Abstract
Psoriasis is a chronic inflammatory disease believed to be correlated with numerous cardiovascular risk factors including increased blood pressure, elevated blood cholesterol level, diabetes, inactivity, high body mass index (obesity) and dyslipidaemia. The present meta-analysis intends to assess the association between psoriasis and cardiovascular risk factors. Three hundred and fifty articles were primarily screened using NCBI MEDLINE/PubMed and Cochrane library from its inception until June 30, 2018. Of these, 26 observational studies depending upon the inclusion and exclusion criteria were included in the study with 17,672 psoriasis patients and 66,407 non-psoriasis subjects. The psoriasis patients were found to be at significantly increased risk of systolic blood pressure (SBP) [ORs 2.31 (95% CI 1.12, 4.74)], diastolic blood pressure (DBP) [ORs 2.31 (95% CI 1.58, 3.38)], abdominal obesity [ORs 1.90 (95% CI 1.45, 2.50)] and triglycerides [ORs 1.80 (95% CI 1.29, 2.51)] as compared to non-psoriasis subjects. The subgroup analyses of studies based on the continents revealed that psoriasis patients from Middle East are prone to higher risk factors of CVD including increased levels of triglyceride, cholesterol, DBP, SBP, fasting blood sugar, body mass index and decreased HDL levels, whereas psoriasis patients from European population reported increased LDL-C and waist circumference. The present study supports a significant association between psoriasis and incidence of major adverse cardiovascular events. Contrary to the previous literature, our finding suggests that hypertension is a highly associative condition in psoriasis. The findings of this study could be validated amongst well-defined cohorts of patients with psoriasis individually in different regions to confirm the implication of the study.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2089-6) contains supplementary material, which is available to authorized users.
Keywords: Psoriasis, Meta-analysis, CVD, Hypertension, Cholesterol, Obesity
Introduction
Psoriasis is an immune-mediated chronic inflammatory disease of the skin affecting nearly 2–3% of the world’s population (Gisondi et al. 2010). The exact etiology of the disease is unknown; however, genetic susceptible markers and environmental predisposition are important during psoriasis pathogenesis (Aggarwal et al. 2017; Raimondo et al. 2018; Singh et al. 2019). Moreover, the immune system plays a crucial role in overall disease progression, with various innate and adaptive immune cells and pro-inflammatory mediators involved at different stages of the disease (Schiattarella et al. 2019). In recent years, various studies from different countries have shown that psoriasis is a systemic inflammatory disease, which is often associated with various comorbidities. In particular, there is a greater risk of developing severe vascular events such as cardiovascular and cerebrovascular diseases (Gelfand 2016; Furue et al. 2017; Takeshita et al. 2017).
Cardiovascular disease (CVD) refers to a number of heart conditions including atherosclerosis, heart attack, ischemic stroke, haemorrhagic stroke, arrhythmia and heart failure (Ma et al. 2014; Shah et al. 2017). The association between CVD and psoriasis could be ascribed to the pro-inflammatory molecules released during chronic inflammation (Mallbris et al. 2004). It has also been reported that treatments for psoriasis such as retinoids and cyclosporine may induce hyperlipidaemia that can promote future CVD (Grossman et al. 1991; Katz et al. 1999).
The available evidences present a controversial depiction of the risk of CVD in psoriasis patients. Numerous studies reported on the higher risk of CVD in psoriasis patients (Sommer et al. 2006; Tablazon et al. 2013; Richard et al. 2013; Karoli et al. 2013; Baeta et al. 2014; Parodi et al. 2014; Edson-Heredia et al. 2015). Furthermore, previous studies have shown increased mortality rates in psoriasis patients as compared to healthy controls (Mehta et al. 2010; Masson Regnault et al. 2017) and the life expectancy of patients with moderate to severe psoriasis is decreased by approximately 5 years, mainly due to cardiovascular comorbidities (Siegel et al. 2013). However, several reports (Piskin et al. 2003; Farshchian et al. 2007; Gelfand 2016) have elucidated that the risk of CVD, transient ischemic attacks, or cerebrovascular accidents remained unchanged between psoriatic patients and controls. Therefore, to answer these conflicting reports, we have performed a systematic review and meta-analysis of the association between psoriasis and cardiovascular risk factors. Depending on these results, the attributable cardiovascular risk was determined depending upon covariates such as region, study types, year of study and smoking behaviour.
Methodology
The criteria of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) were followed in conduction and reporting of the results of this systematic review and meta-analysis.
Search strategy
NCBI MEDLINE/PubMed and Cochrane library were primarily searched from inception until June 30, 2018, using combinations of the keywords: psoriasis and triglyceride, psoriasis and HDL, psoriasis and LDL, psoriasis and total cholesterol, psoriasis and systolic and diastolic blood pressure, psoriasis and fasting blood glucose, psoriasis and body mass index, psoriasis and waist circumference, psoriasis and dyslipidaemia, psoriasis and metabolic syndrome, psoriasis and comorbidities, psoriasis and cardiovascular disease. Other databases such as Google Scholar and Science Direct were also searched to identify the articles not indexed in the NCBI PubMed and Cochrane library. Additionally, the cross-references were also searched to identify additional eligible articles.
Selection criteria
The studies with following inclusion criteria were considered for the present meta-analysis: (1) studies provided original data; (2) study subjects were human adults with psoriasis and control group; (3) studies reported at least three or more parameters out of triglyceride (TGY), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), total cholesterol (TC), systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood glucose (FBG), body mass index (BMI) and waist circumference (WC); (4) the study provided method description of various parameters with means and standard deviation. The exclusion criteria adopted were (1) studies reporting different parameters namely TGY, HDL-C, LDL-C, TC, SBP, DBP, FBG, BMI, WC with no numerical data; (2) studies reporting the discussed parameters on diseases other than psoriasis; (3) studies with reports on various discussed parameters only in psoriasis, without control group.
End points of cardiovascular risk factors
According to the National Cholesterol Education Program Adult Training Panel-III (NCEP ATP III) updated from 2005, a person is said to be under cardiovascular risk factor if (1) fasting glucose is 100 mg/dl or higher (or receiving drug therapy for hyperglycaemia), (2) blood pressure is 130/85 mmHg or higher (or receiving drug therapy for hypertension), (3) triglycerides are 150 mg/dl or higher (or receiving drug therapy for hypertriglyceridaemia), (4) high-density lipoprotein cholesterol complex (HDL-C) is less than 40 mg/dl in men or less than 50 mg/dl in women (or receiving drug therapy for reduced HDL-C), and (5) waist circumference is 102 cm (40 inches) or greater in men or 88 cm (35 inches) or greater in women; if Asian American, 90 cm (35 inches) or greater in men or 80 cm (32 inches) or greater in women.
Data extraction and study quality assessment
The included articles were thoroughly evaluated. Two review authors [SC] and [RP] extracted the data which were cross-checked by the third author [DP]. Extracted data included author's names, year of publication, region, means and SD level of age, TGY (Uyanik et al. 2002; Piskin et al. 2003; Farshchian et al. 2007, 2015; Akhyani et al. 2007; Dreiher et al. 2008; Balci et al. 2010; Choi et al. 2010; Langan et al. 2012; Vayá et al. 2013; Pehlevan et al. 2014; Akcali et al. 2014; Taheri Sarvtin et al. 2014; Parodi et al. 2014; Irimie et al. 2015; Barrea et al. 2016; Ražnatović-Đurović et al. 2016; Coban et al. 2016; Praveenkumar et al. 2016; Uczniak et al. 2016; Sharma et al. 2016; Kothiwala et al. 2016; Singh et al. 2017; Girisha and Thomas 2017; Milčić et al. 2017; Ganguly et al. 2018), HDL-C (Piskin et al. 2003; Farshchian et al. 2007, 2015; Akhyani et al. 2007; Dreiher et al. 2008; Balci et al. 2010; Choi et al. 2010; Langan et al. 2012; Vayá et al. 2013; Pehlevan et al. 2014; Akcali et al. 2014; Taheri Sarvtin et al. 2014; Parodi et al. 2014; Irimie et al. 2015; Barrea et al. 2016; Ražnatović-Đurović et al. 2016; Coban et al. 2016; Praveenkumar et al. 2016; Uczniak et al. 2016; Sharma et al. 2016; Kothiwala et al. 2016; Singh et al. 2017; Girisha and Thomas 2017; Milčić et al. 2017), LDL-C (Uyanik et al. 2002; Balci et al. 2010; Choi et al. 2010; Pehlevan et al. 2014; Irimie et al. 2015; Barrea et al. 2016; Coban et al. 2016; Singh et al. 2017), TC (Piskin et al. 2003; Balci et al. 2010; Langan et al. 2012; Akcali et al. 2014; Taheri Sarvtin et al. 2014; Parodi et al. 2014; Coban et al. 2016; Kothiwala et al. 2016; Singh et al. 2017; Milčić et al. 2017), SBP and DBP (Langan et al. 2012; Pehlevan et al. 2014; Akcali et al. 2014; Parodi et al. 2014; Farshchian et al. 2015; Barrea et al. 2016; Ražnatović-Đurović et al. 2016; Coban et al. 2016; Praveenkumar et al. 2016; Uczniak et al. 2016; Sharma et al. 2016; Kothiwala et al. 2016; Singh et al. 2017; Girisha and Thomas 2017; Milčić et al. 2017), FBG (Farshchian et al. 2007, 2015; Balci et al. 2010; Langan et al. 2012; Vayá et al. 2013; Pehlevan et al. 2014; Parodi et al. 2014; Barrea et al. 2016; Ražnatović-Đurović et al. 2016; Coban et al. 2016; Praveenkumar et al. 2016; Uczniak et al. 2016; Sharma et al. 2016; Kothiwala et al. 2016; Singh et al. 2017; Girisha and Thomas 2017; Milčić et al. 2017), BMI (Farshchian et al. 2007, 2015; Akhyani et al. 2007; Balci et al. 2010; Langan et al. 2012; Vayá et al. 2013; Pehlevan et al. 2014; Akcali et al. 2014; Taheri Sarvtin et al. 2014; Parodi et al. 2014; Barrea et al. 2016; Ražnatović-Đurović et al. 2016; Coban et al. 2016; Praveenkumar et al. 2016; Sharma et al. 2016; Kothiwala et al. 2016; Singh et al. 2017; Girisha and Thomas 2017; Milčić et al. 2017; Ganguly et al. 2018), WC (Vayá et al. 2013; Pehlevan et al. 2014; Akcali et al. 2014; Parodi et al. 2014; Barrea et al. 2016; Ražnatović-Đurović et al. 2016; Praveenkumar et al. 2016; Uczniak et al. 2016; Sharma et al. 2016; Kothiwala et al. 2016; Girisha and Thomas 2017; Milčić et al. 2017; Ganguly et al. 2018), and other relevant qualitative data are shown in Tables 1 and 2. The Newcastle–Ottawa Scale (NOS) was used to evaluate the methodological quality of the included studies. This scale allocates scores up to nine according to the assessment of selection, comparability of cases or controls, and ascertainment of exposure or outcome. Studies that received a score of ≥ 7 stars were judged as high quality.
Table 1.
Demographic profile of studies included in the meta-analysis
| S. no | Study | Year | Case population | Control population | Study type | Smoker | Region | Mean age of case (SD) | Mean age of control (SD) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Uyanik | 2002 | 72 | 30 | Case–control | No | Middle East | 38.4 ± 4.7 | 38.4 ± 4.7 |
| 2 | Piskin | 2003 | 100 | 100 | Case–control | No | Middle East | 45.1 ± 16.4 | 44 ± 16.9 |
| 3 | Farshchian | 2007 | 30 | 30 | Cross-sectional | No | Middle East | 34.6 ± 11.47 | 34.63 ± 12.11 |
| 4 | Akhyani | 2007 | 50 | 50 | Case–control | No | Middle East | 41.82 ± 17.37 | 43.34 ± 20.73 |
| 5 | Dreiher | 2008 | 10,669 | 22,969 | Cross-sectional | Yes | Middle East | 57.8 ± 15.6 | 54.8 ± 17.9 |
| 6 | Choi | 2010 | 197 | 401 | Case–control | Yes | Asia | 45.04 ± 16.64 | 46.89 ± 14.62 |
| 7 | Balci | 2010 | 46 | 46 | Cross-sectional | Yes | Middle East | 39.5 ± 14.2 | 39.8 ± 13.5 |
| 8 | Langan | 2012 | 4065 | 40,650 | Cross-sectional | No | Europe | 45.74 ± 15.21 | 48.03 ± 13.77 |
| 9 | Vaya | 2013 | 91 | 101 | Case–control | No | Europe | 52.02 ± 13.56 | 50.6 ± 10.96 |
| 10 | Akcali | 2014 | 50 | 40 | Cross-sectional | No | Middle East | 38.6 ± 13.2 | 40.5 ± 14.6 |
| 11 | Pehlevan | 2014 | 59 s | 82 | Case–control | Yes | Middle East | 46.8 ± 11.5 | 36.9 ± 11.5 |
| 12 | Parodi | 2014 | 390 | 344 | Cross-sectional | Yes | Europe | 52.9 ± 16 | 54.8 ± 18.1 |
| 13 | Sarvtin | 2014 | 50 | 50 | Case–control | No | Middle East | 43.8 ± 5.4 | 44.9 ± 7.1 |
| 14 | Farshchian | 2015 | 55 | 55 | Case–control | Yes | Middle East | 47.3 ± 18.4 | 45.16 ± 15.9 |
| 15 | Irimie | 2015 | 142 | 167 | Case–control | Yes | Europe | 49.51 ± 8.26 | 47.87 ± 16.43 |
| 16 | Coban | 2016 | 35 | 50 | Prospective cohort | No | Middle East | 44.43 ± 11.59 | 40.48 ± 13.47 |
| 17 | Kothiwala | 2016 | 140 | 140 | Cross-sectional | Yes | Asia | 37.9 ± 13.26 | 36.1 ± 11.63 |
| 18 | Sharma | 2016 | 100 | 100 | Case–control | Yes | Asia | 44.94 ± 11.1 | 43.28 ± 12.1 |
| 19 | Uday | 2016 | 30 | 30 | Case–control | No | Asia | 45.77 ± 12.09 | 42.07 ± 12.58 |
| 20 | Djurovic | 2016 | 101 | 126 | Case–control | Yes | Europe | 50 ± 14.39 | 43.7 ± 14.62 |
| 21 | Uczniak | 2016 | 246 | 75 | Case–control | No | Europe | 46 ± 13 | 46 ± 13 |
| 22 | Barrea | 2016 | 180 | 180 | Cross-sectional | Yes | Europe | 50 ± 11 | 48 ± 9 |
| 23 | Singh | 2017 | 334 | 230 | Case–control | Yes | Asia | 39.1 ± 14 | 35.4 ± 14.7 |
| 24 | Milcic | 2017 | 244 | 163 | Cross-sectional | Yes | Europe | 53.54 ± 15.16 | 43.69 ± 14.68 |
| 25 | Girisha | 2017 | 156 | 156 | Case–control | Yes | Asia | 45.5 ± 12.6 | 45.4 ± 12.5 |
| 26 | Ganguly | 2018 | 40 | 42 | Case–control | No | Asia | 44.83 ± 12.83 | 46.36 ± 10.11 |
The information about the case and control population of individual studies, region of the study, mean age of the case and control population and study setting
Table 2.
Individual level of reported parameters in studies included in the meta-analysis
| Study | Year | BMI | FBS | Systolic BP | Diastolic BP | Triglyceride | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | ||
| Uyanik | 2002 | NM | NM | NM | NM | NM | NM | NM | NM | 150.13 ± 25.86 | 115.05 ± 21.78 |
| Piskin | 2003 | NM | NM | NM | NM | NM | NM | NM | NM | 130.68 ± 67.59 | 111.65 ± 47.38 |
| Farshchian | 2007 | 22.96 ± 4.23 | 23.23 ± 3.08 | 95.36 ± 12.38 | 92 ± 11.28 | NM | NM | NM | NM | 121.63 ± 54.56 | 127.03 ± 65.39 |
| Akhyani | 2007 | 25.56 ± 2.26 | 25.2 ± 2.39 | NM | NM | NM | NM | NM | NM | 140.3 ± 55.24 | 115.84 ± 47.28 |
| Dreiher | 2008 | NM | NM | NM | NM | NM | NM | NM | NM | 148.8 ± 15.6 | 139.3 ± 86.1 |
| Choi | 2010 | NM | NM | NM | NM | NM | NM | NM | NM | 152.17 ± 76.44 | 119.16 ± 70.32 |
| Balci | 2010 | 26.5 ± 4.2 | 26.8 ± 4.1 | 101.8 ± 43.5 | 83.4 ± 9.1 | NM | NM | NM | NM | 139.7 ± 129.9 | 119.1 ± 74.2 |
| Langan | 2012 | 27.1 ± 1.87 | 28.4 ± 1.12 | 97.2 ± 6.66 | 100.8 ± 7.56 | 144 ± 8 | 149 ± 8.25 | 90 ± 5 | 90 ± 4.5 | 150.57 ± 30.99 | 168.28 ± 35.43 |
| Vaya | 2013 | 28.9 ± 5.4 | 26.2 ± 3.9 | 104 ± 33 | 97 ± 17 | NM | NM | NM | NM | 152 ± 121 | 101 ± 50 |
| Akcali | 2014 | 26.92 ± 4.11 | 25.73 ± 5.89 | 110.92 ± 57.58 | 95.66 ± 37.63 | 130 ± 17 | 120 ± 15 | 83 ± 14 | 77 ± 11 | 177.43 ± 173.91 | 146.17 ± 84.93 |
| Pehlevan | 2014 | 29 ± 4.7 | 27 ± 5.6 | 101 ± 32 | 93.6 ± 18.8 | 122.7 ± 12.8 | 113.5 ± 16.4 | 81 ± 9 | 70 ± 16.6 | 142 ± 94 | 101 ± 52 |
| Parodi | 2014 | 27.3 ± 4.8 | 25.5 ± 4.2 | 98.3 ± 25 | 92.8 ± 24 | 128.6 ± 14 | 126.3 ± 12.7 | 79.4 ± 9.6 | 77.3 ± 8.6 | 126.4 ± 69.8 | 108.7 ± 61.3 |
| Sarvtin | 2014 | 24.4 ± 2.2 | 23.8 ± 2.3 | NM | NM | NM | NM | NM | NM | 156.3 ± 56.1 | 117 ± 41.8 |
| Farshchian | 2015 | 26.36 ± 4.71 | 24.6 ± 3 | 101 ± 25.7 | 96 ± 14.4 | 135.83 ± 19 | 118.1 ± 16 | 81.11 ± 11.3 | 74.04 ± 10.9 | 152.6 ± 72 | 107.05 ± 35 |
| Irimie | 2015 | NM | NM | NM | NM | NM | NM | NM | NM | 163.21 ± 56.72 | 109.47 ± 45.29 |
| Coban | 2016 | 27.18 ± 4.8 | 26.88 ± 4.28 | 106.11 ± 45.37 | 93.64 ± 11.77 | 121.57 ± 14.39 | 118.5 ± 9.8 | 79 ± 12.11 | 74 ± 8.3 | 134.83 ± 62.46 | 136.74 ± 137.3 |
| Kothiwala | 2016 | 24 ± 4.43 | 22.6 ± 3.71 | 98.5 ± 16.82 | 91.1 ± 12.82 | 129.4 ± 14.42 | 121.5 ± 11.9 | 82.3 ± 9.3 | 77.8 ± 9.14 | 130.2 ± 69.83 | 142.3 ± 102.88 |
| Sharma | 2016 | 24.42 ± 3.96 | 23.91 ± 3.66 | 103.44 ± 32.46 | 90.95 ± 16.29 | 125.43 ± 11.01 | 121.98 ± 9.67 | 80.83 ± 7.17 | 78.32 ± 6.7 | 156.89 ± 58.47 | 133.34 ± 29.85 |
| Uday | 2016 | 24.56 ± 4.76 | 24.23 ± 3.91 | 134.17 ± 60.04 | 118.4 ± 41.78 | 122.86 ± 14.97 | 121.66 ± 14.85 | 80.86 ± 11.68 | 78.88 ± 12.67 | 120.5 ± 23.81 | 145 ± 24.31 |
| Djurovic | 2016 | 26.81 ± 3.17 | 25.45 ± 5.12 | 97.2 ± 21.6 | 86.22 ± 14.94 | 146.19 ± 18.88 | 119 ± 11.01 | 93.56 ± 9.96 | 75.29 ± 8.4 | 116.03 ± 47.83 | 131.97 ± 92.11 |
| Uczniak | 2016 | NM | NM | 96.71 ± 16.02 | 92.64 ± 13.17 | 135.06 ± 12.82 | 128.8 ± 9.6 | 83.26 ± 6.16 | 83 ± 6.92 | 145.68 ± 29.95 | 110.92 ± 28.83 |
| Barrea | 2016 | 30.2 ± 6.1 | 29.6 ± 7.3 | 105 ± 33 | 96 ± 32.5 | 130 ± 16.25 | 125 ± 17.5 | 80 ± 12.5 | 75 ± 12.5 | 164 ± 91.5 | 139 ± 51.75 |
| Singh | 2017 | 24.8 ± 5.1 | 23.1 ± 4.4 | 89.7 ± 21.2 | 85.7 ± 11.4 | 125.8 ± 14.5 | 119.5 ± 11.8 | 80.4 ± 8.8 | 77.7 ± 7.5 | 151.2 ± 78 | 133.3 ± 52.5 |
| Milcic | 2017 | 27.15 ± 4.87 | 25.45 ± 4.89 | 92.88 ± 36.54 | 87.12 ± 24.48 | 132.08 ± 17.59 | 119 ± 11.61 | 80.62 ± 10.62 | 75.04 ± 8.27 | 147.03 ± 79.65 | 126.55 ± 85.84 |
| Girisha | 2017 | 24 ± 4.8 | 25.2 ± 2.2 | 97.07 ± 14.7 | 97.01 ± 15.78 | 124.26 ± 13.17 | 124.76 ± 13.27 | 81.48 ± 6.77 | 80.62 ± 7.34 | 138.49 ± 38.98 | 129.78 ± 31.26 |
| Ganguly | 2018 | 24.12 ± 3.48 | 23.75 ± 3.79 | NM | NM | NM | NM | NM | NM | 158.4 ± 125.53 | 125.93 ± 53.01 |
| Study | Year | Total cholesterol | LDL-cholesterol | HDL | Waist circumference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | Case | Control | ||
| Uyanik | 2002 | 161.44 ± 43.23 | 145.79 ± 31.67 | 49.73 ± 10.69 | 45.16 ± 10.42 | 37.97 ± 10.015 | 42.03 ± 8.24 | NM | NM |
| Piskin | 2003 | NM | NM | NM | NM | 47.3 ± 10.68 | 48.8 ± 13.4 | NM | NM |
| Farshchian | 2007 | NM | NM | NM | NM | 37.3 ± 4.96 | 35.5 ± 4.17 | NM | NM |
| Akhyani | 2007 | NM | NM | NM | NM | 39.64 ± 7.91 | 41.32 ± 7.73 | NM | NM |
| Dreiher | 2008 | NM | NM | NM | NM | 48.3 ± 12.9 | 49.9 ± 13.4 | NM | NM |
| Choi | 2010 | 187.01 ± 38.26 | 187.4 ± 32.7 | 105.11 ± 32.81 | 109.41 ± 29.45 | 52.66 ± 11.68 | 53.02 ± 13.57 | NM | NM |
| Balci | 2010 | 178.8 ± 36.6 | 170.1 ± 39 | 107.8 ± 34.4 | 103.4 ± 355.6 | 43.6 ± 10.5 | 42.1 ± 11.6 | NM | NM |
| Langan | 2012 | NM | NM | NM | NM | 132.85 ± 13.28 | 123.99 ± 10.62 | NM | NM |
| Vaya | 2013 | 215 ± 41 | 218 ± 32 | NM | NM | 55 ± 13 | 60 ± 14 | 99.86 ± 13.7 | 92.39 ± 12.3 |
| Akcali | 2014 | NM | NM | NM | NM | 41.69 ± 10.5 | 43.88 ± 10.76 | 97.2 ± 10.48 | 94.44 ± 11.07 |
| Pehlevan | 2014 | 196 ± 47 | 184 ± 44 | 119 ± 36 | 113 ± 37 | 51 ± 15 | 52 ± 14 | 94 ± 13 | 93 ± 14 |
| Parodi | 2014 | 201.3 ± 40 | 193.1 ± 40.1 | NM | NM | 49.4 ± 13.8 | 53.8 ± 14.5 | 95.4 ± 17.7 | 88.6 ± 15.3 |
| Sarvtin | 2014 | NM | NM | NM | NM | 47.6 ± 8.8 | 53.8 ± 6.6 | NM | NM |
| Farshchian | 2015 | NM | NM | NM | NM | 66 ± 10.4 | 49 ± 9 | NM | NM |
| Irimie | 2015 | 223.42 ± 142.72 | 204.3 ± 82.51 | 118.62 ± 36.79 | 104.26 ± 31.86 | 44.63 ± 11.39 | 52.46 ± 8.65 | NM | NM |
| Coban | 2016 | 194.43 ± 43.65 | 176.44 ± 36.22 | 119.71 ± 34.83 | 102.94 ± 30.78 | 48.48 ± 10.81 | 47.92 ± 14.1 | NM | NM |
| Kothiwala | 2016 | NM | NM | NM | NM | 42.4 ± 6.79 | 42.2 ± 5.46 | 89.7 ± 11.92 | 83.6 ± 9.22 |
| Sharma | 2016 | NM | NM | NM | NM | 42.77 ± 12.46 | 42.91 ± 6.23 | 83.47 ± 10.27 | 83.04 ± 8.02 |
| Uday | 2016 | NM | NM | NM | NM | 38.93 ± 10.53 | 37.77 ± 11.03 | 89.28 ± 11.77 | 85.36 ± 8 |
| Djurovic | 2016 | NM | NM | NM | NM | 91.22 ± 36.31 | 118.68 ± 38.97 | 88.98 ± 13.01 | 85.63 ± 13.7 |
| Uczniak | 2016 | NM | NM | NM | NM | 58.74 ± 14.56 | 58.17 ± 13.49 | 97.03 ± 13.71 | 92 ± 14.42 |
| Barrea | 2016 | 197.5 ± 54.5 | 171 ± 57.5 | 132.6 ± 44.7 | 121.7 ± 51.8 | 43.3 ± 11 | 45.7 ± 7.9 | 109.6 ± 21.65 | 95.7 ± 22.85 |
| Singh | 2017 | 176.8 ± 38 | 168.1 ± 35.6 | 111.9 ± 30.6 | 106.6 ± 30.1 | 45.7 ± 9.3 | 45.8 ± 10.7 | NM | NM |
| Milcic | 2017 | NM | NM | NM | NM | 45.24 ± 11.99 | 52.2 ± 16.24 | 95.4 ± 13.62 | 84.54 ± 13.62 |
| Girisha | 2017 | NM | NM | NM | NM | 44.42 ± 9.02 | 46.53 ± 7.7 | 86.27 ± 13.14 | 87.35 ± 31.26 |
| Ganguly | 2018 | NM | NM | NM | NM | NM | NM | 86.47 ± 8.9 | 85.17 ± 10.45 |
The mean and standard deviation value of different parameters reported in the individual study. NM implies not mentioned
Statistical analysis
In this meta-analysis, the mean and SD values of TGY, HDL-C, LDL-C, TC, SBP, DBP, FBG, BMI and WC were compared between psoriasis patients and controls. Standardized mean difference (SMD) and its 95% CI [UL, LL] in conjunction with odds ratio (ORs) was used as a summary statistic to measure the effect of various defined parameters. The overall effect size for SMD was presented as Z-score. The Z-score with a p value of ≤ 0.05 was considered statistically significant. Heterogeneity across studies was detected using I2 statistics. Subgroup analysis was also performed to understand the prevalence of individual risk factors of metabolic syndrome among psoriasis patients and control. Meta-regression analysis was used to determine the effect of region, study type, smoking pattern and age-matched studies on various discussed parameters in psoriasis case and non-psoriasis control subjects. To assess the influence of individual study and result of the pooled estimate and to explain heterogeneity, a one-study removed sensitivity analysis was performed by excluding each study at a time. Risk of publication bias was adjudged using a funnel plot. All analyses were performed using Comprehensive Meta-analysis Version 3.3.070, USA.
Results
Literature search
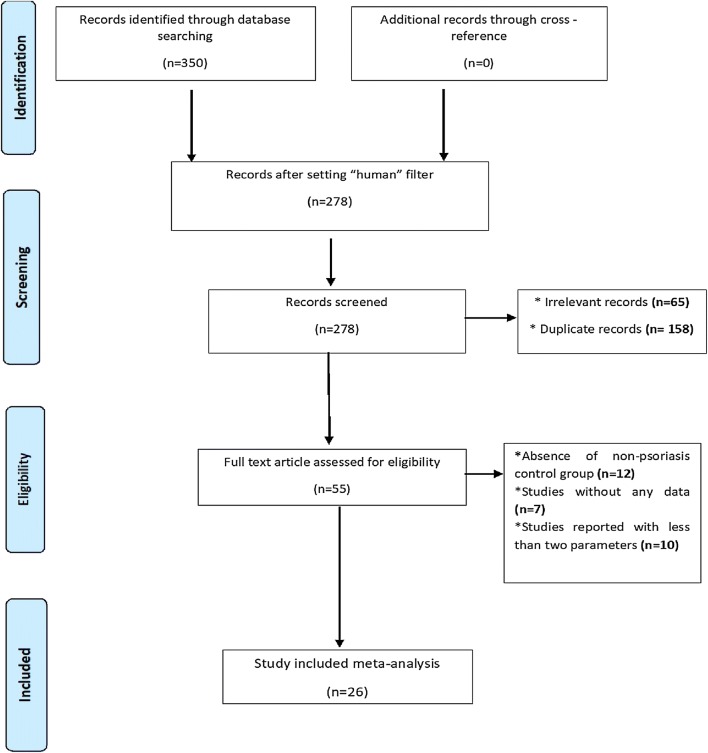
The systematic search resulted in 350 relevant citations. Seventy-two articles were filtered for non-human models and, of the remaining 278 articles, only 55 full-text articles were screened for inclusion and exclusion criteria. Among 55 articles, only 26 articles were included in this meta-analysis (Fig. 1).
Fig. 1.
Schematic representation of the study selection process. Different databases were screened with suitable keywords to retrieve relevant records. Using the inclusion and exclusion criteria, 26 studies were finally selected for study
Description of studies and study quality assessment
Among the 26 included studies 84,079 subjects were enrolled, out of which 17,672 subjects were psoriasis patients (case) as compared to 66,407 non-psoriasis subjects (control). Various parameters, namely TGY, HDL-C, LDL-C, TC, SBP, DBP, FBG, BMI and WC were studied and are summarized in Tables 1 and 2. The details of the study quality included in the meta-analysis have been elucidated in Table 3.
Table 3.
Quality assessment of studies included in the meta-analysis
| S. no | Study | Year | Is the case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls on the basis of design or analysis | Ascertainment of exposure | Same method of ascertainment for both groups | Overall NOS scores |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Uyanik | 2002 | * | * | * | * | ** | * | * | 8 |
| 2 | Piskin | 2003 | * | * | * | * | * | * | * | 7 |
| 3 | Farshchian | 2007 | * | * | * | * | ** | * | * | 8 |
| 4 | Akhyani | 2007 | * | * | * | * | ** | * | * | 8 |
| 5 | Dreiher | 2008 | * | * | * | * | ** | * | * | 8 |
| 6 | Choi | 2010 | * | * | * | * | * | * | * | 7 |
| 7 | Balci | 2010 | * | * | * | * | ** | * | * | 8 |
| 8 | Langan | 2012 | * | * | * | * | ** | * | * | 8 |
| 9 | Vaya | 2013 | * | * | * | * | ** | * | * | 8 |
| 10 | Akcali | 2014 | * | * | * | * | ** | * | * | 8 |
| 11 | Pehlevan | 2014 | * | * | * | * | ** | * | * | 8 |
| 12 | Parodi | 2014 | * | * | * | * | ** | * | * | 8 |
| 13 | Sarvtin | 2014 | * | * | * | * | ** | * | * | 8 |
| 14 | Farshchian | 2015 | * | * | * | * | ** | * | * | 8 |
| 15 | Irimie | 2015 | * | * | * | * | * | * | * | 7 |
| 16 | Coban | 2016 | * | * | * | * | ** | * | * | 8 |
| 17 | Kothiwala | 2016 | * | * | * | * | * | * | * | 7 |
| 18 | Sharma | 2016 | * | * | * | * | ** | * | * | 8 |
| 19 | Uday | 2016 | * | * | * | * | * | * | * | 7 |
| 20 | Djurovic | 2016 | * | * | * | * | ** | * | * | 8 |
| 21 | Uczniak | 2016 | * | * | * | * | ** | * | * | 8 |
| 22 | Barrea | 2016 | * | * | * | * | * | * | * | 7 |
| 23 | Singh | 2017 | * | * | * | * | * | * | * | 7 |
| 24 | Milcic | 2017 | * | * | * | * | ** | * | * | 8 |
| 25 | Girisha | 2017 | * | * | * | * | ** | * | * | 8 |
| 26 | Ganguly | 2018 | * | * | * | * | * | * | * | 7 |
Triglyceride levels
Mean and standard deviation of triglyceride levels in psoriasis patients and non-psoriasis subjects of 26 studies were pooled to give an estimate of overall effect. With high heterogeneity across studies (I2 = 98.5%), the random effect model was used to pool the effect size. Moderate and statistically significant spike in the effect of triglyceride level was evident in psoriasis patients. The pooled SMD (95% CI) was found to be 0.325 (0.141, 0.508) (sfig1a) which was statistically significant from the control (p < 0.001).
HDL levels
HDL levels were reported in 25 studies. With substantially high heterogeneity across the studies (I2 = 99.016%), the random effect model was used. The result based on the model showed lowered HDL levels, SMD (95% CI) – 0.081 (–0.323, 0.161) (sfig1b) was recorded in psoriasis patients. The SMD value for HDL-C depicts that impaired level of HDL-C is not an associative cardiovascular risk factor in psoriasis patients.
LDL levels
Eight studies reported the mean and SD values for LDL levels. The measured heterogeneity (I2) was found to be 81.68%; hence, the random effect model was used. The overall SMD (95% CI) was calculated to be 0.215 (0.070, 0.360) (sfig1c). The result depicted moderately increased LDL levels in psoriasis patients as compared to the control population with statistically significant p value < 0.05.
Total cholesterol levels
Mean and SMD values of ten studies were used to comprehend the total cholesterol levels. The measured heterogeneity between trials (I2 = 45.75%) was recorded. Based upon the random effect model, the overall SMD (95% CI) was estimated to be 0.276 (0.190, 0.363) (sfig1d) and the difference from the control was significant (p < 0.001).
Systolic and diastolic blood pressure levels
Fifteen studies reported systolic and diastolic blood pressure levels. High heterogeneity (I2 = 98.54% and I2 = 94.45%) was noticed across the studies for systolic and diastolic blood pressure levels, respectively. The effect size was measured using random effect model. The pooled SMD (95% CI) for diastolic blood pressure levels was found to be 0.463 (0.254, 0.672) (sfig1e) and the difference from the control was significant at p < 0.05, whereas the pooled SMD (95% CI) for systolic blood pressure was 0.462 (0.065, 0.859) (sfig1f) and the difference from control was significant at p < 0.01. The increased SMD for both SBP and DBP indicates that hypertension is the most associative CVD risk factor among psoriasis patients.
Fasting blood glucose
Fasting blood glucose level was studied in 18 works. Owing to high heterogeneity (I2 = 96.46%), the random effect model was used for assessing the effect size. The pooled SMD (95% CI) for fasting blood glucose was 0.270 (0.023, 0.517) (sfig1g) and the difference from control was significant at p < 0.05.
BMI levels
Mean and SD values of 20 studies were considered to draw the comparison between the body mass index in the psoriasis case and non-psoriasis control. Across the studies, heterogeneity was significant (I2 = 98.68%); hence, the random effect model was used. The estimated SMD (95% CI) for BMI was found to be 0.136 (– 0.260, 0.533) (sfig1h) with non-significant difference from control (p > 0.05). However; the calculated SMD does not present a clear picture of its association.
Waist circumference
Waist circumference level was interpreted from the analysis of 13 studies. Across the studies, the heterogeneity was significant (I2 = 76.29%); hence, the random effect model was chosen. The overall SMD, 95% CI (UL, LL) for waist circumference was estimated to be 0.356 (0.206, 0.506) (sfig1i) with a significant difference from control. The increased waist circumference depicts a closer association with psoriasis patients.
Subgroup analyses
Subgroup analysis was performed by region, i.e. Asia, Europe and Middle East for selected metabolic factors to reduce the heterogeneity among studies (Table 4).
Table 4.
Summary of subgroup analysis of various parameters
| Parameters | Overall estimate | Region | n | SDM | LL | UL | z value | p value |
|---|---|---|---|---|---|---|---|---|
| TGY | 0.325 (0.141, 0.508) | a Europe | 7 | 0.333 | − 0.184 | 0.851 | 1.262 | 0.207 |
| b Middle East | 11 | 0.429 | 0.206 | 0.651 | 3.774 | < 0.001 | ||
| c Asia | 8 | 0.203 | − 0.024 | 0.43 | 1.75 | 0.08 | ||
| HDL | − 0.081 (− 0.323, 0.161) | a Europe | 8 | − 0.258 | − 0.848 | 0.331 | − 0.86 | 0.39 |
| b Middle East | 11 | 0.019 | − 0.243 | 0.28 | 0.141 | 0.888 | ||
| c Asia | 6 | − 0.044 | − 0.134 | 0.045 | − 0.0973 | 0.33 | ||
| LDL | 0.215 (0.070, 0.360) | a Europe | 2 | 0.317 | 0.127 | 0.507 | 3.26 | 0.001 |
| b Middle East | 4 | 0.262 | 0.044 | 0.479 | 2.36 | 0.018 | ||
| c Asia | 2 | 0.017 | − 0.291 | 0.326 | 0.11 | 0.913 | ||
| TC | 0.276 (0.190, 0.363) | a Europe | 4 | 0.205 | 0.012 | 0.399 | 2.084 | 0.037 |
| b Middle East | 4 | 0.323 | 0.124 | 0.521 | 3.19 | 0.001 | ||
| c Asia | 2 | 0.112 | − 0.219 | 0.353 | 0.911 | 0.362 | ||
| DBP | 0.463 (0.254, 0.672) | a Europe | 6 | 0.522 | 0.135 | 0.909 | 2.646 | 0.008 |
| b Middle East | 4 | 0.621 | 0.425 | 0.818 | 6.206 | < 0.001 | ||
| c Asia | 5 | 0.309 | 0.18 | 0.437 | 4.717 | < 0.001 | ||
| SBP | 0.462 (0.065, 0.859) | a Europe | 6 | 0.498 | − 0.185 | 1.18 | 1.43 | 0.153 |
| b Middle East | 4 | 0.632 | 0.344 | 0.921 | 4.29 | < 0.001 | ||
| c Asia | 5 | 0.308 | 0.062 | 0.554 | 2.454 | 0.014 | ||
| FBS | 0.270 (0.023, 0.517) | a Europe | 7 | 0.185 | − 0.208 | 0.578 | 0.921 | 0.357 |
| b Middle East | 6 | 0.347 | 0.181 | 0.512 | 4.11 | < 0.001 | ||
| c Asia | 5 | 0.292 | 0.098 | 0.485 | 2.959 | 0.003 | ||
| BMI | 0.136 (− 0.260, 0.533) | a Europe | 6 | 0.105 | − 0.681 | 0.891 | 0.262 | 0.793 |
| b Middle East | 8 | 0.205 | 0.063 | 0.347 | 2.836 | 0.005 | ||
| c Asia | 6 | 0.12 | − 0.13 | 0.369 | 0.939 | 0.347 | ||
| WC | 0.356 (0.206, 0.506) | a Europe | 6 | 0.508 | 0.348 | 0.667 | 6.239 | < 0.001 |
| b Middle East | 2 | 0.145 | − 0.116 | 0.406 | 1.09 | 0.276 | ||
| c Asia | 5 | 0.211 | − 0.064 | 0.485 | 1.506 | 0.003 |
Eleven studies from the Middle East, 8 from Asia and 7 from Europe reported that the increased incidence of triglyceride level was highest in the Middle East region (SMD 0.429 [95% CI 0.206, 0.651]), followed by European population (SMD 0.333[95% CI 0.184, 0.851]) and subsequently in Asian residents (SMD 0.203 [95% CI 0.024, 0.43]). The HDL-C level was assessed for 11 Middle Eastern studies, 6 Asian and 8 studies from the European region. The SMD in all the three regions was found to be insignificant, i.e. a similar pattern of HDL level was found among psoriasis patient across the region. Two European, two Asian and four studies from the Middle East region depending on heterogeneity used random effect model to estimate the incidence of increased LDL level. LDL level was significantly higher among psoriasis patients from the European population (SMD 0.317 [95% CI 0.127, 0.507], p < 0.001). The LDL level in psoriasis patients from Middle East and Asia was not an associative condition in the populations. Four studies from the Middle East reported maximum and significant difference in total cholesterol (TC) levels (SMD 0.323 [95% CI 0.124, 0.521], p < 0.001) among psoriasis patients compared to the other two regions of Europe and Asia.
On the basis of the random effect model, six European, five Asian and four studies from the Middle East population were assessed for increased blood pressure levels. Apparently, the value of difference was highest and statistically significant for both SBP (SMD 0.632 [95% CI 0.344, 0.921]) and DBP (SMD 0.621 [95% CI 0.425, 0.818]) in the Middle East population. Both Middle East and Asian populations reported blood pressure as an associative condition in psoriasis patients; however, it was not found to be significantly involved in the European group. A similar result was found for FBS level among the psoriasis population. The difference in FBS level among psoriasis patients was found to be statistically significant at p < 0.001 from Middle East (SMD 0.347 [95% CI 0.181, 0.512]) and Asian countries (SMD 0.292 [95% CI 0.098, 0.485]). Eight Middle East studies reported a higher incidence of increased BMI (SMD 0.205 [95% CI 0.063, 0.347]). Increased waist circumference was found as a distinctive pattern among six studies from the European population with SMD = 0.508 [95% CI 0.348, 0.667].
Effect of drug treatment on psoriasis patients
Three studies (Dreiher et al. 2008; Choi et al. 2010; Pehlevan et al. 2014) considered in the present meta-analysis included 10,925 psoriasis patients on drug treatment with cyclosporine, acitretin, methotrexate, immune-modulators or any other steroidal therapy and 23,452 non-psoriasis patients. Meta-analysis of these studies revealed a moderate impact on TGY (0.198 [0.068, 0.098]) and TC (0.136[0.023, 0.072]) indicating that steroidal treatment might contribute to dyslipidaemia among psoriasis patients. Further analysis was conducted excluding these three studies to assess the occurrence of CVD in psoriasis patients. The analysis indicated moderate to significant impact of TC (0.242 [0.128, 0.356]) and TGY (0.317[0.052, 0.582]). Hence, it can be interpreted that cardiovascular risk factors are precursors for psoriasis patients and the drugs used in the disease treatment also contribute to elevated triglyceride and total cholesterol levels.
Meta-regression
Meta-regression analysis was performed to assess the association of mean difference of triglyceride levels, HDL levels, LDL levels, total cholesterol levels, systolic and diastolic blood pressure, fasting blood glucose, body mass index, waist circumference in psoriasis case vs non-psoriasis subjects with other covariates such as region, study types, year of study and smoking behaviour. No significant relationship was found in the mean difference of the defined parameters by any of the selected covariates independently. Within the study, the test of statistics showed that the association between differences in mean and selected covariates were again confirmed as insignificant in a linear model by weighing each study (Q test statistics for each covariate, p > 0.05). However, the test of heterogeneity between and within the study for each covariate was found to be significant for all defined levels.
Publication bias and sensitivity analysis
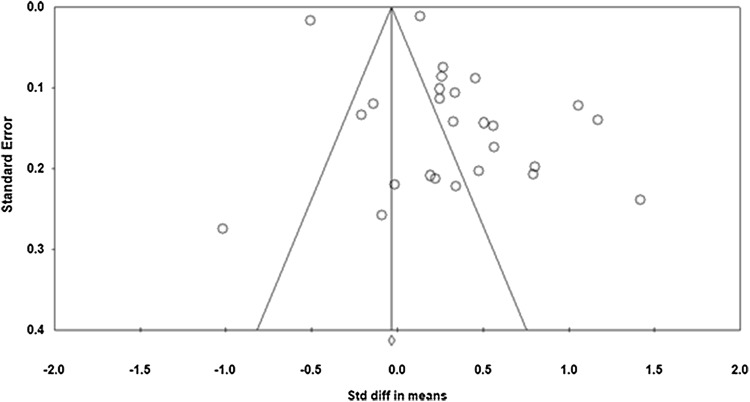
Publication bias was estimated for all included studies in this meta-analysis. From visualization of funnel plot, we confirmed the presence of biasness (Fig. 2). We confirmed the same using Egger’s regression test with biasness between studies at p < 0.05 with 95% CI [− 0.545, 6.453]. Studies with larger than average effects are more likely to be published, and this might lead to an upward bias in the summary effect.
Fig. 2.
Funnel plot for studies included in the meta-analysis
Sensitivity analysis by emitting a single study in each turn showed the pooled SMD of total triglyceride levels, HDL levels, LDL levels, total cholesterol levels, systolic and diastolic blood pressure, fasting blood glucose, body mass index and waist circumference. After emitting each study no difference in overall standard mean difference was found stating that the overall effect sizes were reliable.
Discussion
The conflicting literature associating psoriasis with cardiovascular risk factors is the cornerstone for this study. Psoriasis, which was once believed to be limited to the skin, is presently being studied and diagnosed with multidimensional medical conditions. In this meta-analysis, we analysed several individual cardiovascular risk factors including triglyceride, HDL-C, LDL-C, total cholesterol, SBP, DBP, BMI and waist circumference among psoriasis patients in comparison to non-psoriasis control subjects. Among these defined risk factors, our study reported increased levels of SBP (0.462 [0.065, 0.859]), DBP (0.463 [0.254, 0.672]), waist circumference (0.356 [0.206, 0.506]), triglycerides (0.325 [0.141, 0.508]) and FBS (0.270 [0.023, 0.517]) as highly associative risk factors in psoriasis patients. Apart from these, increased level of TC (0.276 [ 0.19, 0.363]), LDL-C (0.215 [0.070, 0.360]) and BMI (0.136 [–0.260, 0.533]) are moderately prevalent risk factors among patients. However, the role of lowered HDL-C (–0.081 [–0.323, 0.161]) as an associative risk factor remains inconclusive.
The result of the present meta-analysis indicates increased levels of SBP and DBP resulting in hypertension which is in concordance with a meta-analysis by Miller et al. (2013), Armstrong et al. (2011) and Armesto et al. (2012) who reported an analogous association with OR [95% CI] 1.8 [1.6, 2.0], 1.58 [1.42, 1.76] and 1.60 [1.24, 2.05], respectively. However, Cohen et al. (2010) and Langan et al. (2012) suggested a significantly weak association between the two with respective pooled ORs [95% CI] as 1.37 [1.29, 1.46] and 1.20 [1.11, 12.9].
It is interesting to note that increased waist circumference was a highly associative risk factor among psoriasis patients [SMD 0.356, 95% CI 0.206, 0.506]; however, increased BMI was moderately associated 0.136[95% CI -0.260, 0.533] in psoriasis patients. In earlier reports, BMI was found to be a more closely related risk factor as reported by Miller et al. (2013), who found obesity based on abdominal fat 1.6 [95% CI 1.2, 2.3] and obesity based on body mass index OR 1.8 [95% CI 1.4, 2.2]. Setty et al. (2007) found the multivariate relative risks (RR) of psoriasis were 1.40 [95% CI 1.13, 1.73] for a BMI of 25.0—29.9; 1.48 [95% CI 1.15, 1.91] for a BMI of 30.0—34.9; and 2.69 [95% CI 2.12, 3.40] for a BMI of 35.0 or greater. Kumar et al. (2013) reported a higher incidence of increased waist circumference in psoriasis patients from the USA with risk ratio 1.63 (95% CI 1.24, 2.14).
The components of dyslipidaemia may include elevated LDL cholesterol, triglycerides, and/or low HDL (protective) cholesterol. These components may occur singly or, more often, in clusters of two or all three. Our study reported standard mean difference [95% CI] for triglyceride, total cholesterol, LDL-C and HDL-C as 0.325[0.141, 0.508], 0.276[0.19, 0.363], 0.215[0.070, 0.360] and − 0.081[− 0.323–0.161], respectively. Among these components, we could not establish any existing relationship of lowered HDL-C as a risk factor in psoriasis patients. Despite several studies suggesting impaired serum levels of lipids and lipoproteins as an important risk factor for CVD among psoriasis patients, many of the reports remain controversial (Farshchian et al. 2007; Toker et al. 2009; Ma et al. 2014). However, our result was found to be in agreement with Cohen et al. (2010), who reported 48.6% dyslipidaemia in psoriasis patients in comparison to 37.9% in control subjects at p < 0.001. In a systematic review, 20 of 25 included studies found significant associations between psoriasis and dyslipidaemia, with ORs ranging from 1.04 to 5.55 (Ma et al. 2014). Miller et al. (2013) in a meta-analysis found association of dyslipidaemia in psoriasis patients at OR 1.5 (95% CI 1.4, 1.7).
The present study establishes increased blood sugar level as an important risk factor in psoriasis patients with measured SMD (95% CI) 0.27 [0.023, 0.517]. The estimate corresponds to the findings of Khalid et al. (2013) on Danish cohorts who estimated the incidence rate ratio (IRR) of diabetes mellitus was increased in all patients with psoriasis, IRR 1.49 [95% CI 1.43, 1.56], and Lee et al. (2014) reported the condition of diabetes mellitus as being prevalent in psoriasis patients with hazard ratio [95% CI] 1.35[1.11,1.65].
The individual risk factors of cardiovascular disease were also studied according to the different geographical locations of Europe, Asia and Middle East. In all, 8 European studies, 2 each from Serbia and Italy, 1 each from Spain, Poland, Romania and the UK; 7 Asian studies, 1 from South Korea and 6 from India; and 11 studies from the Middle East, including 6 from Turkey, 4 from Iran and 1 from Israel, were involved in the present study. The present study conveyed that different cardiovascular disease markers, reportedly elevated triglyceride, total cholesterol, diastolic blood pressure, systolic blood pressure, fasting blood sugar and body mass index, were highest among the Middle East region. The association was found to be statistically significant, other than HDL levels. Our result is in accordance with reports of several authors including Al-Mutairi et al. (2010) who reported higher odds of inflammatory arthritis, coronary heart disease, obesity, diabetes mellitus II, hypertension and dyslipidaemia among psoriasis patients of Kuwait. Dreiher et al. (2008) reported that prevalence of dyslipidaemia was significantly higher in psoriasis patients, OR 1.48 [95% CI 1.40,1.55]. Zindancı et al. (2012) reported that diabetes mellitus and hypertension accompanying in psoriasis patients along with MS in the Turkish population. In our subgroup analysis, increased LDL and obesity by waist circumference were found to be more associative with the European population. Our result agrees with the meta-analysis of Armstrong et al. (2012) who reported the prevalence of obesity in the same population with OR 1.66 [95% CI 1.46–1.89]. Love et al. (2011) also reported abdominal obesity to be associated with the European population with odds of 1.72 [1.03–2.86].
It is also interesting to note that the present psoriasis treatment regimen is associated with cardiovascular risk factors and cardiovascular disorders. Several authors including Hu and Lan (2017), Abuabara et al. (2011) and Prodanovich et al. (2009) in their study on American cohorts reported use of several systemic immunomodulatory therapies in psoriasis patients resulting in increased hypertension, dyslipidaemia and myocardial infarction. The present meta-analysis also points to the association of psoriasis drugs and cardiovascular risk factors. Likewise, Kim et al. (2017), Ahlehoff et al. (2015), Lan et al. (2012) and Chin et al. (2013) also stated the presence of hypertension and dyslipidaemia in psoriasis patients on treatment with methotrexate and retinoid.
In the wake of the reported results and existing literature it becomes extensively important to understand the shared molecular mechanisms responsible for the association between psoriasis and cardiovascular comorbidities. Genome-wide association studies have found the increased inheritance of certain common genetic variants, HLA, FUT2 and UBE2L3, SH2B3, to be associated with cardiovascular risk in patients with psoriasis, indicating shared genetic factors (Lu et al. 2013). Moreover, this association can be discussed in the context of common inflammatory pathways. Karbach et al. (2014) and Owczarczyk-Saczonek and Placek (2017) explained that the overexpression of Th17 cytokines (IL-17, IL-6 and IL-8) may intercede vascular inflammation and the development of atherosclerosis and cardiovascular comorbidities in patients with psoriasis. However, the temporal relationship between psoriatic systemic inflammation and cardiovascular disease remains unclear. It is possible that the systemic inflammation of psoriasis may lead to the development of cardiovascular diseases, or alternatively the cardiovascular risk factors may cause immune dysfunction leading to psoriasis. In the theory known as the “psoriatic march”, it is proposed that psoriasis may induce systemic inflammation leading to insulin resistance, endothelial dysfunction, and development of atherosclerosis and cardiovascular comorbidities (Boehncke et al. 2011).
The strength of this study is inclusion of studies with incidence (not prevalence) of cardiovascular risk factors. We have also stratified the studies into different regions to get a concise picture of cardiovascular incidences among psoriasis patients. Additionally, we also performed meta-regression analysis to assess the association of cardiovascular risk factors in psoriasis case and non-psoriasis subjects with other covariates such as region, study types, year of study and smoking behaviour.
Conclusion
To conclude, the present meta-analysis study was carried out to understand whether psoriasis has any association with cardiovascular risk factor or not. Our results support a significant association between psoriasis and incidence of major adverse cardiovascular events. While the previous literature reported moderate association between hypertension and psoriasis, our findings suggest that hypertension is a highly associative condition in psoriasis. It is noteworthy that the incidence of cardiovascular events among psoriasis patients is dominant in the Middle East population as compared to other geographic locations considered in the present study. This occurrence may be related to variations in genetic structure, environmental exposures, or issues related to health-care use. Patients with psoriasis should be educated regarding the increased risk of cardiovascular disease and aggressively treated for modifiable cardiovascular risk factors. Moreover, it would be best to conduct a study using homogenous designs and sample size to reduce heterogeneity and variability and to get a more precise information of the association between cardiovascular risk factors and psoriasis. The findings of this study could be validated amongst well-defined cohorts of patients with psoriasis individually in different regions to confirm the implication of the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
The authors acknowledge the University Grant Commission, India, for providing doctoral fellowship to Ms. Saumya Choudhary (Grant Number: .2014-15-MANF-2014-15-MUS-UTT-39790).
Abbreviations
- TGY
Triglycerides
- HDL-C
High density lipoprotein-cholesterol
- LDL-Cz
Low density lipoprotein-cholesterol
- TC
Total cholesterol
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- FBG
Fasting blood glucose
- BMI
Body mass index
- WC
Waist circumference
- SMD
Standardized mean difference
- ORs
Odds ratio
- CVD
Cardiovascular disorder
Authors’ contribution
Saumya Choudhary, Rachna Patel, Dibyabhaba Pradhan: acquisition of data. Saumya Choudhary, Rachna Patel, Dibyabhaba Pradhan: analysis or interpretation of data. All authors: concept or design, drafting of the manuscript and critical revision.
Compliance with ethical standards
Conflict of interest
The authors declare there are no competing interests.
Ethical approval
The present meta-analysis is exempted from ethical approval, as data were obtained from previous studies in which informed consent was already obtained by the trial investigator, and the present analysis will be addressing similar questions to the research question for which the data were collected.
Contributor Information
George Thomas, Email: georgethomas@shiats.edu.in.
Arun Kumar Jain, Email: akjain@instpath.gov.in.
References
- Abuabara K, Lee H, Kimball AB. The effect of systemic psoriasis therapies on the incidence of myocardial infarction: a cohort study. Br J Dermatol. 2011;165:1066–1073. doi: 10.1111/j.1365-2133.2011.10525.x. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Nayek A, Pradhan D, et al. dbGAPs: A comprehensive database of genes and genetic markers associated with psoriasis and its subtypes. Genomics. 2017;110(4):240–247. doi: 10.1016/j.ygeno.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol. 2015;29:1128–1134. doi: 10.1111/jdv.12768. [DOI] [PubMed] [Google Scholar]
- Akcali C, Buyukcelik B, Kirtak N, Inaloz S. Clinical and laboratory parameters associated with metabolic syndrome in Turkish patients with psoriasis. J Int Med Res. 2014;42:386–394. doi: 10.1177/0300060513502891. [DOI] [PubMed] [Google Scholar]
- Akhyani M, Ehsani AH, Robati RM, Robati AM. The lipid profile in psoriasis: a controlled study. J Eur Acad Dermatol Venereol. 2007;21:1330–1332. doi: 10.1111/j.1468-3083.2007.02260.x. [DOI] [PubMed] [Google Scholar]
- Al-Mutairi N, Al-Farag S, Al-Mutairi A, Al-Shiltawy M. Comorbidities associated with psoriasis: an experience from the Middle East. J Dermatol. 2010;37:146–155. doi: 10.1111/j.1346-8138.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- Armesto S, Coto-Segura P, Osuna CG, et al. Psoriasis and hypertension: a case-control study. J Eur Acad Dermatol Venereol. 2012;26:785–788. doi: 10.1111/j.1468-3083.2011.04108.x. [DOI] [PubMed] [Google Scholar]
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case–control study. PLoS ONE. 2011;6:e18227. doi: 10.1371/journal.pone.0018227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54. doi: 10.1038/nutd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeta IGR, Bittencourt FV, Gontijo B, Goulart EMA. Comorbidities and cardiovascular risk factors in patients with psoriasis. An Bras Dermatol. 2014;89:735–744. doi: 10.1590/abd1806-4841.20142874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balci A, Balci DD, Yonden Z, et al. Increased amount of visceral fat in patients with psoriasis contributes to metabolic syndrome. Dermatology (Basel) 2010;220:32–37. doi: 10.1159/000254482. [DOI] [PubMed] [Google Scholar]
- Barrea L, Macchia PE, Di Somma C, et al. Bioelectrical phase angle and psoriasis: a novel association with psoriasis severity, quality of life and metabolic syndrome. J Transl Med. 2016;14:130. doi: 10.1186/s12967-016-0889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehncke W-H, Boehncke S, Tobin A-M, Kirby B. The “psoriatic march”: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 2011;20:303–307. doi: 10.1111/j.1600-0625.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- Chin Y-Y, Yu H-S, Li W-C, et al. Arthritis as an important determinant for psoriatic patients to develop severe vascular events in Taiwan: a nation-wide study. J Eur Acad Dermatol Venereol. 2013;27:1262–1268. doi: 10.1111/j.1468-3083.2012.04706.x. [DOI] [PubMed] [Google Scholar]
- Choi WJ, Park EJ, Kwon IH, et al. Association between psoriasis and cardiovascular risk factors in Korean patients. Ann Dermatol. 2010;22:300–306. doi: 10.5021/ad.2010.22.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban M, Tasli L, Turgut S, et al. Association of adipokines, insulin resistance, hypertension and dyslipidemia in patients with psoriasis vulgaris. Ann Dermatol. 2016;28:74–79. doi: 10.5021/ad.2016.28.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AD, Weitzman D, Dreiher J. Psoriasis and hypertension: a case-control study. Acta Derm Venereol. 2010;90:23–26. doi: 10.2340/00015555-0741. [DOI] [PubMed] [Google Scholar]
- Dreiher J, Weitzman D, Davidovici B, et al. Psoriasis and dyslipidaemia: a population-based study. Acta Derm Venereol. 2008;88:561–565. doi: 10.2340/00015555-0510. [DOI] [PubMed] [Google Scholar]
- Edson-Heredia E, Zhu B, Lefevre C, et al. Prevalence and incidence rates of cardiovascular, autoimmune, and other diseases in patients with psoriatic or psoriatic arthritis: a retrospective study using clinical practice research datalink. J Eur Acad Dermatol Venereol. 2015;29:955–963. doi: 10.1111/jdv.12742. [DOI] [PubMed] [Google Scholar]
- Farshchian M, Zamanian A, Farshchian M, et al. Serum lipid level in Iranian patients with psoriasis. J Eur Acad Dermatol Venereol. 2007;21:802–805. doi: 10.1111/j.1468-3083.2006.02099.x. [DOI] [PubMed] [Google Scholar]
- Farshchian M, Ansar A, Sobhan M. Associations between cardiovascular risk factors and psoriasis in Iran. Clin Cosmet Investig Dermatol. 2015;8:437–442. doi: 10.2147/CCID.S86418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furue M, Tsuji G, Chiba T, Kadono T. Cardiovascular and Metabolic Diseases Comorbid with Psoriasis: Beyond the Skin. Intern Med. 2017;56:1613–1619. doi: 10.2169/internalmedicine.56.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Ray L, Kuruvila S, et al. Lipid accumulation product index as visceral obesity indicator in psoriasis: a case–control study. Indian J Dermatol. 2018;63:136–140. doi: 10.4103/ijd.IJD_315_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand JM. Psoriasis, diabetes, and obesity: weighing the evidence. JAMA Dermatol. 2016;152:753–754. doi: 10.1001/jamadermatol.2016.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girisha BS, Thomas N. Metabolic syndrome in psoriasis among urban south Indians: a case control study using SAM-NCEP criteria. J Clin Diagn Res. 2017;11:1–4. doi: 10.7860/JCDR/2017/24717.9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisondi P, Ferrazzi A, Girolomoni G. Metabolic comorbidities and psoriasis. Acta Dermatovenerol Croat. 2010;18:297–304. [PubMed] [Google Scholar]
- Grossman RM, Delaney RJ, Brinton EA, et al. Hypertriglyceridemia in patients with psoriasis treated with cyclosporine. J Am Acad Dermatol. 1991;25:648–651. doi: 10.1016/0190-9622(91)70247-y. [DOI] [PubMed] [Google Scholar]
- Irimie M, Oanţă A, Irimie CA, et al. Cardiovascular risk factors in patients with chronic plaque psoriasis: a case-control study on the Brasov County population. Acta Dermatovenerol Croat. 2015;23:28–35. [PubMed] [Google Scholar]
- Karbach S, Croxford AL, Oelze M, et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol. 2014;34:2658–2668. doi: 10.1161/ATVBAHA.114.304108. [DOI] [PubMed] [Google Scholar]
- Karoli R, Fatima J, Shukla V, et al. A study of cardio-metabolic risk profile in patients with psoriasis. J Assoc Physicians India. 2013;61:798–803. [PubMed] [Google Scholar]
- Katz HI, Waalen J, Leach EE. Acitretin in psoriasis: an overview of adverse effects. J Am Acad Dermatol. 1999;41:S7–S12. doi: 10.1016/s0190-9622(99)70359-2. [DOI] [PubMed] [Google Scholar]
- Khalid U, Hansen PR, Gislason GH, et al. Psoriasis and new-onset diabetes: a Danish nationwide cohort study. Diabetes Care. 2013;36:2402–2407. doi: 10.2337/dc12-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278–285. [PMC free article] [PubMed] [Google Scholar]
- Kothiwala SK, Khanna N, Tandon N, et al. Prevalence of metabolic syndrome and cardiovascular changes in patients with chronic plaque psoriasis and their correlation with disease severity: A hospital-based cross-sectional study. Indian J Dermatol Venereol Leprol. 2016;82:510–518. doi: 10.4103/0378-6323.183638. [DOI] [PubMed] [Google Scholar]
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change, and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293–1298. doi: 10.1111/jdv.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan C-CE, Ko Y-C, Yu H-S, et al. Methotrexate reduces the occurrence of cerebrovascular events among Taiwanese psoriatic patients: a nationwide population-based study. Acta Derm Venereol. 2012;92:349–352. doi: 10.2340/00015555-1283. [DOI] [PubMed] [Google Scholar]
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556–562. doi: 10.1038/jid.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-S, Lin R-Y, Lai M-S. Increased risk of diabetes mellitus in relation to the severity of psoriasis, concomitant medication, and comorbidity: a nationwide population-based cohort study. J Am Acad Dermatol. 2014;70:691–698. doi: 10.1016/j.jaad.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the national health and nutrition examination survey, 2003–2006. Arch Dermatol. 2011;147:419–424. doi: 10.1001/archdermatol.2010.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chen H, Nikamo P, et al. Association of cardiovascular and metabolic disease genes with psoriasis. J Invest Dermatol. 2013;133:836–839. doi: 10.1038/jid.2012.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Li M, Wang H, et al. High prevalence of cardiovascular risk factors in patients with moderate or severe psoriasis in northern China. Arch Dermatol Res. 2014;306:247–251. doi: 10.1007/s00403-013-1437-3. [DOI] [PubMed] [Google Scholar]
- Mallbris L, Akre O, Granath F, et al. Increased risk for cardiovascular mortality in psoriasis inpatients but not in outpatients. Eur J Epidemiol. 2004;19:225–230. doi: 10.1023/b:ejep.0000020447.59150.f9. [DOI] [PubMed] [Google Scholar]
- Masson Regnault M, Konstantinou M-P, Khemis A, et al. Early relapse of psoriasis after stopping brodalumab: a retrospective cohort study in 77 patients. J Eur Acad Dermatol Venereol. 2017;31:1491–1496. doi: 10.1111/jdv.14387. [DOI] [PubMed] [Google Scholar]
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the general practice research database. Eur Heart J. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milčić D, Janković S, Vesić S, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based cross-sectional study. An Bras Dermatol. 2017;92:46–51. doi: 10.1590/abd1806-4841.20175178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller IM, Ellervik C, Yazdanyar S, Jemec GBE. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014–1024. doi: 10.1016/j.jaad.2013.06.053. [DOI] [PubMed] [Google Scholar]
- Owczarczyk-Saczonek A, Placek W. Interleukin-17 as a factor linking the pathogenesis of psoriasis with metabolic disorders. Int J Dermatol. 2017;56:260–268. doi: 10.1111/ijd.13420. [DOI] [PubMed] [Google Scholar]
- Parodi A, Aste N, Calvieri C, et al. Metabolic syndrome prevalence in psoriasis: a cross-sectional study in the Italian population. Am J Clin Dermatol. 2014;15:371–377. doi: 10.1007/s40257-014-0074-8. [DOI] [PubMed] [Google Scholar]
- Pehlevan S, Yetkin DO, Bahadır C, et al. Increased prevalence of metabolic syndrome in patients with psoriatic arthritis. Metab Syndr Relat Disord. 2014;12:43–48. doi: 10.1089/met.2013.0039. [DOI] [PubMed] [Google Scholar]
- Piskin S, Gurkok F, Ekuklu G, Senol M. Serum lipid levels in psoriasis. Yonsei Med J. 2003;44:24–26. doi: 10.3349/ymj.2003.44.1.24. [DOI] [PubMed] [Google Scholar]
- Praveenkumar U, Ganguly S, Ray L, et al. Prevalence of metabolic syndrome in psoriasis patients and its relation to disease duration: a hospital based case-control study. J Clin Diagn Res. 2016;10:1–5. doi: 10.7860/JCDR/2016/17791.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–703. doi: 10.1001/archdermatol.2009.94. [DOI] [PubMed] [Google Scholar]
- Raimondo A, Balato A, Megna M, Balato N. Limitations of current monoclonal antibodies for plaque-type psoriasis and an outlook for the future. Expert Opin Biol Ther. 2018;18:605–607. doi: 10.1080/14712598.2018.1479738. [DOI] [PubMed] [Google Scholar]
- Ražnatović-Đurović M, Janković J, Janković S. Prevalence of metabolic syndrome in Montenegrin patients with psoriasis. Vojnosanit Pregl. 2016;73:1016–1021. doi: 10.2298/VSP150114138R. [DOI] [PubMed] [Google Scholar]
- Richard M-A, Barnetche T, Horreau C, et al. Psoriasis, cardiovascular events, cancer risk and alcohol use: evidence-based recommendations based on systematic review and expert opinion. J Eur Acad Dermatol Venereol. 2013;27(Suppl 3):2–11. doi: 10.1111/jdv.12162. [DOI] [PubMed] [Google Scholar]
- Schiattarella M, Caiazzo G, Di Caprio R, et al (2019) Paraoxonases and psoriasis: negative imbalance of antioxidant endogenous mechanisms. Ital Dermatol Venereol 154:192–196. 10.23736/S0392-0488.17.05537-7 [DOI] [PubMed]
- Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch Intern Med. 2007;167:1670–1675. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287–292.e4. doi: 10.1016/j.jaad.2017.03.037. [DOI] [PubMed] [Google Scholar]
- Sharma YK, Prakash N, Gupta A. Prevalence of metabolic syndrome as per the NCEP and IDF definitions vis-a-vis severity and duration of psoriasis in a semi-urban Maharashtrian population: a case control study. Diabetes Metab Syndr. 2016;10:S72–76. doi: 10.1016/j.dsx.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Siegel D, Devaraj S, Mitra A, et al. Inflammation, atherosclerosis, and psoriasis. Clin Rev Allergy Immunol. 2013;44:194–204. doi: 10.1007/s12016-012-8308-0. [DOI] [PubMed] [Google Scholar]
- Singh S, Dogra S, Shafiq N, et al. Prevalence of metabolic syndrome in psoriasis and levels of interleukin-6 and tumor necrosis factor-α in psoriasis patients with metabolic syndrome: Indian tertiary care hospital study. Int J Appl Basic Med Res. 2017;7:169–175. doi: 10.4103/ijabmr.IJABMR_330_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Pradhan D, Puri P, et al. Genomic alterations driving psoriasis pathogenesis. Gene. 2019;683:61–71. doi: 10.1016/j.gene.2018.09.042. [DOI] [PubMed] [Google Scholar]
- Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321–328. doi: 10.1007/s00403-006-0703-z. [DOI] [PubMed] [Google Scholar]
- Tablazon ILD, Al-Dabagh A, Davis SA, Feldman SR. Risk of cardiovascular disorders in psoriasis patients: current and future. Am J Clin Dermatol. 2013;14:1–7. doi: 10.1007/s40257-012-0005-5. [DOI] [PubMed] [Google Scholar]
- Taheri SM, Hedayati MT, Shokohi T, HajHeydari Z (2014) Serum lipids and lipoproteins in patients with psoriasis. Arch Iran Med 17:343–346. https://doi.org/0141705/AIM.007 [PubMed]
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377–390. doi: 10.1016/j.jaad.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A, Kadi M, Yildirim AK, et al. Serum lipid profile paraoxonase and arylesterase activities in psoriasis. Cell Biochem Funct. 2009;27:176–180. doi: 10.1002/cbf.1553. [DOI] [PubMed] [Google Scholar]
- Uczniak S, Gerlicz ZA, Kozłowska M, Kaszuba A. Presence of selected metabolic syndrome components in patients with psoriasis vulgaris. Postepy Dermatol Alergol. 2016;33:114–119. doi: 10.5114/ada.2016.59153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyanik BS, Ari Z, Onur E, et al. Serum lipids and apolipoproteins in patients with psoriasis. Clin Chem Lab Med. 2002;40:65–68. doi: 10.1515/CCLM.2002.013. [DOI] [PubMed] [Google Scholar]
- Vayá A, Ricart JM, Andino B, et al. Psoriasis and hemorheology: influence of the metabolic syndrome. Clin Hemorheol Microcirc. 2013;55:331–339. doi: 10.3233/CH-2012-1639. [DOI] [PubMed] [Google Scholar]
- Zindancı I, Albayrak O, Kavala M, et al. Prevalence of metabolic syndrome in patients with psoriasis. Sci World J. 2012;2012:312463. doi: 10.1100/2012/312463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.