Significance
While type IV pili (Tfp) are one of the most widespread adhesive factors found in prokaryotes, little is known about their cellular targets. Obtaining a better understanding of the molecular basis of cellular recognition by Tfp remains an important challenge with major implications for the infectious process of pathogenic bacteria, for host tissue tropism, and for the design of novel inhibitors of adhesion to combat infectious diseases. In this study, we identify the complex N-glycan recognized by meningococcal Tfp. We furthermore demonstrate that glycan fucosylation determines selective receptor recognition. This study unravels the molecular basis of receptor recognition by meningococcal Tfp and outlines a strategy to identify carbohydrate motifs that are targeted by Tfp and therefore, constitute attractive therapeutic targets.
Keywords: type IV pili, host–pathogen interaction, virulence, Neisseria meningitidis, glycan
Abstract
Bacterial infections are frequently based on the binding of lectin-like adhesins to specific glycan determinants exposed on host cell receptors. These interactions confer species-specific recognition and tropism for particular host tissues and represent attractive antibacterial targets. However, the wide structural diversity of carbohydrates hampers the characterization of specific glycan determinants. Here, we characterized the receptor recognition of type IV pili (Tfp), a key adhesive factor present in numerous bacterial pathogens, using Neisseria meningitidis as a model organism. We found that meningococcal Tfp specifically recognize a triantennary sialylated poly-N-acetyllactosamine–containing N-glycan exposed on the human receptor CD147/Basigin, while fucosylated derivatives of this N-glycan impaired bacterial adhesion. Corroborating the inhibitory role of fucosylation on receptor recognition, adhesion of the meningococcus on nonhuman cells expressing human CD147 required prior defucosylation. These findings reveal the molecular basis of the selective receptor recognition by meningococcal Tfp and thereby, identify a potential antibacterial target.
Most microorganisms express adherence factors at their surface, also known as “adhesins,” that facilitate the attachment or adherence of bacteria to host cells in order to colonize and infect the host. Many microbial adhesins display lectin activity and bind to specific glycan regions on constituents of the glycocalyx, such as cell surface glycoproteins, glycosphingolipids, or glycosaminoglycans (1). Adhesion may be mediated through terminal sugars or internal carbohydrate motifs present in linear or branched oligosaccharide chains. The wide structural diversity of carbohydrates allows many combinatorial possibilities for fine tuning host–microbial interactions. It can provide relatively strong and species-specific recognition and can confer the tropism of individual bacteria for a particular host tissue (2). Detailed studies of the specificity of such microbial lectins can lead to the identification and synthesis of powerful inhibitors of adhesion that may form the basis for novel therapeutic agents to combat infectious diseases (3). However, due to the structural complexity and functional diversity of carbohydrates, characterization of the exact nature of the specific carbohydrate motifs recognized by microbial adhesins remains difficult. Indeed, there have been only a few cases where human receptors of pathogenic adhesins have been described (1).
In numerous pathogenic and nonpathogenic bacteria, type IV pili (Tfp) are the key adhesive factor (4). Tfp have a conserved architecture and function among bacterial species (4, 5) and are composed of long filaments that are made up of a repeating structural pilin subunit, which is organized in a helical fashion, in addition to minor pilins that mediate binding to the host cell (6, 7). Some evidences suggest that Tfp can interact with carbohydrates. For instance, Tfp of Vibrio parahaemolyticus, a naturally occurring bacterium common in coastal waters, can interact with chitin, a long-chain polymer of N-acetylglucosamine abundantly found in the ocean (8), while Tfp from Pseudomonas aeruginosa, an important opportunistic pathogen causing acute and chronic pulmonary infections, can bind to purified complex N-glycans (9). More recently, Tfp of Neisseria meningitidis (meningococcus) were also shown to bind to complex N-glycans such as GD2 ganglioside on a glycan array (10). However, these studies did not provide accurate information on the exact nature of the glycan determinant recognized by Tfp on host cells. As an example, the highly restricted expression of GD2 ganglioside mainly in the cerebellum and in peripheral nerves (11) cannot account for the ability of meningococci to specifically colonize human vessels and meninges, two interactions at the heart of meningococcal pathophysiology (12, 13). This, therefore, suggests that meningococcal Tfp may potentially recognize a different selective glycan determinant on human cells.
Here, we used N. meningitidis as a model organism to dissect the receptor recognition of Tfp and designed an innovative strategy to unravel the selective glycan structure targeted by meningococcal Tfp. N. meningitidis is exquisitely adapted to humans and normally resides asymptomatically in the human nasopharynx. It becomes one of the most harmful bacterial pathogens when it reaches the bloodstream and colonizes human vessels. This bacterium rapidly causes progressing septic shock, leading, in the worst cases, to purpura fulminans, an acute systemic inflammatory response associated with an intravascular coagulation and endothelial dysfunctions. Meningococci can also interact with brain endothelial cells and cross the blood–brain barrier, causing meningitis (14–16). Adhesion of meningococci to human endothelial cells was shown to be supported by the direct interaction of Tfp with the endothelial receptor CD147/Basigin (13, 17), a member of the Ig superfamily, containing two Ig-like domains. CD147 has three N-glycosylation sites at positions 44, 152, and 186 that carry high mannose and complex N-glycans, which can be fucosylated and sialylated (18). These N-glycans play a pivotal role in CD147 function, regulating receptor oligomerization, metalloproteinase induction activity, or its interaction with other proteins (19).
Using a combination of lectin affinity methods, enzymatic procedure, and mass spectrometry analysis to dissect the intricate interaction of meningococcal Tfp with CD147, we hereby identify the complex sialylated triantennary poly-N-acetyllactosamine (LacNAc)–containing N-glycan specifically recognized by meningococcal Tfp on Asn186 of CD147. We further show that glycan fucosylation determines the specificity of meningococcal adhesion to human CD147 receptor. This approach may have broad applicability to infections by piliated bacteria and highlights the interest of targeting specific glycan determinants recognized by microbial adhesins in the treatment of clinical bacterial infections.
Results
Tfp-Dependent Adhesion of Meningococci to Human Endothelial Cells Relies on Sialyl-LacNAc–Containing N-Glycan.
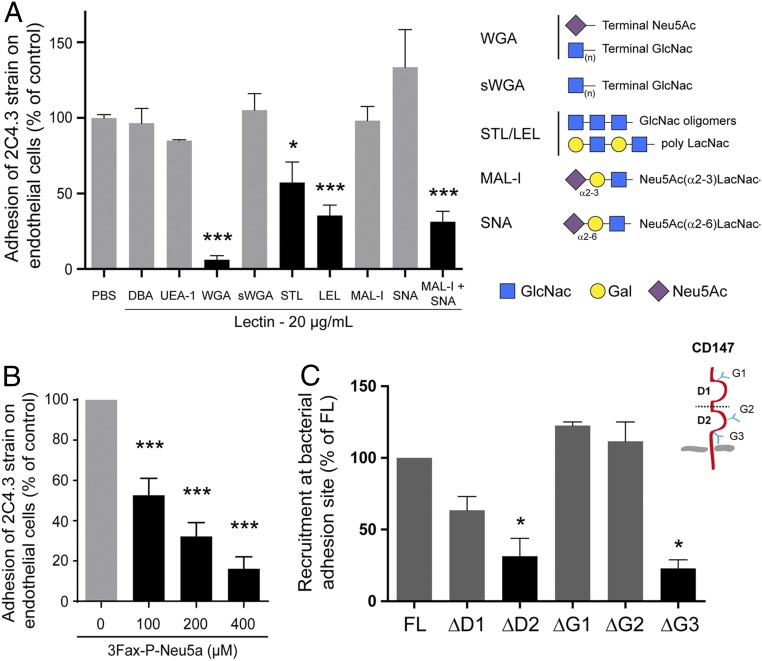
To address the role of CD147-carried N-glycans in the interaction between meningococcal Tfp and endothelial cells, we first qualitatively assessed the inhibitory effect of a library of 23 lectins with characterized glycan-binding specificity on meningococcal adhesion to human brain microvascular endothelial cells (human cerebral microvascular endothelial cells [hCMEC/D3s]) (SI Appendix, Table S1). Among the 23 lectins tested, only 3 inhibited bacterial adhesion to endothelial cells (i.e., wheat germ agglutinin [WGA], which binds both to N-acetylneuraminic acid [Neu5Ac; sialic acid] and N-acetyl-β-d-glucosamine [GlcNAc]; Solanum tuberosum lectin [STL] and Lycopersicon esculentum lectin [LEL], which binds both GlcNAc oligomers and LacNAc). This inhibition was then quantified (Fig. 1A). Dolichos biflorus agglutinin, which binds to terminal N-acetylgalactosamine, and Ulex europaeus agglutinin (UEA-1), which binds to l-fucose, were used as negative control. Addition of WGA inhibited bacterial adhesion to endothelial cells by 90%, whereas succinylated WGA, which only binds to nonsialylated carbohydrates terminating with GlcNAc, had no effect. The addition of chitotriose (a multimer of GlcNAc) as a competitive inhibitor of carbohydrates containing GlcNAc did not affect meningococcal adhesion (SI Appendix, Fig. S1A) and therefore, suggested that Tfp might preferentially interact with sialylated carbohydrates. Furthermore, STL and LEL, which share several substrates, both inhibited adhesion by 50%, suggesting that internal LacNAc and/or LacNAc oligomers may be part of the carbohydrate motif recognized by meningococcal Tfp on the adhesion receptor (Fig. 1A).
Fig. 1.
Tfp-dependent adhesion of N. meningitidis to human endothelial cells relies on a sialyl-LacNAc–containing N-glycan. (A) Inhibition of N. meningitidis adhesion to human endothelial cells using lectins as competitive inhibitors. hCMEC/D3s were incubated with 20 µg/mL of the indicated lectin(s) for 1 h followed by a 30-min infection with the N. meningitidis 2C4.3 strain and subsequent colony forming unit (CFU) determination. Bars represent the percentage of bacterial adhesion in relation to the phosphate buffered saline (PBS) control condition. Statistically significant conditions are indicated in dark gray. Mean ± SEM. DBA, D. biflorus agglutinin; sWGA, succinylated wheat germ agglutinin. *P < 0.01 using one-way ANOVA; ***P < 0.001 using one-way ANOVA. (B) Inhibition of the cellular sialyltransferases impedes bacterial adhesion. 3Fax-P-Neu5a–treated and –nontreated hCMEC/D3s were infected for 30 min before determining CFU. Bars represent the percentage of bacterial adhesion in relation to the no 3Fax-P-Neu5a control condition. Mean ± SEM. ***P < 0.001 using one-way ANOVA. (C) CD147 Asn186 is critical for receptor recognition. hBMECs were transfected with the different engineered HA-tagged human CD147 constructs: full length (FL; CD147-HA-FL); deleted of the distal Ig domain 1 (CD147-HA-ΔD1); deleted of the membrane proximal Ig domain 2 (CD147-HA-ΔD2); or lacking one of the three glycosylation sites by replacement of Asparagine 44, 152, or 186 to Alanine (CD147-HA-ΔG1, -ΔG2, or -ΔG3, respectively). Transfected cells were infected with N. meningitidis 2C4.3 for 2 h and processed for immunofluorescence staining with DAPI and anti-HA antibody before analysis by confocal microscopy. CD147 structure and position of the glycosylation sites are included for clarity. Schematic representation of the different constructs and representative images of the infections are shown in SI Appendix, Fig. S2. Bars represent the percentage of colonies that recruit the truncated or mutated forms of HA-tagged CD147 at the sites of bacterial adhesion as compared with the recruitment of the FL form. Mean ± SEM. *P < 0.05 using one-way ANOVA.
Two sialyl-LacNAc species exist in eukaryotic cells, Neu5Ac-(α2-6)– or -(α2-3)–bound LacNAc. Each one of these species is recognized by a specific lectin (SNA and MAL-I, respectively). While neither SNA nor MAL-I incubated separately inhibited adhesion of meningococci, the combination of both lectins was sufficient to inhibit adhesion by 70% (Fig. 1A), demonstrating that N. meningitidis Tfp may interact specifically with a sialyl-LacNAc–containing N-glycan, regardless of the bond between sialic acid and LacNAc.
To further confirm the role of Neu5Ac in meningococcal adhesion to human endothelial cells, hCMEC/D3s were treated with 3Fax-P-Neu5a, a Neu5Ac analog that inhibits sialyltransferases. As expected, treatment with 3Fax-P-Neu5a at concentrations varying from 100 to 400 µM modified the glycosylation profile of the complex sialylated N-glycans (high glycosylated, HG) fraction of CD147 receptor in a dose-dependent manner, whereas it did not alter the high-mannose N-glycans (low glycosylated, LG) fraction or the membranous expression of CD147 (SI Appendix, Fig. S1 B and C). This effect was accompanied by a dose-dependent inhibition of N. meningitidis adhesion to hCMEC/D3s (by 40 to 80%) (Fig. 1B), thereby demonstrating that the presence of a sialylated N-glycan was critical for adhesion of N. meningitidis to human endothelial cells.
CD147 Asn186 Is Critical for Receptor Recognition by N. meningitidis.
To address the potential role of N-glycans in CD147 receptor recognition by Tfp, we engineered hemagglutinin (HA)-tagged human CD147 lacking one of the three glycosylation sites by replacement of Asparagine 44, 152, or 186 to Alanine (namely CD147ΔG1, -ΔG2, or -ΔG3, respectively) (SI Appendix, Fig. S2A).These constructs were expressed in human bone marrow microvascular endothelial cells (hBMECs) along with full-length CD147 and two truncated forms of CD147, CD147ΔD1 and CD147ΔD2, that lack the first or the second extracellular Ig domain, respectively. The different forms of CD147 were properly expressed at the cell membrane of hBMECs (SI Appendix, Fig. S2B). CD147ΔD2 was not accumulated at bacterial adhesion sites, while full-length CD147 and CD147ΔD1 were massively concentrated under colonies as previously described (17). CD147ΔG1 and CD147ΔG2 were also accumulated under colony. Conversely, the CD147ΔG3 mutant failed to accumulate at N. meningitidis adhesion sites (Fig. 1C and SI Appendix, Fig. S2B), highlighting the key role of the Asn186 N-glycosylation site in Tfp–CD147 interaction.
Direct Interaction of Meningococcal Tfp with CD147 Is Dependent on Sialylated-Containing Carbohydrates.
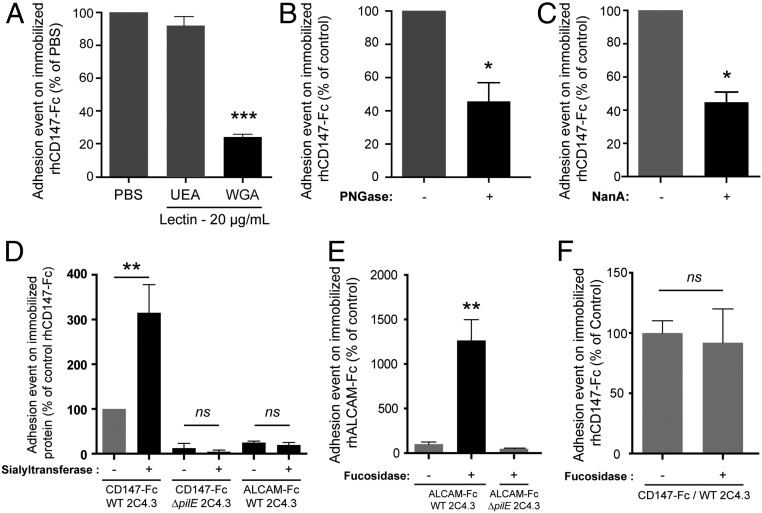
To further explore the recognition of a sialylated N-glycan on CD147 by meningococcal Tfp, we took advantage of the use of a glycosylated recombinant soluble chimeric CD147 molecule in which the extracellular domain of CD147 had been fused to the Fc domain of human IgG1 (CD147-Fc). Consistent with our previous observation (13), wild-type bacteria adhered to immobilized CD147-Fc but not to recombinant ALCAM/CD166-Fc. ALCAM/CD166-Fc is an adhesion molecule of the Ig superfamily that is composed of five Ig domains and nine N-glycosylation sites and is also expressed on endothelial cells (SI Appendix, Fig. S3A) (20). In addition, no binding to immobilized CD147-Fc was observed with a nonpiliated ΔpilE meningococcus derivative or with a nonadherent ΔpilV derivative, thus showing that meningococci bind directly to CD147 in a Tfp-dependent manner (SI Appendix, Fig. S3A). Furthermore, we confirmed that WGA inhibited bacterial adhesion on immobilized CD147-Fc (Fig. 2A) as shown above on whole cells (Fig. 1A).
Fig. 2.
Adhesion of meningococci to immobilized recombinant CD147-Fc is dependent on a sialylated N-glycan. (A) The WGA lectin inhibits adhesion of meningococci to immobilized recombinant CD147-Fc. Adhesion of wild-type (WT) N. meningitidis to immobilized recombinant CD147-Fc was assessed in the presence of 20 µg/mL of UEA-1 agglutinin, WGA, or phosphate buffered saline (PBS) alone as control after 1 h of infection. Bars represent the percentage of adhesion events in relation to the adhesion to immobilized CD147 in the control condition. Mean ± SEM. ***P < 0.001 using one-way ANOVA. (B) Deglycosylation of recombinant CD147-Fc impairs adhesion of meningococci. CD147-Fc recombinant proteins were deglycosylated using peptide-N-glycosydase (PNGase) or left in PNGase buffer alone as control before immobilization on slides. Native and deglycosylated recombinant CD147-Fc were similarly immobilized on slides (SI Appendix, Fig. S4A). Adhesion of WT N. meningitidis to immobilized proteins was assessed after 1 h of infection. Bars represent the percentage of adhesion events on deglycosylated CD147 as compared with native CD147. Mean ± SEM. *P < 0.05 using Student’s t test. (C and D) Adhesion to immobilized CD147-Fc is dependent on sialylation. CD147-Fc and ALCAM-Fc recombinant proteins were either desialylated using α2-3,6,8,9 Neuraminidase A (NanA; C) or oversialylated using sialyltransferase (D) before immobilization on slides. Control native proteins were incubated in the presence of the corresponding buffer alone. Native and enzymatically modified recombinant proteins were similarly immobilized on slides (SI Appendix, Fig. S4 B and C). Adhesion of WT N. meningitidis to immobilized proteins after 1 h of infection was quantified. Bars represent the percentage of adhesion events on modified proteins as compared with native proteins. Mean ± SEM. ns, nonsignificant using one-way ANOVA. *P < 0.05 using Student t test; **P < 0.01 using one-way ANOVA. (E and F) Defucosylation of recombinant ALCAM-Fc is sufficient to promote meningococcal adhesion. Recombinant ALCAM-Fc (E) and CD147-Fc (F) were defucosylated or incubated in buffer alone as the control before being immobilized on slides to similar extent (SI Appendix, Fig. S4 D and E). Adhesion of WT N. meningitidis or nonpiliated ΔpilE derivatives to immobilized proteins was quantified after 1 h of infection. Bars represent the percentage of adhesion events on defucosylated proteins as compared with native proteins. Mean ± SEM. ns, nonsignificant using one-way ANOVA. **P < 0.01 using one-way ANOVA.
We then assessed the adhesion of N. meningitidis on enzymatically deglycosylated recombinant CD147-Fc. Deglycosylation reduced the apparent molecular weight of CD147 and its ability to interact with WGA (SI Appendix, Fig. S3B) and reduced meningococcal adhesion to CD147-Fc by 60% (Fig. 2B). Enzymatic desialylation of recombinant CD147-Fc was followed by a 55% decrease in bacterial adhesion, confirming the role of Neu5Ac in adhesion of meningococci to CD147 (Fig. 2C). Conversely, meningococci adhesion to oversialylated recombinant CD147-Fc was increased by 300% compared with the adhesion to native proteins (Fig. 2D), whereas adhesion of nonpiliated ΔpilE meningococci derivatives was unchanged (Fig. 2D). Importantly, a similar in vitro sialylation of ALCAM-Fc had no effect on bacterial adhesion compared with native ALCAM-Fc (Fig. 2D). These results confirmed that meningococcal Tfp recognize a complex sialylated N-glycan carried by CD147 and that Neu5Ac by itself is not sufficient to mediate adhesion.
Meningococcal Tfp Interact with a Complex Sialylated Triantennary N-Glycan.
To determine precisely which sialylated N-glycan was targeted by meningococcal Tfp on CD147-Fc, we analyzed the N-glycan content of both desialylated and native recombinant CD147-Fc and ALCAM-Fc using matrix-assisted laser desorption/ionization mass spectrometry. Both native recombinant proteins carried a large variety of complex N-glycans ranging from simple biantennary N-glycan (ion 1,835) to complex tetraantennary N-glycan (ion 3,492) (SI Appendix, Fig. S5 and Table S2). The two main N-glycans present on both proteins were ions at mass (m/z) 1,835 and 2,040 that represent 63.9% of the CD147-Fc N-glycans and 32.2% of the ALCAM-Fc N-glycans, respectively (SI Appendix, Table S2). Importantly, we noticed that seven N-glycans released from CD147-Fc were not recovered from ALCAM-Fc (ions are reported in Table 1 and SI Appendix, Table S2). All of these ions corresponded to complex sialylated triantennary N-glycans that were also absent from the desialylated fraction of CD147-Fc, suggesting their potential role in Tfp-dependent adhesion to CD147.
Table 1.
Sialic acid–containing N-glycans are present only in the native recombinant CD147-Fc fraction (expressed as percentage of the total ion population)
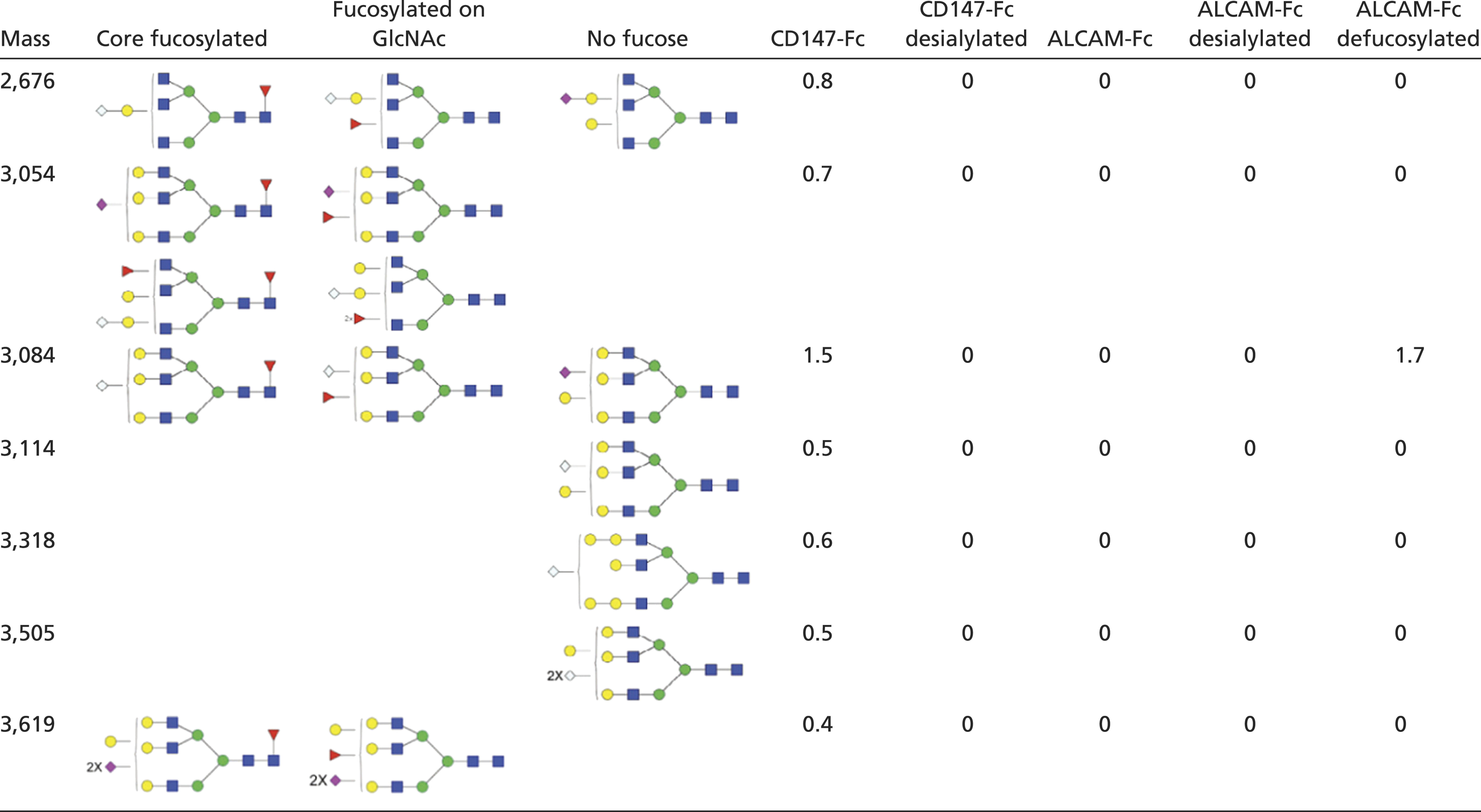 |
Glycan symbols and color coding recommended by the Consortium for Functional Glycomics are used: Blue ■, GlcNAc; green ●, Man; yellow ●, Gal; red ▲, Fuc; pink ◆, Neu5Ac; open ◊, Neu5Gc. Representative laser desorption ionization mass spectra of desialylated and native recombinant CD147-Fc and ALCAM-Fc are shown in SI Appendix, Fig. S5.
Interestingly, we found in the spectra of N-glycans released from ALCAM-Fc some ions closely related to these seven sialylated triantennary N-glycans but carrying additional fucose residues. For instance, while ion 3,084 was absent from the ALCAM-Fc fraction, we found ions 3,258 and 3,432 corresponding to ion 3,084 with the addition of one or two fucose residues (SI Appendix, Table S2). Likewise, ion 3,114 was absent from the ALCAM-Fc fraction, whereas two fucosylated forms of this ion (ions 3,288 and 3,462) were recovered from this receptor (SI Appendix, Table S2). In a general manner, N-glycans present on ALCAM-Fc appeared to be more fucosylated than those present on CD147-Fc since the ALCAM-Fc fraction counted 17.1% of bi- or trifucosylated N-glycans, while the CD147-Fc fraction counted only 3.6% of these N-glycans.
We, therefore, hypothesized that fucosylation may prevent the interaction of meningococcal Tfp with their specific sialylated N-glycan(s). To test this hypothesis, we assessed the ability of meningococci to adhere to enzymatically defucosylated ALCAM-Fc. While meningococci did not adhere to native ALCAM-Fc proteins, they adhered to defucosylated ALCAM-Fc (Fig. 2E). Importantly, this interaction was also dependent on Tfp as the nonpiliated ΔpilE meningococcus derivative did not adhere to defucosylated ALCAM-Fc (Fig. 2E). Conversely, defucosylation of CD147-Fc did not significantly affect bacterial adhesion to CD147-Fc (Fig. 2F). Interestingly, on defucosylation, the percentage of bi- or trifucosylated N-glycans released from ALCAM-Fc dropped from 17.1 to 1.9% (SI Appendix, Table S2), and the loss of ion 3,258 or 3,432 on defucosylated ALCAM-Fc fraction was correlated with the appearance of ion 3,084, the only sialylated triantennary N-glycan also present on CD147 (Table 1). Together, these results indicated a good correlation between bacterial adhesion and the presence of the low-fucosylated triantennary N-glycan ion 3,084 on CD147, which contains a monosialylated LacNAc. They also provide evidence that the fucosylation profile of this specific N-glycan is critical to support bacterial adhesion. Notably, while sialylated triantennary N-glycan recognized by meningococcal Tfp may be naturally present on receptors other than CD147, they may be differently fucosylated.
Fucosylation Alters Recognition of the Human CD147 Receptor by Meningococci on Host Cells.
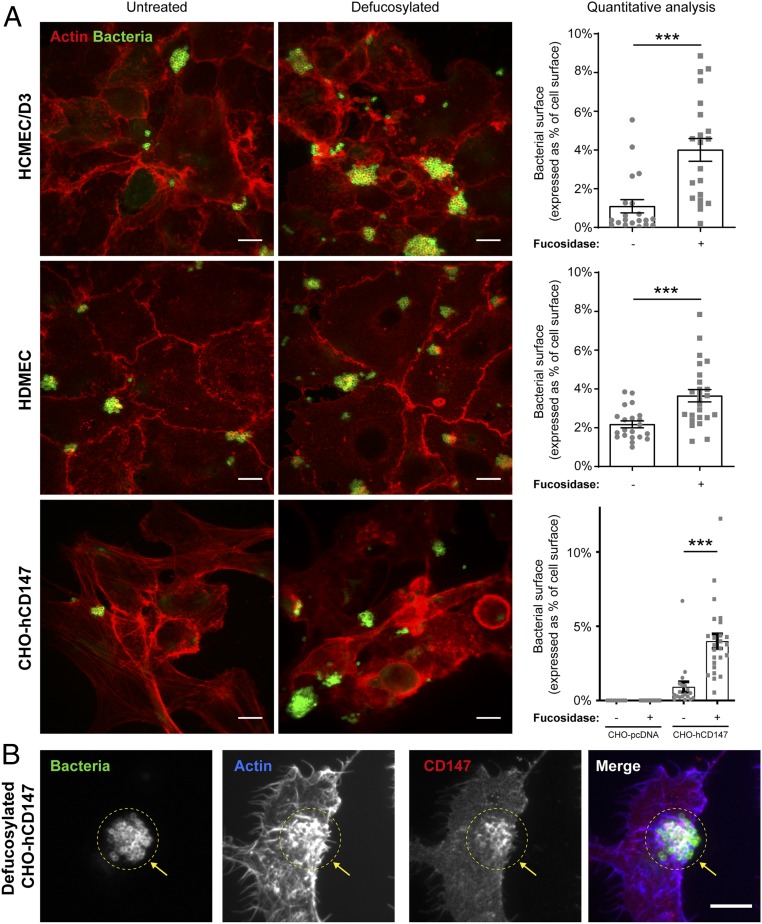
As the above results indicated that N-glycan fucosylation may fine tune receptor recognition by meningococcal Tfp on endothelial cells, we examined meningococcal adhesion to hCMEC/D3s and primary human dermal microvascular endothelial cells (HDMECs) treated with an exoglycosidase involved in the hydrolytic degradation of α-l-fucose from glycoconjugates as previously described (21). α-l-fucosidase treatment induced four- and twofold increases in bacterial adhesion to hCMEC/D3s and HDMECs (Fig. 3A), respectively, demonstrating that fucosylation alters receptor recognition on human host cells. However, α-l-fucosidase treatment was not sufficient to promote meningococcal adhesion to brain endothelial cells from mouse or rat origin (SI Appendix, Fig. S6), even though mouse and rat CD147 share a high sequence homology and similar protein domain organization and N-glycosylation sites with human CD147 (SI Appendix, Table S3).
Fig. 3.
Fucosylation alters receptor recognition on human host cells. (A) Defucosylation of human and nonhuman cells expressing hCD147. hCMEC/D3s, HDMECs, or CHO cells stably expressing human CD147 (CHO-hCD147) or empty vector (CHO-pcDNA) as control (SI Appendix, Fig. S7) were treated for 1 h with 0.08 U/mL human α-l-fucosidase or vehicle as control. Cells were gently washed and infected with N. meningitidis 2C4.3 for 30 min (hCMEC/D3, HDMEC) or 1 h (CHO). Cells were fixed and stained for actin (phalloidin) and bacteria (anti-2C4.3) and analyzed by confocal microscopy. The images are representative of three independent experiments performed in triplicate. Bacterial colonization on defucosylated and control cells was analyzed with ImageJ and defined as the area occupied by the fluorescently labeled bacteria in relation to cell area delineated by actin staining (n = 20 to 30 fields from three independent experiments). Mean ± SEM. (Scale bars, 10 μm.) ***P < 0.001 using one-way ANOVA. (B) hCD147 is recruited at sites of bacterial adhesion on defucosylated CHO cells. CHO-hCD147 cells were stained for actin, human CD147, and bacteria and analyzed by confocal microscopy to visualize CD147 recruitment and actin polymerization occurring at bacterial adhesion sites. The images are representative of three independent experiments performed in triplicate. Arrows point to the recruitment of CD147 and cortical actin polymerization at bacterial adhesion sites. Images of control CHO-pcDNA are available in SI Appendix, Fig. S7. (Scale bars, 10 μm.)
To further dissect the role of N-glycosylation and the carrier protein in receptor recognition by N. meningitidis, we took advantage the hamster CHO cell line that stably express human CD147 (CHO-hCD147) or the empty pcDNA3.1 vector (CHO-pcDNA) as negative control (22). Although CHO cells express endogenous CD147 (23), which presents similar protein domain organization and N-glycosylation sites to human CD147 (SI Appendix, Table S3), and CHO cells are known to produce complex types of recombinant proteins with human-compatible glycosylation (24), they did not support meningococcal adhesion, and α-l-fucosidase treatment was not sufficient to promote adhesion (Fig. 3A and SI Appendix, Fig. S7). In addition, meningococci poorly adhered to CHO-hCD147 cells. However, meningococci displayed extensive adherence and colonization of CHO-hCD147 cells treated with α-l-fucosidase (Fig. 3A). As observed in infected human endothelial cells (13), infection of fucosidase–CHO-hCD147–treated cells was also accompanied by the recruitment of CD147 at sites of bacterial adhesion and by a local cortical actin polymerization (Fig. 3B). These data show that both the human receptor and its glycosylation profile determine the tropism of meningococci for its host cells and identify the fucosylation profile as an important determinant in the glycan recognition by meningococcal Tfp.
Overall, these data demonstrate that meningococcal Tfp specifically interact with human endothelial cells through an unfucosylated triantennary sialylated LacNAc N-glycan on CD147 and that N-glycan fucosylation determines selective receptor recognition.
Discussion
This study provides direct evidence that meningococcal Tfp interact with a specific triantennary sialylated LacNAc–containing N-glycan exposed on the human receptor CD147. Glycoconjugate recognition by Tfp is required for successful adhesion and colonization of target host cells, a step fine tuned by receptor fucosylation. Characterization of the exact nature of the specific carbohydrate motifs recognized by Tfp opens the path to therapeutic receptor blockade through the use of carbohydrate analogs.
Our data argue for the importance of Neu5Ac-terminated N-glycan expression as a major determinant of the specificity of Tfp. Tfp have a unique architecture consisting of a polymeric assembly of the major pilin PilE and of minor pilins, including PilV, in a helicoidal structure presenting a succession of positively and negatively charged grooves at their surface (25). Both PilE and PilV are required for the meningococcal interaction with CD147 and adhesion to host cells (12, 13). Even though the precise N-glycan binding sites on PilE and PilV still remain unknown, it is likely that Neu5Ac, which creates a negative charge at the extremity of N-glycan chains, stabilizes the interaction between Tfp and N-glycan by interacting with positive grooves. Such interaction may imply multivalent binding of Tfp with multiantennary Neu5Ac-terminated N-glycans, further reinforcing adhesion. While we did not detect biantennary N-glycans containing sialylated LacNAc on recombinant CD147-Fc, we cannot exclude the possibility that meningococcal Tfp might also recognize sialylated biantennary LacNAc N-glycan or monosialylated LacNAc N-glycan. Notably, monosialylated LacNAc N-glycan is a quite common glycan and cannot, therefore, account for the tissue specificity of meningococci. This rather suggests that Tfp recognize a sialylated LacNAc in the context of a complex multiantennary N-glycan.
Following initial interaction on CD147, Tfp can then interact with the G protein-coupled β-2-adrenergic receptor (β2AR) (26) that forms a complex with CD147 at the endothelial cell surface (17). Both pilins PilE and PilV are also required to interact with the extracellular N-terminal domain of β2AR. In parallel to this study, we have shown that terminal sialic acid is also determinant for the specific interaction of meningococcal Tfp with the complex glycan structure formed by the two close glycosylation sites exposed on the extracellular N-terminal domain of β2AR (27). Recognition of sialylated LacNAc in the context of a complex multiantennary N-glycan may then favor the interaction of Tfp with both receptors, a process that is further strengthened by the assembly of CD147–β2AR complexes in highly ordered clusters at bacterial adhesion sites (17). Due to the importance of sialic acid in promoting interaction with both receptors, it will be interesting to consider possible in vivo applications of recently developed sialic acid inhibitors (28) to reduce meningococcal vascular colonization.
Remarkably, we showed that fucosylation dramatically inhibits Tfp-dependent adhesion of meningococci to host cells and that defucosylation of a different human receptor other than CD147 is sufficient to promote adhesion. Fucose is found in a broad range of glycans and is increasingly recognized as critical to many cell–cell interactions and signaling processes, including cell adhesion, tissue development, infection, inflammation, and tumor metastasis (29). In human N-linked glycans, fucose is most commonly attached to sugars at the base of glycan chains in the α (1, 6) configuration (known as core fucosylation). However, in the gut, fucose is predominantly displayed near the terminal end of glycans in the α (1, 2), (1, 3), or (1, 4) configuration, where it is well positioned to engage in interactions with other cells and its environment (30). It is interesting to note that intestinal fucose was shown to play a major role in host–microbe symbiosis as it is induced by the resident microbiota and produced more abundantly during infection and inflammation. Diverse gut bacterial species possess the enzymes necessary to metabolize fucose and use them as an energy source; this process improves host health by altering microbial metabolic pathways and reducing the expression of bacterial virulence factors (31, 32). Furthermore, fucose can expand the beneficial members of gut microbiota and promote colonization resistance to opportunistic pathogens (33). In endothelial cells, one of the characteristic roles of fucose is its regulation of selectin-dependent leukocyte adhesion, allowing leukocyte extravasation induced by inflammation (29). Interestingly, we show here that fucosylation on vascular cells also contributes to the barrier function of the glycocalyx to N. meningitidis, a pathogen that does not produce any fucosidase. In addition, as endothelial exposure to inflammatory cytokines further increases the transcription of α1‐2‐fucosyltransferase I (34), this process is also likely to contribute to reducing vascular colonization by meningococci.
In addition, our data reveal that the specificity of Tfp-mediated adhesion to host cells resides in both protein and carbohydrate moieties. These results highlight a degree of complexity in the recognition of host receptors by Tfp that might play a determinant role in both species-specific recognition and the tropism for a particular host tissue by piliated meningococci.
Altogether, our results point to a key role of a specific glycan structure in the interaction of piliated meningococci with its host and to the inhibitory effect of fucosylation in this interaction. Due to the well-conserved set of proteins involved in Tfp biosynthesis in gram-negative bacteria, it is likely that our findings can be applied more broadly to other Type IV-piliated pathogenic bacteria. Our results thereby suggest that targeting glycosylation may be used as a therapeutic strategy to reduce colonization and to treat infections caused by Tfp-expressing bacteria.
Materials and Methods
Details are in SI Appendix, Supplementary Materials and Methods.
Bacterial Strain and Infection.
N. meningitidis 2C4.3 strain (formerly referred to as clone 12) is a piliated and capsulated Opa- Opc- variant of the serogroup C meningococcal clinical isolate 8013. This strain and its isogenic nonpiliated PilE and nonadherent PiV mutants were previously described (13). Bacteria grown on gonococcal broth (GCB) agar plates were inoculated into GCB medium, incubated overnight in GCB medium, subcultured to OD600 = 0.05 in prewarmed cell culture medium, and incubated for 2 h at 37 °C. Cells were infected with bacteria at a multiplicity of infection of 100 bacteria per cell (optical density, OD = 0.1) for 30 min and washed twice with medium to remove nonadherent bacteria, and infection was allowed to proceed for variable times. For adhesion assays on recombinant proteins immobilized on Lab-Tek Chamber slides, experiments were performed during 30 min with a meningococcal suspension of OD600 = 0.1 as previously described (13).
Cell Lines.
hCMEC/D3s and Rat Brain Endothelial cells clone 4 (RBE4) are fully differentiated brain endothelial cell lines derived from human and rat brain capillaries, respectively (35, 36). BEND3 are endothelioma derived from mice brain cortex and were purchased from ATCC (CRL-2299). hBMECs are a human bone marrow capillary endothelial cell line provided by B. Weksler, Weill Cornell Medical College, New York City, NY (37). Primary HDMECs are isolated from the dermis of juvenile foreskin and adult skin (Promocell). CHO-K1 cells stably expressing CD147 (CHO-hCD147) or the empty vector (CHO-pcDNA) as control were provided by Michael Bukrinsky, George Washington University, Washington, DC (22). Cells were grown as detailed in SI Appendix, Supplementary Materials and Methods.
N-Glycan Analysis by Mass Spectrometry.
N-glycans were released by peptide-N-glycosidase F according to the manufacturer’s instructions (New England Biolabs). Samples were purified using extract-clean SPE C18 columns (Grace Davison Discovery Sciences) activated with methanol and equilibrated in water. The flow through was lyophilized, and the samples were permethylated for 2 h in 200 µL dimethylsulfoxyde (DMSO), 10 mg NaOH, and 300 µL ICH3 under argon and strong shaking. The reaction was stopped with 1 mL of 5% acetic acid, and permethylated N-glycans were purified on an Oasis HBL 6-cc 200-mg (Waters) column activated in methanol and equilibrated in water. The column was washed with 5% methanol, and the samples were eluted in 100% methanol. Permethylated N-glycans were analyzed in the positive ion mode by MALDI-TOF-MS using an Ultraflex II (Bruker Daltonics). A total of 5,000 shots were accumulated per spectrum.
Data Availability.
The raw data generated in this work have been deposited on the publicly accessible database Zenodo, https://zenodo.org/record/3600936#.XiBmGCN7nGg.
Supplementary Material
Acknowledgments
We thank Dr. Bukrinsky for providing cells; the Research Federation FRABio (Université Lille, CNRS, FR 3688, FRABio, Biochimie Structurale et Fonctionnelle des Assemblages Biomoléculaires) for providing the scientific and technical environment conducive to achieving this work; and the imaging facility and cytometry facility of the Institut Cochin for their expert technical help. We thank Dr. Pamela Schnupf for her careful reading of the manuscript and helpful comments and suggestions. L.L.G. and Z.V. were supported by doctoral fellowships from the Fondation pour la Recherche Médicale and the University Paris Descartes, respectively. This work was supported by Agence Nationale de la Recherche of France (ANR) Collaborative Research Grant ANR-14-CE14-0010-01 (to X.N. and S.B.) and ANR Grant ANR-15-CE15-0002 (to M.C.). X.N., S.B., and M.C. are supported by INSERM, CNRS, Université Paris Descartes, and the Fondation pour la Recherche Médicale.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The raw data generated in this work have been deposited on the publicly accessible database Zenodo, https://zenodo.org/record/3600936#.XiBmGCN7nGg.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1919567117/-/DCSupplemental.
References
- 1.Ielasi F. S., et al. , Lectin-glycan interaction network-based identification of host receptors of microbial pathogenic adhesins. MBio 7, e00584-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucior I., Burger M. M., Carbohydrate-carbohydrate interactions in cell recognition. Curr. Opin. Struct. Biol. 14, 631–637 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Bucior I., Abbott J., Song Y., Matthay M. A., Engel J. N., Sugar administration is an effective adjunctive therapy in the treatment of Pseudomonas aeruginosa pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L352–L363 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry J. L., Pelicic V., Exceptionally widespread nanomachines composed of type IV pilins: The prokaryotic Swiss Army knives. FEMS Microbiol. Rev. 39, 134–154 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelicic V., Type IV pili: E pluribus unum? Mol. Microbiol. 68, 827–837 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Kolappan S., et al. , Structure of the Neisseria meningitidis Type IV pilus. Nat. Commun. 7, 13015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F., et al. , Cryoelectron microscopy reconstructions of the Pseudomonas aeruginosa and Neisseria gonorrhoeae Type IV pili at sub-nanometer resolution. Structure 25, 1423–1435.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frischkorn K. R., Stojanovski A., Paranjpye R., Vibrio parahaemolyticus type IV pili mediate interactions with diatom-derived chitin and point to an unexplored mechanism of environmental persistence. Environ. Microbiol. 15, 1416–1427 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Bucior I., Pielage J. F., Engel J. N., Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog. 8, e1002616 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mubaiwa T. D., et al. , The glycointeractome of serogroup B Neisseria meningitidis strain MC58. Sci. Rep. 7, 5693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed M., Cheung N. K., Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett. 588, 288–297 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Join-Lambert O., et al. , Meningococcal interaction to microvasculature triggers the tissular lesions of purpura fulminans. J. Infect. Dis. 208, 1590–1597 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Bernard S. C., et al. , Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat. Med. 20, 725–731 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandtzaeg P., van Deuren M., Classification and pathogenesis of meningococcal infections. Methods Mol. Biol. 799, 21–35 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Lécuyer H., et al. , An ADAM-10 dependent EPCR shedding links meningococcal interaction with endothelial cells to purpura fulminans. PLoS Pathog. 14, e1006981 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coureuil M., Lécuyer H., Bourdoulous S., Nassif X., A journey into the brain: Insight into how bacterial pathogens cross blood-brain barriers. Nat. Rev. Microbiol. 15, 149–159 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Maïssa N., et al. , Strength of Neisseria meningitidis binding to endothelial cells requires highly-ordered CD147/β2-adrenoceptor clusters assembled by alpha-actinin-4. Nat. Commun. 8, 15764 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang W., et al. , Modulation of CD147-induced matrix metalloproteinase activity: Role of CD147 N-glycosylation. Biochem. J. 449, 437–448 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Bai Y., Huang W., Ma L. T., Jiang J. L., Chen Z. N., Importance of N-glycosylation on CD147 for its biological functions. Int. J. Mol. Sci. 15, 6356–6377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohneda O., et al. , ALCAM (CD166): Its role in hematopoietic and endothelial development. Blood 98, 2134–2142 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Ali S., et al. , Leukocyte extravasation: An immunoregulatory role for alpha-L-fucosidase? J. Immunol. 181, 2407–2413 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pushkarsky T., et al. , CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc. Natl. Acad. Sci. U.S.A. 98, 6360–6365 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yurchenko V., et al. , CD147 is a signaling receptor for cyclophilin B. Biochem. Biophys. Res. Commun. 288, 786–788 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Goh J. B., Ng S. K., Impact of host cell line choice on glycan profile. Crit. Rev. Biotechnol. 38, 851–867 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Craig L., et al. , Type IV pilus structure by cryo-electron microscopy and crystallography: Implications for pilus assembly and functions. Mol. Cell 23, 651–662 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Coureuil M., et al. , Meningococcus Hijacks a β2-adrenoceptor/β-Arrestin pathway to cross brain microvasculature endothelium. Cell 143, 1149–1160 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Virion Z., et al. , Sialic acid mediated mechanical activation of β2 adrenergic receptors by bacterial pili. Nat. Commun. 10, 4752 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rillahan C. D., et al. , Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 8, 661–668 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J., Hsu H. C., Mountz J. D., Allen J. G., Unmasking fucosylation: From cell adhesion to immune system regulation and diseases. Cell Chem. Biol. 25, 499–512 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Goto Y., Uematsu S., Kiyono H., Epithelial glycosylation in gut homeostasis and inflammation. Nat. Immunol. 17, 1244–1251 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Pacheco A. R., et al. , Fucose sensing regulates bacterial intestinal colonization. Nature 492, 113–117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickard J. M., Chervonsky A. V., Intestinal fucose as a mediator of host-microbe symbiosis. J. Immunol. 194, 5588–5593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham H., et al. , Inhaled NO prevents hyperoxia-induced white matter damage in neonatal rats. Exp. Neurol. 252, 114–123 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Moehler T. M., et al. , Involvement of alpha 1-2-fucosyltransferase I (FUT1) and surface-expressed Lewis(y) (CD174) in first endothelial cell-cell contacts during angiogenesis. J. Cell. Physiol. 215, 27–36 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Durieu-Trautmann O., et al. , Immortalized rat brain microvessel endothelial cells. II. Pharmacological characterization. Adv. Exp. Med. Biol. 331, 205–210 (1993). [DOI] [PubMed] [Google Scholar]
- 36.Weksler B. B., et al. , Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19, 1872–1874 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Schweitzer K. M., et al. , Characterization of a newly established human bone marrow endothelial cell line: Distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab. Invest. 76, 25–36 (1997). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data generated in this work have been deposited on the publicly accessible database Zenodo, https://zenodo.org/record/3600936#.XiBmGCN7nGg.