Abstract
Introduction:
Lately, the use of biological therapy in various autoimmune diseases is increasing. The ideal marker for monitoring the effects of modern therapy is still non-existent.
Aim:
To investigate early response biomarkers of SLE and RA patients under the rituximab treatment are in research phase and each new investigations offer new and original useful data.
Material and Methods:
Immunophenotyping of cells was carried out by a standard method of sample preparation. We investigated by flow cytometric analyses expression of NK and CD19+ cells at ten SLE and five RA patients before and after treatment with rituximab, in laboratory of Department of Clinical immunology in the Clinical Centre University of Sarajevo.
Results:
In both cases, SLE and RA patients, reduced number of CD16+ parameter indicates lower cytotoxic activity of NK cells. Increased number of B cells indicates higher pathological activity leading to severe autoimmune disease allegation.
Conclusion:
Determining the proportion of NK and B will be useful diagnostic tool in therapeutic strategy, and also in monitoring of effect of biological therapy.
Keywords: Autoimmune disease, Biological drugs, B cells and NK cells, therapy
1. INTRODUCTION
Early response biomarkers of SLE and RA patients under the rituximab treatment are still in research phase and each new investigations offer new and original useful data. Due that fact, the leukocyte cell subpopulations analyzes of peripheral blood specimens taken from SLE and RA patients under rituximab treatment, are under intensively researches. For this purpose, the method of choice is immunophenotypization by flow cytometry Usually, analyses take place 6 weeks before and after rituximab intake based on doctor’s evaluation. Rituximab depletes already increased number of NK cells and CD19+ B-cells. CD20 antigen is found on surface of B lymphocytes and it is main binding site for rituximab which is a CD20-directed, IgG1-chimeric monoclonal antibody (mAb). Natural Killer (NK) cells constitute approximately 15% of the peripheral blood ant they expressed specific CD16 (FcyRIII- Fragment crystallizable region, RIII), CD56 molecules and receptors for activation an inhibition. The main phenotype of NK cells is CD3-CD16+CD56+. Antibody dependent cellular cytotoxicity (ADCC) mediated by NK cells, may be a primary mechanism of Rituximab functions. Furthermore, responses to rituximab is depend on CD16 molecule polymorphisms. Activation of NK cells begins by binding CD16+ receptor for Fc region IgG molecules in antigen-antibody complex. This activation mediates antibody-dependent cellular cytotoxicity (ADCC). However, CD16+ receptor can be linked to free circulating IgG antibody causing inhibition of NK cell functions (1-3).
FCyRIIIA gen encodes for CD16+ and is located on chromosome 1. Mutations in this gene have been linked to susceptibility to recurrent viral infections, susceptibility to systemic lupus erythematosus, and alloimmune neonatal neutropenia. In the case of lower expression of this gene, not enough amount of CD16 receptor is producing, which results lower NK cell activity at SLE and RA patients (4-6).
The FCYRIIIA gene displays a functional allelic dimorphism generating allotypes with either a phenylalanine (F) or a valine (V) residue at amino acid position 158. Mutation of FCyRIIIa gene marked as rs396991(T)–FCGR3A-176V/F, occurs when aminoacid valine switch place with phenilalanin and due that is called F allotype, while rs396991(G) polymorphism encodes for valin (V). Valin isoform encoding for CD16 has greater affinity for Fc region IgG molecules unlike F isoform with lower affinity. If some patient have F isoform of that gene, better respond to rituximab is expected (7, 8).
2. AIM
Aim of article was to investigate by flow cytometric analyses expression of NK and CD19+ cells at SLE and RA patients before and after treatment with rituximab.
3. METHODS
Blood collection
Based on clinical and laboratory parameters, doctors rhemuatologist selected patients for further analyses. Their blood was collected into EDTA Vacutainer tubes and transprted to the Flow cytometry laboratory of Department of Clinical immunology in the Clinical Centre University of Sarajevo. Ethical approval was obtained from Ethical Committee Clinical Center University of Sarajevo.
Flow cytometry
Flow cytometry is multiparametric analysis of morphological, biochemical and functional cell features with diameter in range of 0,2-150 μm. By flow cytometry is possible to determine the frequence of T lymphocytes (CD3+, CD4+, CD8+, CD4+/CD8+ ratio), B lymphocytes (CD19+), NK cells (CD16+CD56+), activated lymphocytes (CD8+CD38+) and absolute number of CD4+ T and CD8+ T lymphocytes.
Immunophenotyping of cells was carried out by a standard method of sample preparation. After lysis of erythrocytes, the leukocytes of peripheral blood were analyzed for the expression of specific leukocyte markers using a panel of monoclonal antibodies and flow cytometry (flow cytometer–BD FACS Canto II). 10,000-50,000 events were recorded per tube and analyzed using the BD FACSDiva™ software. The best results will be achieved if analysis of the cells on the flow cytometer are performed as soon as possible.
Monoclonal Antibodies
Combinations of surface markers that are determined by monoclonal antibody conjugated with FITC (i.e. florescin isothiocyanate), PE (i.e. phycoerythrin) and PerCP (i.e. Peridinin-chlorophyll-protein complex) or APC (i.e. alofikocianin) (9,10,11) as follows:
Tube 1: CD3–FITC/ CD8–PE/ CD45-PerCP/ CD4–APC;
Tube 2 : CD3 – FITC / CD16+56–PE/ CD45–PerCP/ CD19–APC.
4. RESULTS
Percentages of CD16 and CD19 receptor molecules on NK cells and B lymphocytes obtained by immunophenotypisation analyses were the main guidance of treatment efficiency.
The number of peripheral blood CD16+56 NK cells and CD19+ B cells were analyzed by flow cytometry. We analyzed 10 samples with SLE diagnosis and 5 samples with RA diagnosis.
Out of 10 samples, 4 samples showed significantly lower number of CD19 B lymphocytes, 4 samples showed higher number of this investigated parameter while 2 of them have value within the referral boundaries. On the contrary, majority of samples showed normal values for CD16+56 (60%). Four samples showed lower number of it with accent on sample 9 and 10 which showed seriously reduced number of this parameter.
Out of 5 samples selected for biological therapy sample 1 showed increased number of CD19+ parameter while sample 3 showed slightly lower number of it. Other samples had values within the referral limit. On the other side, just one sample had slightly reduced number regarding CD16+56 investigated parameter.
In both cases, SLE and RA patients, reduced number of CD16+ parameter indicates lower cytotoxic activity of NK cells. Those kind of NK cells have reduced ability of binding with immune complex connected with antigens and therefore their ADCC activity is reduced. Increased number of B cells indicates higher pathological activity leading to severe autoimmune disease allegation.
5. DISCUSSION
Today is a era if biological drugs. One of the most important of them is Rituximab, a monoclonal recombinant antibody strongly specific to B cell CD20 receptor molecules. In fact, the active place for rituximab adhesion is placed on extracellular region these molecules. CD20 molecules must have normal conformation but in contrary, in the case of specific mutation of CD20 gene, this active region can missed. However, CD20 human gene is placed on 11 chromosome (11q12-q13.1) and has 8 exons in coding region (E1,E2, E3, E4, E5, E6, E7, E8). This gene coding three mRNA splice isoforms or variants. The dominant CD20 mRNA isoform has all exons, but minor isoform is without 3-7 exons and final translational product have no active place specific for rituximab (7, 8, 9). Because of that, before rituximab therapy, it is necessary to perform molecular selection of patients by PCR detection of CD20 splice variant. Minor splice CD20 mRNA isoform is not feture of healthy persons but only appeared in pathological cases such as autoimmune disorders, lymphomas or malignant diseases. This splice form can be unique biomarker in the selection of biological drugs in rheumatology. However, molecular-genetic predictive rheumatological diagnostics is based on determination of specific genetic and epigenetic biomarkers. According to obtained data it is possible to select the the most effective drug for each patient personaly. On this way it is possible to avoid unnecessary costs and increase the effectiveness of the therapy to the highest possible level (10, 11). This means, that the molecular analyzes of specific genes are the base of thargeted precise personilized rheumatology. Actually, now is the era of personalized molecular medicine (12-17).
Rituximab has wide application in clinical management of rheumatic disorders such as for example Rheumatic arthritis (RA). These specific monoclonal antibodies recognize CD20 active sites on B cells, and then, these rituximab-coated B cells, are lyzing by NK cells. On this way, is possible to acchieve desired therapeutic depletion of B cells. The rituximab-induced phenomenon of B cells depletion by NK cells activity is object of many investigations.
However, NK cells are actually cytotoxic lymphocytes targeted specially to antibody coated cells, but until now, there is very little informations about antibody mediated cellular cytotoxity (AMCC) phenomenon, after rituximab binding to B cell CD20 receptor molecules, during biological therapy. The patients which showed incomplete B cell depletion, upon rituximab treatement, must receive an extra dose of this biological drug (18-23).
In this artical is represented and discussed the the obtained results of flow cytometric immunophenotyping analysis of serum specimens taken from clinically selected SLE and RA patients, under RTX therapy. The main goal of our investigations was possible determination of specific molecular biomarkers for individual clinical management improvement if these autoimmune diseases. Out of 10 samples, 4 samples showed significantly lower number of CD19 B lymphocytes, 4 samples showed higher number of this investigated parameter while 2 of them have value within the referral boundaries. On the contrary, majority of samples showed normal values for CD16+56 (60%). Four samples showed lower number of it with accent on sample 9 and 10 which showed seriously reduced number of this parameter.
Out of 5 samples selected for biological therapy sample 1 showed increased number of CD19+ parameter while sample 3 showed slightly lower number of it. Other samples had values within the referral limit. On the other side, just one sample had slightly reduced number regarding CD16+56 investigated parameter.
In both cases, SLE and RA patients, reduced number of CD16+ parameter indicates lower cytotoxic activity of NK cells. Those kind of NK cells have reduced ability of binding with immune complex connected with antigens and therefore their ADCC activity is reduced. Increased number of B cells indicates higher pathological activity leading to severe autoimmune disease allegation. This is the one of first evaluation of NK cells as biomarkers of clinical response after rituximab therapy in rheumatic diseases. The results showed lower level of NK cell killing activity. The determined relative percentage of NK cell is strongly in the correlation for their cilling activity.
The finding of low killing activity in relatives and a correlation between their activity and that of their patients support the view that NK cell deficiency is a genetic determinant of SLE. NK cells in SLE may produce insufficient levels of cytokines required for the regulation of IgG production.
B-cell depletion with unconjugated CD20 monoclonal antibody (mAb) is used to treat rheumatoid arthritis and other autoimmune diseases. This treatement cause deplete mature B cells through monocyte-mediated antibody-dependent cellular cytotoxicity. On the other hand, there is anti-CD19 monoclonal antibodies for therapeutical reduction of B lymphocytes. These humanized recombinant antibodies start used in clinical trials from 2009. and offer new possibilities in B-cell depletion therapeutical effects in treatement of SLE and RA (24-27).
6. CONCLUSION
Determining the proportion of NK and B will be useful diagnostic tool in therapeutic strategy, and also in monitoring of effect of biological therapy.
Figure 1. Samples of RA patients. Treatment with rituximab proved to be successful within the majority of specimens expect sample 2.
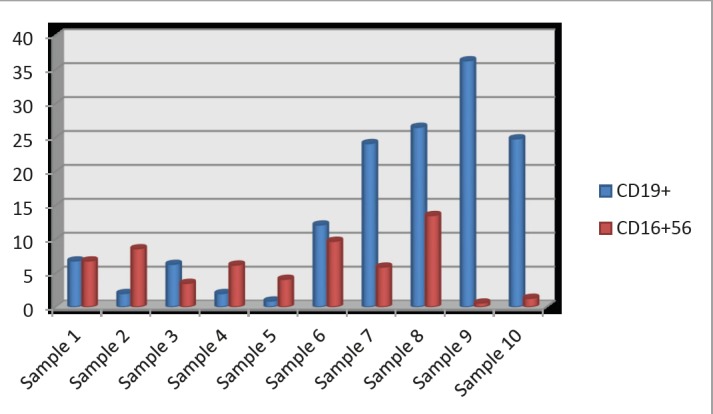
Figure 2. 10 samples of SLE patients. This diagram shows that samples 2, 5, 9 and 10 have seriously decreased number of CD16+56 cells while samples 7, 8, 9, and 10 have seriously increased number of CD19+ B cells. From these results we can conclude that the worst results had samples 9 and 10.
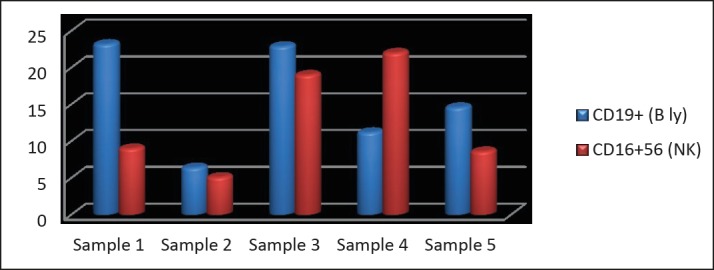
Table 1. Obtained results of immunophenotypization analyses for 10 SLE patient specimens.
| CD19+ (B Ly) | CD16+56 (NK) | |
|---|---|---|
| Referral values (%) | 6.4–23 | 5.6–31 |
| Sample 1 | 6.8 | 6.8 |
| Sample 2 | 2.0 | 8.6 |
| Sample 3 | 6.3 | 3.5 |
| Sample 4 | 2.0 | 6.2 |
| Sample 5 | 0.9 | 4.1 |
| Sample 6 | 12.1 | 9.7 |
| Sample 7 | 24.1 | 5.9 |
| Sample 8 | 26.5 | 13.5 |
| Sample 9 | 36.3 | 0.6 |
| Sample 10 | 24.8 | 1.3 |
Table 2. Obtained number of immunophenotypization analyses for 5 RA patient specimens.
| CD19+ (B Ly) | CD16+56 (NK) | |
|---|---|---|
| Referral values (%) | 6.4-23 | 5.6-31 |
| Sample 1 | 23.2 | 9.0 |
| Sample 2 | 6.4 | 5.1 |
| Sample 3 | 22.9 | 19.0 |
| Sample 4 | 11.2 | 22.0 |
| Sample 5 | 14.6 | 8.6 |
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent forms.
Author’s contribution:
L.Z., M.M. and Dj.S. gave substantial contribution to the conception or design of the work and in the acquisition, analysis and interpretation of data for the work. Each author had role in drafting the work and revising it critically for important intellectual content. E.B. gave final approval of the version to be published and they agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interest:
There are no conflicts of interest.
Financial support and sponsorship:
Nil.
REFERENCES
- 1.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 2.Strand V, Kimberly R, Isaacs JD. Biologic therapies in rheumatology: lessons learned, future directions. Nat Rev Drug Discov. 2007;6:75–92. doi: 10.1038/nrd2196. [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Keystone EC, Emery P, et al. Consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:143–150. doi: 10.1136/ard.2006.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toubi E, Kessel A, Slobodin G, et al. Changes in macrophage function after rituximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:818–820. doi: 10.1136/ard.2006.062505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faurschou M, Jayne DR. Anti-B cell antibody therapies for inflammatory rheumatic diseases. Annu Rev Med. 2014;65:263–278. doi: 10.1146/annurev-med-070912-133235. [DOI] [PubMed] [Google Scholar]
- 6.Vital EM, Dass S, Buch MH, et al. An extra dose of rituximab improves clinical response in rheumatoid arthritis patients with initial incomplete B cell depletion: a randomised controlled trial. Ann Rheum Dis. 2015;74(6):1195–1201. doi: 10.1136/annrheumdis-2013-204544. [DOI] [PubMed] [Google Scholar]
- 7.Anolik JH, Campbell D, Felgar RE, et al. The relationship of Fc gamma RIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum. 2003;48(2):455–459. doi: 10.1002/art.10764. [DOI] [PubMed] [Google Scholar]
- 8.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatachalam V, Morrissey P. Cellular image analysis and imaging by flow cytometry. Clin. Lab. Med. 2007;27(3):653–670. doi: 10.1016/j.cll.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo K, Brinkman RR, Gottardo R. Automated gating of flow cytometry data via robust model-based clustering. Cytometry A. 2008;73(4):321–332. doi: 10.1002/cyto.a.20531. [DOI] [PubMed] [Google Scholar]
- 11.FACS MultiSET System (PDF). Becton Dickinson. Archived from the original (PDF) on October 18, 2006. Retrieved 2007-02-09.
- 12.Kohrt HE, Houot R, Weiskopf K, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122(3):1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor Fc gamma RIIIa gene. Blood. 2002;99(3):754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 14.Treon SP, Hansen M, Branagan AR, et al. Polymorphisms in Fc gamma RIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenstrom’s macroglobulinemia. J Clin Oncol. 2005;23(3):474–481. doi: 10.1200/JCO.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 15.Veeramani S, Wang SY, Dahle C, et al. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118(12):3347–3349. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurati A, Bertani L, Marazza M, et al. NK cell count as predictor of clinical response in patients with rheumatoid arthritis treated with rituximab. Biologics. 2012;6:83–87. doi: 10.2147/BTT.S29079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyake S, Yamamura T. NKT cells and autoimmune diseases: unraveling the complexity. Curr Top Microbiol Immunol. 2007;314:251–267. doi: 10.1007/978-3-540-69511-0_10. [DOI] [PubMed] [Google Scholar]
- 18.Tedder TF. CD19: a promising B cell target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:572–577. doi: 10.1038/nrrheum.2009.184. [DOI] [PubMed] [Google Scholar]
- 19.Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer cell activity in families of patients with systemic lupus erythematosus: demonstration of a killing defect in patients. Clin Exp Immunol. 2005;141(1):165–173. doi: 10.1111/j.1365-2249.2005.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breedveld F, Agarwal S, Yin M, et al. Rituximab pharmacokinetics in patients with rheumatoid arthritis: B-cell levels do not correlate with clinical response. J Clin Pharmacol. 2007;47(9):1119–1128. doi: 10.1177/0091270007305297. [DOI] [PubMed] [Google Scholar]
- 21.Bowles JA, Wang SY, Link BK, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108(8):2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 23.Reis EA, Athanazio DA, Lima I, et al. NK and NKT cell dynamics after rituximab therapy for systemic lupus erythematosus and rheumatoid arthritis. Rheumatol Int. 2009;29(4):469–475. doi: 10.1007/s00296-008-0719-0. [DOI] [PubMed] [Google Scholar]
- 24.Bowles JA, Wang SY, Link BK, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108:2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman GJ. Therapeutic B cell depletion and regeneration in rheumatoid arthritis: emerging patterns and paradigms. Arthritis Rheum. 2006 Aug;54(8):2356–2367. doi: 10.1002/art.22020. [DOI] [PubMed] [Google Scholar]
- 26.Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 27.Mendes R, Bromelow KW, Westby M, et al. Flow cytometric visualization of cytokine production by CD3-CD56+ NK cells and CD3+CD56+NK-T cells in whole blood. Cytometry. 2000;39(1):72–78. doi: 10.1002/(sici)1097-0320(20000101)39:1<72::aid-cyto10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]


