Summary
Background
There is a scarcity of published data on the global prevalence of obstructive sleep apnoea, a disorder associated with major neurocognitive and cardiovascular sequelae. We used publicly available data and contacted key opinion leaders to estimate the global prevalence of obstructive sleep apnoea.
Methods
We searched PubMed and Embase to identify published studies reporting the prevalence of obstructive sleep apnoea based on objective testing methods. A conversion algorithm was created for studies that did not use the American Academy of Sleep Medicine (AASM) 2012 scoring criteria to identify obstructive sleep apnoea, allowing determination of an equivalent apnoea-hypopnoea index (AHI) for publications that used different criteria. The presence of symptoms was not specifically analysed because of scarce information about symptoms in the reference studies and population data. Prevalence estimates for obstructive sleep apnoea across studies using different diagnostic criteria were standardised with a newly developed algorithm. Countries without obstructive sleep apnoea prevalence data were matched to a similar country with available prevalence data; population similarity was based on the population body-mass index, race, and geographical proximity. The primary outcome was prevalence of obstructive sleep apnoea based on AASM 2012 diagnostic criteria in individuals aged 30–69 years (as this age group generally had available data in the published studies and related to information from the UN for all countries).
Findings
Reliable prevalence data for obstructive sleep apnoea were available for 16 countries, from 17 studies. Using AASM 2012 diagnostic criteria and AHI threshold values of five or more events per h and 15 or more events per h, we estimated that 936 million (95% CI 903–970) adults aged 30–69 years (men and women) have mild to severe obstructive sleep apnoea and 425 million (399–450) adults aged 30–69 years have moderate to severe obstructive sleep apnoea globally. The number of affected individuals was highest in China, followed by the USA, Brazil, and India.
Interpretation
To our knowledge, this is the first study to report global prevalence of obstructive sleep apnoea; with almost 1 billion people affected, and with prevalence exceeding 50% in some countries, effective diagnostic and treatment strategies are needed to minimise the negative health impacts and to maximise cost-effectiveness.
Introduction
Obstructive sleep apnoea is a common disorder that can present with or without symptoms and is accompanied by major neurocognitive and cardiovascular sequelae.1–3 At present, care of patients with obstructive sleep apnoea varies by country and depends on a patient’s symptoms. In well resourced settings, considerable efforts are being made to diagnose and treat individuals with obstructive sleep apnoea, but available data suggest that most cases of obstructive sleep apnoea remain undiagnosed and untreated, even in developed countries. In developing countries, there is generally little awareness of obstructive sleep apnoea, and diagnostic and treatment options are often not available or have not been adapted for resource-poor settings.4 Because of the multifactorial and social consequences of obstructive sleep apnoea, the disorder is associated with a high economic and societal burden. In 2015, the cost of diagnosing and treating obstructive sleep apnoea in the USA was approximately US$12·4 billion.5 The global cost of diagnosing and treating obstructive sleep apnoea has not been estimated because information about global prevalence is required first.
Evidence suggests that obstructive sleep apnoea is an important contributor to poor health outcomes and that treatment of this condition is generally beneficial in minimising the associated adverse clinical outcomes and improving sleep-related quality of life.6 Thus, focusing on effective treatment of obstructive sleep apnoea might be one approach for reducing associated health-care costs and the negative impact of the condition, such as the cognitive impact of sleepiness.7,8 Additionally, given the shift in focus from issues around malnutrition and basic hygiene towards chronic health conditions, such as the obesity pandemic and its associated metabolic complications,9–12 the ageing population demographic,13 and the association between obstructive sleep apnoea and various non-communicable diseases,14 obstructive sleep apnoea is likely to be a rising global problem over the coming years.15,16
Planning for effective diagnosis and management strategies requires accurate and country-specific estimates of disease prevalence. In 2007, WHO estimated that more than 100 million individuals were affected by obstructive sleep apnoea worldwide,17 but this estimate was acknowledged to be only an approximation based on data available at the time. In this study we used a new approach and the latest publicly available data to estimate the global prevalence of obstructive sleep apnoea. We aimed to estimate the total number of affected individuals around the world and the proportion of those with moderate or severe obstructive sleep apnoea because positive airway pressure is recommended in all patients with excessive sleepiness, impaired sleep-related quality of life, and comorbid hypertension, which are more likely with an apnoea-hypopnoea index (AHI) of 15 or more events per h.18 The aim of our analysis was to raise awareness of the global burden of obstructive sleep apnoea by providing data to help guide strategies and health policies to address this important health and societal problem, and to highlight substantial gaps in rigorously assessed obstructive sleep apnoea prevalence data that are currently unavailable for much of the global population. These gaps should be of great concern because of the wide-ranging negative sequelae of obstructive sleep apnoea
Methods
Search strategy and selection criteria
We searched PubMed and Embase to identify prevalence studies of obstructive sleep apnoea done in the general or community population, in adults (aged 18 years and older), where obstructive sleep apnoea was measured objectively with a sleep study. We selected general population prevalence studies rather than those done in selected groups on the basis of concomitant diseases or those done in clinical settings where referral bias could be an important limitation. Only studies that used objective testing (rather than questionnaires) to diagnose obstructive sleep apnoea were included in our analysis. This included use of either home sleep testing (including airflow and oximetry) or in-laboratory polysomnography and reporting the AHI. We had no specific requirement for how obstructive sleep apnoea symptoms were evaluated or reported. The timeframe for studies was not limited in the search and the key words used in all combinations were “sleep disordered breathing”, “sleep apnoea”, “sleep apnoea syndrome”, “obstructive”, “prevalence”, and “population”. The original search was done in April, 2017, and then rechecked in February, 2019 (no new papers were identified in the second search). Reference lists of identified papers were manually reviewed. We applied no language exclusions so papers published in other languages were translated into English. Authors of the major studies identified were invited to collaborate. Our estimates focused on individuals aged 30–69 years because data for this group were generally available from the published studies and related to information from the UN for all countries. Therefore, the resulting prevalence estimates relate specifically to a subset of the global population aged 30–69 years.
Data collection and analysis
For studies that did not use the American Academy of Sleep Medicine (AASM) 2012 scoring criteria19 to identify obstructive sleep apnoea, we created a conversion algorithm based on the study by Duce and colleagues20; this approach allowed us to determine an equivalent AHI for publications that used different criteria:21
Thus, we were able to report AHI figures based on the AASM 1999, AASM 2007, and AASM 2012 criteria, even if these criteria were not used in the original studies.19,22 The main differences between the criteria relate to scoring of hypopnoeas (table 1).
Table 1:
| Hypopnoea criteria | |
|---|---|
| 1999 guideline | ≥50% decrease in flow OR a clear reduction in flow that does not reach ≥50% AND is associated with either an oxygen desaturation of ≥3% or an arousal |
| 2007 guideline | ≥30% decrease in flow from baseline with an associated oxygen desaturation of ≥4% |
| 2012 guideline | ≥30% decrease in flow from baseline with an associated oxygen desaturation of ≥3% OR an associated arousal |
An algorithm was developed in consultation with all authors to estimate global prevalence of obstructive sleep apnoea in individuals aged 30–69 years from the available published data. Prevalence for men and women was estimated separately and then combined to provide total prevalence. For countries with published prevalence estimates for obstructive sleep apnoea, these data were used. If no country-specific estimates were available, we matched countries without prevalence data to similar countries with prevalence data; determination of country population similarity was based on the population body-mass index (BMI; within 1 kg/m2), race (smallest difference in proportion of white, black, and Asian populations between the country being matched and the reference country or countries), and geographical proximity based on distance (appendix p 1). Age, sex, BMI, and race were used to match countries because these factors are clinically recognised to affect prevalence of obstructive sleep apnoea and these data were available for all countries. We obtained data on global estimates of BMI from the WHO Global Health Observatory data repository, data on race from the Central Intelligence Agency’s World Factbook, and data on population-based age and sex from the UN World Population Prospects 2015. We evaluated the performance of our estimation procedure by comparing its performance in countries where prevalence data were available. Goodness of fit for these comparisons is shown in the appendix (pp 1–3). In two studies, the overall number of individuals used for the obstructive sleep apnoea prevalence estimates were reported, but not sex-specific numbers. In these cases, we assumed a 50:50 distribution between the sexes.
Our confidence intervals for the global estimate incorporate the sampling variability in the estimates of obstructive sleep apnoea prevalence from 17 studies, but do not incorporate the modelling error in estimating each country’s prevalence estimate (since the algorithm was not a statistical model). A sensitivity analysis based on a regression model is described below, which does incorporate modelling error into the confidence interval. The sampling variability confidence interval assesses how the variability in each study’s prevalence estimate affects the global obstructive sleep apnoea estimate. Specifically, the global estimate can be defined as follows:
where Ni is the population size of the ith subgroup (each subgroup is a sex in a specific country), K=386 (193 countries multiplied by 2 for both sexes) and pi is the obstructive sleep apnoea prevalence estimate applied to the ith subgroup. Since M=32 prevalence estimates were applied to the 386 population sizes (M is the number of reference prevalence estimates; 15 studies produced estimates for men and women, and two studies produced a single-sex estimate), the estimate can be rewritten by summing the population sizes of all subgroups to which each estimate was applied:
where Mk is the total population size to which the kth obstructive sleep apnoea prevalence estimate was applied. The obstructive sleep apnoea prevalence estimate pk has variance pk(1 − pk)/nk, where nk is the number of individuals used to estimate pk. The individuals used in each prevalence estimate are independent; thus, the variance of the global estimate is Var(X), and the 95% CI is
As a sensitivity analysis, an over-dispersed logistic regression model was fit to data from the prevalence estimate studies (each sex was modelled separately). The prevalence estimate of obstructive sleep apnoea was treated as the dependent variable; BMI and proportion of the white population were treated as continuous independent variables, continent was treated as a categorical independent variable, and an over-dispersion factor was added to the model to account for the wide variability across countries. This model then allowed for construction of a 95% CI that incorporated sampling variability in each study as well as the modelling errors in predicting each country’s prevalence estimate. Since we found no studies of obstructive sleep apnoea prevalence in Africa, Africa was grouped with Oceania (similar estimates were obtained if Africa was grouped with Europe, North America, or South America). Statistical analyses were done with R software, version 3.3.
Role of the funding source
This work represents an academic and industry partnership. The study sponsor was involved in study design, data collection, data analysis, data interpretation, and writing of the report. All authors had full access to all the data and collectively made the decision to submit for publication.
Results
17 articles were included in the analyses (table 2).23–39 Reliable prevalence estimates were obtained on the basis of available data for 16 of 193 countries in the world; prevalence estimates for the remaining countries were extrapolated as described above.
Table 2:
Studies reporting country-specific data on obstructive sleep apnoea prevalence
| Sample size | Year | Age range, years | Men (%) | Scoring criteria | Nasal pressure | AHI ≥5 events per h |
AHI ≥15 events per h |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||||
| Australia24 | 380 | 2008 | 40–65 | 73% | AASM 2012 | Yes | 25·5% | 23·5% | 4·7% | 4·9% |
| Brazil25 | 1042 | 2010 | 20–80 | 45% | AASM 2007 | Yes | 46·5% | 30·6% | 24·8% | 9·6% |
| China38 | 3648 | 2005 | ≥20 | 50% | Chicago 1999 | Unspecified | 24·2% | 24·2% | 9·5% | 9·5% |
| Germany39 | 1208 | 2018 | 20–81 | 54% | AASM 2007 | Yes | 59·4% | 33·2% | 29·7% | 13·2% |
| Hong Kong27 | 153 | 2001 | 30–60 | 100% | AASM 2007 | No | 8·8% | ·· | 5·3% | ·· |
| Hong Kong28 | 106 | 2004 | 30–60 | 0% | AASM 2007 | No | ·· | 3·7% | ·· | 1·9% |
| Iceland29 | 415 | 2016 | 40–65 | ·· | AASM 2007 | Yes | 13·3% | 10·8% | 10·6% | 4·8% |
| India30 | 365 | 2009 | 30–65 | ·· | Chicago 1999 | Yes | 13·5% | 6·1% | 5·5% | 6·1% |
| Japan32 | 322 | 2008 | 23–59 | ·· | AASM 2012 | Yes | 59·7% | ·· | 22·3% | ·· |
| New Zealand31 | 364 | 2009 | 30–59 | ·· | AASM 2007 | Yes | 12·5% | 3·4% | 3·9% | 0·2% |
| Norway33 | 518 | 2011 | 30–65 | 55% | AASM 2007 | No | 21·0% | 13·0% | 11·0% | 6·0% |
| Poland34 | 676 | 2008 | 41–72 | 54% | AASM 2007 | Yes | 36·2% | 18·4% | 15·8% | 7·6% |
| South Korea35 | 457 | 2004 | 40–69 | 69% | AASM 2007 | No | 27·1% | 16·8% | 10·1% | 4·7% |
| Singapore36 | 242 | 2016 | 21–79 | 50% | AASM 2007 | Yes | 62·3% | 62·3% | 26·1% | 26·1% |
| Singapore36 | 242 | 2016 | 21–79 | 50% | AASM 2012 | Yes | 70·8% | 70·8% | 30·5% | 30·5% |
| Spain37 | 2148 | 2001 | 30–70 | 49% | AASM 2007 | No | 26·2% | 28·0% | 14·2% | 7·0% |
| Switzerland23 | 2121 | 2015 | 40–85 | 48% | AASM 2012 | Yes | 83·8% | 60·8% | 49·7% | 23·4% |
| USA26 | 1520 | 2013 | 30–70 | 55% | AASM 2007 | Yes | 33·9% | 17·4% | 13·0% | 6·0% |
Based on two different apnoea-hypopnoea index (AHI) cutoff values.
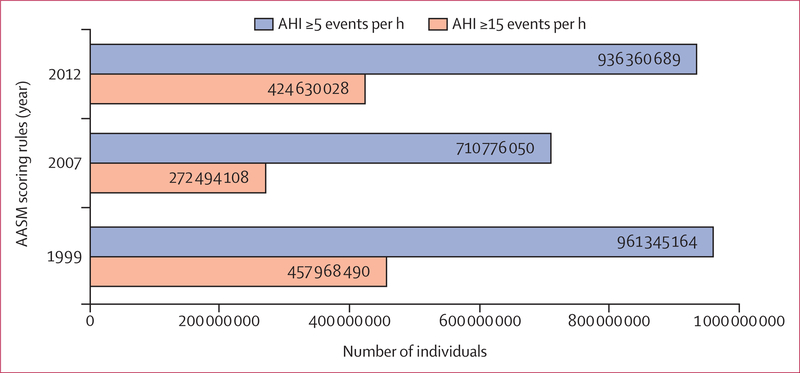
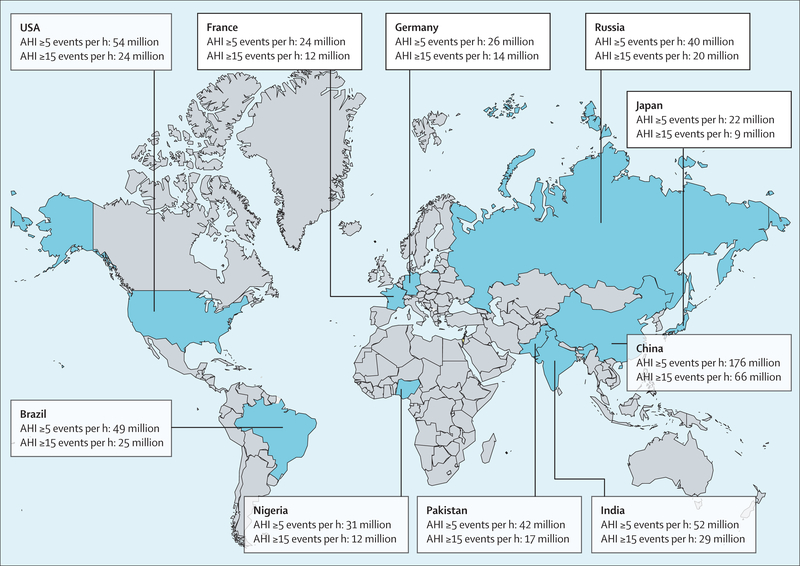
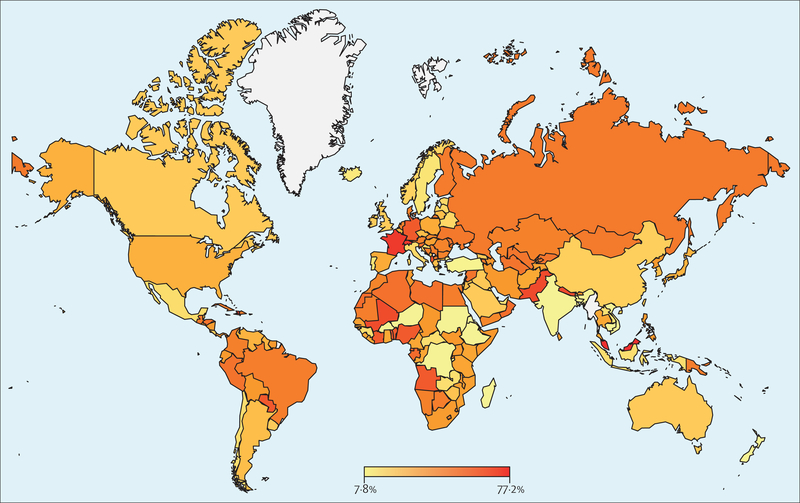
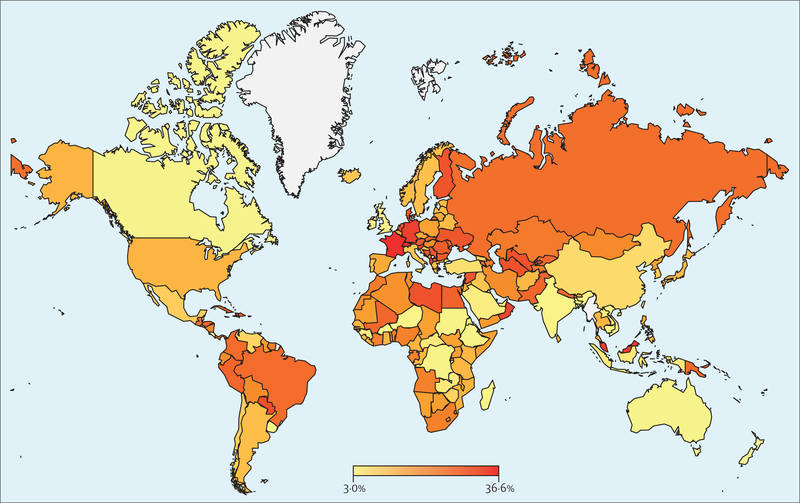
Using an AHI criterion of five or more events per h and the AASM 2012 criteria, an estimated 936 million (95% CI 903–970) individuals aged 30–69 years (men and women) worldwide were found to have obstructive sleep apnoea; the corresponding figure for an AHI of 15 or more events per h was 425 million (399–450; figure 1). The logistic regression model yielded a global obstructive sleep apnoea (AHI of five or more events per h) estimate of 730 million (618–842). Numbers of affected individuals were lower when the AASM 2007 criteria were applied and highest with the 1999 AASM criteria (figure 1). The estimated number of individuals with obstructive sleep apnoea was highest in China, followed by the USA, Brazil, and India; other countries in the top ten for the number of individuals with obstructive sleep apnoea were Pakistan, Russia, Nigeria, Germany, France, and Japan (figure 2), which predominantly reflects the overall size of the population of these countries. When expressed as a prevalence, the obstructive sleep apnoea burden for each country is shown as a heat map for an AHI of five or more events per h and an AHI of 15 or more events per h (figures 3, 4). A summary of the number of affected individuals and overall obstructive sleep apnoea prevalence for each country, region, or territory is provided in table 3.
Figure 1: Estimated prevalence of obstructive sleep apnoea based on different scoring rules.
AASM=American Academy of Sleep Medicine. AHI=apnoea-hypopnoea index.
Figure 2: Top ten countries with the highest estimated number of individuals with obstructive sleep apnoea based on the American Academy of Sleep Medicine 2012 criteria19.
AHI=apnoea-hypopnoea index.
Figure 3: Global heat map of estimated prevalence of obstructive sleep apnoea (AHI five or more events per h) for each country.
AHI=apnoea-hypopnoea index.
Figure 4: Global heat map of estimated prevalence of obstructive sleep apnoea (AHI 15 or more events per h) for each country.
AHI=apnoea-hypopnoea index.
Table 3:
Number of patients with obstructive sleep apnoea and prevalence of obstructive sleep apnoea by country
| Population aged 30–69 years | AHI ≥5 events per h | AHI ≥15 events per h | |
|---|---|---|---|
| Afghanistan | 8 429 549 | 3 040 802 (36·1%) | 1 171 173 (13·9%) |
| Albania | 1 357 655 | 850 606 (62·7%) | 467 163 (34·4%) |
| Algeria | 16 435 999 | 8 807 804 (53·6%) | 3 389 447 (20·6%) |
| Angola | 6 091 184 | 3 670 768 (60·3%) | 1 392 665 (22·9%) |
| Antigua and Barbuda | 42 485 | 10 181 (24·0%) | 3010 (7·1%) |
| Argentina | 19 016 260 | 5 443 083 (28·6%) | 2 490 616 (13·1%) |
| Armenia | 1 513 141 | 768 580 (50·8%) | 380 977 (25·2%) |
| Aruba | 56 900 | 13 896 (24·4%) | 2736 (4·8%) |
| Australia | 12 110 362 | 2 966 536 (24·5%) | 581 348 (4·8%) |
| Austria | 4 601 766 | 2 241 500 (48·7%) | 1 306 180 (28·4%) |
| Azerbaijan | 4 622 249 | 1 975 143 (42·7%) | 1 116 199 (24·1%) |
| Bahamas | 189 235 | 45 418 (24·0%) | 13 296 (7·0%) |
| Bahrain | 679 684 | 496 137 (73·0%) | 214 997 (31·6%) |
| Bangladesh | 62 623 678 | 5 983 546 (9·6%) | 3 381 433 (5·4%) |
| Barbados | 147 687 | 35 406 (24·0%) | 10 439 (7·1%) |
| Belarus | 5 155 802 | 1 068 785 (20·7%) | 708 401 (13·7%) |
| Belgium | 5 917 763 | 1 801 591 (30·4%) | 931 859 (15·7%) |
| Belize | 128 677 | 30 922 (24·0%) | 8982 (7·0%) |
| Benin | 3 096 334 | 1 865 770 (60·3%) | 707 741 (22·9%) |
| Bhutan | 310 278 | 137 202 (44·2%) | 51 229 (16·5%) |
| Bolivia | 3 816 716 | 1 592 038 (41·7%) | 812 240 (21·3%) |
| Bosnia and Herzegovina | 2 185 242 | 1 075 346 (49·2%) | 626 902 (28·7%) |
| Botswana | 826 982 | 410 657 (49·7%) | 152 857 (18·5%) |
| Brazil | 98 118 248 | 48 729 844 (49·7%) | 25 481 720 (26·0%) |
| Brunei | 205 295 | 158 488 (77·2%) | 69 390 (33·8%) |
| Bulgaria | 4 008 872 | 1 943 121 (48·5%) | 1 132 070 (28·2%) |
| Burkina Faso | 4 632 827 | 434 092 (9·4%) | 250 858 (5·4%) |
| Burundi | 2 912 564 | 276 938 (9·5%) | 157 378 (5·4%) |
| Cape Verde | 181 546 | 99 348 (54·7%) | 40 543 (22·3%) |
| Cambodia | 5 791 914 | 537 020 (9·3%) | 314 087 (5·4%) |
| Cameroon | 6 304 367 | 2 313 265 (36·7%) | 929 799 (14·7%) |
| Canada | 19 273 831 | 4 721 439 (24·5%) | 925 209 (4·8%) |
| Central African Republic | 1 443 196 | 267 053 (18·5%) | 100 838 (7·0%) |
| Chad | 3 268 977 | 1 384 009 (42·3%) | 586 746 (17·9%) |
| Chile | 8 809 775 | 1 671 811 (19·0%) | 954 857 (10·8%) |
| China | 744 511 252 | 175 704 655 (23·6%) | 65 516 990 (8·8%) |
| Colombia | 22 074 042 | 10 953 050 (49·6%) | 5 721 534 (25·9%) |
| Comoros | 235 465 | 116 985 (49·7%) | 43 546 (18·5%) |
| Congo | 1 344 345 | 498 468 (37·1%) | 191 977 (14·3%) |
| Costa Rica | 2 237 598 | 548 017 (24·5%) | 107 424 (4·8%) |
| Côte d’Ivoire | 6 367 814 | 3 835 249 (60·2%) | 1 453 687 (22·8%) |
| Croatia | 2 312 850 | 473 908 (20·5%) | 276 871 (12·0%) |
| Cuba | 6 188 715 | 3 096 611 (50·0%) | 1 633 275 (26·4%) |
| Curacao | 81 939 | 27 359 (33·4%) | 8404 (10·3%) |
| Cyprus | 603 532 | 175 258 (29·0%) | 101 317 (16·8%) |
| Czech Republic | 5 973 449 | 1 726 302 (28·9%) | 994 554 (16·6%) |
| Democratic Republic of the Congo | 19 722 105 | 1 870 886 (9·5%) | 1 066 027 (5·4%) |
| Denmark | 2 927 893 | 1 430 836 (48·9%) | 833 901 (28·5%) |
| Djibouti | 310 917 | 115 180 (37·0%) | 44 360 (14·3%) |
| Dominican Republic | 4 125 868 | 2 055 301 (49·8%) | 1 078 533 (26·1%) |
| Ecuador | 6 442 389 | 2 682 640 (41·6%) | 1 366 852 (21·2%) |
| Egypt | 34 241 981 | 17 065 251 (49·8%) | 9 373 686 (27·4%) |
| El Salvador | 2 385 301 | 930 046 (39·0%) | 518 118 (21·7%) |
| Equatorial Guinea | 269 554 | 151 455 (56·2%) | 62 203 (23·1%) |
| Eritrea | 1 444 877 | 136 808 (9·5%) | 78 120 (5·4%) |
| Estonia | 686 893 | 141 780 (20·6%) | 94 804 (13·8%) |
| eSwatini | 348 096 | 158 683 (45·6%) | 67 383 (19·4%) |
| Ethiopia | 27 021 835 | 2 555 467 (9·5%) | 1 461 244 (5·4%) |
| Federated States of Micronesia | 31 811 | 7651 (24·1%) | 2211 (6·9%) |
| Fiji | 382 118 | 159 154 (41·7%) | 90 765 (23·8%) |
| Finland | 2 894 948 | 1 458 419 (50·4%) | 853 928 (29·5%) |
| France | 32 613 385 | 23 506 723 (72·1%) | 11 836 999 (36·3%) |
| French Polynesia | 133 978 | 56 102 (41·9%) | 32 053 (23·9%) |
| Gabon | 543 329 | 302 567 (55·7%) | 124 000 (22·8%) |
| Gambia | 503 578 | 303 519 (60·3%) | 115 181 (22·9%) |
| Georgia | 2 021 511 | 510 200 (25·2%) | 212 087 (10·5%) |
| Germany | 43 751 645 | 26 279 946 (60·1%) | 14 393 964 (32·9%) |
| Ghana | 8 651 157 | 3 100 709 (35·8%) | 1 251 736 (14·5%) |
| Greece | 5 966 188 | 1 708 436 (28·6%) | 782 555 (13·1%) |
| Grenada | 41 701 | 17 361 (41·6%) | 9899 (23·7%) |
| Guam | 76 175 | 49 640 (65·2%) | 20 063 (26·3%) |
| Guatemala | 5 115 784 | 2 619 198 (51·2%) | 1 303 428 (25·5%) |
| Guinea | 3 554 378 | 655 324 (18·4%) | 247 498 (7·0%) |
| Guinea-Bissau | 544 150 | 203 608 (37·4%) | 78 415 (14·4%) |
| Guyana | 315 281 | 101 440 (32·2%) | 60 003 (19·0%) |
| Haiti | 3 652 569 | 2 586 019 (70·8%) | 1 114 034 (30·5%) |
| Honduras | 2 770 841 | 1 473 684 (53·2%) | 746 876 (27·0%) |
| Hong Kong | 4 377 697 | 340 901 (7·8%) | 229 985 (5·3%) |
| Hungary | 5 445 718 | 2 104 733 (38·6%) | 1 139 523 (20·9%) |
| Iceland | 162 564 | 25 372 (15·6%) | 19 136 (11·8%) |
| India | 534 676 709 | 51 556 642 (9·6%) | 28 831 856 (5·4%) |
| Indonesia | 114 334 042 | 21 020 883 (18·4%) | 7 940 317 (6·9%) |
| Iran | 36 179 787 | 15 136 458 (41·8%) | 7 739 991 (21·4%) |
| Iraq | 10 771 896 | 2 734 061 (25·4%) | 1 303 288 (12·1%) |
| Ireland | 2 447 445 | 599 525 (24·5%) | 117 487 (4·8%) |
| Israel | 3 465 330 | 848 255 (24·5%) | 166 411 (4·8%) |
| Italy | 33 020 571 | 6 774 829 (20·5%) | 3 959 253 (12·0%) |
| Jamaica | 1 176 758 | 483 534 (41·1%) | 274 482 (23·3%) |
| Japan | 67 496 374 | 22 092 507 (32·7%) | 9 435 205 (14·0%) |
| Jordan | 2 636 480 | 751 598 (28·5%) | 413 338 (15·7%) |
| Kazakhstan | 7 774 654 | 3 779 915 (48·6%) | 1 862 950 (24·0%) |
| Kenya | 13 024 588 | 4 838 661 (37·2%) | 1 863 525 (14·3%) |
| Kiribati | 38 538 | 12 573 (32·6%) | 5486 (14·2%) |
| Kuwait | 2 012 453 | 377 748 (18·8%) | 146 612 (7·3%) |
| Kyrgyzstan | 2 226 975 | 1 170 950 (52·6%) | 590 266 (26·5%) |
| Laos | 2 144 108 | 377 558 (17·6%) | 132 705 (6·2%) |
| Latvia | 1 037 979 | 215 042 (20·7%) | 142 707 (13·7%) |
| Lebanon | 2 446 505 | 709 282 (29·0%) | 334 845 (13·7%) |
| Lesotho | 607 425 | 299 273 (49·3%) | 111 379 (18·3%) |
| Liberia | 1 306 827 | 478 462 (36·6%) | 192 392 (14·7%) |
| Libya | 2 692 584 | 437 964 (53·4%) | 796 612 (29·6%) |
| Lithuania | 1 492 888 | 426 074 (28·5%) | 243 247 (16·3%) |
| Luxembourg | 308 327 | 78 855 (25·6%) | 48 029 (15·6%) |
| Macao | 347 288 | 27 507 (7·9%) | 18 609 (5·4%) |
| Macedonia | 1 116 387 | 561 099 (50·3%) | 328 508 (29·4%) |
| Madagascar | 6 863 789 | 651 925 (9·5%) | 370 938 (5·4%) |
| Malawi | 4 141 023 | 1 743 713 (42·1%) | 739 079 (17·8%) |
| Malaysia | 13 270 264 | 10 244 644 (77·2%) | 4 485 349 (33·8%) |
| Maldives | 140 459 | 104 079 (74·1%) | 45 157 (32·1%) |
| Mali | 4 343 219 | 2 837 614 (65·3%) | 1 149 316 (26·5%) |
| Malta | 225 879 | 79 359 (35·1%) | 36 769 (16·3%) |
| Mauritania | 1 252 071 | 621 950 (49·7%) | 231 508 (18·5%) |
| Mauritius | 658 995 | 328 627 (49·9%) | 172 660 (26·2%) |
| Mexico | 52 649 824 | 9 921 240 (18·8%) | 5 655 904 (10·7%) |
| Moldova | 2 144 482 | 1 116 710 (52·1%) | 560 328 (26·1%) |
| Mongolia | 1 238 490 | 632 863 (51·1%) | 267 839 (21·6%) |
| Montenegro | 324 337 | 98 333 (30·3%) | 59 139 (18·2%) |
| Morocco | 14 584 119 | 6 974 795 (47·8%) | 3 472 365 (23·8%) |
| Mozambique | 7 104 990 | 2 763 293 (38·9%) | 1 064 150 (15·0%) |
| Namibia | 773 988 | 379 296 (49·0%) | 141 145 (18·2%) |
| Nepal | 10 032 271 | 6 585 332 (65·6%) | 2 677 534 (26·7%) |
| Netherlands | 9 050 266 | 4 430 942 (49·0%) | 2 582 583 (28·5%) |
| New Caledonia | 125 207 | 51 992 (41·5%) | 29 620 (23·7%) |
| New Zealand | 2 256 063 | 227 248 (10·1%) | 68 590 (3·0%) |
| Nicaragua | 2 282 569 | 919 626 (40·3%) | 518 443 (22·7%) |
| Niger | 4 811 710 | 463 647 (9·6%) | 259 493 (5·4%) |
| Nigeria | 51 068 452 | 30 766 820 (60·2%) | 11 667 219 (22·8%) |
| North Korea | 12 477 221 | 5 303 362 (42·5%) | 2 248 695 (18·0%) |
| Norway | 2 684 446 | 593 951 (22·1%) | 351 443 (13·1%) |
| Oman | 2 028 410 | 1 110 702 (54·8%) | 643 631 (31·7%) |
| Pakistan | 63 098 158 | 41 569 793 (65·9%) | 17 041 309 (27·0%) |
| Panama | 1 685 593 | 697 036 (41·4%) | 396 546 (23·5%) |
| Papua New Guinea | 2 560 672 | 1 281 720 (50·1%) | 676 302 (26·4%) |
| Paraguay | 2 441 919 | 1 476 456 (60·5%) | 745 739 (30·5%) |
| Peru | 12 928 630 | 6 873 395 (53·2%) | 3 482 848 (26·9%) |
| Philippines | 37 976 672 | 15 791 279 (41·6%) | 5 895 648 (15·5%) |
| Poland | 21 519 587 | 7 554 166 (35·1%) | 3 823 093 (17·8%) |
| Portugal | 5 691 681 | 967 049 (17·0%) | 713 458 (12·5%) |
| Puerto Rico | 1 807 598 | 892 217 (49·4%) | 463 203 (25·6%) |
| Qatar | 1 154 042 | 359 550 (31·2%) | 214 436 (18·6%) |
| Romania | 10 861 099 | 5 223 068 (48·1%) | 3 041 952 (28·0%) |
| Russia | 78 239 383 | 40 203 912 (51·4%) | 20 043 199 (25·6%) |
| Rwanda | 3 315 948 | 1 511 839 (45·6%) | 642 777 (19·4%) |
| Saint Lucia | 84 561 | 27 871 (33·0%) | 12 180 (14·4%) |
| Saint Vincent and the Grenadines | 49 135 | 20 548 (41·8%) | 11 735 (23·9%) |
| Samoa | 64 569 | 21 696 (33·6%) | 9509 (14·7%) |
| São Tomé and Principe | 52 190 | 28 630 (54·9%) | 11 691 (22·4%) |
| Saudi Arabia | 14 300 262 | 3 491 177 (24·4%) | 913 235 (6·4%) |
| Senegal | 4 064 706 | 1 577 592 (38·8%) | 607 536 (14·9%) |
| Serbia | 4 730 407 | 2 343 842 (49·5%) | 1 371 614 (29·0%) |
| Seychelles | 47 600 | 19 944 (41·9%) | 11 397 (23·9%) |
| Sierra Leone | 1 824 011 | 684 252 (37·5%) | 263 523 (14·4%) |
| Singapore | 3 237 710 | 2 292 299 (70·8%) | 987 502 (30·5%) |
| Slovakia | 3 059 176 | 1 415 361 (46·3%) | 824 991 (27·0%) |
| Slovenia | 1 162 317 | 632 758 (54·4%) | 360 969 (31·1%) |
| Solomon Islands | 183 167 | 100 926 (55·1%) | 41 256 (22·5%) |
| Somalia | 2 669 125 | 1 138 978 (42·7%) | 483 018 (18·1%) |
| South Africa | 20 931 899 | 8 448 087 (40·4%) | 4 765 612 (22·8%) |
| South Korea | 28 715 868 | 8 164 156 (28·4%) | 3 255 814 (11·3%) |
| South Sudan | 3 418 246 | 1 448 320 (42·4%) | 614 029 (18·0%) |
| Spain | 26 158 266 | 9 195 448 (35·2%) | 4 233 728 (16·2%) |
| Sri Lanka | 9 950 827 | 4 054 922 (40·7%) | 1 513 847 (15·2%) |
| Sudan | 12 004 972 | 1 141 322 (9·5%) | 648 692 (5·4%) |
| Suriname | 237 958 | 98 649 (41·5%) | 56 170 (23·6%) |
| Sweden | 4 918 210 | 836 190 (17·0%) | 626 258 (12·7%) |
| Switzerland | 4 518 615 | 3 269 301 (72·4%) | 1 654 232 (36·6%) |
| Syria | 6 085 585 | 3 204 881 (52·7%) | 1 770 598 (29·1%) |
| Taiwan | 13 539 701 | 3 195 369 (23·6%) | 1 191 494 (8·8%) |
| Tajikistan | 2 797 823 | 1 074 632 (38·4%) | 657 685 (23·5%) |
| Tanzania | 14 004 347 | 5 225 916 (37·3%) | 2 012 654 (14·4%) |
| Thailand | 37 728 597 | 13 743 556 (36·4%) | 5 531 503 (14·7%) |
| Timor Leste | 331 218 | 31 787 (9·6%) | 17 873 (5·4%) |
| Togo | 2 088 857 | 1 259 007 (60·3%) | 477 774 (22·9%) |
| Tonga | 34 943 | 11 485 (32·9%) | 5017 (14·4%) |
| Trinidad and Tobago | 699 011 | 167 969 (24·0%) | 48 803 (7·0%) |
| Tunisia | 5 283 180 | 2 187 476 (41·4%) | 1 109 698 (21·0%) |
| Turkey | 35 176 270 | 3 714 957 (10·6%) | 2 797 599 (8·0%) |
| Turkmenistan | 2 163 412 | 1 136 509 (52·5%) | 645 683 (29·8%) |
| Uganda | 8 850 820 | 3 751 125 (42·4%) | 1 590 342 (18·0%) |
| Ukraine | 24 593 547 | 12 691 100 (51·6%) | 7 194 877 (29·3%) |
| United Arab Emirates | 5 433 364 | 950 096 (17·5%) | 360 948 (6·6%) |
| UK | 32 936 962 | 8 065 555 (24·5%) | 1 581 374 (4·8%) |
| USA | 163 246 772 | 54 131 654 (33·2%) | 23 678 109 (14·5%) |
| Uruguay | 1 578 418 | 386 221 (24·5%) | 75 813 (4·8%) |
| Uzbekistan | 11 817 125 | 6 182 507 (52·3%) | 3 510 756 (29·7%) |
| Vanuatu | 89 478 | 44 758 (50·0%) | 23 599 (26·4%) |
| Venezuela | 12 968 894 | 4 243 846 (32·7%) | 1 269 241 (9·8%) |
| Vietnam | 43 025 626 | 4 070 306 (9·5%) | 2 326 560 (5·4%) |
| Virgin Islands | 53 922 | 12 913 (23·9%) | 3833 (7·1%) |
| Western Sahara | 271 213 | 149 225 (55·0%) | 58 160 (21·4%) |
| Yemen | 7 284 023 | 3 422 350 (47·0%) | 1 424 088 (19·6%) |
| Zambia | 3 950 721 | 729 005 (18·5%) | 275 312 (7·0%) |
| Zimbabwe | 4 064 530 | 1 092 368 (26·9%) | 408 656 (10·1%) |
Data are n (%). Based on apnoea-hypopnoea index (AHI) cutoff values of five or more events per h and 15 or more events per h.
Discussion
Our estimates suggest that nearly 1 billion adults aged 30–69 years worldwide could have obstructive sleep apnoea, and the number of people with moderate to severe obstructive sleep apnoea, for which treatment is generally recommended, is estimated to be almost 425 million. To the best of our knowledge, this analysis represents the first set of global estimates of the prevalence of obstructive sleep apnoea.
Based on the published literature and by matching countries based on population demographics where specific data were not available, we estimated that almost 1 billion people worldwide aged between 30–69 years have an AHI of five or more events per h. The overall prevalence of obstructive sleep apnoea is likely to be higher given that the analysis focused only on adults aged 30–69 years and we used AASM 2012 criteria rather than the more liberal AASM 1999 criteria.22,40 The prevalence of obstructive sleep apnoea will be lower in adults younger than 30 years, and higher in adults aged 70 years and older, than we have estimated in this analysis, since previous publications show a linear relationship between age and obstructive sleep apnoea prevalence.25 The number of individuals with moderate to severe obstructive sleep apnoea, based on an AHI of 15 or more events per h, was 425 million. This group could be considered the clinically important obstructive sleep apnoea population for whom treatment would be recommended, even though symptoms were not included in our estimate. We found that obstructive sleep apnoea prevalence estimates were dependent on the respiratory event scoring criteria used, but we focused on AASM 201219 because these guidelines are in widespread clinical use, and standardised data from different studies can be generated with a conversion factor. The optimal criteria for scoring is likely to depend on the technology used and the clinical outcomes of interest, but these variations are subtle compared with the large burden of disease that is undiagnosed at present. Based on the magnitude of these results, re-evaluation of the current AHI thresholds might be prudent when the latest sleep study scoring criteria and technologies are used in relation to clinical outcomes to identify the thresholds at which treatment is most appropriate. In 2007, WHO estimated that more than 100 million people worldwide were likely to have obstructive sleep apnoea.17 However, to our knowledge, this value was acknowledged to be a gross approximation based on the data available at the time. We believe our approach of a country-imputation algorithm is a legitimate attempt to quantitatively synthesise the available evidence to estimate the global prevalence of obstructive sleep apnoea. Our approach used the major known factors that influence prevalence of obstructive sleep apnoea by matching on age, sex, BMI, and race, and, if required, geographical proximity, to identify the most suitable reference study to be applied to a country without introducing further assumptions. Both the primary country-imputation algorithm and the secondary logistic modelling approach led to global estimates that were much larger than those previously reported by WHO, although they were similar in magnitude to previously reported estimates. Increasing prevalence of obstructive sleep apnoea globally is consistent with single-country trends.26 Various complex reasons probably underlie the discrepancy between our estimates and those from WHO in 2007, including the more precise methodological approach in our study and advances in detection technology.41 Various population and population health factors are also likely to have had a role. Obstructive sleep apnoea is common in patients with high BMI26,42 and with increasing age.26,37,42,43 Therefore, the worldwide obesity epidemic and the ageing population demographic are likely to contribute to the rising global prevalence of obstructive sleep apnoea. Additionally, the high prevalence seen in countries such as China is likely to be due to racial and genetic differences in common anatomical features that increase the likelihood of obstructive sleep apnoea, such as a narrower airway.44 The increasing burden of non-communicable diseases, such as diabetes, and more sedentary lifestyles could also have a role in the rising global prevalence of obstructive sleep apnoea.45
Our analysis highlights the importance of considering how the burden of obstructive sleep apnoea should be managed. In countries where a diagnosis of obstructive sleep apnoea is less well recognised, education and advocacy are required to inform both patients and health-care providers about this condition and its associated complications.4,46 In settings where technologies for diagnosing and treating obstructive sleep apnoea are not available, efforts to leverage alternative technologies to facilitate provision of cost-effective care need to be considered, along with strategies to improve accessibility. For example, wearable technologies, validated questionnaires, and connected devices could be used to optimise diagnosis.47,48 In this context, efforts are underway to try to diagnose obstructive sleep apnoea with simple devices that can be used anywhere in the world.49,50 Moreover, cloud-based technologies allow centralised reading and scoring of sleep tests so that local expertise is no longer needed to deliver appropriate diagnostic care.48,51 The high estimated global prevalence in this study suggests that the development of new and cheaper alternatives to diagnose and treat obstructive sleep apnoea are required.
For countries with well resourced health-care systems, delivery models whereby many patients can receive high-quality care without the need for multiple office visits with subspecialists need to be considered, along with alternative payment models.52 For uncomplicated obstructive sleep apnoea, models of care are being developed that allow excellent clinical outcomes while potentially minimising costs. For example, Antic and colleagues53 have shown that primary care physicians and nurse practitioners, under appropriate supervision, can achieve good outcomes in management of obstructive sleep apnoea. With the push towards cost effective care across the entire health-care system, efficient care delivery models are being increasingly discussed52 and resources reallocated to multimorbid obstructive sleep apnoea.54 Notably, although the diagnosis and treatment of sleep disorders can be viewed as a large economic burden, some health economic studies have suggested that management of obstructive sleep apnoea is not just cost effective but potentially cost saving as a result of prevention of major complications.54–56 Nevertheless, the cost of treatment devices might be an important barrier to treatment in low-income and middle-income countries. To improve health equity, it might be necessary for global health bodies, including WHO, the UN, and non-governmental organisations, to lobby for provision of treatment devices for obstructive sleep apnoea at costs substantially lower than current pricing to facilitate access in developing regions.
The findings of our study need to be interpreted in the context of various limitations. First, although the goal of our analysis was to include as much published data from several countries and be as accurate as possible, we found no published prevalence data for obstructive sleep apnoea for most countries, including no countries from Africa. We therefore used techniques designed to provide the best estimates possible using available data from groups with similar demographic characteristics. This led to the reference studies being represented many times because of the nature of our country-imputation algorithm (ie, we used data from 16 countries to make estimates for 177 countries worldwide). Although we believe our global estimates are robust when compared with the country-specific extrapolated estimates, individual country estimates were needed to generate the global estimate, so the country-specific estimates for those countries where no prevalence study was previously done should not be used in any formal way because these estimates might not be as precise as those for countries with available prevalence data. Additionally, we have not provided specific data for men and women or data about disease burden in different age groups. We hope that the data gaps identified in our analysis will help encourage more scrupulous epidemiological studies, particularly from regions where rigorous data are not currently available. Our sensitivity analysis, based on a logistic regression of the available data, yielded a lower estimate of the prevalence of obstructive sleep apnoea than the country-imputation algorithm (730 million vs 936 million) although it still confirmed a high prevalence of obstructive sleep apnoea. The logistic regression is lower because estimates from countries such as Singapore, Germany, and Switzerland are attenuated toward the means of their continent; the country-imputation algorithm does not reduce these estimates. Furthermore, logistic regression weights all predictor variables equally, whereas the country-imputation algorithm weights BMI as the most important factor in matching countries, racial distribution is a secondary factor, and geographical proximity is only used to resolve multiple matches on the first two predictors. This weighting of factors allows Australian men to be matched to Canadian men, for example, which would not be feasible in the logistic regression model. The logistic regression model also estimated that Asian countries similar to Singapore (BMI 24·2 kg/m2, 9% white) have an obstructive sleep apnoea prevalence of 16·7%, whereas the country-imputation algorithm estimated these countries’ prevalence as being 62·3%, as reported by Tan and colleagues.36 These were the major drivers for the difference in the global estimate between the country-imputation algorithm and the more generalised logistic regression model.
Second, we recognise that the parent studies from which we derived our estimates also have their own limitations, including first night effect, degree of sleep deprivation, body position, night to night variability, and participation and selection bias. Participation bias is an issue with many epidemiological studies and cannot easily be addressed with changing study design. Selection bias reflects the fact that people who are invited to participate might not be representative of the general population. This issue is amplified by the reliance on convenience samples rather than efforts to make sampling truly representative of the broader population. In acknowledging this limitation, we hope that new technologies will become available that might allow more consistent assessments of the general population.
Third, issues around methodological variability are clearly important, given the improved sensitivity observed with nasal pressure measurements versus with thermistor signals alone.41,57 Furthermore, pulse oximeters have variable time constraints and thus varying sensitivity and specificity for desaturation depending on equipment characteristics. Manual scoring of sleep studies is also likely to result in interscorer variability, especially in scoring arousals, which could affect hypopnea scoring and partly explain the variation seen in the published prevalence data for obstructive sleep apnoea. Re-scoring the raw data from the original studies according to consistent criteria would be challenging and standardisation of equipment from previous studies is not possible. Instead, we attempted to make estimates based on these known sources of variance. As a result, there are wide confidence intervals around our estimates based on strict versus liberal criteria. Nonetheless, we are aware that much higher prevalence figures could be generated depending on the assumptions used. None of the limitations mentioned here were considered in calculation of the 95% CIs; these intervals only reflect the sampling variability due to the study sizes used to estimate obstructive sleep apnoea prevalence. The confidence intervals do not reflect uncertainty in the obstructive sleep apnoea prevalence estimates for each country. The goodness of fit of the country-imputation algorithm was assessed as a cross-validation; this analysis indicates a potential underestimation of the global obstructive sleep apnoea prevalence (of up to −48%). A sensitivity analysis, based on a logistic regression model, indicated a potential overestimation of up to 28%. However, the logistic regression models severely underestimated prevalence of obstructive sleep apnoea in Singapore, Germany, and Switzerland. Clearly, estimating obstructive sleep apnoea prevalence in 193 countries based on sleep studies from just 16 countries will be subject to some error. These estimates can be refined when further data become available. Despite these limitations, our findings address an important gap in the published literature with regard to reporting comprehensive prevalence data for obstructive sleep apnoea.
In conclusion, this analysis highlights the high worldwide prevalence of obstructive sleep apnoea and variations by country and region. Additional, well designed studies are needed to investigate the prevalence of obstructive sleep apnoea, particularly in countries where published data are not available. This high prevalence and the documented association between obstructive sleep apnoea and numerous adverse clinical outcomes, including cardiovascular disease morbidity and mortality, mean that health-care systems around the world need to consider strategies to raise awareness of obstructive sleep apnoea and to diagnose and treat the condition to have a positive impact on population health and health-care expenditures.
Supplementary Material
Research in context.
Evidence before this study
Despite increasing recognition of obstructive sleep apnoea as a contributor to poor health outcomes, our literature searches identified a scarcity of data about global prevalence of obstructive sleep apnoea. We searched PubMed and Embase using search terms of “adult”, “sleep disordered breathing”, “sleep apnoea”, “sleep apnoea syndrome”, “obstructive”, “prevalence”, and “population”, with no limits on timeframe nor any language restrictions. The original search was done in April, 2017, and then rechecked in February, 2019 (no new papers were identified in the second search). Relevant and local prevalence data are important to facilitate implementation of effective and efficient strategies for diagnosis and management of obstructive sleep apnoea.
Added value of this study
To the best of our knowledge, this is the first study to report a global estimate of obstructive sleep apnoea prevalence and to estimate the number of individuals affected by this condition worldwide. Nearly 1 billion adults aged 30–69 years worldwide were estimated to have obstructive sleep apnoea, with and without symptoms, based on an apnoea-hypopnoea index (AHI) cutoff value of five or more events per h, with 425 million (>45%) of these individuals having an AHI of 15 or more events per h (defined as a moderate to severe disorder requiring treatment). Wide geographical variation exists in the prevalence of obstructive sleep apnoea, with prevalence exceeding 50% in some countries.
Implications of all the available evidence
Given the high burden of obstructive sleep apnoea worldwide, health-care systems need to adopt effective diagnostic and management strategies so that the negative health impacts of obstructive sleep apnoea can be minimised
Acknowledgments
Statistical assistance with the generation of confidence interval values was provided by Colleen Kelly, an independent statistician, from Kelly Statistical Consulting, funded by ResMed. Medical writing assistance for editing was provided by Nicola Ryan, an independent medical writer, funded by ResMed.
Funding ResMed.
Declaration of interests
AVB, KV, and CMN are employees of ResMed. NTA reports being on the advisory board of Bresotec. PRE is supported in part by a Senior Research Fellowship from the NHMRC (grant number 1042341). RH reports grants from the Swiss National Science Foundation (grants 3200B0–105993, 3200B0–118308, 33CSCO-122661, 33CS30–139468, and 33CS30–148401), the Leenaards Foundation, the Ligue Pulmonaire Vaudoise, GlaxoSmithKline, and the Faculty of Biology and Medicine of Lausanne University, and personal fees for medical advisory board work from NightBalance. MJM was a principal investigator on a multicentre trial funded by ResMed. SRP received grant funding through his institution from the American Sleep Medicine Foundation, the ResMed Foundation, Bayer Pharmaceuticals, and Philips Respironics. TP reports personal funding by ICRC St. Anne Hospital Brno Czech Republic, and institutional funding by Heel, Bioprojet, ResMed, Philips Respironics, and Löwenstein Medical Technology. J-LDP is supported by a research grant from the French National Research Agency (ANR-12-TECS-0010), in the framework of the Investissements d’avenir programme (ANR-15-IDEX-02) and reports participation in medXcloud—an academic and industry partnership sponsored by ResMed. AM relinquished all outside personal income as an Officer of the American Thoracic Society in 2012. ResMed provided a philanthropic donation to UC San Diego and reports participation in medXcloud—an academic and industry partnership sponsored by ResMed. MSMI, PEP, SS, and ST report no competing interests.
Footnotes
Data sharing
All data included in this study are available in the public domain. Specific requests or questions should be submitted to the corresponding author for consideration.
Contributor Information
Adam V Benjafield, ResMed Science Center, San Diego, CA, USA.
Najib T Ayas, Department of Medicine, University of British Columbia, Vancouver, BC, Canada.
Peter R Eastwood, Centre for Sleep Science, School of Human Sciences, University of Western Australia, and Department of Pulmonary Physiology and Sleep Medicine, West Australian Sleep Disorders Research Institute, Sir Charles Gairdner Hospital, Nedlands, Perth, WA, Australia.
Raphael Heinzer, Center for Investigation and Research in Sleep (CIRS), University Hospital of Lausanne, Lausanne, Switzerland.
Mary S M Ip, Department of Medicine, University of Hong Kong, Hong Kong Special Administrative Region, China.
Mary J Morrell, National Heart and Lung Institute, Imperial College London, Royal Brompton and Harefield NHS Foundation Trust, London, UK.
Carlos M Nunez, ResMed Science Center, San Diego, CA, USA.
Sanjay R Patel, Division of Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Thomas Penzel, Charité - Universitätsmedizin Berlin, Berlin, Germany.
Jean-Louis D Pépin, HP2 Laboratory, INSERM U1042, Univ. Grenoble Alpes, and EFCR laboratory, Grenoble Alpes University Hospital, Grenoble, France.
Paul E Peppard, Department of Population Health Sciences, University of Wisconsin, School of Medicine and Public Health, Madison, WI, USA.
Sanjeev Sinha, All India Institute of Medical Sciences, New Delhi, India.
Sergio Tufik, Universidade Federal de Sao Paulo, Sao Paulo, Brazil.
Kate Valentine, ResMed Science Center, San Diego, CA, USA.
Atul Malhotra, University of California, San Diego, La Jolla, CA, USA.
References
- 1.Redline S, Young T. Epidemiology and natural history of obstructive sleep apnea. Ear Nose Throat J 1993; 72: 20–21, 24–26. [PubMed] [Google Scholar]
- 2.Levy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 2015; 1: 15015. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra A, Orr JE, Owens RL. On the cutting edge of obstructive sleep apnoea: where next? Lancet Respir Med 2015; 3: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaiswal SJ, Owens RL, Malhotra A. Raising awareness about sleep disorders. Lung India 2017; 34: 262–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson NF. Health Care Savings: The economic value of diagnostic and therapeutic care for obstructive sleep apnea. J Clin Sleep Med 2016; 12: 1075–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaheri S, Somers VK. Cardiovascular diseases and sleep apnea. Handb Clin Neurol 2011; 98: 327–45. [DOI] [PubMed] [Google Scholar]
- 7.Weaver TE, Mancini C, Maislin G, et al. CPAP Treatment of sleepy patients with milder OSA: results of the CATNAP randomized clinical trial. Am J Respir Crit Care Med 2012; 186: 677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med 1999; 340: 847–51. [DOI] [PubMed] [Google Scholar]
- 9.McTigue K, Kuller L. Cardiovascular risk factors, mortality, and overweight. JAMA 2008; 299: 1260–61. [DOI] [PubMed] [Google Scholar]
- 10.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 2005; 352: 1138–45. [DOI] [PubMed] [Google Scholar]
- 11.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA 2018; 319: 1723–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017; 390: 2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UN Department of Economic and Social Affairs Population Division. World Population Ageing; 2015. http://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf (accessed Nov 26, 2018). [Google Scholar]
- 14.Farrell PC, Richards G. Recognition and treatment of sleep-disordered breathing: an important component of chronic disease management. J Transl Med 2017; 15: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malhotra A, Morrell MJ, Eastwood PR. Update in respiratory sleep disorders: epilogue to a modern review series. Respirology 2018; 23: 16–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014; 383: 736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Global surveillance, prevention and control of chronic respiratory diseases. 2007. https://www.who.int/gard/publications/GARD%20Book%202007.pdf?ua=1 (accessed May 17, 2019).
- 18.Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5: 263–76. [PMC free article] [PubMed] [Google Scholar]
- 19.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8: 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duce B, Milosavljevic J, Hukins C. The 2012 AASM respiratory event criteria increase the incidence of hypopneas in an adult sleep center population. J Clin Sleep Med 2015; 11: 1425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep 2009; 32: 150–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AASM. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in adults. Sleep 1999; 22: 667–89. [PubMed] [Google Scholar]
- 23.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015; 3: 310–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008; 31: 1079–85. [PMC free article] [PubMed] [Google Scholar]
- 25.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med 2010; 11: 441–46. [DOI] [PubMed] [Google Scholar]
- 26.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest 2001; 119: 62–69. [DOI] [PubMed] [Google Scholar]
- 28.Ip MS, Lam B, Tang LC, Lauder IJ, Ip TY, Lam WK. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest 2004; 125: 127–34. [DOI] [PubMed] [Google Scholar]
- 29.Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B, Gislason T. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J 2016; 47: 194–202. [DOI] [PubMed] [Google Scholar]
- 30.Reddy EV, Kadhiravan T, Mishra HK, et al. Prevalence and risk factors of obstructive sleep apnea among middle-aged urban Indians: a community-based study. Sleep Med 2009; 10: 913–18. [DOI] [PubMed] [Google Scholar]
- 31.Mihaere KM, Harris R, Gander PH, et al. Obstructive sleep apnea in New Zealand adults: prevalence and risk factors among Maori and non-Maori. Sleep 2009; 32: 949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakayama-Ashida Y, Takegami M, Chin K, et al. Sleep-disordered breathing in the usual lifestyle setting as detected with home monitoring in a population of working men in Japan. Sleep 2008; 31: 419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hrubos-Strom H, Randby A, Namtvedt SK, et al. A Norwegian population-based study on the risk and prevalence of obstructive sleep apnea. The Akershus Sleep Apnea Project (ASAP). J Sleep Res 2011; 20: 162–70. [DOI] [PubMed] [Google Scholar]
- 34.Plywaczewski R, Bednarek M, Jonczak L, Zielinski J. Sleep-disordered breathing in a middle-aged and older Polish urban population. J Sleep Res 2008; 17: 73–81. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med 2004; 170: 1108–13. [DOI] [PubMed] [Google Scholar]
- 36.Tan A, Cheung YY, Yin J, Lim WY, Tan LW, Lee CH. Prevalence of sleep-disordered breathing in a multiethnic Asian population in Singapore: a community-based study. Respirology 2016; 21: 943–50. [DOI] [PubMed] [Google Scholar]
- 37.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 2001; 163: 685–89. [DOI] [PubMed] [Google Scholar]
- 38.Li MX, Wang Y, Hua SC, et al. The prevalence of obstructive sleep apnea-hypopnea syndrome in adults aged over 20 years in Changchun city. Zhonghua Jie He He Hu Xi Za Zhi 2005; 28: 833–35 (in Chinese). [PubMed] [Google Scholar]
- 39.Fietze I, Laharnar N, Obst A, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-Trend. J Sleep Res 2018; 2018: e12770. [DOI] [PubMed] [Google Scholar]
- 40.AASM. International classification of sleep disorders, 2nd edn Diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine, 2005. [Google Scholar]
- 41.Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer. Am J Respir Crit Care Med 1998; 157: 1461–67. [DOI] [PubMed] [Google Scholar]
- 42.Young T, Peppard P, Gottlieb D. The epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165: 1217–39. [DOI] [PubMed] [Google Scholar]
- 43.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep 1991; 14: 486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lam B, Ip M, Tench E, Ryan C. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax 2005; 60: 504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jehan S, Myers AK, Zizi F, Pandi-Perumal SR, Jean-Louis G, McFarlane SI. Obesity, obstructive sleep apnea and type 2 diabetes mellitus: epidemiology and pathophysiologic insights. Sleep Med Disord 2018; 2: 52–58. [PMC free article] [PubMed] [Google Scholar]
- 46.Iber C, Ancoli-Israel S, Chesson AL Jr, Quan Sea. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, first edition Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 47.Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest 2017; 153: 843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penzel T, Schobel C, Fietze I. New technology to assess sleep apnea: wearables, smartphones, and accessories. F1000Res 2018; 7: 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goverdovsky V, von Rosenberg W, Nakamura T, et al. Hearables: multimodal physiological in-ear sensing. Sci Rep 2017; 7: 6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Villegas E, Chen G, Radcliffe J, Duncan J. A pilot study of a wearable apnoea detection device. BMJ Open 2014; 4: e005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuna ST, Benca R, Kushida CA, et al. Agreement in computer-assisted manual scoring of polysomnograms across sleep centers. Sleep 2013; 36: 583–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freedman N Doing it better for less: incorporating OSA management into alternative payment models. Chest 2019; 155: 227–33. [DOI] [PubMed] [Google Scholar]
- 53.Antic NA, Buchan C, Esterman A, et al. A randomized controlled trial of nurse-led care for symptomatic moderate-severe obstructive sleep apnea. Am J Respir Crit Care Med 2009; 179: 501–08. [DOI] [PubMed] [Google Scholar]
- 54.Ayas NT, FitzGerald JM, Fleetham JA, et al. Cost-effectiveness of continuous positive airway pressure therapy for moderate to severe obstructive sleep apnea/hypopnea. Arch Intern Med 2006; 166: 977–84. [DOI] [PubMed] [Google Scholar]
- 55.Frost & Sullivan, American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating sleep apnea draining health care system. 2016. https://aasm.org/resources/pdf/sleep-apnea-economic-crisis.pdf (accessed Nov 26, 2018).
- 56.Pendharkar SR, Povitz M, Bansback N, George CFP, Morrison D, Ayas NT. Testing and treatment for obstructive sleep apnea in Canada: funding models must change. CMAJ 2017; 189: E1524-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hosselet J, Ayappa I, Norman RG, Krieger AC, Rapoport DM. Classification of sleep-disordered breathing. Am J Respir Crit Care Med 2001; 163: 398–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.