Abstract
Background
Mixed dyslipidemia [elevated non-high-density lipoprotein cholesterol (non-HDL-C) and triglycerides (TGs), and decreased HDL-C] is common in type 2 diabetes mellitus (T2DM) and is associated with increased cardiovascular risk. Non-HDL-C and apolipoprotein B (ApoB) are the preferred therapeutic targets for mixed dyslipidemia. Alirocumab is a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 (PCSK9) that effectively reduces low-density lipoprotein cholesterol (LDL-C), non-HDL-C, ApoB, and lipoprotein(a) (Lp[a]), and is well-tolerated in individuals with T2DM.
Methods
The previously reported open-label ODYSSEY DM-DYSLIPIDEMIA trial data demonstrated the effects of alirocumab on individuals with non‐HDL-C ≥ 100 mg/dL and TGs ≥ 150 and < 500 mg/dL receiving stable maximally tolerated statin (n = 413). This post hoc subgroup analysis of the primary trial investigated the effects of alirocumab [75 mg every 2 weeks (Q2W) with possible increase to 150 mg Q2W at Week 12] versus usual care [ezetimibe, fenofibrate, or no additional lipid-lowering therapy (LLT)] on non-HDL-C and other lipids in individuals with T2DM and baseline TGs ≥ 200 mg/dL and HDL-C < 40 mg/dL (men) or < 50 mg/dL (women).
Results
Alirocumab significantly reduced non-HDL-C [LS mean difference (standard error (SE)), − 35.0% (3.9)], ApoB [LS mean difference (SE), − 34.7% (3.6)], LDL-C [LS mean difference (SE), − 47.3% (5.2)], LDL particle number [LS mean difference (SE), − 40.8% (4.1)], and Lp(a) [LS mean difference (SE), − 29.9% (5.4)] versus usual care from baseline to Week 24 (all P < 0.0001). Results were similar for alirocumab versus usual care. TG reductions were similar between alirocumab and usual care (no significant difference), but greater with fenofibrate versus alirocumab (P = 0.3371). Overall, alirocumab significantly increased HDL-C versus usual care [LS mean difference (SE), 7.9% (3.6); P < 0.05], although differences with alirocumab versus ezetimibe or fenofibrate were non-significant. Most individuals receiving alirocumab achieved ApoB < 80 mg/dL (67.9%) and non-HDL-C < 100 mg/dL (60.9%). Adverse event frequency was similar between alirocumab (67.2%) and usual care (70.7%). Additionally, no clinically relevant effect of alirocumab on change in glycemic parameters or use of antihyperglycemic agents was observed.
Conclusions
Alirocumab is an effective therapeutic option for individuals with T2DM, TGs ≥ 200 mg/dL, and HDL-C < 40 mg/dL (men) or < 50 mg/dL (women). Atherogenic lipid (ApoB and non-HDL) reductions were greater with alirocumab than ezetimibe, fenofibrate, or no LLT. Consistent with previous studies, alirocumab was generally well tolerated.
Trial registration Clinicaltrials.gov, NCT02642159. Registered December 24, 2015, https://clinicaltrials.gov/ct2/show/NCT02642159
Keywords: Alirocumab, PCSK9, Diabetes mellitus, Non-HDL-C, HDL-C, Triglycerides, ODYSSEY, DM-DYSLIPIDEMIA, Type 2 diabetes, Usual care
Background
Individuals with type 2 diabetes mellitus (T2DM) are at increased risk of atherosclerotic cardiovascular disease (ASCVD) [1]. Mixed dyslipidemia, i.e. elevated plasma triglycerides (TGs), TG-rich lipoprotein (TRL) and TRL cholesterol (TRL-C) levels and decreased levels of high-density lipoprotein cholesterol (HDL-C) [2], is a major contributor to ASCVD risk in individuals with diabetes [3, 4]. Individuals with mixed dyslipidemia may also have an elevated number of small, dense low-density lipoprotein (LDL) particles [2, 5], as reflected by higher levels of ApoB-100 (ApoB); however, these individuals may not necessarily have elevated LDL cholesterol (LDL-C) levels [6].
ApoB-containing lipoproteins have been demonstrated to be directly associated with the risk of coronary heart disease [7], as indicated by the reduction in cardiovascular risk associated with statin therapy, proportional with the reduction in ApoB [8]. In addition, when LDL-C and ApoB are discordant, as commonly occurs in insulin-resistant states [9], non-HDL-C and ApoB are considered to be stronger predictors of cardiovascular risk than LDL-C [2, 10, 11]. TGs are not a specific therapeutic target in cardiovascular disease; however, when TGs are 200–499 mg/dL, the primary targets of lipid therapy are non-HDL-C and LDL-C [2]. The National Lipid Association recommends non-HDL-C targets of < 130 mg/dL for individuals at high ASCVD risk and < 100 mg/dL for those at very-high ASCVD risk, and ApoB targets of < 90 mg/dL for primary prevention, and < 80 mg/dL for those with very-high cardiovascular risk [2]. The American Association of Clinical Endocrinologists recommends targets of < 100 mg/dL for non-HDL-C and < 80 mg/dL for ApoB in individuals at very-high cardiovascular risk, and targets of < 80 mg/dL for non-HDL-C and < 70 mg/dL for ApoB for individuals at extreme risk [12].
Alirocumab is a monoclonal antibody that binds to circulating proprotein convertase subtilisin/kexin type 9 (PCSK9), which was previously investigated in individuals with mixed dyslipidemia in the ODYSSEY DM-DYSLIPIDEMIA trial [13, 14]. This trial was a Phase 3, randomized, open-label, parallel group, multinational study (NCT02642159) that compared alirocumab with usual care [ezetimibe, fenofibrate, omega-3, niacin, and no additional lipid-lowering therapy (LLT)] in adults with T2DM and mixed dyslipidemia (non‐HDL-C ≥ 100 mg/dL and TGs ≥ 150 and < 500 mg/dL) receiving stable maximally tolerated statin dose (n = 413) [13]. The trial showed that the primary endpoint of reduction in non-HDL-C with alirocumab was superior to usual care overall (mean difference of − 32.5% vs usual care at Week 24; P < 0.0001) and versus fenofibrate (mean difference of − 33.3% vs fenofibrate at Week 24; P < 0.0001), and that alirocumab was generally well tolerated [14].
The aim of this post hoc analysis of the DM-DYSLIPIDEMIA study was to focus on a higher risk and more difficult to treat subpopulation compared with the primary trial population, and provide analyses of other lipids beyond the primary endpoint.
Methods
Primary analysis
Detailed methods of the DM-DYSLIPIDEMIA study have been reported previously [13]. Briefly, individuals were randomized 2:1 to receive alirocumab or usual care for 24 weeks. All individuals were receiving maximally tolerated statin. Usual care included addition of ezetimibe, fenofibrate, no additional LLT, omega-3 fatty acid, or nicotinic acid. Randomization was stratified by the investigator’s choice of usual care therapy, which was prespecified prior to randomization.
The primary analysis included adults with T2DM and mixed dyslipidemia (non‐HDL-C ≥ 100 mg/dL; TGs ≥ 150 and < 500 mg/dL) receiving stable maximally tolerated statin dose for at least 4 weeks prior to screening, without other LLT, and who had a documented history of ASCVD or at least one additional cardiovascular risk factor, and glycated hemoglobin (HbA1c) < 9.0%. The maximally tolerated dose of statin was based on investigator judgement. Individuals with documented statin intolerance and therefore not receiving statin therapy could also be enrolled.
Post hoc subgroup analysis
In this post hoc subgroup analysis, the effect of alirocumab versus usual care on non-HDL-C and other lipids was investigated in a subgroup of individuals with baseline levels of non-HDL-C ≥ 100 mg/dL, TGs ≥ 200 mg/dL, and HDL-C < 40 mg/dL (men) or < 50 mg/dL (women). The thresholds for TGs and HDL-C in this analysis reflect those used in previous analyses of the effects of fenofibrate on cardiovascular events in the ACCORD (TGs ≥ 204 mg/dL, HDL-C ≤ 34 mg/dL) [15] and FIELD trials [TGs ≥ 150.6 mg/dL, HDL-C ≤ 39.8 mg/dL (men) or ≤ 49.9 mg/dL (women)] [16], and icosapent-ethyl in the amended REDUCE-IT trial protocol [TGs 200–499 mg/dL and HDL-C ≤ 40 mg/dL (men) or ≤ 50 mg/dL (women)] [17].
This analysis provides an overall comparison of alirocumab versus usual care (ezetimibe, fenofibrate, no additional LLT, omega-3 fatty acid, and nicotinic acid), and separate analyses of alirocumab versus ezetimibe, fenofibrate, and no LLT. Due to low participant numbers, nicotinic acid and omega-3 fatty acid strata were not compared separately to alirocumab.
Endpoint
The primary efficacy endpoint of the DM-DYSLIPIDEMIA study was the percentage change in non-HDL-C from baseline to Week 24. In this analysis, percentage change from baseline in LDL-C (measured by beta-quantification), non-HDL-C, ApoB, LDL particle number, Lp(a), TGs, TRL-C (i.e. non-HDL-C minus LDL-C), and HDL-C with alirocumab and usual care at Week 24 was analyzed in the intention-to-treat population); additionally, estimates for alirocumab versus ezetimibe, fenofibrate, and no LLT differences were derived from the same model with appropriate contrasts.
Statistical analyses
For LDL-C, non-HDL-C, ApoB, LDL particle number, and HDL-C, least squares (LS) mean difference [standard error (SE)] with alirocumab versus usual care was analyzed by a mixed-effect model with repeat measurements to manage missing data. For Lp(a) and TGs, which are not normally distributed, a combined estimate for adjusted mean difference (SE) with alirocumab versus usual care was calculated by using multiple imputation to manage missing data, followed by robust regression. The combined estimates for proportions (%) of individuals reaching ApoB < 80 mg/dL at Week 24 and non-HDL-C < 100 mg/dL at Week 24 were obtained by using multiple imputation to manage missing data, followed by logistic regression. The safety analysis was descriptive and based on the safety population and was analyzed according to the treatment group (alirocumab or usual care).
Results
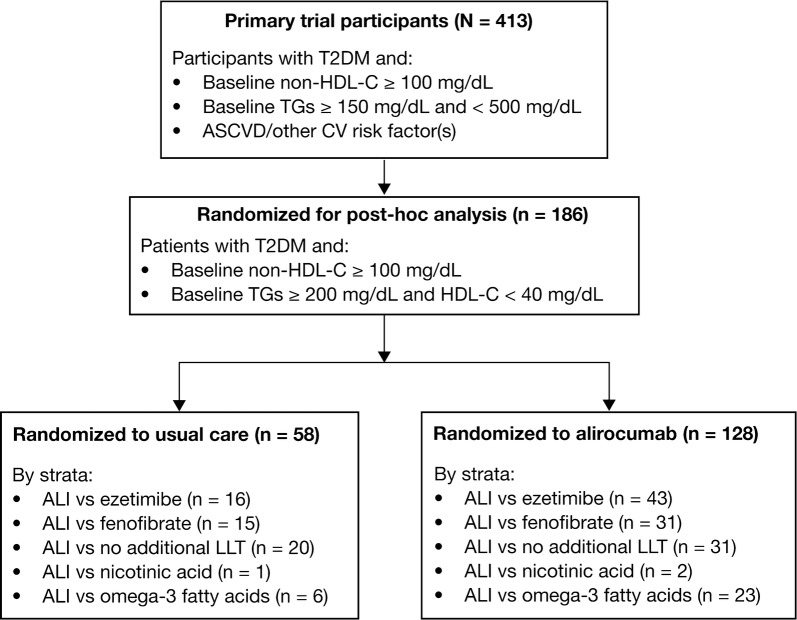
Of the 413 individuals included in the primary DM-DYSLIPIDEMIA study, this post hoc analysis included 186 individuals with TGs ≥ 200 mg/dL and HDL-C < 40 mg/dL (men) or < 50 mg/dL (women), randomized to alirocumab (n = 128) or usual care (n = 58). Figure 1 provides the patient flow chart for the ODYSSEY DM-DYSLIPIDEMIA post hoc analysis, showing the number of individuals randomized to each usual care stratum. Separate analyses were conducted for alirocumab versus ezetimibe (n = 16), fenofibrate (n = 15), and no LLT (n = 20). Due to low participant numbers, nicotinic acid (n = 1) and omega-3 fatty acid (n = 6) strata were not compared separately to alirocumab.
Fig. 1.
Patient flow chart for the ODYSSEY DM-DYSLIPIDEMIA post hoc analysis. ALI, alirocumab; ASCVD, atherosclerotic cardiovascular disease; CV, cardiovascular; HDL-C, non-high-density lipoprotein cholesterol; LLT, lipid-lowering therapy; TG, triglyceride; T2DM, type 2 diabetes mellitus
Baseline characteristics were generally similar across treatment groups (Table 1). Similar proportions of individuals in the alirocumab and usual care groups had ASCVD (defined as coronary heart disease, peripheral arterial disease, or ischemic stroke; 36.7% vs 41.4%) and were receiving insulin (40.6% vs 44.8%) at baseline. Mean (standard deviation [SD]) HbA1c at baseline was also similar between groups [7.0% (0.9) in the alirocumab group and 7.3% (0.8) in the usual care group].
Table 1.
Baseline demographics (ITT population)
| Alirocumab (n = 128) | Usual carea (n = 58) | |
|---|---|---|
| Age, years, mean (SD) | 62.1 (9.5) | 63.4 (9.0) |
| Male, n (%) | 65 (50.8) | 28 (48.3) |
| Race, n (%) | ||
| American Indian or Alaska Native | 3 (2.3) | 0 |
| Asian/Oriental | 2 (1.6) | 6 (10.3) |
| Black | 4 (3.1) | 2 (3.4) |
| Other | 3 (2.3) | 0 |
| White/Caucasian | 116 (90.6) | 50 (86.2) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 17 (13.3) | 8 (13.8) |
| Not Hispanic or Latino | 110 (85.9) | 50 (86.2) |
| Not reported/unknown | 1 (0.8) | 0 |
| Weight, kg, mean (SD) | 93.6 (19.7) | 93.8 (16.4) |
| BMI, kg/m2, mean (SD) | 32.6 (4.9) | 33.0 (4.6) |
| Systolic blood pressure, mmHg, mean (SD) | 130.0 (14.4) | 134.9 (15.7) |
| Diastolic blood pressure, mmHg, mean (SD) | 76.3 (9.8) | 77.2 (9.2) |
| ASCVD (CHD, ischemic stroke, PAD), n (%) | 47 (36.7) | 24 (41.4) |
| HbA1c, %, mean (SD) | 7.0 (0.9) | 7.3 (0.8) |
| Fasting plasma glucose, mg/dL, mean (SD) | 150.2 (39.2) | 153.2 (41.1) |
| Individuals receiving insulin at baseline, n (%) | 52 (40.6) | 26 (44.8) |
| Baseline lipids, mean (SD) | ||
| Non-HDL-C, mg/dL | 170.3 (48.2) | 166.3 (48.8) |
| mmol/L | 4.41 (1.25) | 4.31 (1.26) |
| LDL-C (beta-quantification), mg/dL | 112.8 (43.7) | 110.7 (42.1) |
| mmol/L | 2.92 (1.13) | 2.87 (1.09) |
| ApoB, mg/dL | 109.3 (27.4) | 108.1 (28.9) |
| LDL particle number, nmol/L | 1497.3 (532.0) | 1491.5 (545.1) |
| Lipoprotein(a), mg/dL, median (Q1:Q3) | 18.0 (5.0:55.0) | 9.5 (4.0:30.0) |
| TGs, mg/dL, median (Q1:Q3) | 281.5 (245.0:369.0) | 269.0 (232.0:328.0) |
| mmol/L | 3.19 (2.77:4.17) | 3.04 (2.62:3.71) |
| HDL-C, mg/dL | 34.4 (6.2) | 34.3 (5.9) |
| mmol/L | 0.89 (0.16) | 0.89 (0.15) |
ASCVD atherosclerotic cardiovascular disease, ApoB apolipoprotein B, BMI body mass index, CHD coronary heart disease, HbA1c glycated hemoglobin, HDL-C high-density lipoprotein cholesterol, ITT intention-to-treat, LDL low-density lipoprotein, LDL-C low-density lipoprotein cholesterol, PAD peripheral artery disease, SD standard deviation, TG triglyceride
aOptions included ezetimibe, fenofibrate, no additional lipid-lowering therapy, omega-3 fatty acid, and nicotinic acid
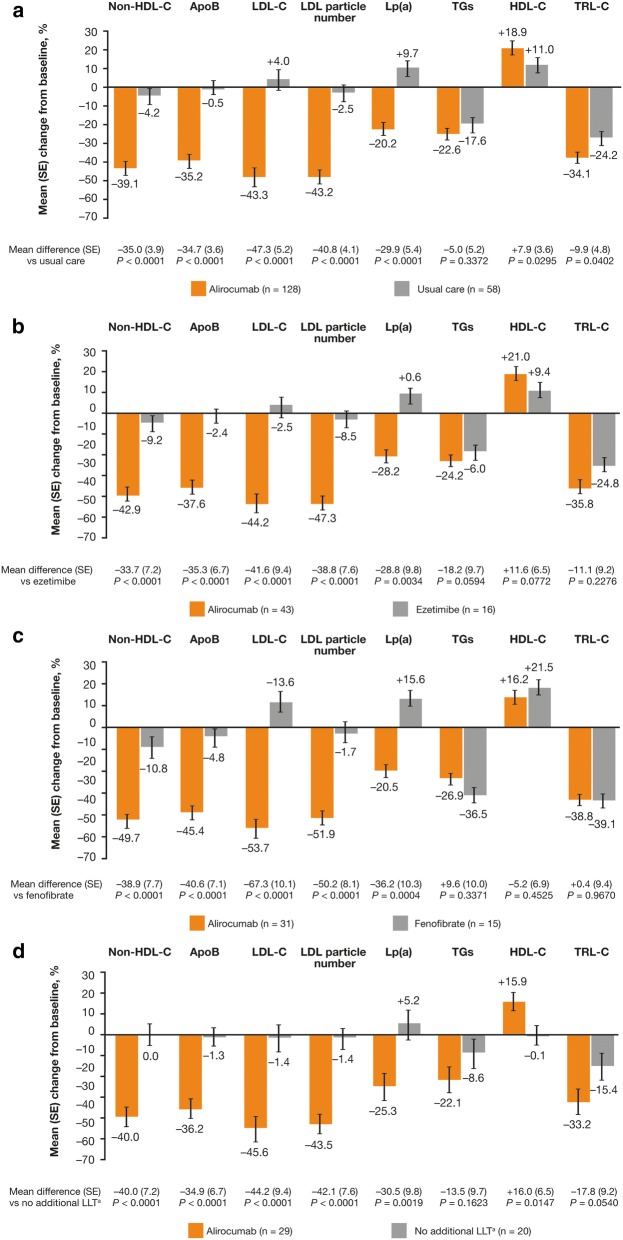
Overall, alirocumab significantly reduced non-HDL-C [LS mean difference (SE): − 35.0% (3.9)] and ApoB [LS mean difference (SE): − 34.7% (3.6)], as well as LDL-C [LS mean difference (SE): − 47.3% (5.2)], LDL particle number [LS mean difference (SE) − 40.8% (4.1)], and Lp(a) [adjusted mean (SE]) − 29.9% (5.4)] from baseline to Week 24 versus usual care (all P < 0.0001; Fig. 2a). In addition, all comparisons for percentage change from baseline in non-HDL-C, ApoB, LDL-C, LDL particle number, and Lp(a) with alirocumab versus ezetimibe, fenofibrate, or no additional LLT were significant (P < 0.01; Fig. 2b–d, respectively).
Fig. 2.
Percent change from baseline in LDL-C, non-HDL-C, ApoB, LDL particle number, Lp(a), TGs, HDL-C, and TRL-C at Week 24 with alirocumab versus usual care (panel a), ezetimibe (panel b), fenofibrate (panel c), and no LLT (panel d) (ITT population). ApoB, apolipoprotein B; HDL-C, high-density lipoprotein cholesterol; ITT, intention-to-treat; LDL, low-density lipoprotein; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; Lp(a), lipoprotein(a); SE, standard error; TG, triglyceride; TRL-C, triglyceride-rich lipoprotein cholesterol. Usual care options were selected by the investigator prior to stratified randomization to alirocumab or usual care. Usual care options included ezetimibe, fenofibrate, no additional LLT, omega-3 fatty acid, and nicotinic acid; due to low participant numbers, nicotinic acid and omega-3 fatty acid strata are not analyzed separately here. a No additional LLT on top of background maximally tolerated statin dose
Alirocumab reduced TGs to a greater extent than usual care overall, ezetimibe, or no LLT [adjusted mean difference (SE): − 5.0% (5.2) vs usual care; − 18.2% (9.7) vs ezetimibe; − 13.5% (9.7) vs no LLT]; however, mean TG reductions were greater with fenofibrate than alirocumab [adjusted mean difference (SE): 9.6% (10.0)]. P-values were not significant for any comparison (Fig. 2).
Overall, alirocumab significantly increased HDL-C compared with usual care [LS mean difference (SE): 7.9% (3.6); P < 0.05] and no LLT strata [LS mean difference (SE): 16.0% (6.5); P < 0.05]; however, LS mean difference (SE) with alirocumab versus ezetimibe or alirocumab versus fenofibrate was non-significant [11.6% (6.5) vs ezetimibe; − 5.2% (6.9) vs fenofibrate; Fig. 2]. The LS mean difference (SE) in TRL-C was significantly reduced with alirocumab versus usual care overall [− 9.9% (4.8); P = 0.0402; Fig. 2a]. However, P-values were not significant for alirocumab versus ezetimibe, fenofibrate, or no additional LLT (Fig. 2b–d).
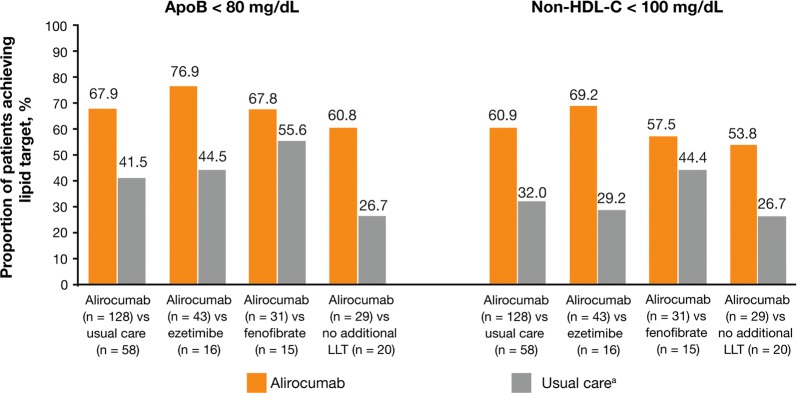
ApoB < 80 mg/dL was achieved by 67.9% of alirocumab-treated individuals compared with 41.5% of individuals in the usual care group (Fig. 3). Similar trends were observed by strata (alirocumab vs ezetimibe: 76.9% vs 44.5%; alirocumab vs fenofibrate: 67.8% vs 55.6%; alirocumab vs no additional LLT: 60.8 vs 26.7%). In addition, non-HDL-C < 100 mg/dL was achieved in 60.9% of alirocumab-treated individuals compared with 32.0% of individuals in the usual care group. Likewise, similar trends were observed by strata (alirocumab vs ezetimibe: 69.2% vs 29.2%; alirocumab vs fenofibrate: 57.5% vs 44.4%; alirocumab vs no additional LLT: 53.8% and 26.7%).
Fig. 3.
Proportion of individuals achieving ApoB < 80 mg/dL and non-HDL-C < 100 mg/dL for alirocumab versus usual care, alirocumab versus ezetimibe, alirocumab versus fenofibrate, and alirocumab versus no LLT (ITT population). ApoB, apolipoprotein B; HDL-C, high-density lipoprotein cholesterol; ITT, intention-to-treat; LLT, lipid-lowering therapy. a Usual care options included continuing on maximally tolerated dose of statins (or no statin if intolerant) with no additional LLT, or with the addition of ezetimibe, fenofibrate, omega-3 fatty acids, or nicotinic acid
The frequency of treatment-emergent adverse events (TEAEs) was similar in the alirocumab and usual care groups (67.2% vs 70.7%, respectively; Table 2). Treatment-emergent serious adverse events occurred in 6.3% and 5.2% of individuals receiving alirocumab and usual care, respectively. No deaths occurred in either treatment group. Discontinuations due to adverse events were similar in the alirocumab and usual care groups (3.1% vs 3.4%, respectively; Table 2). TEAEs occurring in ≥ 2% of individuals are also shown in Table 2. The most common TEAEs in the alirocumab group were urinary tract infection (8.6%), viral upper respiratory tract infection (5.5%), and influenza and diarrhea (both 4.7%). The most common TEAEs in the usual care group were bronchitis (8.6%), diarrhea (6.9%), and arthralgia (6.9%).
Table 2.
Overview of TEAEs (safety population)
| n (%) | Alirocumab (n = 128) | Usual carea (n = 58) |
|---|---|---|
| Any TEAE | 86 (67.2) | 41 (70.7) |
| Any treatment-emergent SAE | 8 (6.3) | 3 (5.2) |
| Any TEAE leading to death | 0 | 0 |
| Any TEAE leading to permanent treatment discontinuation | 4 (3.1) | 2 (3.4) |
| TEAEs occurring in ≥ 2% of individuals (preferred level term)b | ||
| Anemia | 3 (2.3) | 0 |
| Arthralgia | 2 (1.6) | 4 (6.9) |
| Back pain | 1 (0.8) | 2 (3.4) |
| Bronchitis | 1 (0.8) | 5 (8.6) |
| Cellulitis | 3 (2.3) | 0 |
| Cough | 1 (0.8) | 3 (5.2) |
| Diarrhea | 6 (4.7) | 4 (6.9) |
| Dizziness | 2 (1.6) | 2 (3.4) |
| Dyspnea | 1 (0.8) | 2 (3.4) |
| Fall | 3 (2.3) | 2 (3.4) |
| Fatigue | 5 (3.9) | 1 (1.7) |
| Headache | 2 (1.6) | 3 (5.2) |
| Hyperinsulinemic hypoglycemia | 3 (2.3) | 0 |
| Hypoglycemia | 2 (1.6) | 2 (3.4) |
| Hypotension | 1 (0.8) | 2 (3.4) |
| Influenza | 6 (4.7) | 2 (3.4) |
| Injection-site bruising | 3 (2.3) | 0 |
| Injection-site pruritus | 4 (3.1) | 0 |
| Injection-site reaction | 5 (3.9) | 0 |
| Muscle spasms | 3 (2.3) | 1 (1.7) |
| Musculoskeletal pain | 4 (3.1) | 2 (3.4) |
| Myalgia | 3 (2.3) | 1 (1.7) |
| Nausea | 4 (3.1) | 2 (3.4) |
| Osteoarthritis | 0 | 2 (3.4) |
| Pain in extremity | 1 (0.8) | 2 (3.4) |
| Sinusitis | 1 (0.8) | 2 (3.4) |
| Type 2 diabetes mellitus | 1 (0.8) | 2 (3.4) |
| Upper respiratory tract infection | 5 (3.9) | 2 (3.4) |
| Urinary tract infection | 11 (8.6) | 2 (3.4) |
| Viral upper respiratory tract infection | 7 (5.5) | 1 (1.7) |
| Vitamin D deficiency | 0 | 2 (3.4) |
SAE serious adverse event, TEAE treatment-emergent adverse event
aOptions included ezetimibe, fenofibrate, no additional lipid-lowering therapy, omega-3 fatty acid, and nicotinic acid
bGiven in alphabetical order
Mean (SD) change from baseline at Week 24 in fasting plasma glucose was + 11.3 (51.5) mg/dL and + 2.9 (50.3) mg/dL, and in HbA1c was + 0.3 (0.7)% and + 0.3 (0.7)%, in the alirocumab and usual care groups, respectively. The number of antihyperglycemic agents being used was similar at baseline and Week 24 in both the alirocumab group [1.9 (1.0) and 2.0 (1.0), respectively] and the usual care group [2.0 (1.0) and 2.1 (1.0), respectively].
Discussion
Individuals with T2DM are at increased risk of ASCVD [1], and mixed dyslipidemia further increases this risk [3, 18]. A recent analysis of 9593 statin-treated adults in the US National Health and Nutrition Examination Surveys found that the prevalence of TGs < 150, 150–199, and ≥ 200 mg/dL was 68.4%, 16.2%, and 15.4%, respectively [19]. In those on statin therapy with TGs ≥ 200 mg/dL, approximately half a million ASCVD events were estimated to occur in the next 10 years, with an estimated 10-year ASCVD risk score of 14.4%, compared to 11.3% for those with TGs < 150 mg/dL [19]. Furthermore, individuals with low HDL-C levels, despite receiving statin therapy, have been shown to have higher residual cardiovascular risk [20, 21]. There is therefore an opportunity for cardiovascular outcomes to be improved in individuals with T2DM and dyslipidemia who are receiving statin therapy.
Current approaches to reducing cardiovascular risk are tackling production of TG or ApoB particles [22, 23]. An alternative way to reduce residual risk is to reduce atherogenic lipoproteins. We tested this hypothesis in this subgroup of individuals with T2DM, elevated TGs, and low HDL-C. In these individuals, alirocumab significantly reduced LDL-C, non-HDL-C, ApoB, Lp(a), and LDL particle number compared with usual care. These results were comparable with the primary trial [14]; however, this analysis provides insight to the effects of alirocumab in the subgroup of individuals with high TG and low HDL-C despite statins, and who have higher residual cardiovascular risk than those without dyslipidemia. Most individuals receiving alirocumab achieved ApoB < 80 mg/dL and non-HDL-C < 100 mg/dL (67.9% and 60.9%, respectively). As these lipid parameters are associated with increased cardiovascular risk [2], the improvements observed with alirocumab may result in decreased cardiovascular risk. Similar findings have been obtained with evolocumab: the BANTING trial (NCT02739984) demonstrated that evolocumab significantly reduced LDL-C and non-HDL-C compared with placebo in adults with T2DM and hypercholesterolemia/dyslipidemia on a maximally tolerated oral dose of statin over 12 weeks [24].
Consistent with previous findings in participants with T2DM [14, 25], alirocumab resulted in non-significant TG reductions. These data confirm that blocking extra-cellular PCSK9 pathways with PCSK9 monoclonal antibodies does not affect hepatic ApoB production, and that the modest reduction in TGs is likely due to an increased uptake/catabolism of large very-low-density lipoprotein particles through the LDL receptor [26]. In previous studies with gemfibrozil in the Helsinki Heart Study [27] and fenofibrate in the ACCORD trial [15], TG lowering was generally not associated with overall cardiovascular benefit, but improvements were observed in subgroups with high TGs and low HDL-C (Helsinki Heart Study: TGs > 200 mg/dL, LDL-C/HDL-C ratio > 5.0; ACCORD: TGs ≥ 204 mg/dL, HDL-C ≤ 34 mg/dL).
This post hoc analysis provides useful data for comparison with several recently completed or ongoing cardiovascular outcome trials with similar thresholds for TGs and HDL-C. The REDUCE-IT trial demonstrated a reduction in cardiovascular events with 4 g of icosapent ethyl versus placebo (hazard ratio, 0.75; 95% confidence interval, 0.68–0.83; P < 0.001) over a median follow up of 4.9 years [17, 28]. In the ASCEND trial (NCT00135226), 1 g of eicosapentaenoic acid once daily did not reduce the risk of cardiovascular events versus placebo [29]. Other trials are currently ongoing with 4 g of omega-3 carboxylic acids (STRENGTH, NCT02104817) and pemafibrate (PROMINENT, NCT03071692).
Consistent with previous studies, alirocumab was well tolerated in individuals with T2DM [14, 25, 30, 31]. In addition, no clinically relevant effect of alirocumab on change in glycemic parameters or in use of antihyperglycemic agents was observed, in accordance with previous data [32]. A recent study in patients with stable CAD demonstrated that low PCSK9 plasma levels are associated with low HDL-C, metabolic syndrome, obesity, insulin resistance, and diabetes, and diffuse non-obstructive coronary atherosclerosis [33]. However, studies to date have not shown an association between PCSK9 inhibitors and low HDL-C or increased risk of diabetes, metabolic syndrome, or obesity [34–36]. Furthermore, similar safety findings were observed with alirocumab across BMI subgroups, with no difference in percentage change from baseline in body weight observed between alirocumab and control at Weeks 12, 24, and 52 [34]. However, studies of longer duration are required to further assess these potential effects.
This subgroup analysis was limited by the post-randomization nature of post hoc analyses, the relatively low number of participants, and the short duration of the study (24 weeks). In addition, comparisons were made with no adjustment on the type I error rate. Although DM-DYSLIPIDEMIA was not designed to assess cardiovascular outcomes, these findings are supported by the results of the ODYSSEY OUTCOMES cardiovascular outcomes trial [37]. A prespecified analysis of the ODYSSEY OUTCOMES trial showed that alirocumab treatment targeting LDL-C 25–50 mg/dL produced approximately twice the absolute reduction in cardiovascular events in individuals with diabetes as in those without diabetes. In addition, alirocumab treatment did not increase the risk of new-onset diabetes [31]. Since the objective of this post hoc analysis was to analyze a subgroup of patients with TGs > 200 mg/dL and low HDL-C, no analysis by stratification by baseline TG levels were conducted; however, in the primary paper, reduction in non-HDL-C was similar at TG thresholds of < 150, 150–< 200, and ≥ 200 mg/dL [14].
Conclusions
In individuals with T2DM and mixed dyslipidemia, alirocumab significantly reduced LDL-C, non-HDL-C, ApoB, Lp(a), and LDL particle number, and significantly increased HDL-C, compared with usual care overall. Reduction with alirocumab in atherogenic lipids (ApoB and non-HDL) was greater than with ezetimibe, fenofibrate, or no additional LLT. In addition, alirocumab was effective for achieving target non-HDL-C and ApoB, compared with usual care, in this high cardiovascular risk subgroup population. Consistent with previous studies, alirocumab was generally well tolerated.
Acknowledgements
The authors would like to thank the study participants, their families, and all investigators involved in the original studies.
Abbreviations
- ApoB
Apolipoprotein B
- ASCVD
Atherosclerotic cardiovascular disease
- HbA1c
Glycated hemoglobin
- HDL-C
High-density lipoprotein cholesterol
- LDL
Low-density lipoprotein
- LDL-C
Low-density lipoprotein cholesterol
- LLT
Lipid-lowering therapy
- Lp(a)
Lipoprotein (a)
- LS
Least squares
- PCSK9
Proprotein convertase subtilisin/kexin type 9
- SD
Standard deviation
- SE
Standard error
- T2DM
Type 2 diabetes mellitus
- TEAE
Treatment-emergent adverse event
- TG
Triglyceride
- TRL
Triglyceride-rich lipoprotein
- TRL-C
Triglyceride-rich lipoprotein cholesterol
Authors’ contributions
All authors contributed to the study design or concept and the interpretation of the data, and critically reviewed and edited the manuscript. In addition, FJT and SPD were investigators who contributed to the data acquisition. All authors read and approved the final manuscript.
Funding
This sub-analysis and the ODYSSEY trials were funded by Sanofi and Regeneron Pharmaceuticals, Inc. Medical writing assistance and editorial support, under the direction of the authors, was provided by Kate Carolan, PhD, of Prime (Knutsford, UK), funded by Sanofi and Regeneron Pharmaceuticals, Inc. according to Good Publication Practice guidelines (Link). The sponsors were involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. The authors had unrestricted access to study data, were responsible for all content and editorial decisions, and received no honoraria related to the development of this publication.
Availability of data and materials
Data sharing is not applicable to this article as it reports secondary analyses from primary data previously published, as cited in the reference list.
Ethics approval and consent to participate
The ODYSSEY DM-DYSLIPIDEMIA clinical trial was conducted in accordance with the ethical principles laid down by the 18th World Medical Assembly (Helsinki, 1964) and all applicable amendments laid down by the World Medical Assemblies, and the International Conference on Harmonization guidelines. The trial protocol was approved by the relevant institutional review boards or independent ethics committees, and all participating individuals provided written informed consent.
Consent for publication
Not applicable.
Competing interests
HMC has received speaker’s bureau and consultant/advisory board fees from Sanofi Aventis, Regeneron Pharmaceuticals, Inc., Novartis Pharmaceuticals, Novo-Nordisk, and Eli Lilly; has received non-binding research support from Pfizer Inc., AstraZeneca LP, and Novo-Nordisk; and is a shareholder of Roche Pharmaceuticals and Bayer. LAL has received grants and personal fees from Amgen, AstraZeneca, Eli Lilly and Company, Esperion, HLS, Merck, Regeneron Pharmaceuticals, Inc., and Sanofi; and grants from Kowa and the Medicines Company. DM-W has received consultant fees/honoraria from Amgen Inc., AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc., Merck & Co., Inc., Novartis Corporation, Novo Nordisk Inc., and Sanofi-Aventis; and participated in speaker’s bureau for Amgen Inc., AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc., Eli Lilly and Company, Merck & Co., Inc., Novartis Corporation, Novo Nordisk Inc., and Sanofi-Aventis. BC has received research funding and personal fees from Sanofi and Regeneron Pharmaceuticals, Inc.; research funding from Amgen and Pfizer; and honoraria from Amgen, Akcea, AstraZeneca, Pierre Fabre, Genfit, Gilead, Eli Lilly and Company, MSD (Merck & Co.), Novo Nordisk, Sanofi, and Servier. KKR has received research grants from Pfizer Inc., Amgen, Sanofi, Regeneron Pharmaceuticals, Inc., and MSD; honoraria from Dr Reddy’s Laboratories, Zuellig Pharma, Sanofi, Amgen, Boehringer Ingelheim, Novo Nordisk, and Pfizer Inc.; and consultant/advisory board fees from Medco, AstraZeneca, Resverlogix, Kowa, Abbvie, Sanofi, Amgen, Boehringer Ingelheim, Esperion, Akcea, and Regeneron Pharmaceuticals, Inc. FJT has received speaker’s bureau and consultant/advisory board fees from AstraZeneca, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, MSD (Merck & Co.), Novartis Pharmaceuticals Co., Novo Nordisk, and Sanofi-Aventis. CD and AL are employees of and stockholders in Sanofi. MKI and RS are employees of and stockholders in Regeneron Pharmaceuticals, Inc. SDP has received research funding from AstraZeneca, Boehringer Ingelheim, Novartis Pharmaceuticals Co., and MSD (Merck & Co.); and has been a consultant for or received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Laboratoires Servier, MSD (Merck & Co.), Novartis Pharmaceuticals Co., Novo Nordisk, Sanofi-Aventis, and Takeda Pharmaceuticals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bays HE, Jones PH, Orringer CE, Brown WV, Jacobson TA. National Lipid Association annual summary of clinical lipidology 2016. J Clin Lipidol. 2016;10:S1–S43. doi: 10.1016/j.jacl.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Mach F, Baigent C, Catapano AL, et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019 doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 5.Verges B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58:886–899. doi: 10.1007/s00125-015-3525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 7.Ference BA, Kastelein JJP, Ray KK, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321:364–373. doi: 10.1001/jama.2018.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson JG, Wang S, Jacobson TA. Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol. 2012;110:1468–1476. doi: 10.1016/j.amjcard.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Thanassoulis G, Williams K, Ye K, et al. Relations of change in plasma levels of LDL-C, non-HDL-C and apoB with risk reduction from statin therapy: a meta-analysis of randomized trials. J Am Heart Assoc. 2014;3:e000759. doi: 10.1161/JAHA.113.000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Association for Cardiovascular Prevention & Rehabilitation. Reiner Z, Catapano AL, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 11.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Mancini GB, Hegele RA, Leiter LA. Dyslipidemia. Can J Diabetes. 2013;37(Suppl 1):S110–S116. doi: 10.1016/j.jcjd.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2019 executive summary. Endocr Pract. 2019;25:69–100. doi: 10.4158/CS-2018-0535. [DOI] [PubMed] [Google Scholar]
- 13.Müller-Wieland D, Leiter LA, Cariou B, et al. Design and rationale of the ODYSSEY DM-DYSLIPIDEMIA trial: lipid-lowering efficacy and safety of alirocumab in individuals with type 2 diabetes and mixed dyslipidaemia at high cardiovascular risk. Cardiovasc Diabetol. 2017;16:70. doi: 10.1186/s12933-017-0552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray KK, Leiter LA, Muller-Wieland D, et al. Alirocumab vs usual lipid-lowering care as add-on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: the ODYSSEY DM-DYSLIPIDEMIA randomized trial. Diabetes Obes Metab. 2018;20:1479–1489. doi: 10.1111/dom.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ACCORD Study Group. Ginsberg HN, Elam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott R, O’Brien R, Fulcher G, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 18.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 19.Fan W, Philip S, Granowitz C, Toth PP, Wong ND. Hypertriglyceridemia in statin-treated US adults: the National Health and Nutrition Examination Survey. J Clin Lipidol. 2019;13:100–108. doi: 10.1016/j.jacl.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 21.Vallejo-Vaz AJ, Fayyad R, Boekholdt SM, et al. Triglyceride-rich lipoprotein cholesterol and risk of cardiovascular events among patients receiving statin therapy in the TNT trial. Circulation. 2018;138:770–781. doi: 10.1161/CIRCULATIONAHA.117.032318. [DOI] [PubMed] [Google Scholar]
- 22.Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41:1281–1288. doi: 10.1002/clc.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pradhan AD, Paynter NP, Everett BM, et al. Rationale and design of the pemafibrate to reduce cardiovascular outcomes by reducing triglycerides in patients with diabetes (PROMINENT) study. Am Heart J. 2018;206:80–93. doi: 10.1016/j.ahj.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Rosenson RS, Daviglus ML, Handelsman Y, et al. Efficacy and safety of evolocumab in individuals with type 2 diabetes mellitus: primary results of the randomised controlled BANTING study. Diabetologia. 2019;62:948–958. doi: 10.1007/s00125-019-4856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leiter LA, Cariou B, Muller-Wieland D, et al. Efficacy and safety of alirocumab in insulin-treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: the ODYSSEY DM-INSULIN randomized trial. Diabetes Obes Metab. 2017;19:1781–1792. doi: 10.1111/dom.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dijk W, Le May C, Cariou B. Beyond LDL: what Role for PCSK9 in triglyceride-rich lipoprotein metabolism? Trends Endocrinol Metab. 2018;29:420–434. doi: 10.1016/j.tem.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt DL, Steg PG, Brinton EA, et al. Rationale and design of REDUCE-IT: reduction of cardiovascular events with icosapent ethyl-intervention trial. Clin Cardiol. 2017;40:138–148. doi: 10.1002/clc.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ASCEND Study Collaborative Group. Bowman L, Mafham M, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379:1540–1550. doi: 10.1056/NEJMoa1804989. [DOI] [PubMed] [Google Scholar]
- 30.Leiter LA, Tinahones FJ, Karalis DG, et al. Alirocumab safety in people with and without diabetes mellitus: pooled data from 14 ODYSSEY trials. Diabet Med. 2018;35:1742–1751. doi: 10.1111/dme.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray KK, Colhoun HM, Szarek M, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:618–628. doi: 10.1016/S2213-8587(19)30158-5. [DOI] [PubMed] [Google Scholar]
- 32.Colhoun HM, Ginsberg HN, Robinson JG, et al. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY Phase 3 studies. Eur Heart J. 2016;37:2981–2989. doi: 10.1093/eurheartj/ehw292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caselli C, Del Turco S, Ragusa R, et al. Association of PCSK9 plasma levels with metabolic patterns and coronary atherosclerosis in patients with stable angina. Cardiovasc Diabetol. 2019;18:144. doi: 10.1186/s12933-019-0949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tinahones FJ, Laufs U, Cariou B, et al. Alirocumab efficacy and safety by body mass index: a pooled analysis from 10 Phase 3 ODYSSEY trials. Diabetes Metab. 2019 doi: 10.1016/j.diabet.2019.101120:101120. [DOI] [PubMed] [Google Scholar]
- 35.Henry RR, Muller-Wieland D, Taub PR, et al. Effect of alirocumab on lipids and lipoproteins in individuals with metabolic syndrome without diabetes: pooled data from 10 phase 3 trials. Diabetes Obes Metab. 2018;20:1632–1641. doi: 10.1111/dom.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leiter LA, Muller-Wieland D, Baccara-Dinet MT, Letierce A, Samuel R, Cariou B. Efficacy and safety of alirocumab in people with prediabetes vs those with normoglycaemia at baseline: a pooled analysis of 10 phase III ODYSSEY clinical trials. Diabet Med. 2018;35:121–130. doi: 10.1111/dme.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as it reports secondary analyses from primary data previously published, as cited in the reference list.