Abstract
Backgroud: Severe pneumonia is one of the most common causes for mechanical ventilation. We aimed to early identify severe pneumonia patients with high risk of extubation failure in order to improve prognosis.
Methods: From April 2014 to December 2015, medical records of intubated patients with severe pneumonia in intensive care unit were retrieved from database. Patients were divided into extubation success and failure groups, and multivariate logistic regressions were performed to identify independent predictors for extubation failure.
Results: A total of 125 eligible patients were included, of which 82 and 43 patients had extubation success and failure, respectively. APACHE II score (odds ratio (OR) 1.141, 95% confident interval (CI) 1.022–1.273, P = 0.019, cutoff at 17.5), blood glucose (OR 1.122, 95%CI 1.008–1.249, P = 0.035, cutoff at 9.87 mmol/l), dose of fentanyl (OR 3.010, 95%CI 1.100–8.237, P = 0.032, cutoff at 1.135 mg/d), and the need for red blood cell (RBC) transfusion (OR 2.774, 95%CI 1.062–7.252, P = 0.037) were independent risk factors for extubation failure.
Conclusion: In patients with severe pneumonia, APACHE II score > 17.5, blood glucose > 9.87 mmol/l, fentanyl usage > 1.135 mg/d, and the need for RBC transfusion might be associated with higher risk of extubation failure.
Keywords: APACHE II score, blood glucose, extubation failure, fentanyl, RBC transfusion, severe pneumonia
Introduction
Pneumonia is a common respiratory infectious disease and is one of the top six causes of death [1]. Approximately 10% of hospitalized patients with pneumonia require intubation for invasive mechanical ventilation (IMV) due to respiratory failure, which is termed as severe pneumonia [2,3]. IMV provides essential life support and a treatment window by increasing arterial oxygenation, rebalancing ventilation/perfusion ratio, and decreasing respiratory workload, and eventually improves prognosis and reduces mortality. However, the long-term use of IMV may result in hazardous complications, such as ventilator-associated pneumonia, barotrauma, oxygen toxicity, and increased use of sedatives and cost [4,5]. Furthermore, it has been reported that prolonged weaning was associated with higher hospital mortality [6]. Therefore, early identification of pneumonia patients with risk of extubation failure is of great clinical significance because interventions might be planned and implemented when risk could be predicted and monitored.
Previous studies have found a variety of factors are associated with extubation failure, including: (1) demographics and clinical status, such as age, fluid balance, comorbidity burden, cardiac function, mental status, and severity of illness; (2) laboratory abnormalities, such as hypercapnia, serum albumin, and blood urea nitrogen (BUN); (3) respiratory mechanics, such as maximal inspiratory pressure and diaphragmatic function; and (4) treatment interventions, such as sedatives [5,7–9]. However, these predictors are identified from a cluster of diseases including pneumonia, chronic obstructive pulmonary disease, heart failure, and trauma. Moreover, pneumonia and acute respiratory distress syndrome (ARDS) have been elucidated to be independent predictors for extubation failure [9].
Based on the lack of specific investigations of predictors and risks for extubation failure predictors in patients with severe pneumonia, we retrospectively analyzed the data from intubated patients with severe pneumonia and aimed to identify the independent predictors for extubation failure.
Methods
From April 2014 to December 2015, medical records of the patients, who were admitted to Respiratory Intensive Care Unit (RICU) of West China Hospital, Sichuan University, were retrieved from the hospital inpatient database system. Study protocol was approved by the Institutional Ethical Committee for Clinical and Biomedical Research of West China Hospital, and all patients or their legal substitute decision makers were consented for publication of in-hospital data. All methods were performed in accordance with the relevant guidelines and regulations released by the Chinese National Institutes of Health and the Clinical Trial Center of West China Hospital.
Patients
Patients were eligibly included if they were diagnosed of severe pneumonia upon admission and needed IMV via oral intubation or tracheostomy for more than 24 h due to acute respiratory failure. Severe pneumonia was defined if patients met either one of the major criteria (acute respiratory failure requiring IMV and septic shock with need for vasopressors) or at least three minor criteria (respiratory rate ≥ 30 bpm, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ≤ 250, BUN ≥ 20 mg/dl, white blood cell count < 4 × 109/l, platelet count < 100 × 109/l, body temperature < 36°C, multilobar infiltrates, confusion/disorientation, and hypotension requiring aggressive fluid resuscitation) [2]. The indication for IMV was evaluated based on the arterial blood gas (ABG) analysis indicating refractory hypoxemia with PaO2 less than 50 mmHg although on oxygen supplement.
We excluded patients if their pneumonia was secondary to known pulmonary diseases such as acute exacerbation of chronic obstructive pulmonary disease (COPD), asthma, and pulmonary thromboembolism, or if they were in fatal and life-threatening comorbidities including, but not limited to, aggressive carcinoma with unstable clinical conditions and cerebral injury. Patients with immunosuppressive therapy were also excluded in our study.
Study design
The present study was designed as a retrospective cohort study, in which eligible patients were classified into extubation success and failure groups. Patients’ baseline demographics and clinical characteristics, laboratory measurements, imaging information, and treatment details at the time of ICU admission were compared between these two groups to identify potential independent risk factors for the prediction of extubation failure in patients with severe pneumonia.
Definition of extubation success and failure
Successful extubation was defined as removal of endotracheal tube for more than 72 h regardless of the need for sequential non-invasive positive pressure ventilation (NPPV); while extubation failure was defined as unavoidable reintubation within 72 h of extubation [10]. Failure of IMV discontinuation was not treated as extubation failure only if the patients failed the extubation afterward. All patients who fell into the extubation failure criteria were mechanically ventilated after the trial of extubation or ventilator discontinuation.
Ventilation and treatment strategies
In general, mechanical ventilation was initiated with a tidal volume (VT) of 8–10 ml/kg of predicted body weight and a minimal positive end-expiratory pressure (PEEP), which was titrated with FiO2 to target an oxygen saturation of pulse oximetry (SpO2) ≥ 90% or PaO2 ≥ 60 mmHg using pressure-controlled or volume-controlled assist/control mode [11,12]. However, “lung protective ventilation” and “open-lung approach” were given if patients developed ARDS, in which the patients’ lungs were protected by a lower VT of 4–8 ml/kg of predicted body weight with a plateau airway pressure ≤ 30 cm H2O and the collapsed alveoli were opened by intermittent recruitment maneuvers and maintained open by a PEEP of 2 cm H2O higher than pressures at which an abrupt increment was detected in the upward slope of the pressure–volume curve [11,12]. Respiratory therapists up- or down-regulated the support of ventilation based on the ABG analysis every day.
Empirical antibiotics treatment was administered in accordance with the consensus by the Infectious Diseases Society of America and American Thoracic Society, and reasonable changes were made once a specific pathogen was identified and considered as clinical importance [2]. All patients were sedated with midazolam and (or) propofol on the basis of analgesia with fentanyl to meet a goal of Richmond Agitation-Sedation Scale between -2 and 0 and a target of critical care pain observation tool < 3. Daily interruption by temporarily ceasing the sedative infusions was also implemented to avoid potential delirium, which was defined if Richmond Agitation-Sedation Scale was between −3 and +4 and Confusion Assessment Method for Intensive Care Unit was positive [13,14]. Blood glucose level was measured every 4 h routinely and insulin dosing was commenced when two consecutive blood glucose levels are greater than 10 mmol/l with a target upper blood glucose ≤ 10 mmol/l [15]. Other treatments including but not limited to organ function supports, airway clearance by bedside bronchoscopy, circulation and nutrition maintenance, as well as internal environment homeostasis stabilization were also performed when necessary according to patients’ clinical conditions.
Extubation procedures
Spontaneous breathing trial (SBT) was conducted by respiratory therapists if the following criteria were met: (1) the causes of mechanical ventilation were relieved or alleviated; (2) PaO2/FiO2 > 150–200 at FiO2 ≤ 0.4–0.5 and PEEP ≤ 5–8 cm H2O; (3) stable hemodynamics without need for vasopressors or dopamine/dobutamine <5–10 μg/kg/min; and (4) the capability of spontaneous breathing. [16]. During SBT, a low level of pressure support between 5 and 7 cm H2O was used for 30 min, and patients were considered as readiness for extubation if they have no following conditions: (1) agitation, anxiety, depressed mental status, and diaphoresis; (2) evidence of increased accessory muscle activity; (3) PaO2 ≤ 50–60 mmHg at FiO2 ≥ 0.5 or arterial oxygen saturation (SaO2) < 90%; (4) partial pressure of arterial carbon dioxide (PaCO2) > 50 mmHg or an increase of PaCO2 > 8 mmHg; (5) pH < 7.32 or a decrease of pH ≥ 0.07 pH units; (6) respiratory rate (RR) > 35 breaths per minute (bpm) or increased by ≥ 50%; (7) rapid shallow breathing index (RSBI = ratio of RR to VT) > 105 bpm; (8) heart rate > 140 beats per minute or increased by ≥20%; (9) systolic blood pressure < 90 mmHg or > 180 mmHg or increased by ≥ 20%; and (10) cardiac arrhythmias [17]. Otherwise, the previous ventilation mode or a non-fatiguing mode would be switched back and any possible reversible etiologies for failure would also be reviewed before the next SBT every day.
Recording of clinical conditions and laboratory measurements
Patients’ demographics including age, gender, body mass index (BMI), and smoking and disease histories were recorded. The disease severities were assessed upon the admission to ICU by different scoring systems including acute physiology and chronic health evaluation II (APACHE II) scores, multiple organ dysfunction (Marshall) scores, sequential organ failure assessment (SOFA) scores, lung injury scores (LIS), and gas exchange, organ failure, cause, and associated disease (GOCA) scores. APACHE II is a comprehensive scoring system to identify acutely ill patients with high risk of death based on 14 parameters such as body temperature, mean arterial pressure, RR, PaO2, ABG, and mental status [18]. Marshall and SOFA scores are used to evaluate the organ function or rate of failure, which consists of 4 or 6 items involving cardiovascular, hepatic, coagulation, renal, respiratory, and neurological systems [19]. LIS scoring system specifically stratifies the extent of lung injury into three categories from no lung injury to severe lung injury by the infiltration area on chest X-ray, PaO2/FiO2, PEEP, and static compliance of lung [20]. Similar to LIS, GOCA score was developed for patients with ARDS to define the disease but not to predict prognosis [21].
Clinical conditions as well as the laboratory measurements at the time of ICU admission rather than extubation were recorded, including vital signs, ABG, blood cell count, blood electrolytes, myocardial, and inflammatory biomarkers. The worst (highest) level of blood glucose measured within 24 h of ICU admission was recorded. Pathogenic microorganisms were generally screened in sputum (including bronchoalveolar lavage fluid, BALF), blood, and urine by culture or chemiluminescence. We also recorded details of treatment during ICU such as mechanical ventilation settings, types and dose of analgesics and sedatives, usage of vasopressors and muscle relaxants, as well as component and amount of blood transfusions.
Statistical analysis
Statistical analysis was performed by SPSS 21.0 [Copyright (c) SPSS Inc. 1989-2007]. Continuous data were presented as mean ± standard deviation (SD), while dichotomous data were reported as frequency and proportion. Independent Student’s t-test and Chi-square test or Fisher’s exact test were performed between extubation success and failure groups. Variables with two-sided P value of < 0.05 from the independent Student’s t-test and Chi-square test were individually analyzed by univariate logistic regression model, and were assessed for potential correlations and multicollinearity among individual variables by correlation matrixs and variance inflation factors (VIF). Thereafter, significant predictors with P value of < 0.10 were further analyzed in the multivariate logistic regression to identify the independent risk factors associated with extubation failure, and the adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated. Receiver operating characteristic (ROC) curves were conducted to depict the area under the curve (AUC) for the accuracy of each independent risk factor in recognizing extubation failure, and cutoff points together with sensitivity, specificity, positive and negative predictive value (PPV and NPV), and likelihood ratio (LR) were also calculated by maximal Youden index (= sensitivity + specificity − 1).
Results
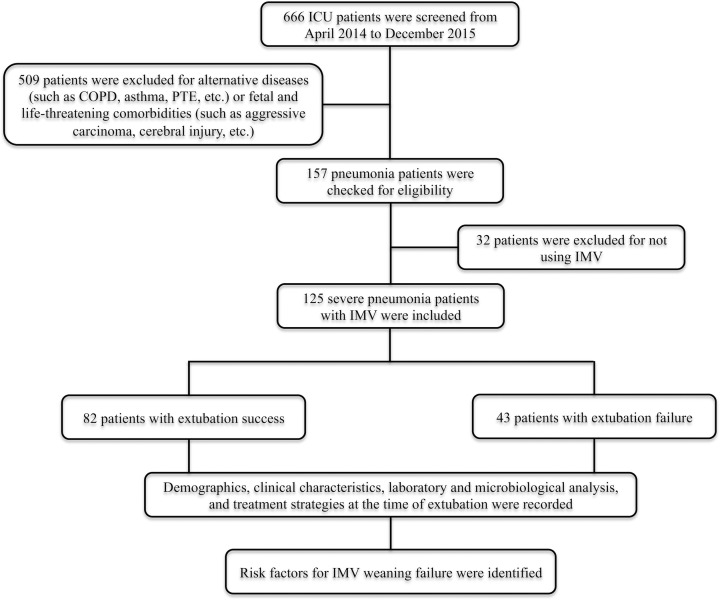
A total of 666 patients with RICU admission were found, of which 509 patients were excluded according to the pre-defined exclusion criteria and 32 patients were excluded for not using IMV. Finally, 125 patients with severe pneumonia were included and divided into extubation success (n = 82) and failure (n = 43) groups (Figure 1). The rate of extubation failure was as high as 34.4% in patients with severe pneumonia, and the total mortality reached up to 58.4% (n = 73). The average days to extubation were 13.4 days.
Figure 1. Study flow diagram.
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IMV, invasive mechanical ventilation; PTE, pulmonary thromboembolism.
Baseline demographics and clinical characteristics between extubation success and failure
Tables 1 and 2 summarized patients’ baseline characteristics and clinical and laboratory measurements, respectively. Compared with patients with extubation success, patients with extubation failure were associated with significantly higher mortality (97.7% vs. 37.8%, P < 0.001), but no significant difference was found in ICU stay (14.42 ± 12.87 vs. 18.29 ± 15.67 days, P = 0.166).
Table 1. Baseline characteristics for patients with extubation success and failure.
| Characteristics | Extubation success (n = 82) | Extubation failure (n = 43) | P |
|---|---|---|---|
| Demographics | |||
| Gender (Male, %) | 57 (69.5) | 24 (55.8) | 0.128 |
| Age (years) | 60 ± 18 | 61 ± 18 | 0.776 |
| BMI (kg/m2) | 22.01 ± 2.85 | 21.41 ± 2.51 | 0.489 |
| Smoking (%) | 26 (31.7) | 19 (44.2) | 0.167 |
| Disease severity | |||
| APACHE II | 16.30 ± 4.87 | 18.30 ± 4.23 | 0.025 |
| Marshall | 2.17 ± 2.30 | 2.56 ± 2.26 | 0.370 |
| SOFA | 5.18 ± 2.80 | 5.98 ± 2.75 | 0.132 |
| LIS | 2.19 ± 0.74 | 2.44 ± 0.73 | 0.047 |
| GOCA | 4.89 ± 1.49 | 5.47 ± 1.18 | 0.030 |
| Comorbidities | |||
| Cardiovascular diseases (%) | 30 (36.6) | 20 (46.5) | 0.282 |
| Diabetes (%) | 17 (20.7) | 12 (27.9) | 0.367 |
| Renal diseases (%) | 17 (20.7) | 9 (20.9) | 0.979 |
| Connective tissue diseases (%) | 5 (6.1) | 6 (14.0) | 0.185 |
| Gastrointestinal diseases (%) | 20 (24.4) | 15 (34.9) | 0.215 |
| Metabolic diseases (%) | 4 (4.9) | 1 (2.3) | 0.659 |
| AIDS (%) | 4 (4.9) | 1 (2.6) | 1.000 |
| Syphilis (%) | 4 (4.9) | 0 (0) | 0.304 |
| Hepatitis B (%) | 4 (4.9) | 2 (5.1) | 1.000 |
| Outcomes | |||
| Mortality (%) | 31 (37.8) | 42 (97.7) | <0.001 |
| Length of ICU stay (days) | 18.29 ± 15.67 | 14.42 ± 12.87 | 0.166 |
Abbreviations: AIDS, acquired immune deficiency syndrome; APACHE II, acute physiology, age, chronic health evaluation; BMI, body mass index; GOCA, gas exchange, organ failure, cause, associated disease; ICU, intensive care unit; LIS, lung injury score; SD, standard deviation; SOFA, sequential organ failure assessment.
Table 2. Clinical characteristics and laboratory analysis at the time of extubation between patients with extubation success and failure.
| Parameters | Extubation success (n = 82) | Extubation failure (n = 43) | P |
|---|---|---|---|
| Clinical characteristics | |||
| Lower extremities edema (%) | 26 (31.7) | 17 (39.5) | 0.381 |
| Body temperature (°C) | 37.1 ± 0.9 | 37.1 ± 1.1 | 0.999 |
| Respiratory rate (bpm) | 23 ± 6 | 26 ± 7 | 0.012 |
| Heart rate (bpm) | 105 ± 24 | 112 ± 28 | 0.121 |
| SBP (mmHg) | 116 ± 22 | 121 ± 22 | 0.251 |
| DBP (mmHg) | 67 ± 15 | 70 ± 20 | 0.484 |
| SpO2 (%) | 96 ± 5 | 94 ± 8 | 0.162 |
| Delirium (%) | 14 (17.1) | 4 (9.3) | 0.240 |
| New-onset septic shock (%) | 14 (17.1) | 16 (37.2) | 0.012 |
| New-onset atrial fibrillation (%) | 2 (2.4) | 1 (2.3) | 1.000 |
| ARDS development (%) | 25 (30.5) | 14 (32.6) | 0.812 |
| Imaging | |||
| Echocardiography | |||
| Left ventricle (cm2) | 44.63 ± 5.02 | 45.33 ± 7.07 | 0.571 |
| Right ventricle (cm2) | 20.40 ± 2.71 | 20.59 ± 3.78 | 0.779 |
| Ejection fraction (%) | 64 ± 7 | 60 ± 15 | 0.070 |
| Pericardial effusion (%) | 18 (24.3) | 7 (20.0) | 0.616 |
| Chest CT | |||
| Pleural effusion (%) | 55 (67.1) | 29 (69.0) | 0.824 |
| Head CT | |||
| Cerebral lacunar infarction | 20 (54.1) | 10 (62.5) | 0.569 |
| Laboratory analysis | |||
| Blood types | |||
| Type A (%) | 16 (32.0) | 5 (17.2) | 0.152 |
| Type B (%) | 10 (20.0) | 8 (27.6) | 0.438 |
| Type AB (%) | 3 (6.0) | 4 (13.8) | 0.413 |
| Type O (%) | 21 (42.0) | 12 (41.4) | 0.957 |
| Arterial blood gas | |||
| pH | 7.383 ± 0.069 | 7.350 ± 0 .105 | 0.039 |
| PaO2/FiO2 (mmHg) | 151.14 ± 87.92 | 121.14 ± 64.39 | 0.050 |
| PaCO2 (mmHg) | 43.1 ± 10.4 | 43.7 ± 14.7 | 0.779 |
| HCO3− (mmol/l) | 25.0 ± 5.1 | 23.0 ± 5.1 | 0.055 |
| Blood cell analysis | |||
| Hemoglobin (g/l) | 101 ± 23 | 102 ± 24 | 0.772 |
| Hematocrit (%) | 35 ± 38 | 31 ± 7 | 0.475 |
| Platelet (109/l) | 164 ± 92 | 152 ± 98 | 0.505 |
| WBC (109/L) | 13.00 ± 7.58 | 14.10 ± 9.10 | 0.475 |
| Neutrophils (%) | 89.2 ± 7.4 | 87.7 ± 12.6 | 0.385 |
| Laboratory analysis | |||
| Biochemical analysis | |||
| TB (μmol/l) | 10.8 ± 7.3 | 15.2 ± 17.7 | 0.048 |
| ALT (IU/l) | 56 ± 160 | 84 ± 230 | 0.434 |
| AST (IU/l) | 86 ± 223 | 237 ± 895 | 0.150 |
| ALP (IU/l) | 121 ± 90 | 106 ± 83 | 0.356 |
| GGT (IU/l) | 86 ± 125 | 116 ± 344 | 0.475 |
| Albumin (g/l) | 27.5 ± 4.8 | 27.8 ± 4.4 | 0.769 |
| BUN (mmol/l) | 10.95 ± 6.72 | 12.35 ± 8.24 | 0.307 |
| Creatinine (μmol/l) | 105.9 ± 94.2 | 109.1 ± 90.7 | 0.857 |
| Glucose (mmol/l) | 8.93 ± 3.96 | 10.47 ± 5.24 | 0.038 |
| LDH (IU/l) | 470 ± 289 | 793 ± 1823 | 0.128 |
| Na+ (mmol/l) | 141.3 ± 6.4 | 139.4 ± 6.8 | 0.129 |
| K+ (mmol/l) | 4.05 ± 0.57 | 4.21 ± 0.67 | 0.173 |
| Cl− (mmol/l) | 105.7 ± 8.1 | 104.3 ± 8.5 | 0.356 |
| Ca2+ (mmol/l) | 1.98 ± 0.16 | 1.97 ± 0.21 | 0.690 |
| Coagulation analysis | |||
| PT (s) | 14.1 ± 2.6 | 15.0 ± 5.2 | 0.177 |
| INR | 1.24 ± 0.22 | 1.32 ± 0.45 | 0.178 |
| APTT (s) | 37.5 ± 11.6 | 48.0 ± 37.0 | 0.021 |
| FiB (g/l) | 4.37 ± 2.00 | 4.53 ± 2.29 | 0.687 |
| D-dimer (mg/l FEU) | 9.77 ± 9.77 | 218.16 ± 1374.57 | 0.182 |
| Laboratory analysis | |||
| Myocardial biomarkers | |||
| Myoglobin (ng/ml) | 286.20 ± 557.74 | 530.58 ± 868.80 | 0.066 |
| Troponin (ng/l) | 79.04 ± 187.12 | 339.31 ± 1500.11 | 0.130 |
| BNP (pg/ml) | 3587 ± 6683 | 6904 ± 10049 | 0.034 |
| Inflammatory biomarkers | |||
| Procalcitonin (ng/ml) | 7.22 ± 18.45 | 7.52 ± 16.31 | 0.930 |
| CRP (ng/l) | 144.79 ± 108.25 | 174.69 ± 140.22 | 0.288 |
| Interleukin-6 (pg/ml) | 598.28 ± 1230.68 | 872.13 ± 1432.04 | 0.373 |
| Cellular immunity | |||
| CD3 cell (%) | 58.23 ± 16.28 | 56.33 ± 15.23 | 0.589 |
| CD4 cell (%) | 28.62 ± 14.75 | 26.67 ± 10.46 | 0.507 |
| CD8 cell (%) | 26.69 ± 16.41 | 26.55 ± 14.40 | 0.968 |
| Humoral immunity | |||
| Complement 3 (g/l) | 0.8550 ± 0.2253 | 0.7309 ± 0.2959 | 0.035 |
| Complement 4 (g/l) | 0.2200 ± 0.0709 | 0.1957 ± 0.0958 | 0.193 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; Ca2+, calcium ion; Cl−, chloride ion; CRP, C-reactive protein; CT, computed tomography; DBP, diastolic blood pressure; FiB, fibrinogen; GGT, γ-glutamyltranspetidase; HCO3−, bicarbonate ion; INR, international normalized ratio; K+, potassium ion; LDH, lactate dehydrogenase; Na+, sodium ion; PaCO2; partial pressure of arterial carbon dioxide; PaO2/FiO2, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen; PT, prothrombin time; SBP, systolic blood pressure; SpO2, oxygen saturation of pulse oximetry; TB, total bilirubin; WBC, white blood cell count.
In the group of extubation failure, we found significantly higher APACHE II score (18.30 ± 4.23 vs. 16.30 ± 4.87, P = 0.025) and blood glucose (10.47 ± 5.24 vs. 8.93 ± 3.96 mmol/l, P = 0.038), but significantly lower pH (7.350 ± 0.105 vs. 7.383 ± 0.069, P = 0.039) and plasma complement 3 level (0.7309 ± 0.2959 vs. 0.8550 ± 0.2253 g/l, P = 0.035) compared with the group of extubation success. Significant increase was also found in LIS score, GOCA score, RR, incidence of new-onset septic shock, total bilirubin (TB), activated partial thromboplastin time (APTT), and brain natriuretic peptide (BNP).
Pathogenic microorganisms features between extubation success and failure
Acinetobacter baumannii was the most commonly detected bacterium in sputum (including BALF), blood and urine, while Candida albicans were the most common fungi identified in sputum (including BALF) and urine (Table 3). Pseudomonas aeruginosa and Candida tropicalis could also be easily found in sputum (including BALF) but not in blood or urine. Herpes simplex virus predominated the causes for severe viral pneumonia, followed by Epstein–Barr virus. However, we did not find any significant differences in each pathogenic microorganism from sputum (including BALF), blood and urine between the extubation success and failure.
Table 3. Microbiological analyses at the time of extubation between patients with extubation success and failure.
| Parameters | Extubation success (n = 82) | Extubation failure (n = 43) | P |
|---|---|---|---|
| Sputum (BALF) culture | |||
| Bacteria | |||
| Acinetobacter baumannii (%) | 50 (61.0) | 25 (64.1) | 0.741 |
| Pseudomonas aeruginosa (%) | 16 (19.5) | 5 (12.8) | 0.364 |
| Klebsiella pneumoniae (%) | 6 (7.3) | 4 (10.3) | 0.725 |
| Burkholderia cepacia (%) | 4 (4.9) | 2 (5.1) | 1.000 |
| Stenotrophomonas maltophilia (%) | 7 (8.5) | 3 (7.7) | 1.000 |
| Enterobacter cloacae (%) | 5 (6.1) | 1 (2.6) | 0.663 |
| Serratia marcescens (%) | 4 (4.9) | 0 (0) | 0.304 |
| Escherichia coli (%) | 3 (3.7) | 1 (2.6) | 1.000 |
| Haemophilus influenzae (%) | 0 (0) | 1 (2.6) | 0.322 |
| Bacillus mirabilis (%) | 2 (2.4) | 0 (0) | 1.000 |
| Staphylococcus aureus (%) | 3 (3.7) | 2 (5.1) | 0.657 |
| Fungi | |||
| Candida albicans (%) | 21 (33.9) | 11 (31.4) | 0.806 |
| Candida tropicalis (%) | 8 (12.9) | 1 (2.9) | 0.150 |
| Candida glabrata (%) | 5 (8.1) | 0 (0) | 0.156 |
| Candida krusei (%) | 1 (1.6) | 1 (2.9) | 1.000 |
| Candida parapsilosis (%) | 0 (0) | 1 (2.9) | 0.361 |
| Aspergillus fumigatus (%) | 2 (2.4) | 2 (5.0) | 0.597 |
| Blood culture | |||
| Bacteria | |||
| Acinetobacter baumannii (%) | 9 (13.4) | 5 (13.5) | 1.000 |
| Escherichia coli (%) | 0 (0) | 1 (2.7) | 0.356 |
| Enterococcus faecium (%) | 0 (0) | 1 (2.7) | 0.356 |
| Pseudomonas aeruginosa (%) | 1 (1.5) | 0 (0) | 1.000 |
| Urine culture | |||
| Bacteria | |||
| Escherichia coli (%) | 1 (3.4) | 0 (0) | 1.000 |
| Klebsiella pneumoniae (%) | 2 (6.9) | 0 (0) | 1.000 |
| Acinetobacter baumannii (%) | 2 (6.9) | 0 (0) | 1.000 |
| Fungi | |||
| Candida tropicalis (%) | 1 (3.6) | 0 (0) | 1.000 |
| Candida glabrata (%) | 2 (7.1) | 1 (10.0) | 1.000 |
| Candida albicans (%) | 3 (10.7) | 3 (30.0) | 0.310 |
| Serum viral IgM | |||
| Herpes Simplex virus (%) | 9 (15.5) | 5 (17.2) | 1.000 |
| Rubella virus (%) | 1 (1.7) | 0 (0) | 1.000 |
| Cytomegalovirus (%) | 0 (0) | 1 (3.4) | 0.330 |
| Epstein–Barr virus (%) | 4 (9.1) | 2 (11.1) | 1.000 |
Abbreviation: BALF, bronchoalveolar lavage fluid.
Treatment and outcome characteristics between extubation success and failure
Compared with patients with extubation success, significantly higher PEEP (9 ± 4 vs. 7 ± 3 cmH2O, P = 0.021) and FiO2 (80 ± 22 vs. 72 ± 22%, P = 0.037) during IMV were needed and more midazolam (73.08 ± 55.17 vs. 48.98 ± 48.08 mg/d, P = 0.013) and fentanyl (0.93 ± 0.48 vs. 0.75 ± 0.45 mg/d, P = 0.043) were used in patients with extubation failure (Table 4). Additionally, significantly more patients used vasopressors (86.0% vs. 62.2%, P = 0.006) and needed transfusion of red blood cells (RBC) (44.2% vs. 28.0%, P = 0.040) in patients with extubation failure. The use of muscle relaxants, corticosteroids, diuretics, and insulin were similar in the two groups.
Table 4. Treatment strategies at the time of extubation between patients with extubation success and failure.
| Parameters | Extubation success (n = 82) | Extubation failure (n = 43) | P |
|---|---|---|---|
| IMV (%) | |||
| Mode (A/C, %) | 70 (85.4) | 40 (93.0) | 0.211 |
| Frequency (bpm) | 14 ± 3 | 14 ± 3 | 0.747 |
| Tidal volume (ml) | 461 ± 74 | 449 ± 49 | 0.415 |
| Inspiratory pressure (cmH2O) | 16 ± 4 | 15 ± 3 | 0.669 |
| PEEP (cmH2O) | 7 ± 3 | 9 ± 4 | 0.021 |
| FiO2 (%) | 72 ± 22 | 80 ± 22 | 0.037 |
| Time to extubation (days) | 13.5 ± 13.8 | 14.4 ± 12.9 | 0.739 |
| Sequential withdrawal with NPPV (%) | 31 (37.8) | 0 (0) | 0.000 |
| Analgesics and sedatives | |||
| Midazolam (mg/d) | 48.98 ± 48.08 | 73.08 ± 55.17 | 0.013 |
| Propofol (mg/d) | 395.22 ± 425.31 | 442.19 ± 522.59 | 0.589 |
| Fentanyl (mg/d) | 0.75 ± 0.45 | 0.93 ± 0.48 | 0.043 |
| Vasopressors (%) | 51 (62.2) | 37 (86.0) | 0.006 |
| Muscle relaxants (%) | 10 (12.2) | 6 (14.0) | 0.780 |
| Antibiotics | |||
| Number of antibacterial agents | 4 ± 7 | 4 ± 3 | 0.580 |
| Number of antifungal agents | 1 ± 1 | 1 ± 1 | 0.410 |
| Number of antiviral agents | 0 ± 0 | 0 ± 0 | 0.711 |
| Venous corticosteroids (%) | 54 (65.9) | 31 (72.1) | 0.477 |
| Venous diuretics (%) | 72 (87.8) | 37 (86.0) | 0.780 |
| Insulin (%) | 35 (42.7) | 23 (53.5) | 0.250 |
| Blood products | |||
| Albumin (%) | 75 (91.5) | 38 (88.4) | 0.750 |
| RBC (%) | 23 (28.0) | 19 (44.2) | 0.040 |
| Platelet (%) | 9 (11.0) | 3 (7.0) | 0.543 |
| Plasma (%) | 18 (22.0) | 13 (30.2) | 0.308 |
| Cryoprecipitate (%) | 3 (3.7) | 0 (0) | 0.551 |
Abbreviations: A/C, assist/control; FiO2, fraction of inspired oxygen; IMV, invasive mechanical ventilation; NPPV, non-invasive positive pressure ventilation; PEEP, positive end-expiratory pressure; RBC, red blood cell.
Predictors and risk factors for extubation failure in patients with severe pneumonia
Correlation matrix analysis showed significant correlations among the potential risk factors for extubation failure identified previously except for APACHE II, EF, blood glucose, dose of fentanyl, and percentage of patients using RBC transfusion, and variance inflation factors for these parameters showed no multicollinearity between each other (VIF ranged from 1.011 to 1.037) (data not shown). Multivariate logistic regression found APACHE II score (OR 1.141, 95% CI 1.022–1.273, P = 0.019), blood glucose (OR 1.122, 95% CI 1.008–1.249, P = 0.035), cumulative dose of fentanyl (OR 3.010, 95% CI 1.100-8.237, P = 0.032), and percentage of patients using RBC transfusion (OR 2.774, 95% CI 1.062–7.252, P = 0.037) were independent risk factors for predicting extubation failure but not EF (OR 0.965, 95%CI 0.925–1.007, P = 0.097) (Table 5).
Table 5. Univariate and multivariate logistic regression of risk factors for extubation failure in patients with severe pneumonia.
| Variables | Univariate logistic regression | Multivariate logistic regression | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| APACHEII | 1.096 (1.010–1.188) | 0.027 | 1.141 (1.022–1.273) | 0.019 |
| LIS | 1.596 (0.947–2.688) | 0.079 | ||
| GOCA | 1.356 (1.025–1.794) | 0.033 | ||
| Respiratory rate (bpm) | 1.072 (1.014–1.134) | 0.015 | ||
| New-onset septic shock (%) | 2.878 (1.237–6.698) | 0.014 | ||
| Ejection fraction (%) | 0.963 (0.923–1.006) | 0.092 | 0.965 (0.925–1.007) | 0.097 |
| pH | 0.009 (0.000–0.910) | 0.045 | ||
| PaO2/FiO2 (mmHg) | 0.995 (0.990–1.000) | 0.056 | ||
| HCO3− (mmol/l) | 0.923 (0.849–1.003) | 0.059 | ||
| TB (μmol/l) | 1.035 (0.995–1.076) | 0.087 | ||
| Glucose (mmol/l) | 1.079 (0.992–1.172) | 0.076 | 1.122 (1.008–1.249) | 0.035 |
| APTT (s) | 1.020 (1.000–1.041) | 0.055 | ||
| Myoglobin (ng/ml) | 1.000 (1.000–1.001) | 0.078 | ||
| BNP (pg/ml) | 1.000 (1.000–1.000) | 0.044 | ||
| Complement 3 (g/l) | 0.146 (0.023–0.912) | 0.039 | ||
| PEEP (cmH2O) | 1.132 (1.016–1.263) | 0.025 | ||
| FiO2 (%) | 1.018 (1.001–1.036) | 0.039 | ||
| Sequential withdrawal with NPPV (%) | 0.000 (0.000–) | 0.998 | ||
| Midazolam (mg/d) | 1.009 (1.002–1.017) | 0.016 | ||
| Fentanyl (mg/d) | 2.304 (1.011–5.247) | 0.047 | 3.010 (1.100–8.237) | 0.032 |
| Vasopressors (%) | 3.748 (1.419–9.900) | 0.008 | ||
| RBC transfusion (%) | 2.031 (0.939–4.390) | 0.072 | 2.774 (1.062–7.252) | 0.037 |
Abbreviations: APACHE II, acute physiology, age, chronic health evaluation; APTT, activated partial thromboplastin time; BNP, brain natriuretic peptide; CI, confidence interval; FiO2, fraction of inspired oxygen; GOCA, gas exchange, organ failure, cause, associated disease; HCO3−, bicarbonate ion; LIS, lung injury score; NPPV, non-invasive positive pressure ventilation; OR, odds ratio; PaO2/FiO2, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen; PEEP, positive end-expiratory pressure; RBC, red blood cell; TB, total bilirubin.
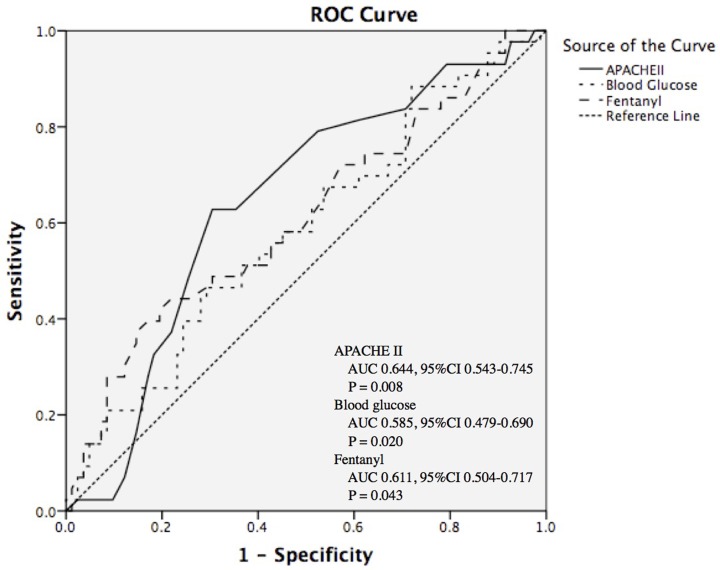
Figure 2 depicted the ROC curves for APACHE II score, blood glucose, and dose of fentanyl, in which all these factors showed acceptable capacity in the identification of extubation failure in patients with severe pneumonia (APACHE II score: AUC 0.644, 95% CI 0.543–0.745, P = 0.008; blood glucose: AUC 0.585, 95% CI 0.479–0.690, P = 0.020; dose of fentanyl: AUC 0.611, 95% CI 0.504–0.717, P = 0.043). A cutoff point of 17.5 for APACHE II score, 9.87 mmol/l for blood glucose, and 1.135 mg/d for fentanyl usage resulted in a sensitivity of 62.79%, 41.86% and 37.21%, and a specificity of 69.51%, 71.95% and 85.37%, respectively, in identifying severe pneumonia patients with high risk of extubation failure, as well as a good negative predictive value (NPV: 78.08%, 70.24%, and 72.16%) (Table 6).
Figure 2. ROC curves of APACHE II, blood glucose, and fentanyl at the time of extubation in distinguishing extubation failure in patients with severe pneumonia.
APACHE II, acute physiology and chronic healthy evaluation II; AUC, area under the curve; CI, confidence interval; ROC, receiver operating curve.
Table 6. Cut-off points of APACHE II, blood glucose, and fentanyl, and the corresponding sensitivity, specificity, PPV, NPV, and LR in distinguishing extubation failure in patients with severe pneumonia.
| Cut-off point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ | LR- | |
|---|---|---|---|---|---|---|---|
| APACHE II | 17.5 | 62.79 | 69.51 | 51.92 | 78.08 | 2.06 | 0.54 |
| Blood glucose (mmol/l) | 9.87 | 41.86 | 71.95 | 43.90 | 70.24 | 1.49 | 0.81 |
| Fentanyl (mg/d) | 1.135 | 37.21 | 85.37 | 57.14 | 72.16 | 2.54 | 0.74 |
APACHE II, acute physiology, age, chronic health evaluation; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
Discussion
It has been recognized that extubation failure is associated with increased mortality, longer ICU and hospital stays, and more nosocomial pneumonia [22,23]. In our study, we also demonstrated that patients with extubation failure had higher risk of death but not longer ICU stays, which mainly resulted from the death of these patients before they met the readiness of extubation. Due to the high incidence of extubation failure as well as the subsequent association of mortality, we are intrigued to explore potential factors associated with extubation failure to improve patients’ outcomes by optimizing treatment strategies and ventilator settings.
APACHE II score is the most commonly used tool to evaluate disease severity in ICU [18,24]. It has also been reported to have potential predictive values in extubation [25]. In our study, we also found that APACHE II score is an independent factor for predicting extubation failure from IMV although with a relatively low sensitivity (62.79%) and specificity (69.51%) at a cut-off point of greater than 17.5. However, we did not find such power of SOFA or Marshall score, which was not consistent with the previous finding that a high SOFA score on day 21 of IMV was associated with extubation and weaning failure in patients requiring prolonged IMV [26]. These differences might be because: (1) the different diseases for mechanical ventilation in different studies. Our study specifically focused on patients with severe pneumonia; (2) the offset effect of APACHE II score on the significance of SOFA as the items of SOFA are also included in APACHE II score. In terms of the LIS and GOCA scores, both of them were shown to be higher in patients with extubation failure, but they were not considered for multivariate logistic regression due to the significant correlations with other variables. Furthermore, LIS and GOCA are designed particularly for ARDS development, and our study did not find significant difference in ARDS development between patients with extubation failure and success.
Hyperglycemia is a common phenomenon in critically ill patients resulted from the development of hyperglycemia or insulin resistance [27,28]. It is found to be associated with increased mortality and adverse events such as postoperative infection and critical-illness polyneuropathy [29]. Studies have demonstrated that lowered blood glucose level rather than the insulin dose could bring beneficial effects [30,31], and the blood glucose control was also recognized as an independent predictor for prolonged mechanical ventilation [32,33] In our study, we also found that blood glucose of greater than 9.87 mmol/l was associated with higher risk of extubation failure, which might be due to the normal immune function maintenance, systematic inflammation reduction, endothelium and mitochondrial ultrastructure production, and skeletal muscle strength improvement after the recovery of normal glucose uptake [34,35].
Overuse and accumulation of opioid agents have been elucidated to be associated with increased adverse events, such as respiratory depression, iatrogenic withdrawal syndrome, and prolongation of time to extubation and weaning [36,37]. In our study, we also found that the use of both fentanyl and midazolam were significantly increased in patients with extubation failure, and the mean dose of fentanyl >1.135 mg/d was an independent predictor in predicting extubation failure. However, midazolam was not further assessed for potential risk factors for extubation failure due to the significant correlation in the correlation matrix although it has been found that unnecessary provision of sedatives is also associated with increased ventilation days [38]. Therefore, further studies are warranted to adjust the interdependence and interruption of sedatives such as midazolam before the conclusive consensus being made.
Previous studies found that increase of blood hemoglobin level rather than RBC transfusion treatment could improve extubation success [39–41]. Moreover, evidence also showed that hemoglobin breakdown products (heme, iron) could induce pro-inflammatory responses through various cellular signals even in the process of acute lung injury and ARDS [42], which suggests that decrease of hemoglobin level might indicate severe lung inflammation. In our study, we found similar baseline hemoglobin levels in the two patient groups, but more patients received RBC transfusion in the group with extubation failure; this implicates that the patients in extubation failure group had severer lung inflammation due to the more need for RBC transfusion, which further explained our finding that RBC transfusion was associated with extubation failure and served as an independent predictor.
Potential limitations in our study included: (1) sample size was relatively low, which might result in inaccuracy or underestimation of the power of the four independent risk factors and missing of other potential risk factors, especially the previously reported risk factor of PaO2/FiO2 for respiratory failure and outcomes, which was calculated to be at the cutoff of a statistical significance; (2) significant correlations were found among the factors identified in univariate logistic regression, which might also miss some factors of potential predictive values; and (3) the study was designed as a retrospective cohort, which disenabled us to (a) evaluate some other critical factors such as RSBI, static lung compliance, and maximal inspiratory pressure, (b) further perform subgroup analysis of different categories of extubation failure such as simple extubation, difficult extubation, and prolonged extubation, and (c) analyze the correlation between the trend of these clinical and treatment outcomes and extubation failure, which might be of greater clinical importance.
Conclusions
In patients with severe pneumonia, APACHE II score of greater than 17.5, blood glucose of greater than 9.87 mmol/l, fentanyl usage of greater than 1.135 mg/day, and the need for RBC transfusion at the time of weaning might potentially discriminate the patients with high risk of extubation failure. Further prospective studies with large patient populations are necessitated to validate more factors, especially the ones without potential correlations, to confirm and determine the exact risk factors, as well as to establish a comprehensive scoring system or algorism for better predicting extubation failure.
Abbreviations
- ABG
arterial blood gas
- APACHE II
acute physiology and chronic health evaluation II
- APTT
activated partial thromboplastin time
- ARDS
acute respiratory distress syndrome
- AUC
area under the curve
- BALF
bronchoalveolar lavage fluid
- BMI
body mass index
- BNP
brain natriuretic peptide
- BUN
blood urea nitrogen
- CI
confidential interval
- COPD
chronic obstructive pulmonary disease
- EF
ejection fraction
- GOCA
gas exchange, organ failure, cause, associated disease
- HCO3−
bicarbonate ion
- IMV
invasive mechanical ventilation
- LIS
lung injury score
- LR
likelihood ratio
- NPPV
noninvasive positive pressure ventilation
- NPV
negative predictive value
- OR
odds ratio
- PaCO2
partial pressure of arterial carbon dioxide
- PaO2/FiO2
ratio of partial pressure of arterial oxygen to fraction of inspired oxygen
- PEEP
positive end-expiratory pressure
- PPV
positive predictive value
- RBC
red blood cell
- RICU
respiratory and infectious intensive care unit
- ROC
receiver operating characteristic
- RR
respiratory rate
- RSBI
rapid shallow breathing index
- SaO2
arterial oxygen saturation
- SBT
spontaneous breathing trial
- SD
standard deviation
- SOFA
sequential organ failure assessment
- SpO2
oxygen saturation of pulse oximetry
- TB
total bilirubin
- VIF
variance inflation factors
- VT
tidal volume
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Sichuan Science and Technology Agency Grant [grant numbers 2017JY0271 and 2015JY0176]; and the Ministry of Science and Technology of the People’s Republic of China [grant number 2015BAI12B10].
Author Contribution
He Yu substantially contributed to the conception and design of the present study, analysis and interpretation of data, and drafting and revising for important intellectual content. Jian Luo substantially contributed to the conception and design of the present study, acquisition, analysis and interpretation of data, and critically revising for important intellectual content. Yuenan Ni acquired and analyzed data in the present study. Yuehong Hu acquired and analyzed data in the present study. Dan Liu acquired and analyzed data in the present study. Maoyun Wang interpreted data in the present study and revised important intellectual content. Binmiao Liang substantially contributed to the conception and design of the present study, revising critically for important intellectual content, and final approval of the version to be published. Zongan Liang interpreted data in the present study and revised important intellectual content.
References
- 1.Petroianni A. and Esquinas A.M. (2017) Noninvasive mechanical ventilation in pneumonia: risks of delayed intubation. Is this “Red Line” well defined and understood? Crit. Care Med. 45, e465–e466 10.1097/CCM.0000000000002230 [DOI] [PubMed] [Google Scholar]
- 2.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C. et al. (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44, S27–S72 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luna C.M., Famiglietti A., Absi R., Videla A.J., Nogueira F.J., Fuenzalida A.D. et al. (2000) Community-acquired pneumonia: etiology, epidemiology, and outcome at a teaching hospital in Argentina. Chest 118, 1344–1354 10.1378/chest.118.5.1344 [DOI] [PubMed] [Google Scholar]
- 4.Slutsky A.S. (1999) Lung injury caused by mechanical ventilation. Chest 116, 9S–15S 10.1378/chest.116.suppl_1.9S-a [DOI] [PubMed] [Google Scholar]
- 5.Nair G.B. and Niederman M.S. (2015) Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med. 41, 34–48 10.1007/s00134-014-3564-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pu L., Zhu B., Jiang L., Du B., Zhu X., Li A. et al. (2015) Weaning critically ill patients from mechanical ventilation: a prospective cohort study. J. Crit. Care 30, 862.e7–e13 10.1016/j.jcrc.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 7.Stieff K.V., Lim F. and Chen L. (2017) Factors influencing weaning older adults from mechanical ventilation: an integrative review. Crit. Care Nurs. Q. 40, 165–177 10.1097/CNQ.0000000000000154 [DOI] [PubMed] [Google Scholar]
- 8.Lu Z., Xu Q., Yuan Y., Zhang G., Guo F. and Ge H. (2016) Diaphragmatic dysfunction is characterized by increased duration of mechanical ventilation in subjects with prolonged weaning. Respir. Care 61, 1316–1322 10.4187/respcare.04746 [DOI] [PubMed] [Google Scholar]
- 9.Yu W.K., Ko H.K., Ho L.I., Wang J.H. and Kou Y.R. (2015) Synergistic impact of acute kidney injury and high level of cervical spinal cord injury on the weaning outcome of patients with acute traumatic cervical spinal cord injury. Injury 46, 1317–1323 10.1016/j.injury.2015.02.025 [DOI] [PubMed] [Google Scholar]
- 10.Epstein S.K., Ciubotaru R.L. and Wong J.B. (1997) Effect of failed extubation on the outcome of mechanical ventilation. Chest 112, 186–192 10.1378/chest.112.1.186 [DOI] [PubMed] [Google Scholar]
- 11.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T. and Wheeler A. (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 342, 1301–1308 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 12.Amato M.B., Barbas C.S., Medeiros D.M., Magaldi R.B., Schettino G.P., Lorenzi-Filho G. et al. (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 338, 347–354 10.1056/NEJM199802053380602 [DOI] [PubMed] [Google Scholar]
- 13.Ely E.W., Inouye S.K., Bernard G.R., Gordon S., Francis J., May L. et al. (2001) Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 286, 2703–2710 10.1001/jama.286.21.2703 [DOI] [PubMed] [Google Scholar]
- 14.Gélinas C., Fortier M., Viens C., Fillion L. and Puntillo K. (2004) Pain assessment and management in critically ill intubated patients: a retrospective study. Am. J. Crit. Care 13, 126–135 10.4037/ajcc2004.13.2.126 [DOI] [PubMed] [Google Scholar]
- 15.Dellinger R.P., Levy M.M., Rhodes A., Annane D., Gerlach H., Opal S.M. et al. (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 39, 165–228 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boles J.M., Bion J., Connors A., Herridge M., Marsh B., Melot C. et al. (2007) Weaning from mechanical ventilation. Eur. Respir. J. 29, 1033–1056 10.1183/09031936.00010206 [DOI] [PubMed] [Google Scholar]
- 17.MacIntyre N.R., Cook D.J., Ely E.W. Jr, Epstein S.K., Fink J.B., Heffner J.E. et al. (2001) Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 120, 375S–395S 10.1378/chest.120.6_suppl.375S [DOI] [PubMed] [Google Scholar]
- 18.Knaus W.A., Draper E.A., Wagner D.P. and Zimmerman J.E. (1985) APACHE II: a severity of disease classification system. Crit. Care Med. 13, 818–829 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 19.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H. et al. (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 22, 707–710 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 20.Murray J.F., Matthay M.A., Luce J.M. and Flick M.R. (1988) An expanded definition of the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 138, 720–723 10.1164/ajrccm/138.3.720 [DOI] [PubMed] [Google Scholar]
- 21.Artigas A., Bernard G.R., Carlet J., Dreyfuss D., Gattinoni L., Hudson L. et al. (1998) The American-European Consensus Conference on ARDS, part 2. Ventilatory, pharmacologic, supportive therapy, study design strategies and issues related to recovery and remodeling. Intensive Care Med. 24, 378–398 10.1007/s001340050585 [DOI] [PubMed] [Google Scholar]
- 22.Epstein S.K., Ciubotaru R.L. and Wong J.B. (1997) Effect of failed extubation on the outcome of mechanical ventilation. Chest 112, 186–192 10.1378/chest.112.1.186 [DOI] [PubMed] [Google Scholar]
- 23.Coplin W.M., Pierson D.J., Cooley K.D., Newell D.W. and Rubenfeld G.D. (2000) Implications of extubation delay in brain-injured patients meeting standard weaning criteria. Am. J. Respir. Crit. Care Med. 161, 1530–1536 10.1164/ajrccm.161.5.9905102 [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman J.E. and Kramer A.A. (2014) A history of outcome prediction in the ICU. Curr. Opin. Crit. Care 20, 550–556 10.1097/MCC.0000000000000138 [DOI] [PubMed] [Google Scholar]
- 25.Schönhofer B., Guo J.J., Suchi S., Köhler D. and Lefering R. (2004) The use of APACHE II prognostic system in difficult-to-wean patients after long-term mechanical ventilation. Eur. J. Anaesthesiol. 21, 558–565 10.1097/00003643-200407000-00010 [DOI] [PubMed] [Google Scholar]
- 26.Shin H.J., Chang J.S., Ahn S., Kim T.O., Park C.K., Lim J.H. et al. (2017) Clinical factors associated with weaning failure in patients requiring prolonged mechanical ventilation. J. Thorac. Dis. 9, 143–150 10.21037/jtd.2017.01.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfe R.R., Herndon D.N., Jahoor F., Miyoshi H. and Wolfe M. (1987) Effect of severe burn injury on substrate cycling by glucose and fatty acids. N. Engl. J. Med. 317, 403–408 10.1056/NEJM198708133170702 [DOI] [PubMed] [Google Scholar]
- 28.Mizock B.A. (1995) Alterations in carbohydrate metabolism during stress: a review of the literature. Am. J. Med. 98, 75–84 10.1016/S0002-9343(99)80083-7 [DOI] [PubMed] [Google Scholar]
- 29.Van den Berghe G., Wilmer A., Hermans G., Meersseman W., Wouters P.J., Milants I. et al. (2006) Intensive insulin therapy in the medical ICU. N. Engl. J. Med. 354, 449–461 10.1056/NEJMoa052521 [DOI] [PubMed] [Google Scholar]
- 30.van den Berghe G., Wouters P., Weekers F., Verwaest C., Bruyninckx F., Schetz M. et al. (2001) Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 345, 1359–1367 10.1056/NEJMoa011300 [DOI] [PubMed] [Google Scholar]
- 31.Van den Berghe G., Wouters P.J., Bouillon R., Weekers F., Verwaest C., Schetz M. et al. (2003) Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit. Care Med. 31, 359–366 10.1097/01.CCM.0000045568.12881.10 [DOI] [PubMed] [Google Scholar]
- 32.Hermans G., Schrooten M., Van Damme P., Berends N., Bouckaert B., De Vooght W. et al. (2009) Benefits of intensive insulin therapy on neuromuscular complications in routine daily critical care practice: a retrospective study. Crit. Care 13, R5 10.1186/cc7694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikaeili H., Yazdchi M., Barazandeh F. and Ansarin K. (2012) Euglycemic state reduces the incidence of critical illness polyneuropathy and duration of ventilator dependency in medical intensive care unit. Bratisl. Lek. Listy 113, 616–619 [DOI] [PubMed] [Google Scholar]
- 34.Hansen T.K., Thiel S., Wouters P.J., Christiansen J.S. and Van den Berghe G. (2003) Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J. Clin. Endocrinol. Metab. 88, 1082–1088 10.1210/jc.2002-021478 [DOI] [PubMed] [Google Scholar]
- 35.Vanhorebeek I., De Vos R., Mesotten D., Wouters P.J., De Wolf-Peeters C. and Van den Berghe G. (2005) Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet 365, 53–59 10.1016/S0140-6736(04)17665-4 [DOI] [PubMed] [Google Scholar]
- 36.Payen J.F., Bosson J.L., Chanques G., Mantz J. and Labarere J. (2009) Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post Hoc analysis of the DOLOREA study. Anesthesiology 111, 1308–1316 10.1097/ALN.0b013e3181c0d4f0 [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y., Wang Y., Du B. and Xi X. (2017) Could remifentanil reduce duration of mechanical ventilation in comparison with other opioids for mechanically ventilated patients? A systematic review and meta-analysis Crit. Care 21, 206 10.1186/s13054-017-1789-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reade M.C., Eastwood G.M., Bellomo R., Bailey M., Bersten A., Cheung B. et al. (2016) Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA 315, 1460–1468 10.1001/jama.2016.2707 [DOI] [PubMed] [Google Scholar]
- 39.Schönhofer B., Böhrer H. and Köhler D. (1998) Blood transfusion facilitating difficult weaning from the ventilator. Anaesthesia 53, 181–184 10.1046/j.1365-2044.1998.00275.x [DOI] [PubMed] [Google Scholar]
- 40.Lai Y.C., Ruan S.Y., Huang C.T., Kuo P.H. and Yu C.J. (2013) Hemoglobin levels and weaning outcome of mechanical ventilation in difficult-to-wean patients: a retrospective cohort study. PLoS ONE 8, e73743 10.1371/journal.pone.0073743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hébert P.C., Blajchman M.A., Cook D.J., Yetisir E., Wells G., Marshall J. et al. (2001) Do blood transfusions improve outcomes related to mechanical ventilation? Chest 119, 1850–1857 10.1378/chest.119.6.1850 [DOI] [PubMed] [Google Scholar]
- 42.Gaggar A. and Patel R.P. (2016) There is blood in the water: hemolysis, hemoglobin, and heme in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 311, L714–L718 10.1152/ajplung.00312.2016 [DOI] [PubMed] [Google Scholar]