Abstract
The receptor tyrosine kinases (RTKs) are a large family of proteins that transduce extracellular signals to the inside of the cell to ultimately affect important cellular functions such as cell proliferation, survival, apoptosis, differentiation, and migration. They are expressed in the nervous system and can regulate behavior through modulation of neuronal and glial function. As a result, RTKs are implicated in neurodegenerative and psychiatric disorders such as depression and addiction. Evidence has emerged that 5 RTKs (tropomyosin-related kinase B (TrkB), RET proto-oncogene (RET), anaplastic lymphoma kinase (ALK), fibroblast growth factor receptor (FGFR), and epidermal growth factor receptor (EGFR)) modulate alcohol drinking and other behaviors related to alcohol addiction. RTKs are considered highly “druggable” targets and small-molecule inhibitors of RTKs have been developed for the treatment of various conditions, particularly cancer. These kinases are therefore attractive targets for the development of new pharmacotherapies to treat alcohol use disorder (AUD). This review will examine the preclinical evidence describing TrkB, RET, ALK, FGFR, and EGFR modulation of alcohol drinking and other behaviors relevant to alcohol abuse.
Keywords: Addiction, alcohol, kinases, ALK, FGFR, EGFR, RET, TrkB
Introduction
Alcohol use disorder (AUD) is a psychiatric disorder defined by uncontrolled excessive alcohol intake, a negative emotional state when not using alcohol, and a compulsion to drink. The lifetime and 12-month prevalence of AUD among adults 18 years and older in the USA in 2013 was 29.1% and 13.9%, respectively, demonstrating that AUD is highly prevalent [1]. Excessive alcohol use is costly on both an individual and societal level and was the 7th leading cause of death worldwide in 2016 [2]. Alcohol use increases the risk for developing cardiovascular disease, gastrointestinal disease, and cancer and is highly comorbid with other psychiatric disorders such as depression [1]. Reducing or abstaining from alcohol use can significantly improve health outcomes for individuals. There are currently four medications (disulfiram, acamprosate, and two formulations of naltrexone) approved by the US Food and Drug Administration (FDA) for the treatment of AUD [3]. Disulfiram inhibits the enzyme aldehyde dehydrogenase that is involved in the metabolism of alcohol, resulting in an accumulation of toxic acetaldehyde that produces nausea, vomiting, palpitations, and other adverse effects that act to prevent alcohol drinking. Naltrexone reduces binge drinking and heavy alcohol use by acting as an antagonist at the opioid receptors, and acamprosate is effective in helping individuals maintain abstinence, likely through modulation of the glutamatergic system. Although these medications are currently underutilized, their effect sizes are small, indicating that there is a great need to discover new pharmacotherapies to treat AUD.
An understanding of the underlying neurobiology that contributes to AUD is essential to developing new medications for this disorder. A conceptual framework describing the neural circuitry involved in the different stages of addiction has been proposed by Koob and Volkow [4]. The binge/intoxication stage involves brain regions involved in reward and motivation, such as the nucleus accumbens and ventral tegmental area (VTA). The withdrawal/negative affect stage is regulated by regions involved in mood and emotional regulation, such as the amygdala and structures of the extended amygdala, and the preoccupation/anticipation (or craving) stage is primarily controlled by frontal cortical areas involved in cognitive function and decision-making. Examination of the molecular and cellular mechanisms that drive both optimal and pathological activity of these brain regions will lead to the identification of new targets that can be used for the development of AUD pharmacotherapies. The receptor tyrosine kinases (RTKs) have been extensively studied for their roles in cancer, and these enzymes are also important for proper brain function during development and in adulthood. This review will examine the evidence for the RTKs in AUD and the currently available small-molecule agonists or antagonists of the RTKs that could conceivably be repurposed for the treatment of AUD.
Receptor Tyrosine Kinases
The RTKs are a superfamily of cell surface receptors that share common structural features, including an extracellular ligand-binding domain, a single transmembrane-spanning domain, a juxtamembrane domain, an intracellular tyrosine kinase domain, and a C-terminal tail. There are 58 members of this family in humans [5] and they are highly conserved between both vertebrate and invertebrate animals. The secreted growth factor ligands for RTKs bind to the extracellular domain and activate downstream signaling cascades [5]. Upon ligand binding, receptors dimerize and autophosphorylate specific tyrosine residues. This recruits scaffolding proteins containing phosphotyrosine-binding domains such as Src-homology 2 (SH2) and phosphotyrosine-binding (PTB) and the subsequent activation of many diverse signal transduction pathways [5, 6]. These pathways include, but are not limited to, the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT), the phospholipase C/protein kinase C (PLC/PKC), and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways. RTKs signal to promote diverse processes such as cellular proliferation, differentiation, survival, and migration.
RTKs are frequently mutated in cancer [6], rendering them constitutively active and leading to pathological cellular proliferation. RTKs are “druggable” targets like the G-protein coupled receptors and ion channels. Because of the importance of RTKs in oncogenesis, major efforts to develop small-molecule inhibitors and monoclonal antibody therapeutics targeting RTKs have advanced over the past two decades [7]. In the nervous system, RTKs have specialized cellular functions such as promoting neuronal differentiation, neurite outgrowth, and axon targeting. In adult animals, RTKs regulate structural and synaptic plasticity [8]. As a result, RTKs are important regulators of learning and memory, mood, and behavioral responses to drugs of abuse [8–10]. Although RTKs have been intensely studied for their roles in cancer, emerging evidence suggests that they can be targeted for neurodegenerative and psychiatric disorders including AUD. Specifically, modulation of the activity of tropomyosin-related kinase B (TrkB), RET proto-oncogene (RET), anaplastic lymphoma kinase (ALK), epidermal growth factor receptor (EGFR), and fibroblast growth factor receptor (FGFR) has been demonstrated to affect behaviors related to alcohol abuse in rodents and other species, suggesting that these RTK targets may yield valuable new therapeutics for AUD.
TrkB
TrkB, encoded by the Ntrk2 gene, is the high-affinity binding receptor for brain-derived neurotrophic factor (BDNF), neurotrophin 4, and neurotrophin 3 (Fig. 1), which are members of the nerve growth factor family [8, 11, 12]. TrkB is expressed in neurons and glia and is found in both the peripheral and central nervous systems [13]. In the brain, it is enriched in the cortex, hippocampus, and specific nuclei of the brainstem. Although homozygous Ntrk2 knockout mice survive to birth, they die as neonates due to an inability to feed because of defects in sensory and motor systems [14]. TrkB signaling is involved many processes such as synaptic plasticity, cell survival, and neurite outgrowth [9, 15] and aberrant TrkB signaling has been implicated in neurodegeneration, cancers, and neuropsychiatric disorders including AUD [9, 16].
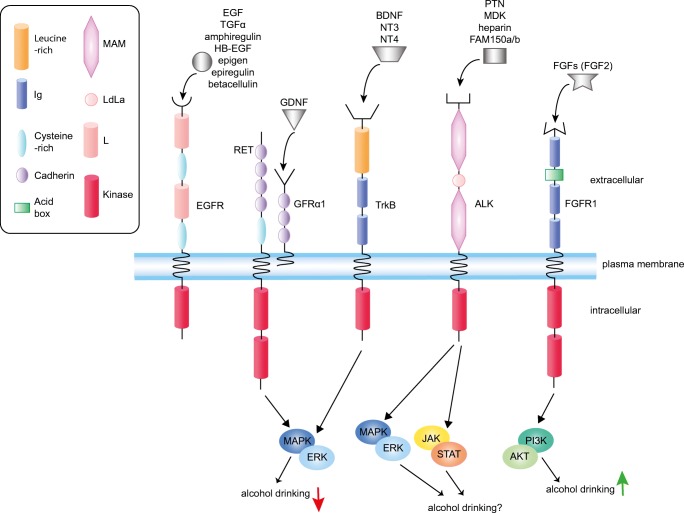
Fig. 1.
Receptor tyrosine kinases (RTKs) and their corresponding ligands that have been investigated for alcohol drinking in animals. The domain structure of each RTK is illustrated. Ligands for each receptor are indicated above the receptor. Also indicated are the relevant downstream signaling pathways involved in alcohol drinking. Note that MAPK/ERK and JAK/STAT signaling are activated in response to ethanol in an ALK-dependent manner in cell lines, but it is not yet known if these pathways are responsible for altering drinking in response to ALK activation. MAM = meprin, A-5 protein, and receptor protein tyrosine phosphatase mu domain; L = receptor L domain; LDLa = low-density lipoprotein receptor domain class A; Ig = immunoglobulin domain; EGF = epidermal growth factor; TGFα = transforming growth factor alpha; HB-EGF = heparin-binding EGF-like growth factor; EGFR = EGF receptor; RET = RET proto-oncogene; GDNF = glial-derived neurotrophic factor; GFRα1 = GDNF family receptor alpha 1; PTN = pleiotrophin; MDK = midkine; ALK = anaplastic lymphoma kinase; BDNF = brain-derived neurotrophic factor; NT3 = neurotrophin 3; NT4 = neurotrophin 4; TrkB = tropomyosin-related kinase B; FGFs = fibroblast growth factors; FGF2 = fibroblast growth factor 2; FGFR = FGF receptor; MAPK/ERK = mitogen-activated protein kinase/extracellular signal-regulated kinase; JAK/STAT = Janus kinase/signal transducer and activator of transcription; PI3K/AKT = phosphoinositide 3-kinase/protein kinase B
Inference that TrkB might be a therapeutic target for the treatment of AUD initially came from several studies on one of its ligands, BDNF. Bdnf gene and protein expression are altered by alcohol exposure both in vitro and in vivo in various brain regions of rats and mice [17–30]. Of note, Bdnf expression was increased in mouse dorsal striatum (DS) after voluntary ethanol intake and after a single binge ethanol drinking session and in rat DS after ethanol self-administration [19, 21, 28]. Direct manipulation of BDNF levels in the dorsolateral striatum (DLS) of rats bidirectionally altered ethanol self-administration, such that infusion of BDNF protein into the DLS reduced lever pressing for ethanol, whereas knockdown of Bdnf expression in the DLS using a virally administered short hairpin (sh)RNA increased ethanol self-administration [28, 31]. The ability of BDNF in the DLS to suppress ethanol self-administration was dependent on MAPK/ERK signaling, because co-infusion of BDNF with the MAPK/ERK inhibitor U0126 blocked the ability of BDNF to suppress ethanol self-administration [31]. Heterozygous Bdnf knockout mice consumed more ethanol than wild-type controls and exhibited enhanced ethanol conditioned place preference (CPP), which is a behavioral measure of the rewarding properties of ethanol [21, 32]. Conditional knockout of BDNF in mouse forebrain neurons increased intake of a sweetened ethanol solution, and knockdown of Bdnf in the DLS of rats using an shRNA increased excessive ethanol consumption in an intermittent access protocol [16]. Systemic injection of the receptor for activated C-kinase 1 (RACK1) protein, which increased Bdnf transcript in the DLS, decreased ethanol intake and preference in mice [21], and intra-DLS administration of RACK1 suppressed ethanol self-administration in rats [33]. Finally, in mice that had been binge drinking for 6 weeks, Bdnf expression was decreased in the prefrontal cortex (PFC) [19]. Similarly, in mice that were ethanol dependent due to chronic exposure to ethanol vapor, BDNF protein levels in the medial PFC were decreased [34]. Infusion of BDNF directly into the medial PFC or overexpression of Bdnf cDNA reduced the higher levels of drinking observed in ethanol-dependent mice, but had no effect on ethanol drinking in mice that were not ethanol-dependent [34].
In addition to its function in the DS and mPFC on behaviors related to AUD, BDNF in the amygdala plays an important role in ethanol intake and related anxiety. Infusion of an antisense oligonucleotide targeting Bdnf into the central or medial nucleus of the amygdala (CeA or MeA, respectively) increased ethanol intake and anxiety-like behavior in rats. BDNF levels in the rat CeA and MeA were decreased during withdrawal from chronic ethanol drinking [30], and infusion of BDNF into the CeA reduced anxiety-like behavior during ethanol withdrawal [30, 35]. Adolescent rats exposed to a chronic intermittent ethanol protocol (intraperitoneal [I.P.] injections of 2 g/kg ethanol once daily for 2 days, then no treatment for 2 days, repeated over a period of 2 weeks) exhibited decreased Bdnf expression in the CeA and MeA as adults, which was correlated with increased anxiety-like behavior and ethanol intake [36]. Finally, BDNF levels were lower in the amygdala of ethanol-naïve alcohol-preferring P rats, which are rats genetically selected for high alcohol preference that exhibit concomitant elevated anxiety-like behavior, when compared with ethanol-naïve nonalcohol-preferring NP rats [26, 37]. These data suggest that low BDNF is a predisposing risk factor for excessive drinking and anxiety. Acute administration of ethanol to P rats increased BDNF levels in the CeA and MeA, but not the basolateral amygdala, and had an associated anxiolytic effect [26, 30]. These effects were not observed in NP rats given an acute ethanol injection [26]. In general, in brain regions such as the DS, amygdala, and mPFC, acute ethanol exposure increases BDNF levels but after chronic ethanol exposure and withdrawal BDNF levels are reduced, contributing to vulnerability to excessive alcohol drinking. Collectively, these data indicate that BDNF in the DS, mPFC, and amygdala plays a behaviorally protective role by reducing ethanol drinking and anxiety-like behavior.
Manipulation of TrkB using both small-molecule agonists and antagonists impacts ethanol drinking in animals. Based on the results described above in which BDNF suppresses ethanol intake, it is logical to assume that an agonist of TrkB could be effective in reducing ethanol drinking, whereas an antagonist of TrkB might increase drinking. Systemic administration of LM22A-4 (Table 1), a TrkB agonist, was effective in decreasing compulsive ethanol consumption in a protocol in which ethanol was adulterated with bitter-tasting quinine. This was done in mice expressing an ortholog of the human polymorphism in BDNF (Met68) that results in decreased activity-dependent secretion of BDNF and increased compulsive-like drinking when compared with the Val68 variant [38]. Studies with TrkB antagonists have yielded mixed results. Systemic administration of the TrkB antagonist ANA-12 (Table 1) did not alter 2-bottle choice voluntary ethanol consumption in mice [39], whereas in another study it reduced both voluntary ethanol intake and binge-like ethanol consumption in the drinking in the dark test [40]. In contrast, systemic treatment with the nonselective kinase inhibitor K252a (Table 1), which also inhibits TrkB, increased voluntary ethanol consumption in Bdnf wild-type mice but was ineffective in heterozygous Bdnf knockout mice [33]. As mentioned above, systemic administration of RACK1, which increases Bdnf expression, suppressed ethanol consumption in mice [21]. K252a was able to block the RACK1-mediated suppression of ethanol intake in mice [33], indicating that the effect of RACK1 on ethanol drinking depends on kinase activity.
Table 1.
Compounds targeting RTKs that have been tested for effects on ethanol drinking
| Compound | Target (activity) | Effect on ethanol consumption | Species | Used clinically? | Route (dose) | Reference(s) |
|---|---|---|---|---|---|---|
| LMA22A-4 | TrkB (agonist) | Decrease (compulsive drinking) | Mouse (Met68 BDNF variant) | No | I.P. (100 mg/kg) | [38] |
| K252a | Pan-kinase (antagonist) | Increase | Mouse | No | I.P. (5 and 25 μg/kg) | [33] |
| ANA-12 | TrkB (antagonist) | Decrease or no effect | Mouse | No | I.P. (0.5 mg/kg) | [39, 40] |
| 7,8 DHF | TrkB (agonist) | No effect | Rat | Yes (dietary supplement) | I.P. (5 mg/kg) | [18] |
| Alectinib | ALK (antagonist) | Decrease | Mouse | Yes* | Oral (60 mg/kg) | [41] |
| NVP-TAE684 | ALK (antagonist) | Decrease | Mouse | No | Oral (10 mg/kg) | [41] |
| Anti-FGF2 antibody | FGF2 (neutralizing antibody) | Decrease | Rat | No | Intra-DMS (750 ng/0.75 μl per hemisphere) | [42] |
| Erlotinib | EGFR (antagonist) | Decrease | Rat | Yes† | I.P. (5, 20, and 40 mg/kg) | [43] |
| PD173074 | FGFR1 | Decrease |
Mouse Rat |
No |
Mouse, I.P. (5 and 15 mg/kg) Rat, intra-DMS (1 and 20 ng per hemisphere) |
[44] |
*FDA approved for the treatment of ALK+ non-small cell lung cancer
†FDA approved for the treatment of non-small cell lung cancer and pancreatic cancer
After chronic ethanol drinking, BDNF infusion into the DLS of rats was no longer able to suppress ethanol intake [45]. This may be due to an upregulation of the low-affinity receptor for BDNF, p75NTR, as knockdown of p75NTR in the DLS of rats using virally administered shRNA targeting p75NTR decreased ethanol intake after chronic drinking [45]. In addition, intra-DLS infusion of LM11A-31, a modulator of p75NTR signaling that blocks the interaction with neurotrophins such as BDNF, reduced binge ethanol drinking in mice [45]. These results indicate that there are neuroadaptations that occur in BDNF/TrkB/p75NTR signaling with repeated cycles of excessive alcohol consumption and withdrawal. The use of pharmacological interventions that activate TrkB signaling should be used with the potential caveat that they might not be effective because of increased brain p75NTR signaling in chronic drinkers.
The relevance of BDNF/TrkB signaling in AUD is provided by studies in humans. Polymorphisms in both the NTRK2 and BDNF genes have been associated with AUD [46–48], although this association does not always exist [49, 50]. Genetic variations in the NTRK2 gene in humans were associated with an AUD diagnosis that co-occurred with antisocial personality disorder (ASPD). Two distinct haplotypes were found to be enriched in AUD subjects, whereas another haplotype was found to be enriched in healthy control subjects suggesting that certain SNPs in the NTRK2 gene may confer resistance to AUD and or AUD/ASPD [46]. Reduced serum levels of BDNF have been detected in individuals with AUD. One study found that AUD subjects had lower serum BDNF levels compared with controls, which correlated with impaired cognitive functioning [51]. Another study found that although BDNF levels were not decreased in the serum of alcohol-dependent subjects during withdrawal, BDNF levels were negatively correlated with the severity of alcohol withdrawal [52]. In addition to decreased BDNF levels in the serum of individuals with AUD, mature BDNF and TrkB protein and mRNA were significantly reduced in the circulating lymphocytes of alcohol-dependent individuals, whereas pro-BDNF and p75NTR mRNA and protein were increased, indicating a dysregulation of the BDNF/TrkB/p75NTR system in alcohol dependence [53]. Notably, the levels of TrkB and mature BDNF protein were negatively correlated with average daily ethanol consumption, whereas the levels of pro-BDNF and p75NTR were positively correlated with daily ethanol intake [53]. Recent analysis of postmortem amygdala from individuals with AUD indicated an increase in an antisense transcript of BDNF and a corresponding decrease in BDNF gene expression specifically in individuals that started drinking during adolescence, suggesting that early-onset alcohol drinking either alters BDNF expression or that low levels of BDNF in the amygdala confers vulnerability to adolescent drinking [54].
The pharmacological manipulation of the BDNF/TrkB signaling pathway is of high interest in many fields. Although BDNF does not readily cross the blood–brain barrier [55], 7,8-dihydroxyflavone (7,8-DHF) a naturally occurring BDNF mimetic and potent TrkB agonist appears to do so [56]. 7,8-DHF has been shown to be protective in animal models of traumatic brain injury, neurodegenerative diseases, and psychiatric disorders including repeated binge drinking-induced depression [18, 57, 58]. Although binge ethanol consumption was not reduced in rats after systemic injection of 7,8-DHF [18], further studies of this compound are needed. Considering the overall beneficial effect of augmenting BDNF/TrkB signaling in reducing ethanol consumption, further investigation using a selective TrkB agonist, 7,8-DHF, or the 7,8-DHF derivative R13 with increased half-life, oral availability, and brain availability [59] should be conducted in multiple animal models of alcohol dependence and excessive drinking.
RET
RET is a receptor for a family of neurotrophic factors known as the glial-derived neurotrophic factors (GDNFs) that include GDNF, neurturin, artemin, and persephin (Fig. 1) [60]. Binding of GDNFs to one of four coreceptors, GDNF family receptor-α 1–4 (GFRα1-4), activates RET signaling. RET is expressed during development and plays a critical role in normal development, because homozygous Ret knockout mice die at birth. Ret knockout mice lack specific neurons in the sympathetic, parasympathetic, and enteric nervous systems and lack kidneys entirely or have severe kidney dysgenesis [61]. Gdnf knockout mice exhibit similar developmental phenotypes as Ret knockout mice [62], indicating that GDNF signaling through RET is important for the development of the kidneys and enteric neurons. Further evidence for the importance of this receptor–ligand interaction in development was demonstrated in explant cultures from Ret knockout mice in which GDNF-stimulated axonal outgrowth was abolished [63].
GDNF is an important neurotrophic factor for midbrain dopaminergic neurons [64]. Treatment of midbrain neuronal cultures with GDNF increased the number of dopamine neurons and stimulated dopaminergic neurite outgrowth [65], an effect that was absent in cultured midbrain neurons from Ret knockout mice [66]. Moreover, GDNF treatment in vivo prevented dopaminergic neuronal loss induced by neurotoxic agents [67, 68]. A recent study demonstrated that RET was essential for the neuroprotective effect of GDNF in dopamine neuron toxicity induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [69], which models the dopamine neuron loss observed in Parkinson’s disease. The protective and restorative effects of GDNF on dopamine neurons have provided the impetus to develop a stable form of GDNF that has better brain biodistribution for the treatment of Parkinson’s disease [70].
GDNF is the most well-studied member of the GDNF family in the context of AUD. Evidence that GDNF could be a potential regulator of alcohol consumption was first demonstrated in 2005 by He et al. [71]. Ibogaine is an alkaloid extract produced from an African shrub that has been reported to reduce addiction to multiple drugs of abuse [72]. He et al. found that systemic treatment of mice and rats with ibogaine increased the expression of Gdnf mRNA in the midbrain and that ibogaine treatment of a dopaminergic human neuroblastoma cell line, SHSY5Y, increased GDNF mRNA, secreted GDNF protein, and activation of the RET signaling pathway, as measured by increased association of RET with GFRα1 and RET tyrosine phosphorylation [71]. Rats treated systemically with the same dose of ibogaine that increased Gdnf mRNA in the midbrain reduced their ethanol drinking in the home cage and decreased lever pressing for ethanol in an operant procedure (self-administration), indicating that ibogaine altered the motivation to consume ethanol. Ibogaine also blocked lever pressing for ethanol in a reinstatement test after extinction, suggesting that it has the potential to reduce relapse to alcohol drinking. The ability of ibogaine to reduce ethanol self-administration was at least partially dependent on increased VTA GDNF levels, because infusion of a blocking antibody to GDNF directly into the VTA was able to attenuate the reduction in ethanol self-administration by ibogaine [71]. Despite its anti-addiction properties, ibogaine has several unappealing side effects including hallucination, bradycardia, ataxia, and tremor. It is also classified as a schedule I category narcotic. Noribogaine, an active metabolite of ibogaine, and 18-MC, a synthetic ibogaine derivative, are two compounds that closely resemble and have similar properties to ibogaine. Carnicella et al. found that rats infused with noribogaine, but not 18-MC, into the VTA reduced operant ethanol self-administration [73]. In SHSY-5Y cells, noribogaine more potently induced Gdnf gene expression compared with 18-MC [73]. The effectiveness of noribogaine to increase Gdnf levels may explain, in part, the difference in the ability of these two drugs to modulate drinking behavior.
Similar to the results obtained with ibogaine, treatment of rats systemically with cabergoline, a dopamine D2 receptor-like agonist used for the treatment of hyperprolactinemia and Parkinson’s disease [74], increased Gdnf mRNA expression in the midbrain and decreased operant ethanol self-administration [75]. Cabergoline also reduced responding for ethanol during extinction sessions and decreased reinstatement to ethanol self-administration. Direct infusion of cabergoline into the VTA of rats reduced ethanol self-administration, indicating that the VTA is a site of action for cabergoline in reducing ethanol seeking [75]. Systemic injection of cabergoline in mice dose-dependently reduced voluntary ethanol drinking [75]. Studies in human dopaminergic SHSY5Y cells demonstrated that cabergoline increased GDNF mRNA, secreted GDNF, and RET phosphorylation [75]. One intracellular signaling pathway that is activated by GDNF/RET is the MAPK/ERK pathway. Cabergoline treatment increased phosphorylated ERK in rat midbrain and in SHSY5Y cells at the same time as the observed increase in RET phosphorylation [75]. The findings with both ibogaine and cabergoline suggest that these drugs are acting through GDNF/RET signaling in the VTA to decrease ethanol drinking in rats and mice.
Direct evidence that activation of GDNF/RET signaling in the VTA reduces alcohol drinking was provided by experiments in which GDNF was infused into the VTA of rats. Intra-VTA GDNF reduced operant ethanol self-administration [71, 75, 76], without affecting sucrose self-administration, and reduced reinstatement of ethanol seeking after extinction [76], suggesting that it may be useful in preventing relapse to alcohol drinking. The ability of intra-VTA GDNF to suppress ethanol self-administration was blocked by intra-VTA infusion of a MAPK/ERK inhibitor, U0126 [76], indicating that GDNF/RET signaling through the MAPK/ERK pathway is responsible for the effect on ethanol self-administration. Infusion of GDNF into rat VTA also decreased home cage ethanol drinking in an intermittent access schedule that promotes high levels of drinking [77, 78]. The effect of intra-VTA GDNF on drinking was sustained for at least 24 h and was blocked by either a protein synthesis inhibitor or downregulation of Gdnf using RNA interference [78]. The ability of an acute infusion of GDNF into the VTA to prolong the suppression of ethanol consumption is attributed to a positive self-regulatory mechanism in which GDNF increases the expression of its mRNA through activation of RET and MAPK/ERK signaling [78, 79]. In summary, GDNF in the VTA is able to reduce ethanol drinking in rats in models of ethanol seeking, relapse, and excessive drinking.
GDNF in the VTA may reduce ethanol drinking by decreasing the rewarding properties of ethanol. Studies in heterozygous Gdnf knockout mice and in rats infused with GDNF in the VTA indicate a role for GDNF in limiting ethanol reward. Gdnf heterozygous knockout mice exhibited increased ethanol CPP, whereas rats infused with GDNF in the VTA reduced both the acquisition and expression of ethanol CPP [80, 81]. The effects of GDNF on ethanol consumption and reward might be due to its ability to increase the excitability of dopamine neurons in the VTA. GDNF applied to cultured midbrain neurons and brain slices increased the evoked firing of dopamine neurons by inhibiting A-type potassium channels through a MAPK/ERK-dependent mechanism [82], and GDNF infused into the rat VTA increased the spontaneous firing of dopamine neurons in a MAPK/ERK-dependent manner [83]. The GDNF-mediated increase in firing of VTA dopamine neurons resulted in increased dopamine release in the nucleus accumbens [83], which was dependent on MAPK/ERK signaling. Interestingly, endogeneous GDNF appears to be produced in the nucleus accumbens and retrogradely transported to the VTA, where it increases the spontaneous firing of dopamine neurons, because knockdown of GDNF in the rat nucleus accumbens using a virally delivered shRNA to Gdnf reduced the spontaneous firing of VTA dopamine neurons, whereas infusion of GDNF into the nucleus accumbens increased the firing rate and ERK phosphorylation in VTA dopamine neurons [83]. Thus, although GDNF protects against ethanol-induced apoptosis [84, 85], it is hypothesized that the mechanism by which GDNF suppresses ethanol consumption and reward is not by reducing the toxic effects of ethanol per se but rather on increasing VTA dopamine neuron activity.
During withdrawal from chronic ethanol drinking, dopamine neuron firing is reduced, causing a hypodopaminergic state. This hypodopaminergic state is associated with anhedonia and depression, which may drive relapse to drinking as a means to restore dopamine tone and a normal emotional state [4]. Rats exposed to a chronic intermittent ethanol drinking protocol had decreased dopamine levels in the nucleus accumbens 24 h after the last drinking session and infusion of GDNF into the VTA restored dopamine tone [80]. Notably, GDNF expression is also decreased in the VTA of rats after withdrawal from chronic intermittent ethanol drinking and knockdown of Gdnf in the VTA of ethanol-naïve rats promotes increased drinking [86]. These results indicate that there is a dysregulation of GDNF expression after chronic drinking that may drive excessive ethanol intake and that restoring GDNF in the VTA is a means to curb drinking. Notably, one study demonstrated that alcohol-dependent patients had reduced serum levels of GDNF [52], pointing to the clinical relevance of GDNF/RET signaling in AUD.
Although efforts are being made to develop more brain bioavailable versions of GDNF, these would still need to be delivered directly to the brain parenchyma [70]. Another possible method of increasing GDNF/RET signaling in the brain would be through small-molecule GDNF-mimetics that bind to the GFRα1 coreceptor and activate RET signaling. One such compound, BT13, activates RET signaling in neuronal cells in culture, promotes neurite outgrowth, and has been demonstrated to reduce neuropathic pain in rats after systemic injection [87]. Other compounds are in development based on the structure of BT13 [88]. Future studies should examine the ability of these compounds to reduce ethanol drinking in animals.
ALK
ALK was discovered as the causative oncogene in anaplastic large-cell non-Hodgkin’s lymphoma, in which it is fused to nucleophosmin through a chromosomal translocation that renders it constitutively active [89]. Since then many activating mutations of ALK have been found and implicated in different types of cancers [90]. Ligands of ALK include midkine (MDK), pleiotrophin (PTN), heparin, ALK and LTK ligand 1 (ALKAL1 or FAM150a), and ALKAL2 (or FAM150b) (Fig. 1) [91–94], although it is not clear which ligands activate ALK signaling in the adult brain. In addition, PTN and MDK bind to and inactivate a protein tyrosine phosphatase, RPTPβ/ζ [95–97]. ALK is a substrate for RPTPβ/ζ; thus, inactivation of this phosphatase by PTN or MDK indirectly activates ALK by increasing its phosphorylation [95]. Alk knockout mice are viable and fertile and exhibit subtle hippocampus-dependent behavioral phenotypes as adults, such as enhanced spatial and novel object recognition memory and decreased depression-like behavior [98, 99]. Alk knockout mice also have less gonadotrophin-releasing hormone (GnRH) neurons in the hypothalamus, which results in decreased circulating testosterone levels and hypogonadotropic hypogonadism [100].
The first evidence that ALK could be involved in AUD came from studies in the fruit fly, Drosophila melanogaster. A loss-of-function mutation in ALK rendered flies less sensitive to the acute sedating effect of ethanol [101]. Parallel studies in mice demonstrated that mice deficient in ALK took longer to recover from ethanol-induced sedation [101]. In addition, Alk knockout mice consumed more ethanol in a voluntary 2-bottle choice ethanol consumption test, in a binge drinking test, and in an operant self-administration procedure [101–103]. Interestingly, although Alk knockout mice consumed more ethanol than Alk wild-type mice, they did not display escalated drinking typically observed after ethanol dependence induced by chronic ethanol vapor exposure [103].
In contrast to what was observed in the Alk knockout mice, systemic inhibition of ALK in adult mice using the ALK inhibitors, NVP-TAE684 and alectinib (Table 1), caused mice to drink less ethanol in a binge drinking test [41], without having any effect on sucrose drinking, suggesting a selective effect of ALK inhibition on ethanol intake. Treatment with the ALK inhibitors did not alter ethanol-induced sedation or ethanol clearance [41]. Furthermore, local knockdown of Alk using a virally delivered shRNA in the VTA, but not the nucleus accumbens, reduced binge ethanol consumption in mice [41]. The opposite phenotypes in ethanol drinking between Alk knockout mice and treatment with the ALK inhibitors may be explained by compensatory mechanisms in Alk knockout mice, which are deficient in ALK throughout embryonic development. Analysis of MAPK/ERK signaling in the Alk knockout mice indicated that they have increased phosphorylated ERK in the brain compared with Alk wild-type mice [101], providing potential evidence that there is overcompensation in the Alk knockout mice. In addition to decreasing ethanol intake in mice, ALK inhibition by TAE684 decreased ethanol CPP [41], indicating that ALK inhibition may reduce binge drinking by attenuating the rewarding properties of ethanol. Overall, the effect of ALK inhibitors on reducing ethanol drinking in mice suggest that they could be used as a therapeutic approach to reduce drinking in humans with AUD. Polymorphisms in ALK were associated with ethanol-induced ataxia, subjective high, and alcohol dependence [101, 104], suggesting that ALK signaling may be relevant in humans with AUD.
Mechanisms by which ALK regulates ethanol drinking are not known, although ALK is expressed in dopamine neurons in the VTA and was found to regulate the desensitization of the dopamine D2 autoreceptor [41]; therefore, the effects of ALK inhibitors on reducing binge drinking and ethanol CPP could be due to modulation of dopamine neuron activity. ALK is also implicated in regulating both GABAergic and glutamatergic neurotransmission. Basal and ethanol-stimulated GABA release were enhanced in the CeA of Alk knockout mice [103]. After chronic ethanol vapor exposure, wild-type mice exhibit an augmented basal GABA release that has been linked to escalated drinking [105]. However, after chronic ethanol vapor exposure, Alk knockout mice did not demonstrate this enhancement in GABA release, which might explain the lack of escalated drinking in Alk knockout mice after the induction of ethanol dependence [103]. Alk knockout mice also had enhanced spontaneous excitatory neurotransmission and reduced long-term depression in nucleus accumbens dopamine D1 receptor-expressing neurons [102]. More experiments are required to understand the relevant mechanisms by which ALK regulates binge and dependence-related alcohol drinking.
Acute ethanol treatment activated ALK signaling in neuroblastoma cell lines, increasing the phosphorylation of both ERK and STAT3 in an ALK-dependent manner [106, 107]. Activation of ALK signaling in response to ethanol in vitro was dependent on the presence of its ligand MDK [107]. Interestingly, Mdk knockout mice also exhibited altered behavioral responses to ethanol, demonstrating ethanol CPP at low doses of ethanol that do not normally induce CPP, increased sensitivity to ethanol-induced ataxia, and increased ethanol intake in both moderate (2-bottle choice) and binge drinking (drinking in the dark) tests [108, 109]. Local knockdown of Mdk in the mouse VTA also increased binge-like drinking [108].
As indicated above, both MDK and PTN are endogenous inhibitors of RPTPβ/ζ which dephosphorylates and inactivates ALK [95–97]. It is possible that ethanol-induced activation of ALK signaling through MDK is indirectly caused by MDK binding to and inactivating RPTPβ/ζ. Of note, systemic treatment of mice with the RPTPβ/ζ inhibitor, MY10 [110], decreased binge ethanol consumption, reduced ethanol CPP, and enhanced the sedative effect of ethanol [106, 111]. Treatment of neuroblastoma cells with MY10 or ethanol increased ALK phosphorylation as expected, but combined treatment of cells with MY10 and ethanol blocked the ethanol-induced increase in ALK phosphorylation [106], which might explain how MY10 is able to reduce drinking even though it is an inhibitor of RPTPβ/ζ. In total, these results indicate a complicated relationship between MDK, ALK, and RPTPβ/ζ signaling in response to ethanol exposure and in alcohol drinking. More experiments are needed to sort out the in vivo relationship between these proteins and other ALK ligands. Nonetheless, the ALK inhibitor, alectinib, which was FDA approved for the treatment of non-small cell lung cancer in 2017, has shown promise preclinically in reducing alcohol drinking and is a potential candidate for clinical studies. In addition, other ALK inhibitors such as lorlatinib and ceritinib, which are able to cross the blood–brain barrier, could be investigated for their role in ethanol drinking.
FGFR and EGFR
FGFRs are receptors for the fibroblast growth factors (FGFs; Fig. 1). There are 18 mammalian secreted FGFs that bind to 4 FGFRs (FGFR1-4) and 4 intracellular FGFs that interact with voltage-gated sodium channels and other proteins [112]. Each of the secreted FGFs associates with multiple FGFRs, resulting in much complexity in the biological function of FGFs and their receptors. Adding to this is that alternative splicing of the FGFR genes in exons encoding the extracellular immunoglobulin-like domains occurs. Differential exon use in the extracellular domain alters the affinity of FGFs with their receptors [112]. FGFs are also bound to heparin sulfate and heparin sulfate proteoglycans, constituents of the extracellular matrix that function as cofactors for FGF binding to their receptors [113–116]. The first discovered FGF was FGF2, which is also known as basic FGF (bFGF). As indicated by its name, FGF2 was originally isolated because of its ability to stimulate the proliferation of fibroblasts [117], but this growth factor has multiple functions in the nervous system, including promoting proper cerebral cortex development [118].
Regarding the role of FGF2 in AUD, Fgf2 mRNA expression was increased in the dorsomedial striatum (DMS) of both mice and rats after 5 weeks of drinking ethanol in an intermittent access schedule [42]. The effect was specific to the DMS, because no change in Fgf2 mRNA was observed in the DLS. Importantly, ethanol drinking was bidirectionally regulated by FGF2. Systemic injection of FGF2 into mice or infusion of FGF2 into the DMS of rats increased ethanol intake and preference, whereas infusion of an FGF2 neutralizing antibody (Table 1) into the rat DMS decreased ethanol drinking [42]. The regulation of FGF2 on ethanol consumption and preference relies on the downstream activation of the PI3K/AKT pathway as pre-infusion of PI3K inhibitor, wortmannin, into the DMS of rats prior to FGF2 blocked the ability of FGF2 to increase drinking [44]. The MAPK/ERK inhibitor, U0126, did not block ability of FGF2 to increase drinking, indicating that activating the PI3K pathway and not the MAPK pathway promotes drinking downstream of FGFR activation [44]. The effect of FGF2 on drinking is specific to ethanol, because systemic FGF2 injections into mice did not alter drinking of sucrose or saccharin solutions [42]. Evidence that FGFR1 might be involved in AUD was demonstrated by Even-Chen and Barak, who found that 7 days of 2.5 g/kg ethanol injections in mice increased Fgfr1 gene expression in the DS and dorsal hippocampus 24 h after the final injection. Similar to what was observed with FGF2, Fgfr1 gene expression was increased in the DMS of mice after 5 weeks of voluntary ethanol consumption using an intermittent access 2 bottle-choice protocol [44]. Treatment with the FGFR1 inhibitor, PD173074 (which also inhibits FGFR3 and FGFR4), via systemic injection in mice or by direct infusion into the DMS of rats decreased ethanol consumption and preference in the intermittent access 2-bottle choice test (Table 1) [44]. PD173074 is not currently clinically approved, but second-generation pan-FGFR antagonists such as AZD4547, BGJ398, LY2874455, and JNJ-42756493 (erdafitinib) that are selective for FGFR over other tyrosine kinases are currently in clinical trials for cancer treatment [119, 120]. For example, BGJ398 (infigratinib) has been tested for its efficacy in treating glioblastoma, suggesting that it can cross the blood–brain barrier and may be a candidate for treatment of AUD. Future experiments should evaluate the utility of these FGFR inhibitors for their ability to decrease alcohol drinking in animals.
EGFR (also known as ERBB or ERBB1) is a receptor for epidermal growth factor (EGF; Fig. 1) and was the first RTK identified [121]. There are four members of the EGFR RTK subfamily, which in addition to EGFR includes ERBB2, ERBB3, and ERBB4. In addition to binding EGF, EGFR is also a receptor for 6 other ligands (TGFα, amphiregulin, heparin-binding (HB)-EGF-like growth factor, epigen, epiregulin, and betacellulin) [122]. Knockout of EGFR is lethal at different times depending on the mouse background strain [123]. EGFR knockout mice in the CD-1 background exhibit defects in the skin, kidney, liver, brain, and gastrointestinal tract and only live up to 3 weeks, indicating the importance of EGFR in the normal development of several organ systems. Mutations in EGFR have long been known to be associated with lung cancer and in fact several inhibitors of EGFR have been developed and are in clinical use for the treatment of lung cancer [122]. In the adult nervous system, EGF is expressed in neurons of the cerebral cortex, hippocampus, and cerebellum [124]. EGF promotes the proliferation of astrocytes and is also neurotrophic [124].
Evidence that EGFR may play a role in AUD emerged from studies in the fruit fly, Drosophila melanogaster. A genetic screen for sensitivity to ethanol-induced sedation in Drosophila determined that a mutation in a gene called happyhour (hppy) decreased sensitivity to ethanol-induced sedation [43]. Hppy encodes a serine/threonine kinase that is homologous to the mammalian GCK-1 family of kinases and was found to be an inhibitor of EGFR signaling in the Drosophila eye [43]. Genetic manipulation of EGFR signaling bidirectionally altered ethanol-induced sedation in Drosophila, with increased EGFR signaling causing resistance and decreased EGFR signaling resulting in sensitivity [43]. Notably, flies given food containing the EGFR inhibitor erlotinib or gefitinib increased their sensitivity to ethanol-induced sedation [43]. To determine the translational relevance of these findings, the authors went on to demonstrate that a systemic injection of erlotinib in mice increased sensitivity to ethanol-induced sedation and that systemic injection of erlotinib (Table 1) in rats reduced voluntary ethanol intake in the home cage using a continuous access 2-bottle choice protocol [43]. Erlotinib treatment in rats did not alter sucrose drinking or water intake, indicating a selective effect on ethanol intake. These results suggest that EGFR inhibitors could be used to reduce drinking in humans, but additional preclinical studies in models of excessive drinking in rodents are necessary. Interestingly, single-nucleotide polymorphisms in the EGF gene were associated with alcohol dependence in the Irish Affected Sib-Pair Study of Alcohol Dependence [125], providing evidence in humans that EGFR signaling may be important in AUD.
Conclusions
Behavioral studies in rats and mice indicate that pharmacological manipulation of the RTKs TrkB, RET, ALK, FGFR, and EGFR alters alcohol drinking. Activation of TrkB by BDNF and RET by GDNF is protective, in that they reduce alcohol consumption, whereas inhibition of ALK, FGFR, and EGFR reduces alcohol drinking. Differences in neuroanatomical sites of action and activation of downstream signaling pathways have been discovered for these RTKs. BDNF/TrkB signaling suppresses drinking through its action in the DLS, CeA, and mPFC. At least in the DLS, suppression of alcohol drinking by BDNF is mediated by activation of the MAPK/ERK pathway. GDNF/RET signaling reduces alcohol drinking through its action in the VTA, also through engagement of MAPK/ERK signaling. In contrast, FGF2 increases drinking by acting in the DMS and activating the PI3K pathway, likely through engagement of FGFR1, because an FGFR1 inhibitor decreases drinking. Finally, reducing ALK expression in the VTA decreases alcohol drinking, but it remains to be determined which pathways activated by ALK in response to ethanol in vivo are responsible for the behavioral effects.
Inhibitors of ALK (alectinib) and EGFR (erlotinib) that are efficacious in reducing drinking in either mice or rats are already FDA approved for the treatment of cancers and could conceivably be repurposed for treatment of AUD. The safety profiles (especially liver toxicity) of these compounds must be taken into consideration when used in individuals with AUD because of the comorbidity with liver disease. More work is clearly necessary in order to develop agonists to TrkB and RET and selective antagonists to FGFR1 that might be used in the clinic. Nonetheless, these kinases represent attractive therapeutic targets for the treatment of AUD. In addition, given that there are 58 members of the RTK family, other RTK targets for AUD are likely to emerge in the future from preclinical studies.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (INIA consortium grant U01 AA020912 and the Center for Alcohol Research in Epigenetics grant P50 AA022538). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grant BF, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators GBDA. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA. 2018;320(8):815–824. doi: 10.1001/jama.2018.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–73. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17(1):58. doi: 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaoka, T., et al., Receptor tyrosine kinase-targeted cancer therapy. Int J Mol Sci, 2018. 19(11). [DOI] [PMC free article] [PubMed]
- 8.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 9.Gupta VK, et al. TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders. Int J Mol Sci. 2013;14(5):10122–42. doi: 10.3390/ijms140510122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner CA, et al. Dysregulated fibroblast growth factor (FGF) signaling in neurological and psychiatric disorders. Semin Cell Dev Biol. 2016;53:136–43. doi: 10.1016/j.semcdb.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R, et al. The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron. 1992;8(5):947–56. doi: 10.1016/0896-6273(92)90209-V. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, et al. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991;66(2):395–403. doi: 10.1016/0092-8674(91)90628-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein R, et al. Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development. 1990;109(4):845–50. doi: 10.1242/dev.109.4.845. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, et al. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75(1):113–22. doi: 10.1016/S0092-8674(05)80088-1. [DOI] [PubMed] [Google Scholar]
- 15.Deinhardt K, Chao MV. Trk receptors. Handb Exp Pharmacol. 2014;220:103–19. doi: 10.1007/978-3-642-45106-5_5. [DOI] [PubMed] [Google Scholar]
- 16.Logrip ML, et al. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015;1628(Pt A):60–7. doi: 10.1016/j.brainres.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alele PE, Devaud LL. Expression of cFos and brain-derived neurotrophic factor in cortex and hippocampus of ethanol-withdrawn male and female rats. J Pharmacol Pharmacother. 2013;4(4):265–74. doi: 10.4103/0976-500X.119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briones TL, Woods J. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience. 2013;254:324–34. doi: 10.1016/j.neuroscience.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logrip ML, Janak PH, Ron D. Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem. 2009;109(5):1459–68. doi: 10.1111/j.1471-4159.2009.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacLennan AJ, Lee N, Walker DW. Chronic ethanol administration decreases brain-derived neurotrophic factor gene expression in the rat hippocampus. Neurosci Lett. 1995;197(2):105–8. doi: 10.1016/0304-3940(95)11922-J. [DOI] [PubMed] [Google Scholar]
- 21.McGough NN, et al. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24(46):10542–52. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MW. Repeated episodic exposure to ethanol affects neurotrophin content in the forebrain of the mature rat. Exp Neurol. 2004;189(1):173–81. doi: 10.1016/j.expneurol.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Miller MW, Mooney SM. Chronic exposure to ethanol alters neurotrophin content in the basal forebrain-cortex system in the mature rat: effects on autocrine-paracrine mechanisms. J Neurobiol. 2004;60(4):490–8. doi: 10.1002/neu.20059. [DOI] [PubMed] [Google Scholar]
- 24.Somkuwar SS, et al. Alcohol dependence-induced regulation of the proliferation and survival of adult brain progenitors is associated with altered BDNF-TrkB signaling. Brain Struct Funct. 2016;221(9):4319–4335. doi: 10.1007/s00429-015-1163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tapia-Arancibia L, et al. Effects of alcohol on brain-derived neurotrophic factor mRNA expression in discrete regions of the rat hippocampus and hypothalamus. J Neurosci Res. 2001;63(2):200–8. doi: 10.1002/1097-4547(20010115)63:2<200::AID-JNR1012>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Moonat S, et al. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16(2):238–50. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moonat S, et al. Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry. 2013;73(8):763–73. doi: 10.1016/j.biopsych.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeanblanc J, et al. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29(43):13494–502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You C, et al. Reversal of deficits in dendritic spines, BDNF and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. Int J Neuropsychopharmacol. 2014;17(2):313–22. doi: 10.1017/S1461145713001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandey SC, et al. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008;28(10):2589–600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeanblanc J, et al. BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. Eur J Neurosci. 2013;37(4):607–12. doi: 10.1111/ejn.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hensler JG, Ladenheim EE, Lyons WE. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/-) mice. J Neurochem. 2003;85(5):1139–47. doi: 10.1046/j.1471-4159.2003.01748.x. [DOI] [PubMed] [Google Scholar]
- 33.Jeanblanc J, et al. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. J Neurosci. 2006;26(5):1457–64. doi: 10.1523/JNEUROSCI.3786-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haun HL, et al. Increasing Brain-derived neurotrophic factor (BDNF) in medial prefrontal cortex selectively reduces excessive drinking in ethanol dependent mice. Neuropharmacology. 2018;140:35–42. doi: 10.1016/j.neuropharm.2018.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandey SC, et al. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26(32):8320–31. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandey SC, et al. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis. 2015;82:607–619. doi: 10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakash A, Zhang H, Pandey SC. Innate differences in the expression of brain-derived neurotrophic factor in the regions within the extended amygdala between alcohol preferring and nonpreferring rats. Alcohol Clin Exp Res. 2008;32(6):909–20. doi: 10.1111/j.1530-0277.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- 38.Warnault V, et al. The BDNF valine 68 to methionine polymorphism increases compulsive alcohol drinking in mice that is reversed by tropomyosin receptor kinase B activation. Biol Psychiatry. 2016;79(6):463–73. doi: 10.1016/j.biopsych.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stragier E, et al. Ethanol-induced epigenetic regulations at the Bdnf gene in C57BL/6 J mice. Mol Psychiatry. 2015;20(3):405–12. doi: 10.1038/mp.2014.38. [DOI] [PubMed] [Google Scholar]
- 40.Leggio GM, et al. Dopamine D3 receptor is necessary for ethanol consumption: an approach with buspirone. Neuropsychopharmacology. 2014;39(8):2017–28. doi: 10.1038/npp.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutton JW, 3rd, et al. Anaplastic lymphoma kinase regulates binge-like drinking and dopamine receptor sensitivity in the ventral tegmental area. Addict Biol. 2017;22(3):665–678. doi: 10.1111/adb.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Even-Chen O, et al. Fibroblast growth factor 2 in the dorsomedial striatum is a novel positive regulator of alcohol consumption. J Neurosci. 2017;37(36):8742–8754. doi: 10.1523/JNEUROSCI.0890-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corl AB, et al. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell. 2009;137(5):949–60. doi: 10.1016/j.cell.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Even-Chen, O. and S. Barak, Inhibition of FGF receptor-1 suppresses alcohol consumption: role of PI3 kinase signaling in dorsomedial striatum. J Neurosci, 2019. [DOI] [PMC free article] [PubMed]
- 45.Darcq E, et al. The neurotrophic factor receptor p75 in the rat dorsolateral striatum drives excessive alcohol drinking. J Neurosci. 2016;36(39):10116–27. doi: 10.1523/JNEUROSCI.4597-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu K, et al. Nucleotide sequence variation within the human tyrosine kinase B neurotrophin receptor gene: association with antisocial alcohol dependence. Pharmacogenomics J. 2007;7(6):368–79. doi: 10.1038/sj.tpj.6500430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klimkiewicz A, et al. COMT and BDNF gene variants help to predict alcohol consumption in alcohol-dependent patients. J Addict Med. 2017;11(2):114–118. doi: 10.1097/ADM.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wojnar M, et al. Association between Val66Met brain-derived neurotrophic factor (BDNF) gene polymorphism and post-treatment relapse in alcohol dependence. Alcohol Clin Exp Res. 2009;33(4):693–702. doi: 10.1111/j.1530-0277.2008.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nedic G, et al. Brain-derived neurotrophic factor Val66Met polymorphism and alcohol-related phenotypes. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:193–8. doi: 10.1016/j.pnpbp.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Grzywacz A, et al. Family-based study of brain-derived neurotrophic factor (BDNF) gene polymorphism in alcohol dependence. Pharmacol Rep. 2010;62(5):938–41. doi: 10.1016/S1734-1140(10)70354-6. [DOI] [PubMed] [Google Scholar]
- 51.Silva-Pena, D., et al., Alcohol-induced cognitive deficits are associated with decreased circulating levels of the neurotrophin BDNF in humans and rats. Addict Biol, 2018. [DOI] [PubMed]
- 52.Heberlein A, et al. BDNF and GDNF serum levels in alcohol-dependent patients during withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1060–4. doi: 10.1016/j.pnpbp.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 53.Zhou L, et al. ProBDNF/p75NTR/sortilin pathway is activated in peripheral blood of patients with alcohol dependence. Transl Psychiatry. 2018;7(11):2. doi: 10.1038/s41398-017-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohnsack JP, et al. The lncRNA BDNF-AS is an epigenetic regulator in the human amygdala in early onset alcohol use disorders. Transl Psychiatry. 2019;9(1):34. doi: 10.1038/s41398-019-0367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10(3):209–19. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 56.Jang SW, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107(6):2687–92. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korkmaz OT, et al. 7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis. Neurosci Lett. 2014;566:286–91. doi: 10.1016/j.neulet.2014.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang YJ, et al. Small-molecule TrkB agonist 7,8-dihydroxyflavone reverses cognitive and synaptic plasticity deficits in a rat model of schizophrenia. Pharmacol Biochem Behav. 2014;122:30–6. doi: 10.1016/j.pbb.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Chen C, et al. The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer’s disease. Proc Natl Acad Sci U S A. 2018;115(3):578–583. doi: 10.1073/pnas.1718683115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3(5):383–94. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 61.Schuchardt A, et al. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367(6461):380–3. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 62.Pichel JG, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382(6586):73–6. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 63.Durbec P, et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381(6585):789–93. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- 64.Kramer ER, Liss B. GDNF-Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Lett. 2015;589(24 Pt A):3760–72. doi: 10.1016/j.febslet.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Lin LF, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 66.Taraviras S, et al. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999;126(12):2785–97. doi: 10.1242/dev.126.12.2785. [DOI] [PubMed] [Google Scholar]
- 67.Hoffer BJ, et al. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci Lett. 1994;182(1):107–11. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 68.Tomac A, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373(6512):335–9. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 69.Drinkut A, et al. Ret is essential to mediate GDNF’s neuroprotective and neuroregenerative effect in a Parkinson disease mouse model. Cell Death Dis. 2016;7(9):e2359. doi: 10.1038/cddis.2016.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grondin R, et al. GDNF revisited: a novel mammalian cell-derived variant form of GDNF increases dopamine turnover and improves brain biodistribution. Neuropharmacology. 2019;147:28–36. doi: 10.1016/j.neuropharm.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 71.He DY, et al. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci. 2005;25(3):619–28. doi: 10.1523/JNEUROSCI.3959-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Popik P, Layer RT, Skolnick P. 100 years of ibogaine: neurochemical and pharmacological actions of a putative anti-addictive drug. Pharmacol Rev. 1995;47(2):235–53. [PubMed] [Google Scholar]
- 73.Carnicella S, et al. Noribogaine, but not 18-MC, exhibits similar actions as ibogaine on GDNF expression and ethanol self-administration. Addict Biol. 2010;15(4):424–33. doi: 10.1111/j.1369-1600.2010.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newman-Tancredi A, et al. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor. J Pharmacol Exp Ther. 2002;303(2):805–14. doi: 10.1124/jpet.102.039875. [DOI] [PubMed] [Google Scholar]
- 75.Carnicella S, et al. Cabergoline decreases alcohol drinking and seeking behaviors via glial cell line-derived neurotrophic factor. Biol Psychiatry. 2009;66(2):146–53. doi: 10.1016/j.biopsych.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carnicella S, et al. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105(23):8114–9. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43(1):35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barak, S., et al., Positive autoregulation of GDNF levels in the ventral tegmental area mediates long-lasting inhibition of excessive alcohol consumption. Transl Psychiatry, 2011. 1. [DOI] [PMC free article] [PubMed]
- 79.He DY, Ron D. Autoregulation of glial cell line-derived neurotrophic factor expression: implications for the long-lasting actions of the anti-addiction drug, Ibogaine. FASEB J. 2006;20(13):2420–2. doi: 10.1096/fj.06-6394fje. [DOI] [PubMed] [Google Scholar]
- 80.Barak S, et al. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J Neurosci. 2011;31(27):9885–94. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carnicella S, et al. GDNF is an endogenous negative regulator of ethanol-mediated reward and of ethanol consumption after a period of abstinence. Alcohol Clin Exp Res. 2009;33(6):1012–24. doi: 10.1111/j.1530-0277.2009.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang F, et al. GDNF acutely modulates excitability and A-type K(+) channels in midbrain dopaminergic neurons. Nat Neurosci. 2001;4(11):1071–8. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- 83.Wang J, et al. Nucleus accumbens-derived glial cell line-derived neurotrophic factor is a retrograde enhancer of dopaminergic tone in the mesocorticolimbic system. J Neurosci. 2010;30(43):14502–12. doi: 10.1523/JNEUROSCI.3909-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McAlhany RE, Jr, West JR, Miranda RC. Glial-derived neurotrophic factor (GDNF) prevents ethanol-induced apoptosis and JUN kinase phosphorylation. Brain Res Dev Brain Res. 2000;119(2):209–16. doi: 10.1016/S0165-3806(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 85.Villegas SN, et al. Glial-derived neurotrophic factor (GDNF) prevents ethanol (EtOH) induced B92 glial cell death by both PI3K/AKT and MEK/ERK signaling pathways. Brain Res Bull. 2006;71(1-3):116–26. doi: 10.1016/j.brainresbull.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 86.Ahmadiantehrani S, Barak S, Ron D. GDNF is a novel ethanol-responsive gene in the VTA: implications for the development and persistence of excessive drinking. Addict Biol. 2014;19(4):623–33. doi: 10.1111/adb.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sidorova YA, et al. A novel small molecule GDNF receptor RET agonist, BT13, promotes neurite growth from sensory neurons in vitro and attenuates experimental neuropathy in the rat. Front Pharmacol. 2017;8:365. doi: 10.3389/fphar.2017.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ivanova L, et al. Small-molecule ligands as potential GDNF family receptor agonists. ACS Omega. 2018;3(1):1022–1030. doi: 10.1021/acsomega.7b01932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morris SW, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263(5151):1281–4. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 90.Yau NK, et al. A pan-cancer review of ALK mutations: implications for carcinogenesis and therapy. Curr Cancer Drug Targets. 2015;15(4):327–36. doi: 10.2174/1568009615666150225123712. [DOI] [PubMed] [Google Scholar]
- 91.Murray PB, et al. Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal. 2015;8(360):ra6. doi: 10.1126/scisignal.2005916. [DOI] [PubMed] [Google Scholar]
- 92.Reshetnyak AV, et al. Augmentor alpha and beta (FAM150) are ligands of the receptor tyrosine kinases ALK and LTK: hierarchy and specificity of ligand-receptor interactions. Proc Natl Acad Sci U S A. 2015;112(52):15862–7. doi: 10.1073/pnas.1520099112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stoica GE, et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276(20):16772–9. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- 94.Stoica GE, et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002;277(39):35990–8. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- 95.Perez-Pinera P, et al. Anaplastic lymphoma kinase is activated through the pleiotrophin/receptor protein-tyrosine phosphatase beta/zeta signaling pathway: an alternative mechanism of receptor tyrosine kinase activation. J Biol Chem. 2007;282(39):28683–90. doi: 10.1074/jbc.M704505200. [DOI] [PubMed] [Google Scholar]
- 96.Maeda N, et al. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J Biol Chem. 1999;274(18):12474–9. doi: 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- 97.Deuel TF. Anaplastic lymphoma kinase: “ligand independent activation” mediated by the PTN/RPTPbeta/zeta signaling pathway. Biochim Biophys Acta. 2013;1834(10):2219–23. doi: 10.1016/j.bbapap.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 98.Bilsland JG, et al. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology. 2008;33(3):685–700. doi: 10.1038/sj.npp.1301446. [DOI] [PubMed] [Google Scholar]
- 99.Weiss JB, et al. Anaplastic lymphoma kinase and leukocyte tyrosine kinase: functions and genetic interactions in learning, memory and adult neurogenesis. Pharmacol Biochem Behav. 2012;100(3):566–74. doi: 10.1016/j.pbb.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 100.Witek B, et al. Targeted disruption of ALK reveals a potential role in hypogonadotropic hypogonadism. PLoS One. 2015;10(5):e0123542. doi: 10.1371/journal.pone.0123542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lasek AW, et al. An evolutionary conserved role for anaplastic lymphoma kinase in behavioral responses to ethanol. PLoS One. 2011;6(7):e22636. doi: 10.1371/journal.pone.0022636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mangieri RA, et al. Anaplastic lymphoma kinase is a regulator of alcohol consumption and excitatory synaptic plasticity in the nucleus accumbens shell. Front Pharmacol. 2017;8:533. doi: 10.3389/fphar.2017.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schweitzer P, et al. Dependence-induced ethanol drinking and GABA neurotransmission are altered in Alk deficient mice. Neuropharmacology. 2016;107:1–8. doi: 10.1016/j.neuropharm.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang KS, et al. A meta-analysis of two genome-wide association studies identifies 3 new loci for alcohol dependence. J Psychiatr Res. 2011;45(11):1419–25. doi: 10.1016/j.jpsychires.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 105.Roberto M, Varodayan FP. Synaptic targets: chronic alcohol actions. Neuropharmacology. 2017;122:85–99. doi: 10.1016/j.neuropharm.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fernandez-Calle R, et al. Pharmacological inhibition of receptor protein tyrosine phosphatase beta/zeta (PTPRZ1) modulates behavioral responses to ethanol. Neuropharmacology. 2018;137:86–95. doi: 10.1016/j.neuropharm.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He D, et al. Ethanol activates midkine and anaplastic lymphoma kinase signaling in neuroblastoma cells and in the brain. J Neurochem. 2015;135(3):508–21. doi: 10.1111/jnc.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen H, He D, Lasek AW. Midkine in the mouse ventral tegmental area limits ethanol intake and Ccl2 gene expression. Genes Brain Behav. 2017;16(7):699–708. doi: 10.1111/gbb.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vicente-Rodriguez M, et al. Genetic inactivation of midkine modulates behavioural responses to ethanol possibly by enhancing GABA(A) receptor sensitivity to GABA(A) acting drugs. Behav Brain Res. 2014;274:258–63. doi: 10.1016/j.bbr.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 110.Pastor M, et al. Development of inhibitors of receptor protein tyrosine phosphatase beta/zeta (PTPRZ1) as candidates for CNS disorders. Eur J Med Chem. 2018;144:318–329. doi: 10.1016/j.ejmech.2017.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fernandez-Calle, R., et al., Inhibition of RPTPbeta/zeta blocks ethanol-induced conditioned place preference in pleiotrophin knockout mice. Behav Brain Res, 2019: p. 111933. [DOI] [PubMed]
- 112.Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–66. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ornitz DM, et al. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992;12(1):240–7. doi: 10.1128/MCB.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252(5013):1705–8. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 115.Spivak-Kroizman T, et al. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell. 1994;79(6):1015–24. doi: 10.1016/0092-8674(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 116.Yayon A, et al. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64(4):841–8. doi: 10.1016/0092-8674(91)90512-W. [DOI] [PubMed] [Google Scholar]
- 117.Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975;250(7):2515–20. [PubMed] [Google Scholar]
- 118.Dono R, et al. Impaired cerebral cortex development and blood pressure regulation in FGF-2-deficient mice. EMBO J. 1998;17(15):4213–25. doi: 10.1093/emboj/17.15.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hierro C, Rodon J, Tabernero J. Fibroblast growth factor (FGF) receptor/FGF inhibitors: novel targets and strategies for optimization of response of solid tumors. Semin Oncol. 2015;42(6):801–19. doi: 10.1053/j.seminoncol.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 120.Katoh M. Therapeutics targeting FGF signaling network in human diseases. Trends Pharmacol Sci. 2016;37(12):1081–1096. doi: 10.1016/j.tips.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 121.Carpenter G, King L, Jr, Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature. 1978;276(5686):409–10. doi: 10.1038/276409a0. [DOI] [PubMed] [Google Scholar]
- 122.Roskoski R., Jr Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol Res. 2019;139:395–411. doi: 10.1016/j.phrs.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 123.Threadgill DW, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269(5221):230–4. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 124.Yamada M, Ikeuchi T, Hatanaka H. The neurotrophic action and signalling of epidermal growth factor. Prog Neurobiol. 1997;51(1):19–37. doi: 10.1016/S0301-0082(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 125.Kalsi G, et al. A systematic gene-based screen of chr4q22-q32 identifies association of a novel susceptibility gene, DKK2, with the quantitative trait of alcohol dependence symptom counts. Hum Mol Genet. 2010;19(12):2497–506. doi: 10.1093/hmg/ddq112. [DOI] [PMC free article] [PubMed] [Google Scholar]