Abstract
No consensus regarding optimal treatment or etiology of Preiser disease exists. We described the epidemiology, classification and treatment characteristics of 18 patients with Preiser disease. Patients with changes related to previous trauma, and without radiographs were excluded. Based on the radiographs at diagnosis, we classified 13 scaphoids as Herbert Lanzetta stage II, four as stage III, and one as stage IV. In 12 patients nonspecific treatment was offered and only two patients received surgical treatment. We found that chosen treatment is not associated with the severity of Herbert Lanzetta stage and the outcome is not influenced by chosen treatment.
Key Words: Avascular necrosis, Osteonecrosis scaphoid, Preiser disease, Retrospective study, Treatment
Main body
Idiopathic avascular necrosis (AVN) of the scaphoid was first described by Georg Preiser in 1910 (1-3). It is a rare condition that can cause pain and sometimes swelling around the anatomical snuffbox, which may be associated with loss of strength and reduced range of motion in the wrist (4).
The scaphoid receives the blood supply mainly from branches of the radial artery, most entering through the distal portion of the scaphoid (5). Few nutritional vessels enter the scaphoid proximal pole directly, therefore the proximal pole is a vascular terminal zone dependent largely on intraosseous blood flow (6). Preiser disease is theorized to result from a disruption of this blood supply (6, 7). Radiographs may demonstrate scaphoid sclerosis without visible fracture, and magnetic resonance imaging shows signal changes. Late radiographic changes include cystic changes, fragmentation, and collapse (4, 8, 9).
Publications are limited to small clinical series or case reports and there is no consensus regarding optimal treatment or etiology (2, 10). In this case series we describe the epidemiology, classification, and treatment characteristics of patients diagnosed and treated with Preiser disease at three large area hospitals with associated specialty offices.
Study design and population
After institutional review board approval of this retrospective study, we identified adult patients with avascular necrosis of the scaphoid between January 2000 and April 2015 using the institute’s Research Patient Data Registry database covering the data of three large area hospitals with associated specialty offices.
Data was retrieved through a combination of Current Procedural Terminology (CPT) procedure codes for the scaphoid, the International Classification of Diseases, 9th revision (ICD-9) codes for Preiser disease and avascular necrosis, and text-search for avascular necrosis of the scaphoid and Preiser disease in radiology reports and operational notes. After reviewing medical records and radiographs (n= 1,062), the following patients were excluded: patients with changes related to previous trauma (n=1,036), and patients without radiographs or radiology reports (n=8). The final cohort included 18 patients with Preiser disease; an incidence of about one per year among three hospitals.
Outcome measures and explanatory variables
We reviewed the medical records for age, sex, side of the affected wrist, duration of evaluation by a hand surgeon, duration from diagnosis to last record within the hospital system, offered treatment, obesity (we used a coded problem of obesity. BMI was not available), smoking, osteoporosis, diabetes mellitus, vascularity disorder, auto-immune disease, connective tissue disorder, steroid use, hydroxychloroquine use, and treatment. Nonspecific treatment included no treatment, non-specific pain medication, physiotherapy and wrist splint or cast.
Radiographs were used to identify the involved portion of the scaphoid (proximal, whole bone) and the severity of Preiser disease. Severity was staged according to the Herbert-Lanzetta Classification (4). Stage I represents normal radiographs, but a positive bone scan; stage II an increased density of the proximal pole and generalized osteoporosis; stage III fragmentation of the proximal pole with or without a pathological fracture; and stage IV a pattern of carpal collapse and osteoarthritis.
A total of 18 patients with Preiser disease were identified [Table 1]. The mean age was 46 years (range: 26-68 years) at the time of diagnosis. Three patients were considered incidental Preiser as they had no wrist symptoms at the time of diagnosis. In 11 patients, the avascular necrosis involved the entire scaphoid bone and, in seven patients, only the proximal pole was involved. Based on the available radiographs at diagnosis, we classified 13 scaphoids as Herbert Lanzetta stage II, four as stage III, and one as stage IV Preiser disease. Figure 1 shows radiographs of a patient with Lanzette stage II and Lanzetta stage III.
Table 1.
Preiser’s disease cases
| |
Gender, age in years | Wrist affected | Part of scaphoid involved | Herbert-Lanzetta stage | Follow-up hand in months | Follow-up within the hospital in months | Treatment offered | Treatment chosen |
|---|---|---|---|---|---|---|---|---|
| 1 | female, 68 | ( R ) | proximal pole | 2 | 0 | 37 | Nonspecific | Nonspecific |
| 2 | male, 27 | L | proximal pole | 2 | 6 | 40 | Nonspecific | Nonspecific |
| 3 | female, 36 | ( R ) | whole-bone | 3 | 6 | 100 | Arthroscopy | Nonspecific |
| 4 | female, 26 | L | proximal pole | 4 | 47 | 87 | Core decompression | Nonspecific |
| 5 | male, 35 | L | proximal pole | 3 | 1 | 52 | Reevaluate after arthroscopy & MRI | Nonspecific |
| 6 | female, 34 | ( R ) | whole-bone | 2 | 6 | 109 | Nonspecific | Nonspecific |
| 7 | female, 67 | ( R ) | whole-bone | 2 | 7 | 37 | Nonspecific | Nonspecific |
| 8 | female, 63 | L | whole-bone | 3 | 29 | 102 | Scaphoid excision and intercarpal fusion | Scaphoid excision and intercarpal fusion |
| 9 | female, 55 | ( R ) | proximal pole | 2 | 16 | 125 | Nonspecific | Nonspecific |
| 10 | male, 30 | ( R ) | proximal pole | 2 | 56 | 191 | Nonspecific | Nonspecific |
| 11* | female, 53 | ( R ) | whole-bone | 2 | 3 | 73 | Nonspecific | Nonspecific |
| 12 | female, 61 | ( R ) | proximal pole | 3 | 34 | 43 | Core decompression | Core decompression |
| 13 | female, 29 | R | whole-bone | 2 | 22 | 174 | Nonspecific | Nonspecific |
| 14 | female, 61 | ( R ) | proximal pole | 2 | 105 | 106 | Nonspecific | Nonspecific |
| 15 | male, 38 | L | whole-bone | 2 | 1 | 47 | Nonspecific | Nonspecific |
| 16 | male, 42 | R | proximal pole | 3 | 30 | 169 | Nonspecific | Nonspecific |
| 17 * | female, 66 | L | proximal pole | 2 | 0 | 149 | Nonspecific | Nonspecific |
| 18 * | female, 35 | ( L ) | proximal pole | 2 | 92 | 130 | Arthroscopy | Nonspecific |
Figure 1.
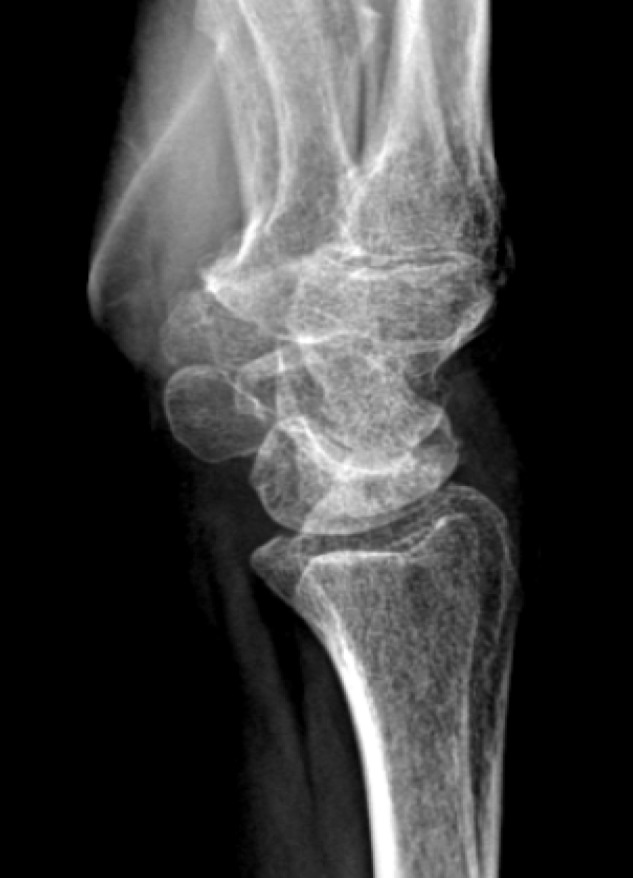
Radiographs of patients with Preiser disease
Medical records addressing the wrist were available for an average of 26 months after diagnosis and the patient record extended an average of 98 months after diagnosis. For two patients there was no follow-up. In 12 patients (67%) only nonspecific treatment was offered. Only two patients received surgical treatment. One patient underwent drilling of the radius and the one patient with advanced arthritis (stage IV) was treated with scaphoid excision and intercarpal fusion. Four patients were offered surgery (wrist arthroscopy for three patients and drilling for one patient) but all declined.
Nine of the 18 patients diagnosed with Preiser disease were obese (50%) and five were smokers (28%). Seven patients had a vascular disorder (39%) and 11 had an autoimmune disorder at time of diagnosis (58%). One patient had Henoch-Schonlein purpura, one patient had ulcerative colitis and the other patients had rheumatoid arthritis. Four patients had a history of steroid use (22%) and two used hydroxychloroquine (11%) [Table 2]. One patient with rheumatoid arthritis used oral steroids and one patient with ulcerative colitis. Two patients only had a steroid injection. There were no patients with symptoms of the opposite wrist. There was one patient with avascular necrosis of the femoral head and one patient with avascular necrosis in the femoral head and knee.
Table 2.
Possible factors associated with Preiser’s disease
| Obesity | Smoking | Osteoporosis | Diabetes Mellitus | Vascularity disorder | Auto-immune disease | Connective tissue disorder | Steroid use | Hydroxychloroquine use | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | X | X | |||||||
| 2 | X | X | X | X | |||||
| 3 | X | X | |||||||
| 4 | X | X | X | X | |||||
| 5 | X | ||||||||
| 6 | X | ||||||||
| 7 | X | X | X | X | |||||
| 8 | X | X | |||||||
| 9 | X | X | X | X | |||||
| 10 | |||||||||
| 11 | X | X | X | ||||||
| 12 | X | X | |||||||
| 13 | X | ||||||||
| 14 | X | X | X | X | X | X | |||
| 15 | X | ||||||||
| 16 | X | X | |||||||
| 17 | X | X | X | X | X | ||||
| 18 | X |
Only small clinical series or case reports are published about Preiser disease; therefore, there is no consensus regarding optimal treatment or etiology (2, 10, 11). In this case series we described the epidemiology, classification and treatment characteristics of patients diagnosed with Preiser disease. We found that the severity on the Herbert-Lanzetta classification is not associated with received treatment.
This study has several limitations. First, we used ICD-9 and CPT codes to identify the initial diagnoses and procedures rather than review of the medical records. There is likely a minor element of miscoding as is typical of studies based on databases. Second, the incidence of Preiser disease is low making it difficult to study the etiology. The study design is retrospective and therefore more susceptible to data loss, bias, and confounding than a prospective study. Lastly, patient follow-up was unavailable in several of our patients, being shorter than six months in five of 18 patients.
Our data determined that the Herbert-Lanzetta classification stage is not associated with offered and chosen treatment. Based on the available radiographs at diagnosis, we classified 13 scaphoids as Herbert-Lanzetta stage II, four as stage III, and one as stage IV Preiser disease. Only two of the six patients that had offered surgery received surgical treatment. All the other patients received nonspecific treatment, despite of the Herbert and Lanzetta stage. The 13 patients that had follow-up of six months or more did not have any symptoms. The five patients that had follow-up of less than six months were still seen by other specialties within the hospital, which suggests that their symptoms were not severe enough warrant hand surgical consultation.
Four patients in our cohort had a history of steroid use (22%). The use of steroid use in Preiser patients is a known factor associated with osteonecrosis, due to possible disruption of the blood flow (3, 7-9, 12-19).
Eleven patients had an autoimmune disorder at time of diagnosis (58%). Autoimmune disorders have also been described as factors associated with Preiser disease (3, 12, 15, 16).
We found that five patients were smokers (28%). Smoking is a known risk factor for Preiser disease as it may inhibit the vascularity to the scaphoid, which might also explain the high percentage of patients with a vascular disorder (cardiovascular or peripheral vascular) (39%) in our cohort (9, 17, 18, 20).
In conclusion, we found that the chosen treatment is not associated with the severity of Herbert and Lanzetta stage and the outcome is not influenced by the chosen treatment. Future studies on Preiser disease should ideally investigate the etiology in order to prevent Preiser disease. This is difficult to study because it is uncommon. We do not understand the etiology of Preiser disease and it is not clear what the best treatment is.
Conflicts of interest:
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Acknowledgements
None.
References
- 1.Preiser G. Eine typische posttraumatische und zur spontanfraktur fuhrende ostitis des naviculare carpi. Fortschr Geb Roentgenstr. 1910;15:189–97. [Google Scholar]
- 2.Kallen AM, Strackee SD. On the history and definition of Preiser’s disease. J Hand Surg Eur Vol. 2014;39(7):770–6. doi: 10.1177/1753193413501099. [DOI] [PubMed] [Google Scholar]
- 3.Green N, Osmer JC. Small bone changes secondary to systemic lupus erythematosus. Radiology. 1968;90(1):118–20. doi: 10.1148/90.1.118. [DOI] [PubMed] [Google Scholar]
- 4.Herbert TJ, Lanzetta M. Idiopathic avascular necrosis of the scaphoid. J Hand Surg Br. 1994;19(2):174–82. doi: 10.1016/0266-7681(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 5.Gelberman RH, Menon J. The vascularity of the scaphoid bone. J Hand Surg Am. 1980;5(5):508–13. doi: 10.1016/s0363-5023(80)80087-6. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt R, Frohner S, van Schoonhoven J, Lanz U, Golles A. Idiopathic osteonecrosis of the scaphoid (Preiser’s disease)--MRI gives new insights into etiology and pathology. Eur J Radiol. 2011;77(2):228–34. doi: 10.1016/j.ejrad.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Vidal MA, Linscheid RL, Amadio PC, Dobyns JH. Preiser’s disease. Ann Chir Main Memb Super. 1991;10(3):227–35. doi: 10.1016/s0753-9053(05)80287-x. [DOI] [PubMed] [Google Scholar]
- 8.De Smet L, Aerts P, Walraevens M, Fabry G. Avascular necrosis of the carpal scaphoid: Preiser’s disease: report of 6 cases and review of the literature. Acta Orthop Belg. 1993;59(2):139–42. [PubMed] [Google Scholar]
- 9.Kalainov DM, Cohen MS, Hendrix RW, Sweet S, Culp RW, Osterman AL. Preiser’s disease: identification of two patterns. J Hand Surg Am. 2003;28(5):767–78. doi: 10.1016/s0363-5023(03)00260-0. [DOI] [PubMed] [Google Scholar]
- 10.Lenoir H, Coulet B, Lazerges C, Mares O, Croutzet P, Chammas M. Idiopathic avascular necrosis of the scaphoid: 10 new cases and a review of the literature Indications for Preiser’s disease. Orthop Traumatol Surg Res. 2012;98(4):390–7. doi: 10.1016/j.otsr.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Lin JD, Strauch RJ. Preiser disease. J Hand Surg Am. 2013;38(9):1833–4. doi: 10.1016/j.jhsa.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Aptekar RG, Klippel JH, Becker KE, Carson DA, Seaman WE, Decker JL. Avascular necrosis of the talus, scaphoid, and metatarsal head in systemic lupus erythematosus. Clin Orthop Relat Res. 1974;101:127–8. [PubMed] [Google Scholar]
- 13.Milgram JW, Riley LH Jr. Steroid induced avascular necrosis of bones in eighteen sites: a case report. Bull Hosp Joint Dis. 1976;37(1):11–23. [PubMed] [Google Scholar]
- 14.Kawai H, Tsuyuguchi Y, Yonenobu K, Inoue A, Tada K. Avascular necrosis of the carpal scaphoid associated with progressive systemic sclerosis. Hand. 1983;15(3):270–3. [PubMed] [Google Scholar]
- 15.Klipper AR, Stevens MB, Zizic TM, Hungerford DS. Ischemic necrosis of bone in systemic lupus erythematosus. Medicine (Baltimore) 1976;55(3):251–7. doi: 10.1097/00005792-197605000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Urman JD, Abeles M, Houghton AN, Rothfield NF. Aseptic necrosis presenting as wrist pain in SLE. Arthritis Rheum. 1977;20(3):825–8. doi: 10.1002/art.1780200311. [DOI] [PubMed] [Google Scholar]
- 17.Moran SL, Cooney WP, Shin AY. The use of vascularized grafts from the distal radius for the treatment of Preiser’s disease. J Hand Surg Am. 2006;31(5):705–10. doi: 10.1016/j.jhsa.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Chang CC, Greenspan A, Gershwin ME. Osteonecrosis: current perspectives on pathogenesis and treatment. Semin Arthritis Rheum. 1993;23(1):47–69. doi: 10.1016/s0049-0172(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 19.Virik K, Karapetis C, Droufakou S, Harper P. Avascular necrosis of bone: the hidden risk of glucocorticoids used as antiemetics in cancer chemotherapy. Int J Clin Pract. 2001;55(5):344–5. [PubMed] [Google Scholar]
- 20.Lauder AJ, Trumble TE. Idiopathic avascular necrosis of the scaphoid: Preiser’s disease. Hand Clin. 2006;22(4):475–84. doi: 10.1016/j.hcl.2006.07.005. [DOI] [PubMed] [Google Scholar]


