Abstract
Objective:
Dietary lapses drive weight loss failure, and specific factors influence risk of lapse. Physical activity (PA) may be one such risk factor, though whether PA increases or decreases appetite, and thus risk of lapse, is unclear. In fact, most studies examining the relation between PA and energy intake are limited by use of laboratory-based settings, intensive PA manipulations, and healthy-weight samples. This study aimed to maximize ecological validity by examining the extent to which free-living PA of various intensities prospectively predicts same-day dietary lapses among individuals enrolled in a weight loss program.
Methods:
Participants were 130 adults with overweight/obesity in a behavioral weight loss treatment instructed to follow a PA and dietary prescription. At mid-treatment, moderate-to-vigorous PA (MVPA) and light PA were measured using hip-worn Actigraph GT3X+ accelerometers. Lapses were assessed using ecological momentary assessment.
Results:
Within-subject total PA (b = −0.012, SE = 0.005, p =.01) and light PA (b = −0.014, SE = 0.006, p =.01) negatively predicted lapse. MVPA followed the same pattern, but the effect was not statistically significant (b = −0.013, SE = 0.009, p =.12).
Conclusions:
This study was the first to investigate if objectively measured PA prospectively predicts lapse from a weight loss program. Results suggested that for every additional 10 minutes of total PA one engaged in, the risk of lapse decreased by 1%.
Keywords: dietary lapse, physical activity, ecological momentary assessment, sensor technology
Over 70% of American adults have overweight or obesity (Centers for Disease Control and Prevention, 2014), but weight loss outcomes in behavioral weight loss programs are often suboptimal (Wilson, D’agostino, Sullivan, Parise, & Kannel, 2002). Non-adherence to negative energy balance diets, i.e., dietary lapse, drives weight loss failure in these programs (Forman et al., 2017). Dietary lapses do not occur at random; specific factors, including affective (e.g., feeling deprived) and physical (e.g., hunger) states have been shown to predict lapse (Carels, Douglass, Cacciapaglia, & O’Brien, 2004; Carels et al., 2001; Forman et al., 2017).
Physical activity (PA) is an especially notable predictive factor of dietary lapse, as PA could either increase or decrease appetite, and thus risk of dietary lapse. For example, some evidence suggests that following PA, hormones are released that reduce hunger and energy intake (Beaulieu, Olver, Abbott, & Lemon, 2014; Hagobian et al., 2012). However, other studies have observed increases in appetite, hunger, and energy intake following PA (compensation; e.g., Beaulieu, Hopkins, Blundell, & Finlayson, 2018; Blundell, Gibbons, Caudwell, Finlayson, & Hopkins, 2015; Martin et al., 2019; Verger, Lanteaume, & Louis-Sylvestre, 1992).
These disparate findings could be due to methodological differences and shortcomings. For instance, some studies only included healthy weight individuals who, relative to individuals with overweight, have been shown to engage in more intense exercise and show different hormonal and appetitive response to PA (Heden, Liu, Park, Dellsperger, & Kanaley, 2013; Tudor-Locke, Brashear, Johnson, & Katzmarzyk, 2010). For example, exercise has been shown to reduce acylated ghrelin (an orexigenic hormone; Heden et al., 2013) and increase GLP-1 (an anorexigenic hormone; Adam & Westerterp-Plantenga, 2004) in normal weight individuals, but not in individuals with obesity, and individuals with obesity report reduced fullness following exercise (versus no exercise), whereas normal weight individuals do not (Heden et al., 2013). In addition, some previous studies have focused only on the effect of laboratory-based, high-intensity exercise paradigms; yet, PA of lower and higher levels (duration and intensity) appear to affect satiety, appetite, and energy intake differently (Beaulieu et al., 2018). Specifically, a recent review found low levels of PA lead to appetite dysregulation and overconsumption, whereas high levels of PA enhance the drive to eat but also increase post-meal satiety, resulting in overall better energy intake regulation (Beaulieu et al., 2018). In addition, in laboratory-based studies, the intensity and duration of exercise is determined by the researcher and eating behavior may be altered by demand characteristics and participant reactivity; thus, these results may not be accurate proxies for real-world relations between PA and eating. Therefore, there is a need to evaluate whether PA influences risk of lapse and thus weight control failure, and if so, if free-living PA of higher and lower intensities and durations differentially influence eating behavior and thus risk of dietary lapse.
Only two known studies (Carels et al., 2001; Goldstein, 2016; Goldstein et al., 2018) have examined the relation between PA and lapse among individuals with overweight and obesity following a weight loss dietary prescription using ecological momentary assessment (EMA; Stone, Shiffman, Atienza, & Nebeling, 2007)—a data collection method gathering user experiences multiple times a day in an individual’s natural environment, usually via a smartphone. In one study, participants used a paper-and-pencil EMA diary to report dietary lapses and engagement in certain activities, including exercise, prior to the lapse. Findings indicated lapses were less likely to occur immediately following exercising. However, the EMA diary did not measure duration of PA and may only have captured structured, intense exercise (Carels et al., 2001). In the other study (Goldstein, 2016; Goldstein et al., 2018), an end-of-day EMA question assessed (yes/no) whether a participant had engaged in structured exercise. This PA variable was not associated with dietary lapses, but its predictive ability was limited because PA was assessed 1) just once daily, precluding examination of intraday relations; 2) operationalized as a structured activity, thus not capturing non-structured PA; 3) measured dichotomously (yes/no), preventing measurement of intensity or time spent exercising (Goldstein, 2016; Goldstein et al., 2018).
This study aims to address the current literature’s limitations by examining the extent to which objectively- and continuously recorded, free-living PA of various intensities prospectively predicts same-day lapses among individuals with overweight who are seeking weight loss. The current study used accelerometry to assess features of PA frequency, intensity, and duration and EMA to assess dietary lapse. This methodology allowed for investigation of intraday, temporal relations between PA and lapses and the within-subjects effects of PA on dietary lapse in the context of a behavioral weight loss intervention prescribing a diet and PA. Moderate-to-vigorous PA (MVPA; activity that burns 3 or more times as much energy per minute as resting levels of energy), light PA (activity that burns between 1.5 and 3 times resting levels), and total PA (sum of MVPA and light PA) were used to assess PA intensity (Troiano et al., 2008; Tudor-Locke et al., 2010). This study focused on within-subject PA to examine the temporal impact of PA (a within-subject increase in PA) on lapse risk, which can elucidate if PA is a momentary risk or protective factor of lapse. Between-subject PA, or differences in average PA between participants, illustrates whether individuals engaging in more (or less) PA overall were at higher or lower risk of lapse, which may be confounded by motivation to adhere to the program generally (e.g., adhering to the PA and dietary prescription). Despite mixed evidence, we hypothesized that increased total PA, MVPA, and light PA would reduce lapse risk at a subsequent time point, consistent with the most ecologically valid study examining intraday relations between PA and dietary lapses (Carels et al., 2001).
Method
Participants
The current study is a secondary analysis of a larger, year-long randomized controlled trial of a behavioral weight loss treatment (Forman et al., 2016). Data from the six-month assessment were used because the graduated PA prescription reached its maximum level of 250 MVPA minutes/week at that point. Inclusion criteria included: aged 18–70 years, body mass index (BMI) between 27–50 kg/m2, and ability to engage in PA (i.e., walk unaided for two city blocks). Exclusion criteria included major medical issues, being pregnant, and having recently lost 5% or more of one’s body weight. Of the 190 participants enrolled in the trial, 130 provided adequate data on PA and dietary lapse for inclusion in analyses; the other 60 participants’ accelerometer and EMA data were not collected concurrently, precluding examination and testing of this study’s hypotheses.
Procedures
Participants received a year-long, 25-session behavioral weight loss treatment which prescribed a reduced calorie diet and a graduated PA prescription. Further details of the trial can be found elsewhere (Forman et al., 2016). All procedures were approved by the Institutional Review Board at Drexel University.
Measures
Physical activity.
Participants wore a hip-worn Actigraph GT3X+ accelerometer, a reliable, well- validated, and objective measure of PA (J. A. Lee, Williams, Brown, & Laurson, 2015; Ozemek, Kirschner, Wilkerson, Byun, & Kaminsky, 2014). Accelerometers measured 10+ minute bouts of MVPA, light PA, and total PA. Participants were told to wear accelerometers during all waking hours for seven days at the assessment point. Non-wear time was defined as ≥ 60 minutes of consecutive zero counts, and days with ≥ 10 hours of wear time were considered valid. ActiLife (ActiLife 6.13.4, 2019) software was used to aggregate and clean the data.
Dietary lapses.
Participants were prescribed a daily calorie goal (1,200–1,800 calories/day) based on their BMI and taught that exceeding this calorie goal constituted a dietary lapse. A dietary lapse was defined as eating or drinking likely to cause weight gain, and/or put weight loss/maintenance at risk, specifically eating a larger portion than intended, eating at a time when one had not intended to eat, or eating an energy dense food one intended to avoid. In group sessions, participants received written and oral instruction on recognizing lapses and how to report lapses via EMA. Participants were given an Android Samsung Galaxy Player 4.0 with a preloaded, customized EMA application (DrexelEMA). The EMA app delivered six surveys at semi-random intervals across the day. At each survey, participants were instructed to respond to the following prompt, “Since the last time you completed this survey, did you have a dietary lapse?” and to self-initiate an EMA survey when a lapse occurred (Forman et al., 2017). DrexelEMA automatically recorded the date and time of each survey, allowing for precise analysis of the temporal relation between PA and dietary lapse.
Analyses
Data were analyzed using SPSS v. 25. To be included in analyses, a participant needed to have a day with ≥ 10 hours of valid accelerometer wear time and ≥ 3 completed EMA surveys; only 130 participants had these data. Minutes of bouted total PA, MVPA, and light PA were calculated for every interval between same- day EMA surveys. Because this interval varied in length, time between surveys was entered as a covariate. To examine whether within-subject PA (i.e., the amount of PA completed between EMA surveys compared to an individual’s average; centered within person) predicted lapse at the next survey, GEE models based on a negative binomial distribution with a logit link function and an AR(1) matrix structure were used. All models also controlled for grand-mean centered between-subject effects (i.e., participants’ mean level of PA) and if a lapse was reported at the previous survey, which has been shown to predict lapse (e.g., Forman et al., 2017).
Results
Participants (N = 130) were adults with overweight (84.6% female; 72.3% Caucasian, MBMI = 36.75 ± 5.98 kg/m2; Mage = 52.06 ± 9.52 years) who provided adequate data on PA and lapse. In total, 4,358 EMA surveys and 725 participant days were examined.
Main effect of PA
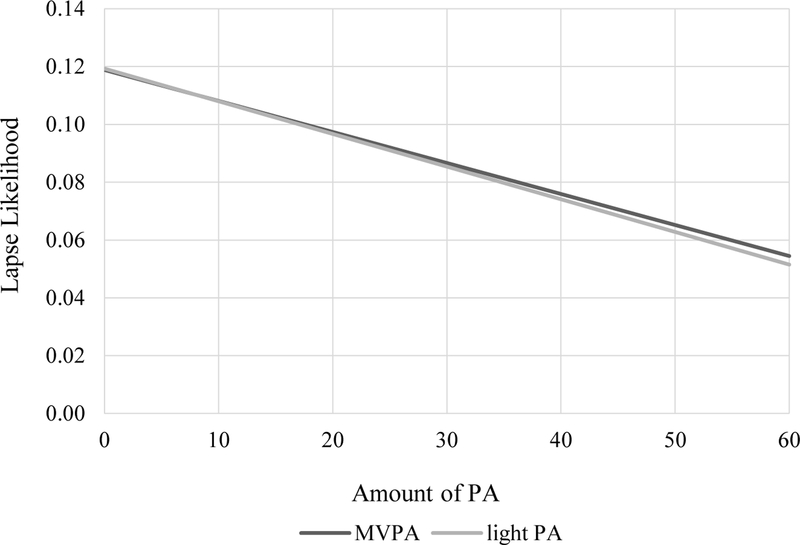
Within-subject total PA (b = −0.012, SE = 0.005, p =.01) and light PA (b = −0.014, SE = 0.006, p =.01) negatively predicted lapse risk. The pattern of effect was similar for MVPA but was not statistically significant (b = −0.013, SE = 0.009, p =.12). See Figure 1 for lapse risk by light PA and MVPA.
Figure 1.
The effects of moderate-to-vigorous physical activity (MVPA) and light physical activity (light PA) on dietary lapse likelihood.
Discussion
This was the first study to examine the relation between objective, continuously-measured, free-living PA of various intensities and lapse among individuals with overweight seeking weight loss. Results partially supported our hypothesis in that for every additional 10 minutes of light PA one engaged in, risk of lapse decreased by approximately 1%. In fact, following no PA, the risk of lapse was 12%, and that was reduced by more than half, i.e., to 5%, following 60 minutes of PA. The effect of MVPA on lapse risk was almost identical to that of light PA, but more variable, which may have precluded the detectection of a significant relation. Participants were less likely to engage in MVPA than light PA during the study period; thus, relatively fewer cases of MVPA in these data may have contributed to increased variability of the effect of MVPA on lapse risk.
These findings are consistent with research suggesting that PA stimulates the release of hormones that reduce hunger and energy intake (Beaulieu et al., 2014; Hagobian et al., 2012). Other potential mechanisms include that PA improves mood and/or self-concept (Peluso & Andrade, 2005), which in turn improves motivation to eat well. In addition, engaging in PA may temporarily increase commitment to dietary goals because individuals do not want to “undo” the hard work they put into PA.
Results showing light PA reduces lapse risk are in contrast with other findings that suggest light PA may instigate energy overcompensation (Beaulieu et al., 2014; Hagobian et al., 2012), i.e., that individuals overcompensate for PA by increasing energy intake beyond what was expended in PA (Martin et al., 2019; Verger et al., 1992). Instead, this study found that light PA lowered risk of dietary lapse, suggesting improved regulation of energy consumption. A difference between samples in these previous studies and the current study is that participants in the present study were attempting to adhere to a weight loss diet, and individuals may respond differently to PA depending on the degree to which they are restraining their eating. Also, this study examined the relation between PA and lapse in an ecologically-valid environment and lapses were self-reported. Thus, if actual calories were decreased or increased following PA remains unknown and should be investigated.
Interestingly, our findings suggest that light PA may aid in dietary adherence, perhaps through improved energy intake regulation. If these findings were replicated, it would raise questions about the near-universal prescription of MVPA versus light PA in weight loss programs. Although light PA is unlikely to directly contribute to weight loss, it could conceivably be a better recommendation than MVPA because light PA might bolster dietary adherence and be more sustainable (Egan, 2017). Still, MVPA produces greater energy expenditure and its own unique health benefits, e.g., reduced cardiovascular disease risk (Yu, Yarnell, Sweetnam, & Murray, 2003) and longevity (I.-M. Lee & Paffenbarger, 2000). Thus, further research is needed to explore the relative benefits of light PA versus MVPA in weight loss programs.
The present study had several limitations. Generalizability is limited by a primarily Caucasian and female sample; further, participants likely were highly motivated individuals, given their enrollment in a long- term weight loss trial. Nevertheless, these individuals still reported lapses 2–3 times/week, indicating even these highly motivated participants need assistance in better adhering to a weight-loss diet. Lapses were self-reported, not objectively-measured deviations in calorie intake and may be biased (Hebert, Clemow, Pbert, Ockene, & Ockene, 1995). For example, PA may impact the perception of what constitutes a lapse, making an individual less likely to report an overeating episode as a lapse because she felt her PA made subsequent overeating not a true lapse. Last, given the design of this study, the time intervals between PA and lapse were variable.
Future work evaluating the latency between PA and eating that predicts non-lapse (e.g., how long before eating would PA need to take place to reduce lapse risk) may help to improve PA prescriptions. Additionally, examination of individual differences in the association between PA and lapse risk may help to individualize treatment recommendations. Investigating the types of dietary lapses (e.g., consuming larger portions, more calorie dense food) influenced by PA may also help to better guide preventative interventions. With replication, these findings can inform ongoing work aiming to predict dietary lapse using machine learning and sensor technology. Continued understanding of what contributes to lapse can inform personalized, tailored interventions that can improve weight loss. Future work should build upon these findings by examining: 1) relations between PA and dietary lapse over time in a weight loss program (e.g., do the associations change as individuals increase PA and/or decrease weight); 2) these relations in more diverse samples; 3) relations between different PA regimens (e.g., weight training) and dietary prescriptions (e.g., a low-carbohydrate diet).
Acknowledgments
This research was supported by an R01 grant (R01DK095069) to Dr. Forman from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Crosby reports personal fees from Health Outcome Solutions, outside the submitted work. None of the remaining authors report a conflict of interest.
Footnotes
ClinicalTrials.gov Identifier:
References
- Adam TC, & Westerterp-Plantenga MS (2004). Activity-induced GLP-1 release in lean and obese subjects. Physiology & Behavior, 83(3), 459–466. [DOI] [PubMed] [Google Scholar]
- Beaulieu K, Hopkins M, Blundell J, & Finlayson G (2018). Homeostatic and non-homeostatic appetite control along the spectrum of physical activity levels: An updated perspective. Physiology & Behavior, 192, 23–29. [DOI] [PubMed] [Google Scholar]
- Beaulieu K, Olver TD, Abbott KC, & Lemon PW (2014). Energy intake over 2 days is unaffected by acute sprint interval exercise despite increased appetite and energy expenditure. Applied Physiology, Nutrition, and Metabolism, 40(1), 79–86. [DOI] [PubMed] [Google Scholar]
- Blundell J, Gibbons C, Caudwell P, Finlayson G, & Hopkins M. J. O. r. (2015). Appetite control and energy balance: impact of exercise. 16, 67–76. [DOI] [PubMed] [Google Scholar]
- Carels RA, Douglass OM, Cacciapaglia HM, & O’Brien WH (2004). An ecological momentary assessment of relapse crises in dieting. J Consult Clin Psychol, 72(2), 341–348. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15065966. doi: 10.1037/0022-006X.72.2.341 [DOI] [PubMed] [Google Scholar]
- Carels RA, Hoffman J, Collins A, Raber AC, Cacciapaglia H, & O’Brien WH (2001). Ecological momentary assessment of temptation and lapse in dieting. Eating Behaviors, 2(4), 307–321. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2014). National Health and Nutrition Examination Survey Data. Retrieved from https://www.cdc.gov/nchs/fastats/obesity-overweight.htm
- Egan B (2017). Physical Activity and Hypertension: Knowing Is Not Enough; We Must Apply. Willing Is Not Enough; We Must Do-von Goethe. Hypertension (Dallas, Tex.: 1979), 69(3), 404. [DOI] [PubMed] [Google Scholar]
- Forman EM, Butryn ML, Manasse SM, Crosby RD, Goldstein SP, Wyckoff EP, & Thomas JG (2016). Acceptance-based versus standard behavioral treatment for obesity: Results from the mind your health randomized controlled trial. Obesity, 24(10), 2050–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman EM, Schumacher LM, Crosby R, Manasse SM, Goldstein SP, Butryn ML, … Thomas GJ (2017). Ecological momentary assessment of dietary lapses across behavioral weight loss treatment: Characteristics, predictors, and relationships with weight change. Annals of Behavioral Medicine, 51(5), 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SP (2016). A Preliminary Investigation of a Personalized Risk Alert System for Weight Control Lapses. Drexel University, [Google Scholar]
- Goldstein SP, Zhang F, Thomas J, Butryn M, Herbert J, & Forman E (2018). Application of Machine Learning to Predict Dietary Lapses During Weight Loss. Journal of diabetes science and technology, 1932296818775757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagobian TA, Yamashiro M, Hinkel-Lipsker J, Streder K, Evero N, & Hackney T (2012). Effects of acute exercise on appetite hormones and ad libitum energy intake in men and women. Applied Physiology, Nutrition, and Metabolism, 38(999), 66–72. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Clemow L, Pbert L, Ockene IS, & Ockene JK (1995). Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. International journal of epidemiology, 24(2), 389–398. [DOI] [PubMed] [Google Scholar]
- Heden TD, Liu Y, Park Y, Dellsperger KC, & Kanaley JA (2013). Acute aerobic exercise differentially alters acylated ghrelin and perceived fullness in normal-weight and obese individuals. Journal of Applied Physiology, 115(5), 680–687. [DOI] [PubMed] [Google Scholar]
- Lee I-M, & Paffenbarger RS (2000). Associations of light, moderate, and vigorous intensity physical activity with longevity: the Harvard Alumni Health Study. American journal of epidemiology, 151(3), 293–299. [DOI] [PubMed] [Google Scholar]
- Lee JA, Williams SM, Brown DD, & Laurson KR (2015). Concurrent validation of the Actigraph gt3x+, Polar Active accelerometer, Omron HJ-720 and Yamax Digiwalker SW-701 pedometer step counts in lab-based and free-living settings. Journal of Sports Sciences, 33(10), 991–1000. [DOI] [PubMed] [Google Scholar]
- Martin CK, Johnson WD, Myers CA, Apolzan JW, Earnest CP, Thomas DM, … Harris M (2019). Effect of different doses of supervised exercise on food intake, metabolism, and non-exercise physical activity: The E-MECHANIC randomized controlled trial. The American journal of clinical nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozemek C, Kirschner MM, Wilkerson BS, Byun W, & Kaminsky LA (2014). Intermonitor reliability of the GT3X+ accelerometer at hip, wrist and ankle sites during activities of daily living. Physiological measurement, 35(2), 129. [DOI] [PubMed] [Google Scholar]
- Peluso MAM, & Andrade L. H. S. G. d. (2005). Physical activity and mental health: the association between exercise and mood. Clinics, 60(1), 61–70. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S, Atienza AA, & Nebeling L (2007). Historical roots and rationale of ecological momentary assessment (EMA). The science of real-time data capture: Self-reports in health research, 3–10. [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell MJM, … exercise. (2008). Physical activity in the United States measured by accelerometer. 40(1), 181. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Brashear MM, Johnson WD, & Katzmarzyk PT (2010). Accelerometer profiles of physical activity and inactivity in normal weight, overweight, and obese US men and women. International Journal of Behavioral Nutrition and Physical Activity, 7(1), 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger P, Lanteaume M, & Louis-Sylvestre J (1992). Human intake and choice of foods at intervals after exercise. Appetite, 18(2), 93–99. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D’agostino RB, Sullivan L, Parise H, & Kannel WB (2002). Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Archives of internal medicine, 162(16), 1867–1872. [DOI] [PubMed] [Google Scholar]
- Yu S, Yarnell J, Sweetnam P, & Murray L (2003). What level of physical activity protects against premature cardiovascular death? The Caerphilly study. Heart, 89(5), 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]