Abstract
Background
Vitamin D (VD) may increase sirtuin 1 (SIRT1) and subsequently PPAR-γ coactivator 1α (PGC-1α) and irisin levels and these improvements may reduce insulin resistance (IR). The aim was to assess the effects of vitamin D supplementation on SIRT1, irisin, and IR in overweight/obese type 2 diabetes (T2D) patients.
Methods
Ninety T2D males and females were recruited as a clinical trial study (mean of age and body mass index (BMI) of intervention and placebo groups were 50.05 ± 10.17 and 50.36 ± 10.2 yrs. and 31.37 ± 3.4 and 30.43 ± 3.2 kg/m2, respectively). The inclusion criteria were T2D, VD deficient, BMI > 25 kg/m2, and serum HbA1c < 8.5%. The exclusion criteria were using vitamin and mineral supplements, having any acute disease, recent modifying dose or type of drugs. The supplementation was 50,000 IU/week VD or placebo for 8 weeks. The demographic characteristics, anthropometrics, dietary intakes and physical activity status, sun exposure status, fasting blood sugar (FBS) and insulin, glycosylated hemoglobin (HbA1c), irisin, SIRT1, 25-hydroxy D3 (25(OH)VD), homeostasis model assessment of insulin resistance (HOMA-IR), and quantitative insulin sensitivity check index (QUICKI) were determined. The significant P-value was ≤0.05.
Results
The increase of serum VD, SIRT1, and irisin in the intervention group was significant (p < 0.001). HbA1c was decreased significantly by 1%. The changes in the other glucose indices (FBS, insulin, and IR) were non-significant.
Conclusions
VD supplementation may improve T2D by decreasing HbA1c and increasing SIRT1 and irisin in VD deficient T2D patients. Further trials are suggested.
Trial registration
Iranian Registry of Clinical Trials, IRCT201604202365N11. Registered 21/08/2016, http://en.irct.ir/trial/2019.
Keywords: Vitamin D; SIRT1; Irisin; Glucose indices, type 2 diabetes; Overweight/obesity
Background
Diabetes mellitus is a metabolic disease characterized by hyperglycemia resulting from defects in insulin secretion or action or both [1]. Obesity is an important risk factor increases cellular oxidative stress, insulin resistance (IR), and pancreatic beta cells malfunction [2]. In addition, vitamin D (VD) deficiency is highly prevalent in many societies and ages. According to Iranian Multi-Centric Osteoporosis Study (IMOS), 2009, 72.1% of men and 75.1% of women had mild to severe VD deficiency. Globally, about 50% of the population are VD deficient. It is estimated that about 1 billion people all over the world are affected by different degrees of vitamin D deficiency [3–6].
According to recent studies, IR as the most important cause of T2D was inversely related to VD [6–8]. Moreover, serum 25-OH VD is significantly lower among diabetic patients compared with non-diabetic ones [9, 10]. Also, the relationship between serum 25-OH VD and insulin secretion was significant according to some studies [11].
The hyperglycemia, hyperlipidemia, and inflammatory cytokines in diabetes can likely increase oxidative stress and decrease silent mating type information regulation 2 homolog 1 (sirtuin 1 [SIRT1]) levels [12].
Sirtuins as class III histone deacetylases are categorized into 7 types among which, SIRT1 has been studied more than other categories in humans. SIRT1 may play a role in chronic diseases such as diabetes. It affects glucose metabolism in the liver, pancreas, muscle and adipose tissue. The expression and activity of SIRT1 are reduced in hunger, calorie restriction, and some chronic diseases such as diabetes. Accordingly, SIRT1 activation may improve glucose indices and mitochondria function [13–16].
In addition, VD may increase human endothelial SIRT1 level and activity that can be downregulated by Hydrogen Peroxide. Anti-cancer effects of VD are related to VD receptor (VDR) and Forkhead box O (FOXO) protein interaction, phosphorylation stimulation, and SIRT1. The insulin-sensitizing effect of VD is likely in relation to SIRT1 [17].
Irisin is a myokine regulates energy metabolism in exercise and improves insulin resistance [18]. It is secreted in response to Peroxisome Proliferator-Activated Receptor (PPAR)-γ Coactivator (PGC-1α), and low irisin levels may decrease expression and activation of PGC-1α. Also, T2D patients have low serum levels of irisin and PGC-1α [19–22].
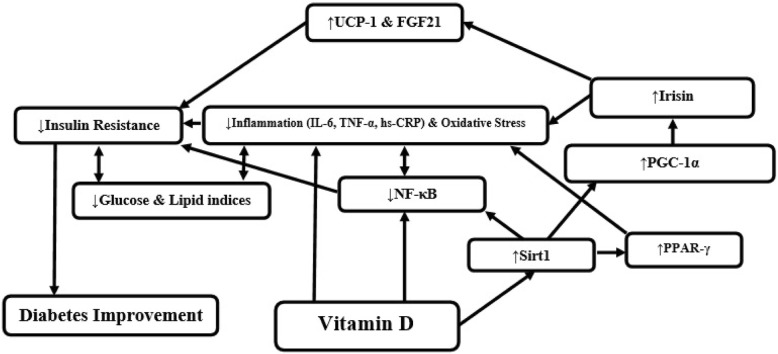
According to the separate studies, VD may increase SIRT1, and subsequently PGC-1α and Irisin. These changes can improve insulin resistance. Thus, VD may decrease insulin resistance by increasing SIRT1 and Irisin (Fig. 1). So, this trial was planned to assess the effects of vitamin D on serum glucose, SIRT1, and Irisin levels in overweight/obese T2D patients.
Fig. 1.
A schematic for the relationship of vitamin D (VD), sirtuin1 (SIRT1), PPAR-gamma coactivator 1-alpha (PGC-1α), insulin resistance (IR), Irisin, and diabetes. (UCP1: Uncoupling Protein 1; FGF21: Fibroblast Growth Factor 21; PPAR-γ: Peroxisome Proliferation Activated Receptor-Gamma; IL-6: Interleukin 6; TNF-α: Tumour Necrosis Factor-Alpha; hs-CRP: high-sensitivity C-Reactive Protein; NF-κB: Nuclear Factor kappa-light-chain-enhancer of activated B cells)
Methods
Study design
This double-blinded randomized clinical trial was conducted on 90 obese T2D patients at Shahid Beheshti Diabetes Clinic, Bandar-E-Anzali, Gilan, Iran. The study lasted from the beginning of autumn 2016 to the end of autumn 2016.
The participants signed a “written informed consent form” at the beginning. Ninety patients were randomly divided into placebo or intervention groups with a 1:1 ratio. The intervention group took 8 VD pearls (50,000 IU/week, Zahravi Co®), and the placebo group took similar pearls containing oral paraffin without VD (50,000 IU/week, Zahravi Co®). The duration of the intervention was 8 weeks. During the intervention, the compliance status including follow-up, side effects, and complications was weekly checked by calling. At the end, the number of the used pearls, returned blisters, and packs were recorded.
Inclusion criteria were 25–65 years old, having T2D, serum HbA1c < 8.5%, 25 kg/m2 ≤ BMI, and the written informed consent form. Exclusion criteria were the inability to cooperating, acute diseases influencing the intervention, intake of the antioxidant and multivitamin-mineral supplements during the past 3-months so far, changing the type and dose of T2D medications, and taking less than about 90% of the study’s supplements.
The outcomes were serum 25-OH VD, SIRT1, Irisin, HbA1c, IR indexes, FBS, and serum insulin.
Measures
At the beginning and end, the demographic characteristics, anthropometrics, and dietary intakes by two 24-h food recall (a weekend and a working day) were determined. Physical activity was measured by a short form of the international physical activity questionnaire (IPAQ) [23]. The sun exposure status was assessed using a valid questionnaire [24]. At the beginning and end, the blood taking from the brachial vein for measuring the serum factors was done. Serum glucose was measured by the Olympus® device and Delta® kits.
The glycosylated hemoglobin was determined using Nycocard® kits in Nyco Card Reader® (Made in Norway). Insulin was measured by the Cobas e 411® device and Roche® kit. Irisin was measured by Zellbio GmbH kit Cat.No: ZB-13253 J-H9648 (Germany)®, with a normal range of 2–80 ng/ml and sensitivity of 0.1 ng/ml. SIRT1 was measured by Zellbio GmbH kit Cat.No: ZB-12557 J-H9648(Germany)®, with a normal range of 5–160 ng/ml and sensitivity of 1 ng/ml. ELISA method; ELISA reader (Model: Tecan A-5082 Made in Austria®).
25-OH VD was measured by 25-(OH) D3 quantitative diagnostic kit (Immuno Diagnostic Systems (IDS), UK)®, which is an Enzyme Immunoassay kit with a sensitivity of 5 nmol/L. The ELISA method and ELISA reader (Model: Tecan A-5082 Made in Austria®) were used for Irisin, SIRT1, and 25-OH D3. The insulin resistance was determined by HOMA-IR and QUICKI indexes [25, 26] as the following formula:
Statistical analysis
According to the Baziar et al study with 1.33 mean difference of HOMA-IR, 1.18 standard deviation for vitamin D group, and 2.84 standard deviations for placebo group [27], the sample size was calculated 45 peoples in any groups using G-Power software and considering α = 5%, β = 20%, and dropout percentage = 12.5%.
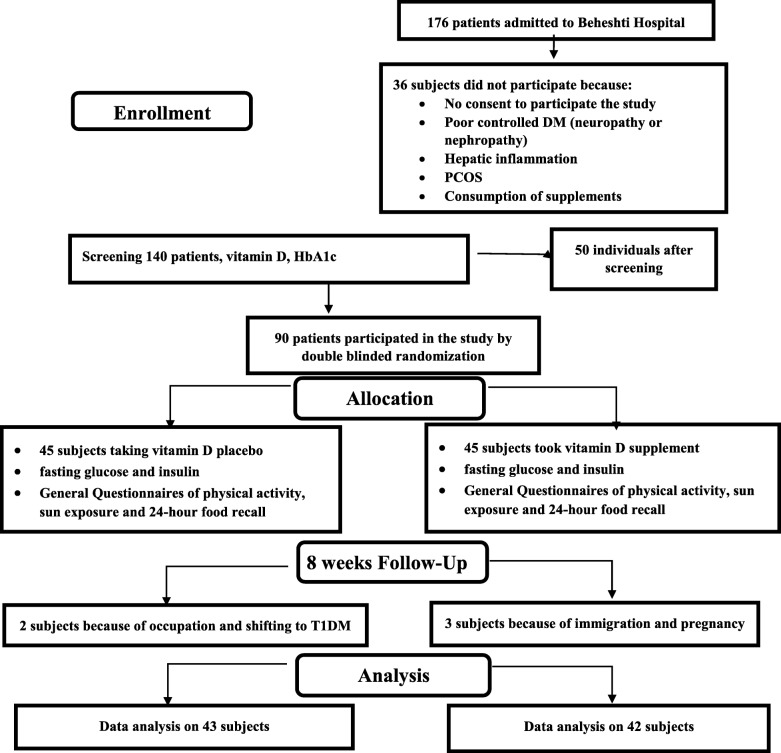
Randomization was done using 23 quadratic blocks and www.sealedenvelope.com. The blinding was according to unique codes produced by software for each subject. Non-parametric, t-test, Mann-Whitney, Chi-square, and ANCOVA tests and SPSS16 statistical software were used for the analyses. The final analysis model was adjusted for the baseline values as potential confounders. P-value < 0.05 was statistically considered significant. The dietary intakes were analyzed by the Nutritionist(IV) software. The modified intention-to-treat (m-ITT) method [28] was used for the missing data (3 participants in the intervention group and 2 ones in the placebo group) (Fig. 2).
Fig. 2.
Flow diagram of participants with type 2 diabetes and overweight/obesity grade 1
This clinical trial was approved by the ethics committee of Iran University of Medical Sciences (IUMS) (Code: IR.IUMS.REC.1395.9223475201), and Iranian Registry of Clinical Trials (IRCT) (Registration No: IRCT201604202365N11). An informed consent form (in Persian) obtained from all the participants. Participation was free, and a participant could withdraw at whatever point the person feels he/she was unable to continue. The dose of VD was for insufficient VD status that side-effects had not been reported previously [29]. All of the ethical codes were according to the Helsinki agreement [30]. The personal information of participants was kept secret before, during, and after the trial. After measurements, at the end, a VD supplement was given to the placebo group according to the protocol.
Results
According to the flow diagram of participants, a total of 176 T2D patients referred to the diabetes clinic were screened (informed consent, medical history, questionnaire), of whom 86 patients did not meet the eligibility criteria. Ninety participants were randomized that 5 patients did not complete the study (for personal reasons; VD group n = 3; placebo n = 2). Thus 85 participants completed the trial (VD group n = 42; placebo n = 43) and the data of them were analyzed (Fig. 2).
Most of the participants were women with type 1 obesity (30 ≤ BMI < 35 [BMI = 34.25 kg/m2, weight: 59–115 kg, height: 163–184.5 cm]), the mean age of 50 years (25–65 year), recent diagnosed T2D nearly less than 6 years, and consumption of maximum 2 types of anti-hyperglycemic medications. The baseline characteristics including age, gender, weight, height, BMI, duration of diabetes, type and dose of medications, and employment status were similar between two groups before intervention (Table 1).
Table 1.
The mean and standard deviation of the baseline characteristics of type 2 diabetes patients
| Variable | Category | Group | P-value | |
|---|---|---|---|---|
| Vitamin D (N = 42) |
Placebo (N = 43) |
|||
| Age (Y) | – | 50.36 (10.2) | 50.05 (10.7) | 0.892* |
| Weight (kg) | – | 77.38 (10.9) | 79.27 (11.6) | 0.442* |
| Height (cm) | – | 159.35 (8.0) | 158.77 (7.6) | 0.736* |
| BMI (kg/m 2)) | – | 30.43 (3.2) | 31.37 (3.4) | 0.194* |
| Disease duration (months) | – | 65.52 (38.5) | 67.02 (35.4) | 0.664* |
| Medications (per day) | Metformin (500 mg/d) | 2.11 (1.13) | 2.51 (1.26) | 0.135* |
| Glibenclamide (5 mg/d) | 1.31 (0.94) | 0.95 (0.78) | ||
| Gender | Male | 7.00 (16.7) | 8.00 (18.6) | 0.815** |
| Female | 35.00 (83.3) | 35.00 (81.4) | ||
| Employment status | Unemployed | 32.00 (76.2) | 32.00 (74.4) | 0.850** |
| employed | 10.00 (23.8) | 11.00 (25.6) | ||
*Independent t-test; **Chi-square test, Y Year, kg Kilogram, cm Centimeter, BMI Body mass index, mg Milligram
Physical activity status (MET-min/week) was not significantly different between the two groups. Within the placebo group, weight and BMI decreased significantly (P < 0.05). However, these differences were not significant between the two groups (P > 0.05) (Table 2).
Table 2.
The mean and standard deviation of physical activity, weight, and BMI in type 2 diabetes patients
| Variable | Vitamin D (N = 42) |
Placebo (N = 43) |
P-value* | |
|---|---|---|---|---|
| Physical activity (MET. Minutes/week) | Baseline | 685.50 (99–34,755) | 462.00 (99–28,560) | 0.346 |
| After intervention | 693.00 (99–33,831) | 462.00 (99–28,659) | 0.333 | |
| P-value** | 0.104 | 0.811 | ||
| Weight (kg) | Baseline | 77.38 (10.92) | 79.27 (11.67) | 0.442 |
| After intervention | 77.13 (10.81) | 78.77 (11.53) | 0.502 | |
| P-value** | 0.076 | 0.011 | ||
| BMI (kg/m2) | Baseline | 30.43 (3.23) | 31.37 (3.40) | 0.194 |
| After intervention | 30.34 (3.32) | 31.18 (3.42) | 0.254 | |
| P-value** | 0.097 | 0.009 |
*Independent t-test, **Paired t-test, kg Kilogram, cm Centimeter, BMI Body mass index, mg Milligram, MET Metabolic equivalent
Energy, macronutrients, micronutrients, and antioxidant intakes were not significantly different within and between groups (Tables 3 and 4). Within the VD group, zinc intake increased significantly (P < 0.05), but within the placebo group, zinc intake decreased significantly (P < 0.05). However, this difference was not significant between the two groups (P > 0.05, Table 4).
Table 3.
The mean and standard deviation of energy intake, macronutrients, and micronutrients before and after intervention in type 2 diabetes patients
| Vitamin D (N = 42) |
Placebo (N = 43) |
P-value* | ||
|---|---|---|---|---|
| Energy (kcal/day) | Baseline | 1638.59 (377.82) | 1578.38 (399.64) | 0.478 |
| After intervention | 1701.36 (485.61) | 1564.68 (392.75) | 0.157 | |
| P-value** | 0.381 | 0.871 | ||
| Protein (g/day) | Baseline | 82.27 (23.34) | 78.79 (31.82) | 0.568 |
| After intervention | 89.31 (35.83) | 80.31 (32.16) | 0.226 | |
| P-value** | 0.190 | 0.823 | ||
| Carbohydrate (g/day) | Baseline | 232.69 (67.62) | 220.85 (54.71) | 0.377 |
| After intervention | 231.41 (62.34) | 214.21 (51.19) | 0.168 | |
| P-value** | 0.891 | 0.522 | ||
| Total fat (g/day) | Baseline | 47.61 (11.25) | 46.83 (12.22) | 0.760 |
| After intervention | 51.31 (17.33) | 47.41 (14.13) | 0.258 | |
| P-value** | 0.199 | 0.830 | ||
| SFA (g/day) | Baseline | 16.10 (4.58) | 15.35 (3.75) | 0.413 |
| After intervention | 16.01 (6.10) | 15.74 (4.54) | 0.819 | |
| P-value** | 0.913 | 0.639 | ||
| MUFA (g/day) | Baseline | 16.06 (3.85) | 15.59 (5.07) | 0.636 |
| After intervention | 17.13 (5.85) | 15.56 (4.86) | 0.182 | |
| P-value** | 0.265 | 0.976 | ||
| PUFA (g/day) | Baseline | 9.98 (3.04) | 10.04 (3.45) | 0.836 |
| After intervention | 10.49 (2.47) | 9.97 (3.86) | 0.480 | |
| P-value** | 0.365 | 0.919 | ||
| Calcium (mg/day) | Baseline | 835.33 (279.75) | 838.41 (301.51) | 0.961 |
| After intervention | 804.44 (324.89) | 833.63 (292.95) | 0.665 | |
| P-value** | 0.487 | 0.929 | ||
| Phosphorus (mg/day) | Baseline | 1444.29 (402.18) | 1364.80 (372.84) | 0.347 |
| After intervention | 1459.15 (467.88) | 1355.23 (388.99) | 0.268 | |
| P-value** | 0.814 | 0.897 | ||
| Vitamin D (μg/day) | Baseline | 1.73 (1.28) | 1.84 (1.41) | 0.710 |
| After intervention | 1.58 (1.18) | 1.64 (1.22) | 0.835 | |
| P-value** | 0.386 | 0.334 | ||
| Total fiber (g/day) | Baseline | 29.23 (10.67) | 26.58 (7.56) | 0.192 |
| After intervention | 29.96 (8.25) | 25.91 (9.05) | 0.578 | |
| P-value** | 0.127 | 0.603 |
*Independent t-test, **Paired t-test, SFA Saturated fatty acid, MUFA Mono unsaturated fatty acid, PUFA Poly unsaturated fatty acid
Table 4.
The mean and standard deviation of the dietary antioxidants before and after intervention in type 2 diabetes patients
| Vitamin D (N = 42) |
Placebo (N = 43) |
P-value* | ||||
|---|---|---|---|---|---|---|
| Beta-Carotene (micg/day) | Baseline | 3780.76 | 3117.63 | 5440.75 | 6623.31 | 0.242 |
| After intervention | 4379.33 | 4073.18 | 4419.51 | 4235.22 | ||
| P-value** | 0.362 | 0.963 | ||||
| Vitamin A (micg/day) | Baseline | 878.74 | 663.17 | 893.10 | 723.97 | 0.952 |
| After intervention | 757.20 | 433.10 | 781.86 | 489.78 | ||
| P-value** | 0.059 | 0.947 | ||||
| Vitamin E (mg/day) | Baseline | 15.79 | 4.15 | 15.96 | 4.63 | 0.951 |
| After intervention | 15.42 | 4.18 | 15.67 | 4.13 | ||
| P-value** | 0.882 | 0.522 | ||||
| Vitamin C (mg/day) | Baseline | 122.87 | 81.12 | 139.18 | 108.88 | 0.596 |
| After intervention | 120.19 | 74.06 | 123.56 | 76.57 | ||
| P-value** | 0.101 | 0.528 | ||||
| Selenium (mg/day) | Baseline | 238.09 | 54.43 | 235.76 | 88.75 | 0.386 |
| After intervention | 213.06 | 61.64 | 230.75 | 83.54 | ||
| P-value** | 0.696 | 0.887 | ||||
| Zinc (mg/day) | Baseline | 20.61 | 5.82 | 20.46 | 5.85 | 0.306 |
| After intervention | 20.96 | 6.40 | 19.39 | 6.05 | ||
| P-value** | 0.003 | 0.005 | ||||
| Manganese (mg/day) | Baseline | 12.57 | 3.61 | 12.83 | 5.54 | 0.381 |
| After intervention | 11.48 | 4.04 | 12.79 | 4.62 | ||
| P-value** | 0.240 | 0.052 | ||||
| Copper (micg/day) | Baseline | 2.79 | 0.75 | 2.67 | 1.05 | 0.268 |
| After intervention | 2.54 | 0.75 | 2.73 | 0.93 | ||
| P-value** | 0.364 | 0.988 | ||||
| Magnesium (mg/day) | Baseline | 717.30 | 168.48 | 725.50 | 247.19 | 0.461 |
| After intervention | 680.08 | 191.00 | 732.94 | 224.49 | ||
| P-value** | 0.695 | 0.284 | ||||
*Independent t-test, **Paired t-test
According to the final analysis model, the differences of serum VD, SIRT1, Irisin, and HbA1c were significant within and between groups (P < 0.05). Serum VD level was doubled compared to the baseline level, and HbA1c decreased by 1% among the VD group (Table 5). Within the VD group, irisin and SIRT1 increased significantly (P < 0.05). The differences in fasting glucose and insulin, HOMA-IR, and QUICKI were not significant between the two groups (Table 6). The sun-exposure status was not significantly different within and between groups.
Table 5.
The mean and standard deviation of the primary variable before and after intervention in type 2 diabetes patients
| Vitamin D (N = 42) |
Placebo (N = 43) |
crude P-value* | Adjusted P-value*** | ||
|---|---|---|---|---|---|
| VitaminD (ng/ml) | Baseline | 17.24 (7.83) | 17.56 (7.82) | 0.853 | < 0.001 |
| After intervention | 38.86 (10.76) | 14.79 (7.08) | < 0.001 | ||
| P-value** | < 0.001 | < 0.001 | |||
| HbA1c (%) | Baseline | 7.51 (0.87) | 7.15 (1.12) | 0.103 | < 0.001 |
| After intervention | 6.76 (0.98) | 7.21 (1.11) | 0.051 | ||
| P-value** | < 0.001 | 0.657 | |||
| SIRT1 (ng/ml) | Baseline | 24.94 (4.38) | 25.57 (3.37) | 0.455 | < 0.001 |
| After intervention | 27.90 (5.58) | 23.40 (3.88) | < 0.001 | ||
| P-value** | < 0.001 | < 0.001 | |||
| Irisin (ng/ml) | Baseline | 18.15 (5.96) | 20.13 (7.17) | 0.171 | |
| After intervention | 21.99 (5.93) | 14.98 (2.76) | < 0.001 | < 0.001 | |
| P-value** | < 0.001 | < 0.001 | |||
| HOMA-IR (N) | Baseline | 5.36 (3.23) | 5.83 (3.09) | 0.499 | 0.421 |
| After intervention | 6.44 (3.73) | 7.39 (4.58) | 0.295 | ||
| P-value** | 0.006 | 0.003 | |||
| QUICKI (N) | Baseline | 0.76 (0.08) | 0.73 (0.06) | 0.149 | 0.645 |
| After intervention | 0.73 (0.07) | 0.71 (0.07) | 0.217 | ||
| P-value** | 0.005 | 0.003 |
*Independent t-test, **Paired t-test, ***ANCOVA
Table 6.
The mean and standard deviation of the secondary variable before and after intervention in type 2 diabetes patients
| Vitamin D (N = 42) |
Placebo (N = 43) |
P-value* | Adjusted P-value*** | ||
|---|---|---|---|---|---|
| FBS (mg/dl) | Baseline | 172.48 (62.16) | 178.28 (71.74) | 0.629 | 0.130 |
| After intervention | 175.52 (65.44) | 162.72 (61.36) | 0.355 | ||
| P-value** | 0.742 | 0.053 | |||
| Insulin (micIU/ml) | Baseline | 12.64 (5.94) | 14.09 (6.43) | 0.284 | 0.101 |
| After intervention | 15.31 (7.24) | 18.77 (9.02) | 0.055 | ||
| P-value** | 0.002 | < 0.001 |
*Independent t-test, **Paired t-test, ***ANCOVA
Safety
The patients reported no side effects associated with treatment.
Discussion
Vitamin D supplementation (50,000 IU/week) for 8 weeks increased serum VD levels in T2D patients with VD deficiency. Compared to placebo, VD increased SIRT1 and Irisin and decreased HbA1c by 1% significantly. The differences in fasting insulin and glucose, HOMA-IR, and QUICKI were not significant. Within the placebo group, serum VD levels decreased significantly.
The results of some studies of the effects of VD supplementation on diabetes showed controversy that the following is referred to some of them.
In some studies, VD effects on insulin and glucose levels of diabetic patients were similar to our trial [31–36].
In line with our results, Polidoro et al, 2013 reported that SIRT1 and Irisin levels increased in the VD group and decreased in the placebo group significantly [17]. The decreases can likely be attributed to progressing VD deficiency in the placebo group after 2 months.
In Anastasilakis et al, 2013 study, irisin levels after osteoporotic fractures in postmenopausal women with low bone mineral density for 3 months didn’t show a significant correlation with serum 25(OH)VD levels. The decrease of Irisin was related to physical inactivity and a sedentary lifestyle during fracture [37]. In another study on healthy peoples, a one-time injection of 100,000 IU VD increased serum 25(OH)VD levels and the changes of serum irisin were non-significant. Only in one subject out of 28, the serum irisin increased by three times; which may be related to genetic variations in response to the intervention [38].
A study in 2017 showed serum irisin levels were decreased after 2 weeks’ mountain climbing. The possible reasons were exposure to hypoxia, the energy-related mechanisms, and the revitalization of musculoskeletal cells in response to hypoxia [39].
The changes in physical activity in our study were not significant. Accordingly, the improvement of serum irisin levels in the VD group was independent of physical activity levels. On the other hand, the decrease and increase of irisin can be related to serum VD levels or the dietary intake of VD in T2D patients.
According to the different studies, the effects of VD on glucose indices are controversial.
Similar to our study, two separate studies with 50,000 IU/d VD didn’t find significant effects on FBS levels [33, 34]. However, in two other studies, the increased serum VD levels were significantly related to FBS [40, 41]. The likely reasons may be the dietary patterns, timing or type of hyperglycemia medications, and the genetic differences of the populations.
In contrast, some studies of VD effects reported a significant improvement in fasting serum insulin [34, 42–44]. The potential mechanisms may be the presence of VD receptors (VDRs) in pancreas cells and expression of 1-alpha-hydroxylase in them [37] and the existence of some elements on human insulin promotors that respond to VD and can activate insulin gene transcription [38–40].
In line with this trial, the different studies showed a significant decrease in HbA1c of diabetics’ patients [33, 34, 43, 44]. However, the effect of VD on HbA1c was non-significant in two separate studies [45, 46]. The reasons may be the unadjusted potential confounders and the different design of these studies. The potential mechanisms are unknown.
No significant changes in insulin resistance and sensitivity of our trial were similar to Jorde et al, 2010 study [40], and SUNNY trial [34]. However, some studies reported a significant improvement in HOMA-IR or QUICKI with vitamin D [43, 44, 47]. The reason may be changing fasting serum glucose and insulin levels after the intervention. Vitamin D may decrease blood glucose by increasing insulin sensitivity, glucose uptake of peripheral tissues, and glycogen synthesis in liver [14]. The existence of VDR and expression of 1-α-hydroxylase in pancreatic cells may be a probable mechanism of the VD effect on insulin secretion [48]. Also, response elements to VD in human insulin promoters may activate insulin gene transcription [15, 16].
The lower serum 25(OH)-VD level is associated with abdominal and peripheral obesity, physical inactivity, smoking, alcohol intake history, and the lower dietary intake of fish. In addition, obesity and physical inactivity are important risk factors for T2D. However, in this trial, differences in physical activity levels were non-significant. Serum VD levels can be directly related to central adiposity [49] wasn’t measured in our participants. Accordingly, the relationship between Vitamin D and glucose profiles can also be interpreted.
Vitamin D can increase insulin receptor gene expression in beta cells, glucose transport in the intestine [50], and intestinal calcium absorption may serve as stimuli for insulin release. In addition, some VDRs on beta cells can turn 25(OH)D3 (calcidiol) into the active VD [51, 52].
The more reasons for the differences between our trial and other studies may be unadjusted confounders, the different designs, the variety of dietary patterns, and the dose or timing of hyperglycemia medications.
This study has several strengths. First, the double-blinded stratified blocked randomization design; Second, considering multiple eligibility criteria; Third, the determining of dietary intakes and physical activity status and adjusting the statistical analysis for them and other potential confounders; Fourth, considering control group. These strengths are likely preferable in comparison with the few other clinical trials that have evaluated the effects of VD in T2D.
However, our study had some limitations. First, the sample size was small; Second, the intervention duration was likely short to understand the real effects of VD supplementation on the different markers in T2D patients; Third, disregarding non-obese patients. Fourth, self-reporting of diet and physical activity; Fifth, failure to measuring serum parathyroid hormone (PTH), Apo-lipoprotein A1, and the more sensitive inflammatory factors; Sixth, 24-h food recall is not a good index for assessing the usual food intake; Seventh, failure to measuring body composition. Even so, this study is the first trial of VD effects on serum SIRT1 and irisin levels in overweight/obese T2D patients.
We suggest similar trials measuring adipose tissue distribution and considering more potential confounders and the longer intervention period.
Conclusion
Vitamin D supplementation improves serum 25-(OH) VD, SIRT1, irisin, and glycosylated hemoglobin in VD deficient T2D patients. The decrease of HbA1c may be related to the increased serum VD and irisin levels. Further trials are suggested.
Supplementary information
Additional file 1: Table S1. The measurement tools and descriptions of companies.
Acknowledgments
The supports of Iran University of Medical Sciences and the cooperation of the Dr. Abrishami clinical laboratory, Bandar-E-Anzali, and all healthcare professionals and patients participated in the study are acknowledged.
Consent fo publication
Not applicable.
Abbreviations
- ANCOVA
Analysis of covariance
- BMI
Body mass index
- ELISA
Enzyme-linked immunosorbent assay
- FBS
Fasting blood sugar
- FGF21
Fibroblast Growth Factor 21
- FOXO
Forkhead box O
- HbA1c
Glycosylated hemoglobin
- HOMA-IR
Homeostasis model assessment-insulin resistance
- hs-CRP
High-sensitivity C-Reactive Protein
- IL-6
Interleukin 6
- IMOS
Iranian Multi-Centric Osteoporosis Study
- IPAQ
International physical activity questionnaire
- IR
Insulin resistance
- ITT
Intention to treat
- NF-κB
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- PGC-1α
PPAR-γ coactivator-1 alpha
- PPAR
Peroxisome proliferation activated receptor
- PPAR-γ
Peroxisome Proliferation Activated Receptor-Gamma
- QUICKI
Quantitative insulin sensitivity check index
- SIRT1
Sirtuin-1
- T2D
Type 2 diabetes
- TNF-α
Tumour Necrosis Factor-Alpha
- UCP1
Uncoupling Protein 1
- VD
Vitamin D
- VDR
Vitamin D receptor
Authors contributions
PS and MV conceived and developed the idea for the paper and revised the manuscript. MN, MM, FM, FSA, and MDM contributed to data collection. PS and MDM wrote numerous drafts. LJ and HS contributed to statistical interpretations. All authors read and approved the final manuscript.
Funding
Funding (PhD grant) was supported by the Iran University of Medical Sciences (grant no. 1395.9223475201). The funding body did not have a role in the design of the study, collection, analysis, and interpretation of data, and writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on a reasonable request.
Ethics approval and consent to participate
This trial was approved by the ethics committee of the Iran University of Medical Sciences (Ethical Code: IR.IUMS.REC.1395.9223475201). A written informed consent form (in Persian) obtained from all the patients. Participation was free, and a patient could withdraw at whatever point the person feels he/she was unable to continue. There was no bar for the patients to receiving the other health care services of the center. The personal information of patients was kept secret before, during, and after the study.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peivasteh Safarpour, Email: peivastehsafarpour@yahoo.com.
Milad Daneshi-Maskooni, Email: miladdaneshi@gmail.com.
Mohammadreza Vafa, Email: rezavafa@yahoo.com.
Mitra Nourbakhsh, Email: nourbakhsh.m@iums.ac.ir.
Leila Janani, Email: leila_Janani@yahoo.com.
Mohsen Maddah, Email: maddahm@yahoo.com.
Fatemeh-Sadat Amiri, Email: f.amiri58@yahoo.com.
Fereshteh Mohammadi, Email: dr_f.mohamadi@yahoo.com.
Homa Sadeghi, Email: homa.sadeghi@gmail.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12875-020-1096-3.
References
- 1.Association AD Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(suppl 1):s5–s10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 2.Himmetoglu S, Teksoz S, Zengin K, Yesim T, Taskın M, Dincer Y. Serum levels of fetuin a and 8-hydroxydeoxyguanosine in morbidly obese subjects. Exp Clin Endocrinol Diabetes. 2013;121(08):505–508. doi: 10.1055/s-0033-1345162. [DOI] [PubMed] [Google Scholar]
- 3.Kimiagar M, Pourshams A, Majd SK, Goglani G, Jaafari E, Semnani S, et al. Vitamin deficiency in Golestan Province, northern Iran: a high-risk area for esophageal cancer. Arch Iran Med. 2010;13(5):391. [PubMed] [Google Scholar]
- 4.Heshmat R, Mohammad K, Majdzadeh S, Forouzanfar M, Bahrami A, Ranjbar Omrani G, Nabipour I, Rajabian R, Hossein-Nezhad A, Rezaei Hemami M, Keshtkar A, Pajouhi M. Vitamin D Deficiency in Iran: A Multi-center Study among Different Urban Areas. Iran J Public Health. 2008;37(sup):72-78. http://ijph.tums.ac.ir/index.php/ijph/article/view/2901.
- 5.Baz-Hecht M, Goldfine AB. The impact of vitamin D deficiency on diabetes and cardiovascular risk. Curr Opin Endocrinol Diabetes Obes. 2010;17(2):113–119. doi: 10.1097/MED.0b013e3283372859. [DOI] [PubMed] [Google Scholar]
- 6.Nimitphong H, Chanprasertyothin S, Jongjaroenprasert W, Ongphiphadhanakul B. The association between vitamin D status and circulating adiponectin independent of adiposity in subjects with abnormal glucose tolerance. Endocrine. 2009;36(2):205–210. doi: 10.1007/s12020-009-9216-9. [DOI] [PubMed] [Google Scholar]
- 7.Roth CL, Elfers C, Kratz M, Hoofnagle AN. Vitamin D deficiency in obese children and its relationship to insulin resistance and adipokines. J Obes. 2011;2011:495101. doi: 10.1155/2011/495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forouhi NG, Luan JA, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance. Diabetes. 2008;57(10):2619–2625. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu KC, Chu A, Go VLW, Saad MF. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am J Clin Nutr. 2004;79(5):820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385. doi: 10.1155/2010/351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48(7):1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 12.Iyer A, Panchal S, Poudyal H, Brown L. Potential health benefits of Indian spices in the symptoms of the metabolic syndrome: a review. 2009. [PubMed] [Google Scholar]
- 13.Salminen A, Kaarniranta K, Kauppinen A. Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int J Mol Sci. 2013;14(2):3834–3859. doi: 10.3390/ijms14023834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guclu A, Erdur F, Turkmen K. The emerging role of sirtuin 1 in cellular metabolism, diabetes mellitus, diabetic kidney disease and hypertension. Exp Clin Endocrinol Diabetes. 2016;124(03):131–139. doi: 10.1055/s-0035-1565067. [DOI] [PubMed] [Google Scholar]
- 15.Chung S, Yao H, Caito S, Hwang J-W, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501(1):79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayoral R, Osborn O, McNelis J, Johnson AM, Izquierdo CL, Chung H, et al. Adipocyte SIRT1 knockout promotes PPARγ activity, adipogenesis and insulin sensitivity in chronic-HFD and obesity. Mol Metab. 2015;4(5):378–391. doi: 10.1016/j.molmet.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polidoro L, Properzi G, Marampon F, Gravina G, Festuccia C, Di Cesare E, et al. Vitamin D protects human endothelial cells from H2O2 oxidant injury through the Mek/Erk-Sirt1 axis activation. J Cardiovasc Transl Res. 2013;6(2):221–231. doi: 10.1007/s12265-012-9436-x. [DOI] [PubMed] [Google Scholar]
- 18.Irving BA, Still CD, Argyropoulos G. Does IRISIN have a BRITE future as a therapeutic agent in humans? Curr Obes Rep. 2014;3(2):235–241. doi: 10.1007/s13679-014-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.J-q C, Y-y H, Gusdon AM, Qu S. Irisin: a new molecular marker and target in metabolic disorder. Lipids Health Dis. 2015;14(1):2. doi: 10.1186/1476-511X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C, Ding Z, Lv G, Li J, Zhou P, Zhang J. Lower irisin level in patients with type 2 diabetes mellitus: a case-control study and meta-analysis. J Diabetes. 2016;8(1):56–62. doi: 10.1111/1753-0407.12256. [DOI] [PubMed] [Google Scholar]
- 22.Binay Ç, Paketçi C, Güzel S, Samancı N. Serum Irisin and oxytocin levels as predictors of metabolic parameters in obese children. J Clin Res Pediatr Endocrinol. 2017;9(2):124. doi: 10.4274/jcrpe.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craig CL, Marshall AL, Sjorstrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 24.Nikooyeh B, Neyestani TR, Farvid M, Alavi-Majd H, Houshiarrad A, Kalayi A, et al. Daily consumption of vitamin D–or vitamin D+ calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr. 2011;93(4):764–771. doi: 10.3945/ajcn.110.007336. [DOI] [PubMed] [Google Scholar]
- 25.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metabol. 2000;85(7):2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 27.Baziar N, Jafarian K, Shadman Z, Qorbani M, Nikoo MK, Mishani MA. Effect of therapeutic dose of vitamin D on serum adiponectin and glycemia in vitamin D-insufficient or deficient type 2 diabetic patients. Iran Red Crescent Med J. 2014;16(9):e21458. doi: 10.5812/ircmj.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph R, Sim J, Ogollah R, Lewis M. A systematic review finds variable use of the intention-to-treat principle in musculoskeletal randomized controlled trials with missing data. J Clin Epidemiol. 2015;68(1):15–24. doi: 10.1016/j.jclinepi.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metabol. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 30.Association WM. World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79(4):373. [PMC free article] [PubMed] [Google Scholar]
- 31.Borissova A, Tankova T, Kirilov G, Dakovska L, Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. 2003;57(4):258–261. [PubMed] [Google Scholar]
- 32.Heshmat R, Tabatabaei-Malazy O, Abbaszadeh-Ahranjani S, Shahbazi S, Khooshehchin G, Bandarian F, et al. Effect of vitamin D on insulin resistance and anthropometric parameters in type 2 diabetes; a randomized double-blind clinical trial. DARU J Pharmaceut Sci. 2012;20(1):10. doi: 10.1186/2008-2231-20-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasri H, Behradmanesh S, Maghsoudi AR, Ahmadi A, Nasri P, Rafieian-Kopaei M. Efficacy of supplementary vitamin D on improvement of glycemic parameters in patients with type 2 diabetes mellitus; a randomized double blind clinical trial. J Renal Inj Prev. 2014;3(1):31. doi: 10.12861/jrip.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krul-Poel YH, Westra S, ten Boekel E, ter Wee MM, van Schoor NM, van Wijland H, et al. Effect of vitamin D supplementation on glycemic control in patients with type 2 diabetes (SUNNY trial): a randomized placebo-controlled trial. Diabetes Care. 2015;38(8):1420–1426. doi: 10.2337/dc15-0323. [DOI] [PubMed] [Google Scholar]
- 35.Ryu O-H, Chung W, Lee S, Hong K-S, Choi M-G, Yoo HJ. The effect of high-dose vitamin D supplementation on insulin resistance and arterial stiffness in patients with type 2 diabetes. Korean J Intern Med. 2014;29(5):620. doi: 10.3904/kjim.2014.29.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor A, Wise P. Vitamin D replacement in Asians with diabetes may increase insulin resistance. Postgrad Med J. 1998;74(872):365–366. doi: 10.1136/pgmj.74.872.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anastasilakis A, Polyzos S, Makras P, Gkiomisi A, Bisbinas I, Katsarou A, et al. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int. 2014;25(5):1633–1642. doi: 10.1007/s00198-014-2673-x. [DOI] [PubMed] [Google Scholar]
- 38.Cavalier Étienne, Mismetti Valentine, Souberbielle Jean-Claude. Evaluation of circulating irisin levels in healthy young individuals after a single 100,000IU vitamin D dose. Annales d'Endocrinologie. 2014;75(3):162–164. doi: 10.1016/j.ando.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Śliwicka E, Cisoń T, Kasprzak Z, Nowak A, Pilaczyńska-Szcześniak Ł. Serum irisin and myostatin levels after 2 weeks of high-altitude climbing. PLoS One. 2017;12(7):e0181259. doi: 10.1371/journal.pone.0181259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Daghri N, Al-Attas O, Alokail M, Alkharfy K, Draz H, Clerici M. Serum 25-hydroxy-vitamin D is associated with adiponectin and insulin resistance in diabetic Saudi adults. 2011. [Google Scholar]
- 41.Nielsen NO, Bjerregaard P, Rønn PF, Friis H, Andersen S, Melbye M, et al. Associations between vitamin D status and type 2 diabetes measures among Inuit in Greenland may be affected by other factors. PLoS One. 2016;11(4):e0152763. doi: 10.1371/journal.pone.0152763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eftekhari M, Akbarzadeh M, Dabbaghmanesh M, Hasanzadeh J. Effects of treatment with oral calcitriol on plasma glucose and insulin levels in non-insulin dependent diabetes mellitus patients. Iranian J Nutr Sc Food Technol. 2011;6(3):1–10. [Google Scholar]
- 43.Tabesh M, Azadbakht L, Faghihimani E, Tabesh M, Esmaillzadeh A. Effects of calcium-vitamin D co-supplementation on metabolic profiles in vitamin D insufficient people with type 2 diabetes: a randomised controlled clinical trial. Diabetologia. 2014;57(10):2038–2047. doi: 10.1007/s00125-014-3313-x. [DOI] [PubMed] [Google Scholar]
- 44.Rad EY, Djalali M, Koohdani F, Saboor-Yaraghi AA, Eshraghian MR, Javanbakht MH, et al. The effects of vitamin D supplementation on glucose control and insulin resistance in patients with diabetes type 2: a randomized clinical trial study. Iran J Public Health. 2014;43(12):1651. [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Sofiani ME, Jammah A, Racz M, Khawaja RA, Hasanato R, El-Fawal HA, et al. Effect of vitamin D supplementation on glucose control and inflammatory response in type II diabetes: a double blind, randomized clinical trial. Int J Endocrinol Metab. 2015;13(1):e22604. doi: 10.5812/ijem.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheth JJ, Shah A, Sheth FJ, Trivedi S, Lele M, Shah N, et al. Does vitamin D play a significant role in type 2 diabetes? BMC Endocr Disord. 2015;15(1):5. doi: 10.1186/s12902-015-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Ye J, Guo G, Lan Z, Li X, Pan Z, et al. Vitamin D status is negatively correlated with insulin resistance in Chinese type 2 diabetes. Int J Endocrinol. 2016;2016:1794894. doi: 10.1155/2016/1794894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vilà L, Roca C, Elias I, Casellas A, Lage R, Franckhauser S, et al. AAV-mediated Sirt1 overexpression in skeletal muscle activates oxidative capacity but does not prevent insulin resistance. Mol Therapy—Methods Clin Dev. 2016;5:16072. doi: 10.1038/mtm.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buijsse B. Vitamin D for prevention of type 2 diabetes: a clouded forecast for the sunshine vitamin. Lancet Diab Endocrinol. 2015;3(1):5–6. doi: 10.1016/S2213-8587(14)70202-5. [DOI] [PubMed] [Google Scholar]
- 50.Maestro B, Campion J, Davila N, Calle C. Stimulation by 1, 25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47(4):383–391. doi: 10.1507/endocrj.47.383. [DOI] [PubMed] [Google Scholar]
- 51.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metabol. 2007;92(6):2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, et al. Expression of 25-hydroxyvitamin D 3-1α-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89:121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The measurement tools and descriptions of companies.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on a reasonable request.