Abstract
Introduction:
Esophageal cancer is diagnosed with more than 480,000 patients per year and this disease became the eighth most common cancer worldwide.
Aim:
In this study, we tried to investigate the role of chemoradiotherapy in decreasing the severity of dysphagia and increasing the quality of life (QOL) in patients with esophageal cancer.
Methods:
Patients were diagnosed with esophageal cancer, which were proven by pathological studies. Also, all of these patients had no primary surgeries for their esophageal cancer. For determining the cancer staging, the endoscopy, sonography, abdominal and pelvic computed tomography scans were assessed.
Results:
In this study, 81% of patients showed responsiveness to the chemoradiotherapy and their dysphagia significantly was getting improved after treatment in comparison to the initial date (P<0.01). Also, the pain score significantly decreased after chemoradiotherapy. However, the analysis failed to show any significant difference between before and after treatment in 19% of patients who had high degrees of dysphagia and they were the candidate for surgery and stent putting. On the other hand, we demonstrated that there is no correlation between sex, age, tumor type and location with the recovery rate of dysphagia. In addition, we showed that none of the patients showed the recurrence of dysphagia during the study (1.5 years).
Conclusion:
Chemoradiotherapy could be a novel treatment for patients with inoperable esophageal cancer to reduce the severity of dysphagia and increasing the QOL of these individuals.
Keywords: Esophageal cancer, Dysphagia, Chemoradiotherapy, Pathology, Surgery
1. INTRODUCTION
Normal swallowing needs the coordination of various nerves, and muscles which transfers the food from mouth to stomach thorough esophagus (1). Each of head and neck inflammations, surgeries, malignancies, and etc. could induce an abnormality in swallowing process which produces dysphagia especially in patients with head and neck cancer (2). On the other hand, past studies showed that dysphagia decrease quality of life (QOL) in patients who suffer from this condition (3). Also, the severity of head and neck cancer and their side effects including dysphagia are important for the clinician to recognize its impact on patients’ QOL (4).
Esophageal cancer (EC) is diagnosed with more than 480,000 patients per year and this disease became the eighth most common cancer worldwide (5). The morbidity and mortality rate of EC is high and caused more than 400,000 deaths per year (6). Past studies showed that the incidence of esophageal adenocarcinoma is rising in comparison to squamous-cell carcinoma that remains unchanged (7). Also it was estimated that 25% of patients which were treated with primary surgery had a five year survival rates (8). The most (about 60 percent) patients with EC are referred to physician when surgical therapy is unable because of its metastasis (9, 10). Unfortunately, due to this reason less than 5 percent were survived after 5 years (11). In these patients which could not remove the tumor with surgical therapy, chemotherapy with or without radiotherapy will be the first choice to reduce the side effects of EC including dysphagia, weight loss, better QOL, and etc. (12).
Recently, the using of neoadjuvant chemoradiotherapy in patients with EC has been increased in past decades. However, many studies showed that there is no significant difference between two groups (a surgical therapy only and a neoadjuvant chemoradiotherapy) in survival rates. On the other hand, some meta-analyses suggest a survival benefit from neoadjuvant chemoradiotherapy which could decrease the morbidity and mortality (13-16). Although, some benefits of neoadjuvant chemoradiotherapy were investigated, using of chemotherapy and high dose radiation at the same time (without surgery in following) showed as an effective treatment of esophageal cancer, producing significantly improved results over treatment with radiation alone (17). As mentioned above, there are many controversies in treating method of EC.
2. AIM
In this regard, we tried to investigate the role of chemoradiotherapy in decreasing the severity of dysphagia and increasing the QOL in patients with EC.
3. METHODS
The study protocol was approved by the Institutional Review Board (IRB) of the Emam Hossein Hospital by Shahid Beheshti University of Medical Sciences. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Total amount of 46 patients were involved in this study gradually who were hospitalized in Emam Hossein Hospital in Tehran, Iran. All patients were newly diagnosed with EC, which be proven by pathological studies. Also, all of these patients had no primary surgeries for their EC. For determining the cancer staging, the endoscopy, sonography, abdominal and pelvic CT scans were assessed.
In this manuscript we use this inclusion criterion:
Patients should not have any primary surgery for their EC.
Patients should not be under any medications or diagnostic/treating proceedings including endoscopic (dilatation, stent putting, and etc.).
Patients with more than 6 months predictive survivals (after chemoradiotherapy).
Patients should not have any additional cancers.
Patients should have a normal laboratory tests for liver, kidney, and bone marrow functions.
Patients with appropriate general condition for starting the chemoradiotherapy.
Patients should not be pregnant or under breast-feeding.
Patients should not have any allergic reaction to the medications which were used in this study (Cisplatin and 5-Fu).
Also, we investigated the primary tests including: laboratory tests (liver function, urea, creatinine, and CBC diff.), chest x-ray, abdominal and pelvic CT scans, and diagnostic endoscopy for biopsy and pathological studies.
We divided the lesions to three parts:
Superior 1/3= from Cricopharyngeal cartilage to Aortic curve.
Middle 1/3= from Aortic curve to the inferior pulmonary vein.
Inferior 1/3= from the inferior pulmonary vein to the lower esophageal sphincter (LES).
In the next step, patients were investigated for the severity of their dysphagia and their QOL based on the questionnaires. After the chemoradiotherapy, these questionnaires were filled again by patients to assess whatever the treatment was effective or not. These processes repeated every 2 weeks.
Based on prior studies, we use Cisplatin at dose of 100mg/m2 every 21 days alone, or in combination of with 4 doses of 5-FU (18, 19). On the other hand, we used RTOG and EORTC criteria for dysphagia degree which in mentioned below.
| Symptoms | Degree |
|---|---|
| Able to swallow solid foods without any problems | 0 |
| Able to swallow solid food with a bit tricky | 1 |
| Only able to swallow soft food or semi-solid | 2 |
| Able to swallow only watery foods or liquids | 3 |
| Inability to swallow liquids | 4 |
The survival rate of these patients was assessed by Kaplan Meier methods, from diagnostic date till the last visiting date or the end of the study. All data were analyzed by the SPSS application.
4. RESULTS
In this study, 37 individuals of total 46 were investigated and 9 patients excluded during the procedures because of treatment discontinuation, treatment intolerance, lack of follow-up, surgery, and etc. In this study, we divided the patients in two age groups including fewer than 60 and over 60 years old. Sixty-two percent were over 60 and 38 percent were under 60 years old. Also, the ratio of female to male was 21:16 (57% of patients were male and 43% were female).
These patients also divided in two groups through their tumor types (squamous cell carcinoma and adenocarcinoma). Although, patients were chosen randomly, 34 individuals had SCC (92%) and others had adenocarcinoma (8%). Superior endoscopic study showed that 10 patients (27%) had the tumor on their superior 1/3 of esophagus, 21 patients (57%) on middle 1/3, and 6 patients (15%) on inferior 1/3.
Most patients while referred to this hospital had high EC stages or metastatic (stage 3 or 4), which indicated to treat with chemoradiotherapy to decrease their signs and symptoms. Analysis showed that 55% patients had stage 4 and 35% had stage 3 of the EC.
Three individuals had stage 2 of dysphagia, 13 individuals had stage 3, 11 individuals had stage 4, and 10 individuals had stage 5. In Table 1, we had shown the differences between dysphagia staging before and after treatments. Twenty-one patients were on stage 2, 6 individuals were on stage 3, 2 individuals were on stage 4, and 2 patients were on stage 5 of dysphagia.
Table 1. Differences between dysphagia staging before and after treatments .
| Stage | Ore | Status at maximal improvement (weeks) |
|---|---|---|
| I | 0 (0) | 21 (57) |
| II | 3 (8) | 5 (14) |
| III | 13 (35) | 6 (16) |
| IV | 11 (30) | 3 (8) |
| V | 10 (27) | 2 (5) |
| Total | 37 | 37 |
Interestingly, 30 patients (81%) showed an appropriate response to the chemoradiotherapy, and got the normal ability to swallow. Also, these patients did not experience the dysphagia after the treatment till the end of this study or their life. However, 7 individuals (19%) did not response to the treatment and stent was applied for these patients.
The mean scores for the severity of dysphagia in these patients were 4 (according to dysphagia scoring) before treatment; however, after the chemoradiotherapy, the mean scores decrease to 1 after 24 weeks (Table 1 and Figure 1).
Figure 1. The mean of dysphagia severity before and during treatment and its changes.

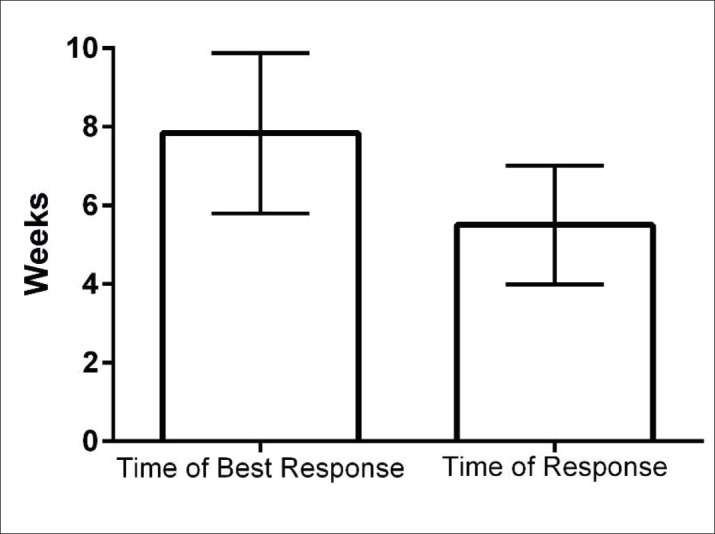
Imaging and laboratory studies showed that 24 individuals (65%) were in stage 3, 13 individuals (35%) were in stage 4 with metastasis (CT scans showed the metastasis). In patients who had no metastasis in CT scans, endosonography was assessed. In this study, we showed that the mean of time to initial responses to the treatments was 6 weeks, and the maximum responses were after 8 weeks (Table 2 and Fig 2).
Table 2. The response to treatment and improve dysphagia during treatment.
| Parameter | ||
|---|---|---|
| Age | Mean ± SD | 63 ± 10 |
| Median (Range) | 64 (42 to 77) | |
| Age Categories | <60 | 14 (38) |
| 60 + | 23 (62) | |
| Sex | Male | 21 (57) |
| Female | 16 (43) | |
| Tumor | Adenocarcinoma | 3 (8) |
| SCC | 34 (92) | |
| Location | Upper | 10 (27) |
| Middle | 21 (57) | |
| Lower | 6 (16) | |
| Stage | III | 24 (65) |
| IV | 13 (35) | |
| FU | Mean ± SD | 30 ± 13 |
| Median (Range) | 24 (2 to 66) | |
| Time To response | Mean ± SD | 6 ± 5 |
| Median (Range) | 4 (2 to 22) | |
| Time to Maximal Response | Mean ± SD | 8 ± 5 |
| Median (Range) | 6 (2 to 22) | |
| Total | 37 |
Figure 2. Initial time of maximum response to treatment during follow-up.
As mentioned in Figure 3, the maximum response was in primary 8 weeks, and after that time the recovery was not significant. Also, we divided the time recovery according to sex and age in Figure 4 and 5. Analysis showed that there is not any correlation between age and recovery time (P=0.217) as well as sex (P=0.437).
Figure 3. Swallowing recovery of patients over time.
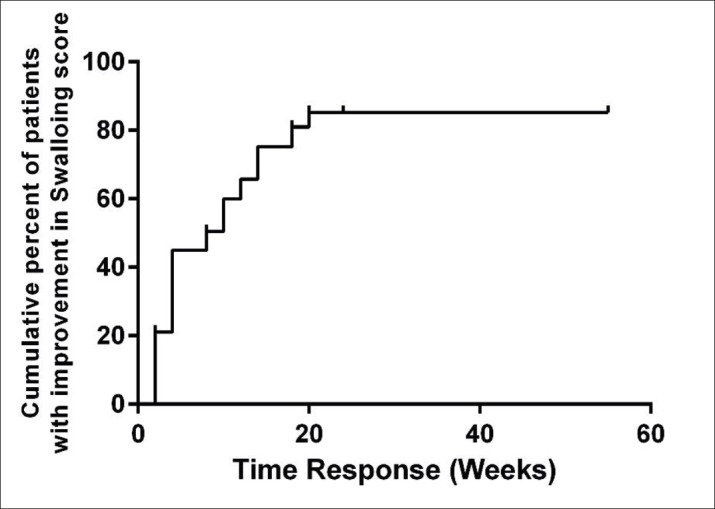
Figure 4. Improvement of swallowing in patients during treatment correlating to age over the period of follow-up.

Figure 5. Improvement of swallowing in patients during treatment correlating to sex over the period of follow-up.

It should be noted that all of three adenocarcinomas were responded to the treatment (100%), however, 27 of 34 individuals with SCC (79%) were responded to the chemoradiotherapy. Also, analysis failed to show any correlation between the tumor type and responsiveness due to the low number of individuals (Table 3).
Table 3. The response to treatment according to age, sex, tumor location and tumor stage, OR: Odds Ratio; 95% CI: 95% Confidence Interval, * Based on Fisher exact test .
| Patients (%) | Response (%) | OR (95% CI) | P* | ||
|---|---|---|---|---|---|
| AgeC | <60 | 14 (38) | 13 (93) | 4.6 (0.5 to 43) | 0.217 |
| 60 + | 23 (62) | 17 (74) | Ref | ||
| Sex | Male | 21 (57) | 18 (86) | 2 (0.4 to 10.6) | 0.437 |
| Female | 16 (43) | 12 (75) | Ref | ||
| Tumor | Adenocarcinoma | 3 (8) | 3 (100) | undefine | >0.99 |
| SCC | 34 (92) | 27 (79) | Ref | ||
| Location | Upper | 10 (27) | 9 (90) | 1.8 (0.1 to 35.4) | 0.843 |
| Middle | 21 (57) | 16 (76) | 0.6 (0.06 to 6.8) | ||
| Lower | 6 (16) | 5 (83) | Ref | ||
| Stage | III | 24 (65) | 19 (79) | 0.7 (0.1 to 4.7) | >0.99 |
| IV | 13 (35) | 11 (85) | Ref | ||
| Total | 37 (100) | 30 (81) | - | - |
Table 4 shows the mean of survival rate in patients which was involved in this study. Eighty-six percent of 14 patients under 60 years old and 61% of 23 patients over 20 years old were alive after 1.5 years in this study (Figure 6). The mean of survival was 40.7 weeks in group under 60 years old and was 38.2 weeks for over 60 years old. Over all, the mortality rate was increased gradually according to sex and age (Figure 7).
Table 4. The mean of survival rates correlated to age, sex, type and location of the tumor and the stage of disease .
| Patients (%) | Survival at 24 Wks | Mean survival (95% CI) | Median survival (95% CI) | HR (95% CI) | P* | ||
|---|---|---|---|---|---|---|---|
| Age | <60 | 14 (38) | 86 | 40.7 (32 to 49) | 50 (30 to 70) | 0.6 (0.2 to 1.80) | 0.36 |
| 60 + | 23 (62) | 61 | 38.2 (28 to 49) | 29 (6 to 52) | Ref | ||
| Sex | Male | 21 (57) | 81 | 44.7 (34 to 55) | 56 (28 to 84) | 0.5 (0.2 to 1.4) | 0.17 |
| Female | 16 (43) | 68 | 32.3 (23 to 42) | 29 (12 to 46) | Ref | ||
| Tumor | Adenocarcinoma | 3 (8) | 67 | 27 (17 to 37) | - | 0.9 (0.2 to 1.7) | 0.95 |
| SCC | 34 (92) | 70 | 40.4 (32 to 49) | 50 (21 to 79) | Ref | ||
| Location | Upper | 10 (27) | 60 | 38 (28 to 48) | - | 1.8 (0.3 to 9.6) | 0.80 |
| Middle | 21 (57) | 76 | 38.2 (29 to 47) | 37 (25 to 49) | 1.5 (0.3 to 7.1) | ||
| Lower | 6 (16) | 67 | 46.8 (25 to 69) | - | Ref | ||
| Stage | III | 24 (65) | 71 | 43.5 (31 to 56) | 50 (20 to 80) | 0.74 (0.4 to 3.7) | 0.64 |
| IV | 13 (35) | 69 | 34.3 (28 to 41 | 37 (21 to 53) | Ref | ||
| Total | 37 (100) | 70 | 40.6 (33 to 49) | 50 (22 to 78) | - | - |
Figure 6. Incidence of death during follow-up in patients under this study.

Figure 7. The cumulative incidence of death in patients over time due to age (A), disease stages (B), and sex (C).
In Table 5, we tried to show the degree of dysphagia in the patients involved in this study. The mean degree of dysphagia before treatment was 30.6 and 72.2 after treatments. Analysis showed that there is a significant difference between these two degrees which is showing that chemoradiotherapy has an impact on the dysphagia and QOL in these patients (P<0.01). Also, the swallowing (P<0.01), reflux (P<0.5), and pain (P<0.01) were recovered after treatments (Table 5).
Table 5. Criteria related to quality of life. Results are presented as Mean ± SD, Median (Range). * Based on Wilcoxon Singed-Rank test.
| Pre | Post | P* | |
|---|---|---|---|
| Dysphagia | 30.6 ± 22.8 | 72.2 ± 20.2 | <0.01 |
| 22.2 (0 to 77.8) | 72.2 (33.3 to 100) | ||
| Eating | 66.3 ± 11.1 | 37.2 ± 12.3 | <0.01 |
| 66.7 (33.3 to 91.7) | 33.3 (0 to 58.3) | ||
| Reflux | 31.2 ± 21 | 28.5 ± 19.3 | 0.40 |
| 33.3 (0 to 66.7) | 33.3 (0 to 66.7) | ||
| Pain | 44.9 ± 19 | 28.9 ± 11.1 | <0.01 |
| 55.6 (0 to 77.8) | 33.3 (0 to 44.4) |
5. DISCUSSION
In this study, we evaluated the changes of dysphagia, 18-month follow-up period, the average survival rate of patients with newly diagnosed with EC. Dysphagia changes during the treatment according to sex and age, tumor type and the location of the tumor was investigated separately. And the average survival of patients according to age, sex and type of tumor and the tumor was expressed.
Patients with dysphagia described a feeling of anger, anxiety, and disappointment, lack of confidence, and depression which could decrease their QOL. Progressive dysphagia and consequently rapid weight loss occur in most patients in the early phase of the EC. Past studies showed that it is necessary for the occurrence of dysphagia, esophageal diameter of about 60% involved by cancer. Dysphagia of solids at an early stage and gradually into semi-solid, materials and liquids are eventually happens (20).
Treatment methods of head and neck cancers usually involve radiotherapy, surgery, and chemoradiotherapy or a combination of them (21, 22). Radiotherapy to the head and neck could increase the risk of xerostomia, trismus, fibrosis of pharyngeal muscles, and pharyngeal stenosis (22). Studies had shown that when radiotherapy combined with chemotherapy (chemoradiotherapy), the severity of dysphagia increases and may lead to aspiration, requiring prolonged gastric tube feeding (23, 24). However, in contrast, this study showed that chemoradiotherapy could significantly decrease the severity of dysphagia after 8 weeks. The treatment of dysphagia in patients with inoperable EC, supportive treatments is using to reduce the severity of the side effects. Top of these treatments is the stent putting by endoscopy (25) which could decrease the severity of dysphagia and improve the QOL of these individuals. Although, there are some other methods such as bipolar electrocoagulation, they had limited indications. However, dysphagia recurred after stent putting and it needs to place multiple stents which could increase the risk of complications such as bleeding (26, 27). In this regard, the supportive chemoradiotherapy is the novel treatment for patients with inoperable EC with appropriate general condition (28).
Simon et al. showed that the tumor volume decreased more than 50% in 19 individuals with total 30 (63.3%) in imaging studies after chemoradiotherapy. Also, they found improvements in endoscopic studies in 20 patients (10 of them with totally removed tumor and other 10 individuals just had some lesions). In addition, they revealed that there is no significant difference between chemoradiotherapy and stent putting in improving dysphagia and also chemoradiotherapy significantly decreased severity of dysphagia in patients with EC (29). In line with past study, some articles demonstrated that chemoradiotherapy decreased the dysphagia degree after a month. Also, they investigated the survival rates of these patients which are about one year survival (about 60%), two years of survival (about 40%), and three years of survival (about 30%) (30, 31). Also, there are some studies which showed the benefits of chemoradiotherapy in decreasing the severity of dysphagia in patients with inoperable EC (32).
In this study, 81% of patients showed responsiveness to the chemoradiotherapy and their dysphagia significantly were getting improved after treatment in comparison to initial date (P<0.01). Also, the pain score significantly decreased after chemoradiotherapy. However, the analysis failed to show any significant difference between before and after treatment in 19% of patients who had high degrees of dysphagia and they were candidate for surgery and stent putting. On the other hand, we demonstrated that there is no correlation between sex, age, tumor type and location with the recovery rate of dysphagia. In addition, we showed that none of the patients showed the recurrence of dysphagia during the study (1.5 year). In the final step, we demonstrated that the survival rate is about 40.7 weeks which are not correlated to sex, age, tumor type and its location
The limitation of current study was small sample size because only 46 patients had inclusion criteria.
6. CONCLUSION
This study suggests that chemoradiotherapy could be a novel treatment for patients with inoperable EC to reduce the severity of dysphagia and increasing the QOL of these individuals. On the other hand, improvements in dysphagia in these patients are not correlated to tumor type and location, sex, and age.
Abbreviation: EC: Esophageal cancer, QOL: quality of life, RTOG: Radiation Therapy Oncology Group, EORTC: European Organization for Research and Treatment of Cancer, LES: lower esophageal sphincter
Authors contribution:
All quoted authors gave substantial contribution in creation of this paper. MF, MT, MF, and MD conceived the project, designed the study, analyzed the data, drafted the manuscript, and generated the data. MD wrote the manuscript. All authors reviewed and approved the final manuscript.
Conflicts of interest:
There are no conflicts of interest.
Financial support and sponsorship:
Nilt
REFERENCES
- 1.Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103(3):823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 2.Stenson KM, MacCracken E, List M, Haraf DJ, Brockstein B, Weichselbaum R, et al. Swallowing function in patients with head and neck cancer prior to treatment. Archives of Otolaryngology, Head & Neck Surgery. 2000;126(3):371–377. doi: 10.1001/archotol.126.3.371. [DOI] [PubMed] [Google Scholar]
- 3.Kulbersh BD, Rosenthal EL, McGrew BM, Duncan RD, McColloch NL, Carroll WR, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. The Laryngoscope. 2006;116(6):883–886. doi: 10.1097/01.mlg.0000217278.96901.fc. [DOI] [PubMed] [Google Scholar]
- 4.Chen AY, Frankowski R, Bishop-Leone J, Hebert T, Leyk S, Lewin J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the MD Anderson dysphagia inventory. Archives of Otolaryngology, Head & Neck Surgery. 2001;127(7):870–876. [PubMed] [Google Scholar]
- 5.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray F, Devesa S. Cancer burden in the year 2000. The global picture. European journal of cancer. 2001;37:4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 7.Eisbruch A, Lyden T, Bradford CR, Dawson LA, Haxer MJ, Miller AE, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. International Journal of Radiation Oncology* Biology Physics. 2002;53(1):23–28. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 8.Gailhoustet L, Goulet O, Cachin N, Schmitz J. Study of psychological repercussions of 2 modes of treatment of adolescents with Crohn’s disease. Archives de pediatrie: organe officiel de la Societe francaise de pediatrie. 2002;9(2):110–116. doi: 10.1016/s0929-693x(01)00717-5. [DOI] [PubMed] [Google Scholar]
- 9.Mori T, Miura K, Aoki T, Nishihira T, Mori S, Nakamura Y. Frequent somatic mutation of the MTS1/CDK4I (multiple tumor suppressor/cyclin-dependent kinase 4 inhibitor) gene in esophageal squamous cell carcinoma. Cancer research. 1994;54(13):3396–3397. [PubMed] [Google Scholar]
- 10.Brücher BL, Weber W, Bauer M, Fink U, Avril N, Stein HJ, et al. Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Annals of surgery. 2001;233(3):300–309. doi: 10.1097/00000658-200103000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conigliaro R, Battaglia G, Repici A, De Pretis G, Ghezzo L, Bittinger M, et al. Polyflex stents for malignant oesophageal and oesophagogastric stricture: a prospective, multicentric study. European journal of gastroenterology & hepatology. 2007;19(3):195–203. doi: 10.1097/MEG.0b013e328013a418. [DOI] [PubMed] [Google Scholar]
- 12.Javle M, Ailawadhi S, Yang GY, Nwogu CE, Schiff MD, Nava HR. Palliation of malignant dysphagia in esophageal cancer: a literature-based review. The journal of supportive oncology. 2006;4(8):365–373. [PubMed] [Google Scholar]
- 13.Logemann JA, Logemann JA. Evaluation and treatment of swallowing disorders. 1983 [Google Scholar]
- 14.Karnell MP, MacCracken E. Database information storage and reporting system for videofluorographic oropharyngeal motility (OPM) swallowing evaluations. American Journal of Speech-Language Pathology. 1994;3(2):54–60. [Google Scholar]
- 15.Samson P, Robinson C, Bradley J, Lockhart AC, Puri V, Broderick S, et al. Neoadjuvant Chemotherapy versus Chemoradiation Prior to Esophagectomy: Impact on Rate of Complete Pathologic Response and Survival in Esophageal Cancer Patients. Journal of Thoracic Oncology. 2016;11(12):2227–2237. doi: 10.1016/j.jtho.2016.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka K, Miyata H, Yamasaki M, Sugimura K, Takahashi T, Kurokawa Y, et al. Circulating miR-200c levels significantly predict response to chemotherapy and prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. Annals of surgical oncology. 2013;20(3):607–615. doi: 10.1245/s10434-013-3093-4. [DOI] [PubMed] [Google Scholar]
- 17.McFarland D, Martin-Harris B, Fortin AJ, Humphries K, Hill E, Armeson K. Respiratory-swallowing coordination in normal subjects: Lung volume at swallowing initiation. Respiratory Physiology & Neurobiology. 2016;234:89–96. doi: 10.1016/j.resp.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loizou LA, Grigg D, Atkinson M, Robertson C, Bown SG. A prospective comparison of laser therapy and intubation in endoscopic palliation for malignant dysphagia. Gastroenterology. 1991;100(5):1303–1310. [PubMed] [Google Scholar]
- 19.Al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle J, Vaitkevicius V, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. Journal of Clinical Oncology. 1997;15(1):277–284. doi: 10.1200/JCO.1997.15.1.277. [DOI] [PubMed] [Google Scholar]
- 20.Tibbling L, Gustafsson B. Dysphagia and its consequences in the elderly. Dysphagia. 1991;6(4):200–202. doi: 10.1007/BF02493526. [DOI] [PubMed] [Google Scholar]
- 21.Wu CH, Ko JY, Hsiao TY, Hsu MM. Dysphagia after radiotherapy: endoscopic examination of swallowing in patients with nasopharyngeal carcinoma. Annals of Otology, Rhinology and Laryngology. 2000;109(3):320–325. doi: 10.1177/000348940010900315. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen NP, Sallah S, Karlsson U, Antoine JE. Combined chemotherapy and radiation therapy for head and neck malignancies. Cancer. 2002;94(4):1131–1141. doi: 10.1002/cncr.10257. [DOI] [PubMed] [Google Scholar]
- 23.Smith RV, Kotz T, Beitler JJ, Wadler S. Long-term swallowing problems after organ preservation therapy with concomitant radiation therapy and intravenous hydroxyurea: initial results. Archives of Otolaryngology–Head & Neck Surgery. 2000;126(3):384–389. doi: 10.1001/archotol.126.3.384. [DOI] [PubMed] [Google Scholar]
- 24.Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. Journal of Clinical Oncology. 2014;32(23):2416–2422. doi: 10.1200/JCO.2013.53.6532. [DOI] [PubMed] [Google Scholar]
- 25.Wen J, Yang Y, Liu Q, Yang J, Wang S, Wang X, et al. Preventing stricture formation by covered esophageal stent placement after endoscopic submucosal dissection for early esophageal cancer. Digestive diseases and sciences. 2014;59(3):658–663. doi: 10.1007/s10620-013-2958-5. [DOI] [PubMed] [Google Scholar]
- 26.Rabenstein T. Palliative endoscopic therapy of esophageal cancer. Visceral Medicine. 2015;31(5):354–359. doi: 10.1159/000441175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakitas MA, Tosteson TD, Li Z, Lyons KD, Hull JG, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. Journal of clinical oncology. 2015;33(13):1438–1445. doi: 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Association JGC. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20(1):1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong SK, Chiu PW, Leung S, Cheung K, Chan AC, Au-Yeung AC, et al. Concurrent chemoradiotherapy or endoscopic stenting for advanced squamous cell carcinoma of esophagus: a case-control study. Annals of surgical oncology. 2008;15(2):576–582. doi: 10.1245/s10434-007-9679-y. [DOI] [PubMed] [Google Scholar]
- 30.Platteaux N, Dirix P, Dejaeger E, Nuyts S. Dysphagia in head and neck cancer patients treated with chemoradiotherapy. Dysphagia. 2010;25(2):139–152. doi: 10.1007/s00455-009-9247-7. [DOI] [PubMed] [Google Scholar]
- 31.Jones DR, Detterbeck FC, Egan TM, Parker LA, Bernard SA, Tepper JE. Induction chemoradiotherapy followed by esophagectomy in patients with carcinoma of the esophagus. The Annals of thoracic surgery. 1997;64(1):185–192. doi: 10.1016/s0003-4975(97)00449-9. [DOI] [PubMed] [Google Scholar]
- 32.Coia LR, Soffen EM, Schultheiss TE, Martin EE, Hanks GE. Swallowing function in patients with esophageal cancer treated with concurrent radiation and chemotherapy. Cancer. 1993;71(2):281–286. doi: 10.1002/1097-0142(19930115)71:2<281::aid-cncr2820710202>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]



