400,000 liver-related deaths worldwide in 2015.24
Highly-effective DAAs were introduced in 2014 and have increased treatment eligibility and success dramatically. The US VA system, Egypt, Republic of Georgia, and Iceland are prime examples of success for large-scale screening, anti-viral treatment and micro-elimination efforts. 17, 29-33 However, in other areas, the potential benefit of DAAs has been blunted by incomplete HCV screening and linkage to care, particularly in rural and socioeconomically disadvantaged areas.34 Despite recommendations for HCV screening in at-risk individuals in many countries, an estimated 80% of chronic HCV infections remain undiagnosed worldwide, ranging from 94% in Africa to 68% in the Americas.35 Even among those who undergo HCV screening, there are suboptimal rates of confirmatory HCV RNA testing and linkage to care.36 Finally, HCV treatment is often not covered by insurance or withheld for high-risk groups such as those who inject drugs, potentially as a result of abstinence requirements from some payers, despite professional society guidance statements recommending otherwise.37-39
Worldwide the number of newly infected individuals (1.75 million) exceeds the sum of patients dying from HCV (399,000) and those being successfully treated and cured (843,000).24, 35 Injection drug use accounts for increasing numbers of HCV infections in the US, particularly among those who reside in rural areas, aged 20-29 years, and are non-Hispanic white. 27, 40-42 HCV seroprevalence is now higher among young adults than baby boomers in several areas of the US.43 Unfortunately, the rapidly changing policy landscape and shifting trends describe above render data inadequate even after one year, limiting our understanding of true contemporary HCV prevalence.44
Chronic HBV Infection
Worldwide, 257 million (3.5%) people had chronic HBV infection in 2015.5, 24, 45 Western Pacific nations (6.2%) and Africa (6.1%) had the highest prevalence of HBV and accounted for more than two-thirds of all cases.24 A recent survey from NHANES estimated the US prevalence of chronic HBV as 840,000 (0.35%) overall in 2011-2016, varying from 3.85% among Asian immigrants and 0.79% among American-born Asians.46
Encouraging trends in HBV incidence have resulted from large-scale neonatal vaccination efforts in many countries.5, 47 Vaccination is particularly important for the estimated 65 million women of childbearing age with chronic HBV.24 Worldwide, HBV prevalence among children <5 years (a surrogate of HBV incidence) has decreased from 4.7% in the pre-HBV vaccination era (1980s-2000s) to 1.3% in 2015. Improvements in vaccination coverage and subsequent decreases in childhood HBV infection have been specifically seen in South East Asia, Western Pacific, and the Eastern Mediterranean region. 7, 24, 48 However, HBV vaccination coverage remains low in some regions, particularly in Africa, and coverage with the initial birth vaccination dose continues to lag (<40%).24 Beyond vaccination programs, efforts to curb HBV mortality are limited by inadequate diagnosis and treatment of chronically infected patients, partly due to the lack of uniform HBV treatment guidelines, poor awareness of active infection (15.2% in one US study), and limited access to risk-assessment tools such as non-invasive tests for fibrosis.35, 46, 49 Overall, among the estimated 250 million individuals with chronic HBV, 10% are aware of their diagnosis and 2% are currently on treatment.50
NAFLD
NAFLD encompasses a spectrum including hepatic steatosis, non-alcoholic steatohepatitis (NASH), and cirrhosis. While accurate estimates of NAFLD are difficult to obtain, it is likely a major driver of the increasing cirrhosis incidence, concordant with trends in obesity and metabolic syndrome.16, 51, 52 The pitfalls of NAFLD epidemiology are partly specific to fibrosis assessment. Hepatic steatosis in the absence of other causes (e.g. significant alcohol use) can be estimated among cohorts receiving abdominal imaging or liver enzyme testing; NASH, a histological diagnosis, can only be estimated from cohorts including histology data or self-reported diagnoses53; cirrhosis can be estimated from cohorts with adequate laboratory, histology or imaging data.
The global prevalence of NAFLD is estimated at approximately 24% with considerable variability from Africa (13.5%) to South America (30.5%), the Middle East (31.8%), and Asia (33.9%).54, 55 Approximately one in three US residents has hepatic steatosis and it is more commonly seen in Hispanics followed by whites and blacks.56, 57 Based on the prevalence of biopsy-proven NASH in patients with NAFLD (6.7-29.9%), it is estimated that approximately 1.5-6.5% of the general US population have NASH.54 A study from 5 European countries estimated the prevalence of NASH as 0.29% using self-reported diagnoses.53 An estimated 178 of 100,000 Americans have NASH cirrhosis based on data from NHANES and its prevalence has increased 2.5-fold from 1999-2002 to 2009-2012.51
Population-based, longitudinal data to track NAFLD incidence are lacking in many places. In studies with varying case definitions and populations, the reported incidence of hepatic steatosis per 100,000 person-years has ranged from 1,850 in Italy, 2,714 in Israel, 3,400 in Hong Kong, 5,090 in Asia, and 9,100 in China.55, 58-62 A population-based study in Minnesota reported that the age- and sex-adjusted NAFLD incidence, defined by diagnostic codes, was 317/100,000 person-years. This is considerably lower than the estimated NAFLD incidence of 2,500/100,000 person-years reported in the US VA system, in which cases were defined by elevated liver enzymes without viral hepatitis or significant alcohol use.16, 52
The increasing burden of obesity in children is particularly concerning given its association with future development of NAFLD, cirrhosis, and HCC.63-65 It is estimated that the pooled mean prevalence of NAFLD in children is 7.6% overall and 34.2% in obese children, with a higher in prevalence in males compared to females.66 Computer simulations based on the current estimates of NAFLD, obesity and diabetes, project that, from 2015-2030, the respective NAFLD and NASH prevalence will rise by 21% and 63%.67 In addition, the prevalence of NAFLD-related decompensated cirrhosis, HCC, and deaths will increase by 168%, 137%, and 178%, respectively.
ALD
ALD encompasses alcoholic hepatitis, steatosis, steatohepatitis and fibrosis/cirrhosis. A lack of accurate reporting and referral bias limit the ability to estimate ALD incidence and prevalence globally but alcohol misuse is responsible for an estimated 27% percent of deaths from liver disease and 30% of liver cancer deaths worldwide.68-70 In the US, the prevalence of ALD is estimated to be 4.7% and alcohol is estimated to account for approximately 20% (NHANES) to 36% (private-insurance claims data) of cirrhosis cases.15, 71-73 Hispanics have a higher prevalence of ALD cirrhosis (16.9/100,000) compared to whites (11.1/100,000) or blacks (9.9/100,000).74
The relationship between alcohol use and liver disease is well established, with prospective cohorts demonstrating that cirrhosis incidence is strongly related to amount of alcohol consumed.75, 76 The estimated burden of ALD can therefore be indirectly estimated by alcohol consumption patterns. An estimated 2.3 billion people drink alcohol worldwide and alcohol is consumed by more than half the population in the Americas, Europe and the Western Pacific.72 The highest per-capita alcohol consumption levels are observed in Eastern Europe (8.1L/woman, 24.9L/man) and lowest in North Africa/Middle East (0.2L/woman, 1.7L/man).68 Existing data suggest ALD prevalence is increasing. In China, the proportion of all hospitalizations for liver disease attributable to alcohol more than doubled from 2002-2013.77 Population-based data from Denmark demonstrated that the incidence of hospitalizations for alcoholic hepatitis increased from 1999-2008.78 Similarly, the prevalence of alcohol-related cirrhosis in the North America is increasing, particularly among young persons.14, 71, 79, 80 The global burden of ALD may continue to escalate given increases in alcohol consumption worldwide (per capita consumption from 5.5L in 2005 to 6.4L in 2016).81, 82
Other CLD Etiologies
Primary biliary cholangitis (PBC) occurs primarily in women and has a reported annual prevalence and incidence of 1.9-40.2 and 0.3-5.8 per 100,000, respectively.83-85 In a systematic review of PBC, all longitudinal studies identified reported increases in the PBC prevalence over time.85 Similarly, data from a multicenter PBC consortium within the US demonstrates a stable incidence (4.2-4.3/100,000 person-years) and rising prevalence (21.7-39.2/100,000 population) from 2006-2014.86 PBC is diagnosed at earlier stages over time suggesting additional data are needed to understand longitudinal differences in its prevalence in the context of widespread antimitochondrial antibody test utilization.87
Similar to PBC, most studies on the epidemiology of primary sclerosing cholangitis (PSC) come from North America and Europe. The reported prevalence of PSC ranges from 0-16.2/100,000 person-years and its incidence ranges from 0-1.3/100,000.85 Prevalence estimates vary from zero cases among Alaskan Natives84 to higher estimates from the UK (12.7/100,000), general US population (13.6/100,000), and Sweden (16.2/100,000).88-90 The incidence of PSC is slowly increasing in populations in both North America and Europe.85
Epidemiologic data on autoimmune hepatitis (AIH) are scant and there are no population-based data from the US. It occurs primarily in women and its prevalence has been reported to be 4/100,000 in Singapore and 16-24 per 100,000 in Europe.91-95 The annual incidence of AIH has been reported to be 0.7/100,000 in Israel and 2.0/100,000 in New Zealand.94, 95 In Denmark, the incidence of AIH has nearly doubled from 1994-2012.91
While hereditary hemochromatosis mutations are relatively common, particularly in Northern Europeans, C282Y homozygosity is weakly penetrant, rarely developing clinical manifestations (<1%), making it a rare cause of CLD.96-99 Wilson Disease also occurs infrequently (worldwide prevalence 3/100,000), although a recent study from the UK reported a higher prevalence of individuals carrying two mutant pathogenic ATP7B alleles (14/100,000).100 This apparent discrepancy may be due to incomplete penetrance of ATP7B mutations or under-diagnosis. The prevalence of Wilson Disease among persons with undergoing evaluation for elevated liver enzymes at a referral center is estimated to be 160/100,000.101
Liver Cancer
Hepatocellular Carcinoma (HCC)
Patients with cirrhosis from any etiology are at high risk for HCC, with annual incidence ranging from 1-4%.102 In 2015, there were an estimated 854,000 incident liver cancer cases (75% increase from 1990) and 810,000 cancer-related deaths worldwide.103 The most significant increases in liver cancer-related mortality were in North America, Europe, and Australia. Many countries, including China, have experienced a decrease in liver cancer burden due to improved HBV vaccination and decreased aflatoxin exposure.104
HCC incidence in the US has been rising in recent years but the rate of rise has slowed.105 Based on data from the US Cancer Statistics registry, the age-adjusted incidence of HCC increased from 4.4/100,000 in 2000 to 6.7/100,000 in 2012.106 However the annual increase of 4.5% between 2000-2009 slowed to 0.7% per year from 2010-2012, suggesting a possible impending plateau of new HCC cases. HCC incidence stratified by age categories demonstrated that increases in HCC incidence were most pronounced in those in the peak-HCV cohort (born 1945-1965) and is decreasing among younger individuals and Asian-Americans.106, 107 However, observed improvements in HCC incidence related to implementation of HBV vaccination and therapy or HCV treatment programs may be mitigated or even overshadowed in the future by increasing childhood obesity and early-onset NASH, and increasing ALD among younger individuals. Finally, HCV therapy is likely to improve survival with cirrhosis, however these patients remain at persistent risk of HCC.108
There are also several notable trends in HCC incidence by race/ethnicity. Age-adjusted incidence rates are higher among Hispanics (6.3/100,000) and blacks (5.0/100,000) compared to non-Hispanic whites (2.4/100,000).109 HCC incidence is also increasing disproportionately in Hispanics (4.7%/year since 2000) compared to other racial/ethnic groups.109, 110 Furthermore, blacks and Hispanics diagnosed with HCC are less likely to have it detected at an early stage.111
There were approximately 150,000 HCC-related deaths (age-adjusted 2.3/100,000) in the US from 1999-2017 (Table 4). Mortality rates in 2017 were highest among males, Asian/Pacific Islanders, and those age 75-84 years. HCC mortality has increased overall (Figure 1) and in nearly every group with the exception of Asian/Pacific Islanders.2 Racial disparities in HCC mortality persist and studies suggest that this may in part be related to treatment process failures, with lower rates of early detection and lower odds of curative treatment, including liver transplantation, among racial/ethnic minorities compared to non-Hispanic whites.111
Table 4.
Potential Data Sources for Liver Disease Epidemiologic Research
| Data source | Country/Region | Strengths | Weaknesses |
|---|---|---|---|
| National Health and Nutrition Examination Survey (NHANES) | United States |
|
|
| Veterans Affairs (VA) | United States |
|
|
| Medicare | United States |
|
|
| Medicaid | United States |
|
|
| Private-insurance claims data |
United States |
|
|
| National Inpatient Sample (NIS) | United States |
|
|
| National Readmissions Database (NRD) | United States |
|
|
| Medical Expenditure Panel Survey (MEPS) | United States |
|
|
| Surveillance Epidemiology, and End Results (SEER) program | United States |
|
|
| US Cancer Statistics registry | United States |
|
|
| Organ Procurement and Transplant Network (OPTN) | United States |
|
|
| National patient registries | Denmark, Finland, Iceland, Norway, Sweden |
|
|
| Clinical Practice Research Datalink (CPRD) | United Kingdom |
|
|
| European Liver Transplant Registry (ELTR) | Europe |
|
|
| NORDCAN database | Denmark, Finland, Faroe Islands, Greenland, Iceland, Norway, Sweden |
|
|
| Global Burden of Disease (GBD) project | Worldwide |
|
|
Complications of CLD and Cirrhosis
Hepatic Decompensation
As CLD progresses, patients develop complications of hepatocellular dysfunction and portal hypertension that contribute to liver-related morbidity and mortality.112 Approximately 4-12% patients with cirrhosis develop at least one decompensating event annually and the most common decompensating events are ascites, variceal hemorrhage, and hepatic encephalopathy (HE). 71, 113, 116 As many as 40% of patients with cirrhosis will develop HE within 5 years of observation.117 The presence of these complications are important prognostic indicators with significantly increased mortality in decompensated compared to compensated cirrhosis.118 Decompensation events also require significant healthcare utilization – hepatic encephalopathy and ascites/spontaneous bacterial peritonitis (SBP) each accounted for 59,000 emergency department visits in 2014.119
Infection
Patients with cirrhosis have a significantly increased risk of sepsis and infection-related mortality, likely as a result of immune dysfunction, changes in gut microbiota and bacterial translocation.120 Among hospitalized patients, the prevalence of bacterial infections ranges from 25-50%, with wide regional variability in the type and microbiology of infections.121 There is a high prevalence of multidrug resistant bacteria in infected cirrhosis patients (34%) with the highest prevalence in India (73%).121
Acute Kidney Injury (AKI)
AKI is common in patients with cirrhosis, particularly those with ascites.112, 122 The physiology of portal hypertension increases the risk of AKI due to splanchnic vasodilation and decreased intravascular blood volume with resulting renal vasoconstriction. Hepatorenal syndrome (HRS), the abrupt and severe form of AKI that occurs in the absence of nephrotoxic medications and shock and despite a trial of volume explanation, portends the worst prognosis.122 Among patients with ascites followed prospectively, AKI will ultimately develop in half, with a 1-year probability of 24%.123 AKI has been reported in approximately 20% of all hospitalized patients with cirrhosis and is more common in those with ascites, SBP and gastrointestinal bleeding.124, 125 The burden of HRS has changed somewhat as definitions have evolved; its annual incidence ranges from 8-18% and 13-27% of patients with AKI qualify as HRS.123, 126, 127
Frailty
Physical frailty is common in cirrhosis due to muscle wasting and poor nutrition. Frailty in those with liver disease is a strong predictor of mortality independent of traditional prognostic tools like the Child Pugh score and Model for End-Stage Liver Disease.128 Frailty can be assessed in a number of ways including grip strength, chair stands per second, gait speed, balance time and activities of daily living.129, 130 Cirrhosis-related frailty, especially in combination with cognitive dysfunction or psychoactive medications, increases the risk of falls and decreased health-related quality of life.131-134 Estimates of its burden are highly variable due to heterogeneity in case definitions, but the reported prevalence of frailty among patients on the liver transplant waitlist ranges from 17-43%, and is more common among older patients and those with NAFLD.130, 135
Acute on Chronic Liver Failure (ACLF)
ACLF, defined as deterioration of liver function and extrahepatic organ failure, is an increasingly recognized entity associated with considerable morbidity and mortality.136, 137 Definitions for ACLF are heterogeneous, with some based on physiologic markers of end-organ dysfunction and others based purely on clinical assessments of organ failure (coma, hemodialysis, mechanical ventilation).138 Algorithms for use in administrative data have been used but have never been validated. The prevalence of ACLF is reported to range from 24-40% among hospitalized patients with cirrhosis.136, 139, 140 In a large cohort of US veterans with ACLF, deaths occurred in 26% and 40% of patients at one and three months, respectively.140 In a multicenter European cohort defining and grading ACLF based on the sequential organ failure assessment (SOFA), 28-day mortality was approximately 30% and ranged from 22-77% depending on the number of organs affected.136, 141
Health Care Utilization, Direct Costs and Disability for CLD and Cirrhosis in the US
Health Care Utilization
In the US in 2014, CLD accounted for over 1,000,000 outpatient and 325,000 emergency department (ED) visits (40% increase in ED visits since 2006).119 Cirrhosis-related hospitalizations have increased every year between 2001-2011, with these increases outpacing both congestive heart failure and chronic obstructive pulmonary disease.142, 143 Inpatient care, which comprises approximately 40% of all costs, accounts for the majority of expenditures in CLD.144 Annual inpatient costs for cirrhosis patients increased from $4.8 billion ($13,079/hospitalization) to $9.8 billion ($15,193/hospitalization) from 2001-2011.142 Readmissions are common (25.8% at 30-days),145conditional on the severity of liver disease,146 and are particularly related to HE and alcohol-use disorder. There are several ongoing efforts to reduce readmissions in the future, although few have proven successful to date.147 In addition to inpatient costs, overall annual health care costs from CLD were $29.9 billion overall, with 3.5-fold higher per-patient costs among patients with ACLF.142, 144
Disability and Health-related Quality of Life (HRQOL)
CLD is also associated with disability and significant decreases in HRQOL.53, 148 Compared to age-matched controls, CLD patients have higher levels of unemployment (65.3% vs 31.4%), inability to work due to disability (30.5% vs 6.6%), and reported days of disability per year (10.2 vs 3.4).144 Among individuals ≥65 years with cirrhosis, approximately one in four report their health as “poor” and 40% have at least one impaired activity of daily living (ADL).133 Decrements in HRQOL can be seen in CLD even without cirrhosis; however, HRQOL is lowest in those with cirrhosis complications including hepatic decompensation.149
Directions for Future Research
Complicating our knowledge of the present burden of disease is the lack of prospective, national registries and our reliance on variable definitions of CLD in administrative data. Existing data sources for identifying burden and trends in CLD each have unique strengths and limitations and there is ample opportunity for improvement (Table 4). Prospective population-based patient registries, such as the Danish National Patient Registry (DNPR), serve as benchmarks for epidemiologic data sources.150 While resource-intensive, these can yield invaluable findings on the incidence, prevalence, natural history, and real-world treatment of CLD.91, 151, 152
In the US, while comprehensive national registries are lacking, there have been efforts to prospectively track specific liver diseases and complications of cirrhosis. For instance, a multisite Cirrhosis Quality Collaborative (CQC), funded by the American Association for the Study of Liver Diseases (AASLD), has set out to track patient-reported outcomes (PROs) in cirrhosis and define standard processes of care and quality metrics.153 There are several specific areas in need of higher quality data such as ALD; its burden is likely underestimated due to late referral of patients with liver disease and underreporting of alcohol use.79
Finally, the paucity of reliable data on CLD in many developing nations cripples efforts to improve care.6 This could be addressed by building infrastructure to define, track and manage cirrhosis through global partnerships. The WHO, for instance, has announced plans to establish centers of excellence to improve data collection on CLD and HCC throughout the world.154 In addition, global surveillance of CLD could be improved by emulating existing models in cancer, where international registries have been immensely important for research on epidemiology, interventions and outcomes.155
Conclusion
Recent data demonstrate that the burden of CLD, cirrhosis, and its complications remains substantial. Improvements in the prevalence and outcomes of viral hepatitis contrast with adverse trends in the prevalence and complications of alcohol-related and nonalcoholic fatty liver diseases. Investment and innovation are vital to maintain adequate surveillance of CLD and develop strategies to reduce its burden. Liver disease, liver cancer, viral hepatitis, organ transplantation, obesity, and alcohol use are all projected to have decreases in NIH funding, which may hamper these efforts.119 Continued attempts to track the burden of liver disease will help identify priorities for clinical care improvements, research investment, and health policy initiatives.
Acknowledgments
Grant Support: This research was supported in part by NIH grant T32 DK007634 (AM). Dr. Singal’s research is supported by U01CA230694 and R01MD12565. Elliot Tapper receives funding from the National Institutes of Health through the Michigan Institute for Clinical and Health Research (KL2TR002241). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ACLF
acute on chronic liver failure
- ADL
activities of daily living
- ALD
alcohol-related liver disease
- APRI
aspartate aminotransferase to platelet ratio index
- CCA
cholangiocarcinoma
- CDC
Centers for Disease Control and Prevention
- CLD
chronic liver disease
- CQC
Cirrhosis Quality Collaborative
- DAAs
direct acting antivirals
- DNPR
Danish National Patient Registry
- ED
emergency department
- FIB-4
fibrosis-4
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCUP
Healthcare Cost and Utilization Project
- HCV
hepatitis C virus
- HRQOL
health-related quality of life
- ICD
International Classification of Diseases
- MELD
model for end-stage liver disease
- MEPS
Medical Expenditure Panel Survey
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NCHS
National Center for Health Statistics
- NEDS
National Emergency Department Sample
- NHANES
National Health and Nutrition Examination Survey
- NRD
National Readmission Database
- PEth
phosphatidylethanol
- PROs
patient-reported outcomes
- SBP
spontaneous bacterial peritonitis
- SOFA
Sequential Organ Failure Assessment
- UNOS
United Network for Organ Sharing
- USPSTF
United States Preventive Services Task Force
- VA
Veterans Affairs
- WHO
World Health Organization
- YLD
years lived with disability
Footnotes
Disclosures: Amit Singal has received grant funding from Abbvie and has served on advisory boards for Gilead and Abbvie. Elliot Tapper has received grant funding (to the University of Michigan) from Gilead and Valeant, consulted for Novartis and Allergan, and has served on advisory boards for Salix/Bausch and Mallinckrodt.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med 2014;12:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asrani SK, Larson JJ, Yawn B, et al. Underestimation of liver-related mortality in the United States. Gastroenterology 2013;145:375–82 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol 2018;69:718–735. [DOI] [PubMed] [Google Scholar]
- 6.Byass P The global burden of liver disease: a challenge for methods and for public health. BMC Med 2014;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong MCS, Huang JLW, George J, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol 2019;16:57–73. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol 2019;70:531–544. [DOI] [PubMed] [Google Scholar]
- 9.Liangpunsakul S, Haber P, McCaughan GW. Alcoholic Liver Disease in Asia, Europe, and North America. Gastroenterology 2016;150:1786–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol 2019;70:151–171. [DOI] [PubMed] [Google Scholar]
- 11.Choi J, Han S, Kim N, et al. Increasing burden of liver cancer despite extensive use of antiviral agents in a hepatitis B virus-endemic population. Hepatology 2017;66:1454–1463. [DOI] [PubMed] [Google Scholar]
- 12.Hadler SC, Fuqiang C, Averhoff F, et al. The impact of hepatitis B vaccine in China and in the China GAVI Project. Vaccine 2013;31 Suppl 9:J66–72. [DOI] [PubMed] [Google Scholar]
- 13.Flemming JA, Dewit Y, Mah JM, et al. Incidence of cirrhosis in young birth cohorts in Canada from 1997 to 2016: a retrospective population-based study. Lancet Gastroenterol Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 14.Beste LA, Leipertz SL, Green PK, et al. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology 2015; 149:1471–1482 e5; quiz e17-8. [DOI] [PubMed] [Google Scholar]
- 15.Scaglione S, Kliethermes S, Cao G, et al. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol 2015;49:690–6. [DOI] [PubMed] [Google Scholar]
- 16.Kanwal F, Kramer JR, Duan Z, et al. Trends in the Burden of Nonalcoholic Fatty Liver Disease in a United States Cohort of Veterans. Clin Gastroenterol Hepatol 2016;14:301–8 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon AM, Green PK, Berry K, et al. Transformation of hepatitis C antiviral treatment in a national healthcare system following the introduction of direct antiviral agents. Aliment Pharmacol Ther 2017;45:1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Younossi Z, Tacke F, Arrese M, et al. Global Perspectives on Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis. Hepatology 2018. [Google Scholar]
- 19.Sayiner M, Koenig A, Henry L, et al. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin Liver Dis 2016;20:205–14. [DOI] [PubMed] [Google Scholar]
- 20.Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017;153:996–1005. e1. [DOI] [PubMed] [Google Scholar]
- 21.Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013-2016. Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mokdad AH, Forouzanfar MH, Daoud F, et al. Global burden of diseases, injuries, and risk factors for young people's health during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2016;387:2383–401. [DOI] [PubMed] [Google Scholar]
- 23.Falla AM, Hofstraat SHI, Duffell E, et al. Hepatitis B/C in the countries of the EU/EEA: a systematic review of the prevalence among at-risk groups. BMC Infect Dis 2018;18:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Global hepatitis report 2017. Volume 2019, 2017. [Google Scholar]
- 25.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014;160:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161–176. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). Viral Hepatitis Surveillance United States, 2016. Volume 2019, 2018. [Google Scholar]
- 28.Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016;388:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong RJ, Jain MK, Therapondos G, et al. Race/ethnicity and insurance status disparities in access to direct acting antivirals for hepatitis C virus treatment. Am J Gastroenterol 2018;113:1329–1338. [DOI] [PubMed] [Google Scholar]
- 30.IMS Health. Medicines Use and Spending Shifts In: Informatics IIfH, ed. Parsippany, NJ: IMS Institute, 2015. [Google Scholar]
- 31.Elsharkawy A, El-Raziky M, El-Akel W, et al. Planning and prioritizing direct-acting antivirals treatment for HCV patients in countries with limited resources: Lessons from the Egyptian experience. J Hepatol 2018;68:691–698. [DOI] [PubMed] [Google Scholar]
- 32.Scott N, Olafsson S, Gottfreethsson M, et al. Modelling the elimination of hepatitis C as a public health threat in Iceland: A goal attainable by 2020. J Hepatol 2018;68:932–939. [DOI] [PubMed] [Google Scholar]
- 33.Chikovani I, Ompad DC, Uchaneishvili M, et al. On the way to Hepatitis C elimination in the Republic of Georgia-Barriers and facilitators for people who inject drugs for engaging in the treatment program: A formative qualitative study. PLoS One 2019;14:e0216123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain MK, Rich NE, Ahn C, et al. Evaluation of a Multifaceted Intervention to Reduce Health Disparities in Hepatitis C Screening: A Pre-Post Analysis. Hepatology 2019. [DOI] [PubMed] [Google Scholar]
- 35.Cooke GS, Andrieux-Meyer I, Applegate TL, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2019;4:135–184. [DOI] [PubMed] [Google Scholar]
- 36.Yehia BR, Schranz AJ, Umscheid CA, et al. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014;9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiessing L, Ferri M, Grady B, et al. Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS One 2014;9:e103345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall AD, Cunningham EB, Nielsen S, et al. Restrictions for reimbursement of interferon-free direct-acting antiviral drugs for HCV infection in Europe. Lancet Gastroenterol Hepatol 2018;3:125–133. [DOI] [PubMed] [Google Scholar]
- 39.National Viral Hepatitis Roundtable. Hepatitis C: The State of Medicaid Access In: Innovation CfHLaP, ed: Harvard Law School, 2017. [Google Scholar]
- 40.Kim HS, Yang JD, El-Serag HB, et al. Awareness of Chronic Viral Hepatitis in the United States: An Update from National Health and Nutrition Examination Survey. J Viral Hepat 2019. [DOI] [PubMed] [Google Scholar]
- 41.Kasting ML, Giuliano AR, Reich RR, et al. Hepatitis C Virus Screening Trends: Serial Cross-Sectional Analysis of the National Health Interview Survey Population, 2013-2015. Cancer Epidemiol Biomarkers Prev 2018;27:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldberg D, Ditah IC, Saeian K, et al. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology 2017;152:1090–1099 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morse A, Barritt ASt, Jhaveri R Individual State Hepatitis C Data Supports Expanding Screening Beyond Baby Boomers to All Adults. Gastroenterology 2018;154:1850–1851 e2. [DOI] [PubMed] [Google Scholar]
- 44.Moon AM, Green PK, Berry K, et al. Transformation of hepatitis C antiviral treatment in a national healthcare system following the introduction of direct antiviral agents. Alimentary pharmacology & therapeutics 2017;45:1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ott JJ, Horn J, Krause G, et al. Time trends of chronic HBV infection over prior decades - A global analysis. J Hepatol 2017;66:48–54. [DOI] [PubMed] [Google Scholar]
- 46.Le MH, Yeo YH, Cheung R, et al. Chronic hepatitis B prevalence among foreign-born and US-born adults in the United States, 1999-2016. Hepatology. [DOI] [PubMed] [Google Scholar]
- 47.Brown RS Jr, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology 2016;63:319–33. [DOI] [PubMed] [Google Scholar]
- 48.Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386:1546–55. [DOI] [PubMed] [Google Scholar]
- 49.Uribe LA, Nguyen N, Kim L, et al. Rates of Treatment Eligibility in Follow-Up of Patients with Chronic Hepatitis B (CHB) Across Various Clinical Settings Who Were Initially Ineligible at Presentation. Dig Dis Sci 2016;61:618–25. [DOI] [PubMed] [Google Scholar]
- 50.Hutin Y, Nasrullah M, Easterbrook P, et al. Access to Treatment for Hepatitis B Virus Infection - Worldwide, 2016. MMWR Morb Mortal Wkly Rep 2018;67:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kabbany MN, Conjeevaram Selvakumar PK, Watt K, et al. Prevalence of Nonalcoholic Steatohepatitis-Associated Cirrhosis in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am J Gastroenterol 2017;112:581–587. [DOI] [PubMed] [Google Scholar]
- 52.Allen AM, Therneau TM, Larson JJ, et al. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology 2018;67:1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balp M-M, Krieger N, Przybysz R, et al. The burden of nonalcoholic steatohepatitis (NASH) among patients from Europe: a real-world patient-reported outcomes study. JHEP Reports 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 55.Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- 56.Rich NE, Oji S, Mufti AR, et al. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:198–210 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–95. [DOI] [PubMed] [Google Scholar]
- 58.Bedogni G, Miglioli L, Masutti F, et al. Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology 2007;46:1387–91. [DOI] [PubMed] [Google Scholar]
- 59.Zhou YJ, Li YY, Nie YQ, et al. Natural course of nonalcoholic fatty liver disease in southern China: a prospective cohort study. J Dig Dis 2012; 13:153–60. [DOI] [PubMed] [Google Scholar]
- 60.Wong VW, Wong GL, Yeung DK, et al. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. J Hepatol 2015;62:182–9. [DOI] [PubMed] [Google Scholar]
- 61.Zelber-Sagi S, Salomone F, Yeshua H, et al. Non-high-density lipoprotein cholesterol independently predicts new onset of non-alcoholic fatty liver disease. Liver Int 2014;34:e128–35. [DOI] [PubMed] [Google Scholar]
- 62.Chang Y, Jung HS, Cho J, et al. Metabolically Healthy Obesity and the Development of Nonalcoholic Fatty Liver Disease. Am J Gastroenterol 2016;111:1133–40. [DOI] [PubMed] [Google Scholar]
- 63.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 2012;307:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmermann E, Gamborg M, Holst C, et al. Body mass index in school-aged children and the risk of routinely diagnosed non-alcoholic fatty liver disease in adulthood: a prospective study based on the Copenhagen School Health Records Register. BMJ Open 2015;5:e006998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hagstrom H, Stal P, Hultcrantz R, et al. Overweight in late adolescence predicts development of severe liver disease later in life: A 39years follow-up study. J Hepatol 2016;65:363–8. [DOI] [PubMed] [Google Scholar]
- 66.Anderson EL, Howe LD, Jones HE, et al. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013;59:160–8. [DOI] [PubMed] [Google Scholar]
- 69.Sheron N. Alcohol and liver disease in Europe--Simple measures have the potential to prevent tens of thousands of premature deaths. J Hepatol 2016;64:957–67. [DOI] [PubMed] [Google Scholar]
- 70.Collaborators GBDCoD. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mellinger JL, Shedden K, Winder GS, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology 2018;68:872–882. [DOI] [PubMed] [Google Scholar]
- 72.World Health Organization. Global status report on alcohol and health, 2018.
- 73.Wong T, Dang K, Ladhani S, et al. Prevalence of Alcoholic Fatty Liver Disease Among Adults in the United States, 2001-2016. JAMA 2019;321:1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang AL, Vadhavkar S, Singh G, et al. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med 2008;168:649–56. [DOI] [PubMed] [Google Scholar]
- 75.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011;141:1572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simpson RF, Hermon C, Liu B, et al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Health 2019;4:e41–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang A, Chang B, Sun Y, et al. Disease spectrum of alcoholic liver disease in Beijing 302 Hospital from 2002 to 2013: A large tertiary referral hospital experience from 7422 patients. Medicine (Baltimore) 2017;96:e6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Damgaard Sandahl T Alcoholic hepatitis. Dan Med J 2014;61:B4755. [PubMed] [Google Scholar]
- 79.Shah ND, Ventura-Cots M, Abraldes JG, et al. Alcohol-related Liver Disease is Rarely Detected at Early Stages Compared With Liver Diseases of Other Etiologies Worldwide. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flemming JA, Dewit Y, Mah JM, et al. Incidence of cirrhosis in young birth cohorts in Canada from 1997 to 2016: a retrospective population-based study. The Lancet Gastroenterology & Hepatology 2018. [DOI] [PubMed] [Google Scholar]
- 81.Collaborators GBDA. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018;392:1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mehta G, Sheron N. No safe level of alcohol consumption - Implications for global health. J Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- 83.Kim WR, Lindor KD, Locke GR 3rd, et al. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology 2000; 119:1631–6. [DOI] [PubMed] [Google Scholar]
- 84.Hurlburt KJ, McMahon BJ, Deubner H, et al. Prevalence of autoimmune liver disease in Alaska Natives. Am J Gastroenterol 2002;97:2402–7. [DOI] [PubMed] [Google Scholar]
- 85.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol 2012;56:1181–8. [DOI] [PubMed] [Google Scholar]
- 86.Lu M, Zhou Y, Haller IV, et al. Increasing Prevalence of Primary Biliary Cholangitis and Reduced Mortality With Treatment. Clin Gastroenterol Hepatol 2018; 16:1342–1350 e1. [DOI] [PubMed] [Google Scholar]
- 87.Murillo Perez CF, Goet JC, Lammers WJ, et al. Milder disease stage in patients with primary biliary cholangitis over a 44D-year period: A changing natural history. Hepatology 2018;67:1920–1930. [DOI] [PubMed] [Google Scholar]
- 88.Kingham JG, Kochar N, Gravenor MB. Incidence, clinical patterns, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology 2004;126:1929–30. [DOI] [PubMed] [Google Scholar]
- 89.Lindkvist B, Benito de Valle M, Gullberg B, et al. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology 2010;52:571–7. [DOI] [PubMed] [Google Scholar]
- 90.Bambha K, Kim WR, Talwalkar J, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology 2003;125:1364–9. [DOI] [PubMed] [Google Scholar]
- 91.Gronbaek L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol 2014;60:612–7. [DOI] [PubMed] [Google Scholar]
- 92.Feld JJ, Heathcote EJ. Epidemiology of autoimmune liver disease. J Gastroenterol Hepatol 2003;18:1118–28. [DOI] [PubMed] [Google Scholar]
- 93.Werner M, Prytz H, Ohlsson B, et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol 2008;43:1232–40. [DOI] [PubMed] [Google Scholar]
- 94.Ngu JH, Bechly K, Chapman BA, et al. Population-based epidemiology study of autoimmune hepatitis: a disease of older women? J Gastroenterol Hepatol 2010;25:1681–6. [DOI] [PubMed] [Google Scholar]
- 95.Delgado JS, Vodonos A, Malnick S, et al. Autoimmune hepatitis in southern Israel: a 15-year multicenter study. J Dig Dis 2013; 14:611–8. [DOI] [PubMed] [Google Scholar]
- 96.Adams PC, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 2005;352:1769–78. [DOI] [PubMed] [Google Scholar]
- 97.Beutler E, Felitti VJ, Koziol JA, et al. Penetrance of 845G--> A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet 2002;359:211–8. [DOI] [PubMed] [Google Scholar]
- 98.Adams PC, Passmore L, Chakrabarti S, et al. Liver diseases in the hemochromatosis and iron overload screening study. Clin Gastroenterol Hepatol 2006;4:918–23; quiz 807. [DOI] [PubMed] [Google Scholar]
- 99.McCune CA, Al-Jader LN, May A, et al. Hereditary haemochromatosis: only 1% of adult HFEC282Y homozygotes in South Wales have a clinical diagnosis of iron overload. Hum Genet 2002;111:538–43. [DOI] [PubMed] [Google Scholar]
- 100.Coffey AJ, Durkie M, Hague S, et al. A genetic study of Wilson's disease in the United Kingdom. Brain 2013;136:1476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tapper EB, Rahni DO, Arnaout R, et al. The overuse of serum ceruloplasmin measurement. Am J Med 2013;126:926 e1–5. [DOI] [PubMed] [Google Scholar]
- 102.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745–50. [DOI] [PubMed] [Google Scholar]
- 103.Global Burden of Disease Liver Cancer C, Akinyemiju T, Abera S, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun Z, Chen T, Thorgeirsson SS, et al. Dramatic reduction of liver cancer incidence in young adults: 28 year follow-up of etiological interventions in an endemic area of China. Carcinogenesis 2013;34:1800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Altekruse SF, Henley SJ, Cucinelli JE, et al. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.White DL, Thrift AP, Kanwal F, et al. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology 2017;152:812–820 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rich NE, Yopp AC, Singal AG, et al. Hepatocellular Carcinoma Incidence Is Decreasing Among Younger Adults in the United States. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ioannou GN, Green PK, Beste LA, et al. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol 2018;69:1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El-Serag HB, Lau M, Eschbach K, et al. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med 2007;167:1983–9. [DOI] [PubMed] [Google Scholar]
- 110.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264–1273 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rich NE, Hester C, Odewole M, et al. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol 2019;17:551–559 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217–31. [DOI] [PubMed] [Google Scholar]
- 113.D'Amico G, Morabito A, D'Amico M, et al. Clinical states of cirrhosis and competing risks. J Hepatol 2018;68:563–576. [DOI] [PubMed] [Google Scholar]
- 114.D'Amico G, Pasta L, Morabito A, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther 2014;39:1180–93. [DOI] [PubMed] [Google Scholar]
- 115.Ratib S, Fleming KM, Crooks CJ, et al. Causes of death in people with liver cirrhosis in England compared with the general population: a population-based cohort study. Am J Gastroenterol 2015;110:1149–58. [DOI] [PubMed] [Google Scholar]
- 116.Fleming KM, Aithal GP, Card TR, et al. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther 2010;32:1343–50. [DOI] [PubMed] [Google Scholar]
- 117.Tapper EB, Parikh ND, Sengupta N, et al. A risk score to predict the development of hepatic encephalopathy in a population-based cohort of patients with cirrhosis. 2018;68:1498–1507. [DOI] [PubMed] [Google Scholar]
- 118.Fleming KM, Aithal GP, Card TR, et al. All-cause mortality in people with cirrhosis compared with the general population: a population-based cohort study. Liver Int 2012;32:79–84. [DOI] [PubMed] [Google Scholar]
- 119.Peery AF, Crockett SD, Murphy CC, et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019;156:254–272 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Piano S, Brocca A, Mareso S, et al. Infections complicating cirrhosis. Liver Int 2018;38 Suppl 1:126–133. [DOI] [PubMed] [Google Scholar]
- 121.Piano S, Singh V, Caraceni P, et al. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology 2019;156:1368–1380 e10. [DOI] [PubMed] [Google Scholar]
- 122.Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol 2015;62:968–74. [DOI] [PubMed] [Google Scholar]
- 123.Montoliu S, Balleste B, Planas R, et al. Incidence and prognosis of different types of functional renal failure in cirrhotic patients with ascites. Clin Gastroenterol Hepatol 2010;8:616–22; quiz e80. [DOI] [PubMed] [Google Scholar]
- 124.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008;48:2064–77. [DOI] [PubMed] [Google Scholar]
- 125.Piano S, Rosi S, Maresio G, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol 2013;59:482–9. [DOI] [PubMed] [Google Scholar]
- 126.Gines A, Escorsell A, Gines P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993;105:229–36. [DOI] [PubMed] [Google Scholar]
- 127.Martin-Llahi M, Guevara M, Torre A, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology 2011;140:488–496 e4. [DOI] [PubMed] [Google Scholar]
- 128.Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Laube R, Wang H, Park L, et al. Frailty in advanced liver disease. Liver Int 2018;38:2117–2128. [DOI] [PubMed] [Google Scholar]
- 131.Tapper EB, Risech-Neyman Y, Sengupta N. Psychoactive Medications Increase the Risk of Falls and Fall-related Injuries in Hospitalized Patients With Cirrhosis. Clin Gastroenterol Hepatol 2015;13:1670–5. [DOI] [PubMed] [Google Scholar]
- 132.Tapper EB, Baki J, Parikh ND, et al. Frailty, Psychoactive Medications, and Cognitive Dysfunction Are Associated With Poor Patient-Reported Outcomes in Cirrhosis. Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rakoski MO, McCammon RJ, Piette JD, et al. Burden of cirrhosis on older Americans and their families: analysis of the health and retirement study. Hepatology 2012;55:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lai JC, Dodge JL, Sen S, et al. Functional decline in patients with cirrhosis awaiting liver transplantation: Results from the functional assessment in liver transplantation (FrAILT) study. Hepatology 2016;63:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lai JC, Volk ML, Strasburg D, et al. Performance-Based Measures Associate With Frailty in Patients With End-Stage Liver Disease. Transplantation 2016;100:2656–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–37, 1437 e1-9. [DOI] [PubMed] [Google Scholar]
- 137.Arroyo V, Moreau R, Jalan R, et al. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J Hepatol 2015;62: S131–43. [DOI] [PubMed] [Google Scholar]
- 138.Hernaez R, Sola E, Moreau R, et al. Acute-on-chronic liver failure: an update. Gut 2017;66:541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bajaj JS, O'Leary JG, Reddy KR, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014;60:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hernaez R, Kramer JR, Liu Y, et al. Prevalence and short-term mortality in a national US cohort with acute-on-chronic liver failure. J Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 141.Gustot T, Fernandez J, Garcia E, et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015;62:243–52. [DOI] [PubMed] [Google Scholar]
- 142.Allen AM, Kim WR, Moriarty JP, et al. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology 2016;64:2165–2172. [DOI] [PubMed] [Google Scholar]
- 143.Asrani SK, Hall L, Hagan M, et al. Trends in Chronic Liver Disease-Related Hospitalizations: A Population-Based Study. Am J Gastroenterol 2019;114:98–106. [DOI] [PubMed] [Google Scholar]
- 144.Stepanova M, De Avila L, Afendy M, et al. Direct and Indirect Economic Burden of Chronic Liver Disease in the United States. Clin Gastroenterol Hepatol 2017;15:759–766 e5. [DOI] [PubMed] [Google Scholar]
- 145.Tapper EB, Bonder A, Cardenas A. Preventing and treating acute kidney injury among hospitalized patients with cirrhosis and ascites: a narrative review. The American journal of medicine 2016;129:461–467. [DOI] [PubMed] [Google Scholar]
- 146.Tapper EB, Halbert B, Mellinger J. Rates of and Reasons for Hospital Readmissions in Patients With Cirrhosis: A Multistate Population-based Cohort Study. Clin Gastroenterol Hepatol 2016;14:1181–1188 e2. [DOI] [PubMed] [Google Scholar]
- 147.Tapper EB, Volk M. Strategies to Reduce 30-Day Readmissions in Patients with Cirrhosis. Curr Gastroenterol Rep 2017; 19:1. [DOI] [PubMed] [Google Scholar]
- 148.Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol 2011;106:1646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tapper E, Kanwal F, Asrani S, et al. Patient Reported Outcomes in Cirrhosis: A Scoping Review of the Literature. Hepatology (Baltimore, Md.) 2017. [DOI] [PubMed] [Google Scholar]
- 150.Boscarino JA, Lu M, Moorman AC, et al. Predictors of poor mental and physical health status among patients with chronic hepatitis C infection: the Chronic Hepatitis Cohort Study (CHeCS). Hepatology 2015;61:802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jepsen P, Ott P, Andersen PK, et al. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010;51:1675–82. [DOI] [PubMed] [Google Scholar]
- 152.Deleuran T, Vilstrup H, Jepsen P. Decreasing Mortality Among Danish Alcoholic Cirrhosis Patients: A Nationwide Cohort Study. Am J Gastroenterol 2016;111:817–22. [DOI] [PubMed] [Google Scholar]
- 153.Kanwal F, Volk M, Singal A, et al. Improving quality of health care for patients with cirrhosis. Gastroenterology 2014; 147:1204–7. [DOI] [PubMed] [Google Scholar]
- 154.World Health Organization. WHO calls for better monitoring of viral hepatitis and liver cancer. Volume 2019, 2018. [Google Scholar]
- 155.Parkin DM. The evolution of the population-based cancer registry. Nat Rev Cancer 2006;6:603–12. [DOI] [PubMed] [Google Scholar]
- 156.Ratib S, West J, Fleming KM. Liver cirrhosis in England-an observational study: are we measuring its burden occurrence correctly? BMJ Open 2017;7:e013752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577–1586. [DOI] [PubMed] [Google Scholar]
- 158.Kanerva N, Sandboge S, Kaartinen NE, et al. Higher fructose intake is inversely associated with risk of nonalcoholic fatty liver disease in older Finnish adults. Am J Clin Nutr 2014;100:1133–8. [DOI] [PubMed] [Google Scholar]
- 159.Riquelme A, Arrese M, Soza A, et al. Non-alcoholic fatty liver disease and its association with obesity, insulin resistance and increased serum levels of C-reactive protein in Hispanics. Liver Int 2009;29:82–8. [DOI] [PubMed] [Google Scholar]
- 160.Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology 2016;63:388–97. [DOI] [PubMed] [Google Scholar]