Abstract
While immune checkpoint inhibitors (ICI) have demonstrated clinical activity in multiple tumor types, the majority of patients do not respond to ICI monotherapy. Mounting evidence suggests that ICI-mediated clinical responses rely upon tumor infiltration by T cells that are able to recognize and kill cancer cells. Here we review therapeutic modalities that have been shown to promote T cell infiltration into human tumors in studies to date, and discuss emerging data guiding how these modalities can be sequenced in order to optimize T cell effector function and memory T cell generation, while minimizing over-activation and potential toxicity.
Keywords: Combination immunotherapy, tumor-infiltrating lymphocytes, tumor microenvironment
Introduction
The goal of cancer immunotherapy is to direct the immune system against tumor cells, leveraging its exquisite specificity and capacity for memory to achieve rapid and durable tumor clearance. The clinical success of checkpoint blockade across many solid tumors and hematologic malignancies, has illustrated the promise of this strategy (1–3). However, the overall proportion of patients responding to ICI is low, with single-agent response rates across tumor types generally ranging from 10–35% (with few exceptions: tumors with microsatellite instability (MSI), Hodgkin lymphoma, Merkel cell carcinoma) (4,5). There is mounting evidence indicating that major barriers to efficacy include the absence of a pre-existing tumor-specific T cell response, and exclusion of T cells from the tumor microenvironment. For example, analyses of pre-treatment melanoma biopsies have shown that clinical response to anti-PD-1 (6) and anti-CTLA-4 (7) is correlated with the presence of tumor-infiltrating lymphocytes (TILs) prior to therapy, specifically CD8+ TILs at the invasive tumor margin. Furthermore, an inflamed transcriptional state, defined as expression of interferon-gamma (IFNγ) by activated T cells, and up-regulation of downstream signaling molecules, has been shown to correlate with clinical response to therapy (8). These IFNγ responsive genes are related to chemokine expression, antigen presentation, and cytotoxic effector molecules.
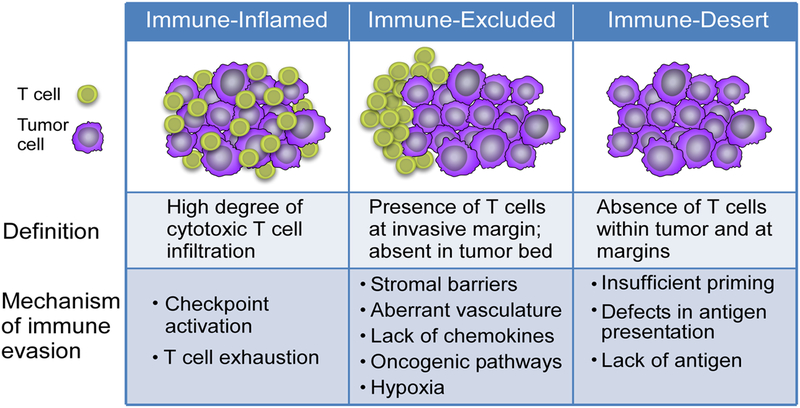
These observations have led to a framework classifying immune profiles of tumors that are unresponsive to immunotherapy into immune-inflamed, immune-excluded, and immune-desert tumors (Figure 1) (9). Immune-inflamed tumors are characterized by the presence of CD4+ and CD8+ T cells within the tumor parenchyma, suggesting the presence of a pre-existing anti-tumor response that has been quelled by an immunosuppressive microenvironment or intrinsic T cell anergy. This phenotype is associated with a type I interferon signature, highlighting the importance of innate immune signaling in successful T cell priming against tumor antigens (10,11). In contrast, cold tumors are characterized by the absence of pre-existing TILs, and can be further subdivided into immune-excluded tumors, in which T cell have been attracted to the periphery of the tumor, but fail to infiltrate, and immune-desert tumors, which are entirely devoid of a T cell infiltrate. Of note, the immune profile of a given individual’s tumors can be heterogeneous – at different sites within the tumor bed, and between primary site and metastases – and may evolve over time with disease progression, recurrence and therapeutic intervention, posing challenges to the use of information from individual tumor biopsies as a guide for therapy selection (12,13).
Figure 1. Tumor Immune Profiles.
Three immune profiles of tumors – inflamed, immune-excluded, and immune-desert – correlate with responsiveness to checkpoint blockade. Features of each profile highlight therapeutic targets for reprogramming the microenvironment.
To increase the clinical benefit of immunotherapy, novel strategies to convert immune-excluded and immune-desert tumors into inflamed microenvironments with increased tumor-infiltrating T cells are needed. To accomplish this goal, we will need to better understand the barriers preventing T cell infiltration, ideally for each individual patient given the heterogeneity described above. In this review, we discuss the therapeutic strategies currently in development that have shown potential to drive T cells into the tumor microenvironment, and describe how early efforts to combine these agents highlight the importance of sequencing therapies to maximize T cell function.
Mechanisms underlying immune phenotype
A productive anti-tumor immune response requires an intricately orchestrated sequence of events: tumor antigens are released, the innate immune system is activated to facilitate antigen processing and presentation, and antigen-presenting cells (APCs) prime naïve T cells in the draining lymph node, resulting in activation and expansion of tumor-specific T cells. These cells must then traffic to the tumor site, infiltrate into the tumor bed, and finally recognize and kill tumor cells (9,14). This can feed forward, resulting in release of additional tumor antigens, and broadening of the T cell response against additional tumor antigens. Preclinical studies and in-depth analysis of the tumor microenvironment from patient samples have begun to elucidate the mechanistic basis underlying T cell exclusion, including intrinsic tumor properties, and extrinsic factors (Figure 1).
(i). Defects in T cell priming
T cell priming is the first step that is required to trigger an effective anti-tumor immune response. Successful priming of a T cell requires recruitment of APCs, innate immune activation, the presence of targetable tumor antigen, and intact antigen presentation machinery. In particular, cross-presentation of tumor antigens by specialized dendritic cells (DCs), such as Batf3-expressing DCs, is crucial for priming of CD8+ T cell responses. Targetable tumor antigens include antigens arising from somatic mutations, known as neoantigens, as well as over-expressed tumor associated antigens and cancer germline antigens. Neoantigens have been shown to be particularly important, evidenced by an association of higher tumor mutational burden (TMB) with improved outcome in patients treated with ICI and other immunotherapies, expansion of neoantigen-specific T cells in patients who receive immunotherapies, and direct evidence of tumor killing by adoptively transferred neoantigen specific T cells (15,16). Given the high response rates of “hypermutated” cancers to ICIs across tumor types, it is conceivable that low mutation burden broadly decreases the likelihood of effective endogenous priming of tumor specific T cells (17). However, TMB and the extent of T cell inflammation (reflected by IFN gene signatures) are not correlated, suggesting that low tumor neoantigen burden does not solely account for lack of T cell infiltration (18–20). Additional mechanisms affecting antigen presentation that can account for lack of tumor T cell inflammation include mutations or epigenetic changes affecting antigen-presentation machinery, such as beta-2-microglobulin (β2m) loss – a subunit required for HLA I surface expression – and mutations in HLA, which have been associated with resistance to checkpoint blockade (21,22). Other innate immune cells also influence T cell priming, notably NK cells, which can exert both positive and negative effects on the generation of anti-tumor immunity. NK cells modulate DC maturation and function via secretion of cytokines (TNF, IFNγ), and enhance cross-presentation by killing target cells and releasing antigen for presentation by DCs (23). NK cells also produce Fms-related tyrosine kinase 3 ligand (FLT3L), an important cytokine for recruitment of intratumoral DCs, providing another axis that could be targeted to enhance T cell priming and activation (24).
(ii). Oncogenic pathway activation
Activation of tumor-intrinsic oncogenic pathways has been associated with T cell exclusion from the microenvironment. This was first observed in patients with metastatic melanoma, with activation of the Wnt/beta-catenin pathway associated with an immune cold phenotype, and subsequently demonstrated to be correlated across tumor types in TCGA (25,26). Studies in mice identified absent recruitment of Batf3-expressing DCs into the tumor bed as a likely mechanism, impairing cross-presentation to CD8+ T cells (27). Upregulation of the mitogen-activated protein kinase (MAPK) pathway has been associated with reduced T cell infiltration in triple-negative breast cancer (28). Targeting MAPK may be particularly attractive, as this pathway can be upregulated in tumor cells, but is also a major pathway downstream of normal T cell receptor (TCR) signaling, and its blockade therefore impacts both tumor cells and T cells. Notably, while inhibition of MEK has been shown to abrogate IL-2 production and priming of naïve T cells, it can also promote the effector phenotype and longevity of tumor-infiltrating CD8+ T cells, suggesting the timing of administration would be an important consideration to achieve maximal anti-tumor effect (29). Loss of PTEN and downstream activation of the PI3K/AKT pathway has also been implicated in T cell exclusion and PI3K-beta inhibitors were shown to sensitize tumors to T cell mediated killing in preclinical studies (30). Moreover, up-regulation of MYC leads to increased PD-L1 and CD47 expression, disrupting T cell activation and priming (31). Another example is CDK4/6 activation, which has been implicated in T cell exclusion and immune evasion in the setting of resistance to checkpoint blockade (32). These oncogenic pathways represent therapeutic targets for inhibiting tumor cell growth and increasing T cell infiltration to improve susceptibility to other immunotherapies.
(iii). Tumor microenvironment: stromal factors, aberrant vasculature, and immunosuppressive factors
T cell infiltration can be impeded by local factors in the tumor microenvironment, including dense stroma, aberrant vasculature, and immunosuppressive factors. For example, Transforming Growth Factor (TGF)-β is an immunosuppressive cytokine that inhibits T cell effector function through multiple mechanisms, including the expansion of T regulatory cells (Tregs) and inhibition of antigen-presenting DCs. TGF-β can also produce stromal modifiers that promote tumor progression and metastasis (33), and its expression has been associated with exclusion of T cells from the tumor microenvironment (34,35). Indoleamine-2,3-dioxygenase (IDO) is an intracellular enzyme involved in tryptophan degradation, which is expressed in the tumor microenvironment and has been implicated in tumor immune escape (36). Adenosine is an immunosuppressive metabolite derived predominantly from ATP catabolism, which under normal conditions protects against excessive immune responses. In the tumor microenvironment, adenosine attenuates DC maturation and effector activity of T cells and NK cells, blunting anti-tumor immune responses (37). Immunosuppressive cell types including FOXP3+ CD4+ Tregs, myeloid derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) can limit effective immune response against tumor cells (38–40). These immunosuppressive cells can be found across the different tumor immune profiles, potentially counteracting effective anti-tumor T cell responses.
Therapeutic approaches to drive T cells into tumors
Since different mechanisms can lead to a lack of T cell infiltration tumors, therapies would ideally target the specific “defect(s)” in a given tumor to overcome T cell exclusion. Here we review the evidence supporting select therapeutic modalities aimed at driving T cells into tumors (Figure 2).
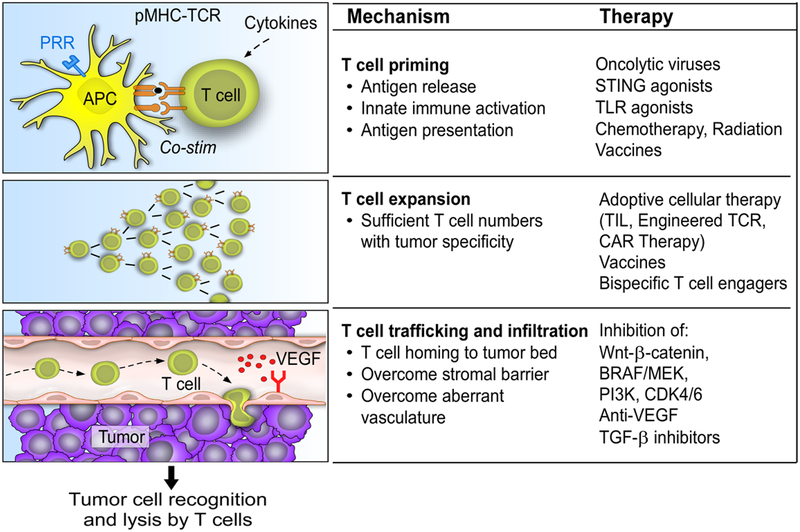
Figure 2. Steps to drive tumor-specific T cells into tumors.
(i) T cell priming, (ii) T cell expansion to achieve sufficient numbers, and (iii) trafficking and infiltration into tumor microenvironment
(i). Therapies to promote T cell priming
Upon activation by innate stimulation pathways, APCs can prime naïve T cells to initiate a tumor-specific T cell response. This process occurs in the draining lymph node, and requires antigen release, uptake by antigen-processing cells and presentation on MHC molecules, and recognition by a cognate TCR. A number of therapeutic approaches have the ability to induce endogenous T cell priming, without needing to target specific tumor antigens.
Oncolytic Viruses
Oncolytic viruses can promote T cell priming through tumor antigen release as well as maturation and trafficking of APCs within the tumor microenvironment. These viruses are modified to selectively infect and replicate within tumor cells, leading to tumor cell lysis, and in turn stimulate an anti-tumor immune response via release of tumor antigens (41). The first FDA approved oncolytic virus, Talimogene laherparepvec (T-VEC), which has demonstrated superior progression-free survival over granulocyte-macrophage colony stimulating factor (GM-CSF) in patients with advanced melanoma (42), is derived from human herpes simplex virus (HSV) and engineered to express GM-CSF. A phase Ib trial of T-VEC in combination with pembrolizumab (anti-PD-1) for patients with advanced melanoma showed an overall response rate of 62%, with a complete response rate of 33% (43). Responses were observed in a number of patients whose tumors had low T cell infiltrates and low IFNγ signatures at baseline. Serial tumor biopsies after single-agent T-VEC showed an increase in the extent of cytotoxic CD8+ T cell infiltration in 8 of 12 injected lesions available for analysis, which increased further after combination therapy; a trend that was not observed in tumor samples from patients who did not have clinical responses. A phase 3 trial of T-VEC combined with pembrolizumab compared to pembrolizumab alone has completed accrual. Several other oncolytic viruses have been evaluated in early phase trials, including Coxackievirus A21, which has shown clinical activity as monotherapy and is being tested with ipilimumab and pembrolizumab in patients with advanced melanoma (44), and the attenuated herpes simplex virus 1 HF-10 (45). Furthermore, both DNX-2401 and H-1 Parvovirus, which have been tested in patients with glioblastoma, mediated increases in cytotoxic CD8+ T cell infiltration (46,47). Given their dual effects of direct tumor-cell killing and T cell priming, oncolytic viruses are likely to play a major role in increasing T cell infiltration for non-inflamed tumors.
Immune Adjuvants
Tumors with a high degree of T cell infiltration are associated with a type I interferon signature, highlighting the importance of innate immune sensing pathways in establishing an anti-tumor T cell response. In order to induce productive T cell responses against tumor antigens, APCs must first be activated by stimulation of danger-associated and pattern-associated molecular pattern (DAMP/PAMP) receptors, enabling presentation of antigen and priming of naïve T cells. A number of therapeutic strategies have been developed to promote innate immune activation, including TLR and STING agonists. Of note, most of these agents are administered intratumorally with the intention to deliver the stimulus of innate immune responses directly at the site of the tumor and to limit. However, their effects may be exerted beyond the injected lesion, by establishing a systemic anti-tumor immune response, particularly when used in combination regimens. Systemically administered agents are in development, for example a small moleculare inhibitor of Ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) which negatively regulates STING (48).
Toll-like receptor agonists.
Toll-like receptors (TLRs) are pattern-recognition receptors that are highly expressed by tumor-infiltrating immune cells, particularly APCs, and upon stimulation have powerful immune adjuvant effects. TLR signaling leads to DC maturation resulting in increased antigen presentation, and type I interferon production. Agonists targeting a variety of TLRs, including TLR3, TLR4, TLR7, TLR8 and TLR9, are under investigation in the clinic. For example, SD-101, a TLR9 agonist administered intratumorally, and given in combination with low-dose radiation for patients with indolent B cell lymphomas, led to reduction in overall tumor burden in 26 of 29 patients, with corresponding increase in CD8+ and CD4+ effector T cells, and decrease of Tregs in the tumor microenvironment (49). Intratumoral SD-101 was also tested in combination with the anti-PD-1 antibody pembrolizumab in patients with metastatic melanoma; the combination mediated increased T cell infiltration and resulted in an overall response rate of 78% in treatment naïve patients and 15% in patients who had previously received prior PD-1 therapy (50). Another TLR9 agonist, CMP001 was tested in combination with anti-PD-1 in patients with advanced melanoma who were refractory to anti-PD-1 monotherapy, and demonstrated objective responses at injected sites as well as non-injected visceral metastases (51). Among patients with paired pre- and post-treatment biopsies available, an increase in CD8+ T cells following treatment was observed.
STING agonists.
In the context of tumor-immunity, the STING (stimulator of interferon genes) pathway responds to tumor-derived DNA within the cytosol of DCs, other innate immune cells, and tumor cells, leading to nuclear factor-kB (NF-kB) activation, JAK-STAT activation and type I IFN production (11,52). As such, agonists of the STING pathway promote cross-presentation of tumor antigens and migration to lymph nodes, and in turn augment the priming and recruitment of T cells. Specifically, type I interferon signaling is crucial for recruiting Batf3+ DCs which are the most potent type of APC for cross-presenting antigen to CD8+ T cells, and necessary for effector T cell function (27). Clinical trials testing these agents are ongoing, with preliminary studies reporting encouraging results. A Phase I dose-finding study of ADU-S100 (MIW815), a STING agonist, was performed and increased T cell infiltration in one of the patients studied (53) was found. Further studies will be required to define the impact of this agent on the extent of T cell infiltration. A phase I study of another STING agonist, MK-1454, in monotherapy or combination with pembrolizumab was recently reported. In the combination arm, 6 of 25 patients had partial response (3 with HNSCC, 2 with anaplastic thyroid carcinoma, 1 with triple-negative breast cancer), with reductions in both target-injected and non-injected lesions; no objective responses were observed with monotherapy (54). STING agonists require local administration, via intratumoral injection, which limits their application to tumors that are accessible to injection, though systemic formulations are also being developed (55).
Cytotoxic Therapies
Traditional cancer therapies such as chemotherapy and radiation may also have a role in augmenting T cell priming. Radiation therapy mediates direct cytotoxicity to cancer cells through lethal DNA damage. In addition, radiation induces a focal inflammatory response at the irradiated site, leading to an increase in DAMPs, type I interferon production, and release of tumor antigens, thereby creating an in situ vaccine effect (56). This has been shown to occur through STING-dependent pathways, and to result in dramatic enhancement of the cross-priming capacity of tumor-infiltrating DCs (57). Of note, the extent of this effect appears to depend upon the dose and fractionation of radiation administered (58). A key factor attenuating the immune response elicited by radiotherapy is Trex1, a DNA exonuclease, which can degrade DNA in the cytosol and therefore preclude activation of STING. Therefore, repeated doses of radiation below the threshold that induces Trex1 (between 12–18Gy in different cancer cells), may optimally stimulate a type I interferon response required to recruit cross-presenting DCs. Radiation therefore has an important role in recruiting inflammatory cells to the tumor site, and in turn has been shown to increase tumor-specific effector T cells infiltrating within the tumor in preclinical models (59). In addition to the type I interferon-mediated effects, radiation therapy may also contribute to enhanced T cell priming via increased tumor antigen release, and increased antigen-recognition through enhanced MHC class I expression on tumor cells, achieving an in situ vaccination effect. For example, a recent clinical trial used local radiation in combination with intratumoral injections of an Fms-like tyrosine kinase 3 ligand agonist (Flt3L, to recruit intratumoral DCs) and a TLR3 agonist (poly-ICLC), in patients with advanced stage indolent non-Hodgkin Lymphoma (iNHL), based on preclinical evidence that this combination achieved robust cross-presentation, priming of CD8+ T cells and increased T cell infiltration (60). In the clinical trial, patients were treated with intratumoral injections and local radiation in a single target lesion, resulting in partial or complete regression of the treated tumor in 8 of 11 patients, and regression of a distant site in three patients, suggestive of generation of systemic anti-tumor effect.
Individual chemotherapeutic drugs may have differential impacts on the tumor microenvironment, shaping the tumor immune microenvironment by affecting immunosuppressive cells, stimulating effector cells, or increasing immunogenicity (61). Some agents have been found to induce T cell infiltration; for example paclitaxel mediated an increase in T cell infiltration in a small prospective study of patients with breast cancer, which was non-inflamed at baseline, following four treatment cycles (62). Other common chemotherapeutic classes, including anthracyclines and alkylating agents, are known to induce immunogenic cell death, and may potentiate responses to ICI. This has been demonstrated in preclinical models, in which oxaliplatin/cyclophosphamide sensitized lung adenocarcinoma lacking T cell infiltration to respond to checkpoint blockade (anti-PD-1 + anti-CTLA-4) (63). In clinical trials, a benefit in combining chemotherapy and checkpoint blockade was demonstrated; for example, the combination of platinum chemotherapy, pemetrexed and pembrolizumab demonstrated improved survival compared to chemotherapy alone (64). Furthermore, neoadjuvant chemotherapy in patients with NSCLC resulted in higher levels of tumor PD-L1 and CD3+ T cell infiltration, which may potentiate response to subsequence checkpoint blockade (65).
It is worth noting that both chemotherapy and radiation can also exert immunosuppressive effects on the tumor microenvironment, highlighting the need for careful selection of individual chemotherapeutic agents, assessing optimal chemotherapy dosing schedules, as well as evaluating optimal dosing and fractionation of radiotherapy.
(ii). Therapies to increase antigen-specific T cells
Additional therapeutic strategies that target specific tumor antigens may be useful to promote expansion of tumor antigen-specific T cells and attain a sufficient number for infiltration into the tumor microenvironment. Alternatively, T cells engineered to target specific tumor antigens can be exogenously infused using adoptive cellular therapy, or T cells can be activated and expanded in a polyclonal fashion using bispecific T cell engagers. These strategies typically require identification of targetable tumor antigen(s), although approaches to broadly target whole tumor cells have been also devised and are promising.
Vaccines
Therapeutic cancer vaccines directed against specific tumor antigens have the ability to prime de novo immune responses, expand existing tumor-specific responses, and ideally establish long-lasting tumor-specific memory T cells (66). Many vaccine formulations and delivery approaches have been tested, including peptide, DNA, RNA, dendritic cell, and whole tumor cell vaccines, targeting over-expressed tumor-associated antigens, cancer-germline antigens, and, more recently, neoantigens. As opposed to native antigens, neoantigens, which are encoded by somatic mutations, are exquisitely tumor-specific and not affected by central tolerance. Some of these strategies have demonstrated capacity to increase T cells infiltration. For example, sipuleucel-T, an autologous cell based vaccine targeting prostatic acid phosphatase (PAP), an enzyme that is overexpressed in prostate cancer, induced a more than three-fold increase of infiltrating CD3+, CD4+ FOXP3−, and CD8+ T cells in radical prostatectomy tissues compared to pre-treatment specimens (67). Clinically, sipuleucel-T increased overall survival by 4 months and improved 3-year survival rates in patients with advanced castration-resistant prostate cancer, leading to its FDA approval in metastatic prostate cancer (68). Additionally, the GM-CSF-transfected autologous tumor cell vaccine, GVAX, for pancreatic cancer was shown to increase tertiary lymphoid structures in the tumor microenvironment – aggregates that resemble lymph nodes and are associated with positive prognosis – and when combined with anti-PD-1, demonstrated enhanced anti-tumor immunity (69–71).
Personalized vaccines targeting neoantigens have recently shown promise as effective tools to expand antigen specific T cells in the periphery and to mediate trafficking of vaccine specific T cells into the tumor. Several preclinical studies demonstrated that neoantigen-directed vaccination can increase tumor antigen-specific T cell infiltration (72,73). The first clinical trials testing neoantigen vaccines in humans were conducted in patients with melanoma (74–76). Extensive immune profiling demonstrated generation of robust, durable, and polyfunctional vaccine-specific CD4+ and CD8+ T cell responses. In a phase I trial testing a personalized long peptide-neoantigen vaccine in patients with glioblastoma, increased infiltration of tumors with CD4+ and CD8+ T cells was seen following vaccination in patients who also developed vaccine-specific circulating T cell responses. Single cell level transcriptomic profiling and TCR sequencing of post-vaccine tumor-infiltrating T cells demonstrated co-expression of multiple inhibitory receptors (PD-1, TIGIT, and TIM3) consistent with a severe exhaustion phenotype and identified vaccine-specific tumor-infiltrating T cells. Another recent study testing vaccines targeting both neoantigens and non-mutated tumor-associated antigens in glioblastoma patients similarly demonstrated the presence of vaccine-specific T cells among tumor-infiltrating lymphocytes following vaccination (77). Taken together, these studies provide evidence that neoantigen vaccines are able to drive antigen-specific T cells into the tumor microenvironment, but also that the functionality of these T cells may be compromised, potentially requiring additional therapeutic intervention such as ICI therapy.
Adoptive Cellular Therapy
Adoptive cellular therapies entail the infusion of large numbers of tumor antigen-specific T cells into the host. These include TIL therapy, in which tumor-infiltrating T cells are isolated, expanded ex vivo, and re-infused peripherally, as well as T cells that are engineered to express tumor antigen-specific TCRs or chimeric antigen receptors (CARs) (78). Patients usually receive lymphodepleting chemotherapy prior to the transfer in order to decrease endogenous lymphocytes which compete with transferred cells for homeostatic cytokines, and eliminate Tregs. IL-2 therapy is given post transfer to support growth and activity of the infused product. Adoptive TIL therapy has shown promise in melanoma (79). Clinical success with CAR-T cells has largely been limited to hematologic malignancies, particularly B-cell leukemias and lymphomas. Efforts to apply this strategy to solid tumors have proved challenging owing to the need for targetable tumor antigen, efficient infiltration, and persistence in the tumor microenvironment. Adjunctive therapies combined with CAR-T cells have been investigated to augment tumor infiltration, including induced expression of chemokines (e.g. CXCL11 (80), CCL19 (81)), as well as alternative delivery methods including regional/local CAR T cell administration. CAR-T cells may be particularly effective for cold tumors with defects in antigen presentation, as the antigen receptor activation is not MHC dependent.
Bispecific T cell engager
Bispecific T cell engagers are soluble chimeric proteins consisting of an antigen recognition domain and a T cell engaging domain, which stimulate polyclonal T cell activation. When the molecule is immobilized on a target cell, T cells can be activated independent of their TCR specificity, recruited into the tumor bed, and release pro-inflammatory cytokines. The antigen recognition domain can be derived from an antibody or a TCR, and ideally targets antigens that are selectively expressed by tumor cells. As with CAR-T cells, this strategy has been most successful in B cell malignancies, targeting CD19, but is also being tested in solid malignancies. One such molecule, IMCgp100, has a TCR-based antigen recognition domain targeting the overexpressed melanoma antigen, gp100. It has been shown to induce lymphocyte mobilization and increased CD8+ PD-1+ T cell infiltration into the tumor bed in uveal melanoma (82). One caveat to this strategy is that by virtue of stimulating T cell activation independent of TCR specificity, it is possible for T cell engagers to activate both cytotoxic and regulatory T cells. This was evidenced by studies of the bispecific T cell engager targeting CD19, blinatumomab, for which the frequency of Tregs determined outcome in patients with B-precusor acute lymphoblastic leukemia. Non-responding patients had significantly higher circulating Tregs, and this effect was mediated by blinatumomab-activated T regs producing IL-10 and suppressing T cell proliferation and tumor lysis (83). Thus, further work is necessary to increase the specificity of this modality.
(iii). Therapies to overcome T cell exclusion
Once tumor-specific T cells have been primed and activated, they must home to the tumor site and infiltrate within the tumor bed. Barriers to infiltration include oncogenic pathway activation, dense stroma, aberrant vasculature, and immunosuppressive factors in the microenvironment. There are a number of therapeutic strategies under development that are designed to target barriers to T cell infiltration:
Oncogenic pathway inhibitors
Activation of select oncogenic pathways has been implicated in T cell exclusion and modulation of T cell function. MAPK signaling is a crucial driver of tumorigenesis, and upregulation of the pathway has also been associated with reduced T cell infiltration, with overlap between this pathway and elements downstream of TCR signaling. Consistent with this mechanism, MEK inhibition has been shown to potentiate antitumor immunity by inducing expansion of antigen-specific CD8+ TILs (via inhibition of signaling that would otherwise lead to T cell exhaustion or apoptosis). Additionally, BRAF or combined MEK/BRAF-inhibition leads to increased expression of melanoma antigens (84), inhibits VEGF production to normalize vasculature, and promotes T cell trafficking in preclinical models (85). BRAF inhibition also has favorable effects on the tumor immune microenvironment in patients, with studies demonstrating increased T cell infiltration, increased cytotoxicity (increased levels of granzyme B, perforin) and immune stimulatory cytokines (IFNγ, TNFα) in posttreatment biopsies (86).
Other examples of T cell exclusion mediated by oncogenic pathway activation include the Wnt-β-catenin pathway, which has been associated with poor T cell infiltration and primary resistance to checkpoint blockade, primarily due to impaired recruitment of cross-presenting DCs (25–27). Preclinical studies of RNAi-based β-catenin inhibition, DCR-BCAT, in combination with checkpoint blockade have shown increased T cell infiltration into tumors and improved tumor growth inhibition compared to monotherapy (87). Early clinical studies of small molecule Wnt inhibitors focused on tumors with upregulated Wnt/β-catenin signaling such as colorectal cancer, but given their immunomodulatory effects, further clinical trials assessing the role of Wnt inhibition in increasing susceptibilty to immunotherapy in other tumor types are anticipated. CDK4/6 inhibitors like palbociclib have been investigated for their immuno-stimulatory potential, and have been shown to enhance T cell activation and increase T cell infiltration, via derepression of nuclear factor of activated T cells (NFAT) activity (88). Alterations in the PI3K-AKT-mTOR pathway have also been associated with modulating the differentiation, homeostasis and functional activity of effector T cells, with loss of PTEN correlating with resistance to checkpoint blockade. PI3K inhibitors are under development to target this pathway (30).
Anti-angiogenesis agents
The tumor microenvironment is characterized by structurally and functionally aberrant vasculature, resulting from an imbalance of pro- versus anti-angiogenic factors. In addition to facilitating tumor growth, this hinders leukocyte-endothelial interactions and impairs infiltration of immune effector cells into the tumor bed. As such, vascular normalization, an attempt to balance pro- and anti-angiogenic factors, has been proposed as a strategy to facilitate immune infiltration. Anti-VEGF agents have been shown to normalize tumor vessels when used at lower doses, and result in improved vessel perfusion, decreased hypoxia and enhanced drug delivery, resulting in overall increase in immune cell access (89). In a clinical trial of bevacizumab and ipilimumab in patients with metastatic melanoma, a qualitative increase in CD8+ T cells and CD163+ DCs was seen following combined treatment, but not with ipilimumab alone (90). In a clinical trial of bevacizumab and atezolizumab in patients with renal cell carcinoma, increased CD8+ T cell infiltration post treatment was observed in all but one of 10 patients. Furthermore, there was a significant increase in CX3CL1, a chemokine that mediates T cell homing, and no change in the ratio of Ki67/CD8 in on-treatment samples, suggesting that the increased infiltration was not due to enhanced intratumoral proliferation, but increased trafficking and infiltration (91). In some cases where T cell exclusion is due to aberrant vasculature, there may be a role for therapeutic manipulation of the vasculature to promote T cell infiltration.
TGFβ inhibitors
TGFβ plays a central role in immune suppression in the tumor microenvironment (33). It has been implicated in T cell exclusion and lack of response to ICI (34,35). In addition, TGFβ has a well-established role in promoting Tregs, suppressing Th1 differentiation, and inhibiting T cell proliferation and effector function. As such, TGFβ blockade using small molecule inhibitors and monoclonal antibodies has been studied as a strategy to convert immune-excluded tumors into immune-inflamed tumors. In a first line trial of patients with unresectable pancreatic cancer, the small molecule inhibitor targeting TGFBR1 kinase, Galunisertib, combined with gemcitabine resulted in improved overall survival, compared to gemcitabine alone (92). The impact of TGFβ inhibition on tumor T cell infiltration has not yet been studied in patients. However, in preclinical studies, TGFβ inhibitors combined with checkpoint blockade or radiation therapy enhanced anti-tumor activity (93,94), providing a rationale for further study in clinical trials.
Sequencing therapies in combination regimens for optimal T cell function
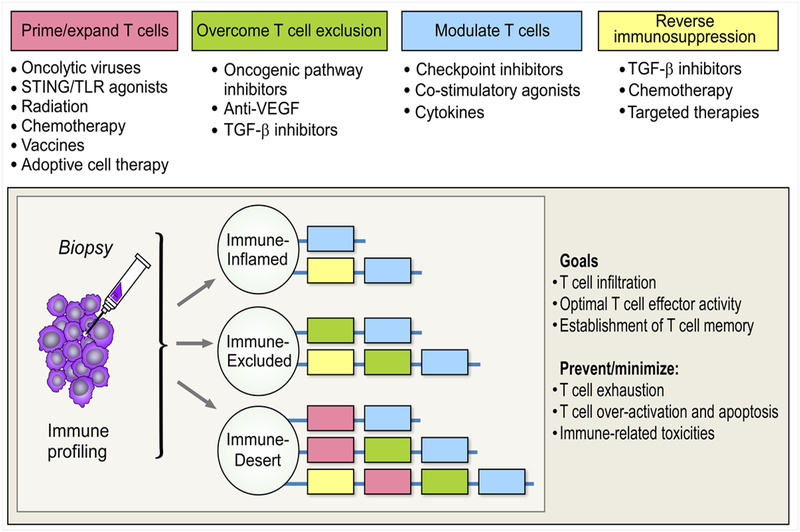
The strategies reviewed above have demonstrated the potential to mobilize T cells into the tumor microenvironment – a critical first step to achieve an effective immune response in patients with immune-cold tumors. However, while infiltration of tumors with antigen-specific T cells is presumably necessary, it is often not sufficient for tumor control, as evidenced by the observation that many tumors with pre-existing TILs still fail to respond to treatment with ICI (8). This may be due to up-regulation of alternative checkpoint molecules, T cell exhaustion, or an immunosuppressive microenvironment. Therefore, additional goals to establish effective anti-tumor immunity in both inflamed and non-inflamed tumors include optimizing T cell effector and memory function, and reducing immunosuppressive factors, while avoiding immune over-activation and potential toxicity (Figure 3). These goals are best achieved in combination regimens.
Figure 3. Rational combination.
Sequencing agents to promote T cell infiltration and optimal functionality
Other agents that optimize T cell functionality are reviewed in detail elsewhere, including checkpoint inhibitors (targeting multiple inhibitory receptors including CTLA-4, PD-1, LAG3, TIM3, and TIGIT) and co-stimulatory agonists (targeting OX40, 4–1BB, GITR, ICOS, CD137, and CD28/27) (95). Likewise, many therapeutic strategies targeting immunosuppresive factors in the microenvironment (Tregs, MDSCs, TAMs etc.) are under investigation (38–40). However, the optimal doses and sequence of administration of these agents when used in combined regimens remains to be defined. Here we highlight some combination regimens that illustrate important considerations for sequencing therapies in order to optimize T cell function – unleashing effector functions, promoting T cell memory, and avoiding over-activation.
(i). Optimal effector T cell activity
Combining multiple agents that augment T cell priming can achieve improved T cell effector function, particularly when using agents that act through multiple mechanisms. However, the increase in T cell infiltration achieved with such agents is frequently accompanied by up-regulation of checkpoint molecules, potentially limiting T cell effector activity. For example, this has been observed in studies of oncolytic viruses, vaccines, radiation, and targeted therapies, particularly PD-1 and PD-L1 (71,84,96,97). These observations provide a rationale for combining T cell priming agents with anti-PD-1 or anti-PD-L1 antibodies. Further research will be necessary to delineate whether agents that augment T cell priming should be given concurrently or sequentially with checkpoint inhibitors to maximize effector function. Given that PD-1 acts primarily on recently activated and exhausted T cells, it is generally thought that concurrent administration or administration following T cell priming agents may be most effective.
However, based on the observation of multiple upregulated co-inhibitory receptors on T cells, the combination of therapies aimed at inducing T cell inflammation with a single checkpoint inhibitor may still be inadequate. This was observed in our own study of neoantigen vaccines for glioblastoma, in which neoantigen-specific T cells detected post-vaccination within the tumor expressed multiple co-inhibitory receptors (98). In such cases, optimal effector function may require targeting multiple checkpoints simultaneously.
(ii). Promoting T cell memory
Another aspect to consider in the design of combination regimens is the impact of these therapies on memory T cell populations. This is particularly relevant for regimens targeting the PD-1/PD-L1 axis, as PD-1 expression has been shown to affect the transition from naïve to effector T cells, and blockade of the PD-1 pathway may impact the maintenance of memory T cells (99,100). In order to avoid a negative impact on memory T cell formation, it may therefore be necessary to administer agents that promote T cell priming, such as cancer vaccines and oncolytic viruses, sequentially with anti-PD-1 rather than concurrently. In contrast, anti-CTLA-4 enhances T cell priming in the draining lymph node and promotes T cell memory, suggesting a potential benefit were CTLA-4 inhibition to be given concurrently or prior to the therapy aimed at priming (101). In an analogous case, the combination of anti-CTLA-4 and radiation was found to be most effective when anti-CTLA-4 was administered prior to radiation, with robust memory formation in a preclinical model (102). As described above, combining checkpoint blockade with cancer vaccines or oncolytic viral therapy is a promising strategy to generate tumor-specific memory T cell populations. For optimal results, careful assessment of timing, sequencing, and duration of these therapies will be necessary.
(iii). Reversing Immunosuppression
To achieve optimal effector function of tumor-infiltrating T cells, additional therapy to counteract immunosuppressive factors in the tumor microenvironment will be needed. Agents that target immunosuppressive cell types (Tregs, MDSCs, TAMs) or inhibit immunosuppressive factors (e.g. TGFβ, IDO) are reviewed in detail elsewhere. Of note, these include some of the agents reviewed here, including chemotherapy, targeted therapies, and TGFβ inhibitors, which have multiple effects in increasing T cell infiltration and depleting immunosuppressive cell types.
There is emerging evidence that therapies targeting immunosuppressive factors should ideally be administered early in the treatment course, to permit optimal T cell priming and activation with subsequent agents. For example, in the CT26 murine colorectal cancer model, CTLA-4 blockade was most effective when given prior to a single radiation dose; this effect was in part attributed to anti-CTLA-4 mediated Treg depletion, via Fc-dependent mechanisms (102). Another study using the same mouse model found that TGFβ inhibition administered prior to radiation resulted in increased intratumoral activated CD8 T cells and fewer CD4 Tregs, with pretreatment demonstrating increased efficacy relative to radiation alone (93). Pre-clinical studies testing BRAF/MEK inhibitors in combination with checkpoint blockade suggest that targeted therapies should be administered first to reprogram the microenvironment, including through decreasing Tregs and MDSCs, followed soon after by checkpoint blockade (103,104).
A number of chemotherapy agents have also been shown to counteract immunosuppressive cell populations including low dose cyclophosphamide for Treg depletion (105), or gemcitabine and 5-fluorouracil for decreasing MDSCs (106). The ideal timing for combining chemotherapy and immunotherapy likely depends on the particular agents and tumor type. For example, in a phase II trial with NSCLC patients the combination of CTLA-4 blockade and paclitaxel/carboplatin improved PFS when chemotherapy was given prior to anti-CTLA-4, but not when the two therapies were given concurrently (107). In contrast, in a preclinical study of mesothelioma, the combination of gemcitabine and CTLA-4 blockade was only synergistic when the 2 therapies were administered concurrently (108). Further work dissecting the mechanisms by which chemotherapy may interact with immunotherapy, including through immunogenic cell death, release of DAMPs, and depletion of immunosuppressive cell types may better inform how to best combine and sequence these therapies.
(iv). Avoiding over-activation and T cell apoptosis
Some combinations can lead to excessive T cell activation, resulting in T cell apoptosis, potentially abrogating the single agent activity of the individual therapeutic agents used in a combinatorial regimen. In other cases, excessive T cell activation may increase immune related toxicities. For example, when administered concurrently with an OX40-agonist and peptide vaccine in the TC-1 tumor model, PD-1 inhibition reversed the therapeutic effect of anti-OX40, abrogating the effect on tumor-growth inhibition and survival, and leading to apoptosis of tumor-infiltrating T cells as a result of excessive activation (109). Another study in a mouse model of mammary cancer confirmed this finding, and further observed that sequential administration of OX40 followed by anti-PD-1 resulted in enhanced anti-tumor activity that was dependent on both CD4+ and CD8+ T cells, while the reverse order abrogated the anti-tumor effect (110). Other therapies, including radiation and chemotherapy, can also cause T cell apoptosis, and careful consideration should be given as to how to sequence them appropriately in combination regimens to avoiding nullifying anti-tumor responses.
An important guiding principle for building combination regimens will be minimizing immune mediated toxicities. We envision this could be accomplished through the use of carefully tailored combination therapy, personalized for a given individual’s tumor, ensuring that the minimum number of therapies necessary is used to achieve an effective immune response. Additional considerations for avoiding T cell over-activation and minimizing toxicity include drug delivery systems, capable of stimulating localized anti-tumor T cell response. Intralesional therapies may be particularly useful for this purpose.
Perspective/Concluding Remarks
Agents capable of driving antigen specific T cells into tumors, as discussed here, are essential elements of effective immunotherapy, particularly for patients with non-T cell inflamed tumors. Optimal clinical efficacy will likely require combination regimens that are able to achieve additional goals, including optimization of T cell effector function and memory T cell formation, and reversal of immunosuppressive mechanisms, addressing the distinct mechanisms underlying therapy resistance in immune cold and immune-inflamed tumors. Ideally, the selection of immunotherapeutic agents and their sequencing should be guided by the specific immune phenotype in a given patient. We acknowledge that this is an ambitious goal given the complexity of these immune phenotypes, but also the toxicity profile of individual therapeutic agents, regulatory requirements, and drug proprietary considerations, and the development of optimal combinatorial approaches is constrained by these realities. Nevertheless, we are confident that ongoing preclinical work as well as intelligently designed, biomarker driven clinical trials will get us closer to this goal. These efforts will continue to advance our understanding of the steps necessary to reprogram the tumor microenvironment to achieve maximal benefit for patients with cancer.
Significance.
The lack of pre-existing T cell inflammation in tumors is a major barrier to effective cancer immunity. A deep understanding of the mechanisms that prevent T cells from trafficking into the tumor in a given individual will be critical for tailoring immunotherapy combinations that can overcome resistance to ICI in patients with cancer.
Financial Support:
This work is supported by grants from the US National Institutes of Health (NCI-2RO1CA155010-02, U24CA224331) (to C.J.W.), (NCI-1R01 CA229261-01) (to P.A.O), the Francis and Adele Kittredge Family Immuno-Oncology and Melanoma Research Fund (to P.A.O.), the Faircloth Family Research Fund (to P.A.O.), the Bender Family Research Fund (to P.A.O), the G. Harold and Leila Y. Mathers Foundation (to C.J. W), the Bridge Project of Harvard and MIT (to C.J.W) and the Howard Hughes Medical Research Fellows Program (to A.J.A). C.J.W. is a Scholar of the Leukemia and Lymphoma Society.
Footnotes
Disclosure of potential conflicts of interest: P.A. Ott reports receiving commercial research grants from BMS, Merck, Neon Therapeutics, Celldex, ArmoBiosciences, AstraZeneca/ MedImmune, Novartis, Pfizer, CytomX, and Genentech and is a consultant/advisory board member for Merck, BMS, Genentech, Novartis, P zer, Neon Therapeutics, Celldex, CytomX, and Array. C.J.W. is a founder of Neon Therapeutics and member of its scientific advisory board. Patent applications have been filed on aspects of the described work entitled as follows: Compositions and Methods for Personalized Neoplasia Vaccines (C.J.W.), Methods for Identifying Tumour Specific Neo-Antigens (C.J.W.) and Combination Therapy for Neoantigen Vaccine (C.J.W.). A.J.A declares no competing financial interests.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine 2010;363(8):711–23 doi 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine 2012;366(26):2443–54 doi 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34(32):3838–45 doi 10.1200/JCO.2016.68.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellmann MD, Friedman CF, Wolchok JD. Combinatorial Cancer Immunotherapies. Advances in immunology 2016;130:251–77 doi 10.1016/bs.ai.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine 2015;372(26):2509–20 doi 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515(7528):568–71 doi 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer immunology, immunotherapy : CII 2012;61(7):1019–31 doi 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. The Journal of clinical investigation 2017;127(8):2930–40 doi 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541(7637):321–30 doi 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 10.Trujillo JA, Sweis RF, Bao R, Luke JJ. T Cell-Inflamed versus Non-T Cell-Inflamed Tumors: A Conceptual Framework for Cancer Immunotherapy Drug Development and Combination Therapy Selection. Cancer immunology research 2018;6(9):990–1000 doi 10.1158/2326-6066.CIR-18-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo SR, Corrales L, Gajewski TF. The STING pathway and the T cell-inflamed tumor microenvironment. Trends in immunology 2015;36(4):250–6 doi 10.1016/j.it.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013;39(4):782–95 doi 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015;350(6257):207–11 doi 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39(1):1–10 doi 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348(6230):124–8 doi 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344(6184):641–5 doi 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357(6349):409–13 doi 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spranger S, Luke JJ, Bao R, Zha Y, Hernandez KM, Li Y, et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proceedings of the National Academy of Sciences of the United States of America 2016;113(48):E7759–E68 doi 10.1073/pnas.1609376113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362(6411) doi 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;37(4):318–27 doi 10.1200/JCO.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 21.Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nature communications 2017;8(1):1136 doi 10.1038/s41467-017-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. The New England journal of medicine 2016;375(9):819–29 doi 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallmer K, Oxenius A. Recognition and Regulation of T Cells by NK Cells. Frontiers in immunology 2016;7:251 doi 10.3389/fimmu.2016.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nature medicine 2018;24(8):1178–91 doi 10.1038/s41591-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015;523(7559):231–5 doi 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 26.Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/beta-catenin pathway activation correlates with immune exclusion across human cancers. Clinical cancer research : an official journal of the American Association for Cancer Research 2019. doi 10.1158/1078-0432.CCR-18-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spranger S, Dai D, Horton B, Gajewski TF. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer cell 2017;31(5):711–23 e4 doi 10.1016/j.ccell.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P, et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clinical cancer research : an official journal of the American Association for Cancer Research 2016;22(6):1499–509 doi 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016;44(3):609–21 doi 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 30.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer discovery 2016;6(2):202–16 doi 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016;352(6282):227–31 doi 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerby-Arnon L, Shah P, Cuoco MS, Rodman C, Su MJ, Melms JC, et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 2018;175(4):984–97 e24 doi 10.1016/j.cell.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batlle E, Massague J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 2019;50(4):924–40 doi 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554(7693):544–8 doi 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018;554(7693):538–43 doi 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 36.Moon YW, Hajjar J, Hwu P, Naing A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. Journal for immunotherapy of cancer 2015;3:51 doi 10.1186/s40425-015-0094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigano S, Alatzoglou D, Irving M, Menetrier-Caux C, Caux C, Romero P, et al. Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Frontiers in immunology 2019;10:925 doi 10.3389/fimmu.2019.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell research 2017;27(1):109–18 doi 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleming V, Hu X, Weber R, Nagibin V, Groth C, Altevogt P, et al. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Frontiers in immunology 2018;9:398 doi 10.3389/fimmu.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pathria P, Louis TL, Varner JA. Targeting Tumor-Associated Macrophages in Cancer. Trends in immunology 2019;40(4):310–27 doi 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nature reviews Drug discovery 2015;14(9):642–62 doi 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33(25):2780–8 doi 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 43.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017;170(6):1109–19 e10 doi 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andtbacka RHI, Curti BD, Kaufman H, Daniels GA, Nemunaitis JJ, Spitler LE, et al. CALM study: A phase II study of an intratumorally delivered oncolytic immunotherapeutic agent, coxsackievirus A21, in patients with stage IIIc and stage IV malignant melanoma. Journal of Clinical Oncology 2014;32(15_suppl):3031- doi 10.1200/jco.2014.32.15_suppl.3031. [DOI] [Google Scholar]
- 45.Andtbacka RHI, Ross MI, Agarwala SS, Taylor MH, Vetto JT, Neves RI, et al. Preliminary results from phase II study of combination treatment with HF10, a replication-competent HSV-1 oncolytic virus, and ipilimumab in patients with stage IIIb, IIIc, or IV unresectable or metastatic melanoma. Journal of Clinical Oncology 2016;34(15_suppl):9543- doi 10.1200/JCO.2016.34.15_suppl.9543. [DOI] [Google Scholar]
- 46.Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36(14):1419–27 doi 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Angelova AL, Barf M, Geletneky K, Unterberg A, Rommelaere J. Immunotherapeutic Potential of Oncolytic H-1 Parvovirus: Hints of Glioblastoma Microenvironment Conversion towards Immunogenicity. Viruses 2017;9(12) doi 10.3390/v9120382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baird JR, Dietsch GN, Florio V, Gallatin M, Knox CD, Odingo J, et al. MV-626, a potent and selective inhibitor of ENPP1 enhances STING activation and augments T-cell mediated anti-tumor activity in vivo. 2018 November 7–11 2018; Washington, D.C. [Google Scholar]
- 49.Frank MJ, Reagan PM, Bartlett NL, Gordon LI, Friedberg JW, Czerwinski DK, et al. In Situ Vaccination with a TLR9 Agonist and Local Low-Dose Radiation Induces Systemic Responses in Untreated Indolent Lymphoma. Cancer discovery 2018;8(10):1258–69 doi 10.1158/2159-8290.CD-18-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribas A, Medina T, Kummar S, Amin A, Kalbasi A, Drabick JJ, et al. SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase Ib, Multicenter Study. Cancer discovery 2018;8(10):1250–7 doi 10.1158/2159-8290.CD-18-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milhem M, Gonzales R, Medina T, Kirkwood JM, Buchbinder E, Mehmi I, et al. Intratumoral toll-like receptor 9 (TLR9) agonist, CMP-001, in combination with pembrolizumab can reverse resistance to PD-1 inhibition in a phase Ib trial in subjects with advanced melanoma [abstract] In: Proceedings of the American Association for Cancer Research Annual Meeting 2018. 2018 Apr 14–18; Chicago, IL. p Philadelphia (PA): AACR; Cancer Res; 2018;78(13 Suppl):Abstract nr CT144. [Google Scholar]
- 52.Corrales L, McWhirter SM, Dubensky TW Jr., Gajewski TF. The host STING pathway at the interface of cancer and immunity. The Journal of clinical investigation 2016;126(7):2404–11 doi 10.1172/JCI86892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meric-Bernstam F, Werner TL, Hodi FS, Messersmith W, Lewis N, Talluto C, et al. Phase I Dose-finding Study of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients With Advanced Solid Tumors or Lymphomas. 2018 November 7–11 2018; Washington DC. [Google Scholar]
- 54.Harrington K, Brody J, Ingham M, Strauss J, Cemerski S, Wang M, et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as mono- therapy or in combination with pembrolizumab. 2018 October 19–23 2018; Munich. [Google Scholar]
- 55.Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 2018;564(7736):439–43 doi 10.1038/s41586-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 56.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer research 2011;71(7):2488–96 doi 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014;41(5):843–52 doi 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nature communications 2017;8:15618 doi 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. Journal of immunology 2005;174(12):7516–23. [DOI] [PubMed] [Google Scholar]
- 60.Hammerich L, Marron TU, Upadhyay R, Svensson-Arvelund J, Dhainaut M, Hussein S, et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nature medicine 2019;25(5):814–24 doi 10.1038/s41591-019-0410-x. [DOI] [PubMed] [Google Scholar]
- 61.Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Annals of oncology : official journal of the European Society for Medical Oncology 2019;30(2):219–35 doi 10.1093/annonc/mdy551. [DOI] [PubMed] [Google Scholar]
- 62.Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research 2001;7(10):3025–30. [PubMed] [Google Scholar]
- 63.Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016;44(2):343–54 doi 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. The New England journal of medicine 2018;378(22):2078–92 doi 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 65.Parra ER, Villalobos P, Behrens C, Jiang M, Pataer A, Swisher SG, et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. Journal for immunotherapy of cancer 2018;6(1):48 doi 10.1186/s40425-018-0368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nature reviews Immunology 2018;18(3):168–82 doi 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fong L, Carroll P, Weinberg V, Chan S, Lewis J, Corman J, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. Journal of the National Cancer Institute 2014;106(11) doi 10.1093/jnci/dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine 2010;363(5):411–22 doi 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 69.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. Journal of immunotherapy 2013;36(7):382–9 doi 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer immunology research 2014;2(7):616–31 doi 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. Journal of immunotherapy 2015;38(1):1–11 doi 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 2014;515(7528):572–6 doi 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 73.Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015;520(7549):692–6 doi 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547(7662):217–21 doi 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017;547(7662):222–6 doi 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 76.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015;348(6236):803–8 doi 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanovic S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019;565(7738):240–5 doi 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 78.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348(6230):62–8 doi 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research 2011;17(13):4550–7 doi 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moon EK, Wang LS, Bekdache K, Lynn RC, Lo A, Thorne SH, et al. Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology 2018;7(3):e1395997 doi 10.1080/2162402X.2017.1395997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nature biotechnology 2018;36(4):346–51 doi 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 82.Sato T, Nathan PD, Hernandez-Aya L, Sacco JJ, Orloff MM, Visich J, et al. Redirected T cell lysis in patients with metastatic uveal melanoma with gp100-directed TCR IMCgp100: Overall survival findings. Journal of Clinical Oncology 2018;36(15_suppl):9521- doi 10.1200/JCO.2018.36.15_suppl.9521. [DOI] [Google Scholar]
- 83.Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia 2017;31(10):2181–90 doi 10.1038/leu.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19(5):1225–31 doi 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19(2):393–403 doi 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2012;18(5):1386–94 doi 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 87.Ganesh S, Shui X, Craig KP, Park J, Wang W, Brown BD, et al. RNAi-Mediated beta-Catenin Inhibition Promotes T Cell Infiltration and Antitumor Activity in Combination with Immune Checkpoint Blockade. Molecular therapy : the journal of the American Society of Gene Therapy 2018;26(11):2567–79 doi 10.1016/j.ymthe.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer discovery 2018;8(2):216–33 doi 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proceedings of the National Academy of Sciences of the United States of America 2012;109(43):17561–6 doi 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer immunology research 2014;2(7):632–42 doi 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nature communications 2016;7:12624 doi 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. British journal of cancer 2018;119(10):1208–14 doi 10.1038/s41416-018-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Young KH, Newell P, Cottam B, Friedman D, Savage T, Baird JR, et al. TGFbeta inhibition prior to hypofractionated radiation enhances efficacy in preclinical models. Cancer immunology research 2014;2(10):1011–22 doi 10.1158/2326-6066.CIR-13-0207. [DOI] [PubMed] [Google Scholar]
- 94.Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, et al. Targeting the TGFbeta pathway with galunisertib, a TGFbetaRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. Journal for immunotherapy of cancer 2018;6(1):47 doi 10.1186/s40425-018-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Popovic A, Jaffee EM, Zaidi N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. The Journal of clinical investigation 2018;128(8):3209–18 doi 10.1172/JCI120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zamarin D, Ricca JM, Sadekova S, Oseledchyk A, Yu Y, Blumenschein WM, et al. PD-L1 in tumor microenvironment mediates resistance to oncolytic immunotherapy. The Journal of clinical investigation 2018;128(4):1413–28 doi 10.1172/JCI98047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bommareddy PK, Aspromonte S, Zloza A, Rabkin SD, Kaufman HL. MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation. Science translational medicine 2018;10(471) doi 10.1126/scitranslmed.aau0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019;565(7738):234–9 doi 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahn E, Araki K, Hashimoto M, Li W, Riley JL, Cheung J, et al. Role of PD-1 during effector CD8 T cell differentiation. Proceedings of the National Academy of Sciences of the United States of America 2018;115(18):4749–54 doi 10.1073/pnas.1718217115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuzefpolskiy Y, Baumann FM, Penny LA, Kalia V, Sarkar S. PD-1 signals instruct a critical metabolic switch for maintenance of T cell memory [abstract]. The Journal of Immunology 2017;198(1 Supplement):151.24-.24. [Google Scholar]
- 101.Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proceedings of the National Academy of Sciences of the United States of America 2011;108(1):266–71 doi 10.1073/pnas.1016791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PloS one 2016;11(6):e0157164 doi 10.1371/journal.pone.0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steinberg SM, Zhang P, Malik BT, Boni A, Shabaneh TB, Byrne KT, et al. BRAF inhibition alleviates immune suppression in murine autochthonous melanoma. Cancer immunology research 2014;2(11):1044–50 doi 10.1158/2326-6066.CIR-14-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Science translational medicine 2015;7(279):279ra41 doi 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abu Eid R, Razavi GS, Mkrtichyan M, Janik J, Khleif SN. Old-School Chemotherapy in Immunotherapeutic Combination in Cancer, A Low-cost Drug Repurposed. Cancer immunology research 2016;4(5):377–82 doi 10.1158/2326-6066.CIR-16-0048. [DOI] [PubMed] [Google Scholar]
- 106.Wang Z, Till B, Gao Q. Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology 2017;6(7):e1331807 doi 10.1080/2162402X.2017.1331807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012;30(17):2046–54 doi 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 108.Lesterhuis WJ, Salmons J, Nowak AK, Rozali EN, Khong A, Dick IM, et al. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PloS one 2013;8(4):e61895 doi 10.1371/journal.pone.0061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shrimali RK, Ahmad S, Verma V, Zeng P, Ananth S, Gaur P, et al. Concurrent PD-1 Blockade Negates the Effects of OX40 Agonist Antibody in Combination Immunotherapy through Inducing T-cell Apoptosis. Cancer immunology research 2017;5(9):755–66 doi 10.1158/2326-6066.CIR-17-0292. [DOI] [PubMed] [Google Scholar]
- 110.Messenheimer DJ, Jensen SM, Afentoulis ME, Wegmann KW, Feng Z, Friedman DJ, et al. Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23(20):6165–77 doi 10.1158/1078-0432.CCR-16-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]