Abstract
Recent observational studies suggest that bisphosphonates (BPs) and anti-diabetes drugs are associated with colorectal cancer (CRC) risk reduction. Hence, we evaluated the CRC preventive effects of BPs (Zometa; Zom; and Fosamax; Fos), individually and when combined with metformin (Met), in azoxymethane (AOM)-induced rat colon cancer model. Rat (30/group) were randomized and treated s.c. with AOM to induce CRC. Dietary intervention with Zom or Fos (0, 20, or 100 ppm) or Met (1000 ppm) or the combinations (Zom/Fos 20 ppm plus Met 1000 ppm) began four weeks after AOM treatment, at premalignant lesions stage. Rats were killed 40 weeks post drug intervention to assess CRC preventive efficacy. Dietary Zom (20 ppm) inhibited non-invasive adenocarcinomas (AdCa) multiplicity by 37% (p<0.03) when compared with control diet fed group. Fosamax at 20 ppm and 100 ppm significantly reduced AdCa incidence (p<0.005) and inhibited the non-invasive AdCa multiplicities by 43.8% (p<0.009) and 60.8% (p<0.004), respectively, compared with the group fed control diet. At 1000 ppm dose, Met failed to suppress colon AdCa formation. However, the lower dose combinations of Zom or Fos with Met resulted in significant inhibition of non-invasive AdCa by 48% (p<0.006) and 64% (p<0.0002), and invasive AdCa by 49% (p<0.0005) and 38% (p<0.006), respectively. Biomarker analysis of combination drug treated tumors showed a decrease in cell proliferation with increased apoptosis when compared to untreated tumors. Overall, our results suggest that the combination of low doses of Zom or Fos with Met showed synergistic effect and significantly inhibited colon AdCa incidence and multiplicity.
Keywords: Colon cancer, Bisphosphonates, Metformin, Chemoprevention, F344 rat model, AOM
INTRODUCTION
Colorectal cancer (CRC) is one of the most frequently diagnosed cancer and is among the leading causes of cancer related mortalities worldwide. In the United States, CRC ranks third in annual cancer incidence cases and second for cancer associated deaths. An estimated 145,600 new cases of CRC and 51,020 deaths are expected to occur in 2019 (1). When CRC is detected early, patients with CRC have better treatment options with an increased five year survival (90%); however, close to two-thirds (61%) of CRCs are at advanced stages when diagnosed and, despite advanced treatment options, patients diagnosed at these stages have a very low survival rate. Early diagnosis and prevention of CRC are crucial to minimize cancer deaths.
Bisphosphonates (BPs; also called diphosphonates) are the analogues of pyrophosphate where oxygen is replaced by a carbon atom with various side chains. BPs have high affinity for calcium hence they accumulate in bone resulting in inhibition of bone resorption. Clinically, BPs are used to treat osteoporosis and osteolytic tumor bone metastases (2). BPs block macrophages to osteoclasts differentiation, activity of mature osteoclasts, and induce osteoclast apoptosis (3–4). While the exact molecular mechanisms behind BPs effects are still not clear, it is noteworthy that polarized macrophages are known to drive the progression of CRC and it is imperative to understand the role of BPs in CRC prevention. Mechanistically, the nitrogen-containing BPs such as Zometa (Zom; zoledronate) and Fosamax (Fos; alendronate) are known to inhibit the mevalonate pathways while non-nitrogen BPs inhibit ATP-dependent enzymes (3). Recent preclinical data showed that Zom can inhibit cancer cells (5–6) by different mechanisms, and highlighted the potential of BPs as anticancer agents, individually and in combination with other agents (2, 7). Accumulating evidence support that BPs suppress bone metastasis due to anti-angiogenic activities and by modulating adhesion and migration of cultured endothelial cells (6).
Metformin (Met) is a clinically approved hypoglycemic agent used for treatment of type II diabetes and is well tolerated in patients (8). Epidemiological studies suggest that patients with diabetes can be benefited by cancer risk reduction effects of Met (9–10). Met exerts its anti-tumor potential through inhibition of tumor proliferation via AMPK pathway activation, and by targeting cancer stem cells selectively (11–12). Met use was found to improve response to chemotherapy (13), with possible improved outcomes in type II diabetic CRC patients (12). There is a strong need to identify effective chemopreventive agents for CRC that can be translated into clinical applications. The above mentioned agents have been tested in various cancers; however, they have not been studied in CRC. In view of the clinically approved advantages and increasing evidence in favor of the antitumor activity of bisphosphonates, we determined the preventive efficacy of the BPs (Zom, Fos) and metformin, individually or in combination, to prevent azoxymethane (AOM)-induced colon cancer in a rat model.
MATERIALS AND METHODS
Animals, Diet, and Care
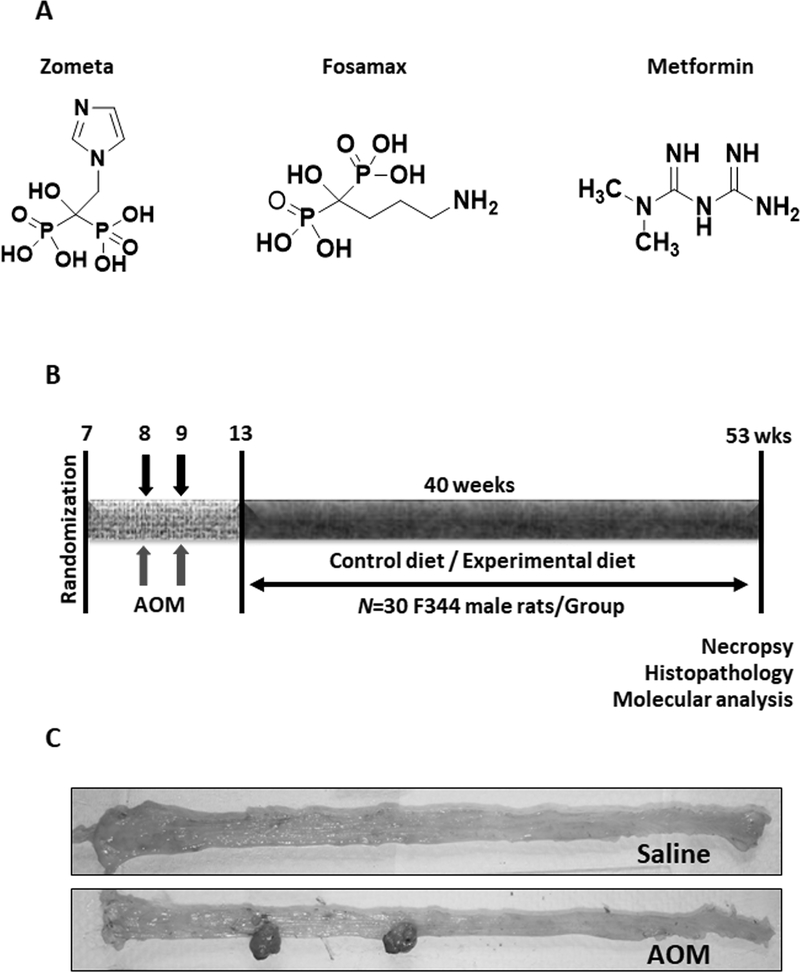
All animal studies were conducted in accordance with, and with the approval of Institutional Animal Care and Use Committee (IACUC). Male F344 rat weanlings (4–6 weeks old) were obtained from Harlan Breeding Laboratories (Frederick, MD) and randomized into control and experimental groups (30 rats/group) based on their weight and provided unrestricted access to food and water. Powdered diet with and without experimental agents was prepared using semi-purified ingredients purchased from Bioserv (Frenchtown, NJ) as described previously (14). Rats were fed experimental diets through food cups which were replenished twice a week with fresh diet. For experimental diet preparation, Zom, Fos, and Met (Fig 1A) were premixed with a small amount of diet initially and then blended into bulk diet using a Hobart mixer for uniform distribution.
Figure 1.
Experimental design to determine the efficacy of bisphosphonates, alone or in combination with metformin. Chemical structures of the test agents Zometa, Fosamax, and Metformin (A). Diagrammatic representation of the experimental design (B). F344 male rats were randomized and administered AOM at a dose of 15 mg/Kg BW once a week for two weeks s.c. and were fed control or experimental diets. Colon tumors were evaluated after termination. AOM-treated rats developed colonic tumors in the distal part of the colon, while saline-treated rats had normal-looking colons (C).
Experimental Bioassay
After randomization, seven weeks old male F344 rats were fed the modified AIN-76A diet for a week, after which colon carcinogen AOM was administered by subcutaneous injections at a dose of 15 mg/kg body weight once weekly for two weeks. Saline was injected into the vehicle group rats. Four weeks after the last AOM injection, animals were fed experimental diets with Zom or Fos at 0 ppm, 20 ppm or 100 ppm; Met 1000 ppm; or combinations (Zom/Fos 20 ppm, + Met 1000 ppm) for 40 weeks (Fig 1B). At this stage carcinogen treated rats develop preneoplastic aberrant crypt foci, representing high-risk individual of CRC. Later, rats were killed by CO2 asphyxiation and the colons were removed, rinsed in PBS, opened longitudinally, and flattened for colon tumor evaluation as previously described (14). The location (proximal and distal), number, and size of all colon tumors were recorded (Fig 1C). Colonic tumors were snap-frozen in liquid nitrogen for molecular analysis or saved in 10% buffered formalin for 24 h, followed by transferring to 80% ethanol for histological processing.
Serum Analysis
Whole blood was collected terminally from the control and experimental group rats by cardiac puncture. Serum was prepared from clot blood by centrifugation (10 minutes at 6,000 rpm). The serum was then analyzed using an IDEXX Catalyst instrument to evaluate the drugs effects on liver function using alanine aminotransferase (ALT) and alkaline phosphatase (ALKP); kidney function using blood urea nitrogen (BUN) and creatinine (CREA); and other serum parameters, such as cholesterol (CHOL), glucose (GLU), amylase (AMYL), lipase (LIPA), total protein (TP), and albumin (ALB), following the manufacturer’s instructions.
Tissue Processing, Histopathology, and Immunohistochemistry
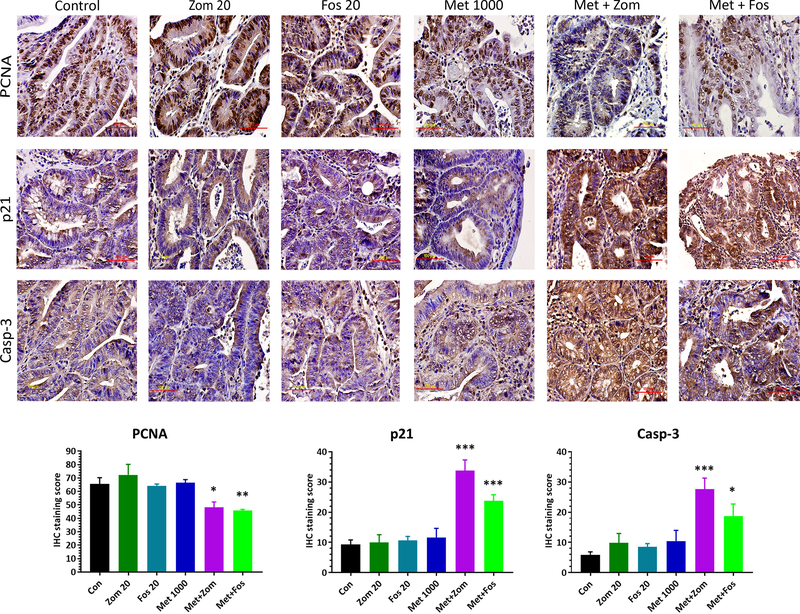
Colon tumor tissues saved in formalin/ethanol were processed, paraffin embedded and cut into 4-μm-thick sections. For histopathology, the tissue sections were deparaffinized in xylene, rehydrated in alcohol gradient, and stained with hematoxylin and eosin (H&E). Stained sections masked for treatment information were analyzed for tumor grade by a pathologist as described previously (14). Formalin fixed paraffin embedded tumor tissue sections were also used for biomarker evaluation using immunohistochemistry (IHC), as described in our previous paper (15). Primary antibodies used were PCNA (ab29; 1:3000), caspase-3 (cs9662; 1:250), and p21 (sc397; 1:50). Detection was conducted using a IHC Detection Kit (Histostain®-Plus, Life Technologies, USA) Primary antibody was replaced with TBS or the respective antibody serum for negative control slide. Digital images of the staining were recorded with an Olympus DP70 camera attached to Olympus microscope IX71. These images were analyzed using IHC profiler following semi-automated analysis protocol (16).
Western blotting
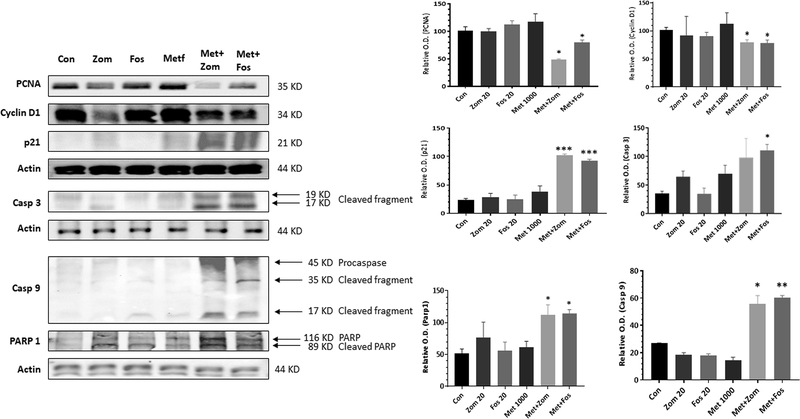
For marker analysis, total cell lysates were prepared from colon tumors from different treatment groups as previously described (14). Proteins were resolved on SDS-PAGE, transferred to a PVDF membrane, and probed with specific antibodies (PCNA [ab-29; 1:1000]; Cyclin D1 [ab-134175; 1:2000]; p21 [cs-2947; 1:1000]; caspase-9 [ab-52298; 1:1000]; caspase-3 [cs-9662; 1:1000]; PARP [cs-9542; 1:1000]; Actin [cs-4970; 1:1000]) overnight at 4°C. The membranes were washed with Tris-buffered saline (TBS; pH 7.4) and were incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:5000, 2.5% skimmed milk in TTBS) and visualized using chemiluminescence reagent, followed by autoradiography as previously described (15). β-actin was used as the loading control.
Statistical Analysis
Colon tumor multiplicity (average number of tumors per rat), was analyzed by unpaired student’s t- test with Welch’s Correction. Fisher’s Exact two-tailed test was used to analyze tumor incidence (percent of rats with colon tumors). Differences between body weights were analyzed by ANOVA. The data are presented as means ± SEM. Differences between groups with p<0.05 were considered significant. GraphPad Prism Software 8.0 (GraphPad Software, Inc., San Diego, CA) was used for statistical analysis.
RESULTS
General observations
Rats fed experimental diets containing Zom, Fos, or Met individually or in combination showed no significant difference in the body weight gain between these groups throughout the study (Suppl. Fig 1A). The average weights of the organs, such as kidneys, liver, and spleen, were also found to be similar among the treatment groups (Suppl. Figs 1B–1D), except for high-dose Zom, for which significant differences were observed in the liver and spleen weights compared with those of controls. At the point of necropsy, no effects of the agents were noted on the gross observations, such as size, shape, color, and texture. Serum from animals (n=4) was analyzed for liver and kidney function parameters ALKP, ALT, CREA, and BUN (Suppl. Fig 2) and various other parameters (Suppl. Table 1). The experimental drugs had no significant effect on these parameters compared to control. Taken together, the gross, histological, and biochemical data suggested that the chemopreventive agents, alone or combination, did not cause any observable toxicity.
Chemopreventive efficacy of the agents when used individually
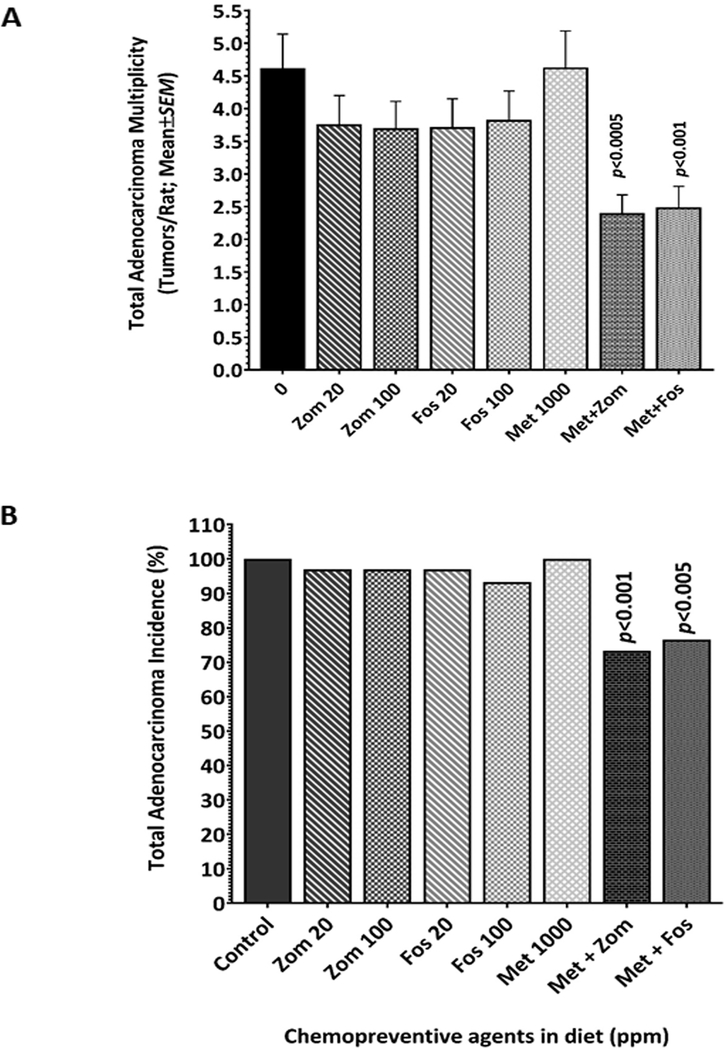
Administration of AOM led to formation of colon tumors in all control group rats, while saline-treated rats were free from colon tumors (Fig 1C). To determine the chemopreventive effect of the test agents, colon tumor multiplicity was compared with the control group animals. Rats of the control group had colon adenocarcinoma (AdCa) multiplicity of 4.62±0.52 (Fig 2A). Rats fed Zom at 20 ppm and 100 ppm had colon tumor multiplicity of 3.76±0.44 (p>0.05) and 3.70±0.41 (p>0.05), respectively. Similarly, rats administered Fos at both doses had colon tumor multiplicity of 3.72±0.43 (p>0.05) and 3.83±0.44 (p>0.05), respectively (Fig 2A). Both BPs showed moderate tumor inhibition (Zom 19–20% inhibition, Fos 17–19% inhibition); with no statistical significance. There was no difference in the inhibitor effects of the two agents and their two tested doses. Met alone had a very poor chemopreventive effect, with average colon AdCa multiplicity of 4.61±0.57 (p>0.05) in this treatment group (Fig 2A). Treatment of the AOM-injected rats with Zom, Fos, or Met individually had no notable effect on colon tumor incidence, although 3–7% of the BPs-treated rats were free from colon tumors; this effect was not significant (p>0.05) (Fig 2B).
Figure 2.
Bisphosphonates and metformin synergized to inhibit colonic AdCa multiplicity and incidence. Comparison of the colonic AdCa multiplicity and incidence between control and experimental groups. Bisphosphonates alone cause non-significant inhibition of the colonic AdCa multiplicity. Importantly, combination of bisphosphonates and metformin led to significant suppression of the colon AdCa multiplicity (A) and incidence (B).
Colon tumor preventive effects of Zom or Fos are significantly enhanced when combined with Met
Although the agents showed marginal tumor inhibition when administered individually, low dose (20 ppm) of either of the BPs (Zom or Fos) when combined with Met (1000 ppm) showed a synergistic chemopreventive effect leading to a significant inhibition of colon tumor multiplicity and incidence. The Zom plus Met combination showed 48% inhibition of colon AdCa (2.4±0.28; p<0.0002), while the Fos plus Met combination showed 46% inhibition (2.49±0.32; p<0.0005) of colon AdCa when compared with the control group (Fig 2A). Interestingly, a significant number of rats given a combination of Met and Zom/Fos were observed to be free from AOM-induced colon AdCa, suggesting that the combination could successfully prevent tumor formation. About 23% (p<0.001) of the rats treated with Zom plus Met, and 27% (p<0.005) of the rats treated with Fos plus Met were free from colon tumors compared to 100% incidence in control group, suggesting that the two agents synergize, leading to prevention of colon tumors (Fig 2B).
The combination inhibits tumor progression – suppression of invasive and non-invasive AdCa
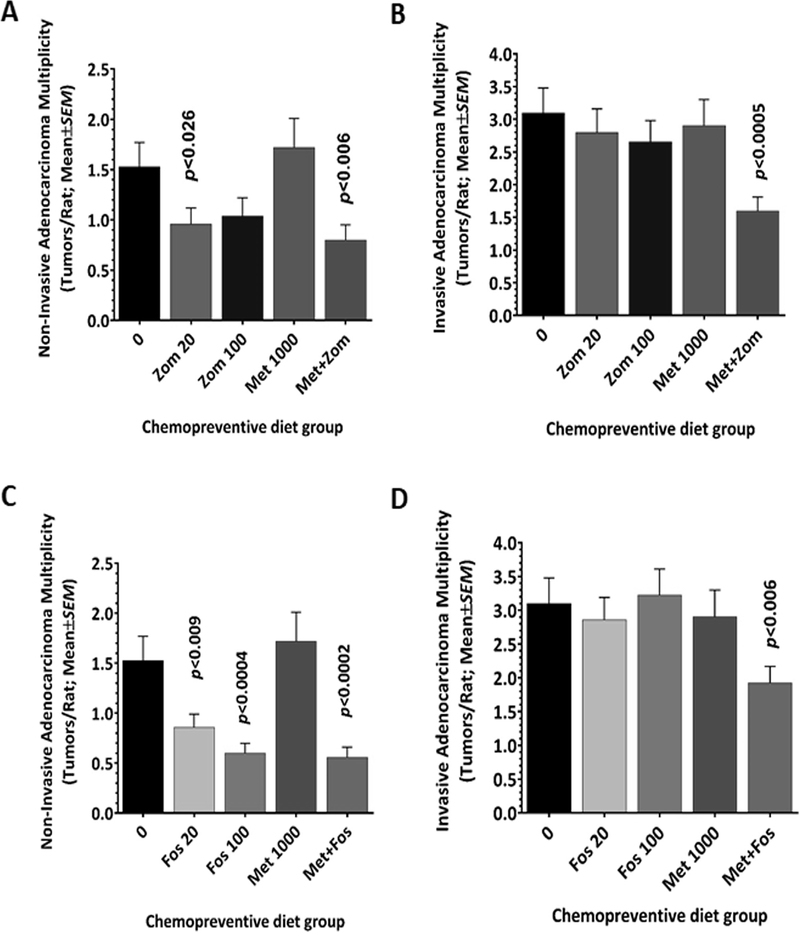
To further evaluate the effect of Zom, Fos, and Met on colon tumor progression, the histopathology grade of the colon tumors from all treatment groups was determined. Total colon tumors of the control-diet-fed animals 4.62±0.52 (Fig 2A) were classified into non-invasive (1.53±0.24; 33%) and invasive AdCa (3.1±0.38; 66%) based on histological criteria (Figs 3 and 4). Zom showed inhibition of the non-invasive AdCa at both dose (0.96±0.16 at 20 ppm; p<0.026, and 1.04±0.18 at 100ppm; p=0.05) (Fig 3A). The invasive AdCa were also slightly inhibited at both doses of Zom (2.80±0.36 and 2.66±0.32, respectively; p>0.05). However, the effect was not significant when compared with controls (Fig 3B). Fos had a stronger chemopreventive effect than did Zom, and there was a dose-dependent inhibitory effect of the non-invasive Adca (0.86±0.13, p<0.009; 0.60±0.10, p<0.0004) (Fig 3C). However, similar to Zom, Fos had a slight and non-significant effect on invasive AdCa multiplicity (2.86±0.33 and 3.23±0.38, respectively; p>0.05) (Fig 3D). Met had no effect on tumor progression when used alone. The combination of Zom and Met inhibited both non-invasive (0.80±0.15; p<0.006) and invasive Adca (1.60±0.21; p<0.0005) (Figs 3A and 3B). Similarly, the Fos and Met combination was effective in preventing both types of colon tumor types (0.56±0.10, p<0.0002; 1.93±0.24 p<0.006) (Figs 3C and 3D). Thus, the results suggest that the BPs are able to inhibit non-invasive AdCa. However, their combination with Met may also have a strong inhibitory effect on invasive AdCa, due to their synergistic effect.
Figure 3.
Combining bisphosphonates and metformin led to significant suppression of the non-invasive and invasive AdCa multiplicity. Effect of bisphosphonates, Zometa and Fosamax, alone or in combination with metformin on the multiplicity of non-invasive adenocarcinoma (A, C) and invasive adenocarcinoma (B, D).
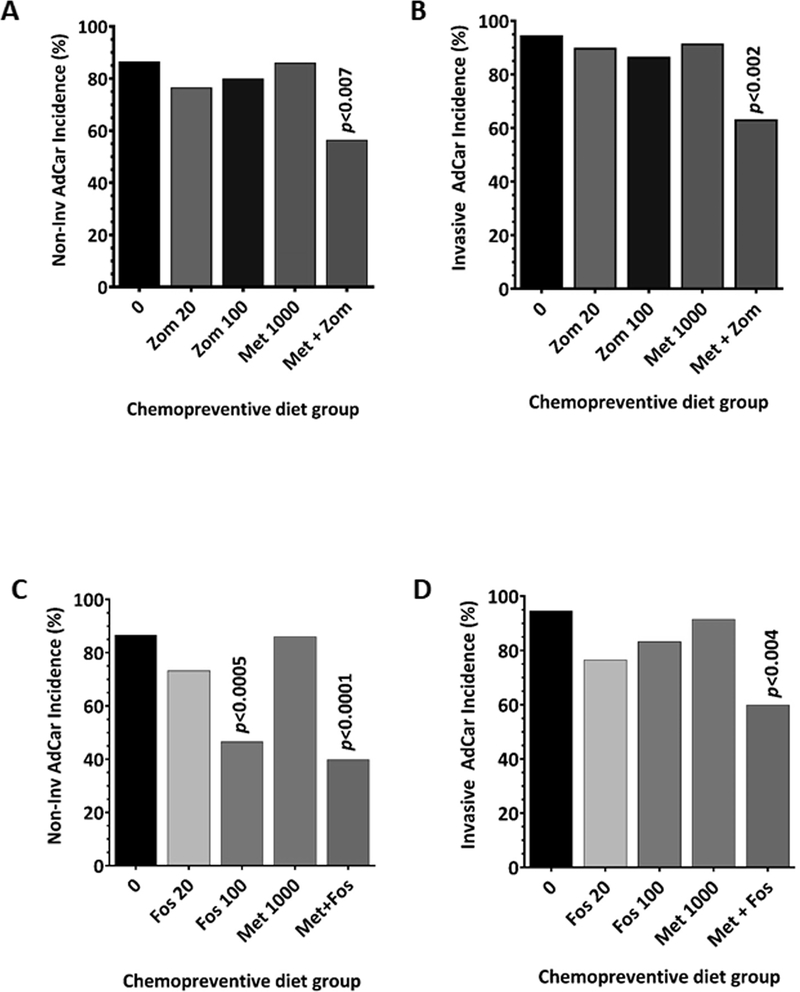
Figure 4.
Combination of bisphosphonates with metformin led to significant suppression of non-invasive and invasive AdCa incidence. Effect of bisphosphonates, Zometa and Fosamax alone or in combination with metformin on the incidence of non-invasive AdCa (A, C) and invasive AdCa (B, D). Individually, only high-dose Fosamax had a significant inhibitory effect on non-invasive adenocarcinomas. However, the low-dose of either Zometa or Fosamax when combined with metformin led to significant suppression of both non-invasive and invasive adenocarcinomas.
Incidence of non-invasive and invasive AdCa is prevented by the combination treatment
We also analyzed the incidence of both histological types of colon tumors among the control and treated animals. Except for 100 ppm Fos, none of the individual treatments of BPs or Met had a significant inhibitory effect on the incidence of non-invasive AdCa (20% to 27% inhibition) and invasive colon AdCa (10% - 24% inhibition) (Fig 4A–4D). Fos alone at the 100-ppm dose showed significant inhibition (46% less; p<0.0005) of only non-invasive AdCa incidence (Fig 4C). The combination of Met with either of the BPs inhibited both types of AdCa; the effect was more profound with the Fos combination. The combination of Zom with Met showed 44% (p<0.05) and 37% (p<0.005) inhibition of the non-invasive and invasive AdCa, respectively (Figs 4A and 4B). On the other hand, the Fos and Met combination had a much stronger inhibition of these two tumor types, non-invasive AdCa 60% inhibition (p<0.0001) and invasive AdCa 40% inhibition (p<0.005). Although both BPs were able to inhibit both types of AdCa, the effect seemed to be more pronounced with Fos.
Proliferation and apoptosis were affected by the chemopreventive combination
Tissue sections from the untreated and treated colon tumors were analyzed to study the expression of some of the proteins that affect tumor growth. There was a correlation between the tumor outcome and biomarker expression. Immunohistochemical and western blotting analysis showed that the control tumors had a strong expression of proliferation markers, PCNA (Figs 5 & 6) and Cyclin D1 (Fig 6). Individual treatment with Fos, Zom, or Met did not show any clear inhibitory effect on the expression of these proteins. However, suppression of PCNA and Cyclin D1 expression was seen in the combination treatment tumors with an increased expression of p21 (Cdk1A) (Figs 5 & 6). Pro-apoptotic markers caspase 3, caspase 9, and Parp-1 were markedly increased in the treatment groups, and particularly in the combination treatment group, while the control group animal tumors showed low expression of these proteins, suggesting an increase in apoptosis upon treatment (Fig 6).
Figure 5.
Representative images (60X) of Immunohistochemical analysis of the colon tumors from control and treated rats showing the modulatory effect of the chemopreventive agents on the expression of PCNA (A), p21 (B), and Caspase 3 (C).
Figure 6.
Western blot analysis showed that the colon tumors of the rats treated with the bisphosphonate and metformin combination had decreased levels of proliferation markers PCNA and Cyclin D1, while there was an increase in p21, Caspase 3, caspase 9, and cleaved Parp-1 pro-apoptotic markers.
DISCUSSION
BPs and Met are two medications that have been widely used for the past several decades. While Met is commonly prescribed for type II diabetes, BPs are used extensively for various indications, such as the osteoporosis treatment, its prevention in the high population, and treating patients with bone metastasis of gastrointestinal and various other cancers. It is however unclear whether these drugs will play a role in the primary and secondary prevention of colorectal cancers.
Developing novel agents for cancer chemoprevention takes several decades, and side effects are a significant concern. Hence, the repurposing of approved agents with demonstrated clinical safety is being pursued as an alternative approach to develop agents for cancer chemoprevention (17). In this context, there is a growing interest in both metformin and BPs as chemopreventives, as recent studies indicated that their use is associated with reduced risk for some cancers (9, 18–19). These agents are also the most commonly prescribed agents for the rapidly increasing elderly population, who are also at higher risk for many cancers. Therefore, it is important to use appropriate models to study the effects of these two agents on one of the most commonly diagnosed cancers. Here, we evaluated the chemopreventive effect of BPs (Zom and Fos), antidiabetic drug Met, and their combination in a preclinical rat model of colorectal cancer.
In vitro studies showed that BPs reduce proliferation and induce apoptosis of colon cancer cells (20), while in vivo BPs were also found to inhibit colorectal carcinogenesis in an ulcerative colitis experimental model (21–22). In the present study, we found a substantial colon tumor inhibition, particularly the non-invasive colon tumors, using BPs alone (Fig 3). While both BPs showed significant inhibition of the non-invasive AdCa, they failed to prevent invasive AdCa (Fig 3). This finding may suggest that BPs may be more effective in the early stages of tumor development in which the cells are much simpler in terms of molecular complexity and aggressive nature. Hence, BPs make a good candidates for chemoprevention, where the objective is to treat the at-risk population who may harbor these types of early-stage polyps or tumors.
Although BPs are used at treat late-stage cancers to prevent cancer metastasis to the bone, we did not see any strong effect on the advanced invasive AdCa. Unfortunately, Met alone failed to show any colon tumor inhibition at the tested dose, and a higher dose may be required to see the chemopreventive effect. These findings are in agreement with earlier studies showing an inverse correlation with BPs use and colon cancer (18–19). Several meta-analysis studies suggest that the use of oral BPs is associated with reduced risk of CRC and that this association is directly dependent on the number of prescriptions and duration of use (18–19, 23). In older populations, particularly post-menopausal women, BP intake was associated with a substantial and significant reduction (40%) in the risk of overall colon cancer deaths, as well as incidence of the colon cancer (24). Moreover, BPs use has been found to be associated with risk reduction of breast and endometrial cancers in women (25–27).
In the current study, we found that although BPs show a moderate colon tumor inhibitory effect, there was a significant inhibition of tumor multiplicity and incidence of the non-invasive and invasive AdCa when BPs were combined with Met (Fig 2 and 3). Thus, our data clearly indicate that BPs given in combination with other agents may synergize to enhance the chemopreventive effects. These findings are consistent with earlier studies that showed similar synergistic / additive effects of the BPs with other targeted agents (28–30).
The antitumor effects of BPs may be attributed to their pharmacological and diverse molecular effects (31). When administered orally, BPs are known to have poor absorption through the GI tract (bioavailability ranges from 0.6 to 1%). Thus, a substantial amount is delivered directly to the colon (32–33). The high concentration of BPs in the colon may have cancer inhibitory effect on the colon cancer cells. Many in vitro studies attribute the antitumor effects of BPs to their ability to inhibit protein prenylation through the inhibition of the mevalonate pathway, which affects cancer cell growth and metastasis. There is substantial evidence that BPs stimulate adaptive and innate immunity (34); inhibit tumor angiogenesis, invasion, and adhesion of tumor cells; and impede overall tumor progression. Although precise molecular MOA for Met effects on cancer cells is not fully elucidated, studies suggest that it can activate AMPK pathways, resulting in energy metabolism aberration thereby inhibiting cell growth (35). The findings from our work, along with strong epidemiological and preclinical data, suggest that BPs may serve as potential chemopreventive agents for colon cancers, particularly in the high-risk older population, and warrant further investigation.
Supplementary Material
ACKNOWLEDGMENTS
Funding (NCI-CN53300; NCI R01 CA213987 to CVR and NCI CCSG P30CA225520) from the NIH. Rodent barrier facility for providing assistance with animal studies. Editorial help from Ms. Kathy Kyler.
Grant Support
This study was funded by NCI-N01-CN53300 to CVR from the NIH/NCI.
Footnotes
Disclosure
The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Heymann D, Ory B, Gouin F, Green JR, Redini F. Bisphosphonates: new therapeutic agents for the treatment of bone tumors. Trends Mol Med 2004; 10:337–43. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs JB, Oliff A. The potential of farnesyltransferase inhibitors as cancer chemotherapeutics. Annu Rev Pharmacol Toxicol 1997; 37:143–66. [DOI] [PubMed] [Google Scholar]
- 4.Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, et al. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res 2000; 15:1467–76. [DOI] [PubMed] [Google Scholar]
- 5.Mackie PS, Fisher JL, Zhou H, Choong PF. Bisphosphonates regulate cell growth and gene expression in the UMR 106–01 clonal rat osteosarcoma cell line. Br J Cancer 2001; 84:951–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ory B, Blanchard F, Battaglia S, Gouin F, Redini F, Heymann D. Zoledronic acid activates the DNA S-phase checkpoint and induces osteosarcoma cell death characterized by apoptosis-inducing factor and endonuclease-G translocation independently of p53 and retinoblastoma status. Mol Pharmacol 2007; 71:333–43. [DOI] [PubMed] [Google Scholar]
- 7.Heymann D, Ory B, Blanchard F, Heymann MF, Coipeau P, Charrier C, et al. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone 2005; 37:74–86. [DOI] [PubMed] [Google Scholar]
- 8.Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334:574–9. [DOI] [PubMed] [Google Scholar]
- 9.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005; 330:1304–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013. August 2; 8(8):e71583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res 2009; 69:7507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Guan M, Zheng Z, Zhang Q, Gao F, Xue Y. Effects of Metformin on CD133+ Colorectal Cancer Cells in Diabetic Patients. PLoS One. 2013. November 21; 8(11):e81264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skinner HD, Crane CH, Garrett CR, Eng C, Chang GJ, Skibber JM, et al. Metformin use and improved response to therapy in rectal cancer. Cancer Med. 2013. February; 2(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammed A, Janakiram NB, Madka V, Zhang Y, Singh A2, Biddick L, Li Q, Lightfoot S, Steele VE, Lubet RA, Suen CS, Miller MS, Sei S, Rao CV. Intermittent Dosing Regimens of Aspirin and Naproxen Inhibit Azoxymethane-Induced Colon Adenoma Progression to Adenocarcinoma and Invasive Carcinoma. Cancer Prev Res (Phila). 2019. September 17. doi: 10.1158/1940-6207.CAPR-19-0312. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar G, Patolla JMR, Madka V, Mohammed A, Li Q, Zhang Y, et al. Simultaneous targeting of 5-LOX-COX and ODC block NNK-induced lung adenoma progression to adenocarcinoma in A/J mice. Am. J. Canc Res. 2016; 6(5): 894–909 [PMC free article] [PubMed] [Google Scholar]
- 16.Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9(5):e96801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stachnik A, Yuen T, Iqbal J, Sgobba M, Gupta Y, Lu P, et al. Repurposing of bisphosphonates for the prevention and therapy of nonsmall cell lung and breast cancer. Proc Natl Acad Sci U S A. 2014. December 16; 111(50):17995–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh S, Singh AG, Murad MH, Limburg PJ. Bisphosphonates are associated with reduced risk of colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013. March; 11(3):232–9.e1 [DOI] [PubMed] [Google Scholar]
- 19.Thosani N, Thosani SN, Kumar S, Nugent Z, Jimenez C, Singh H, et al. Reduced risk of colorectal cancer with use of oral bisphosphonates: a systematic review and meta-analysis. J Clin Oncol. 2013. February 10; 31(5):623–30. [DOI] [PubMed] [Google Scholar]
- 20.Kato J, Futamura M, Kanematsu M, Gaowa S, Mori R, Tanahashi T, et al. Combination therapy with zoledronic acid and cetuximab effectively suppresses growth of colorectal cancer cells regardless of KRAS status. Int J Cancer. 2016. March 15; 138(6):1516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballester I, Daddaoua A, López-Posadas R, Nieto A, Suárez MD, Zarzuelo A, et al. The bisphosphonate alendronate improves the damage associated with trinitrobenzenesulfonic acid-induced colitis in rats. Br J Pharmacol. 2007. May; 151(2):206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Posadas R, Mascaraque C, González R, Suárez MD, Zarzuelo A, Martínez-Augustin O, et al. The Bisphosphonate Pamidronate is an Intestinal Antiinflammatory Agent in Rat and Mouse Experimental Colitis. Inflamm Bowel Dis. 2016. November; 22(11):2549–2561. [DOI] [PubMed] [Google Scholar]
- 23.Rennert G, Pinchev M, Rennert HS, Gruber SB. Use of bisphosphonates and reduced risk of colorectal cancer. J Clin Oncol. 2011. March 20; 29(9):1146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pazianas M, Abrahamsen B, Eiken PA, Eastell R, Russell RG. Reduced colon cancer incidence and mortality in postmenopausal women treated with an oral bisphosphonate--Danish National Register Based Cohort Study. Osteoporos Int. 2012. November; 23(11):2693–701. [DOI] [PubMed] [Google Scholar]
- 25.Newcomb PA, Trentham-Dietz A, Hampton JM. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. Br J Cancer. 2010. March 2; 102(5):799–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newcomb PA, Passarelli MN, Phipps AI, Anderson GL, Wactawski-Wende J, Ho GY, et al. Oral bisphosphonate use and risk of postmenopausal endometrial cancer. J Clin Oncol. 2015. April 1;33(10):1186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rennert G, Pinchev M, Gronich N, Saliba W, Flugelman A, Lavi I, et al. Oral bisphosphonates and improved survival of breast cancer. Clin Cancer Res. 2017. April 1; 23(7):1684–1689. [DOI] [PubMed] [Google Scholar]
- 28.Hattori Y, Shibuya K, Kojima K, Miatmoko A, Kawano K, Ozaki K, et al. Zoledronic acid enhances antitumor efficacy of liposomal doxorubicin. Int J Oncol. 2015. July; 47(1):211–9. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Villasana V, Rodriguez-Aguayo C, Arumugam T, Cruz-Monserrate Z, Fuentes-Mattei E, Deng D, et al. Bisphosphonates inhibit stellate cell activity and enhance antitumor effects of nanoparticle albumin-bound paclitaxel in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2014. November; 13(11):2583–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang JW, Hsieh JJ, Shen YC, Yeh KY, Wang CH, Li YY, Hsu T et al. Bisphosphonate zoledronic acid enhances the inhibitory effects of gefitinib on EGFR-mutated non-small cell lung carcinoma cells. Cancer Lett. 2009. June 8; 278(1):17–26. [DOI] [PubMed] [Google Scholar]
- 31.Clézardin P Mechanisms of action of bisphosphonates in oncology: a scientific concept evolving from antiresorptive to anticancer activities. Bonekey Rep. 2013;2:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fazil M, Baboota S, Sahni JK, Ameeduzzafar Ali J. Bisphosphonates: therapeutics potential and recent advances in drug delivery. Drug Deliv. 2015. January; 22(1):1–9. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura M, Koide M, Udagawa N. Pharmacokinetics of bisphosphonate. Clin Calcium. 2016; 26(11):1561–1570. [PubMed] [Google Scholar]
- 34.Hamilton E1, Clay TM, Blackwell KL. New perspectives on zoledronic acid in breast cancer: potential augmentation of anticancer immune response. Cancer Invest. 2011. October;29(8):533–41. [DOI] [PubMed] [Google Scholar]
- 35.Sui X, Xu Y, Wang X, Han W, Pan H, Xiao M. Metformin: A Novel but Controversial Drug in Cancer Prevention and Treatment. Mol Pharm. 2015. November 2;12(11):3783–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.