Abstract
Atherosclerosis is an inflammatory disease characterized by lipid deposits in the subendothelial space leading to severe inflammation. Nonalcoholic fatty liver disease (NAFLD) shares several risk factors with atherosclerosis, including dyslipidemia, type 2 diabetes mellitus, and metabolic syndrome, all of which lead to lipid deposition in the liver causing inflammation and fibrosis. Several clinical trials have shown that certain Chinese herbal medicines with anti-inflammatory effects can be used as adjuvant therapy to prevent the development of cardiovascular events and liver disease. Ling Zhi 8 (LZ8) is an immunomodulatory protein isolated from a medicinal mushroom and has been well documented to possess a broad range of pharmacological properties. This study aimed to evaluate the protective effects of recombinant Lactococcus lactis expressing LZ8 protein on NAFLD and atherogenesis in a cholesterol-fed rabbit model. Twelve rabbits were divided into three groups and fed with syrup only, L. lactis vehicle, or recombinant L. lactis-LZ8 once a day on weekdays for five weeks, respectively. The gene expression of IL-1β in the aorta was significantly suppressed after oral administration of L. lactis-LZ8. Moreover, in hematoxylin and eosin staining of the aorta, the intima-medial thickness was decreased, and foam cells were significantly reduced in the subendothelial space. LZ8 also inhibited the expression of IL-1β in the liver, decreased fat droplet deposits and infiltration of inflammatory cells, and improved liver function by decreasing liver enzymes in an animal model. Our results suggest that the Lactococcus-expressing LZ8 appears to be a promising medicine for improving both NAFLD and early atherogenesis owing to its anti-inflammatory effect. Furthermore, it is available as a low-cost food-grade product.
1. Introduction
Atherosclerosis is the major cause of myocardial infarction, stroke, and peripheral artery disease. It is a chronic inflammatory process characterized by low-density lipoprotein cholesterol (LDL-C) accumulation in the subendothelial space [1]. Lipid-lowering therapy with statins is the standard treatment for atherosclerotic patients [2]; however, such patients remain at high risk of recurrent atherosclerosis despite aggressive lipid modification treatment. Nonalcoholic fatty liver disease (NAFLD) shares several risk factors with atherosclerosis, including dyslipidemia, type 2 diabetes mellitus, and metabolic syndrome [3, 4]. NAFLD involves a histopathological spectrum including fat accumulation in hepatocytes with different degrees of inflammation and fibrosis. In a large epidemiology cohort study, over 5000 asymptomatic individuals with no history of coronary artery disease or significant alcohol intake received abdominal ultrasonography and coronary computed tomography angiography in a general health examination [5]. The authors reported that NAFLD was consistently associated with coronary artery soft plaque, suggesting early atherosclerosis [5]. There are many etiological and pathological similarities between atherosclerosis and NAFLD, including hypercholesteremia and hypertriglyceridemia, which lead to lipid deposition in tissue and cause inflammation and fibrosis [6, 7].
Several clinical trials have shown that certain Chinese herbal medicines have anti-inflammatory effects and that these medicines could potentially be used as adjuvant therapy to prevent the recurrence of cardiovascular events and liver disease. Ling Zhi 8 (LZ8) is an immunomodulatory protein isolated from the medicinal mushroom known as Ling Zhi, and its nucleotide sequences and structure have been characterized in several studies [8, 9]. It has been well documented that LZ8 possesses a broad range of pharmacological properties, including anti-inflammatory activities [10–13]. However, few studies have investigated its anti-inflammatory effects on atherosclerosis and NAFLD. Over the past two decades, Lactococcus lactis has been used as a food-grade and endotoxin-free genetically engineered vector for protein expression and as an antigen delivery system [14–16]. This study aimed to evaluate the protective effects of recombinant L. lactis expressing LZ8 protein on NAFLD and atherogenesis in a cholesterol-fed rabbit model.
2. Materials and Methods
2.1. Bacterial Strain and Vector
The Lactococcus lactis NZ3900 strain and plasmid pNZ8149 were purchased from MoBiTec (Goettingen, Germany). NZ3900 is the standard strain for food-grade selection due to its ability to grow on lactose. pNZ8149 contains the lacF gene for food-grade selection for growth on lactose and a nisA promoter for nisin-induced gene expression.
2.2. Construction of pNZ8149-LZ8 and Transformation by Electroporation
To construct the recombinant plasmid expressing the fusion gene under the control of the regulatory promoter nisA, the encoding gene of LZ8 from Ganoderma lucidum (described in GenBank M58032.1) was synthesized by modifying its sequence based on the optimized codon usage in L. lactis. 335-bp synthetic fragments of LZ8 were then cloned into the NcoI/XbaI sites of the pNZ8149 vector in-frame and confirmed by DNA sequencing using an automated DNA analyzer (ABI Prism 3700) (Sequence data shown in Supplement ). Computer-assisted homology search revealed that these novel LZ sequences had high identities, 70.57% and 100%, with the published LZ8 in nucleotide and amino acid sequences, respectively. The constructed plasmid was transformed into the L. lactis strain NZ3900 using a Gene Pulser (2500 V, 200 Ω, 25 μF, 5 ms, Bio-Rad, USA). The electroporated mixture was plated onto Elliker plates according to the manufacturer's instructions (MoBiTec). Lactose-positive colonies appeared yellow after 48 h of incubation at 30°C.
2.3. Protein Expression with the Food-Grade Inducer Nisin
The genetically engineered strain NZ3900/pNZ8149-LZ8 was propagated in M17 medium containing 0.5% lactose as the sole carbon source at 30°C. The recombinant clone was grown until an OD600 of 0.5 was reached, which was induced with different concentrations of nisin (0∼50 ng/mL, Sigma, Missouri, USA) for 1∼20 h. The corresponding control strain NZ3900 harboring an empty plasmid was treated in the same way. The harvested cells were monitored for protein expression using immunodetection with a lab-made rabbit anti-LZ8 polyclonal antibody as described previously [17]. For oral vaccination, the harvested cell pellets were washed twice with PBS and the expressed protein levels were determined by immunoblotting using purified E. coli-derived LZ8 as the quantification standard.
2.4. Experimental Design of Oral Live Recombinant LZ8 L. lactis and Hypercholesterolemic Rabbit Model
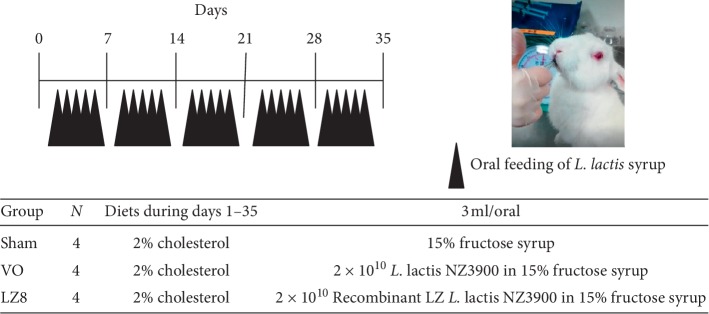
The experimental protocols of oral administration of recombinant L. lactis in rabbits fed with a high cholesterol diet are described in Figure 1. Twelve male 2-month-old New Zealand white rabbits (body weight 2.45 ± 0.30 kg) were purchased from a private farm in Changhua, Taiwan, and housed separately in cages. The experimental rabbits received commercial rabbit chow supplemented with 2% cholesterol, 1% cholic acid, and 0.5% thiouracil for 35 days and were then fasted for 4 hours prior to oral L. lactis. Briefly, the rabbits were divided into three groups and fed with 3 mL of the prepared 15% fructose syrup as depicted in Figure 1 once a day on weekdays for 5 weeks. Blood samples from the fasted rabbits were collected in no-additive tubes via the marginal ear vein weekly and then centrifuged to separate the serum, which was kept frozen at −20°C until use. After 5 weeks, the rabbits were anesthetized with a mixture of ketamine (40 mg/kg) and xylazine (5 mg/kg) given intramuscularly and then sacrificed. The aortas from the ascending aorta, aortic arch to thoracic aorta, and livers were quickly removed for further analyses.
Figure 1.
Experimental scheme of oral recombinant Lactococcus lactis in rabbits fed with a high cholesterol diet. Twelve male New Zealand white rabbits were divided into three groups and fed 3 ml of the prepared fructose syrup as indicated once a day on weekdays. Blood samples were collected weekly via the marginal ear vein and all rabbits were sacrificed on day 35.
2.5. Lipid Profiles in Sera
To evaluate the effects of oral recombinant LZ8 L. lactis on the rabbits, the concentrations of triglycerides (TGs), total cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), aspartate transaminase (AST), and alanine transaminase (ALT) were determined in the sera of fasted rabbits using an automated analyzer with commercially available kits.
2.6. Sudan Red Staining of Rabbit Aortas
To more accurately analyze intima lipid infiltration, Sudan red staining of aortic intima was performed in six rabbits from the three groups. On day 35, the rabbits were sacrificed and the aortas were dissected and carefully cleaned to remove surrounding tissue. The aortas were then washed with PBS and immersed in Sudan IV stain solution (5% (w/v) Sudan IV in 35% ethanol and 50% acetone) at room temperature for 5 min. The tissues were then placed in 80% ethanol for 3 min and washed in running water. The aortas were then longitudinally cut to expose the inner lumen, pinned on a rubber pad with the interior facing up, and photographed using a digital camera (Sony, Japan).
2.7. Hematoxylin and Eosin Staining of Rabbit Livers and Aortas
Twelve liver tissue samples and six aortic arches from the three groups of rabbits were fixed in formalin and embedded in paraffin, dissected into 3 μm sections, deparaffinized with xylene, and then rehydrated in a graded series of ethanol for hematoxylin and eosin (H&E) staining. The extent of atherosclerotic lesions was analyzed by determining the intima to medial thickness ratio histologically using light microscopy, and corresponding images were taken using an Olympus BX51 microscopic/DP71 Digital Camera System (Nagano, Japan).
2.8. RNA Extraction and Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted from rabbit ascending aortas and livers with TRIzol reagent (Invitrogen, CA, USA), and cDNAs were generated from 2 μg of total RNA using a SuperScript III kit (Invitrogen). The oligonucleotide primers used were 5′-TCCAGCTGCGCATCTCCTGC-3′ (sense) and 5′-CTTCTC CTTGCACAAAACTC-3′ (antisense) for IL-1β (the target was 354 bp in length), and 5′-CGAGACCACCTTCAA CTCGATC-3′ (sense) and 5′-CTTCTGCATGCGGTCGG-3′ (antisense) for β-actin (122 bp in length). A total volume of 20 μL of PCR mixture included 10 μl of polymerase chain reaction (PCR) Master Mix (Applied Biosystems, Life Technologies, Carlsbad, CA, USA), 10 pmoles of specific sense and antisense primers for each cytokine gene, and 10 ng of first-strand cDNA. After beginning with a single preincubation step at 95°C for 10 min, PCR was performed under the following conditions: denaturation for 1 min at 94°C, annealing for 1 min at 55°C, and extension for 1 min at 72°C in a thermal cycler (G-STORM, UK). The numbers of cycles used for IL-1β and β-actin amplification were 38 and 35, respectively. The PCR products were analyzed with 2% agarose gel electrophoresis and ethidium bromide staining and captured using a Kodak molecular imaging system (NY, USA). The relative intensities of the bands were quantified using Gel-Pro image analysis software, version 3.1 (Media Cybernetics, Rockville, Maryland).
2.9. Statistical Analysis
All values are expressed as means ± SD, and differences between groups were analyzed by one-way analysis of variance with a Bonferroni multiple comparison test. A p value of <0.05 was considered to be statistically significant.
3. Results
3.1. Expression and Quantification of Recombinant LZ8 by the pNZ8149 Vector and the Host Strain L. lactis NZ3900
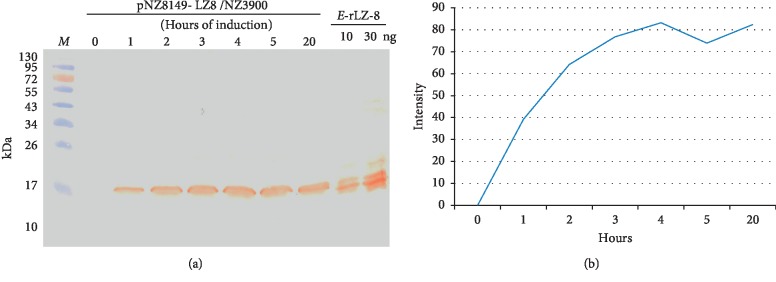
cDNA encoding 110 amino acids of LZ8 was inserted into the pNZ8149 vector and expressed in the L. lactis strain NZ3900. A band around 15 kDa was confirmed by immunoblotting with lab-made rabbit anti-LZ8 polyclonal antibodies (Figure 2(a)). No signal was detected in the cell extract of wild L. lactis NZ3900 strain (data not shown). The optimal yield of nisin-induced rLZ8 in L. lactis was 1 μg/2 × 1010 colony-forming units for 4 hours according to the intensity levels of standard bands (Figure 2(b)).
Figure 2.
(a) Expression analysis of recombinant LZ8-producing L. lactis after nisin induction for 1–20 hours. Immunoblotting of bacterial lysate was performed with lab-made rabbit anti-LZ8-specific antibody. E. coli-derived recombinant LZ8 was used as positive control. Lane M, prestained protein markers. (b) Correlation curve of the induction times and the intensity levels of band signals by video densitometer.
3.2. Body Weight and Serum Biochemical Data
The average body weight was not significantly different among the three groups of rabbits during the entire experimental period (Table 1). At the beginning of the experiment, there were no significant differences in serum lipid levels among the three groups. However, after the rabbits had been fed with a 2% cholesterol diet for 3 weeks, the levels of total cholesterol and LDL-C in the sera were significantly increased in the three groups and reached an even higher level at the end of 5 weeks. The final levels of total cholesterol and LDL-C at W5 were not statistically different between groups. Moreover, no significant differences were found in HDL-C and triglycerides during the cholesterol feeding period (Table 1). Concerning liver function, serum levels of AST and ALT were significantly higher after 3 weeks of the high cholesterol diet in both the sham and VO groups, but not in the LZ8 group (Table 1). LZ8 appeared to improve liver function by decreasing AST and ALT activities.
Table 1.
Effects of oral recombinant Lactococcus lactis in high cholesterol diet of rabbits on body weight and serum parameters.
| Measurement | Sham | VO | LZ8 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| W0 | W3 | W5 | W0 | W3 | W5 | W0 | W3 | W5 | |
| Body weight (Kg) | 2.62 ± 0.32 | 2.75 ± 0.25 | 2.79 ± 0.26 | 2.38 ± 0.30 | 2.51 ± 0.23 | 2.61 ± 0.38 | 2.58 ± 0.25 | 2.72 ± 0.29 | 2.83 ± 0.23 |
| Cholesterol (mg/dL) | 38 ± 8.6 | 1264 ± 214∗∗ | 1487 ± 552∗∗∗ | 41.5 ± 22.9 | 831.3 ± 511.3∗ | 1342 ± 258∗∗∗ | 39.3 ± 8.1 | 1390 ± 602∗∗ | 1980 ± 394.7∗∗∗ |
| HDL-chol (mg/dL) | 19 ± 5.8 | 30 ± 7.9 | 31.5 ± 11.2 | 14.2 ± 4.9 | 39.2 ± 11.9 | 26.5 ± 11.7 | 20.5 ± 4.7 | 29.25 ± 8.7 | 21.8 ± 5.6 |
| LDL-chol (mg/dL) | 8.5 ± 3.6 | 527.9 ± 80.9∗∗ | 540.8 ± 237.5∗∗ | 9.85 ± 7.9 | 291.3 ± 198.8∗ | 520.5 ± 96.1∗∗∗ | 7.05 ± 2.2 | 558.1 ± 262.3∗∗ | 773.2 ± 121.9∗∗∗ |
| Triglyceride (mg/dL) | 56.5 ± 14.5 | 89.5 ± 68.3 | 111.5 ± 52.8 | 44 ± 14.6 | 79.8 ± 58.5 | 71.3 ± 28.7 | 45.8 ± 5.3 | 79 ± 44.0 | 110.8 ± 64.9 |
| AST (U/L) | 24.2 ± 9.1 | 348.5 ± 156.9∗∗ | 107.5 ± 57.1 | 18 ± 5.6 | 179.5 ± 40.9∗∗∗ | 52 ± 25.5 | 36 ± 18.9 | 83.3 ± 73.8 | 73.2 ± 29.2 |
| ALT (U/L) | 44.3 ± 22.6 | 304 ± 94.2∗∗∗ | 134 ± 59.7 | 33.5 ± 5.8 | 183.7 ± 58.7∗∗∗ | 78.5 ± 25.8 | 54 ± 22.8 | 84.3 ± 47.9 | 98.3 ± 57.5 |
HDL, high-density lipoproteins; LDL, low-density lipoproteins; AST, aspartate aminotransferase; ALT, alanine aminotransferase.Values are means ± SD of 4 rabbits from each group. The statistics show comparisons between W0 and W3 or W0 and W5 from each group by the Bonferroni multiple range test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
3.3. Effect of Recombinant LZ8 L. lactis on Preventing the Development of Fatty Liver
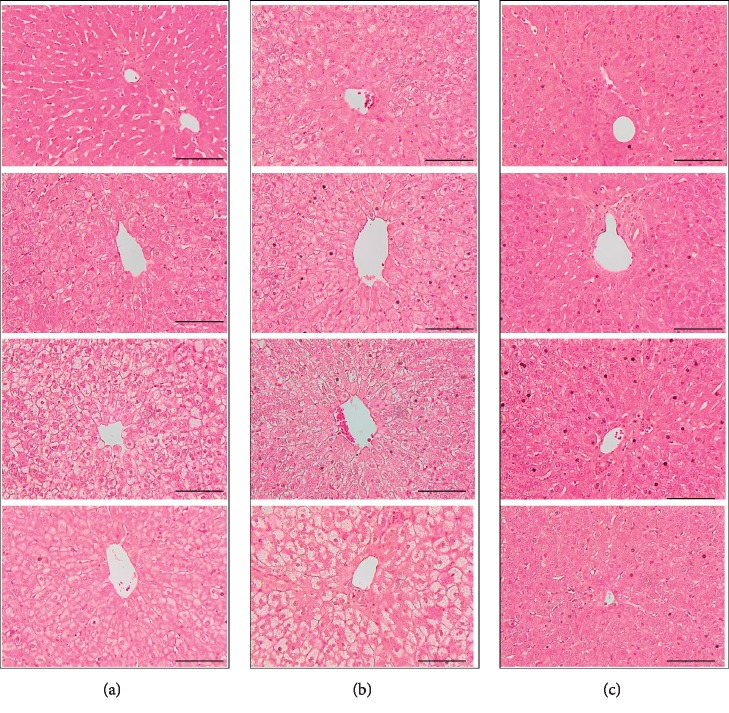
In the sham and VO groups, the hepatocellular fat deposition was seen predominantly after 5 weeks of the 2% cholesterol diet (Figure 3, Sham and VO groups). In contrast, lipid droplets were rarely observed in the liver cells from the four rabbits in the LZ group (Figure 3, LZ8 group). Importantly, the results of serum levels of AST and ALT, and H&E staining of livers suggested that the studied medicine, which was composed of recombinant L. lactis expressing LZ8, had a liver-protecting effect.
Figure 3.
Histological changes in the livers of cholesterol-fed rabbits by H&E staining. Under higher magnification, hepatocyte ballooning and steatosis were observed throughout the sections in the rabbits from the sham and VO groups. H&E staining showed that steatohepatitis was attenuated in rabbits of LZ8 L. lactis treatment group. CV, central vein. Scale bar = 100 μm. Magnification ×400. (a) Sham group.(b) VO group. (c) LZ8 group.
3.4. The Effect of Recombinant LZ8 L. lactis on Preventing the Development of Early Atherosclerosis
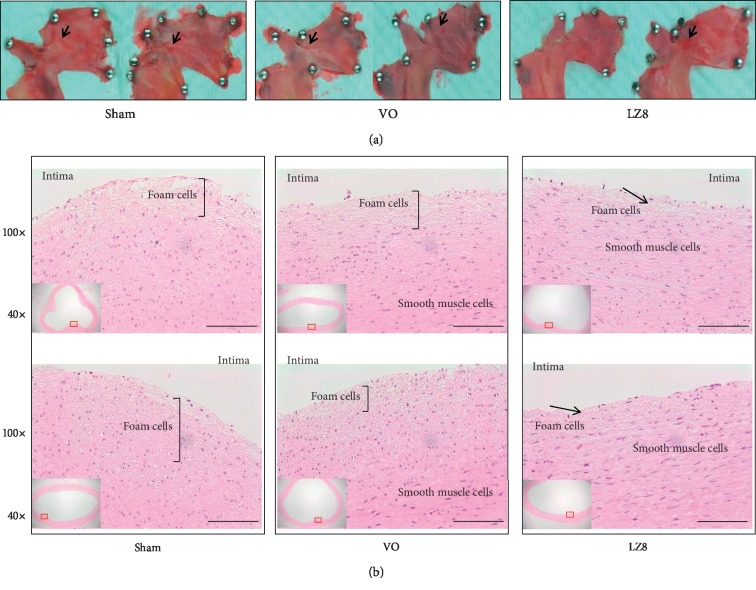
To examine the effect of recombinant LZ8 L. lactis on the development of atherosclerotic lesions after 5 weeks of the 2% cholesterol diet, the harvested aortic arches were separated into two groups and stained with Sudan red and H&E, respectively. After Sudan IV staining of the six aortas from the three groups of rabbits, atherosclerotic lesions were stained red (Figure 4(a)). As expected, aortic internal surfaces in the sham group showed deposition of lipids, and small plaques in fatty streaks were observed, mostly in the aortic arch (Figure 4(a), panel sham). Compared to the sham group, the plaque areas of the aortic arches in the VO and LZ8 groups were significantly reduced (Figure 4(a), panels VO and LZ8). The other six aortas from the three groups of rabbits were stained with H&E (Figure 4(b)). In the sham and VO groups, the atherosclerotic lesions exhibited a thickened intima. The rabbits in the LZ8 group had a lower intima thickness compared to both the sham and VO groups. These results suggest that oral medicine, which was composed of recombinant L. lactis expressing LZ8, had an antiatherosclerotic effect in the rabbit model of atherosclerosis.
Figure 4.
Histological images of aortic arches from three groups of rabbits after 5 weeks of a high cholesterol diet. (a) Sudan IV staining, arrowhead indicates small plaques in fatty streaks were observed, mostly in the aortic arch from sham group. (b) H&E staining, bracket indicates thickness of the intimal layer and arrow indicated foam cell deposition. Scale bar = 100 μm.
3.5. Recombinant LZ8 L. lactis Reduced the mRNA Expression of IL-1β in Cholesterol-Fed Rabbits
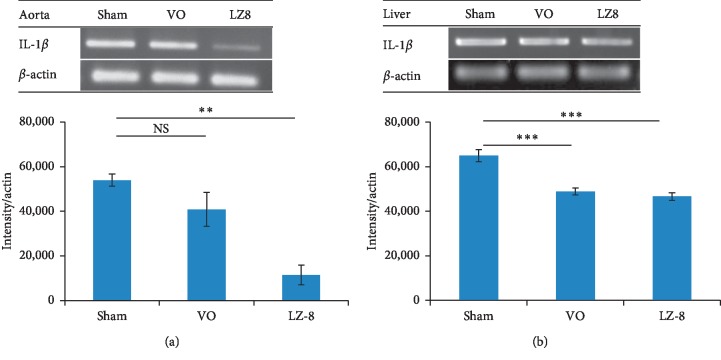
Atherosclerosis is an inflammatory disease, and the proinflammatory cytokine IL-1β has been well studied as a therapeutic target [18, 19]. The effects of recombinant LZ8 L. lactis on the expression levels of IL-1β in aortas and liver tissues from the rabbits were evaluated using reverse transcriptase (RT)-PCR. The results showed that the mRNA expression of IL-1β was significantly lower in aorta homogenates (Figure 5(a)) and livers (Figure 5(b)) in the LZ group than in the sham group.
Figure 5.
Recombinant LZ8 L. lactis vaccine reduced IL-1β mRNA expression. (a) Aortas; and (b) livers of high cholesterol-fed rabbits.RT-PCR data were normalized to β-actin and presented as mean ± SEM of intensity. ∗∗p < 0.01; ∗∗∗p < 0.001, by one-way analysis of variance with the Bonferroni multiple range test.
4. Discussion
Associations between NAFLD and atherosclerosis have been reported in several large epidemiology studies [3, 20, 21]. However, few studies have investigated whether the two diseases share any common pathophysiological mechanisms, which would suggest that they may respond to the same therapy. In the present study, the consumption of a high cholesterol diet for 35 days in a rabbit model resulted in the development of NAFLD and early atherosclerosis. We developed an oral medicine composed of L. lactis expressing the LZ8 protein in a nisin-controlled gene expression system and investigated its anti-inflammatory properties. The mucosal administration of therapeutic molecules offers several advantages, such as easy administration and a reduction in adverse effects. Our results showed that the recombinant LZ8 L. lactis had a promising anti-inflammatory effect on the development of fatty liver and atheroma in cholesterol-fed rabbits.
Our findings further demonstrated that under a continuous high cholesterol diet, serum total cholesterol, LDL-C, and triglycerides remained at high levels. This pattern of dyslipidemia is commonly seen in real-world atherosclerotic patients with a poor diet or receiving medication but with a poor response to lipid-lowering treatment. The LDL-C deposited in the subendothelial space became oxidized LDL, resulting in severe inflammatory processes. IL-1β is an important proinflammatory cytokine that has been associated with the pathogenesis of vascular and immune diseases [22, 23]. In the current study, the aorta expression of IL-1β was significantly suppressed after LZ8 treatment. Moreover, H&E staining of the aorta showed that the intima-medial thickness was decreased and the number of foam cells was significantly reduced in the subendothelial space. In the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) trial [24], the use of a highly selective IL-1β monoclonal antibody (canakinumab) to inhibit inflammation in patients with atherosclerosis led to a significantly lower rate of recurrent cardiovascular events, without inducing any change in the lipid profile. This result suggests that anti-inflammatory therapy may play an important role in treating atherosclerosis, although the molecular mechanism remains unclear. Methotrexate is a nonselective IL-1β inhibitor, and it has been shown to suppress inflammation by diminishing cytokine production [25]. In the Cardiovascular Inflammation Reduction Trial [26], low-dose methotrexate was used for secondary prevention in patients with previous myocardial infarction or multivessel coronary disease. The results showed that methotrexate did not reduce levels of IL-1β, IL-6, or C-reactive protein and did not improve cardiovascular events. These clinical drug trials suggest that only specific inhibition of IL-1β effectively reduces inflammation and progression of atherosclerosis.
The beneficial functions of LZ8, which is also a bioactive component of Ganoderma lucidum, have been reported in several in vitro and in vivo studies. For example, Hsu et al. showed that LZ8 could suppress intestinal inflammation via a CD45-dependent signaling pathway in a mouse model [27]. In addition, You et al. reported that LZ8 may bind to a specific type of N-glycan that suppresses tumor growth and migration of HCC413 cells [26], and Liang et al. reported that Pichia-expressing recombinant LZ8 could induce autophagic cell death in human gastric carcinoma [28]. Furthermore, the oral administration of recombinant LZ8 has been shown to accelerate wound healing in rat livers after monopolar electrosurgery [13]. Recently, Chen et al. demonstrated that LZ8 could reduce nitric oxide synthase and toll-like receptor-4 and suppress the expression of NF-κB in a murine cell line [29].
Recent reports have shown that inflammation plays an important role in the initiation of NAFLD and atherosclerosis. In the present study, we further demonstrated that LZ8 inhibited the expression of IL-1β in the liver, decreased fat droplet deposition and infiltration of inflammatory cells, and improved liver function by decreasing liver enzymes (AST, ALT). However, there were several limitations to this study. First, although feeding rabbits with a high cholesterol diet is a well-established animal model for atherosclerotic heart disease, only a few studies have used it as a model for NAFLD [30]. The rabbits' levels of serum cholesterol and triglycerides increased rapidly after consuming cholesterol. However, atherosclerotic patients seldom have a serum cholesterol level over 1000 mg/dL or an LDL level above 500 mg/dL. Thus, rabbits fed with a 2% cholesterol diet may exhibit physiological phenomena that are somewhat different from those observed in real-world atherosclerosis in human subjects. Second, this preliminary study is the first to demonstrate that the administration of LZ8 protein in an animal model could inhibit NAFLD and early atherogenesis within 5 weeks. Further studies are required to investigate the long-term benefits or adverse effects of this treatment. In future studies, we will include 6–10 animals in each group of rabbits in order to obtain more definitive results.
5. Conclusions
Taken together, our findings suggest that the anti-inflammatory effect of Lactococcus-expressing LZ8, which is available as a safe and low-cost food, might be a promising medicine for improving both atherosclerosis and NAFLD.
Acknowledgments
This study was supported by grants from Taichung Veterans General Hospital (TCVGH-1077313C and TCVGH-1073107C).
Data Availability
All data used to support the findings of this study are included in the article.
Ethical Approval
The protocols used in the rabbit experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Taichung Veterans General Hospital (La-1071566).
Conflicts of Interest
The authors have no conflicts of interest.
Authors' Contributions
MF Lee and WW Lin designed the experiments and wrote the manuscript. CH Chiang and HC Liu performed the experiments and data analysis. SJ Lin and PP Song performed the animal studies. All of the authors read and approved the final version of the manuscript before submitting.
Supplementary Materials
Supplement Figure 1: alignment of nucleotide sequences of modified Ganoderma lucidum immunomodulatory protein (DMR415LZ8) and GenBank no. M58032.1 by MUSCLE version 3.8. Sequences are aligned with translated region and gaps (dashes) are introduced to optimize alignment. ∗represents homology. The similarity between them is 70.57%.
References
- 1.Libby P., Loscalzo J., Ridker P. M., et al. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. Journal of the American College of Cardiology. 2018;72(17):2071–2081. doi: 10.1016/j.jacc.2018.08.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein J. L., Brown M. S. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161(1):161–172. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pais R., Redheuil A., Cluzel P., Ratziu V., Giral P. Relationship among fatty liver, specific and multiple-site atherosclerosis, and 10-year Framingham score. Hepatology. 2019;69(4):1453–1463. doi: 10.1002/hep.30223. [DOI] [PubMed] [Google Scholar]
- 4.Athyros V. G., Alexandrides T. K., Bilianou H., et al. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An expert panel statement. Metabolism. 2017;71:17–32. doi: 10.1016/j.metabol.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Lee S. B., Park G.-M., Lee J.-Y., et al. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: an observational cohort study. Journal of Hepatology. 2018;68(5):1018–1024. doi: 10.1016/j.jhep.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Taylor E., Huang N., Bodde J., et al. MRI of atherosclerosis and fatty liver disease in cholesterol fed rabbits. Journal of Translational Medicine. 2018;16(1):p. 215. doi: 10.1186/s12967-018-1587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan J., Chen Y., Yan H., et al. Genomic and transcriptomic analysis of hypercholesterolemic rabbits: progress and perspectives. International Journal of Molecular Sciences. 2018;19(11):p. 3512. doi: 10.3390/ijms19113512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kino K., Yamashita A., Yamaoka K., et al. Isolation and characterization of a new immunomodulatory protein, Ling Zhi-8 (LZ-8), from Ganoderma lucidium. Journal of Biological Chemistry. 1989;264(1):472–478. [PubMed] [Google Scholar]
- 9.Huang L., Sun F., Liang C., et al. Crystal structure of LZ-8 from the medicinal fungus Ganoderma lucidium. Proteins: Structure, Function, and Bioinformatics. 2009;75(2):524–527. doi: 10.1002/prot.22346. [DOI] [PubMed] [Google Scholar]
- 10.Lin C.-C., Yu Y.-L., Shih C.-C., et al. A novel adjuvant Ling Zhi-8 enhances the efficacy of DNA cancer vaccine by activating dendritic cells. Cancer Immunology, Immunotherapy. 2011;60(7):1019–1027. doi: 10.1007/s00262-011-1016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Y.-L., Liang Y.-C., Tseng Y.-S., et al. An immunomodulatory protein, Ling Zhi-8, induced activation and maturation of human monocyte-derived dendritic cells by the NF-κB and MAPK pathways. Journal of Leukocyte Biology. 2009;86(4):877–889. doi: 10.1189/jlb.0708441. [DOI] [PubMed] [Google Scholar]
- 12.Hsu H. A., Wu C. Y., Chu J. S., et al. Effect of recombinant human bone morphogenetic protein-2 and Ling Zhi-8 on osteogenesis: a comparative study using a rabbit sinus model. Journal of Oral and Maxillofacial Surgery. 2014;72(9):1703.e1–1703.e10. doi: 10.1016/j.joms.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 13.Lin H. J., Chang Y. S., Lin L. H., Haung C.-F., Wu C.-Y., Ou K.-L. An immunomodulatory protein (Ling Zhi-8) from a Ganoderma lucidum induced acceleration of wound healing in rat liver tissues after monopolar electrosurgery. Evidence-Based Complementary and Alternative Medicine. 2014;2014:12. doi: 10.1155/2014/916531.916531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platteeuw C., van Alen-Boerrigter I., van Schalkwijk S., de Vos W. M. Food-grade cloning and expression system for Lactococcus lactis. Applied and Environmental Microbiology. 1996;62(3):1008–1013. doi: 10.1128/aem.62.3.1008-1013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Innocentin S., Guimaraes V., Miyoshi A., et al. Lactococcus lactis expressing either Staphylococcus aureus fibronectin-binding protein A or Listeria monocytogenes internalin A can efficiently internalize and deliver DNA in human epithelial cells. Applied and Environmental Microbiology. 2009;75(14):4870–4878. doi: 10.1128/aem.00825-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pontes D. S., de Azevedo M. S. P., Chatel J.-M., Langella P., Azevedo V., Miyoshi A. Lactococcus lactis as a live vector: heterologous protein production and DNA delivery systems. Protein Expression and Purification. 2011;79(2):165–175. doi: 10.1016/j.pep.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Lee M.-F., Chen Y.-H., Chiang C.-H., Lin S.-J., Song P.-P. Analysis of 10 environmental allergen components of the American cockroach in Taiwan. Annals of Allergy, Asthma & Immunology. 2016;117(5):535–541. doi: 10.1016/j.anai.2016.09.432. [DOI] [PubMed] [Google Scholar]
- 18.Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and Beyond. Journal of the American College of Cardiology. 2017;70(18):2278–2289. doi: 10.1016/j.jacc.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szekely Y., Arbel Y. A review of interleukin-1 in heart disease: where do we stand today? Cardiology and Therapy. 2018;7(1):25–44. doi: 10.1007/s40119-018-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wójcik-Cichy K., Koślińska-Berkan E., Piekarska A. The influence of NAFLD on the risk of atherosclerosis and cardiovascular diseases. Clinical and Experimental Hepatology. 2018;4(1):1–6. doi: 10.5114/ceh.2018.73155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanWagner L. B. New insights into NAFLD and subclinical coronary atherosclerosis. Journal of Hepatology. 2018;68(5):890–892. doi: 10.1016/j.jhep.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alten R., Gram H., Joosten L. A., et al. The human anti-IL-1β monoclonal antibody ACZ885 is effective in joint inflammation models in mice and in a proof-of-concept study in patients with rheumatoid arthritis. Arthritis Research & Therapy. 2008;10(3):p. R67. doi: 10.1186/ar2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W., Yin Y., Zhou Z., He M., Dai Y. OxLDL-induced IL-1beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflammation Research. 2014;63(1):33–43. doi: 10.1007/s00011-013-0667-3. [DOI] [PubMed] [Google Scholar]
- 24.Ridker P. M., Everett B. M., Thuren T., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. The New England Journal of Medicine. 2014;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 25.Brody M., Böhm I., Bauer R. Mechanism of action of methotrexate: experimental evidence that methotrexate blocks the binding of interleukin 1 beta to the interleukin 1 receptor on target cells. European Journal of. Clinical Chemistry and. Clinical Biochemistry. 1993;31(10):667–674. doi: 10.1515/cclm.1993.31.10.667. [DOI] [PubMed] [Google Scholar]
- 26.Ridker P. M., Everett B. M., Pradhan A., et al. Low-dose methotrexate for the prevention of atherosclerotic events. The New England Journal of Medicine. 2018;380(8):752–762. doi: 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu H. Y., Kuan Y. C., Lin T. Y., et al. Reishi protein LZ-8 induces FOXP3+ treg expansion via a CD45-dependent signaling pathway and alleviates acute intestinal inflammation in mice. Evidence-Based Complementary and Alternative Medicine. 2013;2013:11. doi: 10.1155/2013/513542.513542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang C., Li H., Zhou H., et al. Recombinant Lz-8 from Ganoderma lucidum induces endoplasmic reticulum stress-mediated autophagic cell death in SGC-7901 human gastric cancer cells. Oncology Reports. 2012;27(4):1079–1089. doi: 10.3892/or.2011.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S.-J., Lin H.-H., Huang W.-C., et al. Ling-Zhi-8 protein (LZ-8) suppresses the production of pro-inflammatory mediators in murine microglial BV-2 cells. Food and Agricultural Immunology. 2017;28(6):1393–1407. doi: 10.1080/09540105.2017.1346062. [DOI] [Google Scholar]
- 30.Kainuma M., Fujimoto M., Sekiya N., et al. Cholesterol-fed rabbit as a unique model of nonalcoholic, nonobese, non-insulin-resistant fatty liver disease with characteristic fibrosis. Journal of Gastroenterology. 2006;41(10):971–980. doi: 10.1007/s00535-006-1883-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1: alignment of nucleotide sequences of modified Ganoderma lucidum immunomodulatory protein (DMR415LZ8) and GenBank no. M58032.1 by MUSCLE version 3.8. Sequences are aligned with translated region and gaps (dashes) are introduced to optimize alignment. ∗represents homology. The similarity between them is 70.57%.
Data Availability Statement
All data used to support the findings of this study are included in the article.