Abstract
Comparisons of systemic exposure to toxicants during monitored cigarette smoking, electronic cigarette (e-cigarette) use and abstention are needed to enhance our understanding of the risks of e-cigarette use (vaping). In a crossover study, we measured 10 mercapturic acid metabolites of volatile organic compounds (VOCs) in 24-h urine samples collected from 36 dual users (8 women) of e-cigarettes and cigarettes during two days of ad libitum vaping or cigarette-only use, and two days of enforced abstention. Concentrations of VOC metabolites were higher during smoking compared to vaping, except for the methylating agents metabolite. The fold-difference in concentrations when smoking relative to vaping ranged from 1.31 (1.06-1.61) (GM, 95% CI) (1,3-butadiene) to 7.09 (5.88-8.54) (acrylonitrile). Metabolites of acrylamide [fold difference of 1.21 (1.03-1.43)] and benzene [1.46 (1.13-1.90)] were higher during vaping compared to abstention. The 1,3-butadiene and propylene oxide metabolites were higher in variable-power tank users compared to users of cig-a-likes. E-cigarettes expose users to lower levels of toxic VOCs compared to cigarette smoking, supporting their harm reduction potential among smokers. However, some e-cigarettes expose users to VOCs such as acrylamide, benzene, and propylene oxide, and may pose health risks to nonsmoking users. The results of our study will inform regulators in assessing e-cigarettes with respect to the balance between its potential harm reduction for adult smokers and risk to nonsmoking users.
INTRODUCTION
Although nicotine is the primary addictive substance in tobacco smoke (1), and despite concerns about nicotine’s potential deleterious effects (2, 3), the morbidity and mortality of smoking are attributable primarily to non-nicotine toxicants such as volatile organic compounds (VOCs) (4, 5). As such, cigarettes and other combustible tobacco products are the most harmful on the continuum of risk of tobacco products. On the other hand, noncombustible tobacco products, such as electronic cigarettes (e-cigarettes), are believed to be less harmful because their emissions contain low levels or none of the many toxicants present in tobacco smoke (6). Based on these observations, the harm reduction potential of e-cigarettes has been proposed by some, including the Food and Drug Administration (7), as a way to reduce the public health burden of smoking. A further important consideration is the inherent toxicity of e-cigarettes and the potential risks they pose to nonsmokers who vape.
In general, e-cigarette users have lower systemic exposure to toxicants compared to smokers, which supports the idea that e-cigarettes have a lower risk profile relative to cigarettes (6). These findings have been largely derived from studies in which e-cigarette-naïve smokers switched from cigarettes to e-cigarettes and were followed prospectively in their naturalistic settings (i.e., switching studies), and include assessing changes in biomarkers of tobacco-related toxicants (8-11). Additionally, cross-sectional comparisons of biomarkers of toxicant exposure in smokers and e-cigarette users who used their products in their naturalistic settings have been reported (12-15). Important limitations of these studies are that product use (and patterns of use) are not controlled or monitored, are self-reported, and potentially include use of multiple tobacco products, making it difficult to ascertain e-cigarette use and quantify the magnitude of e-cigarette-associated exposures and the risk of e-cigarette-only use. More accurate assessment of toxicant exposure associated with e-cigarette-only use relative to cigarette smoking or no product use will enhance our understanding of the public health risk of e-cigarettes. One between-subject industry study found no significant differences in levels of VOC metabolites in smokers who used blu® e-cigarettes exclusively for 5 days in a research setting compared to smokers who were abstinent over that same period (11).
The primary objective of the present study was to assess one aspect of e-cigarette safety by measuring urinary biomarkers of toxic and/or carcinogenic VOCs in a crossover (within-subject) study where each participant used e-cigarettes only, cigarettes only, and had a period of enforced nicotine and tobacco product abstention. Biochemical measures included mercapturic acid metabolites of acrolein, which is believed to be a major contributor to smoking-induced cardiopulmonary disease (5) and is a thermal breakdown product of glycerin in e-cigarettes (16), benzene, a known human carcinogen (17) that can be formed from e-cigarette constituents such as benzoic acid (18), and propylene oxide, an International Agency for Research on Cancer (IARC) class 2B carcinogen (possibly carcinogenic to humans) (19) that can be formed by thermal degradation of propylene glycol (20).
MATERIALS AND METHODS
Study Design
We conducted a two-arm counterbalanced, crossover study in 36 healthy dual users of e-cigarettes and cigarettes. Participants were asked to smoke cigarettes or vape e-cigarettes only for periods of seven days, each. During each arm, use of the assigned product and subjective measures were tracked by self-report for four days as outpatients, followed by three days on a research ward where product use was monitored or abstention enforced, and biosamples were collected for biomarker measurement. The hospital phase of each arm included a single-dose pharmacokinetic study on the first day of admission (21), followed by two days of ad libitum access to the assigned product. Further, two days of enforced abstention on the research ward were added immediately after the second arm to examine excretion of toxicant biomarkers during a period of no tobacco product use. In this study, we present biomarkers of toxicants measured in spot urine samples collected at baseline (before product assignment), in 24-hour urine samples collected during the two days of ad libitum access to the assigned product during each arm and in 24-hour urine samples collected on the second of 2 days of abstention. Known elimination half-lives of the VOC mercapturic acid metabolites measured are 8 hours for the acrylonitrile metabolite (CNEMA (22)), 9 hours for the acrolein (3-HPMA (23)) and benzene metabolites (PMA, (24)) and 14 hours for the acrylamide metabolite (AAMA, (23) (see the Analytical Chemistry section for full names of the metabolites). Although VOC metabolite levels derived from noncompliant smoking during the at-home period of the e-cigarette arm would potentially carryover to levels measured during e-cigarette use on the research ward, or smoking during the second arm on the research ward would potentially carryover to the abstention arm, 2 to 3 days of abstention from cigarettes are sufficient to observe substantial reductions in urinary mercapturic acid levels to near baseline (25).
Participants
Thirty-six healthy participants recruited via Craigslist.com, Facebook, flyers, and college campus newspapers, completed the study. Participants had to be at least 21 years old, smoke at least 5 cigarettes per day (CPD) over the past 30 days and use the same e-cigarette device at least once daily on 15 of the past 30 days, use e-liquids of at least 6 mg/mL nicotine concentration; have no intention to quit smoking or vaping, and at screening, have saliva cotinine and expired carbon monoxide (CO) of ≥ 50 ng/mL and ≥ 5 ppm, respectively, negative pregnancy test (if a woman), and negative urine illicit drug test, except for cannabis. The study was conducted in accordance with the principles of the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board at the University of California San Francisco. Written, informed consent was obtained from each participant and all participants were financially compensated.
Products
Participants used their usual brands of e-cigarettes and cigarettes, provided by the study. The types of e-cigarettes used by study participants were as follows: cig-a-likes (n=12 participants); fixed-power tanks (n=15), variable-power tanks (n=6) and, pod e-cigarettes (n=3, all JUULs). Details of the products have been described elsewhere (21).
Experimental procedure
We screened participants for eligibility in an outpatient research clinic where consent was obtained, questionnaires completed, and saliva samples were collected for cotinine measurement. Eligible participants returned for an orientation visit at which time the sequence of products was assigned, and a four-day supply of the product assigned to the first arm was dispensed for at-home use. During the orientation visit, we also collected a spot urine sample for baseline assessment of exposure biomarkers.
On Day 5 of each arm, participants were admitted to one of the Clinical Research Center (CRC) research smoking rooms at the Zuckerberg San Francisco General Hospital (ZSFG) between 7:00 to 8:00 AM. We asked participants to abstain from all tobacco product use starting at 10 PM the night before the hospital admission and we measured expired CO to verify abstinence from cigarettes (≤ 5 ppm). An intravenous (IV) line for blood sampling was placed in the forearm followed by a standardized session of product use to examine differences in nicotine pharmacokinetics and subjective effects between e-cigarettes and cigarette use (21).
During the second and third days of admission (of each arm), participants had ad libitum access to the assigned product from 8 AM to midnight (hospital policy prohibits smoking in the hospital smoking rooms after midnight). For these two days of ad libitum use (referred hereafter as ad libitum Day 1 and Day 2), cig-a-like users were given their usual brand of cartridges, fixed-power or variable-power tank users were provided with their usual brand of e-liquid in a vial, which they used with their own device, and JUUL users were provided with their usual flavor of JUUL pods. During the cigarette arm, participants were given their usual brand of cigarettes. Since the participants were dual users, in order to meet their required daily nicotine intake, we anticipated an increased consumption of the assigned product compared to self-reported consumption of that product during the screening visit. Accordingly, participants were given an additional number of cartridges, e-liquid vials, pods or cigarettes during these two ad libitum access days. All remaining products were collected by study nurses at midnight. No participant ran out of their cartridges, e-liquids, pods or cigarettes during the day. After the second study arm, participants remained for an additional two days, during which they abstained from any nicotine or tobacco products. Twenty-four-hour urine was collected on the two days of ad libitum access of each arm and on the last day of abstention.
Analytical chemistry
We measured mercapturic acid metabolites of VOCs in urine samples using liquid chromatography with tandem mass spectrometry (LC-MS/MS) by a method previously described (26). The mercapturic acid metabolites measured were as follows, shown as the mercapturic acid metabolite [abbreviation, parent compound(s), limit of quantitation (LOQ)]: 2-hydroxypropylmercapturic acid [2-HPMA, propylene oxide, 0.5 ng/mL]; 3-hydroxypropylmercapturic acid [3-HPMA, acrolein, 1 ng/mL]; 2-carbamoylethylmercapturic acid [AAMA, acrylamide, 0.5 ng/mL]; 2-cyanoethylmercapturic acid [CNEMA, acrylonitrile, 0.5 ng/mL]; 2-hydroxyethylmercapturic acid [HEMA, acrylonitrile, vinyl chloride, ethylene oxide, 0.5 ng/mL]; 3-hydroxy-1-methylpropylmercapturic acid [HPMMA, crotonaldehyde, 1 ng/mL]; sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid [MHBMA-1+2, 1,3-butadiene, 0.1 ng/mL]; 4-hydroxy-2-buten-1-yl-mercapturic acid [MHBMA-3, 1,3-butadiene, 0.1 ng/mL]; methylmercapturic acid [MMA, methylating agents such as 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone (NNK), N-nitrosodimethylamine (NDMA), and endogenous methylating agents, 5 ng/mL]; and phenylmercapturic acid [PMA, benzene, 0.1 ng/mL].
Statistical analysis
We imputed biomarker values below the LOQ using the LOQ divided by the square root of 2 (LOQ/√2) and we normalized urinary biomarker concentrations by creatinine concentrations, including biomarkers measured in 24-h urine. We normalized the 24-h urine samples for creatinine since the spot urine sample collected at baseline had to be normalized for creatinine. However, differences in 24-h urinary biomarker levels across arms were consistent with or without creatinine correction. Since the concentrations were approximately log-normally distributed, biomarker concentrations were log-transformed.
Our primary analysis was a comparison of Day 2 urinary biomarker concentrations over the three conditions via repeated measures ANCOVA. We focused on Day 2 because, as stated before, VOC metabolite concentrations derived from noncompliant smoking during the at-home phase of the e-cigarette arm would be reduced substantially by Day 2 on the research ward. We conducted post-hoc pairwise comparisons between study arms and applied Bonferonni correction for multiple comparisons. Covariates included sex and treatment order and a random effect of participants. We calculated geometric mean and 95% confidence intervals for the relative ratio of concentrations in cigarette vs e-cigarette arms and e-cigarette vs abstention. We evaluated differences in urinary biomarker levels on Day 2 between e-cigarette device types with Wilcoxon Rank-Sum Tests. We computed Spearman correlation coefficients between 24-h biomarker concentrations and corresponding 24-h area under the plasma nicotine concentration-time curve (AUC) for the e-cigarette and cigarette arms, respectively, as a way to examine the relationship between product use on the research ward and VOC exposure. (Plasma nicotine AUC is reported in another manuscript (27)). Finally, we computed the frequencies of participants with e-cigarette to abstention relative biomarker level ratios of at least 1.25 or at least 1.50 by device type and flavor category, representing at least 25% and 50% higher biomarker levels from e-cigarette use compared to abstention.
All analyses were considered significant at two-tailed p-values of < 0.05 and were conducted in SAS Version 9.4 and R Version 3.4.
RESULTS
Of 36 participants enrolled (8 women), 2 were Asian, 3 were African American/Black, 4 were Latino, 22 were White, and 5 were mixed-race. On average, participants smoked 12.9 ± 6.4 (mean ± SD) CPD, used e-cigarettes on 22.6 ± 7.3 days of the past 30 days, and on days that they used the e-cigarette, they used the e-cigarette 8.1 ± 7.2 times. Average screening saliva cotinine was 189 ± 92.8 ng/mL (range 119 to 248 ng/mL). Eight participants (22.2%) used a dessert/candy flavored e-liquid/e-cigarette, 5 (13.9%) used a fruit flavor, 5 (13.9%) used a menthol flavor and 18 (50%) used a tobacco flavor.
VOC exposure from e-cigarettes vs cigarettes
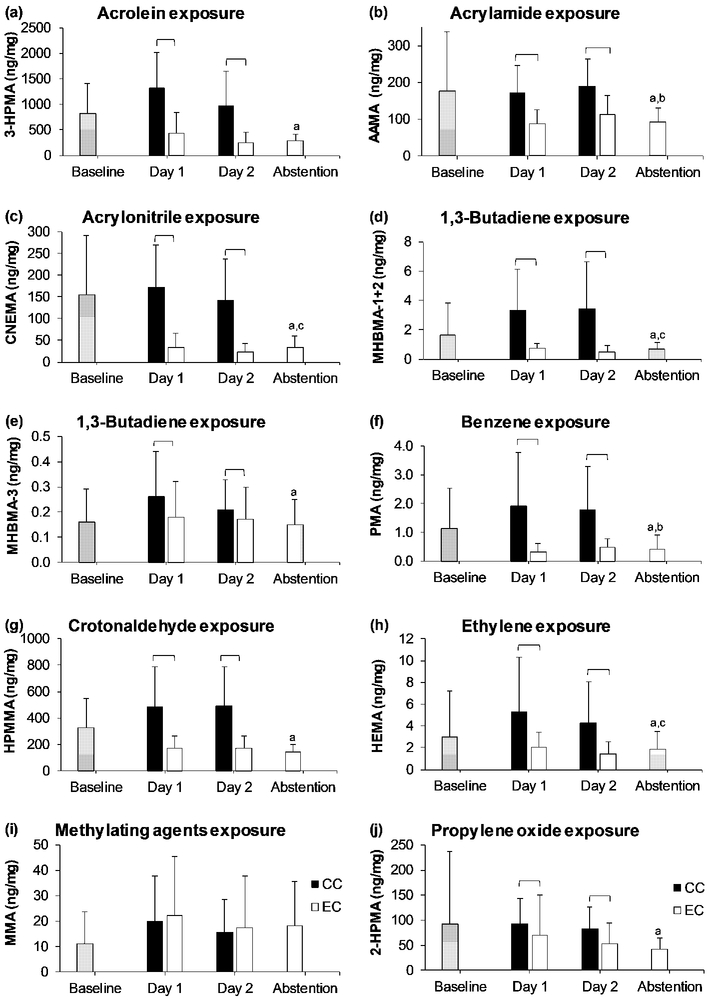
Concentrations of metabolites of VOCs collected in spot urine samples at baseline and in 24-hour urine collected during ad libitum use of the assigned product on Day 1 and Day 2, respectively, and during enforced abstention are shown in Figure 1a-1j. Table 1 shows these VOC metabolite concentrations in 24-hour urine collected on Day 2 during e-cigarette or cigarette use and during abstention. Concentrations of all VOC metabolites were significantly higher during both days of cigarette use compared to e-cigarette use (all p values < 0.001) except for MMA (the metabolite of methylating agents) (Figure 1a-1j). The geometric means of the fold-difference in concentrations of these VOC metabolites when using cigarettes relative to that of e-cigarettes ranged from 1.31 for MHBMA-3 (one of the butadiene metabolites) to 7.09 for CNEMA (acrylonitrile metabolite) (Table 2).
FIGURE 1.
Concentration of metabolites of volatile organic compounds at baseline before study products were assigned, during Days 1 and 2 of each arm, and during abstention from nicotine and tobacco products. Square brackets = significant difference between combustible cigarettes (CC) and e-cigarettes (EC); a = significantly lower than combustible cigarette use on Day 2; b = significantly lower than e-cigarette use on Day 2; c = significantly higher than e-cigarette use on Day 2. 2-HPMA = 2-hydroxypropylmercapturic acid; 3-HPMA = 3-hydroxypropylmercapturic acid; AAMA = 2-carbamoylethylmercapturic acid; CNEMA = 2-cyanoethylmercapturic acid; HEMA = 2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide); HPMMA = 3-hydroxy-1-methyl-propylmercapturic acid; MHBMA-1+2 = sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid; MHBMA-3 = 4-hydroxy-2-buten-1-yl-mercapturic acid; MMA = methylmercapturic acid; and, PMA = phenylmercapturic acid.
TABLE 1.
Concentrations of mercapturic acid metabolites of volatile organic compounds (VOCs) measured in 24-hour urine collected during cigarette smoking, e-cigarette use, and abstention.
| Exposure (Biomarker) (ng/mg creatinine) |
Cigarette (Mean, SD) |
E-cigarette (Mean, SD) |
Abstention (Mean, SD) |
CC to EC ratio (GM, 95% CI) |
Ratio of EC to Abstention (GM, 95% CI) |
||
|---|---|---|---|---|---|---|---|
| All subjects | CC at Arm 2* | EC at Arm 2** | |||||
| Acrolein (3-HPMA) | 965.7 (674.3) | 258.8 (195.2) | 279.9 (140.0) | 3.70 (2.85-4.79) a | 0.82 (0.67-1.01) | 0.81 (0.62-1.04) | 0.83 (0.61-1.14) |
| Acrylamide (AAMA) | 190.2 (72.8) | 112.9 (50.8) | 92.8 (37.2) | 1.70 (1.50-1.92) a | 1.21 (1.03-1.43) b | 1.15 (0.93-1.43) | 1.27 (1.01-1.61) |
| Acrylonitrile (CNEMA) | 140.9 (95.5) | 21.8 (19.7) | 32.9 (27.6) | 7.09 (5.88-8.54) a | 0.64 (0.56-0.74) c | 0.50 (0.42-0.59) | 0.81 (0.71-0.92) |
| 1,3-Butadiene (MHBMA-1+2) | 3.43 (3.23) | 0.51 (0.42) | 0.70 (0.40) | 5.80 (3.73-9.00) a | 0.63 (0.48-0.82) c | 0.47 (0.32-0.68) | 0.83 (0.61-1.13) |
| 1,3-Butadiene (MHBMA-3) | 0.21 (0.12) | 0.17 (0.13) | 0.15 (0.10) | 1.31 (1.06-1.61) a | 1.05 (0.84-1.30) | 0.92 (0.66-1.29) | 1.18 (0.91-1.53) |
| Benzene (PMA) | 1.77 (1.52) | 0.48 (0.31) | 0.42 (0.48) | 3.21 (2.53-4.07) a | 1.46 (1.13-1.90) b | 0.94 (0.64-1.37) | 2.18 (1.71-2.77) |
| Crotonaldehyde (HPMMA) | 489.9 (297.7) | 168.1 (95.36) | 145.6 (55.3) | 2.77 (2.34-3.29) a | 1.08 (0.94-1.25) | 1.23 (0.99-1.54) | 0.97 (0.82-1.14) |
| Ethylene oxide (HEMA) | 4.28 (3.82) | 1.47 (1.06) | 1.84 (1.64) | 2.55 (2.10-3.10) a | 0.82 (0.71-0.95) c | 0.78 (0.66-0.93) | 0.86 (0.69-1.08) |
| Methylating agent (MMA) | 15.51 (12.84) | 17.30 (20.32) | 18.0 (17.5) | 1.01 (0.85-1.20) | 0.94 (0.77-1.15) | 1.18 (0.97-1.44) | 0.77 (0.57-1.04) |
| Propylene oxide (2-HPMA) | 82.7 (43.5) | 53.6 (41.1) | 41.7 (22.9) | 1.69 (1.33-2.16) a | 1.17 (0.98-1.39) | 1.08 (0.88-1.33) | 1.24 (0.95-1.64) |
Notes: * CC at Arm 2 = participants who were assigned to smoke combustible cigarettes during Arm 2 immediately before the abstention days;
EC at Arm 2 = participants who were assigned to vape e-cigarettes during Arm 2, immediately before the abstention days;
= significant difference between combustible cigarettes and e-cigarettes;
= significantly higher during e-cigarette use compared to abstention;
= significantly lower during e-cigarette use compared to abstention; 2-HPMA = 2-hydroxypropylmercapturic acid; 3-HPMA = 3-hydroxypropylmercapturic acid; AAMA = 2-carbamoylethylmercapturic acid; CNEMA = 2-cyanoethylmercapturic acid; HEMA = 2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide); HPMMA = 3-hydroxy-1-methyl-propylmercapturic acid; MHBMA-1+2 = sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid; MHBMA-3 = 4-hydroxy-2-buten-1-yl-mercapturic acid; MMA = methylmercapturic acid; and, PMA = phenylmercapturic acid. During Day 2 of the cigarette arm, per cent below the limit of quantitation (LOQ) for each metabolite was as follows: 3-HPMA (0%); AAMA (0%); CNEMA (0%); MHBMA-1+2 (2.8%); MHBMA-3 (58.3%); PMA (0%); HPMMA (0%); HEMA (0%); MMA (36.1%); 2-HPMA (0%). Per cent below LOQ on Day 2 of the e-cigarette arm were as follows: 3-HPMA (0%); AAMA (0%); CNEMA (0%); MHBMA-1+2 (41.7%); MHBMA-3 (83.3%); PMA (22.2%); HPMMA (0%); HEMA (0%); MMA (33.3%); 2-HPMA (0%). Per cent below LOQ during abstinence were as follows: 3-HPMA (0%); AAMA (0%); CNEMA (0%); MHBMA-1+2 (80.6%); MHBMA-3 (97.2%); PMA (30.6%); HPMMA (0%); HEMA (25.0%); MMA (33.3%); 2-HPMA (0%).
TABLE 2.
Concentrations of mercapturic acid metabolites of volatile organic compounds (VOCs) measured in 24-hour urine during e-cigarette use
| Exposure (Biomarker) (ng/mg creatinine) |
Cig-a-like (mean, SD) (n = 12) |
Fixed-power (mean, SD) (n = 15) |
Variable-power (mean, SD) (n = 6) |
Pod (mean, SD) (n = 3) |
Difference (p value) |
|
|---|---|---|---|---|---|---|
| All included |
Pods excluded |
|||||
| Acrolein (3-HPMA) | 260.6 (236.0) | 238.7 (189.8) | 340.7 (173.1) | 188.0 (49.9) | 0.081 | 0.100 |
| Acrylamide (AAMA) | 117.4 (65.9) | 103.2 (43.6) | 118.8 (35.1) | 131.9 (58.9) | 0.325 | 0.311 |
| Acrylonitrile (CNEMA) | 27.5 (28.6) | 20.8 (15.6) | 17.1 (3.79) | 13.1 (13.4) | 0.442 | 0.950 |
| 1,3-Butadiene (MHBMA-1+2) | 0.51 (0.36) | 0.57 (0.50) | 0.50 (0.43) | 0.19 (0.07) | 0.055 | 0.730 |
| 1,3-Butadiene (MHBMA-3) | 0.16 (0.09) | 0.15 (0.09) | 0.24 (0.23) | 0.08 (0.05) | 0.019 | 0.363 |
| Benzene (PMA) | 0.54 (0.22) | 0.48 (0.41) | 0.38 (0.20) | 0.39 (0.19) | 0.081 | 0.129 |
| Crotonaldehyde (HPMMA) | 152.4 (68.0) | 194.2 (128.6) | 132.5 (30.5) | 171.3 (71.6) | 0.477 | 0.242 |
| Ethylene oxide (HEMA) | 1.56 (0.64) | 1.35 (1.25) | 1.73 (1.31) | 1.27 (1.40) | 0.090 | 0.124 |
| Methylating agent (MMA) | 15.7 (9.3) | 18.8 (27.0) | 18.7 (23.7) | 13.6 (12.8) | 0.601 | 0.865 |
| Propylene oxide (2-HPMA) | 34.2 (17.4) | 62.6 (55.0) | 75.8 (26.4) | 42.2 (19.6) | <0.001 | 0.001 |
Notes: 2-HPMA = 2-hydroxypropylmercapturic acid; 3-HPMA = 3-hydroxypropylmercapturic acid; AAMA = 2-carbamoylethylmercapturic acid; CNEMA = 2-cyanoethylmercapturic acid; HEMA = 2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide); HPMMA = 3-hydroxy-1-methyl-propylmercapturic acid; MHBMA-1+2 = sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid; MHBMA-3 = 4-hydroxy-2-buten-1-yl-mercapturic acid; MMA = methylmercapturic acid; and, PMA = phenylmercapturic acid.
VOC exposure from e-cigarettes vs abstinence
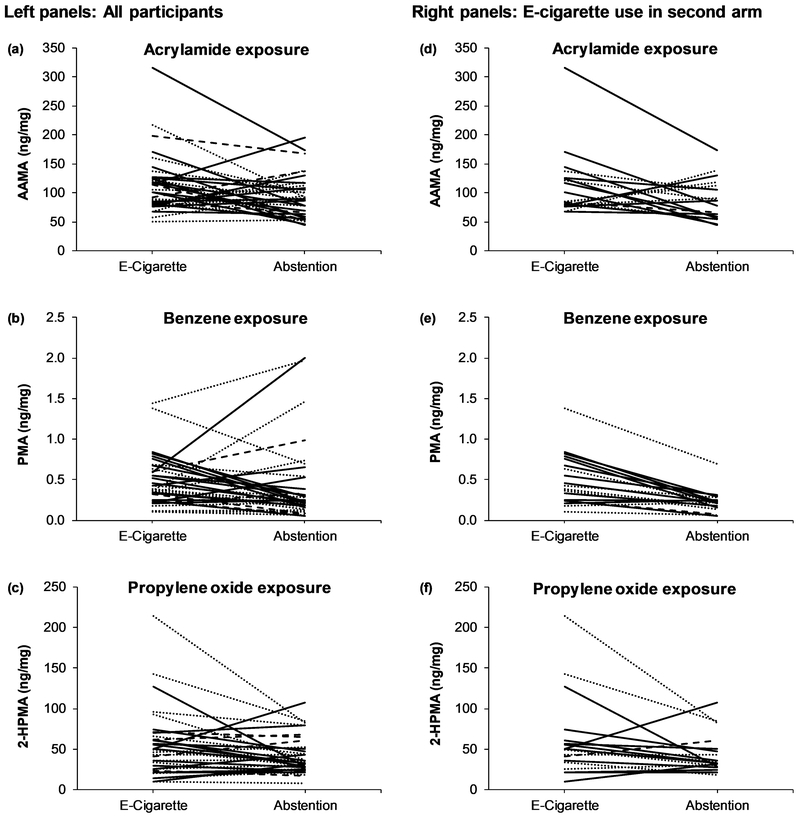
Within-subject concentrations of metabolites of acrylamide (AAMA) and benzene (PMA) were significantly higher during e-cigarette use (Day 2 of the e-cigarette arm) compared to abstention, with average within-subject fold-difference of 1.21 (p = 0.019) and 1.46 (p = 0.006), respectively among all participants (Table 1). Considering the absolute concentration of each metabolite, compared to abstention, most participants had higher levels of AAMA (frequency = 63.9%), PMA (66.7%), and 2-HPMA (58.3%) during e-cigarette use (Figure 2a, 2b, and 2c). Metabolites of acrylonitrile (CNEMA), 1,3-butadiene (MHBMA-1+2), and ethylene oxide (HEMA) were significantly lower during e-cigarette use than during abstention, with average fold-differences of 0.64 (p < 0.001), 0.63 (p = 0.001), and 0.82 (p = 0.010), respectively.
FIGURE 2.
Within-subject changes in mercapturic acid metabolites of acrylamide (a), benzene (b), and propylene oxide (c) for all participants and for participants who used e-cigarettes during arm 2, immediately before two days of abstention, showing acrylamide (d), benzene (e), and propylene oxide (f). Solid black line = cig-a-like; dotted black line = fixed-power tank e-cigarette; solid grey line = variable-power tank e-cigarette; broken grey line = JUUL pod e-cigarette. 2-HPMA = 2-hydroxypropylmercapturic acid; AAMA = 2-carbamoylethylmercapturic acid; and, PMA = phenylmercapturic acid.
The order of assigned products influenced the magnitude of changes in the concentrations of some metabolites measured during e-cigarette use compared to abstention, indicative of potential carryover effect on biomarker levels from smoking cigarettes (Table 1 and Figure 2d, 2e, and 2f). The within-subject fold-difference from e-cigarette use compared to abstention in concentrations of metabolites of acrylamide, benzene and propylene oxide were higher in participants who used e-cigarettes during the second arm (i.e., immediately before the abstinence days) compared to those who smoked cigarettes during the second arm (Table 1). Of note, the average concentration of the benzene metabolite (PMA) was 2.18-fold higher during e-cigarette use relative to abstention among participants who were assigned e-cigarettes during the second arm while it was a 0.94-fold-difference in participants who smoked cigarettes during the second arm.
VOC exposure across different types of e-cigarettes
In Table 2, we present concentrations of VOC metabolites across users of different types of e-cigarettes during the e-cigarette arm. When all participants were considered, the 1,3-butadiene metabolite (MHBMA-3) and the propylene oxide metabolite (2-HPMA) were significantly different across e-cigarette devices, with higher levels in variable-power tank users. When pod users were excluded from the analysis, 2-HPMA was the only VOC metabolite that differed significantly by device type.
Correlations between VOCs and nicotine exposure
Spearman correlation coefficients between VOC metabolite levels and plasma nicotine AUC over 24 h for Day 2 of the cigarette and e-cigarette arms are shown in Table 3. For the cigarette arm, except for MHBMA-1+2 (1,3-butadiene) and MMA (methylating agents), correlation between plasma nicotine AUC and urinary VOC metabolites was moderate and statistically significant. For the e-cigarette arm, the correlation between plasma nicotine AUC and 2-HPMA (propylene oxide) was small and statistically significant but others were not significant.
TABLE 3.
Spearman correlation coefficients between mercapturic acid metabolites of volatile organic compounds (VOCs) measured in urine collected during a day of combustible cigarette smoking or e-cigarette use and area under the plasma nicotine concentration-time curve (AUC) measured during the corresponding day.
| Exposure (Biomarker) | Plasma nicotine during cigarette smoking |
Plasma nicotine during e-cigarette use |
||
|---|---|---|---|---|
| Spearman | p value | Spearman | p value | |
| Acrolein (3-HPMA) | 0.42 | 0.011 | 0.31 | 0.066 |
| Acrylamide (AAMA) | 0.47 | 0.004 | 0.13 | 0.456 |
| Acrylonitrile (CNEMA) | 0.53 | 0.001 | 0.14 | 0.428 |
| 1,3-Butadiene (MHBMA-1+2) | 0.27 | 0.107 | 0.18 | 0.295 |
| 1,3-Butadiene (MHBMA-3) | 0.59 | <0.001 | 0.11 | 0.530 |
| Benzene (PMA) | 0.44 | 0.007 | 0.18 | 0.307 |
| Crotonaldehyde (HPMMA) | 0.43 | 0.008 | 0.29 | 0.084 |
| Ethylene oxide (HEMA) | 0.20 | 0.244 | 0.16 | 0.352 |
| Methylating agent (MMA) | 0.41 | 0.012 | 0.17 | 0.331 |
| Propylene oxide (2-HPMA) | 0.36 | 0.029 | 0.37 | 0.027 |
Notes: Correlations were between mercapturic acid levels measured in 24-hour urine and the area under the plasma nicotine concentration-time curve over 24 hours. 2-HPMA = 2-hydroxypropylmercapturic acid; 3-HPMA = 3-hydroxypropylmercapturic acid; AAMA = 2-carbamoylethylmercapturic acid; CNEMA = 2-cyanoethylmercapturic acid; HEMA = 2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide); HPMMA = 3-hydroxy-1-methyl-propylmercapturic acid; MHBMA-1+2 = sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid; MHBMA-3 = 4-hydroxy-2-buten-1-yl-mercapturic acid; MMA = methylmercapturic acid; and, PMA = phenylmercapturic acid.
Evaluation of elevated exposures from e-cigarette use by device type and flavors
We present the frequency of participants whose biomarker levels were at least 25% (Table 4, Section A) or 50% (Table 4, Section B) higher during e-cigarette use relative to abstention by device types and e-liquid flavors. Notably, 21 (58.3%) of 36 participants had at least 50% higher PMA (benzene) levels during e-cigarette use compared to abstention, including 8 of 12 (66.7%) cig-a-like users, 8 of 15 (53.3%) fixed-power tank users, 3 of 6 (50%) variable-power tank users, and 2 of 3 (66.7%) pod users. Across flavors, 3 of 8 (37.5%) users of dessert/candy e-liquids, 5 of 5 (100%) users of fruit flavors, 3 of 5 (60%) users of menthol flavor, and 11 of 18 (61.1%) users of tobacco flavors had PMA levels that were at least 50% higher during e-cigarette use than abstention.
TABLE 4.
Frequency of participants whose biomarker levels were at least 25% or 50% higher during e-cigarette use relative to abstention
| Exposure (Biomarker) | All | E-cigarette type | Flavor type | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cig-a-like | Fixed- power |
Variable- power |
Pod | Dessert or Candy |
Fruit | Menthol | Tobacco | ||
| Sample size (N) | 36 | 12 | 15 | 6 | 3 | 8 | 5 | 5 | 18 |
| A. Participants with ≥25% increase in VOC biomarker levels during e-cigarette use compared to abstinence (n, %) | |||||||||
| Acrolein (3-HPMA) | 8 (22.2) | 2 (16.7) | 4 (26.7) | 2 (33.3) | 0 (0.0) | 1 (12.5) | 2 (40.0) | 1 (20.0) | 4 (22.2) |
| Acrylamide (AAMA) | 17 (47.2) | 6 (50.0) | 6 (40.0) | 3 (50.0) | 2 (66.7) | 3 (37.5) | 3 (60.0) | 3 (60.0) | 9 (50.0) |
| Acrylonitrile (CNEMA) | 1 (2.8) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (20.0) | 0 (0.0) |
| 1,3-Butadiene (MHBMA-1+2) | 7 (19.4) | 2 (16.7) | 3 (20.0) | 2 (33.3) | 0 (0.0) | 2 (25.0) | 1 (20.0) | 2 (40.0) | 2 (11.1) |
| 1,3-Butadiene (MHBMA-3) | 13 (36.1) | 4 (33.3) | 5 (33.3) | 4 (66.7) | 0 (0.0) | 3 (37.5) | 2 (40.0) | 3 (60.0) | 6 (33.3) |
| Benzene (PMA) | 23 (63.9) | 9 (75.0) | 9 (60.0) | 3 (50.0) | 2 (66.7) | 4 (50.0) | 5 (100.0) | 4 (80.0) | 12 (66.7) |
| Crotonaldehyde (HPMMA) | 15 (41.7) | 5 (41.7) | 6 (40.0) | 2 (33.3) | 2 (66.7) | 5 (62.5) | 2 (40.0) | 5 (100) | 6 (33.3) |
| Ethylene oxide (HEMA) | 6 (16.7) | 2 (16.7) | 2 (13.3) | 2 (33.3) | 0 (0.0) | 1 (12.5) | 2 (40.0) | 1 (20.0) | 1 (5.6) |
| Methylating agent (MMA) | 11 (30.6) | 1 (8.3) | 7 (46.7) | 3 (50.0) | 0 (0.0) | 3 (37.5) | 4 (80.0) | 3 (60.0) | 4 (22.2) |
| Propylene oxide (2-HPMA) | 18 (50.0) | 6 (50.0) | 8 (53.3) | 3 (50.0) | 1 (33.3) | 2 (25.0) | 5 (100.0) | 2 (40.0) | 10 (55.6) |
| B. Participants with ≥50% increase in VOC biomarker levels during e-cigarette use compared to abstinence (n, %) | |||||||||
| Acrolein (3-HPMA) | 4 (11.1) | 0 (0.0) | 2 (13.3) | 2 (33.3) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (20.0) | 3 (16.7) |
| Acrylamide (AAMA) | 12 (33.3) | 5 (41.7) | 4 (26.7) | 2 (33.3) | 1 (33.3) | 3 (37.5) | 0 (0.0) | 3 (60.0) | 6 (33.3) |
| Acrylonitrile (CNEMA) | 1 (2.8) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (20.0) | 0 (0.0) |
| 1,3-Butadiene (MHBMA-1+2) | 6 (16.7) | 2 (16.7) | 2 (13.3) | 2 (33.3) | 0 (0.0) | 2 (25.0) | 1 (20.0) | 2 (40.0) | 1 (5.6) |
| 1,3-Butadiene (MHBMA-3) | 10 (27.8) | 3 (25.0) | 4 (26.7) | 3 (50.0) | 0 (0.0) | 2 (25.0) | 2 (40.0) | 2 (40.0) | 5 (27.8) |
| Benzene (PMA) | 21 (58.3) | 8 (66.7) | 8 (53.3) | 3 (50.0) | 2 (66.7) | 3 (37.5) | 5 (100.0) | 3 (60.0) | 11 (61.1) |
| Crotonaldehyde (HPMMA) | 8 (22.2) | 2 (16.7) | 5 (33.3) | 0 (0.0) | 1 (33.3) | 2 (25.0) | 1 (20.0) | 2 (40.0) | 4 (22.2) |
| Ethylene oxide (HEMA) | 4 (11.1) | 2 (16.7) | 1 (6.7) | 1 (16.7) | 0 (0.0) | 1 (12.5) | 1 (20.0) | 1 (20.0) | 0 (0.0) |
| Methylating agent (MMA) | 7 (19.4) | 1 (8.3) | 5 (33.3) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 4 (80.0) | 0 (0.0) | 3 (16.7) |
| Propylene oxide (2-HPMA) | 11 (30.6) | 3 (25.0) | 5 (33.3) | 3 (50.0) | 0 (0.0) | 1 (12.5) | 4 (80.0) | 1 (20.0) | 6 (33.3) |
Notes: 2-HPMA = 2-hydroxypropylmercapturic acid; 3-HPMA = 3-hydroxypropylmercapturic acid; AAMA = 2-carbamoylethylmercapturic acid; CNEMA = 2-cyanoethylmercapturic acid; HEMA = 2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide); HPMMA = 3-hydroxy-1-methyl-propylmercapturic acid; MHBMA-1+2 = sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid; MHBMA-3 = 4-hydroxy-2-buten-1-yl-mercapturic acid; MMA = methylmercapturic acid; and, PMA = phenylmercapturic acid.
DISCUSSION
The public health burden of e-cigarette use is a balance between their potential benefits as a form of harm reduction for smokers and their direct harms to nonsmokers who vape. The findings of our study support the harm reduction potential of e-cigarettes for smokers but also suggest that e-cigarettes may have deleterious effects in non-smoking vapers. We found that dual users confined to a research ward were exposed to substantially lower levels of toxic and/or carcinogenic VOCs when they used e-cigarettes compared to when they smoked cigarettes. These findings align with previous cross-sectional studies and longitudinal ambulatory switching studies which found that e-cigarette use resulted in lower systemic exposure to toxicants compared to smoking (10, 12).
Of note however, we found higher levels of metabolites of acrylamide, benzene, and possibly propylene oxide during e-cigarette use relative to enforced abstention, suggesting that e-cigarette use results in higher systemic exposure to these toxic/carcinogenic VOCs. These findings are important because, as far as we know, this is the first non-industry-associated assessment of toxicant exposure from use of commercial e-cigarettes in a setting where e-cigarette use is monitored and abstention enforced.
We found no published study explaining how e-cigarette use can lead to increased exposure to acrylamide, an IARC Group 2A carcinogen (probable human carcinogen). Sources of acrylamide exposure include manufacturing, chemical, and agricultural industries, but French fries, potato chips, cereals, and coffee are important dietary sources (28). Acrylamide is generated through the Maillard reactions of food products, which are heat-dependent reactions of glucose with amino acids, particularly asparagine, peptides and aromatic amines (29, 30). These reactions are plausible during e-cigarette use. Amino acids are not a significant constituent of e-liquids but studies have reported greater excretion of aromatic amines from exclusive e-cigarette users compared to controls (31), suggesting that aromatic amines are given off in e-cigarette aerosols and might contribute to acrylamide formation. Another potential pathway of acrylamide formation is the reaction between acrylic acid, formed from oxidation of acrolein, and ammonia, which could be potentially generated by thermal decomposition of nitrogen-containing compounds (29). Of relevance to the latter route, ethyl acrylate, the ethyl ester of acrylic acid, is a major volatile constituent of organic passion fruit pulp (32), is found in pineapples, grapes and vanilla (33), and is a flavor additive (34). Ethyl acrylate would react with ammonia much more readily than would acrylic acid. Furthermore, since acrylamide is used in the manufacture of some plastics and adhesives, we cannot rule out the possibility that acrylamide residues that remain in plastics used in the manufacture of e-cigarette devices or in plastic equipment and containers used in manufacturing and transporting of e-liquids is the source.
We did not see a significant correlation between plasma nicotine AUC over 24 h and 24-h urinary AAMA levels during the e-cigarette arm, suggesting that other sources could have contributed to acrylamide exposure. Also, the elimination half-life of AAMA is 14 hours (23), thus noncompliant smoking during the at-home phase of the e-cigarette arm could have influenced AAMA levels measured during the research ward phase of the e-cigarette arm. Nevertheless, during the smoking arm, there was no increase in AAMA levels from Day 1 to Day 2 (Figure 1a-1j), arguing against substantial carryover effect of smoking on AAMA levels. Further, among those who were assigned the e-cigarette during the second arm, AAMA levels were significantly higher during e-cigarette use compared to abstention, providing evidence of the contribution of e-cigarettes to acrylamide exposure. In summary, acrylamide formation in e-cigarettes is plausible and our findings are suggestive, but a firm conclusion cannot be made that e-cigarette use leads to acrylamide exposure.
Pankow and colleagues demonstrated that benzene can be generated from thermal degradation of the humectants, propylene glycol (PG) and vegetable glycerin (VG), and additives, such as benzoic acid and benzaldehyde (18). Most participants with elevated levels of the benzene metabolite (PMA) during e-cigarette use relative to abstention were users of cig-a-likes or fixed-power e-cigarettes (i.e. low-powered devices); users of fruit or tobacco flavors also showed elevated benzene exposure. Although generation of aldehydes and VOCs in e-cigarette aerosol is known to be temperature dependent (16), and thus higher exposure to these toxicants are expected in users of high-powered devices, our study raises questions about toxicant exposure from use of low-powered devices. Benzene has been detected in some refill e-liquids and cartridges (35), potentially serving as a source of benzene even in devices that operate at low power/temperature settings.
Users of variable-power e-cigarettes, which are typically operated at higher power and temperatures (36), had elevated excretion of the propylene oxide metabolite (2-HPMA) compared to users of the other types of e-cigarettes. Propylene oxide, an IARC Group 2B carcinogen (possibly carcinogenic to humans) (19), can be derived from propylene glycol in the presence of weak bases and heat (37). Aerosol generation is greater in high power e-cigarettes, resulting in greater nicotine intake and potentially more propylene oxide generation. The significant correlation between 2-HPMA levels and plasma nicotine AUC during the e-cigarette arm is evidence for propylene oxide generation in e-cigarettes.
We found no evidence of significant differences in exposure to the other VOCs, including acrolein, across device types. This observation regarding acrolein was surprising since vaping machine studies have reported substantial acrolein generation from e-cigarettes, particularly at higher power settings (38-40). However, it is possible that background exposure to acrolein, primarily from food sources through thermal breakdown of animal and vegetable fats, carbohydrates, and amino acids, or even endogenous production of acrolein (41), could overwhelm the contribution of e-cigarettes to acrolein exposure. To minimize the contribution of food to toxicant exposure during the participants’ stay on the research ward, we did not allow charbroiled meats and fried foods. Further, since participants were admitted to the hospital on the same day of each week, they were served the same meals on each named day, thus reducing variation in diet-related exposures within- and between-participants.
Despite lower risks of e-cigarettes relative to cigarettes, questions remain of the inherent toxicity of e-cigarettes, and risks to nonsmoking adults and children who vape. Our findings of potentially increased systemic exposure to acrylamide, benzene, and propylene oxide from e-cigarette use are particularly concerning given that these VOCs are known or suspected human carcinogens. A previous study found higher levels of another benzene metabolite, trans,trans-muconic acid, in baseline urine samples of e-cigarette users compared to nontobacco users enrolled in a laboratory study, but polyuse of other tobacco products could not be ruled out (15). Our findings also raise concerns about benzene exposure among JUUL users since a major constituent of JUUL pod fluids is benzoic acid. Although the study by Pankow and colleagues did not detect benzene in JUUL aerosol, 2 of 3 JUUL users in our study had elevated PMA excretion during e-cigarette use relative to abstention.
Using the same analytical chemistry methods as used in the current study, we measured all ten VOC metabolites in smokers enrolled in a clinical trial at 10 sites across the U.S. Levels of all metabolites in smokers in the former study were comparable to levels measured at baseline and during the cigarette arm in the current study (42). In addition, of the same eight VOC biomarkers utilized between studies, average levels of 2-HPMA, 3-HPMA, and HEMA measured in e-cigarette-only users from a nationally-representative sample were comparable to levels measured during the e-cigarette arm of the current study; the average level of AAMA was about two times higher and CNEMA was over 5 times higher during the e-cigarette arm of the current study while the average levels of HPMMA, MHBMA-1+2, and PMA were 2.6, 8, and 2 times higher, respectively, in e-cigarette-only users in the former study compared to during the e-cigarette arm of the current study (12).
A strength of our study is its crossover design, in which each participant served as their own control. However, while we counterbalanced the order of e-cigarette and cigarette arms, the two days of abstention always followed the second arm. Since the VOC metabolites have half-lives of several hours, from at least 8 hours for CNEMA (acrylonitrile) (22) to 14 hours for the AAMA (acrylamide) (23), there was likely carryover from product use to abstention, particularly when cigarettes were assigned immediately before the abstention days (see Figure 2a-2f). Thus, we could have underestimated differences in VOC exposure from e-cigarette use compared to abstention. On the other hand, during the cigarette and e-cigarette arms, the levels of biomarkers at Day 1 and Day 2 were consistent, potentially indicating minimal carryover from one day to the next. Another limitation of our study is that most participants were males which limits assessment of sex differences. Further, assessment of differences by device type was limited by the small sample size of variable-power tank users and JUUL pod users enrolled in the study.
In conclusion, e-cigarettes expose users to lower levels of toxic VOCs, supporting their harm reduction potential among smokers. However, some e-cigarettes potentially expose users to VOCs such as acrylamide, benzene, and propylene oxide, and may pose health risks to nonsmoking users. For example, more cig-a-like users had elevated benzene exposure compared to users of other types of e-cigarettes. Further studies are needed to examine what design features of e-cigarettes and user behaviors lead to elevated toxicant exposure. Regulation of e-cigarettes must include a balanced approach to maximize their potential for harm reduction among adult smokers and minimize their risk to nonsmoking users, including minimizing exposure to toxic VOCs.
ACKNOWLEDGEMENTS
We thank Marian Shahid, Annalise Davis, and Jennifer Ko for clinical research coordination; Kristina Bello, Christopher Havel, Trisha Mao, Polly Cheung, and Lisa Yu for performing analytical chemistry; and Zuckerberg San Francisco General Hospital Clinical Research Center nurses and staff for research participant care and study procedures. This study was supported by grants R01DA039264 (supported G. St.Helen, N. Nardone, N. Addo, P. Jacob III, and N.L. Benowitz) and P30DA012393 (supported P. Jacob III, and N.L. Benowitz) from the National Institute on Drug Abuse, 9U54HL147127 (supported G. St.Helen, P. Jacob III, and N.L. Benowitz) from the National Cancer Institute, and was carried out in part at the Clinical Research Center at Zuckerberg San Francisco General Hospital (NIH/NCRR UCSF-CTSI UL1 RR024131). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the Food and Drug Administration (FDA).
Abbreviation list:
- 2-HPMA
2-hydroxypropylmercapturic acid
- 3-HPMA
3-hydroxypropylmercapturic acid
- AAMA
2-carbamoylethylmercapturic acid
- CNEMA
2-cyanoethylmercapturic acid
- HEMA
2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide)
- HPMMA
3-hydroxy-1-methyl-propylmercapturic acid
- LOQ
limit of quantitation
- MHBMA-1+2
sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid
- MHBMA-3
4-hydroxy-2-buten-1-yl-mercapturic acid
- MMA
methylmercapturic acid
- PMA
phenylmercapturic acid
- VOC
volatile organic compound
Footnotes
Potential conflict(s) of interest: NL Benowitz is a consultant to Pfizer and Achieve Life Sciences, companies that market or are developing smoking cessation medications, and has served as a paid expert witness in litigation against tobacco companies.
REFERENCES
- 1.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad S, Zafar I, Mariappan N, Husain M, Wei C-C, Vetal N, et al. Acute pulmonary effects of aerosolized nicotine. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2018;316:L94–L104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schweitzer KS, Chen SX, Law S, Van Demark M, Poirier C, Justice MJ, et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015;309:L175–L87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12:424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haussmann H-J. Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem Res Toxicol. 2012;25:794–810. [DOI] [PubMed] [Google Scholar]
- 6.National Academies of Sciences Engineering and Medicine. Public health consequences of e-cigarettes. Washington, DC; 2018. [Google Scholar]
- 7.Gottlieb S, Zeller M. A nicotine-focused framework for public health. N Engl J Med. 2017;377:1111–4. [DOI] [PubMed] [Google Scholar]
- 8.Cravo AS, Bush J, Sharma G, Savioz R, Martin C, Craige S, et al. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regul Toxicol Pharmacol. 2016;81:S1–S14. [DOI] [PubMed] [Google Scholar]
- 9.Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P, Benowitz NL. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob Res. 2017;19:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulvers K, Emami AS, Nollen NL, Romero DR, Strong DR, Benowitz NL, et al. Tobacco Consumption and Toxicant Exposure of Cigarette Smokers Using Electronic Cigarettes. Nicotine Tob Res. 2016:ntw333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Ruiz CD, Graff DW, Robinson E. Reductions in biomarkers of exposure, impacts on smoking urge and assessment of product use and tolerability in adult smokers following partial or complete substitution of cigarettes with electronic cigarettes. BMC Public Health. 2016;16:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goniewicz ML, Smith DM, Edwards KC, Blount BC, Caldwell KL, Feng J, et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw Open. 2018;1:e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, et al. Nicotine, Carcinogen, and Toxin Exposure in Long-Term E-Cigarette and Nicotine Replacement Therapy UsersA Cross-sectional StudyE-Cigarettes and Toxin Exposure. Ann Intern Med. 2017;166:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubinstein ML, Delucchi K, Benowitz NL, Ramo DE. Adolescent exposure to toxic volatile organic chemicals from e-cigarettes. Pediatrics. 2018;141:e20173557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorkiewicz P, Riggs DW, Keith RJ, Conklin DJ, Xie Z, Sutaria S, et al. Comparison of urinary biomarkers of exposure in humans using electronic cigarettes, combustible cigarettes, and smokeless tobacco. Nicotine Tob Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, et al. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ Sci Technol. 2016;50:9644–51. [DOI] [PubMed] [Google Scholar]
- 17.McMichael A Carcinogenicity of benzene, toluene and xylene: epidemiological and experimental evidence. IARC Sci Publ. 1988:3–18. [PubMed] [Google Scholar]
- 18.Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, et al. Benzene formation in electronic cigarettes. PloS one. 2017;12:e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith C, Perfetti T, Rumple M, Rodgman A, Doolittle D. “IARC Group 2B carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol. 2001;39:183–205. [DOI] [PubMed] [Google Scholar]
- 20.Laino T, Tuma C, Moor P, Martin E, Stolz S, Curioni A. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116:4602–9. [DOI] [PubMed] [Google Scholar]
- 21.St.Helen G, Nardone N, Addo N, Dempsey D, Havel C, Jacob P 3rd, et al. Differences in nicotine intake and effects from electronic and combustible cigarettes among dual users Addiction. 2019;Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakubowski M, Linhart I, Pielas G, Kopecký J. 2-Cyanoethylmercapturic acid (CEMA) in the urine as a possible indicator of exposure to acrylonitrile. Occup Environ Med. 1987;44:834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watzek N, Scherbl D, Feld J, Berger F, Doroshyenko O, Fuhr U, et al. Profiling of mercapturic acids of acrolein and acrylamide in human urine after consumption of potato crisps. Mol Nutr Food Res. 2012;56:1825–37. [DOI] [PubMed] [Google Scholar]
- 24.Van Sittert N, Boogaard P, Beulink G. Application of the urinary S-phenylmercapturic acid test as a biomarker for low levels of exposure to benzene in industry. Occup Environ Med. 1993;50:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacob P, Raddaha AHA, Dempsey D, Havel C, Peng M, Yu L, et al. Comparison of Nicotine and Carcinogen Exposure with Water pipe and Cigarette Smoking. Cancer Epidemiology Biomarkers & Prevention. 2013;22:765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvanko AM, St.Helen G, Nardone N, Addo N, Benowitz N. Circadian subjective and pharmacological effects of ad libitum electronic and combustible cigarette use among dual users. Addiction. 2019;Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharp D Acrylamide in food. The Lancet. 2003;361:361–2. [DOI] [PubMed] [Google Scholar]
- 29.Becalski A, Lau BP-Y, Lewis D, Seaman SW. Acrylamide in foods: Occurrence, sources, and modeling. J Agric Food Chem. 2003;51:802–8. [DOI] [PubMed] [Google Scholar]
- 30.Gokhale MY, Kearney WR, Kirsch LE. Glycosylation of aromatic amines I: Characterization of reaction products and kinetic scheme. AAPS PharmSciTech. 2009;10:317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller TW, Acharya AP, Meyyappan T, Yu M, Bhaskar G, Little SR, et al. Comparison of Bladder Carcinogens in the Urine of E-cigarette Users Versus Non E-cigarette Using Controls. Sci Rep. 2018;8:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janzantti NS, Macoris MS, Garruti DS, Monteiro M. Influence of the cultivation system in the aroma of the volatile compounds and total antioxidant activity of passion fruit. LWT - Food Sci Technol. 2012;46:511–8. [Google Scholar]
- 33.Silano V, Bolognesi C, Castle L, Chipman K, Cravedi JP, Engel KH, et al. Safety of ethyl acrylate to be used as flavouring. EFSA Journal. 2017;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burdock GA. Encyclopedia of food & color additives: CRC Press; 2014. [Google Scholar]
- 35.Lim H-H, Shin H-S. Determination of volatile organic compounds including alcohols in refill fluids and cartridges of electronic cigarettes by headspace solid-phase micro extraction and gas chromatography–mass spectrometry. Anal Bioanal Chem. 2017;409:1247–56. [DOI] [PubMed] [Google Scholar]
- 36.Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control. 2016:tobaccocontrol-2016–053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Z, Xu L, Wei Y, Wang Y, He Y, Xia Q, et al. A new route for the synthesis of propylene oxide from bio-glycerol derivated propylene glycol. Chem Commun (Camb). 2009:3934–6. [DOI] [PubMed] [Google Scholar]
- 38.Havel CM, Benowitz NL, Jacob P 3rd, St.Helen G. An Electronic Cigarette Vaping Machine for the Characterization of Aerosol Delivery and Composition. Nicotine Tob Res. 2017;19:1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conklin DJ, Ogunwale MA, Chen Y, Theis WS, Nantz MH, Fu X-A, et al. Electronic cigarette-generated aldehydes: The contribution of e-liquid components to their formation and the use of urinary aldehyde metabolites as biomarkers of exposure. Aerosol Sci Technol. 2018;52:1219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ, et al. Aldehyde detection in electronic cigarette aerosols. ACS omega. 2017;2:1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abraham K, Andres S, Palavinskas R, Berg K, Appel KE, Lampen A. Toxicology and risk assessment of acrolein in food. Mol Nutr Food Res. 2011;55:1277–90. [DOI] [PubMed] [Google Scholar]
- 42.St.Helen G, Benowitz NL, Ko J, Jacob P 3rd, Gregorich SE, Pérez-Stable EJ, et al. Differences in exposure to toxic and/or carcinogenic volatile organic compounds between Black and White cigarette smokers. J Expo Sci Environ Epidemiol. 2019;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]