Significance
The evolution of sibling rivalry is a classic problem in behavioral ecology. Our approach of observing the experimental evolution of sibling interactions in real time reveals three key insights. First, when parents provide parental care, siblings evolve to compete. Parental care compensates for the costs of sibling rivalry. Second, when parents do not supply care, siblings evolve to cooperate. Sibling cooperation compensates for the loss of parental care. Third, rapid evolutionary switching between sibling rivalry and sibling cooperation is possible because siblings induce greater levels of rivalry (or cooperation) in each other. This generates positive evolutionary feedback, rapidly locking larvae into evolving greater levels of competition (or cooperation) in the presence (or absence) of parental care.
Keywords: indirect genetic effect, cooperation, competition, parental care, burying beetle
Abstract
Sibling rivalry is commonplace within animal families, yet offspring can also work together to promote each other’s fitness. Here we show that the extent of parental care can determine whether siblings evolve to compete or to cooperate. Our experiments focus on the burying beetle Nicrophorus vespilloides, which naturally provides variable levels of care to its larvae. We evolved replicate populations of burying beetles under two different regimes of parental care: Some populations were allowed to supply posthatching care to their young (Full Care), while others were not (No Care). After 22 generations of experimental evolution, we found that No Care larvae had evolved to be more cooperative, whereas Full Care larvae were more competitive. Greater levels of cooperation among larvae compensated for the fitness costs caused by parental absence, whereas parental care fully compensated for the fitness costs of sibling rivalry. We dissected the evolutionary mechanisms underlying these responses by measuring indirect genetic effects (IGEs) that occur when different sibling social environments induce the expression of more cooperative (or more competitive) behavior in focal larvae. We found that indirect genetic effects create a tipping point in the evolution of larval social behavior. Once the majority of offspring in a brood start to express cooperative (or competitive) behavior, they induce greater levels of cooperation (or competition) in their siblings. The resulting positive feedback loops rapidly lock larvae into evolving greater levels of cooperation in the absence of parental care and greater levels of rivalry when parents provide care.
Social interactions between animals typically have fluid fitness outcomes, which depend on the relatedness of the interacting animals, local ecological conditions, and evolved social behaviors. These outcomes can lie anywhere on a spectrum from conflict to cooperation, and they can change within the lifetime of an individual (1). This is especially evident within animal families where siblings are rivals for resources (2, 3) yet may cooperate to obtain more food (4–7), and where parents sometimes abandon their young yet may also care for them diligently (3, 8). An adaptive response to such a variable social environment is for family members to employ flexible behavioral rules for social engagement, so-called negotiation strategies (9–11). Negotiation strategies describe the set of behaviors an individual should deploy in real time to maximize fitness in response to the actions of their social partner. The adaptive response to heightened begging behavior by one offspring, for example, is for its sibling to escalate its competitive prowess by begging more (4, 12). The adaptive response to greater levels of cooperation by a sibling is to cooperate more (4).
In theory, negotiated social strategies within animal families change the scope for any subsequent evolutionary change in social behavior (13, 14). Negotiated strategies mean more than one genotype contributes to a focal individual’s behavior. Consequently, the social behavior expressed by a focal individual is not only due to its own genotype (a direct genetic effect; DGE) but to the genotype of its social partner(s) (an indirect genetic effect; IGE). To see why this matters, imagine a sustained change in the social environment that tips interactions among siblings from largely competitive to largely cooperative. The resulting selection on individual larvae for increased cooperative behavior induces the expression of greater levels of cooperation among their siblings. As the trait evolves, it creates a social environment that is more likely to induce its expression, and this positive feedback cycle can induce very rapid evolution (13). Negative evolutionary feedback that inhibits social evolution is also possible, if the social environment prevents trait expression (13). In theory, therefore, the evolution of socially negotiated traits is strongly influenced by the strength and direction of IGEs, which can potentially tip populations into rapid evolutionary shifts, for example, from one strategy to another (15). Whether this happens in practice, however, is not known. The genetic architecture of negotiated social strategies can be partitioned into DGEs and IGEs to isolate the influence of the IGEs on the evolutionary trajectories of different social types of behaviors (13, 14, 16), but sibling interactions have not been analyzed in this way. More generally, we are unaware of any studies showing that IGEs influence the evolution of social behavior in real time.
The environmental condition that is most likely to tip sibling interactions from conflict to cooperation is the presence or absence of parental care (17). When parents provide care, a negotiated behavioral response among siblings is to exhibit greater levels of competition for the resource that adults supply (2, 12, 18). Conversely, when parents temporarily cease to provide care, a negotiated behavioral response is for siblings to cooperate more with each other (17, 19). An enduring evolutionary change from facultative parental care to obligate care could correspondingly cause the evolution of increased sibling rivalry, whereas the complete cessation of facultative care could cause the evolution of greater sibling cooperation. These predictions have not been explicitly tested before. Indeed, the evolutionary relationship between the supply of parental care and interactions among siblings is poorly understood in general because each type of social interaction is more usually analyzed in isolation (e.g., ref. 3).
Here we show that parental care influences the evolution of sibling interactions, and that IGEs help set the pace of this evolutionary change. Our experiments focus on burying beetles Nicrophorus vespilloides, an insect that breeds on small carrion and provides highly variable levels of care for its larvae. Parents directly provision offspring with regurgitated fluids and maintain the carcass by smearing it with antimicrobial exudates. Despite the benefits of parental care for offspring survival and growth (20, 21), parental care is continuously variable in N. vespilloides. At one extreme, one or both parents may depart before larvae hatch (7), and offspring can survive without any posthatching parental care (21, 22). We established replicate laboratory populations, in which parents were either allowed to supply care to their offspring at each generation (n = 2 Full Care [FC] populations) or prevented from supplying any posthatching care at all (n = 2 No Care [NC] populations: see refs. 6 and 7 for further details of these populations). After 22 generations of experimental evolution, we determined whether interactions among larvae had evolved to be more competitive or more cooperative.
We analyzed larval interactions from a social evolutionary perspective (23), rather than from a purely behavioral perspective. This means that we focused on how sibling interactions influenced larval fitness, rather than deducing the specific behavioral and physiological mechanisms that might have caused the changes in fitness we measured (see Materials and Methods). We defined a competitive sibling interaction as one in which a larva gained fitness at the expense of a rival sibling, and defined a cooperative interaction as one in which larvae caused each other to gain fitness (after ref. 23). We used larval mass at the end of development as our measure of fitness, because this is the best-known predictor of adult burying beetle survival and fecundity (24–26). Larval development ceases approximately 5 d after hatching, when larvae crawl away from the scant remains of the carcass to pupate in the soil.
Results
Larvae Evolve Greater Levels of Cooperation in Response to the Loss of Parental Care.
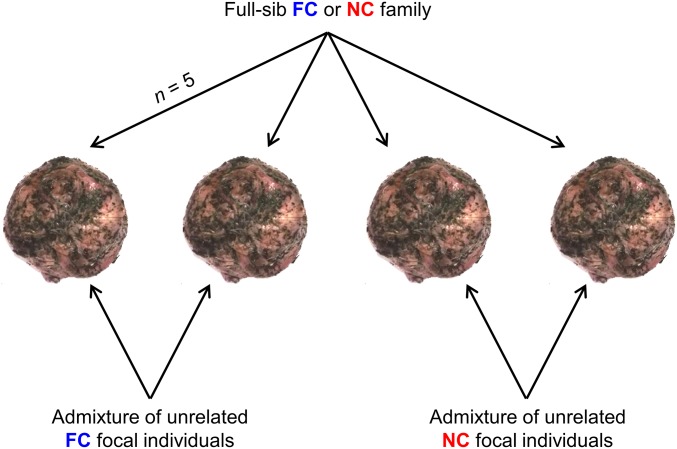
Burying beetle parents convert small carrion into an edible nest for their larvae by removing any fur or feathers, rolling the flesh into a ball, and burying it below ground. Larvae gather in a depression on the top of the ball, after hatching, where they feed themselves and are tended by their parents (22). To evaluate whether larval interactions during development were competitive or cooperative, we established experimental broods of 10 larvae (see Materials and Methods). The focal larvae in each treatment were an admixture of five unrelated individuals, exclusively drawn either from the NC populations or from the FC populations. The remaining five larvae were siblings, and unrelated to the focal larvae. They provided the sibling environment for each treatment, and they too were exclusively drawn either from the NC populations or from the FC populations. Thus, we had a 2 × 2 experimental design, enabling us to test how focal larvae that had evolved under FC or NC fared when developing alongside “siblings” that had evolved under each regime of care (Fig. 1). We placed each brood on a carrion nest of standardized size that had been prepared by unrelated FC beetles to minimize any variation in larval mass that might be attributable to the carrion and left the larvae to develop without receiving any parental care. Five days later, we weighed larvae as they started to disperse away from the carcass.
Fig. 1.
Experimental design to identify whether the sibling environment provided by different experimentally evolving populations influences the development of focal individuals reared with them. A sample of full-sibling FC or NC families was used to generate the interacting sibling environment (n = 5 larvae per carcass). Unrelated FC or NC focal larvae were then added to those carcasses (n = 5 larvae per carcass) to create broods of 10 larvae. Larvae developed together on the carcass without parental care until they dispersed into the soil to pupate, at which point we measured the mass of each dispersing larva. We used larval mass at dispersal as a proxy for fitness, to deduce whether larval interactions were competitive or cooperative.
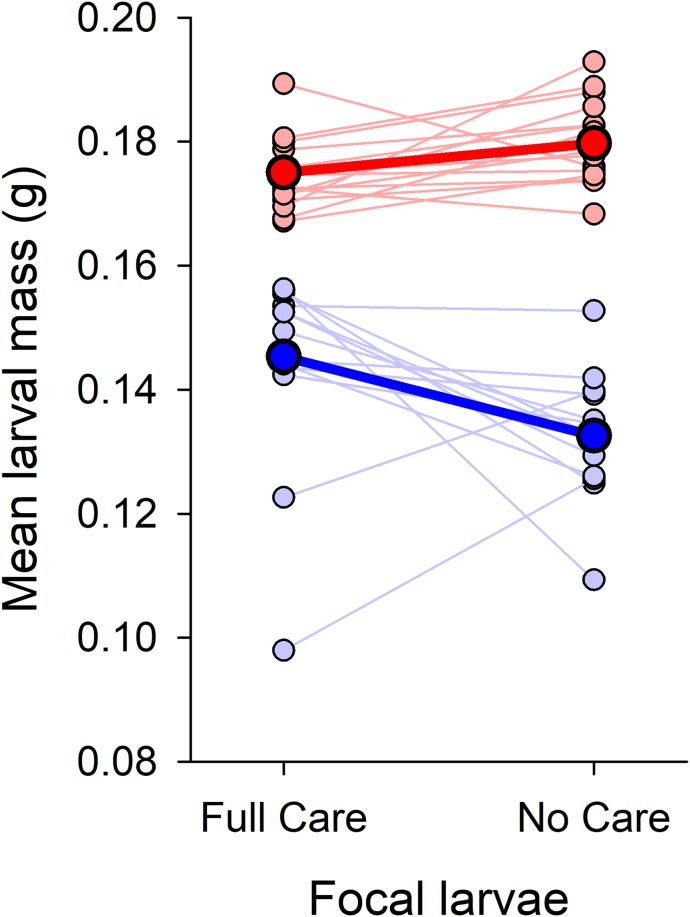
We first focused on the response of focal larvae to developing with different families of FC or NC siblings. We found that the sustained removal of parental care for 22 generations caused larvae to evolve greater levels of cooperation with one another. Conversely, larvae were more competitive if they were drawn from populations where parents had been allowed to care for their young (Fig. 2 and Table 1). Focal larvae that developed alongside NC siblings attained a greater mass at dispersal, regardless of their own evolutionary background (Fig. 2 and Table 1). In contrast, focal larvae that developed alongside FC siblings attained a much lower mass by the end of larval development, regardless of whether they were FC or NC larvae themselves (Fig. 2 and Table 1).
Fig. 2.
The influence of the sibling environment provided by experimentally evolved populations of FC or NC full-sibling families on the larval mass of unrelated FC or NC focal individuals. Blue circle and lines depict the effects of FC siblings on focal larval mass, while red circles and lines depict the effects of NC siblings on focal larval mass. Faint circles and lines show data for individual families, whereas bold circles and lines show the overall population-level influence of FC and NC siblings on focal larval mass.
Table 1.
Model examining influence of the social environment
| Larval mass | Random effects | |||||||
| Factor | Estimate | SE | t value | P | Variance | SD | X2 | P |
| Intercept | 0.1460 | 0.0026 | 55.196 | <0.001 | ||||
| Sibling (NC) | 0.0102 | 0.0033 | 3.109 | 0.003 | ||||
| Focal (NC) | −0.0123 | 0.0024 | −5.035 | <0.001 | ||||
| Sibling (NC) × Focal (NC) | 0.0138 | 0.0033 | 4.135 | <0.001 | ||||
| Block (2) | −0.000002 | 0.0029 | −0.001 | 0.999 | ||||
| Family [Sibling] | 0.000036 | 0.0060 | 10.463 | 0.001 | ||||
| Replicate [Sibling, Family] | 0 | 0 | 0.00 | 1.000 | ||||
| Residual | 0.000441 | 0.0210 | ||||||
Sibling: FC or NC interacting sibling environment larvae on mass attained by unrelated focal larvae. Focal: FC or NC larvae. Significant tests are in boldface.
Larval Interactions Generate Indirect Genetic Effects.
We partitioned sources of variance in the larval mass attained by focal larvae (see Materials and Methods). After statistically controlling for the replicated populations and the random effects of family, we found that the larvae creating the sibling environment for the focal larvae were a significant source of IGEs on larval mass (Table 1). However, the degree to which these IGEs accounted for variation in larval mass depended on the DGEs on larval mass: There was a significant two-way interaction between the evolutionary history of care among the focal larvae and the evolutionary history of care among the sibling environment larvae (a G × G IGE, shown in Table 1). The IGEs caused by larval interactions were therefore different in each of the four experimental treatments.
The Strength and Direction of Larval Indirect Genetic Effects.
To describe how the larval IGEs differed among treatments, we used the interaction coefficient ψ, which relates the expression of traits (in this case, larval mass) in focal individuals to the trait values of their interacting sibling partners (13, 27, 28). ψ is a regression coefficient and analogous to the maternal effect coefficient, m, in quantitative genetic analyses of maternal effects (13). Estimates of ψ vary between [−1, 1], allowing comparisons of the magnitude and direction of IGEs across traits, contexts, and studies (16). When ψ is large and positive, IGEs are strong and reinforcing: Positive values in sibling partners drive increased expression of traits in focal individuals. When ψ is large and negative, IGEs cause antagonistic effects on trait expression in sibling individuals. If there are no IGEs, then ψ is not significantly different from zero. An attractive feature of estimating ψ is that it provides a quantitative method for estimating the evolutionary impact of IGEs on any set of measurable traits (29), and the scope for positive evolutionary feedback from the larval social environment.
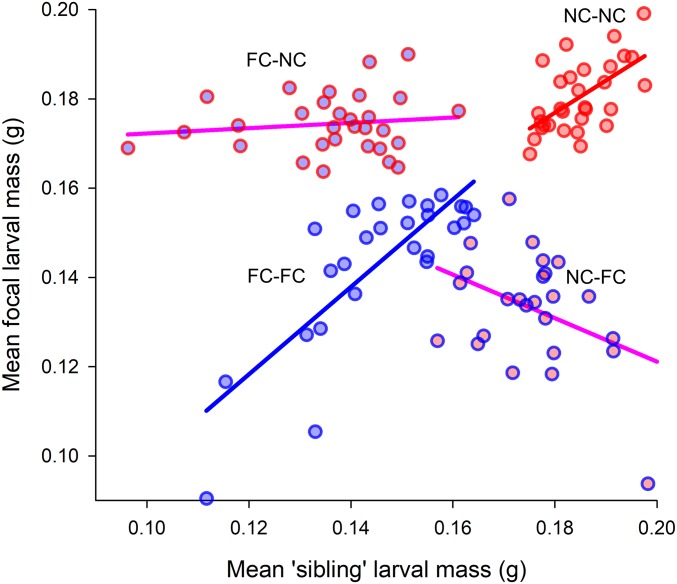
We found that sibling larvae influenced their social partners’ body mass, but only when both sets of larvae within the experimental brood had experienced the same evolutionary history of parental care (Fig. 3 and Table 2). There were significant positive reciprocal IGEs on larval mass in the NC−NC and FC−FC treatments (positive ψ; Fig. 3 and Table 2). No IGEs on larval mass were detected in situations where the evolutionary background of interactors was mismatched (ψ not detectably different from zero in NC−FC and FC−NC; Fig. 3 and Table 2). It has been proposed that estimates of ψ should be corrected when interactions are reciprocal and involve the same trait in both partners (30). In our experiment, uncorrected and corrected values of ψ each indicate potential for rapid evolutionary change (Table 2) (13). The short timeframe of just over 20 generations in which a switch between competitive and cooperative modes of sibling interaction appears to have taken place may be more in line with evolutionary responses predicted by uncorrected ψ values. However, IGEs of even moderate magnitude can drive “social runaway”—that is, the rapid and unstable elaboration or diminution of socially selected traits (15).
Fig. 3.
The influence of the sibling environment on the average mass attained by focal larvae and sibling larvae. Each data point corresponds to a different experimental brood. Unstandardized mean larval masses for focal individuals (y axis) and sibling individuals (x axis) in the four social environments are plotted to compare treatment effects. The color of the circle outline depicts the populations from which the sibling larvae originated (blue circle outlines are for FC larvae; red circle outlines are for NC larvae). Fill color denotes the populations from which the focal were drawn (blue fill is for FC larvae; red fill is for NC larvae). Treatment labels (e.g., FC−FC) show the focal larval population of origin−sibling larval population of origin. Solid lines represent regression slopes, but note that estimates of ψ were calculated separately for each treatment (see main text).
Table 2.
Trait-based estimates of from separate linear mixed models for each of the four focal-interacting sibling treatments
| Treatment* | Effect†,‡ | DF§ | F | P | ψ¶ | ¶ | |
| NC−NC | MLM | (1, 26.790) | 14.075 | <0.001 | 0.553 | 0.302 | |
| Block | (1, 12.764) | 4.541 | 0.0531 | 0.729 | |||
| Family | 0.245 | ||||||
| NC−FC | MLM | (1, 14.170) | 3.615 | 0.078 | −0.430 | −0.226 | |
| Block | (1, 10.638) | 0.124 | 0.732 | −0.166 | |||
| Family | 0.255 | ||||||
| FC−NC | MLM | (1, 25.261) | 0.729 | 0.401 | 0.147 | 0.074 | |
| Block | (1, 12.992) | 1.116 | 0.310 | −0.482 | |||
| Family | 0.518 | ||||||
| FC−FC | MLM | (1, 19.95) | 29.204 | <0.001 | 0.722 | 0.446 | |
| Block | (1, 9.60) | 0.958 | 0.352 | 0.301 | |||
| Family | 0.216 |
(focal)−(sibling).
MLM, mean larval mass.
Fixed effects MLM and Block were estimated using Type III sums of squares. REML was used to estimate covariance parameters for random “family” effects.
Reported as (numerator, denominator).
Significance of both estimates based on the same GLM from which they were derived.
Significant tests are in boldface.
Costs of Competition and Benefits of Cooperation for Larvae.
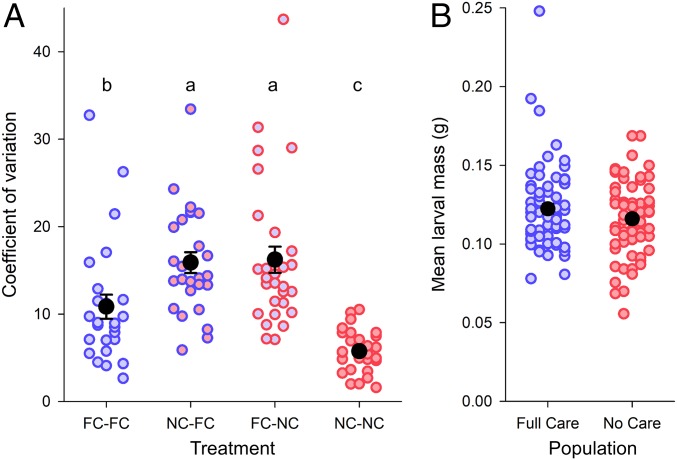
Next, we determined whether the benefits of larval cooperation, and the costs of larval competition, are spread evenly across the brood, by calculating the coefficient of variation (CV) in larval mass at dispersal for each brood. We predicted a greater CV in larval mass in broods where the larvae were drawn from contrasting evolutionary backgrounds (i.e., FC−NC and NC−FC), imagining that more-cooperative larvae would be exploited by more-selfish larvae, resulting in an uneven distribution of resources within each brood. In contrast, we predicted a lower CV in larval mass in broods where the larvae were drawn from the same evolutionary background (i.e., FC−FC and NC−NC) because all larvae had evolved in the same sibling social environment, reducing the scope for temporary exploitation. The results matched our predictions (Fig. 4A and Table 3). Furthermore, we found the lowest CV in larval mass in broods from the NC−NC treatment (Fig. 4A). This finding suggests that, when all of the larvae in a brood are cooperative, they divide the benefits of cooperation evenly among themselves.
Fig. 4.
(A) The CV in the larval mass of individuals on a carcass in each of four treatments where focal and sibling larvae were derived from the different experimental evolution populations. Brood size was experimentally standardized to 10 larvae. The color of the circle outline depicts the populations from which the sibling larvae originated (blue circle outlines are for FC larvae; red circle outlines are for NC larvae). Fill color denotes the populations from which the focal were drawn (blue fill is for FC larvae; red fill is for NC larvae). Solid black circles indicate the overall mean for each treatment, and bars are ±1 SE. Treatment labels (e.g., FC−FC) refer to the population from which the focal larvae were drawn and the sibling larvae were drawn. Treatments not sharing a letter are significantly different in post hoc comparisons (P < 0.05). (B) Larval mass attained by the experimentally evolved populations at generation 23 when reared under their usual environmental conditions (FC, parents present; NC, parents absent), and in brood sizes chosen by parents. Each blue or red datapoint represents the mean larval mass for one brood, whereas solid black circles denote the overall mean with bars ±1 SE.
Table 3.
Differences in the coefficients of variation in larval mass for each of the four focal-interacting sibling treatments, detected using a linear mixed model
| CV | Random effects | |||||||
| Factor | Estimate | SE | t value | P | Variance | SD | X2 | P |
| Intercept | 11.755 | 1.349 | 8.713 | <0.001 | ||||
| Sibling (NC) | 5.563 | 1.717 | 3.240 | 0.002 | ||||
| Focal (NC) | 5.036 | 1.670 | 3.016 | 0.003 | ||||
| Sibling (NC) × Focal (NC) | −15.497 | 2.281 | −6.793 | <0.001 | ||||
| Block (2) | −2.366 | 1.293 | −1.830 | 0.079 | ||||
| Family [Sibling] | 1.991 | 1.411 | 0.126 | 0.253 | ||||
| Residual | 36.065 | 6.005 | ||||||
Significant tests are in boldface.
The experimental data analyzed thus far were collected from larvae that experienced no parental care during their development. In a final analysis, we tested whether parental care compensates for the fitness costs of sibling rivalry. Using data collected from the evolving populations at generation 23, we compared the average larval mass attained by larvae from the two FC populations, when raised by their parents as usual, with the average larval mass attained by larvae from the two NC populations, raised as usual without care. We found that the average larval masses of FC and NC larvae at dispersal did not significantly differ from one another (F1, 131 = 2.28, P = 0.13; Fig. 4B) or between the replicated populations (F1, 131 = 1.35, P = 0.25). Parental care compensates for the negative effects of sibling rivalry, and sibling cooperation compensates for the loss of parental care.
Discussion
Our experiments show how parental care influences the evolution of sibling interactions. We found that, when parents supply care, selection favors the evolution of sibling rivalry. The fitness costs incurred when siblings compete with one another were fully compensated by the fitness benefits that offspring gained from parental care. Sibling rivalry was therefore only sustainable when parents cared for their young. When parents ceased to provide any care for their offspring, we found that selection instead favored cooperation among siblings. Sibling cooperation compensated for the loss of care by parents.
Previous work has suggested that parental care evolves to buffer offspring against harsh environments, potentially enabling niche expansion as a result (31). We have shown that, when parental care is lost, cooperative interactions among larvae can evolve in compensation to serve the same function and continue to buffer offspring against selection from the wider environment. Greater levels of sibling cooperation could thus enable families to persist in the same niche following the loss of parental care. In lineages that have not evolved parental care, increased cooperation among siblings could even enable niche expansion by offering greater levels of defense against predators or better access to food (32–35). Whether it derives from interactions between parents and helpers at the nest (21), or between parents and their young, or among siblings, cooperation among conspecifics appears to be a common factor in enabling individuals to persist in relatively harsh conditions.
Sibling IGEs on mass gain were associated with evolutionary transitions between sibling rivalry and sibling cooperation. We found that ψ was large and positive when interacting siblings were drawn from the same evolutionary background. Relating this measurement back to the theory of IGEs, we infer that increasingly cooperative (or competitive) larval behavior induces greater levels of cooperation (or competition) among siblings, generating a positive feedback loop that not only speeds up the pace of evolutionary change (13) but might also induce the exponential elaboration of socially selected traits such as cooperation under a social runaway process (15). Whereas runaway evolution is more commonly associated with sexual selection, our results suggest that evolutionary elaboration of sibling traits could be similarly fast-paced and have substantial fitness consequences (15). Our work further implies that the evolution of sibling interactions, in general, might be characterized by abrupt transitions between competition and cooperation, rather than more gradual change.
Our results also illustrate an important feature of the influence of IGEs on evolution: We found positive measures of ψ only when the majority of larvae in the brood were cooperative (or competitive). This means positive evolutionary feedback only occurred under these conditions. In the wild, interactions between parents and offspring may modulate the amount of care that a brood receives, which, in turn, may constrain how easily cooperation could evolve in natural populations. When we experimentally mixed broods so that they contained equal numbers of cooperative and competitive larvae, ψ was indistinguishable from zero, and the IGEs of siblings disappeared. In natural populations of burying beetles, it is likely that the supply of care fluctuates greatly from one generation to the next, maintaining a mixture of cooperative and competitive larvae and so preventing any evolutionary runaway to purely competitive or purely cooperative broods.
We are not suggesting that the loss of parental care automatically induces social runaway evolution of greater sibling cooperation. The evolutionary dynamics shown by any particular species will depend on how offspring flexibly respond to a temporary loss of care. We suggest that burying beetle larvae evolved greater levels of cooperation in response to the loss of parental care because this is their preexisting negotiated response to the temporary removal of care (see ref. 5). Thus, the direction of adaptive evolution proceeded in the same direction as adaptive plasticity. In other species, the temporary loss of care has the opposite effect on siblings, sometimes even inducing greater levels of cannibalism among offspring (e.g., refs. 36 and 37). For these species, we predict that a sustained loss of care should induce even greater levels of competition (or cannibalism)—because this is the direction of their preexisting negotiated response.
Although we have unequivocally found evidence of sibling cooperation, both here and in our previous work (experiment 2 in ref. 5), we cannot tell, from these experiments, exactly what form cooperative larval behavior might take. In other species, cooperation between dependent offspring can occur through offspring feeding one another or sharing food (birds: ref. 38; insects: ref. 39) or simply reducing their selfishness (40). Direct provisioning of siblings has not been observed among burying beetle larvae. It is more likely that siblings help each other indirectly to acquire resources from the carcass, through more effective maceration of the flesh with their relatively larger mandibles (7) and by coordinating their actions through greater hatching synchrony (17). Larvae have also been shown to secrete fluids onto the carcass, which arrest microbial growth (41)—presumably to the benefit of the entire brood (42). These larval fluids might further benefit siblings if they contain digestive enzymes or growth factors (43), although this has yet to be discovered.
In conclusion, interactions between parents and offspring can be highly cooperative when parents supply care, or more selfish when parents abandon their offspring. Likewise, siblings can show pronounced rivalry or behave more cooperatively to each other. Our study suggests that adaptive negotiation strategies establish the sign and magnitude of IGEs, and thence determine the combinations of parent and offspring social behavior that can evolve. We found that burying beetle parents can supply care, but then siblings will be rivals. Alternatively, burying beetle parents can abandon their young, and then siblings will be more cooperative.
Materials and Methods
The Experimental Approach.
We manipulated the sibling environment experienced by larvae and measured the effects on larval mass, a key predictor of fitness (24–26). The mass that larvae attain by the time they disperse away from the carcass is due partly to their own foraging efforts (22), and is partly due to care that they receive from their parents (18). Larval foraging might involve some element of competition with siblings for limited resources, but might also involve some cooperation if, for example, maceration of the carcass facilitates sibling foraging success.
We first determined the relative balance of cooperation and competition among siblings on larval mass at dispersal, by removing parents altogether during larval development. We then compared these data to the mass attained by dispersal when larvae developed in the conditions under which they evolved (i.e., FC and NC). This comparison enabled us to determine how parents additionally contribute both to larval mass and to the variance in larval mass within each brood.
Experimental Evolution.
Four populations of N. vespilloides were subjected to experimental evolution (two populations each of FC and NC). They were founded from a genetically diverse stock population created by interbreeding four wild populations of beetles collected in Cambridgeshire, United Kingdom (Byron’s Pool, Gamlingay Woods, Overhall Grove, and Waresley Woods) in the summer of 2014, described in detail in Schrader et al. (6) and Jarrett et al. (7). The experimentally evolving populations were run in two blocks, to be logistically manageable. Block 1 comprised one NC population and one FC population breeding simultaneously. Block 2 comprised the second NC population and the second FC population, again breeding simultaneously. Block 2 was bred 1 wk after Block 1. For the FC populations, a minimum of 30 pairs of unrelated beetles were bred each generation. Pairs were placed in a box containing moistened soil and a small, thawed mouse carcass (8 to 14 g), and then placed in a dark cabinet for 8 d. At that point, the dispersing larvae were placed into individual cells (2 cm3), covered with moistened peat, and left undisturbed to pupate to adults. The newly eclosed adults were housed individually until breeding, a minimum of 17 d after eclosion. For the NC populations, there were two exceptions to this protocol. First, both parents were removed 53 h after pairing, allowing parents to prepare the carcass and allowing the female to lay a clutch of eggs, but ensuring no posthatching parental care occurs, because larvae usually hatch at ∼72 h after pairing (44, 45). Second, a minimum of 50 pairs of unrelated beetles were bred through the first 15 generations of experimental evolution to offset the increased number of complete brood failures (6). All individuals used in this experiment were from generation 23 (i.e., after 22 generations of experimental evolution).
Split-Brood Experimental Design.
The experiment followed a 2 × 2 blocked design (Fig. 1). We performed the experiment in two blocks, corresponding to Block 1 and Block 2 of the experimentally evolving populations. The following four treatments were used: FC focal larvae in an FC sibling environment; FC focal larvae in an NC sibling environment; NC focal larvae in an FC sibling environment; and NC focal larvae in an NC sibling environment. To generate the four treatments, we bred 50 pairs of unrelated individuals from each population, giving each pair a 10- to 11-g mouse carcass under standard conditions (6, 7). Parents and carcass were removed 53 h after pairing. When larvae hatched, we selected 10 broods whose larvae generated the sibling environment (n = 5 larvae per experimental brood). The remaining families of larvae were used to create an admixture of five unrelated FC or NC focal larvae. In this admixed social group, we only used one larva per family to create a genetically diverse pool of focal larvae, and we ensured that none of these larvae were related to the respective treatment family. Thus, each experimental brood comprised 10 larvae, the density on the carcass at which larvae attain peak mass in the absence of parental care (5). We chose this density for our experimental manipulations to increase the likelihood that we would detect an effect of sibling cooperation (or competition) on larval mass. FC and NC populations have experienced highly variable larval densities across the generations since the experiment began (6). Brood size is too inconsistent between the generations and too similar between FC and NC populations for it to have been a consistent source of selection. It is therefore highly unlikely that standardizing brood size introduced any potential confounding effects into our experimental design.
Newly hatched larvae were lightly anesthetized with CO2, and either the left or right hind tarsus was cut off so that we could identify the focal larvae and interacting family larvae within each brood. This marking technique did not affect mortality, larval growth, or development (paired t tests, all P > 0.20), consistent with a previous study that employed this technique (46). Larvae were then placed on a similarly sized carcass (mass = 9.52 ± 0.27 g [mean ± SD]; range = 9.00 to 10.00 g) prepared by unrelated FC parents. From a pool of prepared carcasses, we only used those that were fully prepared but lacked any incision into the carcass. We then cut a small entry hole (1 cm) into the thigh of each prepared carcass, so that all larvae had equal access to these vital resources (17). The experimental broods on their nest were then placed in a small plastic box lined with compost, which was put in a dark cabinet, without parents, until larvae completed development and were dispersing away from the carcass. At this point, 5 d after setting up the broods, we noted the identity of each larva and weighed each one to the nearest 0.1 mg. Note that this means larval mass was measured at the same developmental stage for all larvae. By standardizing carcass quality, carcass size, developmental stage, and brood size experimentally, we could attribute any differences in larval mass to interactions among siblings alone.
Variance Partitioning to Detect IGEs.
Applying a variance partitioning approach, we tested the influence of the evolutionary history of admixed focal larvae plus the influence of their social environment (i.e., the genetic background of the interacting sibling individuals within it) on mass, following previous methods (27, 28, 47, 48). The following statistical analyses were performed in R 3.4.1 (49) using the package lme4 (50). Models were fit using the Restricted Maximum Likelihood (REML) method, and significance testing of random effects was done using ML. To partition variance in larval mass, we used a linear mixed model with the larval mass attained by unrelated focal individuals at dispersal as the dependent variable. The model included the following terms: “focal,” indicating experimental evolution population identity of the admixed focal larvae on the carcass (FC vs. NC); “sibling,” indicating experimental evolution population identity of the full-sib larvae on the carcass (FC vs. NC), and a “sibling × focal” interaction term, and “block,” representing the replicated independently evolving FC and NC populations. We also included “family” nested within “sibling” and “replicate” nested within “family-within-sibling” as random effects. We only retained a treatment family if there were at least two full-sib larvae and two unrelated focal larvae that survived to dispersal on each of the four carcasses. Of the 19 FC and NC treatment families set up, we retained 13 and 15 families, respectively.
IGEs involving a specific trait such as body mass are not expected a priori to be uniform in different evolutionary contexts (47, 48, 51–53). Because we also manipulated the evolved background of focal individuals, it is possible that NC focal larvae would experience different IGEs than FC focal larvae (i.e., G × G IGE; ref. 54). The two-way interaction term between focal and interacting sibling background in this analysis tested specifically for this possibility.
Trait-Based IGE Estimates.
We evaluated IGEs on larval mass, disaggregating our estimates of ψ for each of the four possible treatment combinations of focal and interacting sibling individuals: NC−NC, NC−FC, FC−NC, and FC−FC. A trait-based analysis was used to identify and characterize reciprocal IGEs occurring within these different social environments. Focal trait values were calculated as an average across focal individuals in each brood, so each observation involved focal individuals exposed to a social environment comprising four other individuals from the same background, plus five sibling individuals which were experimentally manipulated to either match or mismatch the focal background (NC or FC). It is possible that exposure of focals to a greater proportion of sibling individuals from opposing environments in the mismatched condition could have stronger effects, so our estimates of social environment effects are therefore likely to be conservative. We made four estimates corresponding to the combinations of evolved populations: , , , and , where terms before and after the hyphen in superscript indicate the evolutionary background of the focal and sibling larvae, respectively. Dependent and independent variables were zero-centered with unit variance prior to analysis (27, 55), and we estimated ψ for each focal−sibling combination using separate general linear models with fixed intercepts α, standardized focal and sibling larval masses, block to account for the replicated independently evolving populations, random effects describing family ID (1|family), and error, ε:
| [1] |
Bijma (30) proposed a correction for estimates of ψ in cases where phenotypic feedback is present, to provide a more directly scalable metric of comparison between IGEs that are reciprocal versus those that are not. Our study focused on IGEs affecting focal and interacting sibling weights, and it is plausible that weight in focal and sibling larvae is reciprocally affected through various feedback loops throughout development on the carcass. We therefore calculated both estimates of ψ, making Bijma’s (30) feedback correction: . Table 2 provides estimates of ψ and for each interacting sibling/focal combination of larvae, and Fig. 3 shows the unstandardized results of all four comparisons (see SI Appendix, Fig. S1 for the unstandardized results separated by block, which illustrates the overall lack of variation attributable to block effects and thus similar evolutionary responses across independently evolving experimental populations).
The Benefits and Costs of Sibling Interactions.
To help interpret ψ estimates, we additionally calculated the CV for larval mass within each brood pairing and compared these across treatments (Table 3). First, the CVs for all larvae per carcass were calculated, and these were then analyzed in a model using the same two main effects as the variance partitioning model above (“focal” and “sibling” population identity, NC or FC, and their interaction, with block to account for the replicated independently evolving populations and “family” as a random effect to account for nonindependence of replicates. The interaction was significant, so we performed post hoc Tukey tests to assess the significance of differences in CVs between each pair of treatment groups. These revealed a pattern consistent with our predictions: When the evolved backgrounds of focals and larvae were different, we found high CVs for larval mass [Fig. 4A, and see SI Appendix, Fig. S2 for CVs separated by block]).
We then used the data collected on generation 23 of the experimentally evolved populations to determine whether parental care compensated for the costs of sibling rivalry. We did not control brood size as in the previous experiments, but rather let parents determine brood size. Therefore, broods were larger than those in our experimental manipulations, and yielded correspondingly smaller larvae at dispersal, due to the well-documented trade-off in N. vespilloides between larval density and larval mass (see ref. 5). We performed a linear model examining the average larval mass of FC and NC broods, which included terms for “population” and “block,” the latter accounting for the replicated experimental populations. We did not detect a significant difference in average larval mass between the two populations (Fig. 4B).
Data Availability.
All data are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank S. Aspinall and C. Swannack for help with animal husbandry and general laboratory assistance. This work was funded by a Consolidator’s Grant from the European Research Council to R.M.K. (310785 Baldwinian_Beetles). R.M.K. was also supported by a Wolfson Merit Award from the The Royal Society. N.W.B. was supported by the UK Natural Environment Research Council (NE/L011255/1).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1911677117/-/DCSupplemental.
References
- 1.Davies N. B., Krebs J. R., West S. A., An Introduction to Behavioural Ecology (Wiley-Blackwell, Oxford, United Kingdom, ed. 4, 2012). [Google Scholar]
- 2.Mock D. W., Parker G. A., The Evolution of Sibling Rivalry (Oxford University Press, Oxford, United Kingdom, 1997). [Google Scholar]
- 3.Royle N. J., Smiseth P. T., Kölliker M., Eds., The Evolution of Parental Care (Oxford University Press, Oxford, United Kingdom, ed. 1, 2012). [Google Scholar]
- 4.Johnstone R. A., Begging and sibling competition: How should offspring respond to their rivals? Am. Nat. 163, 388–406 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Schrader M., Jarrett B. J. M., Kilner R. M., Parental care masks a density-dependent shift from cooperation to competition among burying beetle larvae. Evolution 69, 1077–1084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrader M., Jarrett B. J. M., Rebar D., Kilner R. M., Adaptation to a novel family environment involves both apparent and cryptic phenotypic changes. Proc. R Soc. B Biol. Sci. 284, 20171295 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarrett B. J. M., et al. , A sustained change in the supply of parental care causes adaptive evolution of offspring morphology. Nat. Commun. 9, 3987 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clutton-Brock T. H., The Evolution of Parental Care (Princeton University Press, Princeton, NJ, 1991). [Google Scholar]
- 9.Royle N. J., Russell A. F., Wilson A. J., The evolution of flexible parenting. Science 345, 776–781 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Houston A. I., Székely T., McNamara J. M., Conflict between parents over care. Trends Ecol. Evol. 20, 33–38 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Kilner R. M., Hinde C. A., “Information warfare and parent–offspring conflict” in Advances in the Study of Behavior Brockmann H. J. et al., Eds. (Elsevier, New York, NY, 2008), vol. 38, pp. 283–336. [Google Scholar]
- 12.Roulin A., Dreiss A. N., “Sibling competition and cooperation over parental care” in The Evolution of Parental Care, Royle N. J., Smiseth P. T., Kölliker M., Eds. (Oxford University Press, Oxford, UK, ed. 1, 2012), pp. 133–149. [Google Scholar]
- 13.Moore A. J., Brodie E. D. 3rd, Wolf J. B., Interacting phenotypes and the evolutionary process: I. Direct and indirect genetic effects of social interactions. Evolution 51, 1352–1362 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Wolf J. B., Brodie Iii E. D., Cheverud J. M., Moore A. J., Wade M. J., Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol. 13, 64–69 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Bailey N. W., Kölliker M., Social runaway: Fisherian elaboration (or reduction) of socially selected traits via indirect genetic effects. Evolution 73, 1549–1563 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Bailey N. W., Marie-Orleach L., Moore A. J., Indirect genetic effects in behavioral ecology: Does behavior play a special role in evolution? Behav. Ecol. 29, 1–11 (2018). [Google Scholar]
- 17.Jarrett B. J. M., et al. , Adaptive evolution of synchronous egg-hatching in compensation for the loss of parental care. Proc. R Soc. B Biol. Sci. 285, 20181452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smiseth P. T., Lennox L., Moore A. J., Interaction between parental care and sibling competition: Parents enhance offspring growth and exacerbate sibling competition. Evolution 61, 2331–2339 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Kramer J., Thesing J., Meunier J., Negative association between parental care and sibling cooperation in earwigs: A new perspective on the early evolution of family life? J. Evol. Biol. 28, 1299–1308 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Eggert A-K.., Reinking M., Müller J. K., Parental care improves offspring survival and growth in burying beetles. Anim. Behav. 55, 97–107 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Schrader M., Jarrett B. J. M., Kilner R. M., Using experimental evolution to study adaptations for life within the family. Am. Nat. 185, 610–619 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smiseth P. T., Darwell C. T., Moore A. J., Partial begging: An empirical model for the early evolution of offspring signalling. Proc. Biol. Sci. 270, 1773–1777 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West S. A., Griffin A. S., Gardner A., Social semantics: Altruism, cooperation, mutualism, strong reciprocity and group selection. J. Evol. Biol. 20, 415–432 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Lock J. E., Smiseth P. T., Moore A. J., Selection, inheritance, and the evolution of parent-offspring interactions. Am. Nat. 164, 13–24 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Kilner R. M., et al. , Parental effects alter the adaptive value of an adult behavioural trait. eLife 4, e07340 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascoal S., Jarrett B. J. M., Evans E., Kilner R. M., Superior stimulation of female fecundity by subordinate males provides a mechanism for telegony. Evol. Lett. 2, 114–125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bleakley B. H., Wolf J. B., Moore A. J., “The quantitative genetics of social behaviour” in Social Behaviour: Genes, Ecology, and Evolution, Székely T., Moore A. J., Komdeur J., Eds. (Cambridge University Press, Cambridge, United Kingdom, 2010), pp. 29–54. [Google Scholar]
- 28.Bleakley B. H., Brodie E. D. 3rd, Indirect genetic effects influence antipredator behavior in guppies: Estimates of the coefficient of interaction psi and the inheritance of reciprocity. Evolution 63, 1796–1806 (2009). [DOI] [PubMed] [Google Scholar]
- 29.McGlothlin J. W., Brodie E. D. III, How to measure indirect genetic effects: The congruence of trait-based and variance-partitioning approaches. Evolution 63, 1785–1795 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Bijma P., The quantitative genetics of indirect genetic effects: A selective review of modelling issues. Heredity 112, 61–69 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klug H., Alonzo S. H., Bonsall M. B., “Theoretical foundations of parental care” in The Evolution of Parental Care, Royle N. J., Smiseth P. T., Kölliker M., Eds. (Oxford University Press, Oxford, United Kingdom, 2012), pp. 21−39. [Google Scholar]
- 32.Milinksi M., Do all members of a swarm suffer the same predation? Ethology 45, 373–388 (1977). [Google Scholar]
- 33.Godfray H. C. J., Parker G. A., Clutch size, fecundity and parent-offspring conflict. Philos. Trans. R. Soc. Biol. Sci. 332, 67–79 (1991). [Google Scholar]
- 34.Way M. J., Cammell M., “Aggregation behaviour in relation to food utilization by aphids” in Animal Populations in Relation to Their Food Sources, Watson A., Ed. (Blackwell, Oxford, United Kingdom, 1970), pp. 229–247. [Google Scholar]
- 35.Henry C. S., Eggs and rapagula of Ululodes and Ascaloptynx (Neuroptera: Ascalaphidae): A comparative study. Psyche A J. Entomol. 79, 1–22 (1972). [Google Scholar]
- 36.Kudo S., Nakahira T., Effects of trophic-eggs on offspring performance and rivalry in a sub-social bug. Oikos 107, 28–35 (2004). [Google Scholar]
- 37.Mukai H., Hironaka M., Tojo S., Nomakuchi S., Maternal vibration induces synchronous hatching in a subsocial burrower bug. Anim. Behav. 84, 1443–1448 (2012). [Google Scholar]
- 38.Marti C. D., Food sharing by sibling common barn-owls. Wilson Bull. 101, 132–134 (1989). [Google Scholar]
- 39.Falk J., Wong J. W. Y., Kölliker M., Meunier J., Sibling cooperation in earwig families provides insights into the early evolution of social life. Am. Nat. 183, 547–557 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Romano A., Caprioli M., Boncoraglio G., Saino N., Rubolini D., With a little help from my kin: Barn swallow nestlings modulate solicitation of parental care according to nestmates’ need. J. Evol. Biol. 25, 1703–1710 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Reavey C. E., Beare L., Cotter S. C., Parental care influences social immunity in burying beetle larvae. Ecol. Entomol. 39, 395–398 (2014). [Google Scholar]
- 42.Duarte A., et al. , Social immunity of the family: Parental contributions to a public good modulated by brood size. Evol. Ecol. 30, 123–135 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeBoeuf A. C., et al. , Oral transfer of chemical cues, growth proteins and hormones in social insects. eLife 5, e20375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boncoraglio G., Kilner R. M., Female burying beetles benefit from male desertion: Sexual conflict and counter-adaptation over parental investment. PLoS One 7, e31713 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smiseth P. T., Musa S., Moore A. J., Negotiation between parents: Does the timing of mate loss affect female compensation in Nicrophorus vespilloides? Behaviour 143, 293–301 (2006). [Google Scholar]
- 46.Rauter C. M., Moore A. J., Evolutionary importance of parental care performance, food resources, and direct and indirect genetic effects in a burying beetle. J. Evol. Biol. 15, 407–417 (2002). [Google Scholar]
- 47.Chenoweth S. F., Rundle H. D., Blows M. W., Experimental evidence for the evolution of indirect genetic effects: Changes in the interaction effect coefficient, psi (Ψ), due to sexual selection. Evolution 64, 1849–1856 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Bailey N. W., Zuk M., Socially flexible female choice differs among populations of the Pacific field cricket: geographical variation in the interaction coefficient psi (ψ) Proc. R Soc. B Biol. Sci. 279, 3589–3596 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.R Core Team , R: A language and environment for statistical computing, Version 3.4.1. https://www.r-project.org. Accessed 1 July 2017.
- 50.Bates D., Maechler M., Bolker B. M., Walker S., Fitting linear mixed-effects models using {lme4}. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 51.Kazancıoğlu E., Klug H., Alonzo S. H., The evolution of social interactions changes predictions about interacting phenotypes. Evolution 66, 2056–2064 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Marie-Orleach L., et al. , Indirect genetic effects and sexual conflicts: Partner genotype influences multiple morphological and behavioral reproductive traits in a flatworm. Evolution 71, 1232–1245 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Signor S. A., Abbasi M., Marjoram P., Nuzhdin S. V., Social effects for locomotion vary between environments in Drosophila melanogaster females. Evolution 71, 1765–1775 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Rode N. O., Soroye P., Kassen R., Rundle H. D., Air-borne genotype by genotype indirect genetic effects are substantial in the filamentous fungus Aspergillus nidulans. Heredity 119, 1–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bijma P., Estimating indirect genetic effects: Precision of estimates and optimum designs. Genetics 186, 1013–1028 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are provided in SI Appendix.