Abstract
Introduction:
At night, the pineal gland produces the indoleamines, melatonin, N-acetylserotonin (NAS), and N-acetyltryptamine (NAT). Melatonin is accepted as a hormone of night. Could NAS and NAT serve that role too?
Methods:
Concentration-response measurements with overexpressed human melatonin receptors MT1 and MT2; mass spectrometry analysis of norepinephrine-stimulated secretions from isolated rat pineal glands; analysis of 24-hour periodic samples of rat blood.
Results:
We show that NAT and NAS do activate melatonin receptors MT1 and MT2, although with lower potency than melatonin, and that in vitro, melatonin and NAS are secreted from stimulated, isolated pineal glands in roughly equimolar amounts, but secretion of NAT was much less. All three were found at roughly equal concentrations in blood during the night. However, during the day, serum melatonin fell to very low values creating a high-amplitude circadian rhythm that was absent after pinealectomy, whereas NAS and NAT showed only small or no circadian variation.
Conclusion:
Blood levels of NAS and NAT were insufficient to activate peripheral melatonin receptors and they were invariant, so they could not serve as circulating hormones of night. However, they could instead act in paracrine circadian fashion near the pineal gland or via other higher-affinity receptors.
Keywords: Melatonin, N-acetylserotonin, N-acetyltryptamine, melatonin receptor, pinealectomy
INTRODUCTION
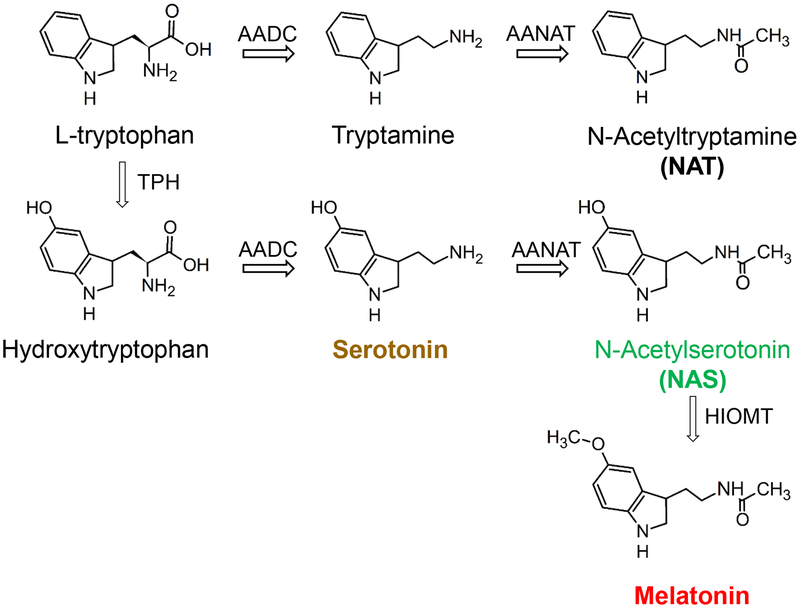
The pineal gland converts circadian clock signals into nocturnal secretion of melatonin, a hormone of darkness. In mammals, retinal ganglion cells send electrical signals to the suprachiasmatic nucleus (SCN), the master biological clock1,2, that are relayed by neurons in several stages to the superior cervical ganglia. These sympathetic neurons project to the pineal gland where they release norepinephrine (NE). Activation of α1 and β1 adrenergic receptors on pinealocytes increases intracellular calcium and cAMP,3–5 upregulating the gateway enzyme arylalkylamine N-acetyltransferase (AANAT). Figure 1 shows the resulting synthesis of melatonin (red): AANAT acetylates serotonin (yellow) to N-acetylserotonin (NAS, green), and hydroxyindole-O-methyltransferase (HIOMT) methylates NAS to melatonin. Like serotonin, the prototypical indoleamine tryptamine is also synthesized from L-tryptophan, and the rate-limiting AANAT enzyme acetylates tryptamine to N-acetyltryptamine (NAT), a structural analog of melatonin6 (Figure 1). Thus, because of nocturnal activation of AANAT, three related indoleamines should be generated in the pineal gland at night: NAT, NAS, and melatonin.
Figure 1. Melatonin synthesis from L-tryptophan in the pineal gland.
Enzymes: TPH, tryptophan hydroxylase; AADC, aromatic amino acid decarboxylase; AANAT, arylalkylamine N-acetyltransferase; HIOMT, hydroxyindole-O-methyltransferase. AANAT is active in the pineal gland only at night and is the gateway enzyme for nocturnal synthesis of melatonin, NAT, and NAS. Those indoleamines and serotonin are color-coded throughout the figures.
NAT and NAS have received less attention than melatonin. In previous elegant studies with pineal microdialysis, NAS and melatonin levels showed parallel sustained nocturnal elevation within the rat pineal gland.7 In other work, NAT levels became a few-fold higher at night than in day in blood plasma of rhesus macaque.6 Could NAT and NAS also serve as signals of night? We evaluate that question in the rat.
Melatonin regulates sleep and seasonal breeding.8–10 The actions on sleep involve the widely distributed G protein-coupled melatonin receptors, MT1 (MTNR1A) and MT2 (MTNR1B).11–14 These receptors with ~55% sequence identity15 couple primarily to signaling by Gi proteins,16 but MT1 may also couple to Gq/11.17 The two melatonin receptors can form homo- and heterodimers in HEK293 cells.18,19
Our experiments test whether NAT and NAS could act as night hormones that activate melatonin receptors in a circadian rhythm using four criteria: They must (i) be agonists of melatonin receptors, (ii) be secreted when NE stimulates the pineal gland, (iii) show large amplitude circadian fluctuations in blood, and (iv) have blood concentrations sufficient to stimulate melatonin receptors. We show that NAT and NAS satisfy the first two criteria well but fail in the last two. These experiments are critical for understanding the activation of melatonin receptors by indoleamines in vivo. They bear on chronobiology and problems of shift work, jet lag, sleeplessness and seasonal depression.
MATERIALS AND METHODS
Cell culture, plasmids, and chemicals
Human embryonic kidney 293 (HEK293) cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY) supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C with 5% CO2. Cells at 70–80% confluency were transiently transfected with human MT1 and/or MT2 cDNA (cDNA Resource Center, Bloomsberg, PA) using 1 mg/mL polyethyleneimine (Polysciences Inc., Warrington, PA) 48 h before the experiments. Melatonin, (±)-norepinephrine-(+)-bitartrate salt (NE), luzindole, and serotonin-hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO), N-acetyltryptamine (NAT) and 4P-PDOT from Tocris Bioscience (Minneapolis, MN), N-acetyl-5-hydroxytryptamine (N-acetylserotonin, NAS) from Enzo Life Sciences (Farmingdale, NY), and Pertussis toxin from List Biological Laboratories, Inc. (Campbell, CA).
Dynamic Mass Redistribution (DMR) assay
After 24-h transient transfection, 105 HEK293 cells were seeded per well with 40 μL DMEM in an Epic-sensor 384-well microplate (Corning Incorporated, Corning, NY) and cultured for another 24 h. Cells were counted by hemocytometer. The plate was transferred to the Corning-Epic-BT reader (Corning Incorporated) inside a 5% CO2 incubator at 37°C. After a stable baseline, concentrated compounds (5X, 10 μL) in HBSS buffer (Gibco) were added by 96-well Benchtop Pipettor (Sorenson BioScience, Salt Lake City, UT) and DMR signals recorded for 1 h using Epic imager software. This method detects redistribution of cellular mass within the evanescent wave formed by oblique illumination of a resonant-waveguide grating-biosensor in the bottom of each well.
For agonist-response relations, graded series of agonist concentrations were applied at the start of DMR measurements (labeled time zero). For receptor-antagonist studies, cells were pre-incubated with various concentrations of antagonists luzindole or 4P-PDOT for 2 h; then, each agonist was added to a final concentration 2 to 10-fold above its measured EC50 and DMR measurements begun. For pertussis toxin inhibition, cells were seeded on the plate with 250 ng/mL toxin for 16–20 h. Then, agonist was applied for DMR measurements. In all experiments, each condition was applied in quadruplicate in adjacent wells. DMR data were analyzed offline with IGOR PRO (Wavemetrics, Lake Oswego, OR) by normalizing and averaging results for identical quadruplicate wells after subtracting control responses and the mean of all experiments was fitted with the Hill equation.
Animals
Male Sprague-Dawley rats (6–18 weeks old, 390–520 g) were housed with a 14:10 light-dark cycle for >2 weeks. For blood sampling, rats were transferred to a different facility with a 12:12 light:dark cycle on the day of surgery to implant a jugular catheter. Animal care, surgeries for catheter installation and pinealectomy, blood sampling, and dissection of the pineal gland were performed by protocols approved by the University of Washington Institutional Animal Care and Use Committee (IACUC).
Catheter implantation
A catheter was installed chronically in the jugular vein to draw blood samples multiple times following published methods20,21 with modifications. Briefly, rats were anesthetized by isoflurane gas, and a commercial sterile catheter with round end (Part No.: C30PU-RJV1402, Instech Inc., Plymouth Meeting, PA) was inserted into the jugular vein and secured by a silk suture around the vein. The catheter tube exited the back side of the neck and was closed by a specially designed plug (Pinport®, Instech Inc.) with an adaptor (Pinport injector®, Instech Inc.) that allowed convenient blood withdrawal.
Pinealectomy
In some animals, the pineal gland was removed surgically following a modified published method.22 After isoflurane anesthesia, the head was secured in a stereotaxic frame. A longitudinal incision (~1.75 cm) was made in the midline of the head extending to the occipital ridge. A circular parietal window (~9 mm diameter) was opened in the skull with a high-speed No. 5 bone-burr bit. Then the dura was cut along the perimeter with a microscissor. The pineal gland under the superior sagittal vein was removed using No. 5 bent forceps. A small piece of Surgifoam® absorbable gelatin sponge (Ethicon, Somervile, NJ) stopped bleeding. The disk of bone removed from the skull was reinstalled and the incision closed using 11-mm Michel wound clips.
Blood collection
Blood samples (100 −300 μL) were collected through the implanted catheter with a 1-ml disposable syringe. The improved Pinport® and Injector® allowed sampling from conscious animals without physical restriction. Heparin (20 unit/mL blood) deposited in the syringe inhibited blood clotting. Samples were transferred to ice-cold Eppendorf tubes and centrifuged at 3,000 rpm for 3 min. Serum in the supernatant was transferred to an ice-cold Eppendorf tube and frozen at −80°C. On the day of mass spectrometry analysis, the frozen serum was thawed. Trichloroacetic acid (TCA) was added to 10% final concentration to precipitate proteins and the tube vortexed thoroughly (~20 s). TCA-treated samples were further centrifuged at 14,000 rpm for 10 min. The clear supernatant was collected for ultra-performance liquid chromatography/mass spectrometry (UPLC/MS) analysis. We refer to this as serum concentration.
Measurement of secretion from pineal glands
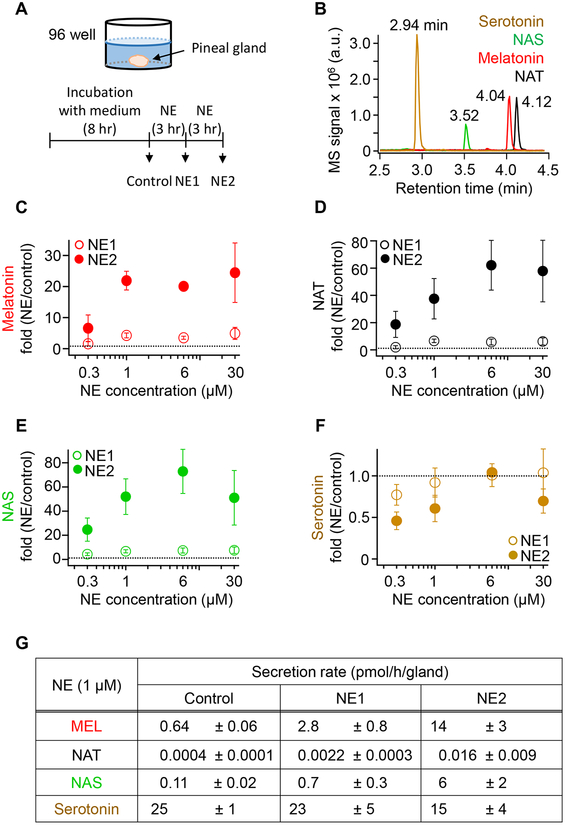
Pineal glands were isolated from rats sacrificed by CO2 during the day. Glands were kept in DMEM (Gibco) supplemented with 10% FBS and 1% penicillin/streptomycin for 48 h at 37°C with 5% CO2. The gland was transferred daily to fresh medium. To estimate pineal secretion, glands were washed twice with M199 medium (Life Technologies, Carlsbad, CA) for 15 min each and transferred into a 96-well plate with 200 μL M199 and incubated at 37°C and 5% CO2. In the experiment of Figure 6, solution was collected (arrows) and new solution added: 8 h in M199 for baseline secretion, 3 h in M199 supplemented with given concentrations of norepinephrine (NE) for stimulated secretion, and 3 h additional in NE for a second stimulated secretion. The samples (~200 μL) were centrifuged at 14,000 rpm for 10 min and frozen at −80°C until the UPLC/MS analysis.
Figure 6. Norepinephrine (NE) stimulates melatonin, NAT, and NAS secretion from isolated rat pineal glands.
(A) Schematic drawing of a pineal gland in one well of a 96-well plate. The gland was incubated in M199 medium (8 h) and then in NE-containing solutions twice for 3h each. (B) Representative chromatographic display of UPLC/MS signals for a mixture of 100 nM serotonin and 1 nM NAS, melatonin, and NAT. Note the shorter retention time of hydrophilic serotonin and subsequent later elution of more hydrophobic NAS, melatonin, and NAT. Mass spectrometry (MS) signals are given in arbitrary units (a.u.). (C-F) Secretion of melatonin (C), NAT (D), NAS (E), and serotonin (F), given as fold value compared to baseline. A thin horizontal line marks 1.0, corresponding to no change. (F) Absolute secretion rates calculated using standard curves for these compounds. The data are means ± SEM (n = 3).
UPLC/MS
Mass spectrometry was performed by Waters Acquity UPLC and Xevo TQ-S MS/MS (Waters Co., Milford, MA) in multiple-reaction-monitoring (MRM) mode. Samples were transferred to 11-mm clear glass crimp-top vials (Thermo Fisher Scientific, Waltham, MA) matched to the 9-mm autosampler. The FTN autosampler, equilibrated at 4°C, injected 10–40 μL volumes onto a C18 column (Waters Acquity UPLC HSS T3 column, 100 Å, 1.8 μm, 2.1 mm × 100 mm). Chromatographic separation at room temperature used 0.3 ml/min of mobile phase combining mixtures of water plus 10 mM formic acid (solution A) and acetonitrile plus 10 mM formic acid (solution B). The elution gradient was initiated with A/B (98:2 v/v) for 1 min, then increased to 2:98 v/v over 4 min, and held for 1 min before re-equilibration with the starting conditions for 2 min. Analytes were ionized in ESI-positive mode with settings: capillary voltage 3.2 kV, source temperature 150°C, and desolvation temperature 500°C. MRM acquisition monitored the following m/z ion fragments (daughter>fragment): melatonin 233.1>174.1, serotonin 177.0>160.1, NAS 219.1>160.1, and NAT 203.2>144.0. Sample concentrations were calibrated with a standard curve using known concentrations (0.003–3 nM for melatonin/NAS/NAT and 0.1–300 nM for serotonin) in UltraPure distilled water (Invitrogen) or in HEPES-buffered Ringer’s solution (in mM): 130 NaCl, 5 KCl, 2 CaCl2, 10 HEPES, 10 glucose, pH adjusted to 7.3. The detection efficiency for the indoleamine molecules was comparable (<20% different at 1 nM) in water versus Ringer’s. The standard curve was typically linear for NAS/NAT/serotonin but occasionally exhibited slight leveling near the lowest concentration (0.003 nM) with melatonin. Because of high indolamine concentrations, samples from isolated pineal gland incubations were diluted 10 times with water before analysis. Deuterated serotonin-d4 (181.0>164.1) and melatonin-d4 (237.1>178.1) were added as internal standards for peak elution times. In preliminary experiments, 1 nM of the internal standard was used to calibrate the injection volume. However, the injection error was negligible (less than 5%). Data acquired by Masslynx software (Waters Co.) were analyzed using QuanLynx software (Waters Co.) to give peak areas.
Statistical analysis
Results are presented as mean±standard error of the mean (SEM). Significance was estimated using Student’s t-test or, for Figure 5, two-way ANOVA (GraphPad Prism). *P < 0.05, **P < 0.01, and ***P < 0.001 were regarded as statistically significant. For DMR measurements, each well was regarded as an independent measurement. For blood samples in Figure 8, all day samples were compared with all night samples to obtain P values using Student’s t-test.
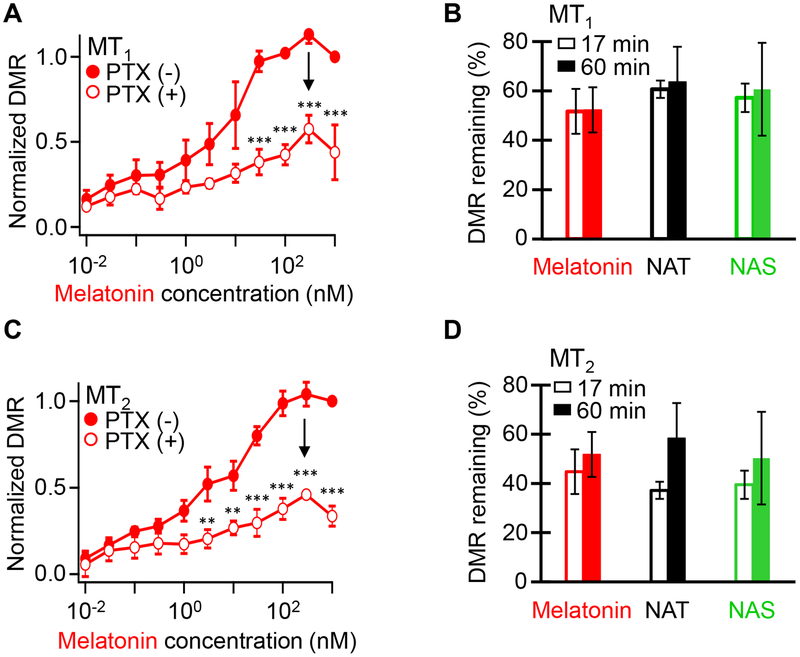
Figure 5. Pertussis toxin (PTX) reduces DMR responses of melatonin receptors.
(A) Concentration-response curves for melatonin in MT1-expressing HEK293 cells. Closed and open circles denote the absence and presence of 250 ng/mL PTX pretreatment for 16–20 h, respectively. These DMR data are collected at 17 min of melatonin-induced time-dependent response curves. (B) The percentage of DMR response remaining after PTX inhibition at 0.3 μM melatonin, 30 μM NAT, or 30 μM NAS in MT1-expressing HEK293 cells. Open and closed bars indicate DMR response at 17 and 60 min, respectively. (C) Concentration-response curves for melatonin in MT2-expressing HEK293 cells. (D) The percentage of DMR response remaining after PTX inhibition at 0.3 μM melatonin, 30 μM NAT, or 30 μM NAS in MT2-expressing HEK293 cells. The data are means ± SEM of 12 wells from 3 independent experiments. DMR responses were compared using two-way ANOVA. **P < 0.01 and ***P < 0.001; points not marked did not differ significantly.
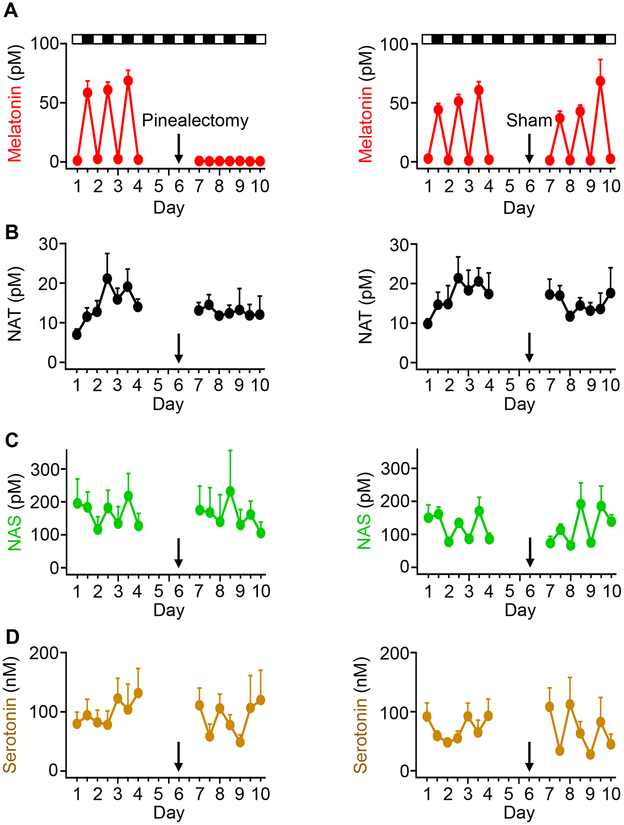
Figure 8. Only the circadian melatonin rhythm is driven by the pineal gland.
Blood was collected from rats through an implanted catheter twice per day for four days (1 PM for day ZT 7 and 1 AM for night ZT 19). Rats had a rest on the 5th day, and then were divided into two groups for pinealectomy or sham operations. The surgery was done on the 6th day (black arrow). From the 7th day, blood collections were done for four days. All collected serum samples were analyzed with UPLC/MS to measure the levels of the four indoleamines: melatonin (A), NAT (B), NAS (C), and serotonin (D). The left column is from the group of 7 animals undergoing pinealectomy, and the right column is from the second group of 7 animals undergoing sham operations. Data are means ± SEM. Each data point includes 3–7 blood samples.
RESULTS
We first determine the sensitivity of melatonin receptors to three indoleamines.
Several indoleamines activate melatonin receptors
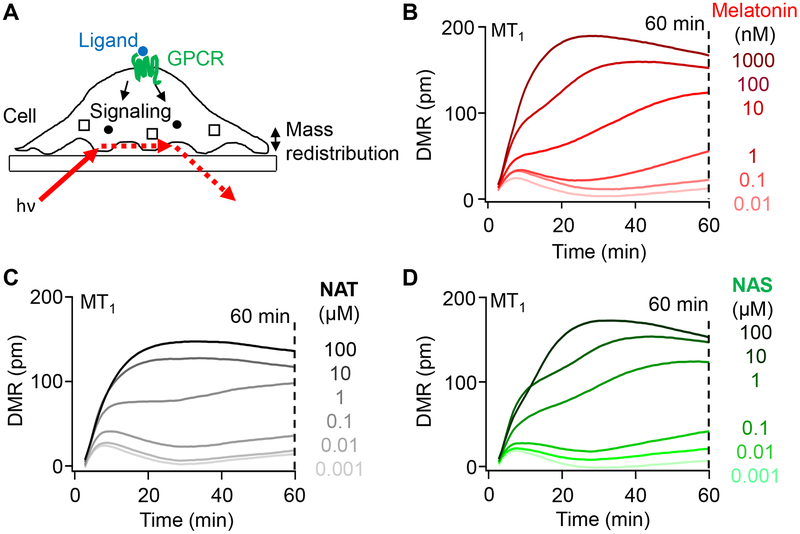
The agonist activity of melatonin, NAT, and NAS on human MT1 and MT2 melatonin receptors was examined by the dynamic mass redistribution (DMR) assay (Figure 2A).23 This high-throughput optical assay records changes of local refractive index in the footprint of plated cells. Figure 2B shows time courses of DMR responses evoked by melatonin in HEK293 cells transfected with MT1 receptors. Responses, measured as reflected wavelength shift in picometers, increased with increasing concentrations of melatonin from 0.01 to 1000 nM, and continued to evolve over 60 min. Similar experiments with NAT and NAS showed similar patterns (Figure 2C,D). For these other indoleamines, concentrations tested were 100-fold higher than for melatonin since they were less potent.
Figure 2. Melatonin, NAT, and NAS activate melatonin receptors, MT1 and MT2.
(A) Principle of dynamic mass redistribution (DMR) measurement showing one cell stimulated by a ligand and illuminated at an angle from below creating an evanescent field in the cell. The reflected light is the DMR response. (B-D) Representative 60-min time courses of DMR responses induced by different concentrations of melatonin (B), NAT (C), and NAS (D). Each curve is the mean of 4 adjacent test wells in a single multiwell plate minus the mean of 4 adjacent control wells (without agonist). HEK293 cells were transfected with MT1 and treated with melatonin ranging from 0.01 nM to 1 μM and NAT and NAS ranging from 1 nM to 100 μM. The DMR response was evaluated at 60 min to estimate all concentration-response curves for Figure 3 and 4.
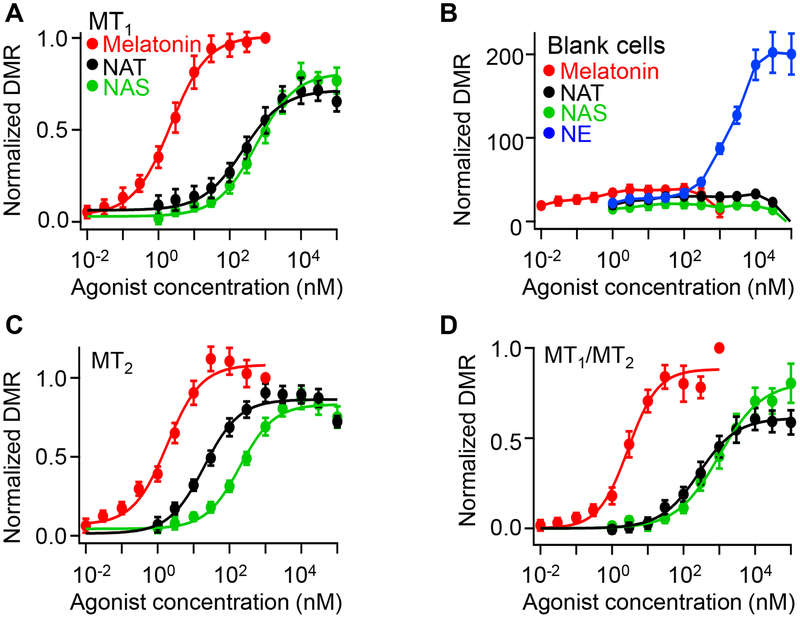
Concentration-response relations for each agonist plotted DMR values at 60 min against concentration. Thus, Figure 3A shows averaged data with MT1 receptors normalized to the highest melatonin response. Compared to melatonin, NAT and NAS were partial agonists with potency order melatonin>>NAT>NAS for this receptor (Table 1). Control experiments with untransfected cells showed that receptor transfection was required to get these large responses (Figure 3B). An additional control in all experiments monitored responses of endogenous α−1B adrenergic receptors24 to a graded series of norepinephrine (NE) concentrations. Endogenous adrenergic responses were always robust (blue symbols in Figure 3B) but indicated much lower potency (micromolar) than for indoleamines acting on melatonin receptors. Cells transfected with MT2 receptors also responded well to the three indoleamines (Figure 3C). For each agonist, the unnormalized magnitudes of the peak DMR responses with MT2 receptors were similar to those with MT1 receptors ± 10%, and the relative order of potency was qualitatively the same (melatonin>NAT>NAS) (Table 1). Because of reports that melatonin receptors can heterodimerize,16,25 we also tried cotransfecting MT1 with MT2 receptors (MT1/MT2) (Figure 3D). The responses of MT1/MT2 cells were not distinguishable from those with MT1 alone.
Figure 3. Melatonin, NAT, and NAS activate melatonin receptors, MT1 and MT2, at different concentrations.
(A) Concentration-response curves for melatonin, NAT, and NAS in MT1 expressing HEK293 cells. DMR data at 60 min from experiments like Figure 2 are shown as symbols. (B) Concentration-response relations for melatonin, NAT, NAS, and NE in untransfected HEK293 cells (blank cells). NE was used as a positive control to activate endogenous adrenergic receptors. (C-D) Concentration-response curves for melatonin, NAT, and NAS in MT2- (C) and MT1/MT2- (D) expressing HEK293 cells. Data are means ± SEM of 20–44 wells from three independent experiments and fitted with a Hill function (smooth curve).
Table 1.
EC50 and IC50 values for Melatonin receptors
| Activation (EC50, nM) | Inhibition (IC50, μM) | |||
|---|---|---|---|---|
| Luzindole | 4P-PDOT | |||
| MEL | 2.2 ± 0.2 | 72 ± 6 | 39 ± 6 | |
| MT1 | NAT | 235 ± 63 | 14 ± 2 | 1.5 ± 0.3 |
| NAS | 555 ± 96 | 8 ± 1 | 8 ± 1 | |
| MEL | 1.8 ± 0.5 | 1.1 ± 0.4 | 0.007 ± 0.002 | |
| MT2 | NAT | 20 ± 6 | 4 ± 2 | 0.003 ± 0.002 |
| NAS | 195 ± 40 | 3 ± 1 | 0.003 ± 0.001 | |
| MEL | 2.8 ± 0.8 | 57 ± 8 | 353 ± 62 | |
| MT1/MT2 | NAT | 266 ± 64 | 8 ± 2 | 2.3 ± 0.7 |
| NAS | 1007 ± 354 | 3.8 ± 0.7 | 6 ± 2 | |
Data are shown as means ± error of Hill equation fit
Taken together, NAT and NAS activate both MT1 and MT2 receptors, although with agonist potencies at least an order of magnitude less than for melatonin. Further, all three receptor agonists were more potent on MT2 than on MT1 receptors.
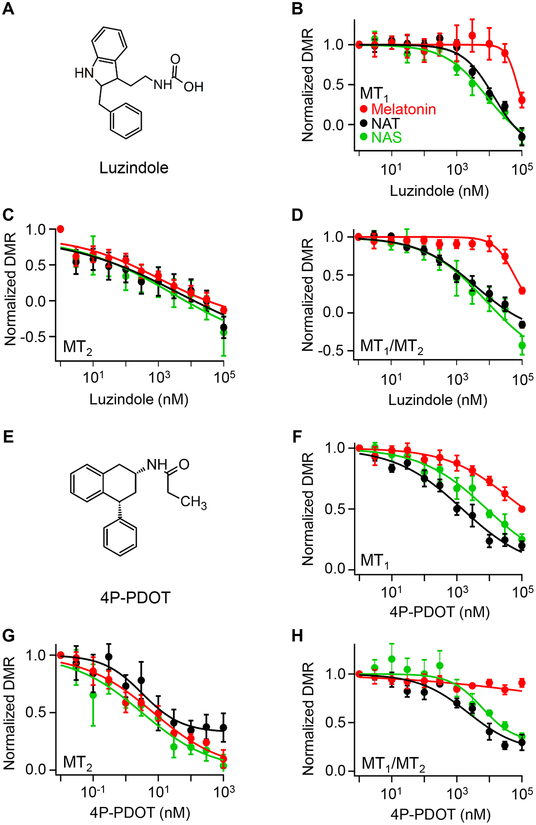
Melatonin receptor antagonists inhibit DMR responses induced by melatonin, NAT, and NAS
To substantiate activation of melatonin receptors by the three agonist indoleamines, we used selective receptor antagonists, luzindole and 4P-PDOT (Figure 4A,E). Previous extensive binding assays using 2-[125I]iodomelatonin26 concluded that luzindole and 4P-PDOT are MT2-preferring competitive antagonists with 25-fold higher (luzindole) or 1000-fold higher affinity for MT2 over MT1. In our work, HEK293 cells transfected with melatonin receptors were pre-treated with different antagonist concentrations for 2 hours. Each indoleamine agonist was then applied at relatively high concentration (Figure 4B–D,F–H). In all examples, there was a graded inhibition of DMR responses as the antagonist concentration was increased. Both antagonists were more potent on MT2 receptors than on MT1 receptors (Table 1), and broadly, 4P-PDOT was more potent than luzindole (note shifted concentration scale in Figure 4G). Again MT1/MT2-coexpressing cells (Figure 4D,H) were most similar to MT1-expressing cells (Table 1). The inhibition of DMR responses by melatonin receptor antagonists confirmed that NAT and NAS were activating classical melatonin receptors in the DMR assay.
Figure 4. MT1 and MT2 receptor antagonists, luzindole and 4P-PDOT, inhibit DMR responses induced by melatonin, NAS, and NAT.
(A) Structure of luzindole. (B-D) Concentration-dependent antagonism by luzindole with MT1- (B), MT2- (C), and MT1/MT2- (D) expressing HEK293 cells. DMR responses were normalized to the value without the antagonist. (E) Structure of 4P-PDOT. (F-H) Concentration-dependent antagonism by 4P-PDOT with MT1- (F), MT2- (G), and MT1/MT2- (H) expressing HEK293 cells. Transfected cells were pre-incubated with antagonist for 2 hours at 37°C with 5% CO2, and then treated with concentrations of melatonin, NAT, and NAS that would activate the receptors strongly: melatonin (μM) 0.25, 0.025, and 0.625; NAT (μM) 5, 0.05, 3.75; NAS (μM) 32.5, 1.25, and 7.5 for MT1, MT2, and MT1/MT2, respectively. The data shown are means ± SEM of 12 to 16 wells from 2 independent experiments fitted with a Hill function.
Pertussis toxin reduces the DMR response
Melatonin receptors couple to the G protein Gi.16 As a final validation of the DMR responses, we verified sensitivity to pertussis toxin (PTX), which inactivates Gi. Cells were incubated with or without 250 ng/mL PTX for 16–20 h. Toxin pretreatment reduced agonist responses with MT1 receptors to ~61% of control measured after 17 min in agonist, and to ~64 % after 60 min in agonist (Figure 5A,B). For MT2 receptors, PTX reduced responses further and slowed them (Figure 5C,D). Thus, all melatonin responses were at least partly sensitive to PTX, and MT2 receptor responses were possibly more sensitive than MT1.
In summary, DMR experiments confirm that NAT and NAS satisfy our first criterion. As had been suggested from competitive receptor binding assays and by a functional transmitter release assay,11,26,27 NAT and NAS are agonists at both melatonin receptors.
Norepinephrine evokes secretion of melatonin, NAT, and NAS from isolated rat pineal glands
We turned to our second criterion by testing for secretion of the three agonist indoleamines from intact isolated pineal glands stimulated by their natural activator, NE. Dissected glands were incubated for two days in M199 medium. During this time, nerve terminals that release NE should degenerate.28 Then secretion was collected using solution changes with one gland per well (Figure 6A). Baseline secretion was collected for 8 h. Then, stimulated secretion was collected in medium containing increasing concentrations of NE for 3 h (called NE1). Finally, stimulated secretion was collected for a second 3-h period in a fresh solution with the same NE concentration (called NE2). Collected media were analyzed by UPLC/MS to calculate the rate of secretion. With the non-polar C18 column and reverse-phase chromatography, the most polar compound eluted first in the order: serotonin, NAS, melatonin, and NAT (Figure 6B).
NE increased secretion of the three agonist indoleamines in a graded manner (Figure 6C–F). In the first 3 h (NE1, open symbols), secretion was modest, and in the second 3 h (NE2, closed symbols), secretion was robust. With 1 μM NE, the secretion of melatonin was elevated to 4.3-fold compared to baseline during NE1, and to 22-fold during NE2. Thus there was a several-hour induction period in NE before full secretion developed. In absolute terms, the melatonin secretion for 1 μM NE was: basal, 0.64±0.06 and NE2, 14±3 pmol/h/gland (Figure 6G). The fold-stimulation and absolute rates of secretion for NAS were similar to melatonin, but for NAT the absolute rate was orders of magnitude smaller than for the other two agonists although the fold-stimulation was again high (Figures 6D,E,G). Thus, melatonin, NAT, and NAS were synthesized, secreted, and easily detected from the NE-stimulated isolated pineal gland. On the other hand, serotonin, the precursor of NAS and melatonin, showed an opposite pattern. Serotonin secretion was always orders of magnitude higher than that of NAS and melatonin (Figure 6G), and it actually decreased slightly during incubation with NE as if the rapid synthesis of serotonin was not quite able to keep up with its stimulated conversion to NAS and melatonin (Figure 6F). In other experiments, the NE stimulation of agonist indoleamine secretion was fully reversed within a few hours of removing NE. We did not study this time course carefully. It may be limited by the rate of diffusion of NE out of an intact gland.
In summary, NAT and NAS satisfy our second criterion for night hormones; They are secreted from the pineal gland in response to NE stimulus
Unlike melatonin, NAS and NAT are not strongly elevated in circulating blood at night
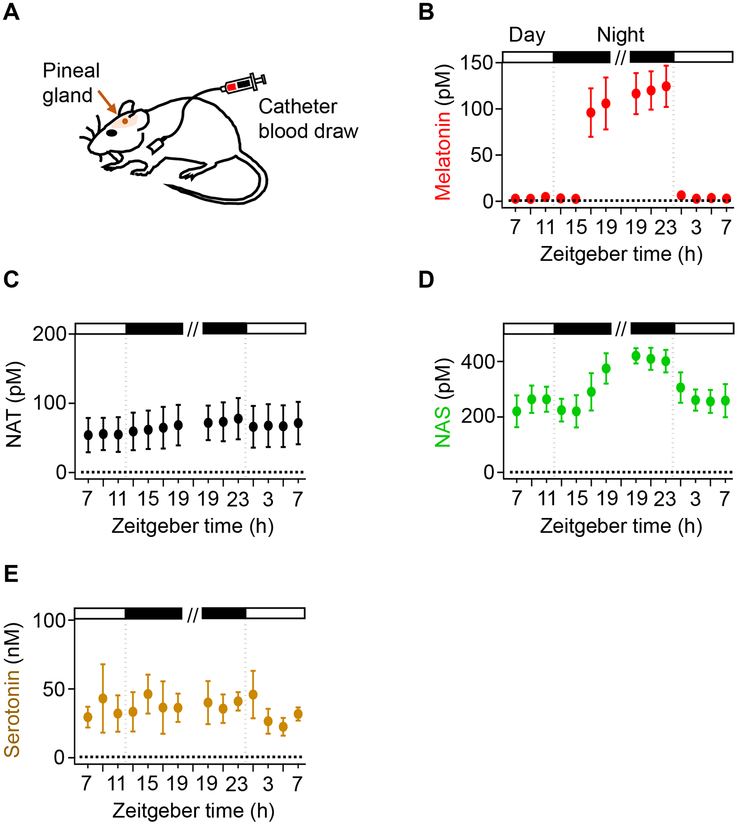
We turn to our third criterion. To evaluate NAT and NAS as potential night hormones, we compared their blood levels to that of melatonin using periodic day and night sampling. A chronic jugular catheter withdrew blood periodically (Figure 7A) in two sessions, each lasting 12 h but separated by a day. These rats were housed with a 12:12 hour light:dark cycle, and time is given in conventional Zeitgeber time (ZT), where ZT 0 is the time of lights on. The first sampling session was between ZT 7 and 19, and, 24 h later, the second session was between ZT 19 and 7. Sera collected every 2 h were analyzed with UPLC/MS. Averaged in the light between ZT 7 and 11, the basal melatonin serum level was 3.4±0.5 pM (Figure 7B). With a 3 – 4 h delay after the onset of dark at ZT 12, serum melatonin increased up to 96±23 pM by ZT 17 and further to 124±18 pM late in the night, but when lights were turned on at ZT 0, serum melatonin dropped within an hour to control levels 3.9±0.6 pM.
Figure 7. Melatonin level is elevated in circulating blood at night.
(A) Schematic drawing showing rat, pineal gland, and indwelling catheter. The pineal gland is indicated with a brown arrow. Blood from the jugular vein was collected through an implanted catheter at various time points. (B-E) Two sets of 12-h blood sampling. For the first set, blood was collected every two hours between Zeitgeber time (ZT) 7 and 19, where by convention ZT = 0 signifies the time of lights on. After 24 hours of rest (as indicated by the broken line), the second set of sampling was done from ZT 19 to ZT 7. The samples were analyzed with UPLC/MS to measure the concentration of melatonin (B), NAT (C), NAS (D), and serotonin (E) in serum. White and black boxes indicate light and dark periods, respectively. Data are means ± SEM from 4 rats in total. Each data point includes 3–4 blood samples.
For NAT, NAS, and serotonin, the serum concentration was already elevated in the day, and any circadian rhythm in circulating blood was weak or absent. The day level for NAT (55±11 pM) was much higher than for day melatonin (3.4±0.5 pM), and showed no significant changes between day and night (Figure 7C). The day level for NAS was even higher (249±24 pM; Figure 7D), and, during the night, it increased by an additional 170 pM with a delayed onset and fast offset. However, the high day level meant that when NAS increased at night, its change was only 1.7-fold, compared to 36-fold for melatonin. Serum serotonin was always several orders of magnitude higher (35±8 nM), and fluctuated without a pronounced day:night pattern (Figure 7E). Overall, our results demonstrated that despite obvious stimulated synthesis of NAT and NAS in isolated pineal glands, a high basal blood level of these indoleamines means that melatonin is the only agonist indolamine that exhibits a wide dynamic range of daily fluctuation in rat blood appropriate for a circulating messenger of night.
Unlike levels of melatonin, serum levels of NAS and NAT are mostly not driven by the pineal gland
We wanted to determine whether the blood levels of indoleamines depended on secretion from the pineal gland. Pinealectomized and sham-operated animals were compared. For 4 days, we sampled blood of unoperated rats every 12 h, once at ZT 7 for a day sample and once at ZT 19 for a night sample. On day 6, rats underwent either pinealectomy or a sham surgery done in the same way except the pineal gland was not removed. Starting the day after the surgery, blood sampling continued for 4 more days. As before, serum melatonin showed significant circadian oscillations from 2.0±0.4 pM in day to 63±5 pM at night (Figure 8A). The sham-operated group continued to show melatonin oscillations, but after pinealectomy, serum melatonin levels fell below previous basal levels even at night (average, 0.6±0.2 pM). Clearly, the pineal gland is the principal source for circulating blood melatonin in night and day, and removal of the gland disrupts any circadian melatonin rhythm. NAS showed a noticeably smaller fluctuation with a considerable basal level during the daytime, albeit any circadian difference lacked statistical significance (Figure 8C). Pinealectomy did not change the average day or night NAS. The sham-operated group showed a small residual oscillation both before the surgery (100±12 pM during day to 155±16 pM during night, p<0.01) and after (88±11 pM during day to 166±31 pM during night, p<0.05), indicating a slight increase of NAS at night. NAT and serotonin did not show significant changes at night or after pinealectomy (Figure 8B,D). Taken together, in circulating blood in the rat the dynamic circadian oscillations of melatonin are driven by the pineal gland, and the flatter concentrations of NAT, NAS, and serotonin reflect other less-cyclic sources.
In summary, NAT and NAS fail our third criterion; Only melatonin shows large daily fluctuation in blood
DISCUSSION
We started with four criteria for a night hormone. Consistent with the hypothesis that NAT and NAS have effects similar to melatonin, we confirmed that they can activate human melatonin receptors and that they are actively secreted from the NE-stimulated rat pineal gland. However, their circadian rhythms in the circulation have low amplitude compared to melatonin, and we show below that their concentrations are too low.
NAT and NAS are lower potency partial agonists of melatonin receptors
The DMR assay reports that NAT and NAS activate human MT1 and MT2 receptors with lower maximum response and 2.5–100-fold lower potency than melatonin. Hence, they are weak partial agonists at these receptors. Inhibition of the DMR agonist responses by two receptor antagonists and by pertussis toxin confirms the receptor requirement of these actions in transfected HEK293 cells; and the greater potency of the two antagonist drugs for block of the MT2 response compared to the MT1 responses concords with reports from other assays.11,26,27 Classical literature gives extensive pharmacological characterization of expressed human and native hamster and rabbit melatonin receptors using displacement of 2-[125I]-iodomelatonin binding and stimulation of [35S]-GTPγS binding in cell lysates, as well as inhibition of dopamine release from retina.11,26,27,29 Our EC50 values obtained with the DMR assay, fall near the top range of the previously reported confidence intervals for Ki values from competitive binding assays.26 The published assays also agree that NAT and NAS are partial agonists.
MT1/MT2 heterodimers
A number of G-protein-coupled receptors including MT1 and MT2 can form homodimers and heterodimers.25 In our DMR assay, cells coexpressing MT1 and MT2 responded as if they were transfected with pure MT1 with no novel properties. Either the fraction of dimers is small or heterodimerization yields receptors whose pharmacology most resembles pure MT1.
Stimulated secretion from the pineal gland
In our experiments, a single isolated and quiescent pineal gland releases only 0.6 pmol/h of melatonin, 0.1 pmol/h of NAS, and also ~25 pmol/h of the precursor serotonin. However, when AANAT activity is stimulated by 1 μM NE, the pineal output increases dramatically after a delay of several hours. Production of NAS must rise to about 20 pmol/h, of which 6 pmol/h escapes to the bath as NAS (Figure 6G), and the remainder becomes methylated to melatonin (Figure 1) before it escapes as well. Adrenergic stimulation of secretion of melatonin and NAS from isolated pineal glands is well known with good evidence for the involvement of α1 and β1 adrenergic receptors.3–5,30,31 The synchronous release of NAS and melatonin is known from pineal cell cultures and microdialysis.7,32,33 We also show here that secretion of NAT from isolated glands rises in parallel with the others, but with much smaller maximum rate. Perhaps the pineal gland generates little tryptamine precursor, or possibly NAT that is produced is rapidly hydroxylated to NAS by tryptophan hydroxylase (TPH), (another untested vertical reaction arrow for the upper right in Figure 1).
We propose that parallel time courses of secretion upon NE application can be rationalized as follows: From the lipid solubility and membrane permeability of melatonin, we showed previously that melatonin cannot be stored in cells after it is synthesized.34 Rather it remains in pinealocytes for only ~3.5 s before it escapes by diffusion; similarly, NAS, which is only slightly less lipid soluble escapes in 18 s.34 When the enzyme AANAT becomes active, it N-acetylates serotonin to make membrane-permeant NAS, more than half of which is converted to membrane-permeant melatonin in the few seconds before the NAS molecules diffuse away. Thus the “secretion” of these two indoleamines is obligately coincident escape. The logic of that paper also applies to NAT with an oil-water distribution coefficient of 40 (compared to 16 for melatonin and 3 for NAS) (PubChem data base: https://pubchem.ncbi.nlm.nih.gov/compound/70547), predicting a cellular retention time of only 1.4 s for NAT. Therefore, when AANAT becomes active and N-acetylates tryptamine to NAT (Figure 1), the secretion of NAT will be synchronous with that of NAS and melatonin.
The circadian time course of melatonin in the circulation
As expected for a hormone of night, the melatonin concentration in circulating rat serum shows a long-known, profound day-night concentration switch (Figure 7). The dynamic changes of concentration parallel the day-night switch seen in pineal microdialysis7 of behaving rats and the NE-stimulated switch seen with isolated pineal glands (Figure 6). Pinealectomy shows that virtually all of the circulating melatonin originates from the pineal gland. Nevertheless, extrapineal melatonin production has been observed in the retina, brain, skin, bone marrow, and gastrointestinal tract.35 Despite a circadian rhythm similar to that of the pineal gland, retinal melatonin is regulated locally by intrinsic clocks entrained by light.36 Melatonin in the gastrointestinal tract is episodic and not circadian, and may comprise more than 400 times more total melatonin than the pineal gland.37 Yet pinealectomy abolished the melatonin rise in serum at night (Figure 8), so melatonin produced in peripheral tissues has little impact on the circulation.
A latent period for melatonin production follows after lights off. With a >3 h delay after lights off, serum melatonin rises relatively abruptly and then stays high through the night (Figure 7). A strain-dependent delay was detailed with high time resolution in careful pineal microdialysis experiments with free-running rats.7,33 That induction period was subdivided in other microdialysis experiments on Wistar rats showing secretion of NE from pineal sympathetic nerve fibers delayed by >1.5 h after lights-off, and then initiation of melatonin secretion delayed by an additional several hours after the rise of NE.38 Presumably, the first delay represents neural processing in the master circadian clock circuits and superior cervical ganglia, and the second represents biochemical events of gene expression and activation of AANAT in the pineal once NE delivery begins. We observed the latter biochemical delay lasting several hours in our experiments with isolated pineal glands (Figure 6). From a medical viewpoint, it is relevant that physiologically, melatonin does not normally rise until several hours after dark.
NAT and NAS in circulating blood
NAT receives little study, but the concept of NAT as a potential night hormone was recently explored by Backlund et al.6 For circulating serum NAT of the rhesus macaque they showed large inter-animal variability but a roughly 3-fold circadian variation with levels of 0.1–1 nM in the day and 0.6–2.5 nM at night. They did not test whether the rhythm had a pineal origin. Finding abundant AANAT transcripts in the macaque retina, Coon et al.39 suggested AANAT is used primarily for synthesis of NAT in retina and for synthesis of melatonin in the pineal. We detected some NE-evoked NAT secretion from the isolated rat pineal gland (Figure 6D) but a significant background and no circadian NAT rhythm in rat serum (Figure 7C,8B). The circulating level remained between 54 and 78 pM, and was unaffected by pinealectomy. Apparently the degree of NAT rhythmicity varies among species. Backlund et al.6 review other N-acetyltransferase enzymes in other tissues that make NAT and may be non-circadian sources of NAS and NAT.
Our fourth criterion for a hormone of night is that blood concentration should be high enough to stimulate melatonin receptors significantly. Our work in rats suggests that circulating melatonin rising to ~124 pM at night would suffice to activate 5% of MT1 receptors and 6% of MT2 receptors with a circadian rhythm, whereas constant NAT levels of ~78 pM could activate only 0.03% of MT1 receptors and 0.04% of MT2 receptors. Thus, circulating NAT accounts for a negligible proportion of peripheral melatonin receptor activation and would not interfere with the response to melatonin. Backlund et al.6 also concluded that NAT was unlikely to reach a blood concentration in rhesus macaque high enough for a peripheral hormone of night. If NAT has circadian physiological actions, they would have to occur locally in or near the pineal where NAT exhibits a circadian rhythm of larger amplitude before it dilutes by distribution into the body.
NAS has seen much more study. Early on, the level of NAS was shown to increase at night in hamsters.40,41
We detected robust NE-evoked NAS secretion from the isolated rat pineal gland (Figure 6E) but only a low-amplitude circadian NAS rhythm in serum superimposed on a high basal background (Figure 7D,8C). This basal level was maintained even after pinealectomy, indicating that most circulating NAS is produced outside the pineal gland. Analogous to NAT, a circulating level around 300 pM of NAS would activate only 0.05% of MT1 receptors and 0.2% of MT2 receptors. Thus both NAT and NAS fail our fourth criterion. Although NAS synthesis is prominent in the pineal gland and retina, AANAT and NAS are also present in the central nervous system.42–44 NAS may act locally in those tissues or it may enter the circulation. NAS is said to have high affinity for another non-G-protein coupled receptor putative melatonin binding site called MT345 and to activate tropomyosin receptor kinase B (TrkB), the receptor for brain derived neurotrophic factor BDNF.46 Thus, NAS may have functions other than as a melatonin precursor.
Despite many studies on melatonin’s circadian rhythm and physiological roles, much less has been learned about its precursor and chemical analogs. We demonstrate secretion of melatonin, NAT, and NAS from rat pineal glands and their ability to activate melatonin receptors. However, in rat, the serum levels of NAT and NAS are too low to activate peripheral receptors even at night, and they are not primarily maintained by secretion from the pineal gland, so they show only weak or no circadian variation. Only melatonin is driven strongly by the pineal gland and satisfies the requirements for a hormone of night.
Acknowledgements
We thank Lea M. Miller for technical assistance, Mr. Mark Moody for participation in preliminary experiments, Dr. Estela Munoz for helpful advice, and Drs. Oscar Vivas and Lizbeth de la Cruz for valuable comments on the manuscript, and the School of Pharmacy Mass Spectrometry Center for assistance.
This work was supported by grants from the National Institutes of Health: National Institute of General Medical Sciences R01GM083913 (BH) and R01GM100893 (CH) and National Institute of Neurological Disease and Stroke R01NS094211 (HdlI), and by the Wayne E. Crill Endowed Professorship (BH).
Footnotes
Competing interests: The authors declare that no competing interests exist.
References
- 1.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69(6):1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201–206. [DOI] [PubMed] [Google Scholar]
- 3.Sugden AL, Sugden D, Klein DC. Essential role of calcium influx in the adrenergic regulation of cAMP and cGMP in rat pinealocytes. The Journal of biological chemistry. 1986;261(25):11608–11612. [PubMed] [Google Scholar]
- 4.Reiter RJ, Tan DX, Manchester LC, Pilar Terron M, Flores LJ, Koppisepi S. Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv Med Sci. 2007;52:11–28. [PubMed] [Google Scholar]
- 5.Klein DC. Arylalkylamine N-acetyltransferase: “the Timezyme”. The Journal of biological chemistry. 2007;282(7):4233–4237. [DOI] [PubMed] [Google Scholar]
- 6.Backlund PS, Urbanski HF, Doll MA, et al. Daily Rhythm in Plasma N-acetyltryptamine. Journal of biological rhythms. 2017;32(3):195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Borjigin J. Relationship between nocturnal serotonin surge and melatonin onset in rodent pineal gland. J Circadian Rhythms. 2006;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006;273(13):2813–2838. [DOI] [PubMed] [Google Scholar]
- 9.Garrido M, Terron MP, Rodriguez AB. Chrononutrition against oxidative stress in aging. Oxid Med Cell Longev. 2013;2013:729804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaral FGD, Cipolla-Neto J. A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab. 2018;62(4):472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62(3):343–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klosen P, Sawarut L, Schuster C, et al. MT1 and MT2 melatonin receptors are expressed in nonoverlapping neuronal populations. Journal of pineal research. 2019:e12575. [DOI] [PubMed] [Google Scholar]
- 13.Pandi-Perumal SR, Trakht I, Srinivasan V, et al. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85(3):335–353. [DOI] [PubMed] [Google Scholar]
- 14.Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A. 1995;92(19):8734–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reppert SM, Weaver DR, Godson C. Melatonin receptors step into the light: cloning and classification of subtypes. Trends Pharmacol Sci. 1996;17(3):100–102. [DOI] [PubMed] [Google Scholar]
- 16.Jockers R, Maurice P, Boutin JA, Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br J Pharmacol. 2008;154(6):1182–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brydon L, Roka F, Petit L, et al. Dual signaling of human Mel1a melatonin receptors via Gi2, Gi3, and Gq/11 proteins. Mol Endocrinol. 1999;13(12):2025–2038. [DOI] [PubMed] [Google Scholar]
- 18.Baba K, Benleulmi-Chaachoua A, Journe AS, et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci Signal. 2013;6(296):ra89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayoub MA, Couturier C, Lucas-Meunier E, et al. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. The Journal of biological chemistry. 2002;277(24):21522–21528. [DOI] [PubMed] [Google Scholar]
- 20.Thrivikraman KV, Huot RL, Plotsky PM. Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Res Brain Res Protoc. 2002;10(2):84–94. [DOI] [PubMed] [Google Scholar]
- 21.Smarr BL, Morris E, de la Iglesia HO. The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology. 2012;153(6):2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuszak J, Rodin M. A new technique of pinealectomy for adult rats. Experientia. 1977;33(2):283–284. [DOI] [PubMed] [Google Scholar]
- 23.Schroder R, Schmidt J, Blattermann S, et al. Applying label-free dynamic mass redistribution technology to frame signaling of G protein-coupled receptors noninvasively in living cells. Nat Protoc. 2011;6(11):1748–1760. [DOI] [PubMed] [Google Scholar]
- 24.Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics. 2011;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayoub MA, Levoye A, Delagrange P, Jockers R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol Pharmacol. 2004;66(2):312–321. [DOI] [PubMed] [Google Scholar]
- 26.Dubocovich ML, Masana MI, Iacob S, Sauri DM. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn Schmiedebergs Arch Pharmacol. 1997;355(3):365–375. [DOI] [PubMed] [Google Scholar]
- 27.Nonno R, Pannacci M, Lucini V, Angeloni D, Fraschini F, Stankov BM. Ligand efficacy and potency at recombinant human MT2 melatonin receptors: evidence for agonist activity of some mt1-antagonists. Br J Pharmacol. 1999;127(5):1288–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afeche SC, Barbosa R, Scialfa JH, Terra IM, Cassola AC, Cipolla-Neto J. Effects of the blockade of high voltage-activated calcium channels on in vitro pineal melatonin synthesis. Cell Biochem Funct. 2006;24(6):499–505. [DOI] [PubMed] [Google Scholar]
- 29.Duncan MJ, Takahashi JS, Dubocovich ML. 2-[125I]iodomelatonin binding sites in hamster brain membranes: pharmacological characteristics and regional distribution. Endocrinology. 1988;122(5):1825–1833. [DOI] [PubMed] [Google Scholar]
- 30.Ganguly S, Coon SL, Klein DC. Control of melatonin synthesis in the mammalian pineal gland: the critical role of serotonin acetylation. Cell Tissue Res. 2002;309(1):127–137. [DOI] [PubMed] [Google Scholar]
- 31.Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55(2):325–395. [DOI] [PubMed] [Google Scholar]
- 32.Morton DJ. Development of an organ culture technique capable of monitoring most pineal gland indole metabolites. Journal of pineal research. 1990;8(4):335–345. [DOI] [PubMed] [Google Scholar]
- 33.Borjigin J, Zhang LS, Calinescu AA. Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol. 2012;349(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu H, Dickson EJ, Jung SR, Koh DS, Hille B. High membrane permeability for melatonin. J Gen Physiol. 2016;147(1):63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? Journal of pineal research. 2007;42(1):28–42. [DOI] [PubMed] [Google Scholar]
- 36.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272(5260):419–421. [DOI] [PubMed] [Google Scholar]
- 37.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47(10):2336–2348. [DOI] [PubMed] [Google Scholar]
- 38.Drijfhout WJ, van der Linde AG, Kooi SE, Grol CJ, Westerink BH. Norepinephrine release in the rat pineal gland: the input from the biological clock measured by in vivo microdialysis. J Neurochem. 1996;66(2):748–755. [DOI] [PubMed] [Google Scholar]
- 39.Coon SL, Del Olmo E, Young WS 3rd, Klein DC. Melatonin synthesis enzymes in Macaca mulatta: focus on arylalkylamine N-acetyltransferase (EC 2.3.1.87). The Journal of clinical endocrinology and metabolism. 2002;87(10):4699–4706. [DOI] [PubMed] [Google Scholar]
- 40.Miguez JM, Recio J, Vivien-Roels B, Pevet P. Daily variation in the content of indoleamines, catecholamines and related compounds in the pineal gland of Syrian hamsters kept under long and short photoperiods. Journal of pineal research. 1995;19(3):139–148. [DOI] [PubMed] [Google Scholar]
- 41.Miguez JM, Recio J, Vivien-Roels B, Pevet P. Diurnal changes in the content of indoleamines, catecholamines, and methoxyindoles in the pineal gland of the Djungarian hamster (Phodopus sungorus): Effect of photoperiod. Journal of pineal research. 1996;21(1):7–14. [DOI] [PubMed] [Google Scholar]
- 42.Bubenik GA, Brown GM, Uhlir I, Grota LJ. Immunohistological localization of N-acetylindolealkylamines in pineal gland, retina and cerebellum. Brain Res. 1974;81(2):233–242. [DOI] [PubMed] [Google Scholar]
- 43.Gaudet S, Palkovits M, Namboodiri MA. Regional distribution of arylamine and arylalkylamine N-acetyltransferase activities in the rat brain. Brain Res. 1991;539(2):355–357. [DOI] [PubMed] [Google Scholar]
- 44.Chae HD, Park TJ, Lee YK, Lee TG, Kim KT. Rapid and simple measurement of serotonin N-acetyltransferase activity by liquid biphasic diffusion assay. Neurochem Int. 1999;35(6):447–451. [DOI] [PubMed] [Google Scholar]
- 45.Nosjean O, Ferro M, Coge F, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. The Journal of biological chemistry. 2000;275(40):31311–31317. [DOI] [PubMed] [Google Scholar]
- 46.Jang SW, Liu X, Pradoldej S, et al. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc Natl Acad Sci U S A. 2010;107(8):3876–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]