Abstract
In this study, we analyzed the Rc and Rd genes that are responsible for the coloration of rice pericarps from six upland rice varieties. We also examined the association of pericarp coloration to the single nucleotide polymorphism in 9-cis-epoxycarotenoid dioxygenase 2 (NCED2), a key gene involved in abscisic acid (ABA) biosynthesis. Our findings demonstrated that all the upland rice varieties analyzed have a Rd gene which encodes a complete dihydroflavonol-4-reductase without early translational termination codon irrespective of their pericarp colors. However, the upland rice varieties with white pericarps were found to have a defective Rc gene with a 14-base deletion at exon 7 which could disrupt the function of a positive regulator of proanthocyanidin biosynthesis. In addition, the NCED2 genes from the upland rice varieties with white pericarps in this study have a C-allele while the NCED2 genes from Pandasan Red, Tomou and Taragang varieties that bear red pericarps were found to have a T-allele which was reported to be associated with a higher ABA level in upland rice. A better understanding of the gene sequences of upland rice varieties with red pericarp may provide important information for rice breeding programs.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2092-y) contains supplementary material, which is available to authorized users.
Keywords: Abscisic acid, Proanthocyanidin biosynthesis, Rice pericarp colors, Single nucleotide polymorphism
Introduction
The rice pericarps can be red, purple, brown, black and white in color. Purple and red pericarps are due to the accumulation of anthocyanins and proanthocyanidins, respectively (Reddy et al. 1995; Finocchiaro et al. 2007). Rice grains with red pericarp may be preferred in some regions of the world for their unique taste, texture, medicinal benefits, and cultural purpose. The red pigment is of interest for nutritional reasons because it has strong antioxidative properties that can potentially reduce cardiovascular disease (Ling et al. 2001).
The biosynthetic pathways of proanthocyanidins and anthocyanin shared two enzymes that are involved in the initial steps, i.e., flavanone-3-hydroxylase which catalyzes the flavonones to dihydroflavonols, and dihydroflavonol-4-reductase (DFR) which catalyzes the conversion of dihydroflavonols to leucoanthocyanidins. In the anthocyanin biosynthetic pathway, leucoanthocyanidins are converted to anthocyanidins by anthocyanidin synthase and subsequently to anthocyanins by anthocyanidin glucosyltransferase. However, anthocyanidin reductase can also change anthocynidins to epicatechins. Leucocyanidins, epicatechins and catechins (from leucocyanidins) are precursors of proanthocyanidins (Xie and Dixon 2005).
The red coloration in rice is primarily regulated by Rc and Rd genes (Furukawa et al. 2006). Rice varieties with both Rc and Rd genes have red grains while rice varieties with Rc but without Rd produce brown grains. Rice varieties with either rc and rd, or rc and Rd produce white rice grains (Furukawa et al. 2006). Rc which encodes a basic helix loop helix (bHLH) protein was mapped to chromosome 7 (Sweeney et al. 2006), corresponding to LOC_Os07g11020 in Nipponbare. Rd which encodes the DFR enzyme involved in both anthocyanin and proanthocyanidin biosynthesis was mapped to chromosome 1 (Furukawa et al. 2006), corresponding to LOC_Os01g44260 in Nipponbare. The Rc gene was reported to have eight exons separated by seven introns with a DNA binding motif bHLH at the seventh exon by Furukawa et al. (2006) and Gu et al. (2011). A 14-bp deletion at the seventh exon which causes a frame shift in the open reading frame and early termination of protein translation of this positive regulator of proanthocyanidin biosynthesis (Furukawa et al. 2006; Gu et al. 2011), was identified in a Nipponbare and other rice varieties that produce white grains. The truncated protein without a functional bHLH domain does not have any transacting regulatory activity. The rice DFR gene consists of three exons and two introns (Chen et al. 1998; Nakai et al. 1998). The occurrence of a stop codon at exon 1 (third codon) and 2 (55th codon) in the DFR of some rice varieties may render the gene non functional (Nakai et al. 1998). The DFR gene was reported to have alternative translation initiation predominantly in developing seeds (Furukawa et al. 2006).
The pigments in rice pericarps have deterrent effects on pathogens or predators in nature (Shirley 1998). The genes involved in the biosynthesis of anthocyanin and proanthocyanidins can be induced by stresses through transcription factors in the basic domain/leucine zipper family and Myb family (Ithal and Reddy 2004; Hartmann et al. 2005). In addition, al. (2011) reported that Rc not only regulated Rd gene but also promoted biosynthesis and accumulation of abscisic acid (ABA) in early developing seeds from a pair of perfect rice isogenic lines. ABA is a phytohormone related to environmental stresses including water deficit (Finkelstein 2013). ABA is also able to promote or inhibit the biosynthesis of anthocyanin in fruits by crosstalking with other phytohormones including jasmonic acid, gibberellin, auxin and cytokinin (Jaakola 2013). The antioxidant properties of anthocyanins and proanthocyanidins may enable the plants to cope better and survive under environmental stresses. The production of xanthoxin through oxidative cleavage of 9-cisepoxyxanthophylls by 9-cis-epoxycarotenoid dioxygenase (NCED) (Finkelstein 2013) is an irreversible reaction and rate-limiting step in ABA biosynthesis. Li et al. (2019) reported that NCED could affect the ABA level in Lycium fruits, which regulates three transcription factors that can improve the production of anthocyanin by upregulating the gene expression of genes related to anthocyanin biosynthesis. ABA-mediated anthocyanin biosynthesis pathways have also been reported in other plants (Xie et al. 2012; An et al. 2018).
The aims of this study were to analyze the Rc, Rd and NCED genes from six upland rice varieties and to examine the association between coloration of rice pericarp and the single nucleotide polymorphism (SNP) in NCED. A better understanding of the gene sequences of upland rice varieties with red pericarp will provide important information for rice breeding programs.
Materials and methods
Plant materials and DNA extraction
Rice seeds of selected Malaysian rice varieties were obtained from Rice Genebank, Malaysian Agricultural Research and Development Institute (MARDI), Kepala Batas, Malaysia; and Kebun Bahagia Bersama, Sungai Buloh, Malaysia (Supplementary Table S1). Rice husk was removed from 20 seeds randomly selected from each rice variety to record the colour of the rice pericarps.
Seeds were germinated on filter paper after sterilization using 40% (v/v) Clorox®-Bleach (The Clorox Company, USA). Fourteen day-old rice seedlings were transplanted into pails containing 15 kg of a mix of top soil and sand (70:30) in a perforated pail. Total genomic DNA was extracted from the leaves of 2 month-old rice using a modified protocol described by Doyle and Doyle (1987); Doyle and Dickson (1987); and Cullings (1992).
Cloning of selected gene regions
Primers were designed based on the Rc, Rd and NCED2 genes of a rice reference sequence from japonica Nipponbare using Primer3 Ver. 0.4.0 (bioinfo.ut.ee/primer3-0.4.0/) (Table 1). PCR was performed in a total volume of 50 μL containing 1 × KAPA HiFi Fidelity buffer, approximately 500 ng DNA template, 0.3 mM dNTPs, 0.3 μM forward and reverse primers, and 0.2 U KAPA HiFi DNA polymerase (KAPA Biosystems, Switzerland). The PCR was conducted using the following parameters: 95 °C for 7 min, followed by 30 cycles of denaturation at 98 °C for 30 s, annealing for 45 s at the optimum annealing temperature (Table 1) and extension at 72 °C for 1 kb per minute. The PCR products were purified using MEGAquick-spin™ Total Fragment DNA Purification Kit (Intron Biotechnology, Korea) according to the manufacturer’s instructions. The PCR products were either sequenced directly or cloned into TA cloning vector before sequencing.
Table 1.
List of primers used in this experiment
| Primer | Sequence (5′ → 3′) |
|---|---|
| NCED-forward primer | TTCGTCTCGCAGTTTACAGG |
| NCED-reverse primer | ACTGGCACTTGGCGTCTTAG |
| Rc-forward primer | AAGCCTACCCTCTCACAGCA |
| Rc-reverse primer | CGGTTCCTTAGCTGCTTCAC |
| Rd-forward primer | CCATCACCAAGTGCAAGGTA |
| Rd-reverse primer | TCTCTTGCTTTGCTGCTTCA |
| Rd-internal forward primer | TGGGTTAGGAACAACGATCC |
| Rd-internal reverse primer | GGGCTCTCGAAGAGGAAGAT |
For TA cloning, 100 ng of purified PCR product was added to 1X PCR buffer, 0.2 mM dATP (New England Biolabs, USA) and 0.2 U Taq DNA polymerase (Genedirex, USA) in a total volume of 100 μL and incubated at 72 °C for 1 h. The A-tailed PCR product was then purified using MEGAquick-spin™ Total Fragment DNA Purification Kit (Intron Biotechnology, Korea) according to the manufacturer’s instructions. The purified A-tailed product was cloned into TA cloning vector (Yeastern Biotech, Taiwan) and transformed into Escherichia coli DH5α competent cells prepared using rubidium chloride (Green and Rogers 2013). The transformed clones were verified by colony PCR with M13 primer pairs and restriction enzyme digestion prior to sequencing.
Sequence analysis of Rc, Rd and NCED2 genes
The sequences were analyzed using BioEdit Sequence Alignment Editor, version 7.2.1 (Hall 1999). Multiple sequence alignment was conducted with ClustalW algorithm (Thompson et al. 1994) with reference sequences from different subpopulations; japonica Nipponbare, indica IR64 and aus Kasalath retrieved from Rice SNP-Seek Database (http://www.snp-seek.irri.org).
Mining of single nucleotide polymorphism (SNP) in NCED2 gene
The single nucleotide polymorphism (SNP) at position 14,233,796 in the NCED2 gene (LOC_Os12g24800) which was reported to be associated with a higher production of ABA (Lyu et al. 2013; Alexandrov et al. 2015) was analyzed in 3024 rice varieties available in the Rice SNP-Seek Database (http://www.snp-seek.irri.org). The frequencies of C and T alleles at the mentioned position were calculated in 184 upland (Supplementary Table S2), 506 japonica and 1154 indica rice varieties with different pericarp colors (brown, light brown, speckled brown, red, purple, variable purple or a mixture colors) available at the Rice SNP-Seek Database (http://www.snp-seek.irri.org).
Results and discussion
Rd and Rc genes from upland rice varieties
The pericarps of Malaysian upland rice varieties analyzed in this study were found to be in red and white colors (Fig. 1). Among the upland rice varieties analyzed in this study, Pandasan was found to have both white and red grains, Tomou and Taragang bear red grains while the other rice varieties bear white grains.
Fig. 1.
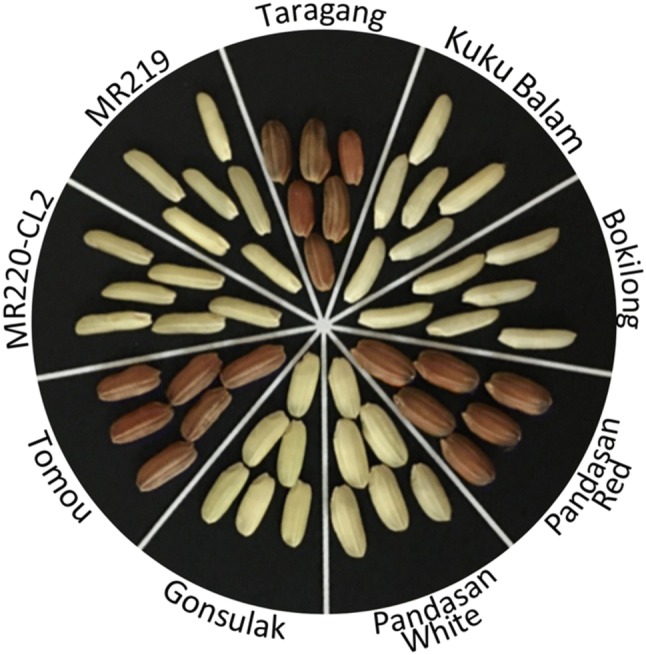
The coloration of rice pericarp from Malaysian rice varieties. Tomou and Taragang have red pericarps while Pandasan has either red or white pericarp. MR220-CL2, MR219, Gonsulak, Bokilong and Kuku Balam have white pericarps
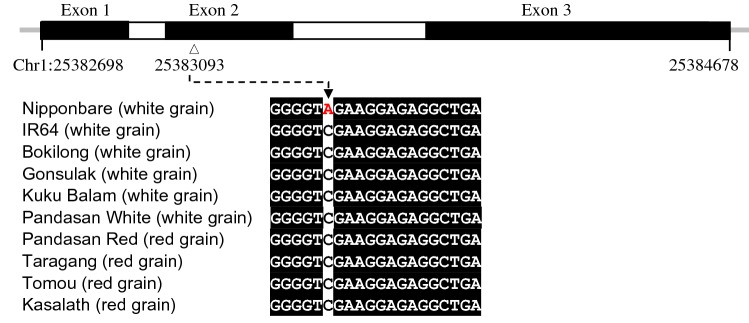
Since the loss of red pigment in rice could be due to the SNP in Rd gene which causes an early termination of DFR protein which is involved in the red pigment biosynthesis, or due to a deletion in Rc gene encoding the BHLH transcription factor which regulates the red pigment biosynthesis, both Rc and Rd genes were analyzed in this study. Nipponbare which has white pericarp was found to have a SNP (A-nucleotide) that causes early translational termination of DFR whereas IR64, Kasalath and all the upland rice varieties analyzed in this study were found to have C-nucleotide at the corresponding SNP position, irrespective of the pericarp colors (Fig. 2). The Rc gene of Bokilong, Gonsulak, Kuku Balam and Pandasan White with white pericarps demonstrated a 14-base deletion (ACGCGAAAAGTCGG) at exon 7, causing a frame shift in protein translation leading to an early truncation of bHLH transcription factor. With a defective rc gene hence a non functional BHLH, these rice varieties could not produce anthocyanin even though the DFR in these rice varieties could be fully functional. Only the Pandasan variety germinated from red grains (Pandasan Red), Tomou and Taragang varieties that bear red pericarps have a non-defective Rc gene as in the Kasalath variety which also produces red pericarp (Fig. 3). In summary, the white grain phenotype in Nipponbare and the upland rice varieties analyzed in this study (Bokilong, Gonsulak, Kuku Balam, and Pandasan White) was caused by different pericarp coloration genes, i.e., mutation of Rd gene in Nipponbare and a 14-bp deletion in Rc gene of the upland rice varieties.
Fig. 2.
The gene structure of Rd gene (LOC_Os01g44260) and the SNP associated with the grain color of rice. The upper panel shows the schematic representation of Rd gene whereby the exons and introns are shown by black and white boxes, respectively. The open triangle indicates the location of single nucleotide substitution from A to C in exon 2 at position 25,383,093 of chromosome 1 that causes an early translational termination of dihydroflavonol-4-reductase
Fig. 3.
The gene structure of Rc gene (LOC_Os07g11020) and the changes in nucleotide sequence associated with the grain color of rice. The upper panel shows the schematic representation of Rc gene whereby the exons and introns are shown by black and white boxes, respectively. The open triangle indicates the location of 14-base deletion (ACGCGAAAAGTCGG) at exon 7 in rice varieties with white grains which causes a frame shift in protein translation leading the truncation of bHLH transcription factor
NCED2 genes from upland rice varieties
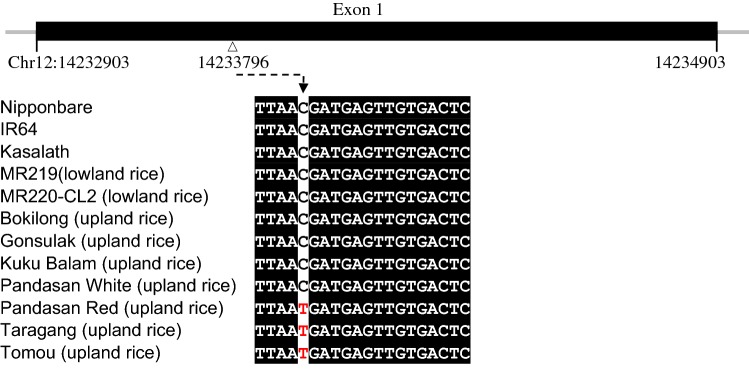
The gene structure of NCED2 gene (LOC_Os12g24800) and SNP associated with drought tolerance of upland rice varieties reported by Lyu et al. (2013) is shown in Fig. 4. A single nucleotide substitution from C to T at position 14,233,796 at chromosome 12 causes the change of valine to isoleucine in the protein. NCED2 with the substitution of isoleucine at the corresponding amino acid position was reported to be dominant in many upland rice varieties and these rice varieties exhibited a higher level of ABA and were more tolerant to drought (Lyu et al. 2013). The NCED2 gene from Bokilong, Gonsulak, Kuku Balam, and Pandasan White belong to the C-type while Pandasan Red, Tomou and Taragang varieties have the T-type NCED2 (Fig. 4). Coincidently, all three rice varieties with the T-type NCED2 were found to bear red pericarps (Fig. 2). Since the T-allele in NCED2 gene was also found to be associated with a higher ABA level in upland rice (Lyu et al. 2013), Pandasan Red, Tomou and Taragang which have the T-allele in their NCED2 genes may have a higher level of ABA. Nevertheless, Kasalath which bears red pericarp was found to have a C-type NCED2 (Fig. 4).
Fig. 4.
The gene structure of 9-cis-epoxycarotenoid dioxygenase 1 (NCED) gene (LOC_Os12g24800) and SNP associated with drought tolerance of upland rice varieties. The upper panel shows the schematic representation of NCED gene whereby the exon is shown by a black box. The open triangle indicates the location of single nucleotide substitution from C to T at position 14,233,796 at chromosome 12 that causes the change of valine to isoleucine in the protein
Since ABA level and the expression of NCED (which is involved in ABA biosynthesis) were associated with pigment production (Li et al. 2019; Karppinen et al. 2018; Jia et al. 2011), we also examined whether the red pigmentation was correlated to the T-allele in NCED2 by analyzing the frequencies of C- and T- alleles in the NCED2 gene of 1967 rice varieties with different pericarp colors available at the Rice SNP-Seek Database (http://www.snp-seek.irri.org), i.e., 1547 white, 2 brown, 14 light brown, 9 speckled brown, 371 red, 18 purple, 1 variable purple, and 5 mixture rice varieties (Supplementary Table S2) (Mansueto et al. 2017). The percentage of T-allele in NCED2 gene was 13.10%, 19.01% and 10.82% for the upland, japonica and indica rice varieties with pigmented grains, respectively (Table 2). The C-allele was found to be the major allele for all three rice groups with pigmented grains (i.e., 86.90%, 80.99% and 89.18% for the upland, japonica and indica varieties, respectively). C-allele was predominant in the NCED gene from rice varieties with white grains, i.e., 84.29%, 86.23% and 92.20% for the upland, japonica and indica, respectively. Our findings did not support the association of the T-type NCED2 with pericarp colour. Since the rice genome has five NCED homologs (NCED1-5; Huang et al. 2018; Hwang et al. 2018), a higher activity of other NCEDs may also increase the ABA level and improve anthocyanin/proanthocyanidin production.
Table 2.
Allele percentage at rice NCED2 at position14233796 in chromosome 12
| Pericarp colora | Uplandb | Japonicab | Indicab | |||
|---|---|---|---|---|---|---|
| C-allele (%) | T-allele (%) | C-allele (%) | T-allele (%) | C-allele (%) | T-allele (%) | |
| White | 84.29 | 15.71 | 86.23 | 13.77 | 92.20 | 7.80 |
| Pigmented | 86.90 | 13.10 | 80.99 | 19.01 | 89.18 | 10.82 |
| Brown | N/A | N/A | 100 | 0 | 100 | 0 |
| Light brown | 100.00 | 0 | N/A | N/A | 90.00 | 10.00 |
| Speckled brown | N/A | N/A | 100 | 0 | 100 | 0 |
| Red | 84.29 | 15.71 | 79.31 | 20.69 | 88.96 | 11.31 |
| Purple | 100 | 0 | 88.89 | 11.11 | 94.44 | 5.56 |
| Variable purple | 100 | 0 | N/A | N/A | 100 | 0 |
| Mixture | 100 | 0 | 75.00 | 25.00 | 90.00 | 10.00 |
aPericarp color was categorized following the Rice Standard Evaluation, IRRI (https://snp-seek.irri.org/phenotype_dict.pdf) based on the Methuen Handbook of Colours (Kornerup and Wanscher 1967). Please refer to the footnote of Supplementary Table S2 for more information
bThe number of upland, japonica and indica varieties analyzed were 184, 506 and 1154; respectively
Conclusions
We conclude that all six upland rice varieties analyzed in this study have a Rd gene encoding a complete DFR (without an early translational termination codon). However, the upland rice varieties with white pericarps were found to have a 14-base deletion at exon 7 in Rc gene which could cause a frame shift in protein translation, leading to early truncation of bHLH transcription factor which regulates the red pigment biosynthesis. The upland rice varieties with white pericarps in this study have C-type NCED2 gene while Pandasan Red, Tomou and Taragang varieties that bear red pericarps were found to have T-type NCED2, which could possibly accumulate a higher level of ABA that upregulates the expression of genes in the anthocyanin/proanthocyanidin biosynthetic pathway. However, not all rice varieties with pigmented grains in the Rice SNP-Seek Database have T-type NCED2. A better understanding of the gene sequences of upland rice varieties with red pericarp may provide important information for rice-breeding programs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The project was supported by Ministry of Education, Malaysia under the Fundamental Research Grant Scheme (Project No. FRGS/1/2016/STG05/UPM/02/18). Teh CY was supported by the UPM post-doctoral scheme. Mohd Kasim NA was supported by FRGS/1/2016/STG05/UPM/02/18 and Graduate Research Fund (GRF) from UPM. Amer Hamzah M was supported by FRGS/1/2016/STG05/UPM/02/18 and MyBrainSc from the Ministry of Education, Malaysia. We acknowledge Gene Bank and Seed Centre, Malaysian Agricultural Research and Development Institute (MARDI), Kepala Batas, Malaysia for providing the rice seeds; and Rice SNP-Seek Database for providing access to the research data.
Author contributions
CLH and CYT designed the experiments; MAH, NAMK, AS, NM. NIMR conducted the experiments and analyzed the data; CLH wrote the article with the contributions of all authors; all authors approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declared no conflict of interest.
Footnotes
Muazr Amer Hamzah and Nur Aini Mohd Kasim contribute equally to the work.
References
- Alexandrov N, Tai S, Wang W, Mansueto L, Palis K, Fuentes RR, Ulat VJ, Chebotarov D, Zhang G, Li Z, Mauleon R, Hamilton RS, McNally KL. SNP-seek database of SNPs derived from 3000 rice genomes. Nucleic Acids Res. 2015;43:D1023–D1027. doi: 10.1093/nar/gku1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JP, Yao JF, Xu RR, You CX, Wang XF, Hao YJ. Apple bZIP transcription factor MdbZIP44 regulates abscisic acid-promoted anthocyanin accumulation. Plant Cell Environ. 2018;41:2678–2692. doi: 10.1111/pce.13393. [DOI] [PubMed] [Google Scholar]
- Chen M, San Miguel P, Bennetzen JL. Sequence organization and conservation in sh2/a1-homologous regions of sorghum and rice. Genetics. 1998;148:435–443. doi: 10.1093/genetics/148.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullings KW. Design and testing of a plant-specific PCR primer for ecological and evolutionary studies. Mol Ecol. 1992;1:233–240. doi: 10.1111/j.1365-294X.1992.tb00182.x. [DOI] [Google Scholar]
- Doyle JJ, Dickson EE. Preservation of plant samples for DNA restriction endonuclease analysis. Taxon. 1987;36:715–722. doi: 10.2307/1221122. [DOI] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- Finkelstein R. Abscisic acid synthesis and response. Arabidopsis Book. 2013;2013:1–36. doi: 10.1199/tab.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finocchiaro F, Ferrari B, Gianinetti A, Dall’Asta C, Galaverna G, Scazzina F, Pellegrini N. Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol Nutr Food Res. 2007;51:1006–1019. doi: 10.1002/mnfr.200700011. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Maekawa M, Oki T, Suda I, Iida S, Shimada H, Takamure I, Kadowaki K. The Rc and Rd genes are involved in proanthocyanidinsynthesis in rice pericarp. Plant J. 2006;49:91–102. doi: 10.1111/j.1365-313X.2006.02958.x. [DOI] [PubMed] [Google Scholar]
- Green R, Rogers EJ. Transformation of chemically competent E. coli. Methods Enzymol. 2013;529:329–336. doi: 10.1016/B978-0-12-418687-3.00028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XY, Foley ME, Horvath DP, Anderson JV, Feng J, Zhang L, Mowry CR, Ye H, Suttle JC, Kadowaki K, Chen Z. Association between seed dormancy and pericarp color is controlled by a pleiotropic gene that regulates abscisic acid and flavonoid synthesis in weedy red rice. Genetics. 2011;189:1515–1524. doi: 10.1534/genetics.111.131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hartmann U, Sagasser M, Mehrtens F, Stracke R, Weisshaar B. Differential combinatorial interactions of cis-acting elements recognized by R2R3-MYB, BZIP, and BHLH factors control light-responsive and tissue-specific activation of phenylpropanoid biosynthesis genes. Plant Mol Biol. 2005;59:155–171. doi: 10.1007/s11103-004-6910-0. [DOI] [PubMed] [Google Scholar]
- Huang Y, Guo Y, Liu Y, Zhang F, Wang Z, Wang H, Wang F, Li D, Mao D, Luan S, Liang M, Chen L. 9-cis-epoxycarotenoid dioxygenase 3 regulates plant growth and enhances multi-abiotic stress tolerance in rice. Front Plant Sci. 2018;9:1–18. doi: 10.3389/fpls.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SG, Lee CY, Tseng CS. Heterologous expression of rice 9-cis-epoxycarotenoid dioxygenase 4 (OsNCED4) in Arabidopsis confers sugar oversensitivity and drought tolerance. Bot Stud. 2018;59:1–12. doi: 10.1186/s40529-018-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ithal N, Reddy AR. Rice flavonoid pathway genes, OsDfr and OsAns, are induced by dehydration, high salt and ABA, and contain stress responsive promoter elements that interact with the transcription activator, OsC1-MYB. Plant Sci. 2004;166:1505–1513. doi: 10.1016/j.plantsci.2004.02.002. [DOI] [Google Scholar]
- Jaakola L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013;18:477–483. doi: 10.1016/j.tplants.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011;157:188–199. doi: 10.1104/pp.111.177311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karppinen K, Tegelberg P, Häggman H, Jaakola L. Abscisic acid regulates anthocyanin biosynthesis and gene expression associated with cell wall modification in ripening bilberry (Vaccinium myrtillus L.) fruits. Front Plant Sci. 2018;9:1–17. doi: 10.3389/fpls.2018.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. Methuen Handbook of Color. 2. London: Methuen; 1967. [Google Scholar]
- Li G, Zhao J, Qin B, Yin Y, An W, Mu Z, Cao Z. ABA mediates development-dependent anthocyanin biosynthesis and fruit coloration in Lycium plants. BMC Plant Biol. 2019;19:1–13. doi: 10.1186/s12870-018-1600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling WH, Cheng QX, Ma J, Wang T. Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. J Nutr. 2001;131:1421–1426. doi: 10.1093/jn/131.5.1421. [DOI] [PubMed] [Google Scholar]
- Lyu J, Zhang S, Dong Y, He W, Zhang J, Deng X, Zhang Y, Li X, Li B, Huang W, Wan W, Yu Y, Li Q, Li Liu X, Wang B, Tao D, Zhang G, Wang J, Xu X, Hu F, Wang W. Analysis of elite variety tag SNPs reveals an important allele in upland rice. Nat Commun. 2013;4:1–9. doi: 10.1038/ncomms3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansueto L, Fuentes RR, Borja FN, Detras J, Abrio-Santos JM, Chebotarov D, Sanciangco M, Palis K, Copetti D, Poliakov A, Dubchak I, Solovye V, Wing RA, Hamilton RS, Mauleon R, McNally KL, Alexandrov N. Rice SNP-seek database update: new SNPs, indels, and queries. Nucleic Acids Res. 2017;45:D1075–D1081. doi: 10.1093/nar/gkw1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K, Inagaki Y, Nagata H, Miyazaki C, Iida S. Molecular characterization of the gene for dihydroflavonol 4-reductase of japonica rice varieties. Plant Biotech. 1998;15:221–225. doi: 10.5511/plantbiotechnology.15.221. [DOI] [Google Scholar]
- Reddy VS, Dash S, Reddy AR. Anthocyanin pathway in rice (Oryza sativa L.): identification of a mutant showing dominant inhibition of anthocyanins in leaf and accumulation of proanthocyanidins in pericarp. Theor Appl Genet. 1995;91:301–312. doi: 10.1007/BF00220892. [DOI] [PubMed] [Google Scholar]
- Shirley B. Flavonoids in seeds and grains: physiological function, agronomic importance and the genetics of biosynthesis. Seed Sci Res. 1998;8:415–422. doi: 10.1017/S0960258500004372. [DOI] [Google Scholar]
- Sweeney MT, Thomson MJ, Pfeil BE, McCouch S. Caught red-handed: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. Plant Cell. 2006;18:283–294. doi: 10.1105/tpc.105.038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie DY, Dixon RA. Proanthocyanidin biosynthesis–still more questions than answers? Phytochemistry. 2005;66:2127–2144. doi: 10.1016/j.phytochem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Xie X, Li S, Zhang R, Zhao J, Chen Y, Zhao Q, Yao Y, You C, Zhang X, Hao Y. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012;35:1884–1897. doi: 10.1111/j.1365-3040.2012.02523.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.