Abstract
The objective of this study is to investigate the immune-enhancing ability of viable and heat-killed Weissella cibaria JW15 (JW15) isolated from Kimchi in RAW 264.7 macrophages. The immune effects were evaluated by measuring the production of NO, cytokines, inflammatory enzyme, and activation of NF-κB. Viable JW15 executed higher activity on stimulating the release of TNF-α as well as activating NF-κB compared to that of heat-killed JW15. Additionally, viable and heat-killed JW15 significantly increased the production of NO, IL-6 and TNF-α more than that of Lactobacillus rhamnosus GG (LGG). Furthermore, viable JW15 induced higher production of iNOS compared with that of viable LGG. Collectively, our finding indicates that viable JW15 had similar, if not more, immune-enhancing activities as heat-killed JW15. In addition, viable JW15 had higher immune-enhancing activity than commercial strain LGG. Therefore, viable JW15 has the potential to be used as a functional food to improve the host immune response.
Keywords: Weissella cibaria JW15 , probiotics, immune-enhancing effect, heat-killed probiotics, viable probiotics
Introduction
In the immune system, macrophages differentiated from monocytes play an important role in host innate and adaptive immune responses as well as immunological homeostasis. Macrophages are activated by microbial components such as lipopolysaccharide (LPS), lipoteichoic acid (LTA), and interferon (IFN). Activated macrophages modulate the host immune system to secrete nitric oxide (NO) or representative pro-inflammatory cytokines such as interleukin (IL)-1β, and tumor necrosis factor-α (TNF-α)[1– 2]. These cytokines contribute to the host immune defense mechanism against intrusion from the outside.
Probiotics are usually defined as living microbial additives that provide health benefits to the host animal by improving the balance between the intestinal microflora. Lactic acid bacteria (LAB) are the most common probiotics[3]. The use of probiotics in foods is common, owing to their beneficial effects such as control of intestinal infections and improvement in allergic diseases and lactose metabolism[4– 5]. In addition, probiotics have been known to increase the immune response of the intestine by acting on immunomodulators[6– 7] and improving the activity of phagocytes[3,5]. Some studies have shown that viable or heat-killed LAB increase the production of IL-6 and TNF-α in macrophage cell lines[8]. Several reports have shown that viable probiotics secrete cytokines more effectively than heat-killed probiotics[9].
At present, 14 officially known species of Weissella as part of lactic acid bacteria family are reported[10]. Weissella are gram-positive, non-spore-forming bacteria with catalase-negative activity. These are obligate heterofermentative organisms that ferment glucose to lactic acid and carbon dioxide through the hetero lactic fermentation pathway[11]. Weissella cibaria, classified by the first taxonomic study in 2002, was isolated from fermented food[12] and is known to exhibit antiviral effects against avian influenza virus through the production of antimicrobial substances such as weissellicin[13]. In addition, W. cibaria displays immune effects by producing inflammatory mediators[14]. W. cibaria JW15 (JW15) isolated from Kimchi was reported to display probiotic properties such as acid tolerance, bile tolerance, heat tolerance, and antimicrobial activities. Heat-killed JW15 increases the production of NO, nuclear factor-κB (NF-κB), IL-1β, and TNF-α in the RAW 264.7 macrophage cell line and mediates immunomodulatory effects[15]. In addition, viable JW15 has been reported to possess the ability to induce immune-enhancing effects against Listeria monocytogenes challenge mouse model and immunosuppressed mouse model[16–18]. Although JW15 has been studied extensively, there is no study of the immunomodulatory effects of viable JW15 cells. In this study, we investigated the immune-enhancing ability of heat-killed JW15 and viable JW15 in RAW 264.7 macrophages.
Materials and methods
JW15 preparation
JW15 (KACC 91811P) isolated from Kimchi (Korean traditional fermented vegetables) was grown in De Man Rogosa and Sharpe (MRS) broth (BD, USA) at 37 °C for 18 hours and viable bacterial cells were counted on MRS plates. The cells were collected by centrifugation at 14 000 g for 10 minutes and the culture supernatant discarded. The pellet was washed twice with sterile phosphate-buffered saline (PBS, pH 7.2). The probiotic cells (1×108 CFU/mL) were heat-killed at 110 °C for 15 minutes and stored at −20 °C until use[15]. The well-known Lactobacillus rhamnosus GG (LGG, ATCC 53103) was used as the reference strain.
Cell culture
The murine macrophage cell line RAW 264.7 (Korean Cell Line Bank, Korea) and RAW BLUE cells (InvivoGen, USA) were grown in Dulbecco's modified Eagle's medium (DMEM; HyClone, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco Laboratories, USA) and 100 units/mL of streptomycin and penicillin (Gibco Laboratories) at 37 °C in a humidified atmosphere with 5% CO2. After subculturing four to five times, RAW BLUE cells were cultured with 100 mg/mL of zeocin (InvivoGen).
Activation of macrophages
RAW 264.7 macrophages (5×105 cells/well) and RAW BLUE (5×105 cells/well) cells were seeded in a 12-well plate. The viable and heat-killed JW15 (100 μL containing 5×108 or 1×108 CFU/mL) were added to each well. The probiotic concentration was adjusted such that each macrophage cell was exposed to either 20 or 100 probiotic cells at 37 °C and 5% CO2. Macrophages incubated with PBS were used as a negative control, while those treated with lipopolysaccharide (LPS) (100, 500, and 1 000 ng/mL; Sigma, USA) in PBS were used as a positive control. For experiments containing viable JW15, RAW 264.7 cells were cultured in gentamycin (50 μg/mL). After 48 hours, the culture supernatants were collected and the concentration of NO, NF-κB, and cytokines (IL-6 and TNF-α) in the supernatant were measured[15].
Cell viability
The cytotoxicity of the viable and heat-killed JW15 against RAW 264.7 cells was evaluated based on the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide MTT (Sigma-Aldrich, USA) method. RAW 264.7 cells were plated at a density of 1×105 cells in a 96-well plate, followed by their treatment with viable or heat-killed JW15 at 37 °C for 48 hours. Cells were washed twice with PBS and incubated with 0.5 mg/mL MTT for 4 hours. The purple-colored formazan was solubilized in dimethyl sulfoxide (DMSO) for at least 1 hour in the dark and the absorbance of the solution measured at 550 nm wavelength by an enzyme-linked immunosorbent assay (ELISA) reader (Tecan, Austria). Cell viability was calculated as follows: Cell viability=[D(550 nm) of sample]/[D(550 nm) of control]×100%.
Measurement of NO production
The level of NO produced by macrophages was determined using Griess reagent (Promega, USA). A total of 50 μL cell culture supernatant or nitrite standard (0–100 μmol/L sodium nitrite) was treated with an equal volume of 1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride at room temperature (20 °C to 25 °C) in the dark for 10 minutes. The absorbance of the reaction solution was measured at 540 nm using a microplate reader. Samples were assayed in triplicates. NO concentrations were calculated using a nitrite standard curve[19].
Assay for NF-κB
To investigate whether JW15 activates NF-κB pathway, RAW BLUE cells stably transfected with the secreted alkaline phosphatase (SEAP) reporter gene placed under the transcriptional control of an NF-κB response element were used. SEAP was secreted in cell culture media upon NF-κB pathway activation. SEAP secretion was measured using alkaline phosphatase substrate QUANTI-Blue (InvivoGen) as per the manufacturer's instructions. The absorbance of the sample was measured at 620 nm with a microplate reader[15].
Induction of cytokine release
The production level of cytokines (IL-6 and TNF-α) in the culture supernatant was analyzed according to the ELISA assay protocol provided by the supplier (eBioscience, USA). Briefly, 96-well plates (SPL, Korea) were coated overnight with captured antibodies against IL-6 and TNF-α in a coating buffer at 4 °C. The plates were washed with PBS containing 0.05% (v/v) Tween 20 (PBST, BioShop, Canada) and incubated at room temperature for 1 hour to prevent any nonspecific protein binding. After washing with PBST, standard IL-6 and TNF-α and supernatant samples were incubated at room temperature for 2 hours. All standards and samples were tested in triplicates. The samples were treated with detection antibodies for 1 hour. After washing, the plate was treated with avidin-horseradish peroxidase (HRP) for 30 minutes and washed seven times with PBST. The wells were incubated with tetramethylbenzidine (TMB) in the dark for 15 minutes and the reaction was stopped with 2 N sulfuric acid (H2SO4). The absorbance was read at 450 nm with a microplate reader and the concentration of cytokines calculated from the standard curves of each cytokine standard (0–1 000 pg/mL for IL-6 and TNF-α)[19].
Quantitative real-time PCR
RAW 264.7 cells cultured in a six-well plate for 24 hours were pretreated with viable and heat-killed JW15 for 24 hours at 37 °C and 5% CO2. After incubation, cells were washed in cold PBS. Total RNA was prepared with TRIzol reagent (Invitrogen, USA) and the concentration of total RNA measured by recording the absorbance at 260 nm using an Epoch Microplate Spectrophotometer (Bio Tek Instruments Inc., USA). One microgram of RNA was reverse transcribed into first-strand cDNAs using a Moloney murine leukemia virus (MMLV) reverse transcriptase (iNtRON Bio, Korea) and random primers (9-mers; TaKaRa Bio Inc., Japan). The relative expression of inducible nitric oxide synthase (iNOS), IL-1β, TNF-α, and IL-6 was detected by ABI 7300 Real-Time PCR system (Applied Biosystems, USA) according to the manufacturer's protocol and normalized to the level of β-actin as the reference gene. Primer sequences are listed in Table 1.
1.
Primers for quantitative real-time PCR
| Gene | Primer sequence (5′ → 3′) |
| iNOS | F: TCCCTTCCGAAGTTTCTGGC
R: CTCTCTTGCGGACCATCTCC |
| IL-1β | F: CCTTGGGCCTCAAAGGAAAGAATC
R: GGAAGACACAGATTCCATGGTGAAG |
| TNF-α | F: GAACTGGCAGAAGAGGCACT
R: AGGGTCTGGGCCATAGAACT |
| IL-6 | F: TCCATCCAGTTGCCTTCTTG
R: GGTCTGTTGGGAGTGGTATC |
| β-actin | F: ATCACTATTGGCAACGAGCG
R: TCAGCAATGCCTGGGTACAT |
Western blotting analysis
RAW 264.7 macrophages were harvested by centrifugation at 6 000 g for 10 minutes and washed in cold PBS. Washed cell pellets were treated with Pro-prep solution (iNtRON Biotechnology, Korea) for 2 hours at 4 °C and the proteins collected by centrifugation at 14 000 g for 15 minutes. The supernatant was transferred to a new tube and the protein concentration was measured with the Bio-Rad protein assay reagent (Bio-Rad Laboratories Inc., USA). An equal amount of protein (50 μg) was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electroblotted onto a polyvinylidene fluoride transfer membrane (Perkin Elmer Co., USA). The membrane was blocked with 5% skim milk at room temperature and incubated overnight with primary antibodies at 4 °C. After washing in Tris-buffered saline with Tween 20 (TBST), the membrane was incubated with secondary antibodies for 1 hour. The antibody-specific protein was detected with an enhanced chemiluminescence reagent (Amersham Biosciences, UK) with ChemiDoc equipment GeneGnome 5 (Syngene, UK). The density measurement of each band was carried out using NIH Image J software.
Statistical analysis
All data were analyzed using SPSS 12.0 for Windows Version (SPSS Inc., USA). Significant differences between groups were tested by ANOVA and compared using Duncan's test (P<0.05).
Results
Effects of viable and heat-killed JW15 on RAW 264.7 proliferation
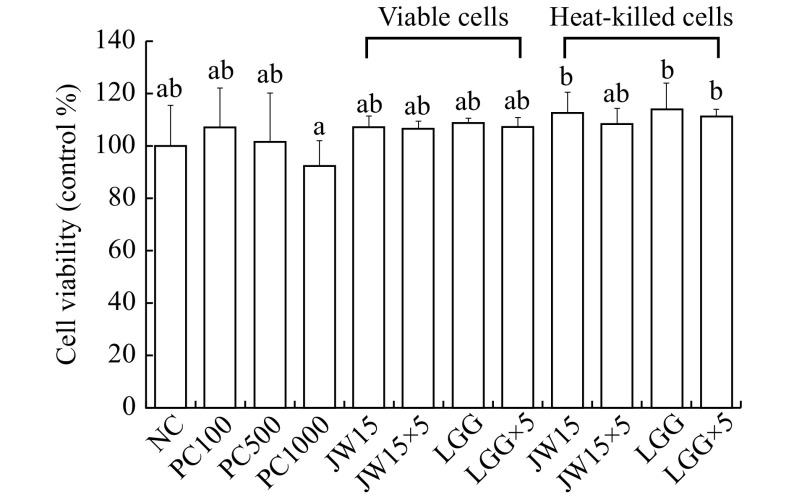
To investigate the cytotoxic effects of viable and heat-killed JW15 on RAW 264.7 macrophage cells, we performed the MTT assay. The treatment of cells with LPS resulted in a decrease in the cell viability in a concentration-dependent manner, but no significant difference was observed. In addition, JW15 displayed no significant inhibitory effects, indicating that the viable and heat-killed JW15 had no cytotoxic effect against RAW 264.7 macrophage cells (Fig. 1).
1. Cell viability analysis of RAW 264.7 macrophages treated with viable or heat-killed Weissella cibaria JW15.
RAW 264.7 cells (5×105 cells/mL) were incubated for 48 hours. The amounts of viable cells were determined by MTT assay. The result is presented as the percentage of values obtained from treated compared to non-treated control. PC100: LPS at 100 ng/mL; PC500: LPS at 500 ng/mL; PC1000: LPS at 1 000 ng/mL; JW15: Weissella cibaria JW15 at 1×108 CFU/mL; JW15×5: Weissella cibaria JW15 at 5×108 CFU/mL; LGG: Lactobacillus rhamnosus GG at 1×108 CFU/mL; LGG×5: Lactobacillus rhamnosus GG at 5×108 CFU/mL. Different superscript letters (a and b) indicate statistical differences as determined by ANOVA (P<0.05).
Production of NO and cytokines and activation of NK-κB by viable and heat-killed JW15
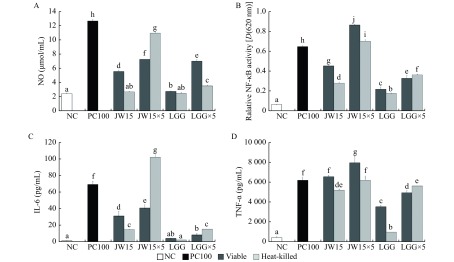
We evaluated the immune-enhancing capacity of viable or heat-killed JW15, in RAW 264.7 macrophages by determining its effects on the production of pro-inflammatory mediators. As shown in Fig. 2, the treatment of RAW 264.7 cells with JW15 and LGG resulted in a dose-dependent increase in NO, cytokine production and NK-κB activation. The viable and heat-killed JW15 showed significant NO-inducing ability compared to that of the commercial strain LGG at either low or high concentrations (P<0.05). The production of NO by heat-killed JW15×5 was higher than that of viable JW15×5, but viable JW15 induced higher production of NO than heat-killed JW15 at a low concentration. Furthermore, JW15 significantly increased NF-κB activity as compared with viable LGG. Both low and high concentrations of viable JW15-treated macrophages significantly induced NF-κB activation compared with heat-killed JW15×5-treated cells (P<0.05). Furthermore, the level of cytokines (IL-6 and TNF-α), triggered by viable and heat-killed JW15 was significantly higher than that induced by viable and heat-killed LGG (P<0.05). The viable JW15×5-treated cells had increased production of TNF-α [(7954.6±625.50) pg/mL], which was significantly different from that in heat-killed JW15×5-treated cells [(6193.3±318.45) pg/mL] (P<0.05). Therefore, viable JW15 was able to contribute to the host defense system by increasing inflammatory mediators, as observed with heat-killed JW15.
2. Effects of viable or heat-killed Weissella cibaria JW15 on NO, NF-κB and cytokine in RAW 264.7 or RAW BLUE cells.
Production of NO (A), NF-κB (B), IL-6 (C), and TNF-α (D) in RAW 264.7 cells. RAW 264.7 cells (5×105 cells/mL) were cultured with viable or heat-killed JW15 or LGG for 48 hours. Culture supernatants were collected and analyzed for NO, NF-κB, and cytokine induction. NC: negative control; PC100: lipopolysaccharide (LPS) at 100 ng/mL; JW15: Weissella cibaria JW15 at 1×108 CFU/mL; JW15×5: Weissella cibaria JW15 at 5×108 CFU/mL; LGG: Lactobacillus rhamnosus GG at 1 ×108 CFU/mL; LGG×5: Lactobacillus rhamnosus GG at 5×108 CFU/mL. Different superscript letters (a, b, c, d, e, f, g, h, i, and j) indicate statistical differences as determined by ANOVA (P<0.05).
Effects of JW15 on iNOS and cytokine mRNA expression in macrophages
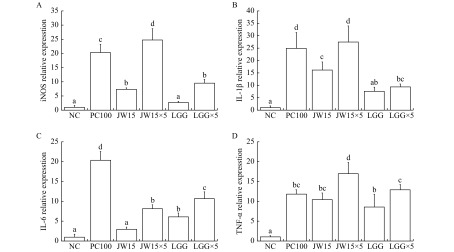
To investigate whether the difference in the enzyme and cytokine production in RAW 264.7 cells was related to the difference in the expression of enzyme and cytokine genes, mRNA levels were measured by real-time PCR (Fig. 3). As shown in Fig. 3A, both JW15 and JW15×5 significantly increased the iNOS mRNA gene expression compared to LGG (P<0.05). In addition, JW15×5 treatment markedly increased the mRNA expression level of IL-1β. Thus, viable JW15 may upregulate NO production in RAW 264.7 cells through the induction of iNOS expression at the transcriptional level. Furthermore, viable JW15 increased the expression of pro-inflammatory cytokine genes at the transcriptional level.
3. Effects of viable Weissella cibaria JW15 on iNOS, and cytokine gene expression in RAW 264.7 macrophage cells.
Production of iNOS(A), IL-1β (B), IL-6 (C), and TNF-α (D) in RAW 264.7 cells. RAW 264.7 cells (5×105 cells/mL) were cultured with viable JW15 or LGG for 48 hours. NC: negative control; PC100: lipopolysaccharide (LPS) at 100 ng/mL; JW15: Weissella cibaria JW15 at 1×108 CFU/mL; JW15×5: Weissella cibaria JW15 at 5×108 CFU/mL; LGG: Lactobacillus rhamnosus GG at 1×108 CFU/mL; LGG×5: Lactobacillus rhamnosus GG at 5×108 CFU/mL. Different superscript letters (a, b, c, d, e, and f) indicate statistical differences as determined by ANOVA (P<0.05).
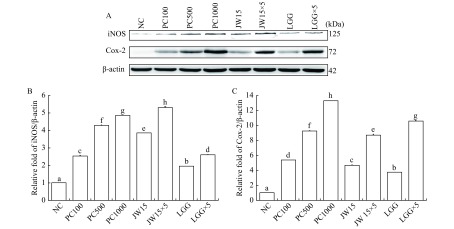
4. Effects of viable Weissella cibaria JW15 on iNOS and Cox-2 protein expression in RAW 264.7 macrophage cells.
A: Western blotting diagram of iNOS and COX-2 levels treated with viable JW15. B: Statistical analysis of iNOS expression. C: Statistical analysis of COX-2 expression. RAW 264.7 cells (5×105 cells/mL) were cultured with viable JW15 or LGG for 48 hours. NC: negative control; PC100: lipopolysaccharide (LPS) at 100 ng/mL; JW15: Weissella cibaria JW15 at 1×108 CFU/mL; JW15×5: Weissella cibaria JW15 at 5×108 CFU/mL; LGG Lactobacillus rhamnosus GG at 1×108 CFU/mL; LGG×5: Lactobacillus rhamnosus GG at 5×108 CFU/mL. Different superscript letters (a, b, c, d, e, f, g, and h) indicate statistical differences as determined by ANOVA (P<0.05).
Effects of JW15 on iNOS and cyclooxygenase-2 (COX-2) protein expression in macrophages
Next, we performed Western blotting analyses to examine whether the viable JW15 were involved in the regulation of expression of the NO synthesizing enzymes iNOS and COX-2 (Fig. 4). The treatment of cells with different concentrations of LPS resulted in an increase in the protein expression of iNOS and COX-2 in a dose-dependent manner. The viable JW15 increased COX-2 expression, similar to viable LGG. Furthermore, iNOS protein expression was significantly higher in cells treated with viable JW15 as compared with those treated with LGG (P<0.05). These results indicate that viable JW15 increase both iNOS and COX-2 protein levels, thereby inducing NO production.
Discussion
A recent study highlighted the promising health-promoting effects of viable and heat-killed LAB. Heat-killed LAB are known to exert rapid effects by inhibiting the intestinal harmful bacteria, owing to the immunomodulatory effects of their cell constituents such as peptidoglycan, β-glucans, teichoic acid, LTA, and LPS[20]. Viable LAB are able to colonize in the gut, as these cells exhibit the ability to survive in the stomach and bile and adhere to the intestine[21]. Therefore, the administration of viable LAB is more effective in improving the intestinal environment and for long-term intestinal health. To evaluate the immune effects in microphages, heat-killed probiotic cells which do not affect the tissue culture medium were generally used. In the study, we conducted the experiment by gentamycin treatment so that viable JW15 did not affect tissue culture media and we compared production of cytokines and activation of NF-κB of viable and heat-killed JW15 in RAW 264.7 cells.
In the present study, the treatment of macrophages with a high concentration of viable JW15 resulted in an increase in the production of NO, iNOS, COX-2, and cytokines. The production of NO is short term and may either kill or inhibit the growth of bacteria and tumor cells and induce cytokine production[22]. The induction of iNOS is associated with the activation of NF-κB, which is involved in the activation of pro-inflammatory genes and plays a key role in the regulation of immune response to prevent infection[23]. Enterococcus faecium JWS833 increase the production of NO in macrophages[19]. Also, some probiotics have been reported to increase the production of iNOS and NF-κB activity in RAW 264.7 cells[24]. These results suggest that viable JW15 modulates the host immune response and improves the immune system. The phagocytosis of macrophages plays an initial and crucial role in host defense against pathogens. Several studies have reported that phagocytosis in macrophages stimulates other mediators (pro-inflammatory cytokines and NO) leading to increased intestinal mucosa immunity and inhibition of pathogen invasion by the administration of probiotics[25]. These results showed that JW15 may help to initiate and enhance the macrophage immune response against pathogens.
Studies have shown that NO and NF-kB control immune activation by upregulating the expression of several cytokines in macrophages[2,26]. Viable JW15 induced NO and NF-kB activity and enhanced the level of cytokines IL-1β, IL-6, and TNF-α in macrophage cells. These cytokines contribute to the host immune defense mechanism against intrusion from the outside. In many cases, cancer and virus-infected patients may be treated with cytokines[27]. Lactobacillus (L.) plantarum increases the production of TNF-α and IL-6 in RAW 264.7 macrophage cells[28] and L. sakei was shown to induce TNF-α and IL-6 expression in macrophages[24]. Similar results were confirmed in this study, suggesting that viable JW15 strain may enhance immune responses against tumors and intracellular pathogenic infections. Excessive or prolonged production of pro-inflammatory cytokines such as TNF-α and IL-1β can promote tissue damage and, in some cases, may lead to sepsis and chronic inflammation[27,29]. JW15 increased the production of TNF-α and IL-1β same as LPS or more than LPS. In the previous study, JW15 increased the production of pro-inflammatory cytokines and IL-10 which caused anti-inflammatory response[17]. JW15 not only increased inflammatory cytokines but also induced the production of IL-10 that inhibited the production of inflammatory cytokines. JW15 modulates immune response by regulating the production of pro-inflammatory and anti-inflammatory cytokines. Therefore, the significantly induced production of TNF-α and IL-1β by JW15 does not appear to cause an excessive inflammatory response.
Lipoteichoic acid (LTA) and peptidoglycan, cell wall components of probiotics belong to gram-positive bacteria, are known as pathogen-associated molecular pattern (PAMP)[30]. There are many reports that peptidoglycan and LTA from various probiotics regulate production of pro-inflammatory cytokine[30]. In addition, LTA of W. cibaria has been shown to induce TNF and IL-1b production[31]. This result is consistent with our results. It is assumed that LTA of JW15 may have influenced productions of pro-inflammatory cytokines. The main pattern recognition receptors that detect PAMPs such as peptidoglycan and LTA are Toll-like receptors (TLR) 2[32]. Some probiotics can increase the expression of TLR2 to modulate immune activity in macrophages and mouse dendritic cells[33]. It suggests that the cell wall component of JW15 can increase the expression of TLR2.
In this experiment, heat-killed JW15 increased NO and IL-6 production, while viable JW15 induced NF-κB activation and TNF-α production. Similarly, various Lactobacillus induced higher levels of IL-6[34] and TNF-α[32]. Chang et al[35] also observed that viable W. cibaria B0145 could induce higher production of NO and TNF-α compared with heat-inactivated B0145. In this experiment, both viable and Heat-killed JW15 activated macrophage by inducing the production of other cytokines, respectively. Further experiments such as animal experiments or molecular studies of JW15 are required for more accurate comparisons. Both viable JW15 and heat-killed JW15 significantly increased NO and cytokines production and NF-κB activation than that of LGG. Although there may be a clear difference between the strains, JW15 appears to have immune-enhancing effects in both conditions, such as viable and heat-killed compared with LGG.
In summary, we demonstrate the immune-enhancing effects of viable and heat-killed JW15 in RAW 264.7 macrophages. Stimulation with heat-killed JW15 induces higher NO and IL-6 production compared to viable JW15 and LGG. The viable JW15 was able to induce significant production of TNF-α, iNOS, IL-1β and activation NF-κB. It was confirmed that viable JW15 had immune enhancement activities as good as heat-killed JW15 or more. Thus, viable JW15 may be useful in functional foods for the facilitation of host immunomodulation.
Acknowledgments
This work was carried out with the support of "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01283407)", Rural Development Administration, Republic of Korea. This work was supported by Post-Doctoral Fellowship Program funded by the Ministry of Education of the Republic of Korea through the Chungbuk National University in 2019.
Funding Statement
This is an open access article under the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited
References
- 1.Marin ML, Lee JH, Murtha J, et al Differential cytokine production in clonal macrophage and T-cell lines cultured with bifidobacteria. J Dairy Sci. 1997;80(11):2713–2720. doi: 10.3168/jds.S0022-0302(97)76232-5. [DOI] [PubMed] [Google Scholar]
- 2.Lorsbach RB, Murphy WJ, Lowenstein CJ, et al Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993;268(3):1908–1913. [PubMed] [Google Scholar]
- 3.Fuller R Probiotics in man and animals. J Appl Bacteriol. 1989;66(5):365–378. doi: 10.1111/j.1365-2672.1989.tb05105.x. [DOI] [PubMed] [Google Scholar]
- 4.Cross ML Microbes versus microbes: immune signals generated by probiotic lactobacilli and their role in protection against microbial pathogens. FEMS Immunol Med Microbiol. 2002;34(4):245–253. doi: 10.1111/j.1574-695X.2002.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 5.Nomoto K Prevention of infections by probiotics. J Biosci Bioeng. 2005;100(6):583–592. doi: 10.1263/jbb.100.583. [DOI] [PubMed] [Google Scholar]
- 6.Isolauri E, Sütas Y, Kankaanpää P, et al Probiotics: effects on immunity. Am J Clin Nutr. 2001;73(2):444S–450S. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 7.Rolfe RD The role of probiotic cultures in the control of gastrointestinal health. J Nutr. 2000;130(2):396S–402S. doi: 10.1093/jn/130.2.396S. [DOI] [PubMed] [Google Scholar]
- 8.Morita H, He F, Fuse T, et al Cytokine production by the murine macrophage cell line J774.1 after exposure to lactobacilli. Biosci Biotechnol Biochem. 2002;66(9):1963–1966. doi: 10.1271/bbb.66.1963. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen M, Vuopio-Varkila J, Varkila K Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect Immun. 1996;64(12):5403–5405. doi: 10.1128/iai.64.12.5403-5405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bruyne K, Camu N, De Vuyst L, et al Weissella fabaria sp. nov., from a Ghanaian cocoa fermentation . Int J Syst Evol Microbiol. 2010;60:1999–2005. doi: 10.1099/ijs.0.019323-0. [DOI] [PubMed] [Google Scholar]
- 11.Padonou SW, Schillinger U, Nielsen DS, et al Weissella beninensis sp. nov., a motile lactic acid bacterium from submerged cassava fermentations, and emended description of the genus Weissella . Int J Syst Evol Microbiol. 2010;60:2193–2198. doi: 10.1099/ijs.0.014332-0. [DOI] [PubMed] [Google Scholar]
- 12.Björkroth KJ, Schillinger U, Geisen R, et al Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food and clinical samples . Int J Syst Evol Microbiol. 2002;52:141–148. doi: 10.1099/00207713-52-1-141. [DOI] [PubMed] [Google Scholar]
- 13.Srionnual S, Yanagida F, Lin LH, et al Weissellicin 110, a newly discovered bacteriocin from Weissella cibaria 110, isolated from plaa-som, a fermented fish product from Thailand . Appl Environ Microbiol. 2007;73(7):2247–2250. doi: 10.1128/AEM.02484-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang MS, Lim HS, Kim SM, et al Effect of Weissella cibaria on fusobacterium nucleatum-induced interleukin-6 and interleukin-8 production in KB cells . J Bacteriol Virol. 2011;41(1):9–18. doi: 10.4167/jbv.2011.41.1.9. [DOI] [Google Scholar]
- 15.Ahn SB, Park HE, Lee SM, et al Characteristics and immuno-modulatory effects of Weissella cibaria JW15 isolated from Kimchi, Korea traditional fermented food, for probiotic use . J Biomed Res. 2013;14(4):206–212. doi: 10.12729/jbr.2013.14.4.206. [DOI] [Google Scholar]
- 16.Park HE, Lee WK Immunomodulatory effects of mixed Weissella cibaria JW15 with water extract of black soybean and burdock on Listeria monocytogenes infection in mice . J Biomed Transl Res. 2017;18(1):1–6. [Google Scholar]
- 17.Park HE, Kang KW, Kim BS, et al Immunomodulatory potential of Weissella cibaria in aged C57BL/6J mice . J Microbiol Biotechnol. 2017;27(12):2094–2103. doi: 10.4014/jmb.1708.08016. [DOI] [PubMed] [Google Scholar]
- 18.Park HE, Lee WK Immune enhancing effects of Weissella cibaria JW15 on BALB/c mice immunosuppressed by cyclophosphamide . J Funct Foods. 2018;49:518–525. doi: 10.1016/j.jff.2018.09.003. [DOI] [Google Scholar]
- 19.Choi HJ, Shin MS, Lee SM, et al Immunomodulatory properties of Enterococcus faecium JWS 833 isolated from duck intestinal tract and suppression of Listeria monocytogenes infection . Microbiol Immunol. 2012;56(9):613–620. doi: 10.1111/j.1348-0421.2012.00486.x. [DOI] [PubMed] [Google Scholar]
- 20.Adams CA The probiotic paradox: live and dead cells are biological response modifiers. Nutr Res Rev. 2010;23(1):37–46. doi: 10.1017/S0954422410000090. [DOI] [PubMed] [Google Scholar]
- 21.Masood MI, Qadir MI, Shirazi JH, et al Beneficial effects of lactic acid bacteria on human beings. Crit Rev Microbiol. 2011;37(1):91–98. doi: 10.3109/1040841X.2010.536522. [DOI] [PubMed] [Google Scholar]
- 22.Marletta MA Nitric oxide synthase structure and mechanism. J Biol Chem. 1993;268(17):12231–12234. [PubMed] [Google Scholar]
- 23.Cato ACB, Wade E Molecular mechanisms of anti-inflammatory action of glucocorticoids. BioEssays. 1996;18(5):371–378. doi: 10.1002/bies.950180507. [DOI] [PubMed] [Google Scholar]
- 24.Jung JY, Shin JS, Lee SG, et al Lactobacillus sakei K040706 evokes immunostimulatory effects on macrophages through TLR 2-mediated activation . Int Immunopharmacol. 2015;28(1):88–96. doi: 10.1016/j.intimp.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 25.Lin WH, Yu B, Lin CK, et al Immune effect of heat-killed multistrain of Lactobacillus acidophilus against Salmonella typhimurium invasion to mice . J Appl Microbiol. 2007;102(1):22–31. doi: 10.1111/j.1365-2672.2006.03073.x. [DOI] [PubMed] [Google Scholar]
- 26.Kopp EB, Ghosh S NF-κB and rel proteins in innate immunity . Adv Immunol. 1995;58:1–27. doi: 10.1016/S0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- 27.Arai KI, Lee F, Miyajima A, et al Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- 28.Chon H, Choi B, Lee E, et al Immunomodulatory effects of specific bacterial components of Lactobacillus plantarum KFCC11389P on the murine macrophage cell line RAW 264•7 . J Appl Microbiol. 2009;107(5):1588–1597. doi: 10.1111/j.1365-2672.2009.04343.x. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Dinarello CA, Molgora M, et al Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019;50(4):778–795. doi: 10.1016/j.immuni.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuguchi T, Takagi A, Matsuzaki T, et al Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2 . Clin Diagn Lab Immunol. 2003;10(2):259–266. doi: 10.1128/CDLI.10.2.259-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong YF, Lee YD, Park JY, et al Lipoteichoic acid isolated from Weissella cibaria increases cytokine production in human monocyte-like THP-1 cells and mouse splenocytes . J Microbiol Biotechnol. 2016;26(7):1198–1205. doi: 10.4014/jmb.1601.01047. [DOI] [PubMed] [Google Scholar]
- 32.Vinderola G, Matar C, Perdigon G Role of intestinal epithelial cells in immune effects mediated by gram-positive probiotic bacteria: involvement of toll-like receptors. Clin Diagn Lab Immunol. 2005;12(9):1075–1084. doi: 10.1128/CDLI.12.9.1075-1084.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujie H, Villena J, Tohno M, et al Toll-like receptor-2-activating bifidobacteria strains differentially regulate inflammatory cytokines in the porcine intestinal epithelial cell culture system: finding new anti-inflammatory immunobiotics. FEMS Immunol Med Microbiol. 2011;63(1):129–139. doi: 10.1111/j.1574-695X.2011.00837.x. [DOI] [PubMed] [Google Scholar]
- 34.Khani S, Motamedifar M, Golmoghaddam H, et al In vitro study of the effect of a probiotic bacterium Lactobacillus rhamnosus against herpes simplex virus type 1 . Braz J Infect Dis. 2012;16(2):129–135. doi: 10.1016/S1413-8670(12)70293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CK, Wang SC, Chiu CK, et al Effect of lactic acid bacteria isolated from fermented mustard on immunopotentiating activity. Asian Pac J Trop Biomed. 2015;5(4):281–286. doi: 10.1016/S2221-1691(15)30346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]