Abstract
We have previously shown that increased and deregulated estrogen receptor α expression in the mammary gland leads to the development of proliferative disease and cancer. To address the importance of cyclin D1 in ERα-mediated mammary tumorigenesis, we crossed ERα-overexpressing mice with cyclin D1 knockout mice. Mammary gland morphogenesis was completely interrupted in the ERα-overexpressing cyclin D1-deficient triple transgenic mice. In addition to a highly significant reduction in mammary epithelial cell proliferation, cyclin E was upregulated resulting in DNA damage checkpoint activation and apoptosis. This imbalance between proliferative and apoptotic rates in conjunction with remarkable structural defects and cellular disorganization in the terminal end buds interrupted ductal morphogenesis. Interestingly, the structure of the mammary fat pad was fundamentally altered by the consequences of overexpressing ERα in the epithelial cells in the absence of cyclin D1 illustrating how alterations in the epithelial compartment can impact surrounding stromal composition. Transplantation of embryonic ERα-overexpressing and cyclin D1-deficient mammary epithelium into the cleared fat pad of wild-type mice did not rescue the aberrant mammary gland phenotype indicating that it was intrinsic to the mammary epithelial cells. In conclusion, although cyclin D1 is not essential for proliferation of normal mammary epithelial cells, ERα-overexpressing cells are absolutely dependent on cyclin D1 for proliferation. This differential requirement for cyclin D1 in normal vs abnormal mammary epithelial cells supports the application of cyclin D1 inhibitors as therapeutic interventions in ERα-overexpressing breast cancers.
Keywords: ERα, cyclin D1, cyclin E, DNA damage, mammary gland
Introduction
The developing mammary gland displays many properties related to tumor progression such as invasion of the terminal end bud (TEB) through the fat pad (Wiseman and Werb, 2002). TEBs are highly mitotic and motile structures, which aggressively advance through the stroma and branch by bifurcation until they reach the end of the fat pad when they regress and disappear (Hinck and Silberstein, 2005). Perturbations in the balance between proliferative activity and apoptosis can contribute to both abnormal development and cancer. These two processes must act in concert in order to provide normal TEB and ductal growth. In addition, the correct cellular organization of the TEB is essential for its development and function. The strategic localization of myoepithelial cells allows the communication between luminal cells and the stroma, playing an indispensable role in cell growth, differentiation, maintenance of the epithelial architecture and carcinogenesis (Deugnier et al., 2002).
Cell cycle progression is tightly controlled to ensure proper proliferation of cells. Cyclins D (D1, D2 and D3) and cyclin E regulate G1 to S phase cell cycle progression. The cyclin D1 protein is overexpressed in 30–50% of human breast cancers and the gene is amplified in approximately 15% of breast cancers (Sutherland and Musgrove, 2004). Cyclin D1 and estrogen receptor α (ERα) are frequently co-expressed in human ductal hyperplasia and breast cancer (Shoker et al., 2001). Moreover, cyclin D1 expression positively correlates with ERα expression in ductal carcinoma in situ (DCIS) (Oh et al., 2001). The cyclin E protein is overexpressed in approximately 40% of breast cancers while gene amplifications are rare (Sutherland and Musgrove, 2004). Cyclin D1 knockout mice undergo normal pubertal mammary gland development but they fail to develop lobuloalveolar structures during pregnancy (Fantl et al., 1995; Sicinski et al., 1995). Cyclin D1 appears to play a very specific role in mammary gland development since the lobuloalveolar defect in cyclin D1 knockout mice occurs even in the presence of cyclin D2 and D3 overexpression (Yu et al., 2001). Genetic replacement of cyclin D1 by cyclin D2 can drive nearly normal mammary gland development even during pregnancy (Carthon et al., 2005). Cyclin D1 and E are considered weak oncogenes since their overexpression results in the formation of long-latency mammary adenocarcinomas (Sutherland and Musgrove, 2004). In addition, these proteins seem to have redundant functions in mammary gland development since the cyclin D1 knockout phenotype can be rescued by genetic replacement with cyclin E (Geng et al., 1999). Previous studies showed that cyclin E overexpression causes DNA damage and checkpoint activation (Spruck et al., 1999; Ekholm-Reed et al., 2004; Bartkova et al., 2005; Minella et al., 2007). Aberrant stimulation of cell proliferation leads to DNA replication stress, double-strand breaks, genomic instability, activation of DNA damage checkpoints and p53-dependent apoptosis or cell cycle arrest to ensure genomic integrity (Gorgoulis et al., 2005).
We have previously shown that increased and deregulated expression levels of nuclear-localized ERα in mammary epithelial cells (MECs) of transgenic mice (conditional estrogen receptor in mammary tissue (CERM) model) leads to the development of DCIS (Frech et al., 2005). This mechanism is consistent with one of the proposed etiology of human DCIS; that is, increased ERα expression in preneoplasia and DCIS is correlated with an increased risk of developing ERα-positive invasive breast cancer (Shoker et al., 1999). Also in correlation with human disease, DCIS lesions in the CERM model are ERα/PR-positive and they demonstrate high proliferative rates and overexpression of cyclin D1.
To investigate whether cyclin D1 is an essential contributor to the development and progression of ERα-induced mammary cancer, cyclin D1 knockout mice were crossed with ERα-overexpressing mice. Understanding how ERα driven breast cancers originate constitutes a major step toward identifying novel molecular targets for developing more effective and less toxic approaches for patients at high risk of developing invasive disease.
Results
Loss of cyclin D1 completely interrupted mammary gland development in CERM mice
Cyclin D1 is not required for pubertal mammary gland development in mice that have normal ERα expression levels (Fantl et al., 1995; Sicinski et al., 1995). However, when loss of cyclin D1 was introduced in mice with deregulated ERα expression (CERM D1−/− mice), mammary gland development was interrupted. This phenotype was specific to CERM D1−/− mice and not observed in any of the control mice (P<0.0001, Fisher’s exact test). We performed a time course study to determine the onset of abnormal development. Neonatal mammary gland development was relatively normal in CERM D1−/− mice (Figures 1a and b). Pubertal CERM D1−/− mammary glands showed significantly reduced numbers of TEBs per gland (6.5 ±1.6) compared to CERM (18.3 ±0.9) (P=0.0006, t-test) (Figures 1c and d). The phenotype progressed such that by 12 weeks, mammary glands were characterized by almost complete absence of fat and epithelial cells, remarkably high numbers of fibroblasts (Figures 1e and f, e and f inserts) and abnormally increased amounts of collagen deposition as demonstrated by Sirius Red staining (Supplementary Data). This phenotype was present throughout the mammary gland fat pad in both whole mounts and H&E sections.
Figure 1.
Loss of cyclin D1 completely interrupted mammary gland development in conditional estrogen receptor in mammary tissue (CERM) mice. Mammary gland whole mounts (a–f large panels and a–d inserts) and H&E sections (e and f inserts) from nulliparous 1-, 6- and 12-week-old female CERM and CERM D1−/− mice. Arrowheads indicate primary duct in CERM (a) and CERM D1−/− (b) mammary glands. Black arrows indicate terminal end buds (TEBs) in CERM (c) and CERM D1−/− (d) glands. Impaired ductal morphogenesis in CERM D1−/− mice (f) characterized by absence of fat and epithelial cells and high numbers of fibroblasts (f insert) compared to adequate ductal development in CERM mice (e and e insert).
Cyclin E was upregulated in epithelial cells composing CERM D1−/− TEBs
We first confirmed that cyclin D1 was expressed in CERM but was undetectable in CERM D1−/− mammary glands (Figures 2a and b). We then hypothesized that low levels of cyclin D2 and D3 expression in CERM D1−/− glands were not able to compensate for the loss of cyclin D1 interrupting ductal development. Immunohistochemical staining demonstrated that nearly all cells within CERM and CERM D1−/− TEB demonstrated nuclear-localized cyclin D2 and D3 (Figures 2c–f). We then investigated cyclin E expression levels in CERM and CERM D1−/− glands. There was a highly significant increase in the mean percentage of cells that demonstrated nuclear-localized cyclin E within CERM D1−/− TEBs (68.7% ± 6.6) compared to CERM TEBs (16.2% ± 8.6) (P<0.0007, Mann–Whitney test; Figures 2g and h). However, pubertal mammary gland development in CERM D1−/− mice was still interrupted.
Figure 2.
Cyclin E was upregulated in epithelial cells within CERM D1−/− terminal end buds (TEBs). Immunohistochemical staining of cyclin D1, D2, D3 and E in 6-week-old CERM (a, c, e and g) and CERM D1−/− (b, d, f and h) TEBs. Black arrows indicate positive nuclear staining.
CERM D1−/− TEBs demonstrated a significantly reduced number of MECs and disorganization of the myoepithelial cell layer
CERM D1−/− TEBs showed a significantly reduced mean number of MECs (42.0 ±3.1) compared to CERM TEBs (218.3 ±14.0) (P<0.0001, Mann–Whitney test) (Figures 3a and b). Immunohistochemical staining of CERM D1−/− TEBs using two myoepithelial cell markers, smooth muscle actin (SMA) and p63, demonstrated structural defects with disorganization of the myoepithelial cell layer and infiltration of these cells into the body of the TEB (Figures 3c–f). Since CERM D1−/− TEBs showed large empty luminal spaces and exaggerated subcapsular spaces, the expression patterns of adhesion proteins were investigated. P-cadherin is selectively expressed by cap cells and E-cadherin is expressed only by body cells (Daniel et al., 1995). Immunohistochemical staining of P- and E-cadherin on CERM D1−/− TEBs demonstrated normal expression patterns of these adhesion proteins (Figures 3g–j). The phenotype of the CERM D1−/− TEB described above was uniformly observed in all serial sections analysed.
Figure 3.
CERM D1−/− terminal end buds (TEBs) demonstrated significantly reduced number of mammary epithelial cells (MECs) and disorganization of the myoepithelial cell layer. H&E (a, b) and immunohistochemical staining of smooth muscle actin (SMA), p63, P- and E-cadherin in 6-week-old CERM (a, c, e, g and i) and CERM D1−/− (b, d, f, h and j) TEBs. Short arrow shows CERM D1−/− TEBs characterized by large empty luminal space and exaggerated subcapsular space (b). Arrowheads indicate infiltration of myoepithelial cells into the body cell compartment as demonstrated by SMA (d) and p63 (f) staining. Black arrows indicate positively stained nuclei (c-h).
MECs within CERM D1−/− TEBs demonstrated significantly reduced proliferative and significantly increased apoptotic rates
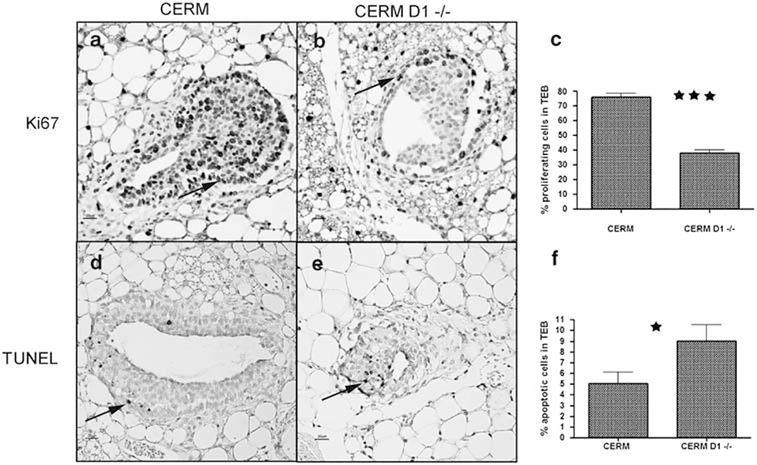
Assessment of proliferation was performed by Ki67 immunostaining in MECs composing the TEBs of CERM and CERM D1−/− mammary glands. There was a highly significant decrease in the mean percentage of MECs within TEBs that demonstrated nuclear-localized Ki67 in CERM D1−/− (38.0% ±2.2) compared to CERM (76.0% ±2.6) glands (P=0.0007; Mann–Whitney test) (Figures 4a–c). Apoptotic rates by TUNEL assay were significantly increased in the MECs within CERM D1−/− TEBs (9.04% ± 1.5) compared to CERM TEBs (5.05% ± 1.0) (P=0.03, Mann–Whitney test) (Figures 4d–f).
Figure 4.
CERM D1−/− terminal end buds (TEBs) demonstrated significantly reduced proliferative and significantly increased apoptotic rates. Assessment of proliferative and apoptotic indices by Ki67 and TUNEL immunostaining in 6-week-old CERM (a, b) and CERM D1−/− (d, e) TEBs. Black arrows indicate Ki67 or TUNEL positively stained nuclei. Bar graph comparing proliferative (c) and apoptotic (f) rates in CERM vs CERM D1−/− TEBs.
MECs within CERM D1−/− TEBs expressed markers of an activated DNA damage response
The occurrence of a DNA damage response can be ascertained by immunohistochemical detection of well-characterized antibodies such as serine 139-phosphorylated histone H2AX (γH2AX), threonine 68-phosphorylated Chk2 (pT-Chk2) and serine 15-phosphorylated p53 (pS-p53) (Bartkova et al., 2005; Gorgoulis et al., 2005). The mean percentage of MECs within TEBs demonstrating nuclear-localized γH2AX was significantly higher in CERM D1−/− (8.3% ± 1.0) compared to CERM glands (0.97% ±0.27) (P=0.001, Mann–Whitney test; Figures 5a–c). The mean percentage of MECs within TEBs with nuclear-localized pT-Chk2 was significantly higher in CERM D1−/− (43.07% ± 6.27) compared to CERM glands (1.38% ±0.28) (P<0.0001, Mann–Whitney test; Figures 5d–f). None of the CERM TEBs showed any epithelial cells with nuclear-localized pS-p53. In contrast, CERM D1−/− glands showed a significantly higher number of TEBs containing 1–10% of pS-p53-positive cells within the TEB (P=0.05, χ2-test; Figures 5g and h).
Figure 5.
Mammary epithelial cells (MECs) within CERM D1−/− terminal end bud (TEB) expressed markers of an activated DNA damage response. Immunohistochemical detection of γH2AX, pT-Chk2 and pS-p53 in 6-week-old CERM (a, d and g) and CERM D1−/− (b, e and h) TEBs. Bar graph comparing the mean percentage of γH2AX-positive cells (c) and pT-Chk2-positive cells (f) in CERM and CERM D1−/− TEBs. Arrows indicate positively stained nuclei.
Downregulation of transgenic ERα before puberty rescued the CERM D1−/− mammary gland phenotype
Rescue experiments were performed to prove that the phenotype was dependent upon the overexpression of transgenic ERα during puberty (Figures 6a and b). CERM D1−/− mice were exposed to doxycycline during their prenatal life until they were 3 weeks of age when doxycycline administration was discontinued; mice were euthanized at 6 weeks of age. The CERM D1−/− phenotype was rescued by downregulation of transgenic ERα at 3 weeks of age indicating that cyclin D1 dependency for normal TEB development was only observed when ERα was overexpressed (Figures 6c and d). Rescued TEBs exhibited normal proliferative capacity and normal organization of body and cap cells (Figures 6e–g). A series of experiments evaluating ovarian function confirmed that the phenotype was not secondary to altered ovarian physiology (Supplementary Data).
Figure 6.
Downregulation of transgenic ERα at 3 weeks of age rescued the normal terminal end bud (TEB) phenotype. Mammary gland whole mounts (a, c), H&E sections (b, d) and immunohistochemical detection of Ki67, smooth muscle actin (SMA) and p63 (e–g) in 6-week-old CERM D1−/− mice which had ERα overexpression either throughout their lifetime (a, b) or until 3 weeks of age (c–g). Aberrant mammary gland phenotype in CERM D1−/− mice that had continuous ERα overexpression throughout their lifetime as demonstrated by interrupted mammary gland morphogenesis (a) and aberrant TEB phenotype (b). Rescued phenotype in CERM D1−/− mice that received doxycycline until 3 weeks of age as demonstrated by adequate mammary gland development (c) and TEB histological appearance (d) and normal expression patterns of Ki67 (e), SMA (f) and p63 (g). Black arrows indicate positively stained nuclei.
Transplantation of CERM D1−/− mammary epithelium into wild-type hosts did not rescue the CERM D1−/− mammary gland phenotype
Mammary epithelial transplants were performed to investigate whether the CERM D1−/− phenotype was intrinsic to the epithelial cell compartment (Robinson et al, 2000). Embryonic CERM D1−/− mammary epithelium was transplanted into the cleared fat pad of wild-type nude mice. Thus, the transplanted epithelia were exposed to normal circulating hormone levels and wild-type stroma throughout puberty. The TEBs present in the transplanted mammary gland showed the same aberrant phenotype as demonstrated by histological analysis of H&E serial sections (Figures 7a and b) and same pattern of immunohistochemical staining of markers previously examined in intact CERM and CERM D1−/− glands (Figures 7c–j). These results demonstrate that the phenotype was intrinsic to the MECs.
Figure 7.
Transplantation of CERM D1−/− mammary epithelium into nude mice did not rescue the normal terminal end bud (TEB) phenotype. H&E sections (a, b) and immunohistochemical staining of cyclin D1 (c, d), SMA (e, f) Ki67 (g, h), TUNEL (i, j) in CERM (a, c, e, g and i) and CERM D1−/− (b, d, f, h and j) transplanted mammary glands. Arrowheads indicate aberrant histological appearance of TEBs from CERM D1−/− transplants (b). Black arrows indicate positively stained nuclei.
Discussion
Cyclins D and E play a key role during G1 to S phase progression of the cell cycle. It has been suggested that modestly elevated levels of cyclin D2 and D3 allow pubertal mammary gland development in cyclin D1−/− mice but the lobuloalveolar defect still persists (Yu et al., 2001). Genetic replacement of cyclin D1 by cyclin D2 compensates for cyclin D1 deficiency in the mammary gland (Carthon et al., 2005). In our studies, we were unable to detect any difference in cyclin D2 and D3 expression between CERM and CERM D1−/− mammary glands. It has been shown that the genetic replacement of cyclin D1 with human cyclin E in transgenic mice rescues the cyclin Dl-deficient phenotype (Geng et al., 1999). It is important to note that in these knock-in mice, the expression of human cyclin E is driven by the cyclin D1 promoter. In contrast, in our study, cyclin E overexpression results from a pathophysiological response to deregulated and increased ERα expression in conjunction with cyclin D1 loss. In this situation, increased cyclin E levels are not able to rescue the cyclin Dl-deficient phenotype. Human breast cancers that demonstrate cyclin E overexpression are associated with lower survival rates (Keyomarsi et al., 2002). Regulation of cyclin E expression is critically important for cells and several groups have shown that cyclin E overexpression causes genetic instability, DNA damage and tumorigenesis (Spruck et al., 1999; Ekholm-Reed et al., 2004; Bartkova et al., 2005; Minella et al., 2007). Interestingly, hepatocellular carcinomas exhibiting both downregulation of cyclin D1 and overexpression of cyclin E have higher frequencies of p53 mutation and tumor invasion, and demonstrate the worst 4-year survival (Peng et al., 1998). Similarly, in our studies, introduction of deregulated and increased ERα expression in the mammary gland in concert with cyclin D1 loss resulted in upregulation of cyclin E and DNA damage checkpoint activation leading to p53-dependent apoptosis.
The correct cellular organization of the TEB is essential for its development and function. Imbalanced proliferative and apoptotic rates in CERM D1−/− TEBs were accompanied by remarkable structural defects and cellular disorganization including exaggerated subcapsular spaces and infiltration of myoepithelial cells into the body cell compartment. Consequently, normal mammary gland morphogenesis was completely interrupted in CERM D1−/− mice. Interestingly, loss of normal epithelial function was followed by remarkable alterations in the stromal compartment including a marked decrease in the number of fat cells and an increase in the number of fibroblasts associated with abnormally high amounts of collagen deposition. Epithelial-stromal crosstalk is crucial for normal mammary gland development (Silberstein, 2001) and altered epithelial-stromal interactions can contribute to cancer progression (Wiseman and Werb, 2002). These experiments represent an example of the fundamental parallels between pubertal mammary gland development and tumor initiation and progression: imbalanced proliferative and apoptotic rates, aberrant cell cycle control, DNA damage checkpoint activation, cellular disorganization and stromal alterations are all characteristics of abnormal development and carcinogenesis.
The CERM D1−/− phenotype was rescued by downregulation of transgenic ERα expression at 3 weeks of age. Those results indicate that cyclin D1 dependency for normal TEB development was only found when ERα was overexpressed. Absence of cyclin D1 in normal MECs results in normal ductal development suggesting that cyclin D1 is dispensable for proliferation of normal MECs. In contrast, cancerous MECs overexpressing ras and neu oncogenes are absolutely dependent on cyclin D1 for malignant transformation (Yu et al., 2001). Similarly, in our study, absence of cyclin D1 in abnormal ERα-overexpressing MECs interrupted cell proliferation and led to apoptosis. The results presented here demonstrate that normal and abnormal MECs can have differential sensitivities to loss of cyclin D1. This differential requirement for cyclin D1 strongly supports the application of cyclin D1 inhibitors as therapeutic interventions in ERα-overexpressing breast cancers.
In summary, cyclin D1 loss in conjunction with ERα deregulation in the mammary gland resulted in a novel and absolute dependency upon cyclin D1 for MEC proliferation. An imbalance between proliferative and apoptotic rates in conjunction with remarkable structural defects in the TEBs interrupted ductal morphogenesis. Interestingly, the structure of the mammary fat pad was fundamentally altered as a consequence of increased ERα levels in the epithelial cell compartment in the absence of cyclin D1 illustrating how alterations in epithelial tissue function can impact surrounding stromal composition.
Materials and methods
Mice
Bigenic MMTV-rtTA/tetop-ERα (CERM) mice were bred to cyclin D1 knockout (D1−/−mice to obtain CERM cyclin D1 ± mice, which were bred to obtain experimental nulliparous female CERM D1−/− mice. Control nulliparous female mice included in the study were non-transgenic, CERM, CERM cyclin D1 ±, cyclin D1 ± and cyclin D1−/−. Details about time course study are included in the Supplementary Data. Mice were maintained on special diet containing 200 mg doxycycline per kilogram food (Bio-Serv, Frenchtown, NJ, USA) during prenatal life until euthanized. A cohort of CERM D1−/− mice were maintained on doxycycline until 3 weeks of age when they were moved to regular diet until they were euthanized at 6 weeks of age. Mice were genotyped using PCR-based assays previously described (Sicinski et al., 1995; Frech et al., 2005). Mice were maintained under the guidelines approved by the Georgetown University Animal Care and Use Committee.
Morphological and histological analyses of mammary glands
One number four mammary gland was collected and whole mounts were prepared as previously published (Frech et al., 2005). The other gland was collected in formalin and embedded in paraffin using the standard techniques. Five micrometers H&E serial sections of the whole mammary gland were analysed. Details about cell counting methodology are included in the Supplementary Data. Digital photographs were taken using the Nikon Eclipse E800M microscope (Nikon Instruments Inc., Melville, NY, USA).
Immunohistochemistry
Detection of protein expression and cellular localization in mammary glands by immunohistochemistry was accomplished using Mouse on Mouse peroxidase kit and Vectastain Elite ABC Rabbit IgG kit (Vector Laboratories Inc.,. Burlingame, CA, USA) following the manufacturer’s recommendations. A list of primary antibodies and cell counting methodology is included in the Supplementary Data. All inmunohistochemical procedures were performed in 6-week-old CERM and CERM D1−/− mammary glands and in CERM and CERM D1−/− mammary gland transplants.
Mammary gland transplantation
Mammary epithelia were transplanted into cleared mammary fat pads as previously described (Robinson et al., 2000). Details presented in Supplementary Data.
Statistical analyses
Means and SE were analysed using t-tests. Non-parametric data were analysed using Mann–Whitney, χ2 and Fisher’s exact tests as appropriate (GraphPad Software, San Diego, CA, USA). Significance was assigned at P-value less than or equal to 0.05.
Supplementary Material
Acknowledgements
This work was supported by grant NCI, NIH 1RO1CA112176 (PAF) and DOD Breast Cancer Research Predoctoral Award W81XWH-05–1-0302 (MSF).
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
References
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K et al. (2005). DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434: 864–870. [DOI] [PubMed] [Google Scholar]
- Carthon BC, Neumann CA, Das M, Pawlyk B, Li T, Geng Y et al. (2005). Genetic replacement of cyclin D1 function in mouse development by cyclin D2. Mol Cell Biol 25: 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel CW, Strickland P, Friedmann Y. (1995). Expression and functional role of E- and P-cadherins in mouse mammary ductal morphogenesis and growth. Dev Biol 169: 511–519. [DOI] [PubMed] [Google Scholar]
- Deugnier MA, Teuliere J, Faraldo MM, Thiery JP, Glukhova MA. (2002). The importance of being a myoepithelial cell. Breast Cancer Res 4: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm-Reed S, Mendez J, Tedesco D, Zetterberg A, Stillman B, Reed SI. (2004). Deregulation of cyclin E in human cells interferes with prereplication complex assembly. J Cell Biol 165: 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. (1995). Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev 9: 2364–2372. [DOI] [PubMed] [Google Scholar]
- Frech MS, Halama ED, Tilli MT, Singh B, Gunther EJ, Chodosh LA et al. (2005). Deregulated estrogen receptor alpha expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res 65: 681–685. [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Whoriskey W, Park MY, Bronson RT, Medema RH, Li T et al. (1999). Rescue of cyclin D1 deficiency by knockin cyclin E. Cell 97: 767–777. [DOI] [PubMed] [Google Scholar]
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T et al. (2005). Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434: 907–913. [DOI] [PubMed] [Google Scholar]
- Hinck L, Silberstein GB. (2005). Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Res 7: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN et al. (2002). Cyclin E and survival in patients with breast cancer. N Engl J Med 347: 1566–1575. [DOI] [PubMed] [Google Scholar]
- Minella AC, Grim JE, Welcker M, Clurman BE. (2007). p53 and SCF(Fbw7) cooperatively restrain cyclin E-associated genome instability. Oncogene 48: 6948–6953. [DOI] [PubMed] [Google Scholar]
- Oh YL, Choi JS, Song SY, Ko YH, Han BK, Nam SJ et al. (2001). Expression of p21Waf1, p27Kip1 and cyclin D1 proteins in breast ductal carcinoma in situ: relation with clinicopathologic characteristics and with p53 expression and estrogen receptor status. Pathol Int 51: 94–99. [DOI] [PubMed] [Google Scholar]
- Peng SY, Chou SP, Hsu HC. (1998). Association of downregulation of cyclin D1 and of overexpression of cyclin E with p53 mutation, high tumor grade and poor prognosis in hepatocellular carcinoma. J Hepatol 29: 281–289. [DOI] [PubMed] [Google Scholar]
- Robinson GW, Accili D, Hennighausen L. (2000). Rescue of mammary epithelium of early phenotypes by embryonic mammary gland transplantation as exemplified with insulin receptor null mice Methods in Mammary Gland Biology and Breast Cancer Research, In: Ip MM, Asch BB (eds). Kluwer Academics/Plenun Publishers: New York, pp 301–316. [Google Scholar]
- Shoker BS, Jarvis C, Davies MP, Iqbal M, Sibson DR, Sloane JP. (2001). Immunodetectable cyclin D(1)is associated with oestrogen receptor but not Ki67 in normal, cancerous and precancerous breast lesions. Br J Cancer 84: 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoker BS, Jarvis C, Sibson DR, Walker C, Sloane JP. (1999). Oestrogen receptor expression in the normal and pre-cancerous breast. J Pathol 188: 237–244. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H et al. (1995). Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82: 621–630. [DOI] [PubMed] [Google Scholar]
- Silberstein GB. (2001). Tumour-stromal interactions. Role of the stroma in mammary development. Breast Cancer Res 3: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruck CH, Won KA, Reed SI. (1999). Deregulated cyclin E induces chromosome instability. Nature 401: 297–300. [DOI] [PubMed] [Google Scholar]
- Sutherland RL, Musgrove EA. (2004). Cyclins and breast cancer. J Mammary Gland Biol Neoplasia 9: 95–104. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. (2002). Stromal effects on mammary gland development and breast cancer. Science 296: 1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Geng Y, Sicinski P. (2001). Specific protection against breast cancers by cyclin D1 ablation. Nature 411: 1017–1021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.