Abstract
Background
Pharmacokinetics (PK) are severely altered in critically ill patients due to changes in volume of distribution (Vd) and/or drug clearance (Cl). This affects the target attainment of antibiotics in critically ill children. We aimed to identify gaps in current knowledge and to compare published PK parameters and target attainment of antibiotics in critically ill children to healthy children and critically ill adults.
Methods
Systematic literature search in PubMed, EMBASE and Web of Science. Articles were labelled as relevant when they included information on PK of antibiotics in critically ill, non-neonatal, pediatric patients. Extracted PK-parameters included Vd, Cl, (trough) concentrations, AUC, probability of target attainment, and elimination half-life.
Results
50 relevant articles were identified. Studies focusing on vancomycin were most prevalent (17/50). Other studies included data on penicillins, cephalosporins, carbapenems and aminoglycosides, but data on ceftriaxone, ceftazidime, penicillin and metronidazole could not be found. Critically ill children generally show a higher Cl and larger Vd than healthy children and critically ill adults. Reduced target-attainment was described in critically ill children for multiple antibiotics, including amoxicillin, piperacillin, cefotaxime, vancomycin, gentamicin, teicoplanin, amikacin and daptomycin. 38/50 articles included information on both Vd and Cl, but a dosing advice was given in only 22 articles.
Conclusion
The majority of studies focus on agents where TDM is applied, while other antibiotics lack data altogether. The larger Vd and higher Cl in critically ill children might warrant a higher dose or extended infusions of antibiotics in this patient population to increase target-attainment. Studies frequently fail to provide a dosing advice for this patient population, even if the necessary information is available. Our study shows gaps in current knowledge and encourages future researchers to provide dosing advice for special populations whenever possible.
Electronic supplementary material
The online version of this article (10.1007/s40262-019-00813-w) contains supplementary material, which is available to authorized users.
Key Points
| This review provides a complete and comprehensive overview of all studies regarding pharmacokinetics and target attainment of all antibiotic agents in critically ill children. |
| Current knowledge gaps include several frequently used antibiotics, such as ceftriaxone, ceftazidime, penicillin, flucloxacillin and metronidazole that lack data altogether in this patient population. |
| This literature overview hopes to inspire researchers to close these gaps, not only by publishing pharmacokinetic data, but also by providing dosing guidance for implementation in the clinic, as this information is vital to optimize antibiotic treatment in this vulnerable population. |
Introduction
Antibiotics make up the most common class of drugs prescribed to hospitalized children, with roughly 50% of patients receiving an antibiotic agent during their hospital stay [1]. In critically ill children, the prophylactic and therapeutic use of intravenous antibiotics is even more prevalent [1].
Currently, critically ill children are generally started on the same (weight-corrected) dose of antibiotic therapy as their non-critically ill counterparts. As a consequence of altered pharmacokinetics (PK) due to critical illness there is a high likelihood that target concentrations, associated with optimal efficacy while minimizing toxicity, are not attained. In intensive care unit (ICU) patients, antibiotic concentrations are outside of the therapeutic window in up to 41% of adult patients [2] and even 95% in a critically ill pediatric ICU (PICU) population [3]. This non-target attainment in critically ill patients is caused by pathophysiological changes in volume of distribution (Vd), protein binding and/or drug clearance (Cl) [4–6]. Contrary to renal dysfunction, an increased renal clearance caused by hemodynamic alterations during critical illness, described as augmented renal clearance (ARC), is reported in up to 65% of critically ill adults [7, 8] and children [9, 10].
In addition to these pathophysiological alterations, young children also show developmental changes in almost all processes involved in drug disposition. Apart from differences in body composition, children also show maturation of drug metabolizing enzymes and glomerular filtration rate (GFR) in the first years of life [11]. Currently, most pediatric dosing guidelines for children older than 1 month (e.g. British National Formulary for Children and Dutch Pediatric Formulary) only present a single body-weight based dose, not accounting for these developmental changes.
Whether these changes in drug disposition lead to non-target attainment of antibiotics in patients obviously also depends on the pharmacodynamic (PD) interaction between antibiotic and micro-organism. The two main parameters in this interaction are the susceptibility of the micro-organism, defined as the minimum inhibitory concentration (MIC), and the kill-characteristic of the antibiotic [4]. Different classes of antibiotics have different kill-characteristics and can be divided in time-dependent, concentration dependent and exposure dependent antibiotics [4]. When the kill-characteristic of an antibiotic are known, PD targets can be established for these antibiotics. Common PD targets for time-dependent, concentration dependent and exposure dependent antibiotics are the time free drug concentrations are above the MIC at the site of infection (fT > MIC), peak concentration over MIC (Cmax/MIC) and area under the curve (AUC) over MIC (AUC/MIC), respectively. Subsequently, the probability of reaching these targets in special populations, such as critically ill children, can be simulated using PK data [12].
Knowledge of altered PK parameters and desired PD targets is essential to serve as the basis for the development of individualized starting dosing guidelines and further individualized dose adjustments using therapeutic drug monitoring (TDM). Multiple studies have shown that the application of TDM improves target attainment of antibiotic agents in critically ill patients [3, 13–16].
The aim of this systematic review is to summarize the reported PK data and target attainment of antibiotics in critically ill children, in relation data from non-critically ill children and/or critically ill adult patients. This may aid to identify gaps in current knowledge for future research, to optimize dosing guidelines and support TDM practice.
Methods
Search Strategy
We performed a systematic search in concordance with PRISMA guidelines in MEDLINE (using PubMed), EMBASE and Web of Science databases from 1900 to April 2017. The PRISMA checklist is included as Supplemental Data File 1. Researchers were alerted to additional results for the search after April 2017 until May 15th 2019 by automatic e-mail alerts, and articles after this period were screened and selected in the same manner as articles in the original search. Duplicate articles within each database and between databases were excluded by using EndNote and manual selection. The main research question was broken down into four domains (Pharmacokinetics, Antibiotics, Critically ill and Children). Keywords were allocated to these domains and as many synonyms for each keyword as possible were included in the search. Whenever possible, keywords were converted to corresponding MeSH-terms and/or subject headings. In the final search, both MeSH-terms, Subject Headings and keywords in the title and abstract were included. In order to include all antibiotic agents for the ‘Antibiotic’ domain in our search, we used the term “Anti-Bacterial Agents” [Pharmacological Action] from the MeSH Database in combination with a free text search built with all the drug names and substance names linked to this MeSH-term [17]. Antiviral and antifungal therapies were left out of the scope of this systematic review. An overview of the final search strategy is depicted in Table 1 and a full list of antibiotic agents in Supplemental Data File 2.
Table 1.
Overview of final search strategy in PubMed with MeSH (Medical Subject Headings) terms and free text keywords for each of the four domains (Pharmacokinetics, Antibiotics, Critically ill, and Children)
| Pharmacokinetics | Antibiotics | Critically ill | Children |
|---|---|---|---|
|
MeSH terms Pharmacokinetics [Mesh] Pharmacokinetics [Subheading] Monte Carlo Method [Mesh] Drug Monitoring [Mesh] Drug Dosage Calculations [Mesh] |
MeSH-terms Anti-Bacterial Agents [Pharmacological Action] Anti-Bacterial Agents [Mesh] |
MeSH terms Intensive Care Units [Mesh] Critical Illness [Mesh] Critical Care [Mesh] |
MeSH terms Adolescent [Mesh] Child [Mesh] Infant [Mesh] |
|
Title/abstract Peak concentration*, Trough concentration*, Area Under Curve, Therapeutic Equivalency, Tissue Distribution, Pharmacokinetic*, PopPK, Target-attainment, Drug monitoring, TDM, Pharmacodynamic*, Dose calculation*, Drug dos* |
Title/abstract See Electronic Supplementary Material |
Title/abstract Severe ill, severe illn*, severely ill, PICU, PICUs, ICU, ICUs, Critical Care, Intensive Care Unit*, serious illn*, serious ill, seriously ill, critical illn*, critical ill, critically ill* |
Title/abstract Child*, schoolchild*, infan*, adolescen*, pediatri*, paediatr*, boy, boys, boyhood, girl, girls, girlhood, youth, youths, baby, babies, toddler*, teen, teens, teenager*, postnat*, puberty, preschool*, suckling*, picu |
Terms within each domain were combined with OR, all domains were combined with AND, as shown in the Electronic Supplementary Material
Study Selection
The title and abstract of every result in the search were screened for eligibility by SH, ND and LO. A study was labelled as eligible when it contained information on all four domains (Pharmacokinetics, Antibiotics, Critically ill and Children). Studies were labelled as ineligible when information on one or more domains was missing, when the study population consisted of only adults or neonates (< 1 month old), and/or when all patients were treated with renal replacement therapy or extracorporeal membrane oxygenation because of the direct influence on Vd and Cl depending on the extracorporeal circuit. Critical illness was based solely on the mention of ICU admission, regardless of disease severity scores, organ dysfunction or diagnoses of patients. This was done in order to best reflect the heterogenous PICU population and identify the impact of (critical) illness on their PK parameters. When the study population consisted of a mix of critically ill children and non-critically ill children, neonates or adults studies were only included when PK data of the critically ill children was reported separately. Other exclusion criteria were non-English articles, conference abstracts, letters to the editor, no full-text availability, animal studies, in vitro studies and/or oral dosing. Review articles were also excluded but the references in these reviews were screened for additional relevant articles not identified by our search.
Overlapping articles were included and non-overlapping articles between the three reviewers were screened again by SH and SdW and included if labelled as eligible by both researchers.
Data Extraction
For each eligible article data was systematically extracted and all the extracted data was entered in a database using Microsoft Excel. The extracted data included type of antibiotic studied, study design, dose, sample size, type of population, disease severity based on validated clinical scores (PELOD, PIM, PRISM-scores or STAT categories), renal dysfunction and age of subjects. In addition, the PK-analysis used in the article was studied, whether a (Pop)PK model was used, how many compartments were included in the model, studied co-variates on PK, what PK-parameters were found, and any additional findings of interest were noted. Lastly, when a dosing advice for critically ill children was provided, this was noted.
The PK-parameters of interest that were collected were Vd, Cl, trough (Cmin) and peak (Cmax) concentrations, AUC, half-life (t½) and elimination rate constant (k). All values of PK-parameters were normalized in order to ease comparability between different studies. Vd was normalized to liters/kilogram (l/kg) and Cl values to l/kg/h (l/kg/h). In case of allometric scaling or covariate contribution to one of these parameters, which is often seen in pharmacometric models, the covariate values of a mean/median study patient were used to normalize PK-parameter values.
PD parameters that were collected included the probability of target attainment (PTA), MIC and PD targets for time-dependent, concentration dependent and exposure dependent antibiotics: fT > MIC, AUC/MIC and Cmax/MIC, respectively.
Results
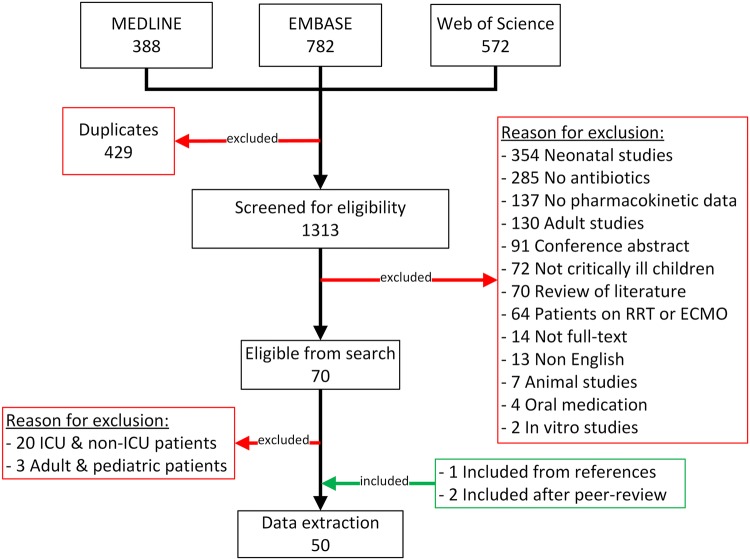
The literature search in PubMed, EMBASE and Web of Science yielded 1742 articles. After the exclusion of duplicate articles within each database and between the 3 databases a total of 1313 articles were screened. From the screening process a total of 70 articles were labelled as eligible (Fig. 1).
Fig. 1.
A total of 1742 articles were screened from the 3 databases. After excluding duplicates and non-eligible articles and including articles from references a total of 48 eligible articles were analyzed for data extraction. RRT renal replacement therapy, ECMO extracorporeal membrane oxygenation, ICU intensive care unit
Twenty-three of the included articles did contain information on the PK of antibiotic agents in a population of critically ill children but results were mixed with either adult data or non-critically ill data and therefore excluded. One article [18] was included from the references of included articles and/or reviews regarding the subject. Additionally, two articles that did not come up during the systematic review and were not mention in references of reviews were added after the peer-review process [19, 20]. Data-extraction was performed for 50 full-text articles with data on the PK of antibiotics in critically ill children. A complete list of all 50 articles and extracted PK-data is presented in Table 2.
Table 2.
Overview of all included articles in our systematic review that met our inclusion criteria
| Author (year) [citation] | Antibiotic | Dose | Study design | N | Type of illness | Age and weight mean (range) | AKI included? | Covariates | PK parameters | PTA | Dose advice |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bressolle (1996) [64] | Amikacin |
1 dd infusion in 30 min. Range of dose from 70 mg to 1500 mg. Dose in mg/kg/day is unknown 2 children received infusion 2 dd and 2 children received infusion 3 dd |
Cohort | 36 | Mixed |
5.66 y (6 mo–15 y) 20.4 kg ± 13.6 (range not given) |
Yes | Weight, height |
Measured concentrations in whole population: Cmax 40.7 mg/l ± 15.8 (n = 44), Cmin 0.97 mg/l ± 0.66 (n = 39) Measured concentrations in validation group: Cmax 16.0 mg/l ± 7.19 (n = 12), Cmin 1.40 ± 1.39 (n = 10) Estimated pediatric parameters after Bayesian estimation: Cmax 16.2 µg/ml ± 5.84, Cmin 1.45 µg/ml ± 1.43 |
Not given | |
| Marik (1991) [63] | Amikacin |
Children < 1 y: 20 mg/kg/day Children > 1 y: 15 mg/kg/day Randomization between once-daily dosing (+ loading dose of 20–25 mg/kg) and twice-daily dosing. Infusion in 3–5 min |
RCT | 60 | Mixed |
0.54 y (6 mo–1 y) Weight not given |
No | Age |
Group 2 (6 mo–1 y): Vd 0.50 l/kg (range 0.22–0.73), 0.18 l/kg central compartment, 0.32 l/kg peripheral compartment, Cl 0.068 l/kg/h (range 0.018–0.129), distribution t½ 0.31 h (range 0.03–0.58), elimination t½ 2.86 h (range 0.63–6.28) Group 3 (1 week–6 mo): Vd 0.58 l/kg (range 0.32–0.98), 0.21 l/kg central compartment, 0.37 l/kg peripheral compartment, Cl 0.063 l/kg/h (range 0.036–0.108), distribution t½ 0.44 h (range 0.12–0.99), elimination t½ 5.02 h (range 1.46–11.89) |
Targets: Cmax 30–40 mg/l for 1 dd and 20–30 mg/l in 2 dd with Cmin of < 5 mg/l for both groups Target attainment for Cmax > 20 mg/l: 44% in 2 dd dosing group, 100% for 1 dd dosing group. Target attainment for Cmin < 5 mg/l: 79% in 2 dd dosing group, 100% for 1 dd dosing group |
Not given |
| Sherwin (2014) [65] | Amikacin | 10–20 mg/kg/day in 2–4 doses (range 4.9–22.3 mg/kg/day). Mean dose 16.4 ± 3.9 mg/kg | Cohort | 70 | Burn |
4.5 y (6 mo–17 y) 20 kg (8–90 kg) |
Yes | Age, sex, weight, height, percent total body area burn, serum creatinine |
Amikacin Cl 5.98 l/h/70 kg (95% CI 4.97–6.99) Amikacin Vd 2 compartments (central 16.7 and peripheral 40.1 l/70 kg (95% CI central 14.0–19.4 and peripheral 15.8–80.4) |
Not given | |
| De Cock (2015) [21] | Amoxicillin + clavulanic acid | 100 mg of amoxicillin/kg/day in 4 dd, 5:1 ratio of amoxicillin and clavulanic acid | Cohort | 50 | Mixed |
2.58 y (1 mo–15 y) 14.4 kg (4.07–65 kg) |
Yes | Weight, post-menstrual age, CysC, vasopressor treatment |
Amoxicillin Vd 3 compartments (9.07, 5.43, and 11.24 l/70 kg) Amoxicillin Cl 17.97 l/h/70 kg Clavulanic acid Vd 2 compartments 11.6 and 9.85 l/70 kg Clavulanic acid Cl 12.2 l/h/70 kg |
PTA for 3 dosing schemes (25 mg/kg every 12 h for 1–3 mo, every 8 h for > 3 mo, 25 mg/kg 4 dd and 25 mg/kg 6 dd) with target fT > MIC > 40% with MIC 8 Clavulanic acid target attainment for different dosing schemes: 48%, 66% and 96% for bolus and 53%, 73% and 99% for 1-h infusion |
150 mg/kg/day of amoxicillin in 6 doses every 4 h. Given as a bolus in children with CysC > 1 mg/l and as a 1-h infusion to children with CysC < 1 mg/l |
| Jones (1990) [22] | Amoxicillin + clavulanic acid | 50 mg/kg amoxicillin + 5 mg/kg clavulanic acid, infusion in 30 min | Cohort | 15 | Mixed |
6.9 y(2y – 14y) Weight not given |
Unknown | None |
Amoxicillin AUC0-last: 130.09 µg/h/ml ± 18.96 Amoxicillin K: 0.807/h ± 0.125 Amoxicillin t½: 0.878 h ± 0.135 Amoxicillin Cl: 283.2 ml/min per 1.7 m2 ± 43.65 Amoxicillin Vd: 469 ml/kg ± 79 Clavulanic acid AUC0-last: 13.82 µg/h/ml ± 4.39 Clavulanic acid K: 0.936/h ± 0.248 Clavulanic acid t½: 0.786 h ± 0.201 Clavulanic acid Cl: 298.16 ml/min per 1.7 m2 ± 138.05 Clavulanic acid Vd: 434 ml/kg ± 179 |
Not given | |
| Cies (2017) [39] | Aztreonam | 8 g/day in 4 doses, infusion in 4 h | Case report | 1 | Pneumonia |
16 y Weight not given |
No | None |
Cmax aztreonam 71 mg/l Cl: 2.3 ml/kg/min Vd not reported Target: fT > MIC 40% for MIC 4 mg/l, PTA of 90% |
Using 2 g every 6 h as prolonged infusion estimated PTA is 100% | 2 every 6 h, as prolonged infusion in 4 h |
| Cies (2019) [20] | Cefazolin |
25 mg/kg/dose, with a maximum of 2000 mg/dose One extra 25 mg/kg (maximum 1000 mg) dose added to CPB circuit during surgery |
Cohort | 41 | Cardiac surgery | Overall median age and weight not given, only in subgroups | No | Weight, age, eGFR |
Mean (SD) population estimation in different age cohorts: Birth–6 mo: Cl 0.00054 l/kg/h (0.00036), Vd 0.598 l/kg (0.26) 7 mo–3 y: Cl 0.0006 l/kg/h (0.0003), Vd 0.786 l/kg (0.15) 4–16 y: 0.00042 l/kg/h (0.00024), Vd 3.4 l/kg (0.94) |
Percentage cefazolin loss in CPB system ranges from 78.2% in the 4–11 y cohort to 95.9% in the youngest cohort from birth to 3 mo | Not given |
| De Cock (2017) [19] | Cefazolin |
25 mg/kg/dose, with a maximum of 2000 mg/dose 4 doses in total before, during and after surgery |
Cohort | 56 | Cardiac surgery |
2.8 y (6 d–15 y) 6.8 kg (2.7–70 kg) |
Yes | Weight, sex, age, eGFR, temperature, comedication, total protein, albumin |
Model parameters for median study patient (6.8 kg, eGFR 91.6 ml/min/1.73 m2 and albumin 39 g/l): Cl = 0.229 l/kg/h Central Vd = 0.284 l/kg Peripheral Vd = 0.351 l/kg Q central-peripheral = 0.8 l/kg/h Model parameters for adult patient (70 kg, eGFR 120 ml/min/1.73 m2 and albumin 39 g/l): Cl = 0.167 LL/kg/h Central Vd = 0.284 l/kg Peripheral Vd = 0.351 l/kg Q central-peripheral = 0.447 l/kg/h |
PTA as 100% fT > MIC during surgery and 50%fT > MIC after surgery.16 PTA was calculated for MICs between 0.125 and 16 mg/l PTA with standard doses 40–54%. A simulated dose regimen of 40 mg/kg, 30 min before incision; 20 mg/kg, at start of CPB; 20 mg/kg, at the start of rewarming on CPB and 2 doses of 40 mg/kg every 8 h after CPB showed the most optimal PTA (88–99%) |
40 mg/kg, 30 min before incision; 20 mg/kg, at start of CPB and at start of rewarming on CPB and 2 doses of 40 mg/kg every 8 h after CPB |
| Beranger (2018) [28] | Cefotaxime | 100–300 mg/kg/day in 4 doses, in patients > 50 kg the adult dose of 3 dd 1000 mg was used | Cohort | 64 | Mixed |
2 y (0–19 y) 10.9–17.7 kg (2.5–68 kg) |
Yes | Weight, age, creatinine, number of failing organs, PELOD-1 and -2 scores |
Targets: fT > MIC 100% and fT > 4×MIC 100% with MIC 0.5 mg/l PTA of different dosing schemes simulated, target PTA > 90% Cl 14.7 l/h (of which 13.7 l/h renal and 1 l/h via desacetyl-cefotaxime). Vd cefotaxime 21.4 l. t½ 0.34–1.15 h (only range was mentioned in text) |
Time above MIC 0.5 = median 66.6% (range 40–100%) and for MIC 2 median 46.7% (range 28.3–85%) | 100 mg/kg/day as continuous infusion |
| Hartman (2019) [29] | Cefotaxime | 100–150 mg/kg/day in 3–4 doses | Cohort | 37 | Meningococcal septic shock |
2 y (0.3–16 y) 13.7 kg (6–70 kg) |
Yes | Age, creatinine, PRISM score, SOFA score |
Median concentration 8.0 mg/l (IQR 2.5–18.7 mg/l, range 0–81.1 mg/l) Cefotaxime concentration weakly correlated with creatinine levels |
Percentage of target attainment depended heavily on MIC, ranging from 14.7% for MIC 16 mg/l to 95.6% for MIC of 0.125 mg/l | Not given |
| Von Hatting-berg (1980) [27] | Cefotaxime | 100 mg/kg/day in 2–3 doses | Cohort | 2 | Mixed |
2.2 y (8 mo–3.7 y) 9.1 kg (7.1–11.1 kg) |
Yes | None |
Cl for 8-month-old child without kidney dysfunction and 3.7-year-old child with kidney dysfunction were 0.479 l/kg/h and 0.109 l/kg/h, respectively Vd 0.31 l/kg and 0.16 l/kg and t½ 0.46 h and 1.02 h, respectively |
For patients without kidney dysfunction and MIC of 2 and 5 mg/l, doses of 10 g/kg/dose and 30 g/kg/dose needed for target attainment | Not given |
| Cies (2018) [32] | Ceftaroline | 60 mg/kg/day (1 patient with 54 mg/kg/day) in 4 doses | Cohort | 7 | MRSA |
7 y (1 mo–13 y) 25.5 kg (12.6–40.1 kg) |
No | None |
PK parameters in study population: Median Vd: 0.41 l/kg Median Cl: 0.218 l/kg/h Median t½: 1.3 h Median k: 0.5/h PK parameters in package insert for pediatric patients: Vd: 0.28 l/kg Cl: 0.138 l/kg/h t½: 2.7 h |
Target: fT > MIC 40% and fT > 4–6 × MIC 40% Estimated fraction unbound 0.8 All patients needed a dose alteration or non-standard dose to reach the target of fT > 4–6 × MIC 40% |
For bloodstream infections, pneumonia, and meningitis with MRSA, dosing every 6 h is advised For patients with increased Vd, a dose of 15 mg/kg/dose is advised |
| Olguin (2008) [30] | Cefuroxime | 400 mg/kg/day in 4 doses, infusion during 30 min | Cohort | 11 | Mixed |
1.42 y (4 mo–14 y) 8.2 kg (5.1–45 kg) |
Yes | Severity of illness |
3 groups: control group of non-critically ill, severely ill group of non-intubated PICU patients and a very severely group of intubated PICU patients Vd (range): control 1.5 l/kg (0.9–1.8), severely ill 1.6 l/kg (1.0–4.0), and very severely ill 3.1 l/kg (0.9–9.5) Cl (range): control 0.55 l/kg/h (0.10–0.96), severely ill 0.48 (0.26–1.96), and very severely ill 1.87 (0.25–0.77) AUC (range): control 116.4 µg/ml/h (84.9–161.7), severely ill 121.6 (59.6–202.1), and very severely ill 190.7 (79.7–729.7) |
Not given | |
| Lipman (2002) [70] | Ciprofloxacin | 20 mg/kg/day in 2 doses for 7–14 d | Cohort | 20 | Pneumonia, bronchiolitis, sepsis |
13.5 mo (3 mo–4.75 y) 8.95 kg (4.2–21.1 kg) |
No | Age |
3 mo–1 y: Cmax 6.08 mg/l ± 1.23, Cmin 0.21 mg/l ± 2.39, t1/2 3.67 h ± 1.15, Vd 2.06 l/kg ± 1.33, Cl 0.56 l/kg/h ± 1.40, AUC0-tau 15.6 mg/l/h ± 1.30 > 1 y: Cmax 7.38 mg/l ± 1.35, Cmin 0.14 mg/l ± 2.03, t1/2 2.84 h ± 1.18, Vd 1.44 l/kg ± 1.17, Cl 0.53 l/kg/h ± 1.22, AUC0-tau 15.9 mg/l/h ± 1.28 |
A dose of 20 mg/kg/day will cover bacteria with an MIC of 0.8 mg/l (with target Cmax/MIC > 8) | 20–30 mg/kg/day in 2–3 doses |
| Akins (2006) [67] | Daptomycin | 1 day of 6 mg/kg/day, afterwards 8 mg/kg/day in 1 dose | Case report | 1 | Endocarditis |
13 y 49.3 kg |
No | Renal function, albumin, blood pressure, respiration rate, temperature, sex |
Elimination rate constant 0.301/h after single dose, 0.151 in steady state Cmax: 83.0 µg/ml after single dose, Cmin: 0.07 µg/ml after single dose, 2.7 in steady state t½: 2.31 h after single dose, 4.58 in steady state Vd: 0.067 l/kg after single dose, 0.089 in steady state Cl: 20.13 ml/kg/h after single dose, 13.47 in steady state AUC: 298 mg/h/l after single dose, 593.92 in steady state |
Not given | |
| Antacho-poulos (2018) [69] | Daptomycin | 10 mg/kg/day in 1 dd, infusion in 30 min | Cohort | 4 | Mixed |
9.75 y (8–14 y) 42.5 kg (26–45 kg) |
No | None |
Day 1: Median Cl: 0.0352 l/kg/h Median Vd: 0.245 l/kg Median AUC0–∞: 296.15 mg/l/h Median Cmax: 34.65 mg/l Day 5: Median Cl: 0.0216 l/kg/h Median Vd: 0.31 l/kg Median AUC0–24h: 505.75 mg/l/h Median Cmax: 41.4 mg/l |
Higher Cl and lower AUC in patients with sepsis (n = 2) than without sepsis on day 1 and day 5 Patients with sepsis show double the Cl than non-critically ill children PTA: despite higher doses used (10 mg/kg instead of 4 mg/kg), similar to lower values for AUC/MIC and Cmax/MIC in patients with sepsis due to higher Cl |
Not given |
| Morris (2017) [68] | Daptomycin | 8 mg/kg every 48 h, infusion in 60 min. Longer dosing interval because of renal failure (Clcr < 30 ml/min and peritoneal dialysis 10 ml/kg/cycle | Case report | 1 | Cardiac |
8 y 17 kg |
Yes | None | Steady-state Cmax of daptomycin 68 mg/l, Cmin 14.6 mg/l. Target Cmin was < 20 mg/l | Not given | |
| Kraus (1993) [62] | Gentamicin | 1.8–3.1 mg/kg/dose, 2–3 dd. Infusion in 30 min | Cohort | 44 | Mixed |
2.2 y (0.8 mo–14 y) 10.2 kg ± 11.6 (range not given) |
No | Age, post-operative status |
Gentamicin Vd 0.424 l/kg ± 0.116 Gentamicin Cl 0.123 l/kg/h ± 0.041 K 0.316/h ± 0.188 t½ 2.6 h ± 1.0 Predicted dosing intervals of 4 h (for 1 patient), 6 h (for 20 patients), 8 h (for 16 patients), and 12 h (for 7 patients) |
Dose simulation predicts an average dose of 2.74 mg/kg/dose ± 0.44 to reach Cmax of 7 mg/l and Cmin of 1 mg/l Average daily dose for these targets 9.1 mg/kg/day ± 2.4 (range 5.2–14.8 mg/kg/day) 30/44 (68%) of patients needed a higher dose than the standard dose of 7.5 mg/kg/day |
9 mg/kg/day |
| Lopez (2010) [61] | Gentamicin | 8 mg/kg every 24–36 h infused in 30 min | Cohort | 36 | Mixed |
5.3 mo (1 d–15 y) 7.9 kg (IQR 4.6–13.8) |
Yes | Weight, age, Clcr |
Gentamicin Cl 2.09 l/h/70 kg and 0.14 l/h/kg Distributional Cl 0.18 l/h Vd central compartment 0.35 l/kg Vd peripheral compartment 3.78 l |
PTA for Cmax/MIC 8 for MIC 2 mg/l for different age groups and doses: PTA 90% for all non-neonates with 7 mg/kg and PTA 100% for all age groups with 8 mg/kg | 8 mg/kg/dose for all age groups. Dosing interval dependent on age |
| Zakova (2014) [60] | Gentamicin | Unknown | Cohort | 140 | Mixed |
0.3 mo (0 d–21 mo) 4.53 kg (range not given) |
Yes | Age, sex, weight, co-medication, PRISM score, creatinine, albumin, |
Median Vd 0.39 l/kg (IQR 0.30–0.50), Median elimination rate constant (k) 0.18/h (IQR 0.11–0.24) |
28.4% of patients within Cmax target of 16–20 mg/l (38.8% below, 32.8% above) 22.4% of patient within AUC target of 70–100 mg/h/l (37.3% below, 40.3% above) 53.7% of patients within drug-free interval target of 4–16 h (6% below, 40.3% above) |
6 mg/kg/day in 1 dose for patients > 5 kg and with creatinine < 20% above the age specific upper limit of normal |
| Haessler (2003) [31] | Gentamicin, cefazoline |
Cefazoline: loading dose of 40 mg/kg at induction of anesthesia and 105 mg/kg/day in 3 doses for 48 h Gentamicin loading dose of 5 mg/kg at induction of anesthesia and 4 mg/kg/day for 48 h |
Cohort | 19 | Cardiac surgery |
9.8 mo (1 d–2.6 y) 6.43 kg (3.8–10.5 kg) |
No | None |
Cefazolin: Plasma concentration at end of surgery 54 µg/ml ± 16, first Cmin 12 µg/ml ± 7, steady-state Cmin 15 µg/ml ± 10, steady-state Cmax 19 µg/ml ± 22 Vd: before bypass patients 0.191 l/kg ± 0.028, during bypass 0.357 l/kg ± 0.096, after bypass 0.127 l/kg ± 0.004 K = before bypass 0.738 ± 0.422, during bypass 0.331 ± 0.226, after bypass 1.429 ± 0.470 Gentamicin: Cmax during surgery 20.8 µg/ml ± 9.5, mean concentrations at the end of surgery 5.9 µg/ml ± 1.5. First Cmin 1.1 µg/ml ± 0.5, steady-state Cmin 0.8 µg/ml ± 0.9, steady-state Cmax 3.2 µg/ml ± 1.2 Vd before bypass 0.237 l/kg ± 0.084, during bypass 0.400 l/kg ± 0.038, after bypass 0.624 l/kg ± 0.036 K = before bypass 0.962 ± 0.402, during bypass 0.336 ± 0.054, after bypass 0.188 ± 0.019 |
Not given | |
| Giannoni (2006) [38] | Imipenem | 100 mg/kg/day in 3–4 doses, infusion in 30 min | Cohort | 19 | Mixed |
0.8 y (9 d–12 y) Weight not given |
Yes | Age, weight, BSA, creatinine, Clcr, urea, albumin, lactate, bicarbonate, PRISM score, MAP, heart rate, central venous pressure |
Terminal t½ after first dose: 1.22 h ± 0.47 Terminal t½ in steady state: 1.35 h ± 0.38 Cl after first dose: 0.27 l/kg/h ± 0.11 Cl in steady state: 0.34 l/kg/h ± 0.14 Vd after first dose: Vd 0.42 l/kg ± 0.13 and Vss 0.30 ± 0.1 Vd in steady state: V 0.64 l/kg ± 0.3 and Vss 0.46 ± 0.25 |
fT > MIC: all children reached fT > MIC of 70–100% for all isolated pathogens | 100 mg/kg/day |
| Cies (2015) [34] | Meropenem | 160 mg/kg/day in 4 doses, infusion in 30 min, later switch to continuous infusion of 200 mg/kg/day | Case report | 1 | Ventriculitis |
2 y Weight not given |
No | None |
Random meropenem plasma concentrations and CSF during intermittent and continuous dosing Calculated Cl 0.612 l/kg/h, higher than healthy volunteers and PICU patients Intermittent dosing: plasma concentration 12 µg/ml after 2 h and immeasurable after 4 h. In CSF, 1 µg/ml after 2 h and 0.5 µg/ml after 4 h Continuous dosing: plasma concentration 13 µg/ml and CSF 0.5 µg/ml |
Continuous infusion gave PTA of 100% in serum and CSF | Not given |
| Cies (2017) [35] | Meropenem |
Range of different doses from 40 mg/kg/day to 160 mg/kg/day over 2–4 doses, infusion in 30 min 1 patient received continuous dosing of 200 mg/kg/day 1 patient received 100 mg/kg/day in 2 doses with prolonged infusion of 4 h |
Cohort | 9 | Mixed |
2 y (1–9 y) 14.1 kg (7.5–40 kg) |
No | Weight, age, Clcr |
Meropenem Cl: 6.99 ml/kg/min ± 2.5 Vc: 0.57 l/kg ± 0.47 Kcp: 2.512/h ± 1.449 Kpc: 3.268/h ± 1.667 Total Vd 0.78 l/kg ± 0.73 |
Target: fT > MIC 40% and 80% for MICs from 0.03 to 32 mg/l PTA of 90% defined as optimal |
120–160 mg/kg/day as continuous infusion |
| Cies (2017) [3] | Multiple (ampicillin, cefazolin, cefepime, cefotaxime, ceftaroline, doripenem, piperacillin/tazobactam, meropenem) | Unknown doses used. Authors refer to Lex-Comp Online, Pediatric and National Lexi-Drugs and Thompson Micromedex as sources for “standard published dosing recommendations” but dose in mg/kg are not given | Cohort | 82 | Mixed |
4.1 y (4 d–18 y) 10 kg (2.7–116 kg) |
Yes | None |
Target: fT > MIC 40%, fT > 4–6 × MIC 40% and fT > MIC 100% No PK parameters calculated, only drug concentrations and PD targets |
78 of 82 patients (95%) no therapeutic concentration: 73/78 subtherapeutic and 5/78 supratherapeutic concentrations (fT > 4–6 × MIC 100% | Not given |
| Wagner (1994) [66] | Netilmicin |
6 mg/kg 1 dd infusion in 5 min For renal insufficiency, reduction of dosage was performed either by extension of dosing interval to 36–72 h and/or reduction of dose to 2.5–3.0 mg/kg |
Cohort | 9 | Mixed |
4.6 y (1 mo–15.5 y) 20 kg (3.4–70 kg) |
No | Age | Cmax 33 µmol/l (range 23–41), Cmin 1.3 µmol/l (range 0.2–3.2) | Not given | |
| Beranger (2018) [26] | Piperacillin + tazobactam | Piperacillin 350 mg/kg/day in 4 doses, infusion in 30 min. Tazobactam 37.5 mg/kg/day in 4 doses, infusion in 30 min | Cohort | 67 | Mixed |
2.3–2.6 y (1–18 y) 11.9–13.7 kg (2.7–53) |
Yes (1 patient) | Weight, eGFR, PELOD score, |
Piperacillin t½ median 0.9 h (range 0.15–4.2 h) Population piperacillin Cl 0.18 l//kg/h Population piperacillin Vd 0.351 l/kg |
Median (range) of time > MIC 53.3% (0–100) and 4 × MIC 18.3% (0–100). Target MIC not known For 18 cases with measured fT > MIC was 100% and 4 × MIC 69.2% Extended or continuous infusions were adequate to attain the 50% fT > MIC target. Only CIs allowed the 100% fT > MIC target to be attained |
400 mg/kg/day in continuous or extended infusions, for children with ARC |
| Cies (2014) [24] | Piperacillin + tazobactam | Mean approximately. 400 mg/kg/day in 4 dd (range 300–425.6 mg/kg/day) | Cohort | 13 | Mixed |
2 y (9 mo–6 y) 13 kg (8.5–30 kg) |
Yes | Weight | Vd (central compartment) 0.249 l/kg (SD 0.211), Cl 0.299 l/kg/h (SD 0.128) | PTA for several dosing schemes: 50 mg/kg 6 dd, 80 mg/kg 3 dd, 100 mg/kg 4 dd infused in 0.5, 3, or 4 h. 400 mg/kg continuous infusion. MIC ranges 0.3–128 µg/ml with target fT > MIC > 50% | 400 mg/kg/day of piperacillin in 4 doses as 3-h infusion or as continuous infusion |
| Nichols (2015) [23] | Piperacillin + tazobactam | 300/37.5 mg/kg/day in 3 doses, infused over 4 h | Cohort | 12 | Mixed |
5 y (1–9 y) 18.3 kg (9.5–30.1 kg) |
No | Weight, Cl, Vd, sex |
Piperacillin Vd 6.58 l Piperacillin Cl 3.51 + (0.0814 × (weight-18)) l/h Tazobactam Vd 5.54 l Tazobactam Cl 3.43 × (1 − (0.285 × female) + 0.0676 × (weight-18) l/h |
T > MIC > 50% and T > MIC > 100% for MIC 8, 16, and 32 for multiple dosing schemes (80 and 100 mg/kg/day in 3 or 4 doses with 0.5, 3, or 4 h infusion) | 100/12.5 mg/kg of piperacillin/tazobactam every 6–8 h as extended infusion |
| De Cock (2017) [25] | Piperacillin + tazobactam | 300 mg/kg/day in 4 doses, infusion in 5–30 min | Cohort | 47 | Mixed |
2.83 y (2 mo–15 y) 14 kg (3.4–45 kg) |
No | Weight, age, maturation, creatinine, CysC, PRISM, PELOD, reason for admission, sex, co-medication |
Piperacillin Cl 0.25 l/kg/h Piperacillin V1 = 0.13 l/kg, V2 0.11 l/kg Tazobactam Cl 0.13 l/kg/h Tazobactam V1 = 0.13 l/kg, V2 0.11 l/kg |
PTA simulated for 75–100 mg/kg every 4, 6, or 8 h with infusion in 15, 30, 60, and 120 min and continuous infusion of 300, 350, and 400 mg/kg/day. Target-attainment is fT > MIC 50% with MIC of 16 mg/l | 6 dd 75–100 mg/kg/dose as prolonged infusion of continuous infusion of 300 mg/kg/day |
| Lukas (2004) [58] | Teicoplanin |
Loading dose: 3 doses of 10 mg/kg every 12 h, infused in 1 h Randomized in 2 groups between 10 mg/kg 1 dd or 15 mg/kg 1 dd as maintenance dose, infused in 1 h |
RCT | 20 | Mixed |
3.1 y (4 mo–10 y) 14 kg (4–28 kg) |
No | Age, weight |
AUC35-last (after the 3 loading doses) 1194 mg/h/l ± 410. For children < 12 mo (n = 4) 1484 ± 315 and children > 12 mo (n = 16) 1121 ± 406 Cmax 59.9 mg/l ± 18.8 for total cohort, for children < 12 mo (n = 4) 71.8 mg/l ± 9.3 and > 12 mo (n = 16) 56.9 ± 9.5 Cmin 9.01 mg/l ± 4.3 for total cohort, for children < 12 mo (n = 4) 12.1 mg/l ± 4.6 and > 12 mo (n = 16) 8.24 ± 4 Model parameters: Cl 0.23 l/h, Vd 3.16 L, V2 4.7 L, Q 0.32 l/h, t1/2 alfa 2.0 h, t½ beta 79.3 h, t½ central compartment 9.5 h PopPK model weight corrected: Cl 0.017 l/kg/h, V 0.26 l/kg PopPK model age corrected: > 12 mo: Cl 0.26 l/h, Vd 4.17 l < 12 mo: Cl 0.04 l/h, Vd 1.43 l |
Subtherapeutic Cmin more frequent in children > 12 mo (35%) vs. children < 12 mo (8%) Simulation with loading dose of 5 mg/kg with 4 mg/kg/day maintenance dose for children < 12 mo and loading dose of 10 mg/kg and 8 mg/kg/day maintenance dose for children > 12 mo gave PTA > 90% |
Loading dose of 5 mg/kg with 4 mg/kg 1 dd maintenance dose for children < 12 mo Loading dose of 10 mg/kg and 8 mg/kg 1 dd maintenance dose for children > 12 mo |
| Reed (1997) [18] | Teicoplanin | 6 mg/kg/day in 1 dose infusion in 20–30 min | Cohort | 12 | Mostly cardiac surgery, 1 patient head surgery |
6 y (2.4–11.4 y) 21.4 kg (9.3–43.5 kg) |
No | None |
After first dose: Vd 0.46 l/kg ± 0.04 (Vc 0.09, V2 0.04, and V3 0.33) Total Cl 2.38 l/kg/h (renal Cl 1.09 l/kg/h) Terminal t½ 11.3 h ± 1.0 Cmin after first dose: 1.8 mg/l ± 0.6 Cmax after first dose: 39.3 mg/l ± 7.6 After 5th dose: Vd 0.56 l/kg ± 0.09 (Vc 0.09, V2 0.08, and V3 0.4) Total Cl 2.19 l/kg/h (renal Cl not determined) Terminal t½ 16.1 h ± 3.4 Cmin after fifth dose: 3.1 mg/l ± 1.2 Cmax after fifth dose: 40.8 mg/l ± 7.4 |
– | 8 mg/kg every 12 h should be effective to achieve Cmin concentrations of 11 mg/l within 48 h. Higher doses (e.g. 15 mg/kg every 12 h) may be needed for the treatment of bacterial endocarditis |
| Sanchez (1999) [59] | Teicoplanin |
Loading dose: 3 doses of 10 mg/kg every 12 h Maintenance dose: 10 mg/kg every 24 h |
Cohort | 21 | Cardiac |
Unknown (7 d–12 y) Weight not given |
Yes | Age | AUC 224.5 mg/l/h, V central compartment 0.38 l/kg, Vd at steady state 1.02 l/kg, Cl 45 ml/kg/h, Cmax 26.2 mg/l, Cmin steady state 5.8 mg/l, t½ 17.41 h |
PTA: 60% of samples were above 5 mg/l, 25% of samples above 10 mg/l. Only 11% of Cmin concentrations > 10 mg/l. No differences in concentrations and PK parameters between different age groups (< 3 mo, 3–12 mo, and > 12 mo) |
Not given |
| Avedissian (2017) [52] | Vancomycin | Median 45 mg/kg/day (IQR 40.0–58.6 mg/kg/day) | Cohort | 250a | Mixed |
9.8 y (unknown) 30.0 kg (IQR 15.0–50.0) |
No | ARC (Clvanco > 130 ml/min), age, weight, sex, serum creatinine |
Vancomycin Vd overall 0.62 l/kg (0.58–0.66) Vancomycin t½ 3.62 h (IQR 3.06–4.51) Vancomycin Cl 97.34 ml/min (IQR 76.1–115.2) |
In patients with ARC 79% subtherapeutic concentrations vs. 58% in no-ARC group | Not given |
| Bonazza (2016) [41] | Vancomycin | Unknown | Cohort | 265 | Mixed |
unknown (0–18 y) 10–12.4 kg (range not given) |
Yes | Age, AKI, co-medication | Cmin: < 10 mg/l = 20%, 10–15 mg/l = 31.7%, 15–20 mg/l = 23%, > 20 mg/l = 25.3% | Not given | |
| Cies (2013) [44] | Vancomycin |
< 2 mo, non-cardiac patient: 45 mg/kg/day in 3 doses < 2 mo and cardiac patient: 30 mg/kg/day in 3 doses > 2 mo: 60 mg/kg/day in 4 doses with maximum of 1 g/dose Renal dysfunction: 10 mg/kg/dose, frequency depended on plasma concentrations |
Cohort | 113 | Sepsis, pneumonia, meningitis |
2 y (1 mo–18 y) 13–13.5 kg (0.68–108 kg) |
Yes | Age, Cmin, length of therapy, ECMO therapy, co-medication, use of vasopressors |
2 groups [1 group in which high Cmin (15–20 mg/l) were desired vs. a control group in which Cmin < 15 mg/l were desired] More sepsis in high group, more pneumonia in control group Mean vancomycin concentration in ‘high’ group 17.8 mg/l ± 3.1 vs. In the control group, 8.4 mg/l ± 3.1 |
All 57 patients (100%) in the high group achieved a Cmin > 15 µg/ml compared with 0 of 56 patients in the control group | Not given |
| De Cock (2017) [42] | Vancomycin | Intermittent dosing: 60 mg/kg/day in 4 dd infused in 60 min; continuous dosing: loading dose 15 mg/kg in 60 min, maintenance dose 40 mg/kg in 24 h | Cohort | 32 | Mixed |
4.1 y (1.3–6.3 y) 17–30 kg (10–64 kg) |
Yes | Total protein, albumin |
Cmin intermittent dosing: 6.7 mg/l (IQR 4.7–8.7) Continuous dosing 14.5 mg/l (IQR 10.2–18.7) AUC0–24h/MIC 425 (IQR 293–497), fAUC0–24h/MIC 294 (IQR 222–357) for MIC 1 mg/l |
Target attainment for Cmin (10–15 mg/l) = 8% AUC/MIC > 400 was reached in 54% of patients fAUC0–24h/MIC > 200 was reached in 83% of patients Cmin of 7 mg/l corresponded with AUC/MIC > 400 |
Not given |
| Fitzgerald (2019) [56] | Vancomycin | 40 mg/kg/day in 4 doses | Cohort | 43 | Post-cardiac arrest |
Unknown (4 mo–9.2 y) Weight unknown |
Yes | AKI at day 5 |
on-AKI patients: median first vancomycin Cmin 6.8 mg/l (IQR 4.3–18.0), median highest Cmin 8.6 mg/l (IQR 5.7–18), 11% Cmin > 20 mg/l AKI patients: median first vancomycin Cmin 15.8 mg/l (IQR 9.6–21.0), median maximum Cmin 19.6 mg/l (IQR 15.0–24.4), 44% concentrations > 20 mg/l |
AKI significantly higher concentrations and higher prevalence of concentrations > 20 mg/l | Not given |
| Genuini (2018) [55] | Vancomycin | Loading dose of 15 mg/kg, infused during 1 h, followed by 45 mg/kg/day as continuous dose | Cohort | 28 | Mixed |
2 y (1 mo–17 y) 11 kg (3–53 kg) |
Yes | Age, weight, creatinine | Target plateau concentration 15–30 mg/l, target AUC0–24h of 400 |
12/28 patients (43%) had their first vancomycin plateau concentration between 15 and 30 mg/l on day 1, 45% had their 2nd concentration between 15 and 30 AUC0–24h > 400 was reached in 7/28 patients (25%) on day 1, 4/23 (17%) on day 2 and 6/19 (32%) on day 3 |
Not given |
| Giachetto (2011) [46] | Vancomycin |
Protocol: 40 mg/kg/day for extrameningeal infections, 60 mg/kg/day for CNS infections Doses used in study: mean 39.92 mg/kg/day (range 33–45) on day 1 (n = 22) and mean 44.17 mg/kg/day (24–60) on day 3 (n = 15) |
Cohort | 22 | Mixed |
3.2 y (1 mo–16 y) Weight not given |
No | Positive fluid balance |
Vancomycin Cl on day 1: 1.95 ml/kg/min ± 1.10 (n = 18) Vancomycin Cl on day 3: 2.47 ml/kg/min ± 1.07 (n = 15) Vancomycin Vd on day 1: 0.51 l/kg ± 0.24 (n = 18) Vancomycin Vd on day 3: 0.86 l/kg ± 0.58 (n = 15) Cmax day 1: 21.80 µg/ml ± 13.6 (n = 18) Cmin day 1: 7.8 µg/ml ± 4.8 (n = 22) Cmax day 3: 21.67 µg/ml ± 8.8 (n = 15) Cmin day 3: 9.36 µg/ml ± 7.8 (n = 15) AUC0–24h day 1: 364 µg/h/ml ± 218.9 (n = 18) AUC0–24h day 3: 364 µg/h/ml ± 212.8 (n = 15) |
AUC0–24h > MIC > 400 on day 1: 50% for MIC 1 mg/l, 5.6% for MIC 2 mg/l (n = 18) AUC0–24h > MIC > 400 for MIC 1 and 2 mg/l on day 3: 46.7% for MIC 1 and 6.7% for MIC 2 (n = 15) |
Loading dose of 18–24 mg/kg in a patient with positive water balance. Maintenance dose adjusted according to serum concentrations |
| Glover (2000) [47] | Vancomycin | Initial dose 47.3 mg/kg/day ± 12.4, final dose 60.6 mg/kg/day ± 14.7 | Cohort | 76 | Mixed |
5.75 y (1 mo–18.8 y) Weight not given |
No | None |
Initial concentrations: Cmax 19.9 mg/l ± 8.1, Cmin 6.2 mg/l ± 3.5 Final concentrations: Cmax 26 mg/l ± 6.2, Cmin 7.8 mg/l ± 2.9 |
Dose of at least 40 mg/kg/day is needed to reach Cmin of 5–10 mg/l | 60 mg/kg/day every 8 h in case of no renal impairment |
| Goboova (2015) [50] | Vancomycin | Started with 2 g/day in 2 doses, later increased the dose to 4 g/day in mg/kg/day because of ARC | Case report | 1 | Trauma |
16 y 89 kg |
No | ARC | Vancomycin Cmin with 2 g/day in 2 doses: 1.5 mg/l, later with 4 g/day in 2 doses initially adequate Cmin (9.93 mg/l) but with further drop of creatinine low Cmin again (4.88 mg/l) | Not given | |
| Gous (1995) [48] | Vancomycin | 40 mg/kg/day in 4 doses, infusion in 60 min | Cohort | 20 | Sepsis, pneumonia, meningitis |
3 mo (0.5–10 mo) 6.4 kg (2.0–10.4 kg) |
No | None |
Vancomycin pharmacokinetics day 2: Cmax 29.1 mg/l ± 12.1 (range 11.0–64.8) Cmin 12.0 mg/l ± 5.9 (range 1.5–21.6) Vd 0.81 l/kg ± 0.6 (range 0.18–2.6) t½ 5.3 h ± 3.2 (range 1.3–12.3) Cl 1.5 ml/kg/min ± 0.5 (range 0.8–2.5) Vancomycin pharmacokinetics day 8: Cmax 35.5 mg/l ± 11.1 (range 17.5–53.3) Cmin 11.7 mg/l ± 6.8 (range 3.3–26.2) Vd 0.44 l/kg ± 0.19 (range 0.2–1.0) t½ 3.4 h ± 1.2 (range 1.5–5.3) Cl 1.2 ml/kg/min ± 0.4 (range 0.6–1.9) |
Not given | |
| Holsen (2017) [54] | Vancomycin |
Median dose 45.0 in vancomycin/piperacillin + tazobactam group 50.0 mg/kg/day in vancomycin/ceftriaxone group IQR in both groups 40–60 mg/kg/day |
Cohort | 93 | Mixed |
6.5– 9 y (unknown) 20.8–25.7 kg (range not given) |
No | Age, sex, height, weight, GFR, PRISM, PIM-2, dehydration, ECMO, past history, sepsis |
Vancomycin trough samples: median 9.5 µg/ml (IQR 7.6–14.2) for vancomycin/piperacillin + tazobactam group, 6.2 (IQR 6.6–14.2) for vancomycin/ceftriaxone group Vancomycin AUC0.24h median 452 mg/h/l (IQR 375–521) for vancomycin/piperacillin + tazobactam group, 540 (IQR 422–617) for vancomycin/ceftriaxone group |
Not given | |
| Moffett (2019) [57] | Vancomycin | Median dose 43.5–58 mg/kg/day in 3–4 doses | Cohort | 261 | Post-cardiac surgery |
3.7 mo (IQR 0.8–9.2 mo) 4.8 kg (IQR 3.4–7.4 kg) |
Yes | Weight, eGFR, age in final model, type of surgery, urine output, post-operative day, sex also tested | For median study patient: vancomycin Cl 0.111 l/kg/h, Vd 0.908 l/kg |
Simulations with 5 dosing regimens from 10–20 mg/kg/dose every 6–12 h (maximum 60 mg/kg/day) and target of AUC0–24/MIC of 400. PTA > 90% for dosing regimens of 60 mg/kg/day in 3–4 doses. Simulated Cmin concentrations were lowest using an 8-h interval (74.4% of Cmin < 15.0 mg/l, vs. 53.2% using a 6-h interval) Other regimens (40 mg/kg/day in 4 doses, 40 mg/kg/day in 2 doses and 45 mg/kg/day) showed in 3 doses and 40 showed PTA of 33.2%, 33.9% and 60%, respectively |
60 mg/kg/day in 3 doses |
| Seixas (2016) [43] | Vancomycin | Mean vancomycin empiric dose 59.23 mg/kg/day ± 49.85 (IQR 39–79). The interval between doses varied from 6 to 48 h | Cohort | 94 | Cancer |
7.28 y (2 mo–17.6 y) 22.3 kg (IQR 11.1–35.7 kg) |
Yes | Age, weight, vancomycin concentration, stem cell transplantation, co-medication |
Cmin 15.6 mg/l (IQR 5.25–19.15) Cmax 25.26 mg/l (IQR 16.5–33.5) Cl 0.16 l/kg/h (IQR 0.08–0.18) Vd 1.04 l/kg (IQR 1.02–1.09) |
AUC0–24h/MIC > 400 reached in 56% | Not given |
| Silva (2012) [45] | Vancomycin | Mean 81 mg/kg/day (range 10–156 mg/kg/day) | Cohort | 31 | Oncologic/hematologic |
7 y (2 mo–13 y) 22 kg (5–62 kg) |
Yes | Clcr, creatinine, albumin |
Vancomycin Cl 0.18 ± 0.11 (range 0.067–0.48) Vancomycin Vd 1.03 ± 0.12 Estimated Cmax 29.33 ± 11.6 |
AUC0.24h/MIC > 400 with MIC of 1 = 55.7% of total population (although also with higher dosing). Target attainment for 40–60 mg/kg/day in 50% of samples (9/18 in 13 patients) | Not given |
| Thomas (2017) [53] | Vancomycin | 36.25 mg/kg/day (median) with IQR of 29–40 mg/kg/day across the whole study population (including neonates) | Cohort | 42 | Cardiac |
2 mo (unknown) 5.1–12.4 kg (IQR 3.8–15.4 kg) |
No | Age, weight, Clcr at start of treatment and at time of sample, total vancomycin dose, cardio-pulmonary bypass | Target = Cmin between 8 and 15 µg/ml | 39/77 therapeutic (50.6%), 24/77 subtherapeutic (31.2%), 14/77 supratherapeutic (18.2%) | 30 mg/kg/day for neonates, 35–40 mg/kg/day for infants, and 45 mg/kg/day in children, with adjustments required for patients with reduced Clcr or aortic cross-clamp time greater than 55 min |
| Totapally (2013) [49] | Vancomycin | Dosing advice in protocol 40 mg/kg/day, mean dose in study population 34.2 mg/kg/day ± 12.4 | Cohort | 284 | Mixed |
2.23 y (unknown) 25.8 kg ± 23.5 (range not given) |
No | AKI |
Cmin for all patients 7.9 µg/ml ± 4.9 Cmax for all patients 27.6 µg/ml ± 18.4 |
Not given | |
| Zane (2017) [51] | Vancomycin | 40 mg/kg/day in 4 doses | Cohort | 52 | Cardiac with hypothermia |
23–43 mo (1.75 mo–17 y) 12–16.4 kg (3.8–88.3 kg) |
Yes | Age, weight, temperature, GFR |
Vancomycin Cl in normothermic patients: 4.48 l/h/70 kg (0.19 l/h/kg^0.75) V1 12.7 l (95% CI 8.05–17.35 l) V2 35.5 l (95% CI 29.11–41.89 l) Q 8.49 l/h (95% CI 6.93–10.05) |
Not given |
Articles are listed alphabetically based on antibiotic agent and first author
95% CI 95% confidence interval, AKI acute kidney injury, ARC augmented renal clearance, AUC (free) area under the curve, BSA body surface area, CPB cardiopulmonary bypass, Cl clearance, Clcr creatinine clearance, Cmax peak concentration, Cmin trough concentration, CNS central nervous system, CysC cystatin C, d days, dd daily doses, ECMO extra-corporal membrane oxygenation, GFR estimated glomerular filtration rate, h hours, IQR interquartile range, K elimination rate constant, Kcp elimination rate constant from central to peripheral compartment, Kpc elimination rate constant from peripheral to central compartment, mo months, MIC minimum inhibitory concentration, min minutes, MRSA methicillin-resistant Staphylococcus aureus, N number of PICU patients in the study, PD pharmacodynamic, PELOD Pediatric Logistic Organ Dysfunction, PICU Pediatric Intensive Care Unit, PIM Pediatric Index of Mortality, PK pharmacokinetic, PRISM Pediatric Risk of Mortality, PTA probability of target attainment, SD standard deviation, Q perfusion rate, RCT randomized controlled trial, T time, t½ half-life, V1 volume of compartment 1, V2 volume of compartment 2, V3 volume of compartment 3, Vd volume of distribution, Vss volume of distribution at steady state, y years
aCohort included patients up to age 21 years
β-Lactam Antibiotics
Penicillins
Amoxicillin and Clavulanic Acid
Both studies on amoxicillin PK included patients that were treated with amoxicillin combined with clavulanic acid as a β-lactamase inhibitor [21, 22]. Doses used in the studies ranged from a single dose of 50 mg/kg amoxicillin and 5 mg/kg clavulanic acid to 100 mg/kg/day of amoxicillin and 20 mg/kg clavulanic acid every 6 h. Patient characteristics within these 2 studies varied: Jones et al. [22] studied 15 children with pneumonia, asthma or pyelonephritis who were slightly older than the 50 patients in the study by de Cock [21]. In addition, data on renal function were not reported by Jones, nor was there any information on disease severity. The study by De Cock et al. included a varied PICU population of which 44% received the combination for postoperative prophylaxis. Patients had a median (range) PRISM-score of 6.5 (0–32) and median (range) PELOD score of 1 (0–31).
Median estimated Vd was 0.368 and 0.469 l/kg for amoxicillin and 0.306 and 0.434 l/kg for clavulanic acid for the De Cock and Jones study, respectively. Cl of amoxicillin, normalized to l/kg/h, was comparable between both studies at 0.242 and 0.257 l/kg/h, whereas clavulanic acid Cl was slightly higher in the study by Jones et al. (0.256 l/kg/h) compared to 0.174 l/kg/h in the study by De Cock et al.
De Cock et al. estimated PK-parameters using population PK (PopPK) modelling. They identified weight, post-menstrual age, cystatin C based estimated GFR (eGFR) and vasopressor treatment as significant co-variates on either Vd and/or Cl of amoxicillin and clavulanic acid. Target attainment, which was defined as fT > MIC of 40% against an MIC of 8 mg/l, was reported only for clavulanic acid for 3 different dosing regimens, including dosing regimens of 25 mg/kg every 4–12 h the authors based on the British National Formulary for Children and Samford Guide for Antibiotic Therapy. Target attainment was estimated at 48–96% for bolus infusions and 53–99% for extended infusions of 1 h. Target attainment for amoxicillin was not reported in exact numbers but was identified from figures. Target attainment of time above MIC of 8 mg/l ranged from 10 to 85% for three different dosing schemes using bolus injections in children with no vasopressors and no renal dysfunction. Prolonged infusion of 1 h increased PTA to 25–100%. The authors propose a daily amoxicillin dose of 150 mg/kg/day in six doses. Duration of infusion is dependent on renal function, with an extended infusion in children with cystatin C concentrations < 1 mg/l.
Piperacillin and Tazobactam
Five studies reported PK-parameters of piperacillin with or without the addition of the β-lactamase inhibitor tazobactam [3, 23–26]. Median doses used ranged from 300 to 400 mg/kg/day in 3–4 doses, with 1 study using a prolonged infusion time of 4 h [23] and 1 study not reporting exact dosing schedules used in the study [3]. In total 153 patients were included in these 5 different studies, with a median age of 0.8–5 years and ages ranging from 0.1 to 18 years. Risk of mortality was reported in 1 study [25], with a median PRISM-score of 8 (range 0–40) and median PELOD scores, reported in 2 studies [25, 26], ranged from 1 to 10. Patients with renal dysfunction were largely excluded from analyses, with only 1 study including 1 patient with renal dysfunction within the cohort [26].
Reported median piperacillin Vd ranged from 0.240 to 0.444 l/kg with the study cohort consisting of predominantly septic, neutropenic and/or burn patients showing the highest Vd [24]. Cl ranged from 0.190 to 0.299 l/kg/h. Patients from the cohort with the lowest median piperacillin Cl still had a particularly high median eGFR of 142 ml/min/1.73 m2 based on serum creatinine[26].
Four of the 5 studies used a PopPK approach and data was best described by a 2-compartment model in 2 studies [24, 25]. In 2 other studies a 1-compartment model best fitted the data, probably because of the use of an extended infusion time [23] or a lack of samples in the distribution phase [26]. All models included weight as a covariate for Vd and/or Cl. Additional covariates that were included in the final models of these studies were post-menstrual age with a maturation coefficient [25] and eGFR [26] as a covariate for piperacillin Cl and PELOD-2 scores for Vd [26]. Nichols et al. [23] included gender as a covariate in their final model for tazobactam Cl. Other covariates that were tested for significance but were not included in the final models include cystatin C based eGFR, PRISM-scores, reason for admission, and co-medication [25].
Target attainment of piperacillin was tested for multiple targets and dosing schemes using Monte Carlo simulations in all four modelling studies. Three of these four used the same target of fT > MIC of 50% against an MIC of 16 mg/l, reflecting the clinical breakpoint of Pseudomonas aeruginosa [23–25]. Simulations in all studies concluded extended infusion over > 1 h is needed to reach a PTA of > 90%, but proposed daily piperacillin doses varied, ranging from 300 mg/kg/day by Nichols et al. up to 600 mg/kg/day by Béranger et al.
Cephalosporins
Cefotaxime
While three studies were found reporting cefotaxime PK in critically ill children [27–29], most children in the study by Von Hattingberg et al. were neonates, with only two patients > 1 month of age included in the PK analysis [27]. Doses used varied from 100 to 300 mg/kg/day in 3–4 daily doses, with patients > 50 kg receiving adult doses of three daily doses of 1000 mg in the study by Béranger et al. [28]. Although the exact reason for admission was only clear from the study by Hartman et al. [29], organ dysfunction scores and length of PICU stay was reported in two studies [28, 29]. Organ dysfunction scores and disease severity were highest among the meningococcal septic shock patients studied by Hartman et al. PELOD scores were included in both the model building and validation cohorts by Béranger et al. [28].
Vd and Cl were reported by Von Hattingberg and Béranger et al. For the typical study patient (weighing 10.9 kg and 23.7 months of age) in the study by Béranger the median Vd and Cl were 0.31 l/kg and 0.334 l/kg/h, respectively. The authors used allometric scaling based on both body weight and postnatal age to predict individual cefotaxime Cl. The two patients in the study by Von Hattingberg et al., one with and one without kidney injury, had a Vd of 0.16 and 0.31 l/kg and Cl of 0.109 and 0.479 l/kg/h, respectively. Elimination t½ of cefotaxime were similar in both studies, ranging from 0.34 to 1.15 h in the study by Béranger et al. and 0.46–1.02 h for the two patients without and with kidney injury by Von Hattingberg et al.
Both studies used a one-compartment model to describe PK parameters, possibly due to the limited sampling strategy with a median of two samples per patient. The co-variates studied by Béranger et al. include weight, age, serum creatinine, and PELOD-scores [28]. Only weight and age were included in the final model as significant covariates on Cl and/or Vd. Monte-Carlo simulations were performed with several dosing regimens to identify the needed dose to reach the target of fT > MIC and fT > 4xMIC of 100%, against MIC values of 0.5 mg/l. The authors concluded that intermittent dosing without prolonged infusion, for patients over 1 month of age, would require a daily dose of 4500 mg/kg to reach these targets. However, continuous dosing of 100 mg/kg/day would be sufficient to reach adequate targets in all age groups. Therefore, the authors advised to use continuous dosing for optimal cefotaxime dosing in critically ill children.
The study by Hartman et al. found a slightly higher percentage of target attainment (71.3%) for the PD-target of fT > 4xMIC of 100% against an MIC of 0.5 mg/l using standard doses. Higher MIC values of 1 and 4 mg/l, that might be more clinically relevant, showed a lower target attainment of 55.1% and 14.7%, respectively. The authors state that this is a best-case scenario of target attainment, as samples were randomly drawn across the dosing interval and no PK model to simulate actual probability of target attainment was developed.
Cefuroxime
We identified only one study on cefuroxime PK in PICU patients [30], 15 patients in total, including 4 non-critically ill patients with pharyngitis. The 11 PICU patients were divided in 2 groups: a severely ill group of 5 PICU patients not requiring mechanical ventilation, and a very severely ill group with 6 patients requiring mechanical ventilation. No severity of illness scores were provided. All patients were treated with a cefuroxime dose of 400 mg/kg/day in four doses as intermittent infusions over 30 min.
Both Vd and Cl were higher in mechanically ventilated patients compared to control and PICU patients that did not require mechanical ventilation. Even though differences between Vd and Cl between control and non-intubated PICU patients were minimal, t½ of cefuroxime was longer in both critically ill groups compared to the control patients (1.0–1.3 h vs. 0.5 h).
The study used both 1- and 2-compartment models to describe the obtained PK-data, for each individual patient. In the majority of patients (8/11) a 2-compartment model gave the best fit of the observed data. No dosing advice was given to account for these PK changes in critically ill patients.
Cefazolin
Three cefazolin studies were included in our review, all conducted before, during or after cardiopulmonary bypass (CPB) [19, 20, 31]. One study included only patients under 10 kg bodyweight and up to 2.6 years of age [31] while the other 2 studies basically covered the whole pediatric age range from birth to 16 years old [19, 20]. Used cefazolin doses varied between the 3 studies with de Cock et al. and Cies et al. both using 25 mg/kg/dose preoperatively and during CPB and Haessler et al. using 40 mg/kg preoperatively and 105 mg/kg/day in 3 doses in the days after surgery. Interestingly, Cies et al. also added a dose of 25 mg/kg bodyweight cefazolin to the CPB primer solution to ensure stable antibiotic levels during CBP.
In the study by Haessler et al. cefazolin concentrations during and after surgery showed a mean Cmax of 166 mg/l and a steady-state Cmin of 15 mg/l was reached [31]. Elimination rate constants of cefazolin were significantly lower during CBP (0.331/h) and significantly higher after surgery (1.429/h) compared to before surgery (0.738/h). In addition, the Vd increased during CBP due to increased blood volume during extracorporeal circulation (0.357 l/kg compared to 0.191 l/kg before surgery). After surgery Vd returned to baseline values (0.127 l/kg). This increase in Vd during CPB was also seen by de Cock et al. who used a CPB-compartment ranging from 150 to 1000 ml in their model. The mean population values for Cl and Vd in their model, 0.229 l/kg/h and 0.635 l/kg respectively, show a comparable elimination rate constant but a higher Vd, possibly due to including older patients in their cohort. PK parameters in the study by Cies et al. are hard to compare with the other studies due to their alternative dosing strategy, giving markedly lower clearance values of roughly 0.0005 l/kg/h. Volume of distribution values found by Cies et al. were comparable with those found by de Cock et al., except for the oldest age group of 4–16 years old.
Both de Cock et al. [19] and Cies et al. [20] used a PopPK approach by using a 2-compartment and 1-compartment model, respectively. Both models included bodyweight and age in their final models, with de Cock et al. also including eGFR as a covariate for Cl and albumin concentrations as a covariate for protein binding. Dosing simulations were performed only by de Cock et al., using a PD-target of 50–100% fT > MIC against MICs ranging from 0.125 to 16 mg/l. They conclude that the standard dosing regimen of 25 mg/kg/dose shows a PTA of roughly 50%, while the optimal dosing regimen that used doses up to 40 mg/kg/dose showed a PTA of 88–99%. Cies et al. conclude that adding cefazolin to the CPB primer solution ensures stable, adequate concentrations of cefazolin throughout surgery, but more research is needed.
Ceftaroline
Cies et al. mentioned TDM outcomes in 7 patients treated with non-standard (higher) doses of 60 mg/kg/day in 4 doses in a paper on multiple antibiotics [3]. The majority of patients did not require an additional dose alteration to achieve target attainment.
Cies and colleagues also published a case series of 7 patients treated with ceftaroline, presumably the same patients as mentioned in the previous publication, which is the only PK-data of ceftaroline we identified [32]. All patients were treated for a suspected MRSA infection and patients with an estimated creatinine clearance below 60 ml/min/1.73 m2 were excluded. Patients started with a non-standard dose of 54–60 mg/kg/day, but dosing regimens were altered to reach the target of fT > 4–6 × MIC for 40% of the dosing interval, with MICs ranging from 0.38 to1 mg/l. Individual PK-parameters for several dosing regimens are mentioned, but for the starting regimen median Vd, Cl and t½ were 0.41 l/kg, 0.218 l/kg/h and 1.3 h. The authors compare their observed PK-parameters to the PK-parameters for healthy pediatric patients in the package insert. The patients in the study showed a higher median Vd (0.41 l/kg), higher Cl (0.218 l/kg/h) and shorter t½ (1.3 h) than the package insert estimates, which were 0.28 l/kg, 0.138 l/kg/h and 2.7 h, respectively [33]. All patients required a dose alteration or a non-FDA-approved dose to reach target attainment, and all patients eventually were cured from their MRSA infections. The authors advise a 6-h dosing interval in bloodstream infections, pneumonia or meningitis with MRSA and a dose of 15 mg/kg/dose in patients with increased Vd.
Carbapenems
Meropenem
For meropenem 1 case report and 1 PopPK model, both by Cies et al., were identified [34, 35]. The case report describes a 2-year-old girl with a Serratia marcescens ventriculitis [34]. She was treated with 150 mg/kg/day intermittent infusions for 30 min, but this resulted in undetectable meropenem plasma concentration 4 h after dosing. Eventually she was successfully treated with an increased dose of meropenem of 200 mg/kg/day as a continuous infusion, reaching plasma concentrations of 13 mg/l and CSF concentrations of 0.5 mg/l. The calculated Cl of this patient was 0.612 l/kg/h. The authors claim this clearance is higher than reported values in healthy adults and critically ill children on extracorporeal membrane oxygenation, but exact numbers are not reported.
The 2017 PopPK model by Cies et al. included data from 9 patients treated with doses ranging from 40 to 200 mg/kg/day [35]. Most patients received intermittent infusions in 30 min, 2–4 times per day. One patient was treated with continuous infusion of 200 mg/kg/day and one patient received 100 mg/kg/day in 2 daily doses as prolonged infusions for 2 h. Median (range) age of patients was 2 (1–9) years. No patients with renal dysfunction were included, and the median eGFR was 168 ml/min/1.73 m2, indicating possible ARC within the study population.
A roughly 2 times larger Vd (0.78 l/kg) and slightly higher Cl (0.419 l/kg/h) were described in this cohort by Cies et al. [35]. compared to other studies in clinically stable pediatric patients by Du et al. [36] and Blumer et al. [37] (Vd 0.2–0.43 l/kg and Cl 0.18–0.34 l/kg/h, respectively).
After a sparse sampling scheme with a total of 16 meropenem concentrations a Pmetrics 2-compartment PopPK model was used to estimate PK. Weight, age and creatinine clearance were considered to be significant covariates for Vd and/or Cl.
Target attainment of 40% fT > MIC was only reached in simulations of dosing regimens with prolonged or continuous infusion of 120–160 mg/kg/day, with MICs ranging from 0.25 up to 2 mg/l. Target attainment of 80% fT > MIC was only reached with continuous infusion for MICs up to 2 mg/l. For higher MICs, the PTA was below 90% in all simulations.
Imipenem
Gianonni et al. studied imipenem PK in 19 critically ill children ranging from 9 days to 12 years of age [38]. Patients were treated with 100 mg/kg/day in 3–4 daily 30-min infusions. The median (range) PRISM score was 9 (0–23) and although patients with renal dysfunction were included, the exact number of patients with impaired renal function is unclear.
A non-compartmental analysis was performed to estimate Cl, t½ and Vd of imipenem in critically ill children after the first dose and at steady state. All PK-parameters slightly increased from first dose to steady state. The authors compared their findings to other studies in (non-critically ill) children with imipenem. PK-parameters were within the reported values for pediatric patients, with a slightly lower Cl, higher Vd and a longer t½ in critically ill patients. Moreover, the correlation between several covariates and the PK-parameters was tested, including age, weight, creatinine clearance, albumin, lactate, disease severity, blood pressure and heart rate. Eventually, Cl parameters correlated best with creatinine clearance, mean arterial pressure and lactate acidosis, although it is unclear how creatinine clearance was measured or estimated.
Other Penicillins
Aztreonam
The only PK-data on aztreonam, a broad-spectrum monobactam antibiotic agent, is found within a case-report by Cies et al. [39]. In this case-report the authors describe a case of a 16-year-old tetraplegic patient with a pneumonia caused by a multi-resistant Pseudomonas aeruginosa [39]. This pathogen was susceptible to aztreonam against an MIC of 6 mg/l and the patient was treated with aztreonam 8 g/day in 4 prolonged infusions of 4 h. Cl was 0.138 l/kg/h, almost double of the adult Cl values reported in the package insert (0.078 l/kg/h) [40]. The used dose resulted in plasma concentrations above 6 mg/l for at least 40% of the time with a Cmax of 71 mg/l.
Glycopeptide Antibiotics
Vancomycin
A total of 17 studies reporting PK-parameters of vancomycin in critically ill children were identified by the search, with more than 1000 PICU patients included in total over the whole pediatric age range [41–57]. Most studies included a mixed population, but also specific populations (such as patients with hematologic/oncologic [43, 45], cardiac [51, 53, 56, 57], traumatic [50] and infectious diseases [44, 48]) being studied separately. Four studies [42, 43, 46, 48] were prospective PK studies and the remaining 13 studies were based on TDM data.
Most studies used intermittent dosing with daily doses of vancomycin ranging from 30 to 60 mg/kg/day, with only 1 study reporting higher mean doses (81 mg/kg/day) [45] and 1 study not mentioning the dose at all [41]. Two studies reported data on continuous dosing schedules, using a loading dose of 15 mg/kg and a maintenance dose of 40–45 mg/kg over 24 h [42, 55].
Vancomycin is predominantly cleared by glomerular filtration and is also associated with nephrotoxicity, therefore 7 studies mainly reported findings on the effect or occurrence of acute kidney injury (AKI) and/or ARC in patients treated with vancomycin [41, 44, 49, 50, 52, 54, 56]. Several studies, including 2 studies with over 250 patients, reported conflicting results regarding vancomycin Cmin in patients with and without AKI. Bonazza et al. [41] showed increased Cmin in patients with AKI whereas Totapally et al. [49] and Holsen et al. [54] both found no significant differences in Cmin and/or Cmax between patients with and without AKI. To identify whether higher vancomycin Cmin lead to kidney injury Cies et al. performed a retrospective study comparing 2 groups, with either a high (15–20 mg/l) or low (10–15 mg/l) target Cmin of vancomycin [44]. The incidence of AKI was not significantly different between these 2 groups.
ARC is the main focus of 2 studies, 1 case report [50] and 1 cohort study with 250 patients aged 0–21 years old [52]. ARC was defined by an estimated creatinine clearance of > 150 and > 160 ml/min/1.73 m2 by Goboova et al. [50] and Avedissian et al. [52], respectively. The patient in the case report required a doubling and tripling of the initial dose (from 2 g/day in 2 doses to 4–6 g/day in 2 doses) to reach therapeutic Cmin of 10–15 mg/l. Avedissian et al. found 29 patients with ARC, 12% of their total cohort [52]. PK data were analyzed using pop-PK with NONMEM. Patients with ARC were significantly older and had lower baseline serum creatinine levels. PK-parameters for vancomycin were significantly different in patients with ARC: they had lower median Cmin, higher median Cl, larger median Vd and a shorter median t½. Subtherapeutic drug concentrations were more common in patients with ARC compared to patients without ARC (79% and 58%, respectively), but this difference was not statistically tested. Both age and serum creatinine were independent covariates for Cl. Weight was the only independent covariate for Vd.
Of the 17 vancomycin articles, 8 reported Cmin and/or Cmax as the only PK-parameters. Six of these have already been discussed in the previous paragraphs regarding AKI or ARC [41, 44, 49, 50, 54, 56]. The 2 remaining articles, by Glover et al. [47] and Thomas et al. [53], both included patients with a normal renal function at the start of treatment. Both studies analyzed PK-parameters in steady state after the initial dose, while Glover et al. also looked at the final doses used after TDM. After the initial therapy with a mean dose of 47.3 mg/kg/day mean Cmin were 6.2 mg/l on average [47]. Mean doses after TDM were roughly 60 mg/kg/day, resulting in higher mean Cmax and Cmin of 26.0 and 7.8 mg/l, respectively. The authors advised to use 60 mg/kg/day in critically ill patients with normal renal function to ensure optimal target attainment, but do not specify the desired target concentrations. Thomas et al. included both neonates, infants and children in their study [53]. They found that roughly 50% (39 of 77) of patients reach adequate Cmin (using a dose of 30–40 mg/kg/day and a target Cmin between 8 and 15 mg/l) and sub- and supratherapeutic concentrations in 31.2% and 18.2% of patients, respectively. Multivariable regression analysis identified age, weight and creatinine clearance at the start of treatment as significant co-variates for vancomycin Cmin.
Cmin measurements are mainly used for TDM as a surrogate parameter of the true PD target of vancomycin used in adults (AUC/MIC > 400) and is commonly extrapolated to pediatric patients. Several studies determined, simulated or estimated AUC/MIC in their analysis [42, 43, 45, 46, 54, 55, 57]. Giachetto et al. reported vancomycin AUC0–24h/MIC on both day 1, using an initial dose of 33–45 mg/kg/day, and after TDM on day 3 for MICs of 1 and 2 mg/l [46]. Mean AUCs for day 1 and day 3 were 364 mg/l/h for both days, with roughly 50% of patients reaching the target of AUC/MIC > 400 for the MIC of 1 mg/l and 5–7% for the MIC of 2 mg/l. Simulations with several dosing regimens in the study by Moffett et al. [57] showed a target attainment of > 90% for regimens using 60 mg/kg/day in 3–4 doses and a target attainment ranging from 33.2 to 60% for dosing regimens with a lower daily dose of 40–45 mg/kg/day in 2–4 doses. Other studies reporting AUC/MIC data all reported similar percentages of target-attainment of approximately 50%, with only Genuini et al. [55] (using a continuous dose of 45 mg/kg/day) reporting lower target attainment of 17–32%. De Cock et al. also included 3 patients with continuous dosing, but no separate PK-parameters for these 3 patients were presented [42]. The study by de Cock et al. was the only study including free AUC/MIC values, with a target of fAUC0–24h/MIC > 200 assuming an unbound fraction of 50% [42]. The measured unbound fraction of vancomycin in their study was higher than this assumption (71.1%). The majority of patients (83%) reached this unbound vancomycin target using a dose of 60 mg/kg/day. When using Cmin between 5 and 10 mg/l as a surrogate target, only 8% of patients reach this target, questioning the validity of this surrogate target in critically ill pediatric patients.
Other PK-parameters presented in several studies include Cl and Vd in 7 studies [43, 45, 46, 48, 51, 52, 57], with 2 studies reporting data on vancomycin t½ [48, 52]. Mean Vd within each study ranged from 0.44 to 1.04 l/kg, with a median Vd among studies of 0.77 l/kg. Mean Cl in each study ranged from 0.072 to 0.19 l/kg/h, with a median of 0.154 l/kg/h found among studies. Avedissian et al. and Gous et al. both reported a similar t½ (3.4 and 3.62 h, respectively) [48, 52].
Teicoplanin
Three prospective studies describe teicoplanin PK in critically ill children with ages ranging from 7 days to a maximum of 12 years old [18, 58, 59]. Doses used in studies varied, with 2 studies [58, 59] using 3 loading doses of 10 mg/kg teicoplanin every 12 h and afterwards a maintenance dose of 10 mg/kg every 24 h. The study by Lukas et al. was designed as a randomized controlled trial where patients in the other study arm received a higher maintenance dose of 15 mg/kg every 24 h [58]. One study, by Reed et al., used lower daily doses of 6 mg/kg in patients undergoing cardiac or head surgery, without information on whether a loading dose was given [18].
Sanchez et al. described 21 patients, mainly post cardiac surgery, including 1 patient with renal dysfunction with an eGFR of 55 ml/min/1.73 m2 [59]. A total of 127 samples were drawn, with a full PK-curve after the first dose of teicoplanin and Cmin 1, 2, 3 and 7 days after the first dose. PK-parameters were determined by a non-compartmental analysis using an open 2-compartment model. Mean Cmax values at 0.5 h were 26.2 mg/l, Cmin at steady state were 5.8 mg/l and mean AUC was 224.5 mg/l/h. Vd at steady state was 1.02 l/kg and total Cl was 0.045 l/kg/h and a terminal t½ of 17.41 h. PK-parameters are presented without an indication of variance, which makes extrapolation to other cohorts difficult. The authors used a target of > 10 mg/l, which was reached in only 11% of Cmin samples. Teicoplanin concentrations and PK-parameters did not differ between patients under 3 months, 3–12 months and over 12 months of age although it is unclear how many patients were represented in each age group and the overall group size was relatively small.
As mentioned before, Lukas et al. performed a randomized controlled trial with half of the patients receiving a larger maintenance dose [58]. However, no significant differences in teicoplanin concentrations were observed between these 2 dosing strategies. The authors did find a significant difference between young infants (aged < 12 months) and older children (> 12 months), with a lower target attainment in older children (65%) compared to young infants (92%). Further analyses in this study focused on the differences in PK-parameters between these 2 age groups, regardless of the randomized maintenance dose. Children > 12 months had a higher Cl, larger Vd and longer t½ (3.9 l/kg/h, 0.29 l/kg and 9.32 h, respectively) compared to younger infants (1.05 l/kg/h, 0.09 l/kg, 8.1 h, respectively). The authors concluded that for younger infants 3 loading doses of 5 mg/kg every 12 h followed by 4 mg/kg once daily would be sufficient to reach adequate target attainment. For older children, the authors advise 3 loading doses of 10 mg/kg every 12 h and subsequently 8 mg/kg once daily as maintenance dose. Interestingly, this is a lower maintenance dose than used in the study population that showed limited target attainment.
Reed et al. [18] included 12 patients with a median age of 6 years; 11 after cardiac surgery and 1 with head surgery. Teicoplanin PK-parameters were determined after the 1st and 5th dose in a 3-compartment model using PCNONLIN. Cmax and Cmin were 39.3 mg/l and 1.8 mg/l, respectively, after the first dose and 40.8 mg/l and 3.1 mg/l, after the fifth dose. After the first dose, Vd was 0.46 l/kg and total Vd in steady state, after the 5th dose, was 0.56 l/kg. Total body Cl after the first dose was 2.38 l/kg/h, with renal Cl contributing for 1.09 l/kg/h of total Cl. After the 5th dose only total body Cl was determined at 2.19 l/kg/h. Terminal t½ was 11.3 and 16.3 h after the first and fifth dose, respectively. No additional covariates were identified for their influence on teicoplanin PK. The authors recommended using higher doses (than the 6 mg/kg once daily used in this study) of teicoplanin in critically ill children, 8 mg/kg every 12 h to reach Cmin of > 10 mg/l and 15 mg/kg every 12 h for Cmin > 20 mg/l (e.g. in case of endocarditis).
Aminoglycoside Antibiotics
Gentamicin
Haessler et al., also determined gentamicin concentrations before, during and after cardiac surgery in children < 10 kg [31]. During surgery, gentamicin Cmax reached 20.8 mg/l and mean Cmin on day 2 and 3 were 1.1 (SD 0.5) mg/l and 0.8 (SD 0.4) mg/l, respectively. Like the results for cefazolin, as discussed above, k for gentamicin declined during surgery (0.336/h) compared to baseline (0.962/h). However, contrary to cefazolin data, the k remained significantly lower after surgery (0.188/h). Vd also showed a different pattern for gentamicin than cefazolin. Vd increased during surgery (0.237 l/kg before surgery to 0.400 l/kg during surgery), in concordance with cefazolin data. However, Vd remained increased after surgery (0.624 l/kg) compared to baseline, and was even larger than the Vd during surgery.
Three other studies report PK-parameters of gentamicin in critically ill children [60–62]. The study by Kraus et al. [62] used a dosing scheme of multiple daily doses, whereas the other 2 studies used a single daily dose [60, 61]. Kraus et al. used doses ranging from 1.8 to 3.1 mg/kg/dose with 2–3 daily doses, with the majority of patients (39/44) receiving 3 daily doses [62]. The group of 44 patients was divided in 2 groups based on the number of gentamicin Cmax and Cmin concentrations that were available. The group with only 1 set of Cmax and Cmin concentrations (n = unknown) was used to determine population-based PK-parameters, which were validated in the other group of patients with multiple sets of Cmax and Cmin concentrations. The population-based values for Vd, Cl and t½ were 0.416 l/kg, 0.114 l/kg/h and 2.8 h, respectively. PK-parameters for the validation group with multiple sets of Cmax and Cmin concentrations were not significantly different from these population-based values. There was no correlation between age or post-operative status and PK-parameters. Dosing simulations were performed using the population-based values, with Cmax = 7 mg/l and Cmin = 1 mg/l as predefined target. A mean daily dose of 9.1 mg/kg/day (range 5.2–14.8 mg/kg/day) divided in 2–6 daily doses was predicted to reach these target concentrations.
Lopez et al. used a dosing scheme of 8 mg/kg every 24–36 h [61]. The actual dose used in the study by Zakova et al. is unknown, but Monte-Carlo simulations for target attainment were performed with a simulated dose of 6 mg/kg every 24 h [60]. Both studies used a mixed patient population; with Zakova mainly using younger children, aged up to 21 months and Lopez et al. including older patients up to 14 years old (and including 14 neonatal patients as well that are not included in this review). Both studies included patients with renal dysfunction, 10% of patients in the study by Lopez et al. required renal replacement therapy, but for both studies it is unclear how many patients actually suffered from any form of renal dysfunction.
Several co-variates were tested for their influence on PK-parameters; both studies identified age and weight as significant co-variates for Vd and/or Cl. Serum creatinine was correlated with the k in the study by Zakova [60], but was not found to improve the model of Lopez et al. [61]. Other tested co-variates by Zakova et al. were gender, co-medication, admission unit, PRISM-scores and serum albumin, with only the admission unit (PICU or Cardiac Critical Care Unit) being significantly correlated with Vd and k in multiple regression analysis [60].
The 2 measured PK-parameters by Zakova et al. are Vd and the k [60]. Values for Cmax, AUC and the drug-free interval were simulated using Monte-Carlo simulations. Median Vd was 0.39 l/kg and k 0.18/h. Using a simulated dose of 6 mg/kg every 24 h, median Cmax, AUC and drug-free interval values were 17.6 mg/l, 78.6 mg/l/h and 15.2 h. The simulated dosing scheme of 6 mg/kg resulted in 28.4% of patients within the Cmax target of 16–20 mg/l (38.8% below, 32.8% above), 22.4% of patients within AUC target of 70–100 mg/h/l (37.3% below, 40.3% above) and 53.7% of patients within the drug-free interval target of 4–16 h (6% below, 40.3% above).
Lopez et al. used non-linear mixed effect modelling to determine population-based PK-parameters in a 2-compartment model [61]. Mean population value for Cl was normalized for a 70 kg patient at 2.09 l/h/70 kg, with age and weight being significant co-variates for individual Cl values. Vd of the peripheral compartment was fixed at 3.78 l and the volume of the central compartment was 0.35 l/kg. Target attainment was defined as Cmax > 16 mg/l and was determined for different daily doses up to 8 mg/kg of gentamicin. This target was reached in all non-neonates using a dose of 7–8 mg/kg, but the authors stated that dosing intervals should be extended in younger infants due to age-related changes in renal Cl and the risk of toxicity in the case of gentamicin accumulation.
Amikacin
One randomized controlled trial (RCT) and 2 cohort studies on the PK of amikacin were found [63–65]. The RCT, performed by Marik et al. included 60 pediatric patients < 1 year of age that were treated with amikacin 20 mg/kg/day [63]. Patients were randomized to either a once daily dosing scheme, including a loading dose of 20–25 mg/kg, or a twice daily dosing scheme without a loading dose. Data was analyzed using a 2-compartment PK model using NONLIN. The pediatric patients under 1 year were split into 2 groups of 30 patients based on age, a group of patients aged < 6 months (median age 8 weeks) and a group of children 6–12 months (median age 28 weeks). Children older than 1 year were included in a group also including adults (median age 34 years). Vd in patients < 1 year was larger (0.58 and 0.50 l/kg for patients < 6 months and 6–12 months, respectively) than older patients (0.33 l/kg). In addition, Cl was higher in younger patients 0.063 and 0.068 l/kg/h vs. 0.051 l/kg/h in older patients. Elimination t½ was longest in children < 6 months (5.02 h) and shortest in children 6–12 months (2.86 h). Target-attainment, defined as Cmax > 20 mg/l and Cmin < 5 mg/l, was 100% in the group with once daily dosing and 79% (Cmax target) and 44% (Cmin target) for twice daily dosing. Children required higher daily doses in mg/kg to reach these targets: 21 mg/kg/day for 6–12 months, 18.6–20.5 mg/kg/day for < 6 months and 13.8–15.5 mg/kg/day for older patients.
The 2 other studies used a cohort of patients treated with amikacin, with Bressolle et al. including both children and adults in their 2-compartment model [64]. Patients received doses ranging from 70 to 1500 mg, however the dose in mg/kg/day the pediatric patients received is not reported. The only PK-data that was separately presented for the pediatric population were Cmax and Cmin values. Mean Cmax and Cmin values in the pediatric study population were 40.7 and 0.97 mg/l, respectively. These values were validated in a test-population of 8 additional children, which showed lower Cmax of 16.0 mg/l and higher Cmin, 1.40 mg/l. After Bayesian estimation for children, predicted concentrations for Cmax and Cmin were 16.2 mg/l and 1.45 mg/l, respectively.
Sherwin et al. only included patients with burns in their study, with a median burned surface area of 43% [65]. Both studies by Bressolle and Sherwin used a wide pediatric age range from 6 months to 15 and 17 years, respectively, and both studies included patients with renal dysfunction [64, 65]. Studied covariates included weight and height, and Sherwin et al. also tested age, gender, percentage of burned surface area and serum creatinine as covariates, but none improved the model significantly [65]. Population parameters for Cl and Vd were normalized for a typical 70 kg patient. Amikacin Cl was 5.98 l/h/70 kg and Vd was 16.7 l/70 kg for the central compartment and 40.1 l/70 kg for the peripheral compartment. These values are higher than the Cl and Vd of the previously discussed study by Marik et al. [63], indicating altered PK in burn patients.
Netilmicin
Only 1 study on netilmicin PK in pediatric patients was available [66]. This study by Wagner et al. mainly included neonatal patients; only 9 of the total of 66 patients, with a mean age of 4.6 years were admitted to the pediatric ICU. Patients were treated with a once daily dose of 6 mg/kg. The authors mentioned a reduced dose and prolonged dosing interval for patients with renal insufficiency, but no patients with renal dysfunction were included in the pediatric cohort. The only PK-data that are presented are Cmax and Cmin values for pediatric patients. Mean Cmax values were 33 mg/l (range 23–41 mg/l) and Cmin values were 1.3 mg/l (range 0.2–3.2 mg/l). The authors concluded that once daily dosing of netilmicin is sufficient to reach adequate targets.
Other Antibiotics Agents with Eligible Studies in Our Search
Daptomycin
2 case-reports and 1 pharmacometric model were available for daptomycin that included PK parameters [67–69]. Both patients in the case reports suffered from cardiac problems, a 13 year old boy with endocarditis [67] and a 8 year old girl with multi-organ failure (including renal insufficiency) awaiting heart transplantation [68]. Similar doses were used in both studies (6–8 mg/kg every 24 h). The dose interval was prolonged to 48 h for the patient with renal insufficiency [68].
Morris et al. only presented steady state Cmax and Cmin values, which were 68 mg/l and 14.6 mg/l, respectively [68]. These values were within the desired target of Cmin below 20 mg/l. Akins et al. provided Cmax and Cmin concentrations after a single dose and in steady state [67]. In addition, Vd, Cl, t½, k and AUC values were presented for both the first dose and in steady state. Cmax were higher and Cmin concentrations were lower than the values reported by Morris et al., both after the first dose as in steady state. Values in steady state for Vd and Cl were 0.089 l/kg and 0.0137 l/kg/h, respectively. t½ in steady state was 4.58 h, with an AUC of 594 mg/l/h.
The pharmacometric model by Antachopoulos included data of 4 patients aged from 8 to 14 years old with mixed disease conditions in their one compartment model [69]. On the first day of treatment, 3 of the 4 patients met pediatric criteria for sepsis. The patients with sepsis had a significantly higher Cl (median Cl 0.0423 l/kg/h) compared to the patient without sepsis (0.0151 l/kg/h). Vd was also higher in patients that met sepsis criteria (0.26 l/kg, compared to 0.16 l/kg for the non-sepsis patient). This resulted in a lower AUC0–∞ and lower Cmax values of daptomycin in sepsis patients compared to non-sepsis patients (AUC0–∞ 236.5 mg/h/l vs. 663.9 mg/h/l and Cmax 35.4 mg/l vs. 59.8 mg/l, respectively). PK parameters on the 5th day were comparable with the first day, with a higher Cl, larger Vd, lower AUC0–24h and Cmax in the 2 patients meeting sepsis criteria.
Ciprofloxacin
Lipman et al. published the only study on ciprofloxacin in 20 patients between 3 months and 5 years with severe sepsis [70]. Patients received a daily dose of 20 mg/kg/day in 2 doses during 1–2 weeks of treatment. The authors used non-compartmental analysis to determine PK-parameters in 2 age groups, 3 months–1 year and 1–5 years of age. No significant differences in PK-parameters between these 2 age groups were seen. When using a target of Cmax/MIC = 8, the authors concluded that a dose of 20 mg/kg/day is sufficient to cover pathogens with an MIC up to 0.8 mg/l. To reach the target of AUC/MIC of 100–150 in PICU patients with a normal renal function infected by more resistant micro-organisms the authors advise a daily dose of 30 mg/kg/day in 3 doses.
Discussion
Although antibiotic use in critically ill children is one of the pillars of intensive care treatment, from our review we can conclude that current knowledge on the PK and target attainment of these drugs in critically ill children is relatively scarce. Nevertheless, an important finding is that target attainment is often suboptimal in this patient population using standard doses of different classes of antibiotics [19, 21, 24–26, 28, 29, 41, 43, 45, 46, 50, 55, 56, 59, 62, 63, 69].
Not unexpectedly, a large number of manuscripts focus on agents where TDM is generally applied during routine care, such as vancomycin and gentamicin, as data are relatively readily available from medical records. In contrast, to the best of our knowledge, other frequently used agents (like ceftriaxone, ceftazidime, penicillin, flucloxacillin, metronidazole) completely lack PK data in the pediatric ICU population [71, 72]. In addition, only the minority of included articles (22/50) provide dosing guidance for clinicians, even though in most publications (38/50) the necessary data on Vd and Cl was available to simulate optimal dosing regimens.
When comparing the findings on these different antibiotics in critically ill children to data in critically ill adults and/or healthy children, it is apparent that PK alterations occur in this patient population. For example, Cl values of amoxicillin are almost double in critically ill children (0.24 l/kg/h) compared to critically ill adults (0.13 l/kg/h) but with a similar Vd (0.37–0.47 l/kg in children vs. 0.37 l/kg in adults) [73]. For piperacillin, two studies in critically ill children show slightly higher Cl values [24, 25] than in critically ill adults with hyperfiltration [74], but this is not supported by two other publications [23, 26]. Cefotaxime Cl was almost 50% higher in critically ill children [28] while mean Vd was markedly smaller than values reported in non-critically ill children [75], resulting in a shorter t½ and higher recommended doses to reach common PD targets. Median values of vancomycin Cl and Vd in the studies in this review exceed values of non-critically ill children, pediatric cancer patients [76, 77] and critically ill adults [78, 79].
These altered PK parameters are most likely the result of a combination of disease-related alterations and age-related changes in PK [5]. Disease-related changes may impact PK by a multitude of pathophysiological mechanisms. Firstly, fluid resuscitation is a common therapy for hemodynamically unstable critically ill patients, that may result in an increase in total body water, larger Vd and therefore dilution of hydrophilic compounds. Systemic inflammatory response syndrome (SIRS), caused by inflammatory cytokines, induces capillary leak and the shift of intravascular fluid to the extravascular space [80]. This ‘third-spacing’ of fluid may reduce drug concentrations, not only in plasma but also at the site of infection. Furthermore, hypoalbuminaemia is common among critically ill children, causing a relatively large fraction of unbound drug that is available to distribute to peripheral tissues, which mostly influences highly protein bound drugs (> 80% protein binding) [42, 81, 82]. These alterations in Vd mainly affect concentration-dependent antibiotics, such as aminoglycosides which require a high Cmax value for optimized bacterial killing [83, 84]. Also, inflammation and critical illness appear to downregulate drug metabolism, such as seen with CYP3A-mediated midazolam metabolism in critically ill children [85] and CYP2C9-mediated warfarin metabolism in critically ill adults [86].
In addition to changes in Vd, protein binding and drug metabolism, renal Cl of drugs can be altered during critical illness. AKI in critically ill children is primarily caused by reduced renal blood flow (e.g. due to volume depletion or decreased arterial blood pressure) and glomerular or tubular damage due to cytokine release, disseminated intravascular coagulation or nephrotoxic agents [87, 88]. AKI is common with a prevalence of around 35% in critically ill children, causing a reduced renal Cl and potential supratherapeutic or toxic concentrations of renally excreted drugs [89]. While a general picture of reduced target-attainment in critically ill children may arise from our review, several studies do report supratherapeutic plasma concentrations for some of the toxic antibiotics like aminoglycosides or glycopeptides in a small proportion of patients [41, 43, 53, 63].
This counter-intuitive finding of reduced exposure in critically ill children, may be explained by a high incidence of ARC. Contrary to AKI, ARC has been identified in up to 67% of both critically ill adults [8] and children [10]. This is in line with several studies in this review showing extremely high drug Cl and/or eGFR in this patient population [21, 26, 50, 52]. A recent review by Dhont et al. on ARC in children shows that time-dependent antibiotics, like β-lactam antibiotics, which require drug concentrations above an MIC-threshold for a prolonged period of time, could be at the highest risk for non-target attainment due to this phenomenon [9]. However, a correlation between ARC and subtherapeutic drug concentrations or clinical outcome could not be made due to multiple confounding factors that also contribute to subtherapeutic drug concentrations and adverse outcome of critically ill children.
Furthermore, age-related changes in the processes involved in disposition, metabolism and excretion of drugs may impact both Vd and Cl. Changes in Vd in neonates and young infants may results from a different body composition, with a higher proportion of body water compared to adults [11], mainly influencing hydrophilic compounds like the majority of antibiotic agents. Developmental changes affecting drug Cl include a lower (absolute) GFR and tubular excretion in neonates and children younger than 2 years of age [11]. Interestingly, when Cl values are weight-corrected the renal clearance reaches adult levels relatively quickly, within the first month of life [90]. In addition, weight-corrected GFR is almost 70% higher in 2–5 year old children than in adults while renal blood flow and tubular secretion stay relatively stable [91]. This relation between age and GFR might be contributed to the relatively larger kidney size in children compared to adults [90]. Since the majority of studies in this review had a median age of included patients between 1 and 5 years of age, this could be a possible explanation of the higher weight-corrected Cl compared to adult populations.
Lastly, although PD targets for antibiotic agents are partially drug-specific, determined by a drugs’ kill-characteristic, and partially defined by pathogenic susceptibility, defined as the MIC value, only the former is accounted for in dosing recommendations. While MIC-based dosing would seem to provide an improvement in the attainment of PD targets, there are numerous drawbacks to using MIC values in dosing guidelines as outlined by Mouton et al. [92]. In summary, the in vitro MIC assay is insufficiently accurate and reproducible to adequately represent conditions in vivo and therefore cannot be used as an exact concentration to strife for during treatment. Doing so possibly leads to an underestimation of the antibiotic effect in vivo [93, 94] or missed treatment options [95]. Secondly, the MIC that is used in the desired PD targets is regarded as static value but should be seen more as a distribution of MICs within a bacterial strain [92]. In addition, PD might also be affected by alterations in PK [96]. In their semi-mechanistic PK/PD model Nielsen et al. have simulated the effects of a wide range of dosing regimens of six antibiotics from in vitro time-kill curve experiments. The authors state that when the PK-profile of benzylpenicillin, a hydrophilic β-lactam antibiotic with a short t½, was used for other antibiotics, fT > MIC was still the best predictor of effect, even for antibiotics that are normally regarded as concentration-dependent or exposure-dependent. Lastly, the susceptibility of pathogens is heavily region-bound, making it difficult to extrapolate dosing advices between regions with different resistance patterns.
A logic next step seems to incorporate both age- and disease related co-variates in dosing guidelines using modelling and simulation. Most of the PopPK modelling studies in this review have incorporated (allometrically scaled) age and/or weight covariates in their final models [21, 25, 26, 28, 51, 52, 55, 57, 61, 65]. However, models incorporating (bio)markers of organ failure (e.g. eGFR, serum creatinin, albumin, C-reactive protein, transaminases) as covariate were limited [21, 25, 26, 28, 51, 52, 55, 57]. Of the 48 studies, only 5 PK models have incorporated eGFR in their estimations for drug Cl [21, 26, 51, 55, 57] and only 3 additional studies found a significant relation between serum creatinine and drug concentration [29, 45, 60]. Other studies have either not investigated this relationship or found the addition of creatinine clearance to be non-significant. This might reflect that serum creatinine is a suboptimal marker for GFR in this population and/or the impact of other factors than GFR on the variability in drug clearance. The inability to accurately estimate drug clearance using biomarkers is one of the fundamental challenges regarding optimizing target attainment in this patient population.
In order to overcome suboptimal target attainment of antibiotics in critically ill children clinicians have several options. Firstly, increasing the dose or dose frequency of an antibiotic could provide an easy solution to increase exposure and therefore increase target attainment. However, a linear dose increase might introduce increased toxicity and will not account for the extremely large within and between subject variability in PK seen in critically ill patients caused by pathophysiological changes, heterogeneity of underlying diseases and extracorporeal circuits seen in PICU patients. Secondly, continuous or extended infusion could be used for time-dependent antibiotics, like β-lactam antibiotics, in order to optimize dosing regimens to the established PK-PD relationship. Continuous or extended infusion has been proposed in almost all β-lactam modelling studies found in this review [21, 23–26, 28, 32, 35, 61] and is recommended in a recent guideline from the French Society of Anaesthesia and Intensive Care Medicine (SFAR) [97]. Additionally, clinicians can be supported by PK-PD software using a combination of PopPK models, Bayesian forecasting and TDM, to ensure optimal target attainment in special populations or individual patients. In the previously mentioned SFAR guideline the use of TDM is also recommend, including an overview of suggested PD targets for several β-lactam antibiotics [97]. However, although several software packages are available [5], successful clinical implementation requires close collaboration between clinicians and pharmacists. Additionally, it is still unknown whether optimizing target attainment of antibiotics will result in a clinical benefit in terms of survival or duration of ICU-stay. Although several meta-analyses and large clinical studies show contradicting evidence in adults [98–103], 1 smaller study showed a low mortality of only 4.2% in a selective pediatric sepsis cohort when TDM and non-standard dosing was applied, even after initial subtherapeutic antibiotic concentrations [3]. Ideally, large clinical trials aiming to identify whether this increased target attainment of antibiotics leads to a reduced morbidity and/or mortality should be conducted in both critically ill adult and pediatric patients.
Conclusion
This systematic review shows that the PK of most antibiotics is significantly altered during critical illness in children. For most drugs both Vd and Cl are increased, putting this population at increased risk for suboptimal target attainment. A second main finding in this review is the lack of knowledge of PK in critically ill children of several, frequently used antibiotic agents such as ceftriaxone, ceftazidime, penicillin, flucloxacillin and metronidazole. Finally, the majority of articles do not provide any dosing guidance for PICU patients, even if the necessary PK-parameters to simulate dosing regimens are presented in the paper.
Adequate antibiotic dosing of critically ill children is challenging, due to a wide range pathophysiological changes, developmental differences between different age groups and great within and between subject variability in PK. This challenging landscape requires close collaboration between clinicians, pharmacists and clinical pharmacologists, as expertise of all these parties is required for an accurate assessment of this patient population. This literature overview hopes to inspire both researchers and clinicians in this field to close these gaps, not only by presenting pharmacokinetic data, but also by providing guidance for implementation in the clinic, as this information is vital to optimize antibiotic treatment in this vulnerable population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with Ethical Standards
Funding
Financial support from institutions Radboudumc, Nijmegen, The Netherlands.
Conflict of interest
Stan J. F. Hartman, Roger J. Brüggemann, Lynn Orriëns, Nada Dia, Michiel F. Schreuder and Saskia N. de Wildt declare that they have no conflict of interest.
References
- 1.van Houten MA, Luinge K, Laseur M, Kimpen JL. Antibiotic utilisation for hospitalised paediatric patients. Int J Antimicrob Agents. 1998;10(2):161–164. doi: 10.1016/S0924-8579(98)00022-3. [DOI] [PubMed] [Google Scholar]
- 2.De Waele JJ, Lipman J, Akova M, Bassetti M, Dimopoulos G, Kaukonen M, et al. Risk factors for target non-attainment during empirical treatment with beta-lactam antibiotics in critically ill patients. Intensive Care Med. 2014;40(9):1340–1351. doi: 10.1007/s00134-014-3403-8. [DOI] [PubMed] [Google Scholar]
- 3.Cies JJ, Moore WS, 2nd, Enache A, Chopra A. Beta-lactam therapeutic drug management in the PICU. Crit Care Med. 2017 doi: 10.1097/ccm.0000000000002817. [DOI] [PubMed] [Google Scholar]
- 4.Tsai D, Lipman J, Roberts JA. Pharmacokinetic/pharmacodynamic considerations for the optimization of antimicrobial delivery in the critically ill. Curr Opin Crit Care. 2015;21(5):412–420. doi: 10.1097/MCC.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3–11. doi: 10.1016/j.addr.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49(1):1–16. doi: 10.2165/11318140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations*. Crit Care Med. 2014;42(3):520–527. doi: 10.1097/CCM.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 9.Dhont E, Van Der Heggen T, De Jaeger A, Vande Walle J, De Paepe P, De Cock PA. Augmented renal clearance in pediatric intensive care: are we undertreating our sickest patients? Pediatr Nephrol. 2018 doi: 10.1007/s00467-018-4120-2. [DOI] [PubMed] [Google Scholar]
- 10.Van Der Heggen T, Dhont E, Peperstraete H, Delanghe JR, Vande Walle J, De Paepe P, et al. Augmented renal clearance: a common condition in critically ill children. Pediatr Nephrol. 2019 doi: 10.1007/s00467-019-04205-x. [DOI] [PubMed] [Google Scholar]
- 11.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 12.Tängdén T, Ramos Martin V, Felton TW, Nielsen EI, Marchand S, Bruggemann RJ, et al. The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med. 2017;43(7):1021–1032. doi: 10.1007/s00134-017-4780-6. [DOI] [PubMed] [Google Scholar]
- 13.De Waele JJ, Carrette S, Carlier M, Stove V, Boelens J, Claeys G, et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: a randomised controlled trial. Intensive Care Med. 2014;40(3):380–387. doi: 10.1007/s00134-013-3187-2. [DOI] [PubMed] [Google Scholar]
- 14.Economou CJP, Wong G, McWhinney B, Ungerer JPJ, Lipman J, Roberts JA. Impact of beta-lactam antibiotic therapeutic drug monitoring on dose adjustments in critically ill patients undergoing continuous renal replacement therapy. Int J Antimicrob Agents. 2017;49(5):589–594. doi: 10.1016/j.ijantimicag.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Jager NG, van Hest RM, Lipman J, Taccone FS, Roberts JA. Therapeutic drug monitoring of anti-infective agents in critically ill patients. Expert Rev Clin Pharmacol. 2016;9(7):961–979. doi: 10.1586/17512433.2016.1172209. [DOI] [PubMed] [Google Scholar]
- 16.Wong G, Briscoe S, McWhinney B, Ally M, Ungerer J, Lipman J, et al. Therapeutic drug monitoring of beta-lactam antibiotics in the critically ill: direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J Antimicrob Chemother. 2018;73(11):3087–3094. doi: 10.1093/jac/dky314. [DOI] [PubMed] [Google Scholar]
- 17.MeSH Database. Anti-bacterial Agents [Pharmacological Action] https://www.ncbi.nlm.nih.gov/mesh/82000900. Accessed 01 Apr 2017.
- 18.Reed MD, Yamashita TS, Myers CM, Blumer JL. The pharmacokinetics of teicoplanin in infants and children. J Antimicrob Chemother. 1997;39(6):789–796. doi: 10.1093/jac/39.6.789. [DOI] [PubMed] [Google Scholar]
- 19.De Cock PA, Mulla H, Desmet S, De Somer F, McWhinney BC, Ungerer JP, et al. Population pharmacokinetics of cefazolin before, during and after cardiopulmonary bypass to optimize dosing regimens for children undergoing cardiac surgery. J Antimicrob Chemother. 2017;72(3):791–800. doi: 10.1093/jac/dkw496. [DOI] [PubMed] [Google Scholar]
- 20.Cies JJ, Moore WS, 2nd, Parker J, Stevens R, Al-Qaqaa Y, Enache A, et al. Pharmacokinetics of cefazolin delivery via the cardiopulmonary bypass circuit priming solution in infants and children. J Antimicrob Chemother. 2019 doi: 10.1093/jac/dky574. [DOI] [PubMed] [Google Scholar]
- 21.De Cock PA, Standing JF, Barker CI, de Jaeger A, Dhont E, Carlier M, et al. Augmented renal clearance implies a need for increased amoxicillin-clavulanic acid dosing in critically ill children. Antimicrob Agents Chemother. 2015;59(11):7027–7035. doi: 10.1128/AAC.01368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones AE, Barnes ND, Tasker TC, Horton R. Pharmacokinetics of intravenous amoxycillin and potassium clavulanate in seriously ill children. J Antimicrob Chemother. 1990;25(2):269–274. doi: 10.1093/jac/25.2.269. [DOI] [PubMed] [Google Scholar]
- 23.Nichols K, Chung EK, Knoderer CA, Buenger LE, Healy DP, Dees J, et al. Population pharmacokinetics and pharmacodynamics of extended-infusion piperacillin and tazobactam in critically ill children. Antimicrob Agents Chemother. 2015;60(1):522–531. doi: 10.1128/AAC.02089-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cies JJ, Shankar V, Schlichting C, Kuti JL. Population pharmacokinetics of piperacillin/tazobactam in critically ill young children. Pediatr Infect Dis J. 2014;33(2):168–173. doi: 10.1097/INF.0b013e3182a743c7. [DOI] [PubMed] [Google Scholar]
- 25.De Cock PA, van Dijkman SC, de Jaeger A, Willems J, Carlier M, Verstraete AG, et al. Dose optimization of piperacillin/tazobactam in critically ill children. J Antimicrob Chemother. 2017 doi: 10.1093/jac/dkx093. [DOI] [PubMed] [Google Scholar]
- 26.Béranger A, Benaboud S, Urien S, Moulin F, Bille E, Lesage F, et al. Piperacillin population pharmacokinetics and dosing regimen optimization in critically ill children with normal and augmented renal clearance. Clin Pharmacokinet. 2018 doi: 10.1007/s40262-018-0682-1. [DOI] [PubMed] [Google Scholar]
- 27.Von Hattingberg HM, Marget W, Belohradsky BH, Roos R. Pharmacokinetics of cefotaxime in neonates and children: clinical aspects. J Antimicrob Chemother. 1980;6(SUPPL.A):113–118. doi: 10.1093/jac/6.suppl_a.113. [DOI] [PubMed] [Google Scholar]
- 28.Béranger A, Oualha M, Urien S, Genuini M, Renolleau S, Aboura R, et al. Population pharmacokinetic model to optimize cefotaxime dosing regimen in critically ill children. Clin Pharmacokinet. 2018;57(7):867–875. doi: 10.1007/s40262-017-0602-9. [DOI] [PubMed] [Google Scholar]
- 29.Hartman SJF, Boeddha NP, Ekinci E, Koch BCP, Donders R, Hazelzet JA, et al. Target attainment of cefotaxime in critically ill children with meningococcal septic shock as a model for cefotaxime dosing in severe pediatric sepsis. Eur J Clin Microbiol Infect Dis. 2019 doi: 10.1007/s10096-019-03535-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olguin HJ, Asseff IL, Vieyra AC, Perez AG, Saldana NG, Quesada AC, et al. Effect of severity disease on the pharmacokinetics of cefuroxime in children with multiple organ system failure. Biol Pharm Bull. 2008;31(2):316–320. doi: 10.1248/bpb.31.316. [DOI] [PubMed] [Google Scholar]
- 31.Haessler D, Reverdy ME, Neidecker J, Brule P, Ninet J, Lehot JJ. Antibiotic prophylaxis with cefazolin and gentamicin in cardiac surgery for children less than ten kilograms. J Cardiothorac Vasc Anesth. 2003;17(2):221–225. doi: 10.1053/jcan.2003.51. [DOI] [PubMed] [Google Scholar]
- 32.Cies JJ, Moore WS, 2nd, Enache A, Chopra A. Ceftaroline for suspected or confirmed invasive methicillin-resistant staphylococcus aureus: a pharmacokinetic case series. Pediatr Crit Care Med. 2018 doi: 10.1097/pcc.0000000000001497. [DOI] [PubMed] [Google Scholar]
- 33.Forest Pharmaceutical IT T, Italy. Ceftaroline [package insert]. 2015.
- 34.Cies JJ, Moore WS, Calaman S, Brown M, Narayan P, Parker J, et al. Pharmacokinetics of continuous-infusion meropenem for the treatment of Serratia marcescens ventriculitis in a pediatric patient. Pharmacotherapy. 2015;35(4):E32–E36. doi: 10.1002/phar.1567. [DOI] [PubMed] [Google Scholar]
- 35.Cies JJ, Moore WS, 2nd, Enache A, Chopra A. Population pharmacokinetics and pharmacodynamic target attainment of meropenem in critically ill young children. J Pediatr Pharmacol Ther. 2017;22(4):276–285. doi: 10.5863/1551-6776-22.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du X, Li C, Kuti JL, Nightingale CH, Nicolau DP. Population pharmacokinetics and pharmacodynamics of meropenem in pediatric patients. J Clin Pharmacol. 2006;46(1):69–75. doi: 10.1177/0091270005283283. [DOI] [PubMed] [Google Scholar]
- 37.Blumer JL, Reed MD, Kearns GL, Jacobs RF, Gooch WM, 3rd, Yogev R, et al. Sequential, single-dose pharmacokinetic evaluation of meropenem in hospitalized infants and children. Antimicrob Agents Chemother. 1995;39(8):1721–1725. doi: 10.1128/aac.39.8.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giannoni E, Moreillon P, Cotting J, Moessinger A, Bille J, Decosterd L, et al. Prospective determination of plasma imipenem concentrations in critically ill children. Antimicrob Agents Chemother. 2006;50(7):2563–2568. doi: 10.1128/aac.01149-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cies JJ, LaCoursiere RJ, Moore WS, 2nd, Chopra A. Therapeutic drug monitoring of prolonged infusion aztreonam for multi-drug resistant Pseudomonas aeruginosa: a case report. J Pediatr Pharmacol Ther. 2017;22(6):467–470. doi: 10.5863/1551-6776-22.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bristol-Myers-Squibb. Aztreonam (Azactam) [package insert]. Revised Sep 2018.
- 41.Bonazza S, Bresee LC, Kraft T, Ross BC, Dersch-Mills D. Frequency of and risk factors for acute kidney injury associated with vancomycin use in the pediatric intensive care unit. J Pediatr Pharmacol Ther. 2016;21(6):486–493. doi: 10.5863/1551-6776-21.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Cock PA, Desmet S, De Jaeger A, Biarent D, Dhont E, Herck I, et al. Impact of vancomycin protein binding on target attainment in critically ill children: back to the drawing board? J Antimicrob Chemother. 2016 doi: 10.1093/jac/dkw495. [DOI] [PubMed] [Google Scholar]
- 43.Seixas GT, Araujo OR, Silva DC, Arduini RG, Petrilli AS. Vancomycin therapeutic targets and nephrotoxicity in critically ill children with cancer. J Pediatr Hematol Oncol. 2016;38(2):e56–e62. doi: 10.1097/MPH.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 44.Cies JJ, Shankar V. Nephrotoxicity in patients with vancomycin trough concentrations of 15–20 mug/ml in a pediatric intensive care unit. Pharmacotherapy. 2013;33(4):392–400. doi: 10.1002/phar.1227. [DOI] [PubMed] [Google Scholar]
- 45.Silva DC, Seixas GT, Araujo OR, Arduini RG, Carlesse FA, Petrilli AS. Vancomycin serum concentrations in pediatric oncologic/hematologic intensive care patients. Braz J Infect Dis. 2012;16(4):361–365. doi: 10.1016/j.bjid.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Giachetto GA, Telechea HM, Speranza N, Oyarzun M, Nanni L, Menchaca A. Vancomycin pharmacokinetic-pharmacodynamic parameters to optimize dosage administration in critically ill children. Pediatr Crit Care Med. 2011;12(6):e250–e254. doi: 10.1097/PCC.0b013e3181fe4047. [DOI] [PubMed] [Google Scholar]
- 47.Glover ML, Cole E, Wolfsdorf J. Vancomycin dosage requirements among pediatric intensive care unit patients with normal renal function. J Crit Care. 2000;15(1):1–4. doi: 10.1053/jcrc.2000.0150001. [DOI] [PubMed] [Google Scholar]
- 48.Gous AG, Dance MD, Lipman J, Luyt DK, Mathivha R, Scribante J. Changes in vancomycin pharmacokinetics in critically ill infants. Anaesth Intensive Care. 1995;23(6):678–682. doi: 10.1177/0310057X9502300603. [DOI] [PubMed] [Google Scholar]
- 49.Totapally BR, Machado J, Lee H, Paredes A, Raszynski A. Acute kidney injury during vancomycin therapy in critically ill children. Pharmacotherapy. 2013;33(6):598–602. doi: 10.1002/phar.1259. [DOI] [PubMed] [Google Scholar]
- 50.Goboova M, Kuzelova M, Kissova V, Bodakova D, Martisova E. An adjustment of vancomycin dosing regimen for a young patient with augmented renal clearance: a case report. Acta Fac Pharm Univ Comen. 2015;62(2):1–4. doi: 10.1515/afpuc-2015-0025. [DOI] [Google Scholar]
- 51.Zane NR, Reedy MD, Gastonguay MR, Himebauch AS, Ramsey EZ, Topjian AA, et al. A population pharmacokinetic analysis to study the effect of therapeutic hypothermia on vancomycin disposition in children resuscitated from cardiac arrest. Pediatr Crit Care Med. 2017;18(7):e290–e297. doi: 10.1097/PCC.0000000000001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avedissian SN, Bradley E, Zhang D, Bradley JS, Nazer LH, Tran TM, et al. Augmented renal clearance using population-based pharmacokinetic modeling in critically ill pediatric patients. Pediatr Crit Care Med. 2017 doi: 10.1097/pcc.0000000000001228. [DOI] [PubMed] [Google Scholar]
- 53.Thomas CA, Picone A, Menon S, Willis BC. Empiric vancomycin dosing in pediatric patients with congenital heart disease and the impact of cardiopulmonary bypass on trough concentrations. Pharmacotherapy. 2017 doi: 10.1002/phar.2019. [DOI] [PubMed] [Google Scholar]
- 54.Holsen MR, Meaney CJ, Hassinger AB, Fusco NM. Increased risk of acute kidney injury in critically ill children treated with vancomycin and piperacillin/tazobactam. Pediatr Crit Care Med. 2017;1:4. doi: 10.1097/pcc.0000000000001335. [DOI] [PubMed] [Google Scholar]
- 55.Genuini M, Oualha M, Bouazza N, Moulin F, Treluyer JM, Lesage F, et al. Achievement of therapeutic vancomycin exposure with continuous infusion in critically ill children. Pediatr Crit Care Med. 2018 doi: 10.1097/pcc.0000000000001474. [DOI] [PubMed] [Google Scholar]
- 56.Fitzgerald JC, Zane NR, Himebauch AS, Reedy MD, Downes KJ, Topjian AA, et al. Vancomycin prescribing and therapeutic drug monitoring in children with and without acute kidney injury after cardiac arrest. Paediatr Drugs. 2019 doi: 10.1007/s40272-019-00328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moffett BS, Resendiz K, Morris J, Akcan-Arikan A, Checchia PA. Population pharmacokinetics of vancomycin in the pediatric cardiac surgical population. J Pediatr Pharmacol Ther. 2019;24(2):107–116. doi: 10.5863/1551-6776-24.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lukas JC, Karikas G, Gazouli M, Kalabalikis P, Hatzis T, Macheras P. Pharmacokinetics of teicoplanin in an ICU population of children and infants. Pharm Res. 2004;21(11):2064–2071. doi: 10.1023/B:PHAM.0000048198.56873.d8. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez A, Lopez-Herce J, Cueto E, Carrillo A, Moral R. Teicoplanin pharmacokinetics in critically ill paediatric patients. J Antimicrob Chemother. 1999;44(3):407–409. doi: 10.1093/jac/44.3.407. [DOI] [PubMed] [Google Scholar]
- 60.Zakova M, Pong S, Trope A, Atenafu EG, Papaioannou V, Bitnun SA, et al. Dose derivation of once-daily dosing guidelines for gentamicin in critically ill pediatric patients. Ther Drug Monit. 2014;36(3):288–294. doi: 10.1097/FTD.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 61.Lopez SA, Mulla H, Durward A, Tibby SM. Extended-interval gentamicin: population pharmacokinetics in pediatric critical illness. Pediatr Crit Care Med. 2010;11(2):267–274. doi: 10.1097/PCC.0b013e3181b80693. [DOI] [PubMed] [Google Scholar]
- 62.Kraus DM, Dusik CM, Rodvold KA, Campbell MM, Kecskes SA. Bayesian forecasting of gentamicin pharmacokinetics in pediatric intensive-care unit patients. Pediatr Infect Dis J. 1993;12(9):713–718. doi: 10.1097/00006454-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Marik PE, Havlik I, Monteagudo FS, Lipman J. The pharmacokinetic of amikacin in critically ill adult and paediatric patients: comparison of once- versus twice-daily dosing regimens. J Antimicrob Chemother. 1991;27 Suppl C:81–89. doi: 10.1093/jac/27.suppl_c.81. [DOI] [PubMed] [Google Scholar]
- 64.Bressolle F, Gouby A, Martinez JM, Joubert P, Saissi G, Guillaud R, et al. Population pharmacokinetics of amikacin in critically ill patients. Antimicrob Agents Chemother. 1996;40(7):1682–1689. doi: 10.1128/AAC.40.7.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sherwin CM, Wead S, Stockmann C, Healy D, Spigarelli MG, Neely A, et al. Amikacin population pharmacokinetics among paediatric burn patients. Burns. 2014;40(2):311–318. doi: 10.1016/j.burns.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 66.Wagner BP, Pfenninger J. Once daily dosing of netilmicin in neonatal and pediatric intensive care. Intensive Care Med. 1994;20(5):365–367. doi: 10.1007/BF01720910. [DOI] [PubMed] [Google Scholar]
- 67.Akins RL, Haase MR, Levy EN. Pharmacokinetics of daptomycin in a critically ill adolescent with vancomycin-resistant enterococcal endocarditis. Pharmacotherapy. 2006;26(5):694–698. doi: 10.1592/phco.26.5.694. [DOI] [PubMed] [Google Scholar]
- 68.Morris S, Gould K, Ferguson LP. The use of daptomycin to treat methicillin-resistant Staphylococcus epidermidis bacteremia in a critically ill child with renal failure. J Pediatr Pharmacol Ther. 2017;22(4):300–303. doi: 10.5863/1551-6776-22.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antachopoulos C, Ilia S, Kadiltzoglou P, Baira E, Dokoumetzidis A, Gikas E, et al. Pharmacokinetics of daptomycin in critically ill pediatric patients. Antimicrob Agents Chemother. 2018;62:6. doi: 10.1128/aac.02462-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lipman J, Gous AG, Mathivha LR, Tshukutsoane S, Scribante J, Hon H, et al. Ciprofloxacin pharmacokinetic profiles in paediatric sepsis: how much ciprofloxacin is enough? Intensive Care Med. 2002;28(4):493–500. doi: 10.1007/s00134-002-1212-y. [DOI] [PubMed] [Google Scholar]
- 71.Malacarne P, Rossi C, Bertolini G, GiVi TIG. Antibiotic usage in intensive care units: a pharmaco-epidemiological multicentre study. J Antimicrob Chemother. 2004;54(1):221–224. doi: 10.1093/jac/dkh299. [DOI] [PubMed] [Google Scholar]
- 72.Abbas Q, Ul Haq A, Kumar R, Ali SA, Hussain K, Shakoor S. Evaluation of antibiotic use in Pediatric Intensive Care Unit of a developing country. Indian J Crit Care Med. 2016;20(5):291–294. doi: 10.4103/0972-5229.182197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carlier M, Stove V, Roberts JA, Van de Velde E, De Waele JJ, Verstraete AG. Quantification of seven beta-lactam antibiotics and two beta-lactamase inhibitors in human plasma using a validated UPLC-MS/MS method. Int J Antimicrob Agents. 2012;40(5):416–422. doi: 10.1016/j.ijantimicag.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 74.Roberts JA, Kirkpatrick CM, Roberts MS, Dalley AJ, Lipman J. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int J Antimicrob Agents. 2010;35(2):156–163. doi: 10.1016/j.ijantimicag.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Kafetzis DA, Brater DC, Kanarios J, Sinaniotis CA, Papadatos CJ. Clinical pharmacology of cefotaxime in pediatric patients. Antimicrob Agents Chemother. 1981;20(4):487–490. doi: 10.1128/aac.20.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krivoy N, Peleg S, Postovsky S, Ben Arush MW. Pharmacokinetic analysis of vancomycin in steady state in pediatric cancer patients. Pediatr Hematol Oncol. 1998;15(4):333–338. doi: 10.3109/08880019809014017. [DOI] [PubMed] [Google Scholar]
- 77.Chang D. Influence of malignancy on the pharmacokinetics of vancomycin in infants and children. Pediatr Infect Dis J. 1995;14(8):667–673. doi: 10.1097/00006454-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 78.Escobar L, Andresen M, Downey P, Gai MN, Regueira T, Borquez T, et al. Population pharmacokinetics and dose simulation of vancomycin in critically ill patients during high-volume haemofiltration. Int J Antimicrob Agents. 2014;44(2):163–167. doi: 10.1016/j.ijantimicag.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Llopis-Salvia P, Jimenez-Torres NV. Population pharmacokinetic parameters of vancomycin in critically ill patients. J Clin Pharm Ther. 2006;31(5):447–454. doi: 10.1111/j.1365-2710.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 80.Teelucksingh S, Padfield PL, Edwards CR. Systemic capillary leak syndrome. Q J Med. 1990;75(277):515–524. doi: 10.1093/oxfordjournals.qjmed.a068460. [DOI] [PubMed] [Google Scholar]
- 81.Wong G, Briscoe S, Adnan S, McWhinney B, Ungerer J, Lipman J, et al. Protein binding of beta-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother. 2013;57(12):6165–6170. doi: 10.1128/AAC.00951-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roberts JA, Stove V, De Waele JJ, Sipinkoski B, McWhinney B, Ungerer JP, et al. Variability in protein binding of teicoplanin and achievement of therapeutic drug monitoring targets in critically ill patients: lessons from the DALI study. Int J Antimicrob Agents. 2014;43(5):423–430. doi: 10.1016/j.ijantimicag.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 83.Mouton JW, den Hollander JG. Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 1994;38(5):931–936. doi: 10.1128/aac.38.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 85.Vet NJ, Brussee JM, de Hoog M, Mooij MG, Verlaat CW, Jerchel IS, et al. Inflammation and organ failure severely affect midazolam clearance in critically ill children. Am J Respir Crit Care Med. 2016;194(1):58–66. doi: 10.1164/rccm.201510-2114OC. [DOI] [PubMed] [Google Scholar]
- 86.Miners JO, Yang X, Knights KM, Zhang L. The role of the kidney in drug elimination: transport, metabolism, and the impact of kidney disease on drug clearance. Clin Pharmacol Ther. 2017;102(3):436–449. doi: 10.1002/cpt.757. [DOI] [PubMed] [Google Scholar]
- 87.Ashraf M, Shahzad N, Irshad M, Hussain SQ, Ahmed P. Pediatric acute kidney injury: a syndrome under paradigm shift. Indian J Crit Care Med. 2014;18(8):518–526. doi: 10.4103/0972-5229.138156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. doi: 10.1136/bmj.k4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11(4):R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen N, Aleksa K, Woodland C, Rieder M, Koren G. Ontogeny of drug elimination by the human kidney. Pediatr Nephrol. 2006;21(2):160–168. doi: 10.1007/s00467-005-2105-4. [DOI] [PubMed] [Google Scholar]
- 91.Hayton WL. Maturation and growth of renal function: dosing renally cleared drugs in children. AAPS Pharm Sci. 2000;2(1):E3. doi: 10.1208/ps020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mouton JW, Muller AE, Canton R, Giske CG, Kahlmeter G, Turnidge J. MIC-based dose adjustment: facts and fables. J Antimicrob Chemother. 2018;73(3):564–568. doi: 10.1093/jac/dkx427. [DOI] [PubMed] [Google Scholar]
- 93.Mouton JW. Soup with or without meatballs: impact of nutritional factors on the MIC, kill-rates and growth-rates. Eur J Pharm Sci. 2018;125:23–27. doi: 10.1016/j.ejps.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 94.Woksepp H, Hallgren A, Borgstrom S, Kullberg F, Wimmerstedt A, Oscarsson A, et al. High target attainment for beta-lactam antibiotics in intensive care unit patients when actual minimum inhibitory concentrations are applied. Eur J Clin Microbiol Infect Dis. 2016 doi: 10.1007/s10096-016-2832-4. [DOI] [PubMed] [Google Scholar]
- 95.Kumaraswamy M, Lin L, Olson J, Sun CF, Nonejuie P, Corriden R, et al. Standard susceptibility testing overlooks potent azithromycin activity and cationic peptide synergy against MDR Stenotrophomonas maltophilia. J Antimicrob Chemother. 2016;71(5):1264–1269. doi: 10.1093/jac/dkv487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nielsen EI, Cars O, Friberg LE. Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization. Antimicrob Agents Chemother. 2011;55(10):4619–4630. doi: 10.1128/AAC.00182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR) Crit Care. 2019;23(1):104. doi: 10.1186/s13054-019-2378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, Rai V, Wong KK, Hasan MS, et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016;42(10):1535–1545. doi: 10.1007/s00134-015-4188-0. [DOI] [PubMed] [Google Scholar]
- 99.Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin Infect Dis. 2013;56(2):272–282. doi: 10.1093/cid/cis857. [DOI] [PubMed] [Google Scholar]
- 100.Hao JJ, Chen H, Zhou JX. Continuous versus intermittent infusion of vancomycin in adult patients: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;47(1):28–35. doi: 10.1016/j.ijantimicag.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 101.Roberts JA, Abdul-Aziz MH, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, et al. Continuous versus intermittent beta-lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med. 2016;194(6):681–691. doi: 10.1164/rccm.201601-0024oc. [DOI] [PubMed] [Google Scholar]
- 102.Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med. 2009;37(6):2071–2078. doi: 10.1097/CCM.0b013e3181a0054d. [DOI] [PubMed] [Google Scholar]
- 103.Teo J, Liew Y, Lee W, Kwa AL. Prolonged infusion versus intermittent boluses of beta-lactam antibiotics for treatment of acute infections: a meta-analysis. Int J Antimicrob Agents. 2014;43(5):403–411. doi: 10.1016/j.ijantimicag.2014.01.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.