Significance
Sudden cardiac death in heart failure is a major unsolved clinical problem that is linked to the development of a spontaneous arrhythmia. Early afterdepolarizations (EADs) are an arrhythmogenic mechanism, but the cellular trigger for EADs in heart failure is unclear. We show that the reduction in synchronous Ca2+ release early in the action potential (AP) of failing cardiac myocytes promotes the appearance of late Ca2+ sparks which can propagate, forming Ca2+ ripples and waves. These, in turn, produce an inward sodium–calcium exchange current which opposes AP repolarization. Restoration of AP phase 1 repolarization improved Ca2+ release synchrony and reduced late Ca2+ spark rate, suggesting a different approach to reducing the risk of sudden death in heart failure.
Keywords: heart, arrhythmia, cardiac myocytes, action potential, Ca2+ sparks
Abstract
Sudden death in heart failure patients is a major clinical problem worldwide, but it is unclear how arrhythmogenic early afterdepolarizations (EADs) are triggered in failing heart cells. To examine EAD initiation, high-sensitivity intracellular Ca2+ measurements were combined with action potential voltage clamp techniques in a physiologically relevant heart failure model. In failing cells, the loss of Ca2+ release synchrony at the start of the action potential leads to an increase in number of microscopic intracellular Ca2+ release events (“late” Ca2+ sparks) during phase 2–3 of the action potential. These late Ca2+ sparks prolong the Ca2+ transient that activates contraction and can trigger propagating microscopic Ca2+ ripples, larger macroscopic Ca2+ waves, and EADs. Modification of the action potential to include steps to different potentials revealed the amount of current generated by these late Ca2+ sparks and their (subsequent) spatiotemporal summation into Ca2+ ripples/waves. Comparison of this current to the net current that causes action potential repolarization shows that late Ca2+ sparks provide a mechanism for EAD initiation. Computer simulations confirmed that this forms the basis of a strong oscillatory positive feedback system that can act in parallel with other purely voltage-dependent ionic mechanisms for EAD initiation. In failing heart cells, restoration of the action potential to a nonfailing phase 1 configuration improved the synchrony of excitation–contraction coupling, increased Ca2+ transient amplitude, and suppressed late Ca2+ sparks. Therapeutic control of late Ca2+ spark activity may provide an additional approach for treating heart failure and reduce the risk for sudden cardiac death.
Worldwide, ∼26 million people suffer from heart failure (HF) (1). More than 50% of HF patients die suddenly, and this sudden cardiac death is most likely due to the spontaneous emergence of arrhythmias (2, 3). At the cellular level, two identified initiators of cardiac arrhythmias are delayed afterdepolarizations (DADs) and early afterdepolarizations (EADs) (4). DADs occur in the resting period between heart beats (diastole) and are due to spontaneous Ca2+ release from the sarcoplasmic reticulum (SR) in the form of Ca2+ sparks (5), which summate to form propagating Ca2+waves (6, 7). These waves cause a depolarizing inward current via the Na+/Ca2+ exchange (NCX) mechanism (6, 8, 9). In contrast to DADs, EADs are less well understood and occur during the repolarization phase of the cardiac action potential (AP) where several ionic currents interact to control repolarization (10). EADs can be produced by reactivation of ionic currents during AP repolarization when the potassium currents forming the “repolarization reserve” (11, 12) are insufficient to maintain the repolarization trajectory of the AP, although why this should occur spontaneously within a steady train of APs is uncertain (13). Spontaneous Ca2+ waves have also been implicated in EAD generation (9), but it is unclear how such waves might be initiated when the SR should be depleted and/or refractory after SR Ca2+ release is triggered by the upstroke of the AP (14).
We recently showed that “late” Ca2+ sparks (LCS) can occur during the decay of the Ca2+ transient in normal rabbit cardiac myocytes (15). Here, we report that stochastic LCS and low-amplitude “Ca2+ ripples” are promoted in HF and can generate sufficient NCX current (INCX) to trigger EADs. In addition, normalizing the early AP waveform in HF promotes a faster and larger SR Ca2+ release (which should improve contractility) and at the same time decreases arrhythmogenic LCS activity.
Results
LCS occur in a wide variety of species (SI Appendix, Fig. S1), but, to study repolarization mechanisms, an AP similar to human is desirable, and we therefore used an established rabbit model of chronic myocardial infarction that causes HF. When left ventricular ejection fraction had fallen to 44 ± 3% (SI Appendix, Fig. S2 and Table S1), indicative of moderate to severe HF, ventricular myocytes exhibited t-tubule remodeling (Fig. 1 A and B) similar to that seen in most models of HF (16). This “t-tubule disease” (17) decreases the efficiency of excitation–contraction coupling and the rate of rise of the Ca2+ transient (SI Appendix, Fig. S3) (18, 19). Although there was no change in ICa density (Fig. 1C) in this HF model, the AP lost phase 1 repolarization (red arrow, Fig. 1D) and became prolonged (SI Appendix, Fig. S3). All of these changes are also seen in human HF (17, 20). The loss of AP phase 1 contributes to the reduced SR Ca2+ release rate (Fig. 1E and SI Appendix, Fig. S3) and increased SR release latency (Fig. 1F) (16, 18) because this phase of the AP affects the magnitude and time course of ICa which triggers SR Ca2+ release (21, 22).
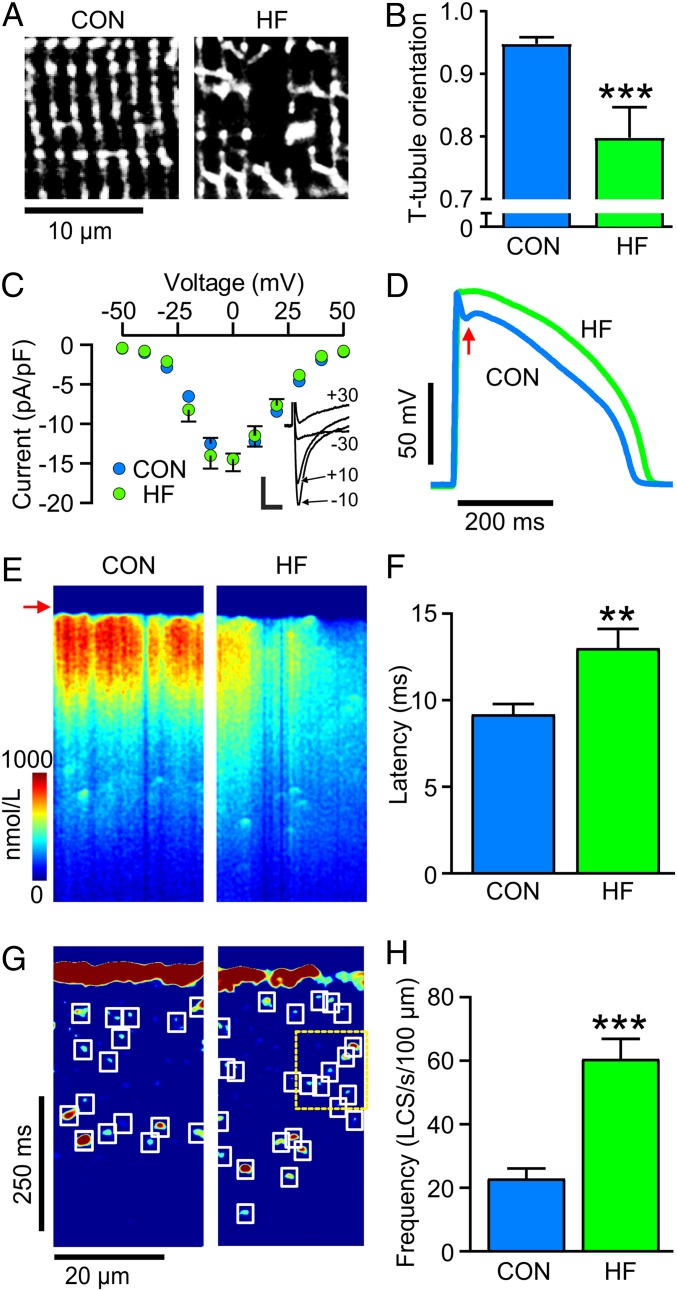
Fig. 1.
Changes in structure, AP time course, t-tubule morphology, and increased LCS in HF. (A) di-8-ANEPPS staining shows reduced tubule regularity and (B) reorientation of t-tubules in HF cells compared to control (CON). n/N = 9/4 CON 12/5 HF. (C) ICa density was unchanged in HF. n/N = 20/4 CON 10/3 HF Representative currents at the indicated test potentials (mV) are shown (Inset). Error bars not shown where error is less than the size of symbols. (Scale bars: 5 pA/pF, 10 ms.) (D) APs in HF myocytes had less AP phase 1 repolarization (arrow). (E) Ca2+ transients in CON myocytes showed synchronous Ca2+ release, while HF showed less uniform Ca2+ release and (F) an increase in latency from electrical stimulation to detectable Ca2+ increase. (G) Image processing reveals increased LCS frequency in HF with some Ca2+ ripples (yellow dashed box). (H) LCS frequency was greater in HF cells than in CON. n/N = 16/6 CON; 14/5 HF. **P < 0.01, ***P < 0.001, unpaired t tests.
The reduction in synchronous Ca2+ release in HF, seen as a less uniform increase in Ca2+ after the AP upstroke (Fig. 1E, arrow), was associated with an increase in the number of LCS (Fig. 1 G and H). This ∼3-fold increase in LCS frequency can be explained by an increase in the number of Ca2+ release sites that were not triggered earlier during the AP (15, 22). The increased availability of Ca2+ release sites also promotes sequential activation of LCS, resulting in Ca2+ ripples (15), an example of which can be seen within the yellow dashed box in Fig. 1G.
Linkage between LCS and Arrhythmogenesis.
LCS activity depends on SR Ca2+ content (15) which generally increases with AP frequency (10, 23). A pacing-pause protocol increases SR Ca2+ and may provoke EADs (24). With this protocol, LCS activity increased, and EADs appeared (Fig. 2A). The high rate of LCS production (and appearance of Ca2+ ripples; see SI Appendix, Fig. S4) in these conditions clearly opposed the normal decline of Ca2+ during the Ca2+ transient.
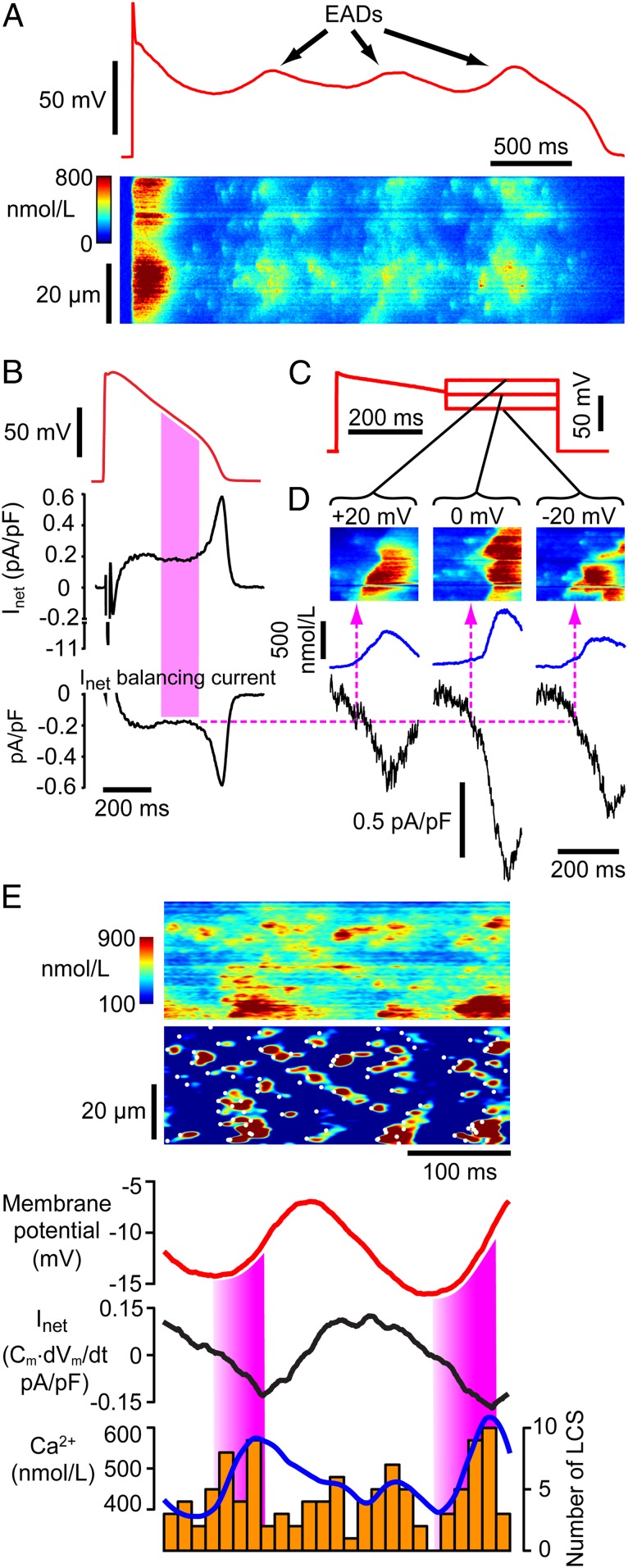
Fig. 2.
LCS can trigger EADs. (A) EADs evoked by pacing-pause protocol. During repolarization, an initial Vm inflection at ∼ −20 mV generates EADs (Top), and numerous LCS create oscillatory Ca2+ release (Bottom). (B) Calculation of Inet for a typical HF AP. The filled region indicates where the greatest number of LCS occur (A and Fig. 4E) and corresponds to an Inet of ∼0.18 pA/pF. Any balancing current that counteracts Inet (i.e., −Inet, Bottom) will stop repolarization (SI Appendix, Fig. S5). (C) Failing AP with voltage-clamp steps used to probe Ca2+ and currents underlying EADs. (D) During the AP steps, LCS-triggered Ca2+ waves (Top) and inward currents (black trace) appear. Dashed line shows the inward current that balances Inet in B and could therefore stop repolarization. This current occurs when LCS are about to initiate Ca2+ waves. (E) Example of apparently chaotic Ca2+ during EADs in an HF cell (Top). Image processing reveals many LCS and Ca2+ ripples during Vm oscillations (red trace). The filled regions show where the depolarizing current (Inet) is increasing, and this corresponds to increasing Ca2+ (blue) and LCS activity (orange bars).
In order to generate an EAD, the net cellular repolarizing current (Inet) and/or repolarization reserve (11, 12) must be decreased and/or counteracted by depolarizing membrane current. While Inet is the sum of all inward and outward currents, the rate of change of membrane potential (dVm/dt) is directly proportional to Inet because Inet = −Cm·dVm/dt (where Cm is membrane capacitance). Applying this equation allows us to determine how much current is responsible for the repolarization phase of the AP, and Fig. 2B illustrates the magnitude and time course of Inet.. In this exemplar, Inet was only ∼0.18 pA/pF during the late AP repolarization phase, and the average Inet during AP repolarization was 0.26 ± 0.08 pA/pF (mean ± SD n/N = 15/4), showing that a small change in membrane currents will affect repolarization trajectory. It follows that any additional inward current of similar amplitude to Inet could stop AP repolarization (SI Appendix, Fig. S5) and thereby initiate an EAD.
An increase in intracellular Ca2+ moves the electrochemical gradient for NCX Ca2+ transport outward, causing an inward shift of INCX (25) which can be up to ∼1 pA/pF in magnitude (26). To examine whether the observed LCS activity could produce sufficient INCX to oppose Inet, we voltage-clamped cells with a failing AP which included steps to different holding potentials around the times (and Vm) where EADs occur (Fig. 2C). During these Vm steps, many LCS occurred which triggered Ca2+ ripples and Ca2+ waves. The average Ca2+ (blue trace) and evoked inward current (black trace) are shown in Fig. 2 D, Lower. This inward current had a linear relationship to mean Ca2+ with a slope of −1.5 pA/pF/µmol/L (SI Appendix, Fig. S6), compatible with the expected Ca2+ dependence of INCX (26). The critical inward current (from Fig. 2B) that will balance Inet and stop repolarization is indicated by the dotted line which, after projection onto the Ca2+ records, reveals the Ca2+ changes associated with this critical current density. It is notable that this current density is developed just before the onset of Ca2+ waves which appear as chevron patterns in line scans (5, 7), showing that LCS and/or Ca2+ ripples can be the initiating events for EADs.
Fig. 2E shows that multiple Ca2+ ripples, rather than Ca2+ waves, can also give rise to EADs in current-clamped cells. In this exemplar, the line scan Ca2+image (upper panel) is rather chaotic with no obvious Ca2+ waves being present. However, after image processing, numerous LCS events are seen, some of which form propagating Ca2+ ripples (cf. SI Appendix, Fig. S4). Vm and Inet can be linked to Ca2+ by two mechanisms: (1) Either Ca2+ activates inward INCX producing an inward shift of Inet and depolarization of Vm or (2) Vm activates L-type Ca2+ channels (LTCCs) which then trigger SR Ca2+ release. As shown by the colored bars in Fig. 2E, the rise in Ca2+ occurred in synchrony with inward Inet (peak value ∼ 0.15 pA/pF) which also supports the idea that the initiation of EADs can result from the summation of LCS and Ca2+ ripples which activate inward INCX.
Model Analysis of the Role of ICa and INCX in EADs.
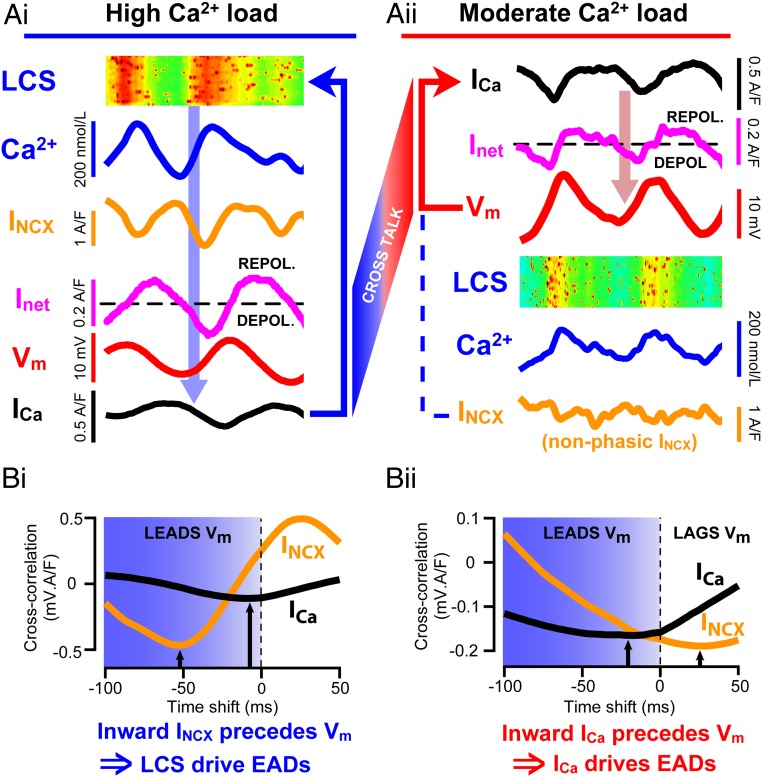
The importance of INCX for EAD generation has also been demonstrated in murine cells by heterozygous NCX knockdown and in failing rabbit ventricle by acute NCX blockade (27, 28). To further test the idea that sufficient INCX can be generated by LCS to trigger an oscillatory EAD, we modified a new computer model of spatially distributed Ca2+ signaling (29) to calculate membrane currents that should occur in response to experimental Vm and Ca2+ signals (Fig. 3A). This approach breaks the intrinsic feedback loop(s) that otherwise make experimental dissection of causation problematic. Utilizing the recorded Ca2+ and Vm data shown in Fig. 2 as exemplar controlling variables, the model exhibited two types of behavior: when Ca2+ and LCS activity was high (Fig. 3Ai), INCX was in phase with dV/dt and of sufficient magnitude to explain the start of the Vm oscillations (cf. Fig. 2 D and E). However, when SR Ca2+ release was slightly reduced, changes in INCX were much smaller because the change in Vm opposed the change in electrochemical gradient for Ca2+ extrusion by NCX (Fig. 3Aii). The relative roles of INCX and ICa in initiating the EAD can be examined by cross-correlation with Vm. The magnitude of the negative peak in the cross-correlogram shows the strength of the linkage between these inward currents and Vm, while the time delay associated with the peak in the correlogram indicates whether they are the consequence of a change in Vm (by lagging behind Vm) or a potential cause (by leading the change in Vm). When the Ca load was high, inward INCX clearly preceded the change in Vm (Fig. 3Bi), consistent with the idea that the EADs were triggered by LCS activity and the consequent change in INCX. In comparison, ICa was both more weakly correlated and almost in phase with Vm. When SR load was decreased, the reduction in LCS activity led to a smaller inward INCX being less well correlated with Vm and with inappropriate phase to explain EAD initiation (Fig. 3Bii). However, ICa became more strongly correlated with Vm (due to a decrease in Ca2+-dependent LTCC inactivation (10)) and had appropriate timing to explain EAD initiation. Thus, while both mechanisms for EAD generation were present in the model, an increase in Ca2+ release caused the dominant inward current trigger for EAD generation to shift from ICa reactivation to LCS-evoked INCX.
Fig. 3.
Computer simulations of experimental traces using a model that generates LCS (29). Panels are ordered to indicate the flow of causation. (Ai) Increased LCS frequency is in phase with inward INCX and dVm/dt, which precedes Vm and ICa forming an oscillatory feedback pathway as shown by the arrows. (Aii) When Ca2+ load is reduced, Vm oscillates because ICa (and possibly INa) supply the inward current to increase dVm/dt and cause the EAD, as shown. In this case, LCS (and Ca2+) are the consequence of the reactivation of ICa. Note that in this simulation, INCX bears no obvious relation to dVm/dt (unlike the results shown in Ai). Cross-talk between the oscillators shown in Ai and Aii can occur because they contain common mechanisms. (B) Cross-correlograms between Vm and both ICa and INCX from the data shown in part A. The negative peaks in the cross-correlogram (black arrows) reveal both the strength of association (amplitude) and temporal relationship (offset from Vm) between Vm and ICa or INCX (see SI Appendix). (Bi) When Ca2+ load was high, inward INCX preceded Vm oscillations and was more strongly correlated with Vm than ICa. (Bii) Under reduced Ca2+ load, INCX lags behind Vm oscillations. ICa reactivation preceded Vm and so has the required temporal relationship to explain EAD initiation, although some inward INCX may still contribute (13).
Improving Ca2+ Signaling and Suppressing LCS in HF Cells.
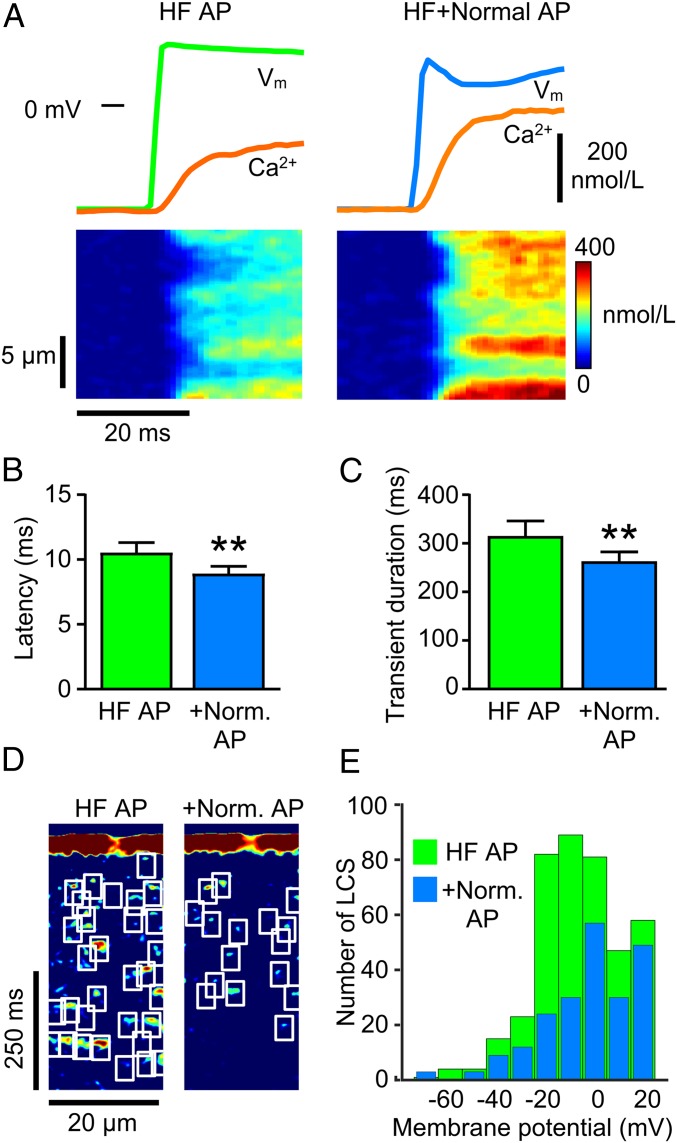
Fig. 4A shows that a normal AP applied to HF cells increased the rate of rise and amplitude of the Ca2+ transient, and this was associated with an increase in Ca2+ release synchrony. On average, the normal AP increased the rate of rise of the Ca2+ transient from 30 ± 8 nmol/L/ms to 40 ± 9 nmol/L/ms (P < 0.01, paired t test), while the time to peak decreased from 75 ± 9 ms to 65 ± 9 ms (P < 0.01, paired t test). This improvement in excitation–contraction coupling efficiency was also reflected by a decrease in the latency for Ca2+ release (Fig. 4B). With the improvement in synchrony of early release, the duration of the Ca2+ transient became shorter (Fig. 4C) due to an ∼50% decrease in LCS frequency (Fig. 4 D and E). These results confirm that at least a part of the nonuniform and delayed SR Ca2+ release seen in the HF model is due to AP phase 1 effects on ICa.
Fig. 4.
Applying a normal AP to HF cells improves excitation–contraction coupling and reduces LCS frequency. (A) Ca2+ transient upstroke velocity, amplitude (orange), and Ca2+ release synchrony all increase in an HF cell (Left) when voltage-clamped with a normal AP (Right). (Lower) Improved synchrony in Ca2+ release. (B) Mean latency for Ca2+ release and (C) Ca2+ transient duration were reduced in HF cells clamped with a normal AP. (D) Processed Ca2+ images show fewer LCS in HF after applying normal AP. (E) Number of LCS detected in HF with failing AP waveform (HF AP, 404 LCS) and with a normal AP (+Norm AP, 217 LCS) from 13 HF cells binned by Vm during the AP repolarization. **P < 0.01, paired t test n/N = 13/5.
It may not be possible to completely restore the maximum rate of rise, time to peak Ca2+, and Ca2+ transient duration to CON values with phase 1 modification alone because (1) the t-tubule disruption associated with HF would still be present and (2) there is reduced SERCA2a expression in HF which decreases SR Ca2+ uptake rate (30). Nevertheless, it is apparent that AP restoration can improve the defective Ca2+ signaling seen in HF and, importantly, reduce the LCS activity that can trigger EADs.
Discussion
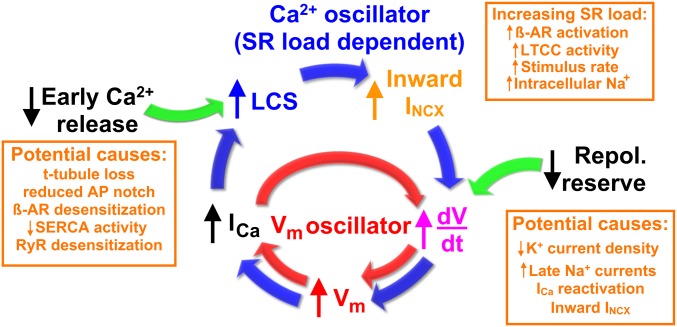
Reactivation of ICa and INa to overcome the repolarization reserve and initiate EADs has been widely considered to be the primary mechanism for EAD generation (illustrated by the inner feedback loop shown in Fig. 5), e.g., refs. 11, 31, and 32. Indeed, when SR Ca2+ release was inhibited by blocking LTCCs, it was still possible to evoke EADs by a simulated ICa (33). Here we show that LCS-activated inward NCX current can initiate EADs and, we suggest, act as a coupled oscillator to further increase the risk for development of multiple EADs (outer loop in Fig. 5). This Ca2+ oscillator arises from two connected mechanisms: (1) Diffusion of Ca2+ from an initiating LCS may trigger additional LCS due to the gain inherent in Ca2+-induced Ca2+ release (CICR). This SR load-dependent process manifests as propagating Ca2+ ripples and, if enough LCS sites are available, more synchronous and larger Ca2+ waves. (2) LCS will increase inward NCX current to depolarize Vm (34) while Vm couples back onto LCS activity via the Vm dependence of LTCC gating (which may explain the Vm dependence of LCS frequency shown in Fig. 4E). The stochastic nature of LCS provides an explanation for the sudden appearance of arrhythmogenic EADs, and, once started, an EAD will promote additional LCS and EADs due to the increased SR Ca2+ load arising from ICa reactivation.
Fig. 5.
Flow diagram for two interacting positive-feedback mechanisms that can drive Vm and Ca2+ oscillations during EADs. The red inner cycle represents the well-established Vm oscillator, wherein an increase in dVm/dt (caused by a relative increase in depolarizing currents compared to repolarizing currents, e.g., text in orange box at Lower Right) leads to voltage-dependent reactivation of ICa, which in turn causes further depolarization. The blue outer cycle represents the stochastic LCS-mediated Ca2+ oscillator mechanism for EAD initiation. Reduced early Ca2+ release and/or increased SR load increases LCS production, and the resulting increase in inward INCX tends to depolarize the membrane which then feeds onto ICa to trigger additional LCS. Green arrows indicate possible factors contributing to EAD initiation. β-AR, beta-adrenoreceptor.
The computer model clearly showed that both the electrical (Vm) and Ca2+ oscillators can couple and synergize (Fig. 4A). While the phase relationship between LCS-dependent NCX currents and dVm/dt directly supports the idea that INCX can initiate Ca2+ dominant oscillations, ICa reactivation is also important (35) because late LTCC openings are a potent trigger for LCS (15). However, when Ca2+ levels are lower, and Ca2+ ripples and waves cannot form, EADs can still arise from instability in the repolarization process due to recruitment of noninactivated inward currents in the presence of insufficient repolarization reserve (11). In the latter case, smaller numbers of LCS may still play a lesser role in EAD initiation, and this is reminiscent of the role played by diastolic Ca2+ sparks in cardiac pacemaking by sino-atrial node cells (36).
Ca2+ Ripples, Ca2+ Waves, and Vm Oscillator Coupling.
Although Ca2+ waves (Fig. 2D) have been implicated in EAD genesis (37), it is notable that the fluctuations in Ca2+ shown in Fig. 2 A and E are not typical Ca2+ waves (as seen in Fig. 2D) (6, 7); instead, the Ca2+ fluctuations came from low-amplitude Ca2+ ripples. In such cases, the local propagation of Ca2+ release (short-range Ca2+ ripples) may not be able to initiate cell-wide Ca2+ waves because the effective amplification of Ca2+ release by CICR is insufficient for full Ca2+ wave support. This lack of sufficient amplification could be explained by the stochastic nature of LCS, coupled with local SR refractoriness (15, 38), decreasing the recruitment of adjacent LCS sites. Nevertheless, some synchronization in Ca2+ ripple initiation must occur for average Ca2+ to oscillate. The mathematical basis for the emergence of macroscopic Ca2+ oscillations from large numbers of independent LCS oscillators is beyond the scope of this study, but Kuramoto model analysis has shown that macroscopic oscillations can develop even when coupling across individual oscillators is weak (39). The relative importance of the Ca2+ and Vm oscillators in EAD generation will be variable because they depend on many factors such as the actual Vm trajectory, K+ current availability, the level of SR Ca2+ loading in the cell, and ICa availability, as well as RYR2 Ca2+ sensitivity, as illustrated in Fig. 5. In connection with this point, increased LTCC activity and SR load associated with β-adrenergic stimulation (10) would almost certainly increase the risk for LCS-stimulated EADs.
It should be noted that the LCS activity seen in our confocal line scans reflects only a small fraction of the actual number of LCS occurring in the entire cell; the confocal line scan surveys ∼2% of the cell volume, so ∼1 LCS/ms (Fig. 2E) would correspond to ∼50 LCS/ms cell-wide, and this much larger number underlies the measured NCX current that can initiate the EAD. This estimate is in reasonable agreement with the computer model used here which predicts that 107 LCS/ms may generate 0.26 pA/pF NCX current (29).
Since LTCC activity is common to both the Vm and Ca2+ oscillators, the idea that LTCC gating modification could be a therapeutic target for EAD prevention (33) becomes even more attractive. In addition, if modifying the late component of ICa is able to reduce net Ca2+ influx into the cell, SR Ca2+ content might be reduced (40), and this would also inhibit LCS activity (15). The improvement in Ca2+ signaling produced by applying a normal AP to HF myocytes is remarkable. This suggests that new therapies should be developed with the aim of improving early Ca2+ release by restoring phase 1 repolarization and/or restoring t-tubule regularity. This would reduce LCS frequency and thereby reduce the risk for potentially lethal LCS-triggered arrhythmias as well as mitigate the defective excitation–contraction coupling seen in HF (41).
Materials and Methods
More extensive details are available in SI Appendix. Briefly, all experiments were performed in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 and institutional approval by the University of Bristol ethics committee. We used an established coronary artery ligation model that leads to heart failure in adult New Zealand White rabbits (3–3.5 kg) which were daily monitored for health status. The target endpoint for HF was an ejection fraction of 40% (as measured by echocardiography SI Appendix, Fig. S2). Some rabbits did not quite reach this endpoint but presented other indicators of heart failure including dilated left ventricle and lung congestion (SI Appendix, Table S1). This model, due to repolarizing current behavior, can be used to gain insight into repolarization reserve with human relevance (42). It was not possible to wait for arrhythmias to start in this model (as they would be fatal), but EADs can be provoked in vitro by suitable interventions. Rather than use pharmacological manipulation to provoke EADs, we chose a pacing-pause method to minimize other possible perturbations of cellular function.
Cardiac Myocyte Isolation.
Left ventricular epicardial myocytes were obtained from rabbit hearts after full anesthesia (50 mg/kg sodium pentobarbital i.v.) and euthanasia. Enzymatic dissociation was carried out using 1 mg/mL collagenase I (Worthington), 0.05 mg/mL protease (type XIV Sigma), and 0.1 mmol/L Ca2+, as described previously (22). Guinea pig, rat, mouse, and zebrafish myocytes were isolated using similar methods to those described previously (22, 43, 44), and methods for zebrafish myocyte isolation are given in SI Appendix.
Electrophysiology.
Electrophysiology experiments were performed in a modified Tyrode’s solution (containing, in mmol/L: 133 NaCl, 5 KCl, 1 NaH2PO4, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10 glucose, 1.8 CaCl2, 1 MgCl2, pH 7.4 with NaOH) at 36 ± 1 °C. Patch pipettes were pulled from borosilicate glass using a P80 micropipette puller (Sutter Instruments). Pipettes were filled with an intracellular solution containing, in mmol/L: 120 aspartic acid, 20 KCl, 10 HEPES, 10 NaCl, 5 glucose, 5 Mg.ATP, 0.05 Fluo-4 pentapotassium salt, with KOH added to adjust to pH 7.2. Tip resistance was typically 1.6–2.0 MΩ when filled with this solution. Membrane potential and currents were recorded using an Axopatch 1D amplifier (Molecular Devices), Power1401 digitizer (Cambridge Electronic Design), and Signal data acquisition software (version 6.04, Cambridge Electronic Design). Cell membrane capacitance was measured by step depolarizations to −75 mV from a holding potential of −80 mV for 25 ms. Series resistance was compensated by ∼70%. Liquid junction potential (10 mV) was subtracted from recordings.
Confocal Imaging.
Ca2+ sparks and transients were recorded in line scan mode (45) from the fluo-4 loaded cells using an inverted confocal microscope (LSM 880, Zeiss) with a 1.4 NA 63× oil immersion lens. Excitation light was provided by a 488-nm argon laser, and fluorescence emission was collected at 492–600 nm. Ca2+ line scans were recorded with the pinhole set to <2 Airy units, at a pixel size of 0.1–0.2 μm/pixel, and with a scan speed of 1 ms per line. GaAsP photodetectors were used to increase the sensitivity of Ca2+ spark detection. The t-tubule system was imaged by labeling the sarcolemma with di-8-ANEPPS from a stock 1 mmol/L solution (in anhydrous dimethyl sulfoxide) added directly to the cell-recording chamber (final concentration 1 μmol/L) for 2–3 min. Excitation was at 488 nm, and emission was collected at >600 nm.
Statistical Analysis.
Statistical analyses were performed at the level of the cell, and statistics on replicates of n individual independent cell experiments from N animals are given in the text as n/N. Data were tested for normality using the Shapiro-Wilk test (Prism7, Graphpad); in any cases where data were skewed, the test was reapplied to log-transformed data. Paired or unpaired t tests were performed on normally and log-normally distributed data. Results are presented as mean ± SEM. The limit of statistical confidence was P < 0.05.
Data Availability Statement.
All data and computer codes are available upon request from the authors.
Supplementary Material
Acknowledgments
We thank Drs. H. Cheng (BHF PG/16/55/32277 to J.C.H.), C. Du (BHF PG/15/106/31915 to J.C.H.), and R. J. Richardson for supplying some of the myocytes used for SI Appendix, Fig. S1 and Prof. G. L. Smith (University of Glasgow) for help and advice on HF model development.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918649117/-/DCSupplemental.
References
- 1.Ponikowski P., et al. , Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 1, 4–25 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E. J., et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee , Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 139, e56–e528 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Tomaselli G. F., Zipes D. P., What causes sudden death in heart failure? Circ. Res. 95, 754–763 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Clusin W. T., Calcium and cardiac arrhythmias: DADs, EADs, and alternans. Crit. Rev. Clin. Lab. Sci. 40, 337–375 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Cheng H., Lederer W. J., Cannell M. B., Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science 262, 740–744 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Berlin J. R., Cannell M. B., Lederer W. J., Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circ. Res. 65, 115–126 (1989). [DOI] [PubMed] [Google Scholar]
- 7.Cheng H., Lederer M. R., Lederer W. J., Cannell M. B., Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am. J. Physiol. 270, C148–C159 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Houser S. R., Piacentino V. 3rd, Weisser J., Abnormalities of calcium cycling in the hypertrophied and failing heart. J. Mol. Cell. Cardiol. 32, 1595–1607 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Shiferaw Y., Aistrup G. L., Wasserstrom J. A., Intracellular Ca2+ waves, afterdepolarizations, and triggered arrhythmias. Cardiovasc. Res. 95, 265–268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bers D. M., Excitation-Contraction Coupling and Cardiac Contractile Force (Kluwer Academic Publishers, Dordrecht, ed. 2, 2001). [Google Scholar]
- 11.Roden D. M., Abraham R. L., Refining repolarization reserve. Heart Rhythm 8, 1756–1757 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegyi B., et al. , Altered repolarization reserve in failing rabbit ventricular myocytes: Calcium and β-adrenergic effects on delayed- and inward-rectifier potassium currents. Circ. Arrhythm Electrophysiol. 11, e005852 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato D., Xie L.-H., Nguyen T. P., Weiss J. N., Qu Z., Irregularly appearing early afterdepolarizations in cardiac myocytes: Random fluctuations or dynamical chaos? Biophys. J. 99, 765–773 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobie E. A., Song L.-S., Lederer W. J., Local recovery of Ca2+ release in rat ventricular myocytes. J. Physiol. 565, 441–447 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler E. D., Kong C. H. T., Hancox J. C., Cannell M. B., Late Ca2+ sparks and ripples during the systolic Ca2+ transient in heart muscle cells. Circ. Res. 122, 473–478 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sipido K. R., Cheng H., T-tubules and ryanodine receptor microdomains: On the road to translation. Cardiovasc. Res. 98, 159–161 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Crossman D. J., et al. , T-tubule disease: Relationship between t-tubule organization and regional contractile performance in human dilated cardiomyopathy. J. Mol. Cell. Cardiol. 84, 170–178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litwin S. E., Zhang D., Bridge J. H., Dyssynchronous Ca2+ sparks in myocytes from infarcted hearts. Circ. Res. 87, 1040–1047 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Song L. S., et al. , Orphaned ryanodine receptors in the failing heart. Proc. Natl. Acad. Sci. U.S.A. 103, 4305–4310 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piacentino V., 3rd, et al. , Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ. Res. 92, 651–658 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Sah R., Ramirez R. J., Backx P. H., Modulation of Ca2+ release in cardiac myocytes by changes in repolarization rate: Role of phase-1 action potential repolarization in excitation-contraction coupling. Circ. Res. 90, 165–173 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Cooper P. J., Soeller C., Cannell M. B., Excitation-contraction coupling in human heart failure examined by action potential clamp in rat cardiac myocytes. J. Mol. Cell. Cardiol. 49, 911–917 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Endoh M., Force-frequency relationship in intact mammalian ventricular myocardium: Physiological and pathophysiological relevance. Eur. J. Pharmacol. 500, 73–86 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Viswanathan P. C., Rudy Y., Pause induced early afterdepolarizations in the long QT syndrome: A simulation study. Cardiovasc. Res. 42, 530–542 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Eisner D. A., Lederer W. J., Na-Ca exchange: Stoichiometry and electrogenicity. Am. J. Physiol. 248, C189–C202 (1985). [DOI] [PubMed] [Google Scholar]
- 26.Ginsburg K. S., Weber C. R., Bers D. M., Cardiac Na+-Ca2+ exchanger: Dynamics of Ca2+-dependent activation and deactivation in intact myocytes. J. Physiol. 591, 2067–2086 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bögeholz N., et al. , Suppression of early and late afterdepolarizations by heterozygous knockout of the Na+/Ca2+ exchanger in a murine model. Circ. Arrhythm Electrophysiol. 8, 1210–1218 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Milberg P., et al. , Acute inhibition of the Na+/Ca2+ exchanger reduces proarrhythmia in an experimental model of chronic heart failure. Heart Rhythm 9, 570–578 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Zhong M., et al. , NCX-Mediated subcellular Ca2+ dynamics underlying early afterdepolarizations in LQT2 cardiomyocytes. Biophys. J. 115, 1019–1032 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flesch M., et al. , Sarcoplasmic reticulum Ca2+ATPase and phospholamban mRNA and protein levels in end-stage heart failure due to ischemic or dilated cardiomyopathy. J. Mol. Med. (Berl.) 74, 321–332 (1996). [DOI] [PubMed] [Google Scholar]
- 31.January C. T., Riddle J. M., Early afterdepolarizations: Mechanism of induction and block. A role for L-type Ca2+ current. Circ. Res. 64, 977–990 (1989). [DOI] [PubMed] [Google Scholar]
- 32.Boutjdir M., el-Sherif N., Pharmacological evaluation of early afterdepolarisations induced by sea anemone toxin (ATXII) in dog heart. Cardiovasc. Res. 25, 815–819 (1991). [DOI] [PubMed] [Google Scholar]
- 33.Madhvani R. V., et al. , Shaping a new Ca2+ conductance to suppress early afterdepolarizations in cardiac myocytes. J. Physiol. 589, 6081–6092 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato D., Dixon R. E., Santana L. F., Navedo M. F., A model for cooperative gating of L-type Ca2+ channels and its effects on cardiac alternans dynamics. PLoS Comput. Biol. 14, e1005906 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss J. N., Garfinkel A., Karagueuzian H. S., Chen P.-S., Qu Z., Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm 7, 1891–1899 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maltsev V. A., Lakatta E. G., Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc. Res. 77, 274–284 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z., et al. , Revisiting the ionic mechanisms of early afterdepolarizations in cardiomyocytes: Predominant by Ca waves or Ca currents? Am. J. Physiol. Heart Circ. Physiol. 302, H1636–H1644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobie E. A., Lederer W. J., Dynamic local changes in sarcoplasmic reticulum calcium: Physiological and pathophysiological roles. J. Mol. Cell. Cardiol. 52, 304–311 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pietras B., Daffertshofer A., Network dynamics of coupled oscillators and phase reduction techniques. Phys. Rep. 819, 1–105 (2019). [Google Scholar]
- 40.Eisner D. A., Choi H. S., Díaz M. E., O’Neill S. C., Trafford A. W., Integrative analysis of calcium cycling in cardiac muscle. Circ. Res. 87, 1087–1094 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Gómez A. M., et al. , Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science 276, 800–806 (1997). [DOI] [PubMed] [Google Scholar]
- 42.Baczkó I., Jost N., Virág L., Bősze Z., Varró A., Rabbit models as tools for preclinical cardiac electrophysiological safety testing: Importance of repolarization reserve. Prog. Biophys. Mol. Biol. 121, 157–168 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Bryant S. M., et al. , Caveolin 3-dependent loss of t-tubular ICa during hypertrophy and heart failure in mice. Exp. Physiol. 103, 652–665 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng H., et al. , Potent hERG channel inhibition by sarizotan, an investigative treatment for Rett Syndrome. J. Mol. Cell. Cardiol. 135, 22–30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannell M. B., Cheng H., Lederer W. J., Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys. J. 67, 1942–1956 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and computer codes are available upon request from the authors.