Abstract
Introduction:
Diabetic nephropathy is the second most common secondary type of glomerular diseases among Saudi patients after systemic lupus erythematosus. Ocimum basilicum (O. basilicum) was reported to have anti-diabetic and antioxidants effects.
Aim:
This study aimed to evaluate the efficacy of O. basilicum in controlling STZ-induced diabetes mellitus in rats and preserving the structure of kidney against diabetes-induced nephropathy.
Methods:
This study utilized forty adult male Spraque-Dawley rats assigned into four groups (n=10 each); control, streptozotocin-induced diabetic, metformin-treated and O. Basilicum-treated groups. The blood glucose level (BGL), total anti-oxidant capacity (TAC), serum creatinine and BUN levels were assessed. Kidneys were dissected out and processed for histopathological and immunohistochemical assessment.
Results:
The BGL significantly decreased in Metformin- and O. basilicum-treated (p=0.02, p=0.01) rats while TAC significantly increased (p=0.01, p=0.003) respectively, compared to the untreated diabetic rats. In addition, O. basilicum significantly reduced (p=0.004, p=0.02) both creatinine and BUN levels compared to the untreated diabetic group, respectively. Examination of kidney of O. basilicum-treated diabetic rats revealed few degenerated renal tubules, with no inflammatory cell infiltrates, peritubular capillaries congestion and minimal peritubular collagen fibers deposition. It also reduced immunoexpression of desmin and αsmooth muscle actin in glomeruli of O. basilicum-treated diabetic rats.
Conclusion:
Glucose lowering and antioxidant effects of O. basilicum was evident biochemically in this study. O. basilicum could protect the kidney against diabetes-induced nephropathy as revealed biochemically and histopathologically. Further exploration of the mechanism and assessment of efficacy in human through clinical study are recommended.
Keywords: Ocimum Basilicum, Basil-diabetes mellitus, kidney-desmin-ASMA-creatinine, histopathology
1. INTRODUCTION
Diabetes mellitus (DM), one of the common metabolic diseases, characterized by chronic hyperglycemia and disturbed metabolism of carbohydrate, fat, and protein. It might result from defects in either insulin secretion, insulin action, or both (1). Type 1 diabetes mellitus (T1DM) is insulin dependent and results when T-cells destroy the insulin-secreting β-cells, while T2DM occurs when there is a rise in glucose levels with mounting cell’s resistant to inadequate insulin (2). Considerable scientific research suggested that under oxidative stress situations, reactive oxygen species (ROS) are produced. However, it is believed that the equilibrium between the oxidation and antioxidation processes results in causing human diseases including diabetes (3).
Among the sequale of diabetes mellitus that occur after long time is the progressive development of the some complications like retinopathy with possibility of development of blindness, nephropathy with possibility of development of renal failure, and/or neuropathy (4). A recent study conducted by AlFaadhel et al. in Saudi Arabia (5). They found that the most common secondary types of glomerular diseases was the systemic lupus erythematosus-associated lupus nephritis followed by diabetic nephropathy. They added that the prevalence of diabetic nephropathy increased from 1.4% in the first era to 10.2% in the last one.
Conventional antidiabetic drugs were reported to be associated with many side effects therefore, research has turned to the traditional remedies in the form of plants (6). There are already 1,200 plants that received acknowledgement being a possible anti-diabetic remedy (7). Medicinal plants contain high levels of flavonoids, carotenoids, antioxidants, terpenoids, and alkaloids needed for the human body. Since medicinal plants are natural sources, they have fewer side effects, and they offer evidence for the synthesis of novel and improved medicines for treating diabetes (8).
Extracts of Ocimum basilicum L (Lamiaceae), also known as Basil or Rehan, have been reported to possess different pharmacological activities due to the high content of phytocompounds as phenolic compounds and essential oils such as estragol, linalool and eugenol. These pharmacological effects include lowering blood glucose, antioxidants, reducing inflammation, and lowering the incidence of cancers and diabetes as well as hepatoprotective properties (9, 10).
Sakr and Al-Amoudi have examined the possible protecting effect induced by Ocimum basilicum extract on renal affection induced after administration of deltamethrin in albino rats (11). They found that O. basilicum extract could alleviate the deltamethrin-induced structural changes in the kidney. Renal functions of Ocimum basilicum-treated rats was also enhanced as evidenced by significant restoration of serum creatinine and urea. El Shahat et al., 2017 reported that basil (Ocimum basilicum L.) can guard against hepatic and renal injury occur as a result of methotrexate administration and might be of therapeutic latent in lessening the systemic side effects of this anticancer drug (12).
2. AIM
Therefore, this study aimed to assess the effect of O. basilicum in controlling streptozotocin-induced diabetes mellitus in rats and to assess its efficacy in preserving the structure of kidney against diabetes-induced nephropathy.
3. METHODS
Ocimum Basilicum (O. Basilicum), also known as Reihan, was obtained from the local gardens of Albaha in April 2018 and was morphologically recognized and verified by c botanist, at Faculty of Science, King Abdulaziz University. The essential oils of O. Basilicum were extracted and prepared according to methods described by Ismail (13). Streptozotocin (STZ), (Sigma Aldrich Chemical Company, Co., St. Louis, MO, USA) was utilized for induction of diabetes mellitus in rats at the dose of 60 mg/kg that was injected intraperitoneally after being dissolved in 0.01 M sodium citrate buffer (14). Metformin hydrochloride (Shanghai Shiguibao Medicine Co., Ltd., China) was used for pharmacological validation of OB. It was dissolved in 0.9% (W/V) sodium chloride at a dose of 500 mg/kg/day, and given to rats through gastric tube for six weeks as was previously described by Pushparaj et al. (15).
This study was revised and permitted by the biomedical research ethics committee at the Faculty of Science, Albaha University. Forty adult male Spraque-Dawley rats weighing 200-250 g were purchased from the Experimental Animal Unit of King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia.
Rats were left to acclimatized fourteen days under the standard laboratory conditions. They were assigned into four groups (n=10 each). The first groups was the control one which was injected once intraperitoneally with 0.01 M sodium citrate buffer. The other three groups were injected with freshly prepared STZ after fasting overnight for 12 hours, followed by 5% sucrose solution in order to avoid hypoglycemia and facilitate STZ entry to β cells (16). On the 7th day of STZ injection, blood samples were obtained from rat tail vein and blood glucose level (BGL) was assessed using Bayer’s Contour meter (Japan). BGL was measured at regular intervals during the 6 weeks. Type 2 DM was consider at BGL < 250 mg/dl (15). The diabetic rats were assigned into 3 groups (n=10), non-treated diabetic group (STZ), diabetic treated with Metformin and diabetic treated with OB. All treatments were continued for six weeks.
At the end of the experiment, the rats were fasted for 12 hour then blood samples were obtained from rat tail vein, centrifuged at 3000 rpm for 15 minutes to separate serum that was stored at -80 °C. The blood glucose level (BGL) was assessed using Bayer’s Contour meter (Japan). The total anti-oxidant capacity (TAC) was estimated using the Bio-diagnostic kit method (17). After obtaining the blood samples, the rats were scarified by decapitation under deep ether anesthesia. The abdomen was opened and the kidneys were dissected out, fixed in neutral buffered formalin overnight and processed to obtain paraffin blocks. Paraffin sections at 4 μm were obtained and stained with hematoxylin and eosin for histopathological examination using light microscope (Olympus, USA) connected to digital camera.
Another group of paraffin sections were stained immunohistochemically using an avidin–biotin technique that was described by Nie et al. (18). Antiα smooth muscle actin (ASMA) and desmin (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was utilized at dilution of 1:500. The primary antibody was omitted in some sections to be utilized as negative controls. All positive reactivity was distinguished as principally nuclear and cytoplasmic brown staining.
A morphometric study included measuring the Surface area of glomeruli (μm2) was done using the Pro Plus image analysis software version 6.0. For each rat, five sections were examined and five readings were obtained and the mean was considered for each rat as was described by Nie et al. (18). Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 16. One-way analysis of variance (ANOVA). Post-hoc Bonferroni test was used to compare data of all the treated groups. Data were presented as mean±standard deviation (SD). A p value less than 0.05 was considered to be significant.
4. RESULTS
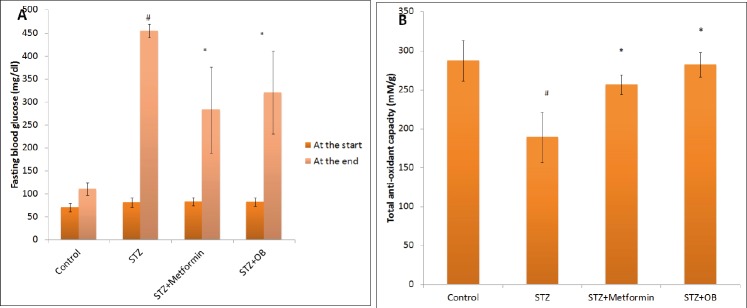
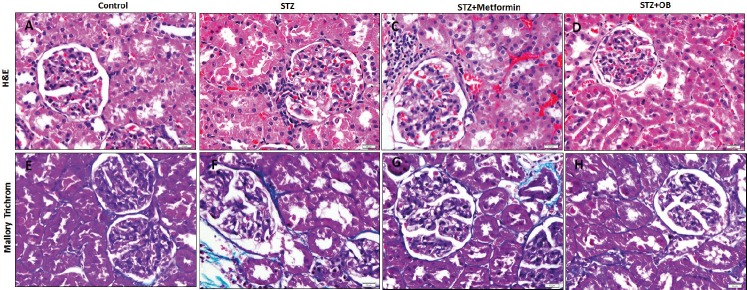
Measuring the fasting BGL at the start of the experiment revealed that it was within the normal rang in all groups. The end of the 1st week after STZ injection, the BGL was increased to above 300 mg/dl in all rats. It was kept increased in the untreated diabetic rats while it significantly decreased in Metformin- (p=0.02) and O. basilicum-treated (p=0.01) rats compared to the untreated diabetic rats (Figure 1A). It was observed that STZ-induced diabetic rats had a significant reduction (p<0.001) in the TAC compared to the control rats while those treated with metformin (p=0.01) or O. basilicum (p=0.003) showed a significant rise in TAC compared to the untreated diabetic rats (Figure 1B). Induction of diabetes in rats induced a significant increase (p=0.002, p=0.02) in creatinine and BUN levels in the serum respectively, compare to the control rat. Administration of metformin resulted in a significant reduction in creatinine level (p=0.02) and non-significant reduction in BUN level while O. basilicum significantly reduced (p=0.004, p=0.02) both creatinine and BUN compared to the untreated diabetic group, respectively (Table 1). Histological assessment of the structure of the renal cortex of the studied groups revealed that control renal cortex appeared with intact glomeruli and renal tubules. On the other hand, renal cortex of the diabetic group showed some degenerated renal tubules, specifically the proximal convoluted tubules (PCT) that possessed dark nuclei and cytoplasm. Inflammatory cell infiltrates were frequently observed in the periglomerular areas and the peritubular capillaries appeared congested. The mean surface area of the glomeruli was significantly increased (p<0.001) in this group compared to the control. Metformin-treated diabetic rats still showed all these pathological changes expect the degenerated cells of the PCT that was less frequently observed. When it came to the O. basilicum-treated diabetic rats, it was observed that most of the PCT appeared intact apart from few degenerated cells. There is no inflammatory cell infiltrate and the congested periglomerular capillaries were less frequently observed. The mean surface area of the glomeruli was significantly increased (p=0.02) in this group compared to the untreated diabetic group (Figure 2 A-D, Table 1).
Figure 1. Effect of Ocimum Basilicum on fasting blood glucose level (A) and total antioxidants capacities (B) in serum of the studied groups. Resulrs are expressed as mean± Standard deviation (SD). Significance is considered at P value<0.05.
Table 1. Effect of OB on kidney function and glomerular surface area in the studied groups. One-way analysis of variance (ANOVA) followed by post-hoc Bonferroni test was used to compare data of all the treated groups. Data are presented as mean±standard deviation (SD). P significance versus control group, P# significance versus STZ group .
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | STZ | Metformin | OB | |
| Creatinin (mg/dl) | 34.13 ± 11.02 | 53.3±12.2 P=0.002 |
43.2±3.13 P#=p=0.02 |
40.2±3.13 P#=0.004 |
| BUN (mmol/L) | 4.9±1.5 | 6.9±1.9 P=0.02 |
5.4±1.4 P#=0.06 |
5.1±1.3 |
| Surface area of glomeruli (μm2) | 8810±531.9 | 15440±1739.6 p<0.001 |
13886±2036 P#=0.08 |
12975±2036 P#=0.02 |
Figure 2. Effect of Ocimum Basilicum on the histological structure of the renal cortex stained with hematoxylin & eosin (A-D) and Mallry trichrome (F-H) in the studied groups. OB:Ocimum Basilicum.
Mallory trichrome-stained sections of renal cortex from control rats showed small amount of collagen fibers in between the glomerulus and tubules while these fibers were obviously increased in diabetic rats. On the other hand, peri-glomerular and peritubular collagen fibers decreased in both metformin- and O. basilicum-treated groups (Figure 2 E-H).
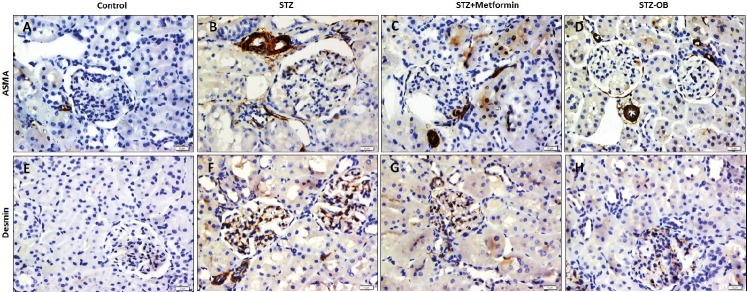
Immunohistochemical assessment of the expression of ASMA in the studied groups revealed that there was very week expression of ASMA in the control renal cortex apart from this observed in the blood vessels. The enlarged glomeruli of the diabetic rats showed moderate positive expression of ASMA in the glomerular and the periglomerular areas. Diabetic rats treated with either metformin or O. basilicum showed moderate to weak ASMA expression with no marked difference between the two groups (Figure A-D).
When it came to the immunoexpression of desmin, it was observed that both glomeruli and renal tubules of the control group showed negative reaction. The enlarged glomeruli of the diabetic rats showed strong positive expression of desmin while some tubules showed moderate reaction compared to the control. Treatment of diabetic rats with metformin resulted in a reduction of glomerular desmin expression to moderate reaction while treatment with O. basilicum resulted in a weak expression of desmin in the renal glomeruli (Figure 3 E-H).
Figure 3. Effect of Ocimum Basilicum on the immunohistochemical expression of ASMA and desmin in the renal cortex of the control (A, E, STZ-treated (B, F), Metformin-treated diabetic (C, G) and OB-treated (D, H) groups (ASMA (A-D), desmin (F-H) X400). ASMA: Alpha smooth muscle actin. Ocimum Basilicum: OB.
5. DISCUSSION
In a recent study performed by Widjaja et al., the hypoglycemic impact of basil was documented (19). They reported that ethanolic extract of basil leaves at the dose of 100, 200 and 400 mg/kg body weight (BW), significantly lower blood glucose and advanced glycation end products in diabetic rats compared to the untreated diabetic rats. Despite of this, no studies have been conducted to assess efficacy of O. basilicum in preserving the structure of kidney against diabetes-induced nephropathy. Therefore this study was conducted to investigate this issue.
In this study, BGL significantly decreased in both Metformin- and O. basilicum-treated rats. This finding, was supported by many previous studies (20, 21). The major phenolic substances extract from Ocimum gratissimum and Ocimum basilicum which included (caftaric, rosmarinic, caffeic, and chicoric acids) have been reported to significantly affected the gene expression pattern of the key insulin regulatory genes and proliferative genes as well as glucose transporter 2 (GLUT2), in treated islets. This explained its efficacy in reducing the blood glucose level (22).
Treating the diabetic rats with O. basilicum, in this study, significantly increased the TAC compared to the untreated diabetic rats. These findings were in agreement with that of Dasgupta et al. (23) who reported that O. basilicum increased the response of the antioxidant enzymes by enhancing “the hepatic glutathione reductase, superoxide dismutase, and catalase activities”. In addition it increased glutathione content and decreased lipid peroxidation and lactate dehydrogenase activity in the liver of mice. Similar findings were also reported by Sakr and Al-Amoudi (11). Basil was described to ameliorate methotrexate-induced nephrotoxicity due to the presence of flavonoids and numerous compounds with high antioxidant activities (24).
In the present work, administration of O. basilicum and metformin induced a significant decrease in creatinine level while O. basilicum only could significantly reduce BUN level compared to the untreated diabetic rats. These findings were supported by previous studies showed that O. basilicum has a protective effect on the kidney of rats receiving a toxic dose of mercury, lead and paracetamol (25-27). Not only that, Suanarunsawat and Songsak concluded that O. basilicum in diet induced a protective effect on renal glomerular filtration ability in diabetic rats as it reduced the elevated level of serum creatinine in the blood (28). In a more recent study, raw or gamma-irradiated basil added to the diet of methotrexate-treated rats markedly decreased the activities of serum liver enzymes, serum urea, creatinine and uric acid with subsequent improvement in the antioxidants level of the liver and kidney (12).
Renal cortex of the diabetic rats, in this study, showed degeneration of some PCTs, inflammatory cell infiltrates, congested peritubular capillaries and peri-glomerular and peritubular fibrosis evident by Mallory stain and ASMA immunohistochemistry. These findings were in accordance with many previous researches (29). Li and Zhang reported that diabetic nephropathy was associated with tubulointerstitial and glomerular fibrosis and sclerosis in STZ-induced diabetic rats (30).
Treatment of diabetic rats with metformin and O. basilicum these alleviated these pathological changes and reduced ASMA and desmin expression in the renal glomeruli. Makwana and Rathore found that the extract of O. basilicum prevented the paracetamol-induced hepatic and renal histopathological changes in the rats (27). “The cytoplasmic vacuolization of the lining epithelial cells of the renal tubules” induced by deltamethrin were markedly improved by extract of Ocimum basilicum (11). They attributed this effect to flavonoids present in excess in the leaves of O. basilicum which proved to have many biological effects related to the antioxidant activities. Zhang et al. reported that the main components of O. basilicum were: linalool (about 30%), (Z)-cinnamic acid methyl ester (about 21 %), cyclohexene, alpha- cadinol, in addition other compounds (31). Increased diameter of renal glomeruli indicating mesangial expansion was observed in diabetic rats, in this study and this was in agreement with previous studies that diabetes in rats was associated with glomerular injuries (32, 33). Several glomeruli showed mesangial widening with mesangial expansion and increased glomerular diameter.
ASMA expression was increased, in this study, in glomeruli and the periglomerular area of the diabetic rats compared to the control rats. This was in agreement with the finding of Li and Zhang who found that the level of immunoexpression of transforming growth factor-β (TGFβ) and alph-smooth muscle actin (αSMA) in the rats suffering from diabetic nephropathy were up-regulated compared with the normal group (30). It was said that fibroblasts have a crucial role in the repair during diabetic nephropathy, and enhanced TGF‑β might result in an enhanced transformation of these cells into activated myofibroblasts, evident by increased expression of α‑SMA that enhances fibroblast contractile activity (34).
Desmin is an intermediate filament protein which was described to be a podocyte injury indicator as injured podocytes undergo epithelial-to-mesenchymal transition (35). The expression of desmin is reported to be induced markedly in pathological conditions that affects podocytes as in diabetic nephropathy (36). Therefore it was assessed in this study. In this study, desmin expression was increased in the glomeruli of diabetic rats and this was in agreement with what was reported by Erfan (37) and Gu et al. (38). Diabetic rats treated with O. basilicum, in this study, showed reduced ASMA and desmin expression. This effect might be attributed to the inhibitory effect induced by O. basilicum on lipid peroxidation. Yamamoto et al. (39) proved that ocimum suppressed hepatic fibrosis and protected liver against parenchymal damage induced by CCL4.
6. CONCLUSION
Glucose lowering and antioxidant effects of O. basilicum was evident biochemically in this study. O. basilicum could protect the kidney against diabetes-induced nephropathy as revealed biochemically and histopathologically. Further exploration of the mechanism and assessment of efficacy in human through clinical study are recommended.
Author’s contribution:
D.A.A. gaves a substantial contribution to the conception and design of the study, substantial contribution of data, and, also, had a part in article preparing for drafting or revising it critically for important intellectual content. Author has given final approval of the version to be published.
Conflicts of interest:
There are no conflicts of interest.
Financial support and sponsorship:
Nil
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diab Care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Copenhaver M, Hoffman RP. Type 1 diabetes: where are we in 2017? Transl. Pediatr. 2017;6(4):359–364. doi: 10.21037/tp.2017.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sur TK, Hazra AK, Bhattacharyya D, Hazra A. Antiradical and antidiabetic properties of standardized extract of Sunderban mangrove Rhizophora mucronata. Pharmacogn. Mag. 2015;11(42):389–394. doi: 10.4103/0973-1296.153094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krentz AJ, Patel MB, Bailey CJ. New drugs for Type 2 diabetes mellitus: What is their place in therapy? Drugs. 2008;68:2131–2162. doi: 10.2165/00003495-200868150-00005. [DOI] [PubMed] [Google Scholar]
- 5.AlFaadhel T, Alsuwaida A, Alsaad K, Almezaini L, et al. Prevalence and 20-year epidemiological trends of glomerular diseases in the adult Saudi population: a multicenter study. Ann Saudi Med. 2019 May-Jun;39(3):155–161. doi: 10.5144/0256-4947.2019.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D, Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: a systematic review. Elec Physician. 2016;8(1):1832–1842. doi: 10.19082/1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang CLT, Lin Y, Bartolome AP, Chen YC, Chiu SC, Yang W. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid. Based Complement. Alternat. Med. 2013:1–33. doi: 10.1155/2013/378657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giovannini P, Howes MJ, Edwards SE. Medicinal plants used in the management of diabetes and its sequelae in Central America: a review. J. Ethnopharmacol. 2016;184:58–71. doi: 10.1016/j.jep.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Mastaneh M, Ahmad M, Taher N, Mehrdad H. Antioxidant effect of Ocimum basilicum cv. dark opal (Lamiaceae) Phenolics. Orient J Chem. 2014;30:1965–1969. [Google Scholar]
- 10.El-Beshbishy HA, Bahashwan SA. Hypoglycemic effect of basil (Ocimum basilicum) aqueous extract is mediated through inhibition of α-glucosidase and α-amylase activities. Toxicol Ind Health. 2012;28:42–50. doi: 10.1177/0748233711403193. [DOI] [PubMed] [Google Scholar]
- 11.Sakr SA, Al-Amoudi WM. Effect of leave extract of Ocimum basilicum on deltamethrin induced nephrotoxicity and oxidative stress in albino rats. J Appl Pharmaceu Sci. 2012;02(05):22–27. [Google Scholar]
- 12.El Shahat AN, El-Shennawy HM, Mohamed Abd el-Megid HM. Studying the protective effect of gamma-irradiated basil (Ocimum basilicum L.) against methotrexate-induced liver and renal toxicity in rats. Indian J Anim Res. 2017;51(1):135–140. [Google Scholar]
- 13.Ismail M. Central Properties and Chemical Composition of Ocimum basilicum. Essential Oil. J Pharmaceu Biol. 2006;44(8):619–626. [Google Scholar]
- 14.Salemi Z, Rafie E, Goodarzi MT, Ghaffari MA. Effect of Metformin, Acarbose and Their Combination on the Serum Visfatin Level in Nicotinamide/Streptozocin-Induced Type 2 Diabetic Rats. Iran Red Crescent Med J. 2016 Mar;18(3):e23814. doi: 10.5812/ircmj.23814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pushparaj P, Tan CH, Tan BKH. Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. J Ethnopharmacol. 2000;72(1):69–76. doi: 10.1016/s0378-8741(00)00200-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, Li Z, Kullin M, Borg LAH, Karlsson FA. Alterations in net glucose uptake and in the pancreatic B-cell GLUT2 transporter induced by diazoxide and by secretory stimuli. J Endocrinol. 2005;185(2):291–299. doi: 10.1677/joe.1.05701. [DOI] [PubMed] [Google Scholar]
- 17.Koracevic D, Koracevic G, Djordjevic V, Androgenic S, Cosic S. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–561. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie R, Zhou Q, Jassim E, et al. Differential expression of estrogen receptors a & b in the reproductive tract of the adult male dog and cat. Biol Reprod. 2002;66:1161–1168. doi: 10.1095/biolreprod66.4.1161. [DOI] [PubMed] [Google Scholar]
- 19.Widjaja SS, Rusdiana, Savira M. Glucose Lowering Effect of Basil Leaves in Diabetic Rats. Open Access Maced J Med Sci. 2019 May 5;7(9):1415–1417. doi: 10.3889/oamjms.2019.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z, Wang X, Zhou M, Ma L, Deng Y, Zhang H, et al. The antidiabetic activity of total lignin from Fructus Arctii against alloxan-induced diabetes in mice and rats. Phytother Res. 2008;22(1):97–101. doi: 10.1002/ptr.2273. [DOI] [PubMed] [Google Scholar]
- 21.Krishnasamy G, Muthusamy K, Chellappan DR, Subbiah N. Antidiabetic, antihyperlipidaemic, antioxidant activity of Syzygium densiflorum fruits in streptozotocin and nicotinamide-induced diabetic rats. Pharm Biol. 2016;54(9):1716–1726. doi: 10.3109/13880209.2015.1125932. [DOI] [PubMed] [Google Scholar]
- 22.Casanova LM, Gu W, Costa SS, Jeppesen PB. Phenolic Substances from Ocimum Species Enhance Glucose-Stimulated Insulin Secretion and Modulate the Expression of Key Insulin Regulatory Genes in Mice Pancreatic Islets. J Nat Prod. 2017 Dec 22;80(12):3267–3275. doi: 10.1021/acs.jnatprod.7b00699. [DOI] [PubMed] [Google Scholar]
- 23.Dasgupta T, Rao A, Yadava P. Chemomodulatory efficacy of basil leaf (Ocimum Basilicum) on drug metabolizing and antioxidant enzymes, and on carcinogen induced skin and forestomach papillomagenesis. Phytomedicine. 2007;11:139–151. doi: 10.1078/0944-7113-00289. [DOI] [PubMed] [Google Scholar]
- 24.Sakr SA, El-Abd SF, Osman M, Kandil AM, Helmy MS. Ameliorative effect of aqueous leave extract of Ocimum basilicum on CCl4–induced hepatotoxicity and apoptosis in Albino rats. J Am Sci. 2011;7:116–127. [Google Scholar]
- 25.Sharma MK, Kumar M, Kumar A. Ocimum sanctum leaves extract provides protection against mercury induced toxicity in Swiss albino mice. Indian J Exp Biol. 2002;40:1072–1082. [PubMed] [Google Scholar]
- 26.Karamala SK, Srilatha C, Anjaneyulu Y, ChandraSekharaRao TS, Sreenivasulu D, Pidugu AP. Haematobiochemical changes of lead poisoning and amelioration with Ocimum sanctum in Wistar albino rats. Vet World. 2011;4:260–263. [Google Scholar]
- 27.Makwana M, Rathore HS. Prevention of hepatorenal toxicity of acetaminophen with Ocimum sanctum in mice. IJPT. 2011;3:1385–1396. [Google Scholar]
- 28.Suanarunsawat T, Songsak T. Anti-hyperglycemic and anti-hyperlipidemic effect of dietary supplement of white Ocimum sanctum Linn. before and after STZ-induced diabetes mellitus. Int J Diabetes and Metabolism. 2005;13:18–23. [Google Scholar]
- 29.Kundu A, Dey P, Sarkar P, Karmakar S. Protective effects of Croton hookeri on streptozotocin-induced diabetic nephropathy. Food Chem Toxicol. 2019 Oct 7;:110873. doi: 10.1016/j.fct.2019.110873. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Zhang W. Protective effect of berberine on renal fibrosis caused by diabetic nephropathy. Molecular Medicine Reports. 2017;16:1055–1062. doi: 10.3892/mmr.2017.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Ji-W, Li Sheng K, Wu Wen J. The main chemical composition and in vitro antifungal activity of the essential oils of ocimum basilicum Linn. Var. pilosum (willd.) Benth. Molecules. 2009;14(1):273–278. doi: 10.3390/molecules14010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao X, Wang J, Chang X, Zhen J, Zhou G, Hu Z. Mycophenolate mofetil ameliorates diabetic nephropathy through epithelial mesenchymal transition in rats. Mol Med Rep. 2015 Sep;12(3):4043–4050. doi: 10.3892/mmr.2015.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Liu Z, Zhou F, Zhao H, Yang Q, Li H, Sun J, Wang S. Evaluating Pharmacological Effects of Two Major Components of Shuangdan Oral Liquid: Role of Danshensu and Paeonol in Diabetic Nephropathy Rat. Biomol Ther (Seoul) 2016 Sep 1;24(5):536–542. doi: 10.4062/biomolther.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou J, Yaoita E, Watanabe Y, Yoshida Y, Nameta M, Li H, Qu Z, Yamamoto T. Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch. 2006 Apr;448(4):485–492. doi: 10.1007/s00428-005-0134-9. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-Mesenchymal Transition Is a Potential Pathway Leading to Podocyte Dysfunction and Proteinuria. AJP. 2008 Feb;172(2):299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erfan OS. Effect of Garlic on Rat Diabetic Renal Cortex. A histological and Immunohistochemical Study. Egyptian Journal of Anatomy. 2017 Jul;40(2):253–264. [Google Scholar]
- 38.Gu L, Gao Q, Ni L, Wang M, Shen F. Fasudil inhibits epithelial-myofibroblast transdifferentiation of human renal tubular epithelial HK-2 cells induced by high glucose. Chem Pharm Bull. 2013;61(7):688–694. doi: 10.1248/cpb.c13-00066. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto T. Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch. 2006;448:485–492. doi: 10.1007/s00428-005-0134-9. [DOI] [PubMed] [Google Scholar]