Abstract
Background
It is unclear whether non-high-density lipoprotein cholesterol (Non-HDL-C) is associated with haemorrhagic transformation (HT) after acute ischaemic stroke (AIS). We aimed to explore the association between Non-HDL-C and HT, as well as compare the predictive values of Non-HDL-C and low-density lipoprotein cholesterol (LDL-C) for HT.
Methods
We consecutively enrolled AIS patients within 7 days of stroke onset. Participants were divided into four categories according to quartiles of Non-HDL-C. HT was assessed by follow-up brain imaging. We assessed the association between Non-HDL-C, LDL-C and HT in multivariate logistic regression analysis.
Results
A total of 2043 patients were included, among whom 232 were identified as HT. Compared with the highest quartiles, the first, second and third quartiles of Non-HDL-C were associated with increased risk of HT (adjusted odds ratios [ORs] 1.74 [95% confidence interval [CI] 1.09–2.78], 2.01[95% CI 1.26–3.20], and 1.76 [95% CI 1.10–2.83], respectively, P for trend = 0.024). Similar results were found for LDL-C. There was significant interaction between Non-HDL-C and age (P for interaction = 0.021). The addition of Non-HDL-C and LDL-C to conventional factors significantly improved predictive values [Non-HDL-C, net reclassification index (NRI) 0.24, 95%CI 0.17–0.31, P < 0.001; LDL-C, NRI 0.15, 95%CI 0.08–0.22, P = 0.03].
Conclusions
Low Non-HDL-C was associated with increased risks of HT. In addition, Non-HDL-C has similar effects as LDL-C for predicting HT.
Keywords: Non-high-density lipoprotein cholesterol, Haemorrhagic transformation, Acute ischaemic stroke
Background
Haemorrhagic transformation (HT) is a common complication after acute ischaemic stroke (AIS), occurring in about 10–40% of patients [1]. The presence of HT may contribute to poor outcome in stroke patients [2]. A number of factors associated with HT have been reported, including age, stroke severity, atrial fibrillation and thrombolysis.
Although dyslipidaemia is known as an important risk factor of stroke [3], the association between the serum lipid levels and HT has not been well established. Prior studies have stated that low level of low-density lipoprotein cholesterol (LDL-C) could increase HT in patients with AIS [4–6], whereas the relationship between non-high-density lipoprotein cholesterol (Non-HDL-C) and HT is still not clear. As a composite marker, Non-HDL-C includes the triglyceride-rich lipoproteins such as chylomicrons, LDL, very-low-density lipoproteins, and their remnants [7]. Recent studies demonstrated that Non-HDL-C was more strongly associated with cardiovascular diseases than LDL-C [8–10]. However, whether Non-HDL-C is superior to LDL-C for predicting HT has not been studied. Therefore, we aimed to explore the association between Non-HDL-C and HT, as well as compare the predictive values of Non-HDL-C and LDL-C for HT in patients with AIS.
Methods
Study population
We consecutively enrolled ischaemic stroke patients within 7 days of stroke onset between January 2016 and September 2018 based on the Chengdu Stroke Registry Database, which has been described in details [11]. All patients were clinically diagnosed as ischaemic stroke based on the World Health Organization criteria, [12] and finally confirmed by computed tomography (CT) or magnetic resonance imaging (MRI) scan. Patients were not eligible if they: (1) were diagnosed with HT based on the initial head CT on admission, or (2) did not undergo later CT or MRI scan; or (3) lacked lipid profile test within 24 h after admission.
Data collection
Baseline information including patients’ demographics, stroke severity on admission, medical history, current smoking, alcohol consumption, systolic blood pressure (SBP), diastolic blood pressure (DBP), blood glucose, lipid parameters, the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification, thrombolysis and thrombectomy were collected. Medical history contained hypertension, diabetes mellitus, hyperlipidaemia and atrial fibrillation. Stroke severity on admission was evaluated using the National Institutes of Health Stroke Scale (NIHSS) [13].
Lipid parameters
Blood samples were collected within 24 h after hospital admission, and serum lipid parameters, including total cholesterol (TC), triglycerides (TG), HDL-C and LDL-C, were tested in the Department of Laboratory Medicine, West China Hospital. Non-HDL-C levels were determined by subtracting serum HDL-C levels from total cholesterol [14].
Assessment of HT
HT was defined as haemorrhage within the infarcted area or parenchyma hemorrhage outside the infarct zone that was present on a second CT or MRI (usually within 7 ± 2 days after admission), but not on head CT or MRI on admission, based on the European Cooperative Acute Stroke Study II criteria [15]. Additionally, HT was classified into symptomatic or no symptomatic HT group based on whether patients experienced any neurological deterioration [16]. HT was identified separately by two researchers (YW and QS), and a third researcher (CW) was consulted when a disagreement occurred.
Statistical analysis
Participants were divided into four categories according to quartiles of Non-HDL-C. Continuous variables are described as means with the standard deviations or median with interquartile ranges, and categorical variables are presented as frequencies with percentages. Differences in continuous data were assessed using Student’s t test, ANOVA test, the Mann–Whitney U test or the Kruskal–Wallis test and differences in categorical data were assessed using the chi-squared test or Fisher’s exact test. Univariate analysis was carried out to identify possible risk factors for HT. We further performed multivariate logistic regression analysis to assess the association between Non-HDL-C or LDL-C and HT. The odds ratio (OR) and 95% confidence interval (CI) was calculated. We created two models. Model 1 adjusted for age and sex. Model 2 adjusted for other potential confounding variables besides age and sex on model 1. Multivariable spline regression model was used to test nonlinear relationship between LDL-C, Non-HDL-C and HT. In addition, C statistics and net reclassification index [17] were calculated to evaluate the predictive value of adding Non-HDL-C or LDL-C to conventional risk factors model. In addition, we performed stratified analyses to explore potential indicators that may modify the relationship between Non-HDL-C and HT. The significance of interaction was tested by the likelihood ratio test. All statistical analyses were performed using SPSS 22.0 (IBM, Chicago, IL, USA), R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA). Two-sided values of P < 0.05 were considered statistically significant.
Results
Baseline characteristics
In all, 2206 consecutive patients with ischaemic stroke within 7 days were admitted to our hospital during the study period and 2043 patients were included in this study (Fig. 1). Of these 2043 patients, the mean age was 65 ± 14 years, and 63.1% were males.
Fig. 1.
Study patients flow chart. HT: haemorrhagic transformation; CT: computed tomography; MRI: magnetic resonance imaging
Demographic and clinical characteristics of participants based on Non-HDL-C quartiles are summarised in Table 1. Non-HDL-C levels ranged from 0.59 to 10.03 mmol/L (mean value, 3.14 mmol/L). Patients were divided into four categories based on Non-HDL-C quartiles: Q1, < 2.35 mmol/L; Q2, 2.35–3.06 mmol/L; Q3, 3.07–3.83 mmol/L and Q4, > 3.83 mmol/L. As shown in Table 1, patients in the lowest quartile were more likely to be older, have a higher proportion of atrial fibrillation, have higher NIHSS scores and HDL-C compared with those in the highest quartile of Non-HDL-C. In addition, patients in the highest quartile were more likely to have a higher proportion of hypertension, diabetes mellitus, have higher SBP, DBP, glucose, TG, TC and LDL-C compared with those in the lowest quartile of Non-HDL-C.
Table 1.
Baseline characteristics of participants according to Non-HDL-C quartiles
| Characteristics | Quartiles of Non-HDL, mmol/l | P-value | |||
|---|---|---|---|---|---|
| Q1: < 2.35 | Q2: 2.35–3.06 | Q3: 3.07–3.83 | Q4: > 3.83 | ||
| n = 514 | n = 510 | n = 511 | n = 508 | ||
| Age, (Mean ± SD), years | 66 ± 15 | 65 ± 14 | 65 ± 14 | 63 ± 14 | < 0.001a |
| Male, n (%) | 327 (63.6%) | 341 (66.9%) | 321 (62.8%) | 301 (59.3%) | 0.093b |
| Medical history | |||||
| Hypertension, n (%) | 269 (52.3%) | 265 (52.0%) | 297 (58.1%) | 301 (59.3%) | 0.030 b |
| Diabetes mellitus, n (%) | 129 (25.1%) | 109 (21.4%) | 91 (17.8%) | 138 (27.2%) | 0.002 b |
| Hyperlipidaemia, n (%) | 14 (2.7%) | 19 (3.7%) | 17 (3.3%) | 25 (4.9%) | 0.293 b |
| Atrial fibrillation, n (%) | 97 (18.9%) | 62 (12.2%) | 44 (8.6%) | 32 (6.3%) | < 0.001 b |
| Smoking, n (%) | 213 (41.4%) | 220 (43.1%) | 223 (43.6%) | 208 (40.9%) | 0.787 b |
| Alcohol consumption, n (%) | 142 (27.6%) | 141 (27.6%) | 142 (27.8% | 135 (26.6%) | 0.971 b |
| TOAST classification | |||||
| Large-artery atherosclerosis, n (%) | 151 (29.4%) | 156 (30.6%) | 179 (35.0%) | 185 (36.4%) | < 0.001b |
| Small-artery occlusion, n (%) | 84 (16.3%) | 112 (22.0%) | 132 (25.8%) | 156 (30.7%) | |
| Cardioembolic, n (%) | 150 (29.2%) | 130 (25.5%) | 97 (19.0%) | 57 (11.2%) | |
| Undetermined aetiology, n (%) | 108 (21.0%) | 93 (18.2%) | 87 (17.0%) | 98 (19.3%) | |
| Other aetiology, n (%) | 21 (4.1%) | 19 (3.7%) | 16 (3.1%) | 12 (2.4%) | |
| NIHSS on admission, median (IQR) | 6 (2–13) | 6 (2–11) | 5 (2–11) | 5 (2–10) | 0.002 c |
| SBP, (Mean ± SD), mmHg | 141 ± 22 | 144 ± 23 | 147 ± 24 | 151 ± 23 | < 0.001a |
| DBP, (Mean ± SD), mmHg | 82 ± 14 | 85 ± 14 | 87 ± 15 | 88 ± 15 | < 0.001 a |
| Glucose, (Mean ± SD), mmol/L | 7.61 ± 2.92 | 7.67 ± 3.06 | 7.62 ± 2.96 | 8.98 ± 4.44 | < 0.001a |
| Thrombolysis, n (%) | 31 (6.0%) | 22 (4.3%) | 21 (4.1%) | 33 (6.5%) | 0.216 b |
| Thrombectomy, n (%) | 33 (6.4%) | 24 (4.3%) | 22 (4.3%) | 23 (4.5%) | 0.386 b |
| Lipid profile | |||||
| TG, (Mean ± SD), mmol/L | 1.11 ± 0.72 | 1.36 ± 0.84 | 1.74 ± 1.03 | 2.53 ± 1.88 | < 0.001a |
| TC, (Mean ± SD), mmol/L | 3.14 ± 0.55 | 3.97 ± 0.41 | 4.63 ± 0.41 | 5.81 ± 0.84 | < 0.001a |
| HDL-C, (Mean ± SD), mmol/L | 1.29 ± 0.41 | 1.25 ± 0.36 | 1.23 ± 0.36 | 1.19 ± 0.35 | 0.001 a |
| LDL-C, (Mean ± SD), mmol/L | 1.58 ± 0.40 | 2.33 ± 0.30 | 2.87 ± 0.36 | 3.79 ± 0.77 | < 0.001a |
| Non-HDL, (Mean ± SD), mmol/L | 1.86 ± 0.36 | 2.72 ± 0.20 | 3.40 ± 0.22 | 4.62 ± 0.78 | < 0.001a |
Abbreviations: TOAST The Trial of ORG 10172 in Acute Stroke Treatment, NHISS National Institutes of Health Stroke Scale, SBP Systolic blood pressure, DBP Diastolic blood pressure, TG Triglyceride, TC Total cholesterol, LDL-C Low-density lipoprotein cholesterol, HDL-C High-density lipoprotein cholesterol, Non-HDL-C Non-high-density lipoprotein cholesterol
a ANOVA test
b Chi-squared test
c Kruskal–Wallis test
Of the 2043 patients, 232 (11.4%) were identified as HT, of whom 34 (1.7%) were symptomatic HT. Incidence of HT was 14.0% in quartile 1, 11.5% in quartile 2, 12.3% in quartile 3, and 7.1% in quartile 4 for LDL-C (P = 0.003), and was 14.8% in quartile 1, 13.1% in quartile 2, 11.2% in quartile 3, and 6.3% in quartile 4 for Non-HDL-C (P < 0.001).
Association of Non-HDL-C and LDL-C with HT, symptomatic HT
In the univariate analysis, age (P < 0.001), males (P < 0.001), atrial fibrillation (P < 0.001), smoking (P = 0.033), alcohol consumption (P = 0.049), TOAST classification (P < 0.001), NIHSS scores on admission (P < 0.001), SBP (P < 0.001), DBP (P = 0.048), thrombolysis (P < 0.001), thrombectomy (P < 0.001), TG (P < 0.001), TC (P = 0.003), HDL-C (P = 0.005), LDL-C (P < 0.001) and Non-HDL-C (P < 0.001) were significantly associated with HT (Table 2). In addition, only age (P = 0.017), atrial fibrillation (P < 0.001), NIHSS scores on admission (P < 0.001), TC (P = 0.047) and Non-HDL-C (P = 0.028) were significantly related to symptomatic HT (Additional file 1: Table S1).
Table 2.
Univariate analysis to identify risk factors associated with haemorrhagic transformation in patients with acute ischaemic stroke
| Characteristic | Total patients (n = 2043) | With HT (n = 232) | Without HT (n = 1811) | P-value |
|---|---|---|---|---|
| Age (Mean ± SD), years | 65 ± 14 | 68 ± 14 | 64 ± 14 | < 0.001a |
| Males, n (%) | 1290 (63.1%) | 113 (48.7%) | 1177 (65.0%) | < 0.001b |
| Medical history, n (%) | ||||
| Hypertension, n (%) | 1132 (55.4%) | 115 (49.5%) | 1017 (56.2%) | 0.864b |
| Diabetes mellitus, n (%) | 467 (22.9%) | 52 (22.4%) | 415 (22.9%) | 0.528b |
| Hyperlipidaemia, n (%) | 75 (3.7%) | 7 (3.0%) | 68 (3.8%) | 0.574b |
| Atrial fibrillation, n (%) | 235 (11.5%) | 69 (29.7%) | 166 (9.2%) | < 0.001b |
| Smoking, n (%) | 864 (42.3%) | 83 (35.8%) | 781 (43.1%) | 0.033b |
| Alcohol consumption, n (%) | 560 (27.4%) | 51 (22.0%) | 509 (28.1%) | 0.049b |
| TOAST classification | ||||
| Large-artery atherosclerosis, n (%) | 671 (32.8%) | 73 (31.5%) | 598 (33.0%) | < 0.001b |
| Small-artery occlusion, n (%) | 484 (23.7%) | 4 (1.7%) | 480 (26.5%) | |
| Cardioembolic, n (%) | 434 (21.2%) | 111 (47.8%) | 323 (17.8%) | |
| Undetermined aetiology, n (%) | 386 (18.9%) | 40 (16.1%) | 346 (19.3%) | |
| Other aetiology, n (%) | 68 (3.3%) | 4 (1.7%) | 64 (3.5%) | |
| NIHSS on admission, median (IQR) | 5 (2–10) | 11(6–18) | 5 (2–10) | < 0.001c |
| SBP, (Mean ± SD), mmHg | 146 ± 23 | 140 ± 23 | 146 ± 23 | < 0.001a |
| DBP, (Mean ± SD), mmHg | 85 ± 15 | 84 ± 16 | 86 ± 15 | 0.048a |
| Glucose, (Mean ± SD), mmol/L | 7.97 ± 3.45 | 8.16 ± 2.52 | 7.94 ± 3.55 | 0.233a |
| Thrombolysis, n (%) | 107 (5.2%) | 27 (11.6%) | 80 (4.4%) | < 0.001b |
| Thrombectomy, n (%) | 102 (5.0%) | 24 (10.3%) | 78 (4.3%) | < 0.001b |
| Lipid profile | ||||
| TG, (Mean ± SD), mmol/L | 1.68 ± 1.32 | 1.37 ± 1.01 | 1.72 ± 1.35 | < 0.001a |
| TC, (Mean ± SD), mmol/L | 4.38 ± 1.13 | 4.18 ± 1.11 | 4.41 ± 1.14 | 0.003a |
| HDL-C, (Mean ± SD), mmol/L | 1.24 ± 0.37 | 1.32 ± 0.44 | 1.23 ± 0.36 | 0.005a |
| LDL-C, (Mean ± SD), mmol/L | 2.64 ± 0.95 | 2.44 ± 0.86 | 2.66 ± 0.95 | < 0.001a |
| Non-HDL-C, (Mean ± SD), mmol/L | 3.14 ± 1.11 | 2.86 ± 1.05 | 3.18 ± 1.11 | < 0.001a |
Abbreviations; NHISS National Institutes of Health Stroke Scale, SBP Systolic blood pressure, DBP Diastolic blood pressure, TG Triglyceride, TC Total cholesterol, LDL-C Low-density lipoprotein cholesterol, HDL-C High-density lipoprotein cholesterol. Non-HDL-C Non-high-density lipoprotein cholesterol
a Student’s t test
b Chi-squared test
c Mann-Whitney Test
Table 3 shows the association between quartiles of Non-HDL-C or LDL-C and HT. After adjusting for age and sex in model 1, patients in the lower Non-HDL-C quartiles were associated with increased risks of HT (P for trend < 0.001). Compared with the highest quartiles, the first, second and third quartiles of Non-HDL-C were associated with increased risk of HT (adjusted ORs 1.74 [95% CI 1.09–2.78], 2.01[95% CI 1.26–3.20], and 1.76 [95% CI 1.10–2.83], respectively) after adjusting for age, sex, NIHSS scores on admission, atrial fibrillation, smoking, alcohol consumption, SBP, thrombolysis, thrombectomy and TOAST classification in model 2. However, the only significant association was found between the third quartiles of Non-HDL-C and symptomatic HT (adjusted ORs 3.82 [95% CI 1.05–13.85]) after adjusting for age and NIHSS scores on admission (Additional file 2: Table S2).
Table 3.
Association of quartiles of Non-HDL-C, LDL-C and haemorrhagic transformation
| Variables | Crude | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Non-HDL-C | |||||||||
| Q1: < 2.35 mmol/L | 2.58 | 1.67–3.98 | < 0.001 | 2.60 | 1.68–4.03 | < 0.001 | 1.74 | 1.09–2.78 | 0.019 |
| Q2: 2.35–3.06 mmol/L | 2.25 | 1.45–3.50 | < 0.001 | 2.34 | 1.50–3.66 | < 0.001 | 2.01 | 1.26–3.20 | 0.003 |
| Q3: 3.07–3.83 mmol/L | 1.87 | 1.19–2.93 | 0.007 | 1.88 | 1.19–2.97 | < 0.001 | 1.76 | 1.10–2.83 | 0.019 |
| Q4: > 3.83 mmol/L | 1.00 | 1.00 | 1.00 | ||||||
| Age (years) | 1.01 | 1.00–1.02 | 0.006 | 1.01 | 1.00–1.02 | 0.136 | |||
| Sex (female/male) | 0.53 | 0.40–0.70 | < 0.001 | 0.52 | 0.35–0.78 | 0.002 | |||
| NIHSS | 1.08 | 1.06–1.10 | < 0.001 | ||||||
| Atrial fibrillation | 1.68 | 1.13–2.50 | 0.011 | ||||||
| Smoking | 1.54 | 0.99–2.38 | 0.054 | ||||||
| Alcohol consumption | 0.99 | 0.65–1.50 | 0.955 | ||||||
| SBP (mmHg) | 0.99 | 0.98–1.00 | 0.002 | ||||||
| Thrombolysis | 2.07 | 1.25–3.43 | 0.005 | ||||||
| Thrombectomy | 1.81 | 1.05–3.12 | 0.032 | ||||||
| TOAST classification (cardioembolic/non cardioembolic) | 2.07 | 1.45–2.94 | < 0.001 | ||||||
| LDL-C | |||||||||
| Q1: < 1.99 mmol/L | 2.20 | 1.45–3.33 | < 0.001 | 2.26 | < 0.001 | 1.48–3.44 | 1.57 | 1.00–2.47 | 0.049 |
| Q2: 1.99–2.56 mmol/L | 1.70 | 1.10–2.62 | 0.017 | 1.77 | 0.011 | 1.14–2.75 | 1.51 | 0.95–2.40 | 0.079 |
| Q3: 2.57–3.24 mmol/L | 1.83 | 1.19–2.81 | 0.006 | 1.85 | 0.005 | 1.20–2.85 | 1.82 | 1.16–2.87 | 0.009 |
| Q4: > 3.24 mmol/L | 1.00 | 1.00 | 1.00 | ||||||
| Age (years) | 1.02 | 1.00–1.03 | 0.004 | 1.01 | 1.00–1.02 | 0.129 | |||
| Sex (female/male) | 0.53 | 0.40–0.70 | < 0.001 | 0.53 | 0.35–0.80 | 0.002 | |||
| NIHSS | 1.08 | 1.06–1.10 | < 0.001 | ||||||
| Atrial fibrillation | 1.67 | 1.12–2.49 | 0.011 | ||||||
| Smoking | 1.53 | 0.99–2.36 | 0.056 | ||||||
| Alcohol consumption | 0.98 | 0.65–1.49 | 0.936 | ||||||
| SBP (mmHg) | 0.99 | 0.00–1.00 | 0.002 | ||||||
| Thrombolysis | 2.03 | 1.23–3.36 | 0.006 | ||||||
| Thrombectomy | 1.81 | 1.05–3.11 | 0.033 | ||||||
| TOAST classification (cardioembolic/non cardioembolic) | 2.13 | 1.50–3.02 | < 0.001 | ||||||
Abbreviations: OR Odds ratio, CI Confidence interval, Non-HDL-C Non-high-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol, NHISS National Institutes of Health Stroke Scale, SBP Systolic blood pressure, TOAST The Trial of ORG 10172 in Acute Stroke Treatment
After adjusting age and sex in model 1, patients in the lower LDL-C quartiles were associated with increased risks of HT (P for trend < 0.001). Compared with the highest quartiles, the first and third quartiles of LDL-C were associated with increased risks of HT (adjusted ORs 1.57 [95%CI 1.00–2.47] and 1.82 [95%CI 1.16–2.87]), but not the second quartiles (adjusted ORs 1.51 [95%CI 0.95–2.40]) after adjusting for age, sex, NIHSS scores on admission, atrial fibrillation, smoking, alcohol consumption, SBP, thrombolysis, thrombectomy and TOAST classification in model 2 (Table 3). No obvious relationship was found between LDL-C and symptomatic HT after adjusting for age and NIHSS scores on admission (Additional file 2: Table S2).
Using a multiple-adjusted spline regression, no nonlinear trend was found between Non-HDL-C, LDL-C and HT (Fig. 2). When adding Non-HDL-C or LDL-C to model 2, the C-statistics were 0.79 (95%CI 0.77–0.80, P < 0.001) for Non-HDL-C and 0.78 (95%CI 0.77–0.80, P < 0.001) for LDL-C (Table 4). In addition, when adding Non-HDL-C, LDL-C to model 2 containing conventional risk factors significantly improved predictive ability (Non-HDL-C, net reclassification index 0.24, 95%CI 0.17–0.31, P < 0.001; LDL-C, net reclassification index 0.15, 95%CI 0.08–0.22, P = 0.03) (Table 4).
Fig. 2.
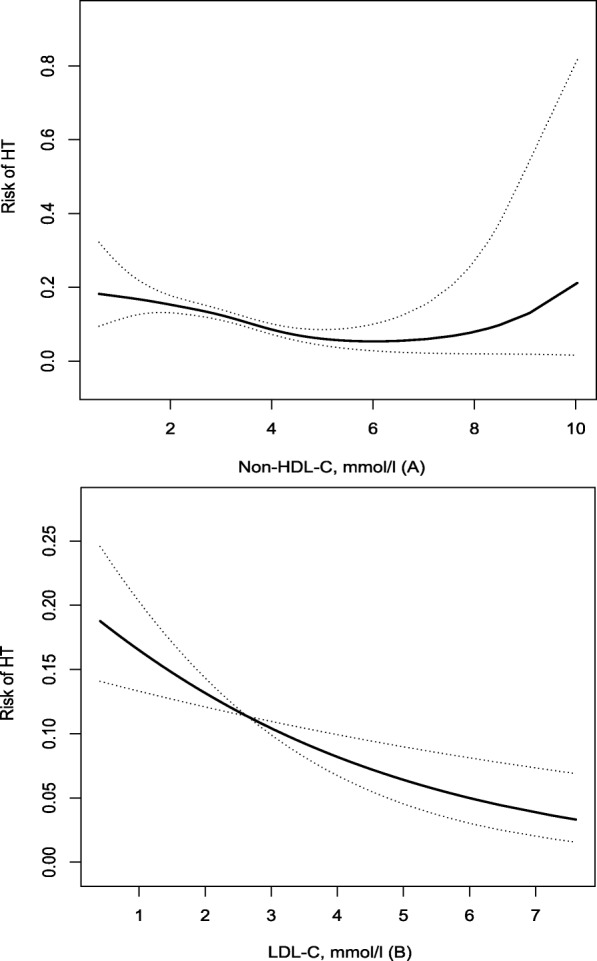
Relationship of Non-HDL-C (A), LDL-C (B) with risk of haemorrhagic transformation after acute ischaemic stroke. Risk of haemorrhagic transformation and 95% confidence intervals determined using the generalized additive model
Table 4.
C statistics and net reclassification index for HT by Non-HDL-C, LDL-C among patients with acute ischaemic stroke
| Variable | C statistics (95%CI) | P-value | NRI (95%CI) | P-value |
|---|---|---|---|---|
| Non-HDL-C | 0.59 (0.55–0.63) | < 0.001 | NA | NA |
| LDL-C | 0.57 (0.53–0.61) | 0.001 | NA | NA |
| Non-HDL-C + Model 2 | 0.79 (0.77–0.80) | < 0.001 | 0.24 (0.17–0.31) | < 0.001 |
| LDL-C + Model 2 | 0.78 (0.77–0.80) | < 0.001 | 0.15 (0.08–0.22) | 0.03 |
Model 2 adjusted for age, sex, National Institutes of Health Stroke Scale scores on admission, atrial fibrillation, smoking, alcohol consumption, systolic blood pressure, thrombolysis, thrombectomy and the Trial of ORG 10172 in Acute Stroke Treatment classification. NRI, net reclassification improvement
Patients' age affects the relationship between non-HDL-C and HT
Age is an interaction factor between Non-HDL-C and HT(P = 0.021). Limiting the analysis to younger patients (< 60 years) showed a significant negative relationship between Non-HDL-C and HT (OR 0.64, 95%CI 0.47–0.87), P < 0.01), whereas this relationship was no longer significant in older patients (≥60 years) (Table 5). After adjustment for potential confounding variables, we found that the relationship between Non-HDL-C and HT did not change by sex, baseline NIHSS score, atrial fibrillation, smoking, alcohol consumption, SBP, reperfusion therapy (thrombolysis/thrombectomy) and stroke subtype (all P for interaction > 0.05) (Table 5).
Table 5.
Stratified logistic regression analysis to identify variables that modify the correlation between Non-HDL-C and haemorrhagic transformation
| OR (95%CI), P-valuea | P for interactiona | |
|---|---|---|
| Age | ||
| < 60 | 0.64 (0.47–0.87), P < 0.001 | 0.021 |
| ≥ 60 | 0.95 (0.81–1.12), 0.523 | |
| Sex | ||
| Male | 0.78 (0.64–0.96) 0.021 | 0.188 |
| Female | 0.95 (0.78–1.15) 0.586 | |
| Baseline NIHSS score | ||
| < 15 | 0.90 (0.76–1.06) 0.217 | 0.363 |
| ≥ 15 | 0.78 (0.59–1.02) 0.069 | |
| Atrial Fibrillation | ||
| No | 0.82 (0.69–0.96) 0.016 | 0.151 |
| Yes | 1.05 (0.78–1.40) 0.760 | |
| Smoking | ||
| No | 0.89 (0.74–1.06) 0.190 | 0.655 |
| Yes | 0.83 (0.66–1.04) 0.109 | |
| Alcohol consumption | ||
| No | 0.91 (0.78–1.07) 0.261 | 0.160 |
| Yes | 0.71 (0.53–0.97) 0.032 | |
| SBP | ||
| < 140 | 0.91 (0.74–1.11) 0.338 | 0.404 |
| ≥ 140 | 0.80 (0.66–0.98) 0.031 | |
| Reperfusion therapy (Thrombolysis/Thrombectomy) | ||
| No | 0.86 (0.74–1.01) 0.070 | 0.944 |
| Yes | 0.88 (0.62–1.24) 0.452 | |
| Stroke subtype | ||
| Non-cardioembolic | 0.79 (0.66–0.95) 0.013 | 0.134 |
| Cardioembolic | 0.99 (0.79–1.25) 0.953 | |
aAbove model adjusted for age, sex, National Institutes of Health Stroke Scale scores on admission, atrial fibrillation, smoking, alcohol consumption, systolic blood pressure, thrombolysis, thrombectomy and Stroke subtype. In each case, the model is not adjusted for the stratification variable
Discussion
In the present study, we found low Non-HDL-C was associated with an increased risk of HT in patients with AIS after adjustment for known risk factors. In addition, Non-HDL-C has similar effects as LDL-C for predicting HT.
Some studies showed that Non-HDL-C was a good biomarker for predicting cardiovascular events [7, 18, 19]. Despite these data, the role of the Non-HDL-C for HT is still not clear in patients with ischaemic stroke. In the present study, low Non-HDL-C was independently associated with increased risks of HT. In addition, no robust association was observed between LDL-C and HT. Prior studies [4, 20] have shown that low LDL-C was related to greater risk for HT; however, these studies included patients with ischaemic stroke receiving intravenous or intra-arterial rt-PA and mechanical recanalization. Conversely, other research [21–23] stated that LDL-C on admission was not associated with intracranial haemorrhage after intravenous thrombolysis. In our study, we found lower LDL-C was significantly related to higher risks of HT in the univariate analysis, but this association was attenuated after adjusting for risk factors, suggesting a possible mediating effect of unmeasured confounders. Further studies are needed to clarify the association especially in general AIS patients.
The mechanisms that explain the association of cholesterol and HT are uncertain, but there are some possible explanations as follows. First, cholesterol may play a great role in keeping the integrity of cerebral vascular vessel. It is reported that low level of cholesterol could cause the increased permeability of the erythrocyte membrane [24], and even contribute to the leakage and rupture of vessels wall [25]. Second, cholesterol is likely to affect aggregation of platelet. Some studies have shown that low level of cholesterol might lead to decreased platelet aggregation, and then increase the risk of bleeding [26]. Third, abnormal blood lipid levels could result in the increased plasma viscosity and whole blood viscosity, and then cholesterol would be accumulated in endothelium, thereby exciting the sympathetic nervous system and renin angiotensin system, with the injury of vascular wall [27, 28]. Further studies are needed to verify the mechanism between serum lipid levels and HT.
Although increasing evidence [8–10] indicated that Non-HDL-C was superior to LDL-C in terms of predicting the risk of cardiovascular disease, we found Non-HDL-C has similar predictive values as LDL-C for HT in AIS. The addition of Non-HDL-C or LDL-C to a conventional risk factor model could improve predictive ability for HT, suggesting that Non-HDL-C could be a potential predictive marker for HT as well as LDL-C. Furthermore, Non-HDL-C is more accurate and reliable when measured in the non-fasting sate compared with LDL-C [29]. In addition, some guidelines on the management of blood cholesterol has recommended that Non-HDL-C could be as a primary goal in the primary and secondary prevention of cardiovascular disease [30–32].
In this study, we found Non-HDL-C was negatively associated with HT in younger stroke patients. In older stroke patients, Non-HDL-C is also negatively related to HT although there was no significant difference. One possible explanation for the difference is that malnutrition is more commonly seen in elderly population [33] and malnutrition might lead to decreased serum cholesterol [34]. In addition, there are other factors that may contribute to the occurrence of HT. Older people usually present arterial stiffness that recently has been recognised as a possible risk factor for HT [35].
Interestingly, our study found that SBP on admission was significantly lower in patients with HT (140 ± 23 mmHg) than those without HT (146 ± 23 mmHg), which is in line with some previous studies [36–38]. A possible explanation might be that slightly elevated blood pressure could provide an adequate cerebral blood supply, and thereby reduce the damage to blood-brain barrier due to ischemia and hypoxia [39], which may prevent the occurrence of HT. A much-debated question is whether blood pressure is related to HT. Some studies [40–43] showed elevated SBP was associated with increased risks of HT, whereas other studies [44–46] did not observe the association. In addition, recent studies have reported that blood pressure variability, rather than the single measure of blood pressure on admission, is an emerging risk factor for HT [47–49]. More research is needed in this field.
Our study has some limitations. First, patients presented with HT at admission were excluded. Therefore, the results might not be generalizable to all ischaemic stroke patients. However, the proportion of patients with HT at admission is low (3.9%) in this study. Second, this study was just an observational, single-centre study, so the findings might not be generalized to the whole Chinese population. Third, although we struggled to obtain medical history, there might be some omissions. Therefore, the results of this study should be interpreted cautiously.
Conclusions
In conclusion, low Non-HDL-C was independently associated with an increased risk of HT. In addition, Non-HDL-C has similar effects as LDL-C for predicting HT. These findings suggest that patients with low Non-HDL-C or LDL-C are prone to haemorrhagic transformation and those might be considered in practice to reduce the risk of haemorrhagic transformation. Further large sample size studies are needed to confirm these findings.
Supplementary information
Additional file 1: Table S1. Univariate analysis to identify risk factors associated with symptomatic haemorrhagic transformation in patients with acute ischaemic stroke.
Additional file 2: Table S2. Association of quartiles of Non-HDL-C, LDL-C and symptomatic haemorrhagic transformation.
Acknowledgements
Not applicable.
Abbreviations
- AIS
Acute ischaemic stroke
- CI
Confidence interval
- CT
Computed tomography
- DBP
Diastolic blood pressure
- HT
Haemorrhagic transformation
- LDL-C
Low-density lipoprotein cholesterol
- MRI
Magnetic resonance imaging
- NIHSS
National Institutes of Health Stroke Scale
- Non-HDL-C
Non-high-density lipoprotein cholesterol
- NRI
Net reclassification index
- OR
Odds ratio
- ORs
Odds ratios
- SBP
Systolic blood pressure
- TC
Total cholesterol
- TG
Triglycerides
- TOAST
The Trial of ORG 10172 in Acute Stroke Treatment
Authors’ contributions
LM and WB designed the study, guided the data analysis and revised the manuscript. WY, SQ, CY and YC collected the clinical data. WY, SQ and WC collected the imaging data. WY and LJ performed statistics analysis. WY and SQ drafted the manuscript. All authors revised the manuscript and approved the final version.
Funding
This study was supported by the Major International (Regional) Joint Research Project, National Natural Science Foundation of China (81620108009), the National Key Research and Development Program of China, Ministry of Science and Technology of China (2016YFC1300500–505) and the 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD18009).
Availability of data and materials
The data used in this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This study was approved by The Biomedical Research Ethics Committee of West China Hospital, Sichuan University, and conformed to local and international ethical criteria. Informed consent was not required since the study was observational and retrospective in nature.
Consent for publication
Participants consent for publication: Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanan Wang and Quhong Song contributed equally to this work.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12883-020-1615-9.
References
- 1.Tran-Dinh A, Levoye A, Lambert G, Louedec L, Journe C, Meilhac O, Amarenco P. Low levels of low-density lipoprotein-C associated with proprotein convertase subtilisin kexin 9 inhibition do not increase the risk of hemorrhagic transformation. Stroke. 2014;45(10):3086–3088. doi: 10.1161/STROKEAHA.114.005958. [DOI] [PubMed] [Google Scholar]
- 2.Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, Kim LJ, Mayer SA, Sheth KN, Schwamm LH. Treatment and outcome of hemorrhagic transformation after intravenous Alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(12):e343–e361. doi: 10.1161/STR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, Sandercock P, Wang Y, Huang Y, Cui L, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurology. 2019;18(4):394–405. doi: 10.1016/S1474-4422(18)30500-3. [DOI] [PubMed] [Google Scholar]
- 4.Bang OY, Saver JL, Liebeskind DS, Starkman S, Villablanca P, Salamon N, Buck B, Ali L, Restrepo L, Vinuela F, et al. Cholesterol level and symptomatic hemorrhagic transformation after ischemic stroke thrombolysis. Neurology. 2007;68(10):737–742. doi: 10.1212/01.wnl.0000252799.64165.d5. [DOI] [PubMed] [Google Scholar]
- 5.Kim BJ, Lee SH, Ryu WS, Kang BS, Kim CK, Yoon BW. Low level of low-density lipoprotein cholesterol increases hemorrhagic transformation in large artery atherothrombosis but not in cardioembolism. Stroke. 2009;40(5):1627–1632. doi: 10.1161/STROKEAHA.108.539643. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Wei C, Song Q, Liu J, Cheng Y, Li Y, Wu B, Liu M. Reduction in the ratio of low-density lipoprotein cholesterol to high-density lipoprotein cholesterol is associated with increased risks of hemorrhagic transformation in patients with acute ischemic stroke. Curr Neurovasc Res. 2019;16(2):266–272. doi: 10.2174/1567202616666190619151914. [DOI] [PubMed] [Google Scholar]
- 7.Puri R, Nissen SE, Shao M, Elshazly MB, Kataoka Y, Kapadia SR, Tuzcu EM, Nicholls SJ. Non-HDL cholesterol and triglycerides: implications for coronary atheroma progression and clinical events. Arterioscler Thromb Vasc Biol. 2016;36(11):2220–2228. doi: 10.1161/ATVBAHA.116.307601. [DOI] [PubMed] [Google Scholar]
- 8.Orakzai SH, Nasir K, Blaha M, Blumenthal RS, Raggi P. Non-HDL cholesterol is strongly associated with coronary artery calcification in asymptomatic individuals. Atherosclerosis. 2009;202(1):289–295. doi: 10.1016/j.atherosclerosis.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Verbeek R, Hovingh GK, Boekholdt SM. Non-high-density lipoprotein cholesterol: current status as cardiovascular marker. Curr Opin Lipidol. 2015;26(6):502–510. doi: 10.1097/MOL.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 10.Sniderman A, McQueen M, Contois J, Williams K, Furberg CD. Why is non-high-density lipoprotein cholesterol a better marker of the risk of vascular disease than low-density lipoprotein cholesterol? J Clin Lipidol. 2010;4(3):152–155. doi: 10.1016/j.jacl.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Zheng L, Cheng Y, Zhang S, Wu B, Wang D, Zhang S, Tao W, Wu S, Liu M. Trends in outcomes of patients with ischemic stroke treated between 2002 and 2016: insights from a Chinese cohort. Circ Cardiovasc Qual Outcomes. 2019;12(12):e005610. doi: 10.1161/CIRCOUTCOMES.119.005610. [DOI] [PubMed] [Google Scholar]
- 12.Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980;58(1):113–130. [PMC free article] [PubMed] [Google Scholar]
- 13.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.STR.20.7.864. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1--full report. J Clin Lipidol. 2015;9(2):129–169. doi: 10.1016/j.jacl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian acute stroke study Investigators. Lancet. 1998;352(9136):1245–1251. doi: 10.1016/S0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 16.National Institute of Neurological D, Stroke rt PASSG Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 17.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 18.Elshazly MB, Martin SS, Blaha MJ, Joshi PH, Toth PP, McEvoy JW, Al-Hijji MA, Kulkarni KR, Kwiterovich PO, Blumenthal RS, et al. Non-high-density lipoprotein cholesterol, guideline targets, and population percentiles for secondary prevention in 1.3 million adults: the VLDL-2 study (very large database of lipids) J Am Coll Cardiol. 2013;62(21):1960–1965. doi: 10.1016/j.jacc.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 19.Holewijn S, den Heijer M, Swinkels DW, Stalenhoef AF, de Graaf J. Apolipoprotein B, non-HDL cholesterol and LDL cholesterol for identifying individuals at increased cardiovascular risk. J Intern Med. 2010;268(6):567–577. doi: 10.1111/j.1365-2796.2010.02277.x. [DOI] [PubMed] [Google Scholar]
- 20.Lin SF, Chao AC, Hu HH, Lin RT, Chen CH, Chan L, Lin HJ, Sun Y, Lin YY, Chen PL, et al. Low cholesterol levels increase symptomatic intracranial hemorrhage rates after intravenous thrombolysis: a multicenter cohort validation study. J Atheroscler Thromb. 2018;26(6):513–527. doi: 10.5551/jat.46151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong CT, Chiu WT, Chi NF, Lai LY, Hu CJ, Hu HH, Chan L. Low-density lipoprotein level on admission is not associated with postintravenous thrombolysis intracranial hemorrhage in patients with acute ischemic stroke. J Investig Med. 2018;67(3):659–662. doi: 10.1136/jim-2018-000827. [DOI] [PubMed] [Google Scholar]
- 22.Lin TC, Lin YK, Chen CI, Chan L, Chi NF, Yuan RY, Sheu JJ, Wei CR, Tsai JP, Yeh TH. Serum lipid level is not associated with symptomatic intracerebral hemorrhage after intravenous thrombolysis for acute ischemic stroke. PeerJ. 2018;6:e6021. doi: 10.7717/peerj.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messe SR, Pervez MA, Smith EE, Siddique KA, Hellkamp AS, Saver JL, Bhatt DL, Fonarow GC, Peterson ED, Schwamm LH. Lipid profile, lipid-lowering medications, and intracerebral hemorrhage after tPA in get with the guidelines-stroke. Stroke. 2013;44(5):1354–1359. doi: 10.1161/STROKEAHA.111.671966. [DOI] [PubMed] [Google Scholar]
- 24.Wieberdink RG, Poels MM, Vernooij MW, Koudstaal PJ, Hofman A, van der Lugt A, Breteler MM, Ikram MA. Serum lipid levels and the risk of intracerebral hemorrhage: the Rotterdam study. Arterioscler Thromb Vasc Biol. 2011;31(12):2982–2989. doi: 10.1161/ATVBAHA.111.234948. [DOI] [PubMed] [Google Scholar]
- 25.Thrift A, McNeil J, Donnan G. Reduced frequency of high cholesterol levels among patients with intracerebral haemorrhage. J Clin Neurosci. 2002;9(4):376–380. doi: 10.1054/jocn.2002.1111. [DOI] [PubMed] [Google Scholar]
- 26.Fessler MB, Rose K, Zhang Y, Jaramillo R, Zeldin DC. Relationship between serum cholesterol and indices of erythrocytes and platelets in the US population. J Lipid Res. 2013;54(11):3177–3188. doi: 10.1194/jlr.P037614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenson RS, McCormick A, Uretz EF. Distribution of blood viscosity values and biochemical correlates in healthy adults. Clin Chem. 1996;42(8 Pt 1):1189–1195. doi: 10.1093/clinchem/42.8.1189. [DOI] [PubMed] [Google Scholar]
- 28.Rosenson RS, Shott S, Tangney CC. Hypertriglyceridemia is associated with an elevated blood viscosity Rosenson: triglycerides and blood viscosity. Atherosclerosis. 2002;161(2):433–439. doi: 10.1016/S0021-9150(01)00656-6. [DOI] [PubMed] [Google Scholar]
- 29.Mahajan N, Ference BA, Arora N, Madhavan R, Bhattacharya P, Sudhakar R, Sagar A, Wang Y, Sacks F, Afonso L. Role of non-high-density lipoprotein cholesterol in predicting cerebrovascular events in patients following myocardial infarction. Am J Cardiol. 2012;109(12):1694–1699. doi: 10.1016/j.amjcard.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Gao R, Zhao S, Lu G, Zhao D, Li J. Guidelines for the prevention and treatment of dyslipidemia in Chinese adults (revised edition 2016) Chin Circul J. 2016;31(10):937–953. [Google Scholar]
- 31.National Cholesterol Education Program Expert Panel on Detection E Treatment of high blood cholesterol in a: third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. doi: 10.1161/circ.106.25.3143. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, De Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the Management of Blood Cholesterol. J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Zhiying, Pereira Suzette, Luo Menghua, Matheson Eric. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients. 2017;9(8):829. doi: 10.3390/nu9080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amirkalali B, Sharifi F, Fakhrzadeh H, Mirarefein M, Ghaderpanahi M, Badamchizadeh Z, Larijani B. Low serum leptin serves as a biomarker of malnutrition in elderly patients. Nutr Res. 2010;30(5):314–319. doi: 10.1016/j.nutres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Acampa M, Camarri S, Lazzerini PE, Guideri F, Tassi R, Valenti R, Cartocci A, Martini G. Increased arterial stiffness is an independent risk factor for hemorrhagic transformation in ischemic stroke undergoing thrombolysis. Int J Cardiol. 2017;243:466–470. doi: 10.1016/j.ijcard.2017.03.129. [DOI] [PubMed] [Google Scholar]
- 36.Bayramoglu M, Karatas M, Leblebici B, Cetin N, Sozay S, Turhan N. Hemorrhagic transformation in stroke patients. Am J Phys Med Rehabil. 2003;82(1):48–52. doi: 10.1097/00002060-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Chen G, Wang A, Zhao X, Wang C, Liu L, Zheng H, Wang Y, Cao Y, Wang Y. Frequency and risk factors of spontaneous hemorrhagic transformation following ischemic stroke on the initial brain CT or MRI: data from the China National Stroke Registry (CNSR) Neurol Res. 2016;38(6):538–544. doi: 10.1080/01616412.2016.1187864. [DOI] [PubMed] [Google Scholar]
- 38.Tan S, Wang D, Liu M, Zhang S, Wu B, Liu B. Frequency and predictors of spontaneous hemorrhagic transformation in ischemic stroke and its association with prognosis. J Neurol. 2014;261(5):905–912. doi: 10.1007/s00415-014-7297-8. [DOI] [PubMed] [Google Scholar]
- 39.Jiang B, Churilov L, Kanesan L, Dowling R, Mitchell P, Dong Q, Davis S, Yan B. Blood pressure may be associated with arterial collateralization in anterior circulation ischemic stroke before acute reperfusion therapy. J Stroke. 2017;19(2):222–228. doi: 10.5853/jos.2016.01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong M, Kaste M. Association of characteristics of blood pressure profiles and stroke outcomes in the ECASS-II trial. Stroke. 2008;39(2):366–372. doi: 10.1161/STROKEAHA.107.492330. [DOI] [PubMed] [Google Scholar]
- 41.Butcher K, Christensen S, Parsons M, De Silva DA, Ebinger M, Levi C, Jeerakathil T, Campbell BC, Barber PA, Bladin C, et al. Postthrombolysis blood pressure elevation is associated with hemorrhagic transformation. Stroke. 2010;41(1):72–77. doi: 10.1161/STROKEAHA.109.563767. [DOI] [PubMed] [Google Scholar]
- 42.Mazya M, Egido JA, Ford GA, Lees KR, Mikulik R, Toni D, Wahlgren N, Ahmed N. Predicting the risk of symptomatic Intracerebral hemorrhage in ischemic stroke treated with intravenous Alteplase. Stroke. 2012;43(6):1524–1531. doi: 10.1161/STROKEAHA.111.644815. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, Lees KR, Toni D, Investigators S. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from safe implementation of thrombolysis in stroke-international stroke thrombolysis register (SITS-ISTR) Stroke. 2009;40(7):2442–2449. doi: 10.1161/STROKEAHA.109.548602. [DOI] [PubMed] [Google Scholar]
- 44.Leonardi-Bee J, Bath PMW, Phillips SJ, Sandercock PAG. Blood pressure and clinical outcomes in the international stroke trial. Stroke. 2002;33(5):1315–1320. doi: 10.1161/01.STR.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- 45.Lindley RI, Wardlaw JM, Sandercock PA, Rimdusid P, Lewis SC, Signorini DF, Ricci S. Frequency and risk factors for spontaneous hemorrhagic transformation of cerebral infarction. J Stroke Cerebrovasc Dis. 2004;13(6):235–246. doi: 10.1016/j.jstrokecerebrovasdis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke. 2012;43(11):2904–2909. doi: 10.1161/STROKEAHA.112.665331. [DOI] [PubMed] [Google Scholar]
- 47.Ko Y, Park JH, Yang MH, Ko SB, Han MK, Oh CW, Lee J, Lee J, Bae HJ. The significance of blood pressure variability for the development of hemorrhagic transformation in acute ischemic stroke. Stroke. 2010;41(11):2512–2518. doi: 10.1161/STROKEAHA.110.595561. [DOI] [PubMed] [Google Scholar]
- 48.Liu K, Yan S, Zhang S, Guo Y, Lou M. Systolic blood pressure variability is associated with severe hemorrhagic transformation in the early stage after thrombolysis. Transl Stroke Res. 2016;7(3):186–191. doi: 10.1007/s12975-016-0458-6. [DOI] [PubMed] [Google Scholar]
- 49.Kim TJ, Park HK, Kim JM, Lee JS, Park SH, Jeong HB, Park KY, Rha JH, Yoon BW, Ko SB. Blood pressure variability and hemorrhagic transformation in patients with successful recanalization after endovascular recanalization therapy: a retrospective observational study. Ann Neurol. 2019;85(4):574–581. doi: 10.1002/ana.25434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Univariate analysis to identify risk factors associated with symptomatic haemorrhagic transformation in patients with acute ischaemic stroke.
Additional file 2: Table S2. Association of quartiles of Non-HDL-C, LDL-C and symptomatic haemorrhagic transformation.
Data Availability Statement
The data used in this study are available from the corresponding author upon reasonable request.


