Abstract
Purpose:
The prospective, multicenter LOCATE (18F Fluciclovine [FACBC] PET/ CT in Patients with Rising PSA after Initial Prostate Cancer Treatment) trial assessed the impact of positron emission tomography/computerized tomography with 18F-fluciclovine on treatment plans in patients with biochemical recurrence of prostate cancer after primary therapy with curative intent.
Materials and Methods:
Men who had undergone curative intent treatment of histologically confirmed prostate cancer but who were suspected to have recurrence based on rising prostate specific antigen levels were enrolled prospectively. Each man had negative or equivocal findings on standard of care imaging. 18F-fluciclovine positron emission tomography/computerized tomography was performed according to standardized protocols. Treating physicians completed a questionnaire regarding the patient treatment plan before and after scanning, recording changes to the treatment modality (eg salvage radiotherapy to systemic androgen deprivation therapy) as major and changes in a modality (eg modified radiotherapy fields) as other.
Results:
Between June 2016 and May 2017, 213 evaluable patients with a median age of 67 years and median prostate specific antigen 1.00 ng/ml were enrolled in study. 18F-fluciclovine avid lesions were detected in 122 of the 213 patients (57%). Overall 126 of the 213 patients (59%) had a change in management after the scan, which were major in 98 of 126 (78%) and in 88 (70%) were informed by positive positron emission tomography/computerized tomography findings. The most frequent major changes were from salvage or noncurative systemic therapy to watchful waiting (32 of 126 cases or 25%), from noncurative systemic therapy to salvage therapy (30 of 126 or 24%) and from salvage therapy to noncurative systemic therapy (11 of 126 or 9%).
Conclusions:
18F-fluciclovine positron emission tomography/computerized tomography detected 1 or more recurrence sites in the majority of men with biochemical recurrence, frequently resulting in major changes to management plans. Future studies will be planned to determine whether a management change leads to improved outcomes.
Keywords: prostatic neoplasms; positron emission tomography computed tomography; fluciclovine F-1; neoplasm recurrence, local; prostate-specific antigen
RELAPSE remains common despite advances in primary treatment and improved overall survival of prostate cancer with BCR developing in 20% to 40% of patients.1–4 Although a significant proportion of men with BCR undergo salvage therapy, localization of recurrence with standard imaging remains challenging, especially when PSA levels are low.5,6 Consequently local therapy guided by nomogram based probabilities is often selected rather than anatomical localization of recurrent disease.7
An imaging modality providing anatomical localization of recurrence in patients with low PSA would facilitate treatment planning, leading to a better chance of cure when tumors are small and amenable to localized therapy, avoiding futile salvage therapy and informing decisions to delay ADT with its associated morbidity.8,9
Currently PET has limited usefulness in prostate cancer. Although widely available, FDG (18F-fluorodeoxyglucose) PET/CT is not routinely used for prostate cancer imaging due to low uptake except in castrate resistant disease.11C-choline is FDA (Food and Drug Administration) approved and some 18F-choline analogues are approved in some European countries. 11C-choline yields slightly better disease staging than FDG but remains suboptimal, particularly for identifying extraprostatic lesions, with limited accuracy in patients with low PSA and slow kinetics.5,6,10–13 Moreover, 11C based PET radiotracers have a 20-minute half-life, making them unsuitable for use at centers without cyclotrons on site. PSMA based radiotracers such as 68Ga-PSMA-11 are in development and show promising results in recurrent prostate cancer cases with meta-analysis data showing a pooled, subject level detection rate of 76%.14
The synthetic amino acid radiotracer 18F-fluciclovine is approved by the FDA and the EU (European Commission) to detect prostate cancer in patients with elevated PSA following prior treatment. Approval was based on encouraging diagnostic performance and histologically confirmed data on patients with BCR showing 68% subject level detection, 62% positive predictive value (greater than 90% for extraprostatic disease) and 70% specificity.15 A recent update to the NCCN Guidelines recommends that 18F-fluciclovine PET/ CT or PET/MRI be considered in the workup of patients with prostate cancer recurrence or progression.16
Given the established performance of 18F-fluciclovine PET/CT and its approved United States indication to document PSA recurrence, we present results from the multicenter LOCATE trial. The trial was done to evaluate the impact of 18F-fluciclovine PET/CT on the planned treatment of patients with prostate cancer BCR after curative intent primary therapy and negative/equivocal standard of care imaging. Ongoing data analysis will provide secondary information on the correlation with biopsy, when performed, and clinical followup.
METHODS
Design
LOCATE (ClinicalTrials.gov NCT02680041) was an open label, multicenter, interventional, single group assignment phase 4 trial across 15 sites in the United States. The primary objective was to measure the proportion of patients with suspected prostate cancer BCR after primary therapy with curative intent in whom the planned treatment was altered after 18F-fluciclovine PET/CT. Secondary end points included analysis of patients whose actual treatment deviated from the revised plan. Groups at participating institutions obtained Institutional Review Board approval before accrual and all patients provided written informed consent (supplementary table, http://jurology.com/).
Patients
Men 18 years old or older with histologically confirmed prostate cancer who were considered for salvage therapy because of rising PSA were eligible if they had negative or equivocal findings on 2015 or 2016 recommended United States standard of care imaging (abdominopelvic CT or MRI and bone scintigraphy) for disease restaging in the preceding 60 days. BCR was diagnosed after prostatectomy with or without adjuvant radiotherapy as detectable or rising PSA 0.2 ng/ml or greater with a second confirmatory level of 0.2 ng/ml or greater. In patients who had undergone radiotherapy or brachytherapy BCR was diagnosed by a PSA increase of 2.0 ng/ml or greater above the nadir.17
Study exclusion criteria were ongoing treatment with any prostate cancer systemic therapy, ADT 3 months or less before screening, bilateral orchiectomy and intolerance of 18F-fluciclovine. Nonsurgical local treatment must have occurred 1 year or more before enrollment and brachytherapy must have occurred 2 or more years previously.
Protocol
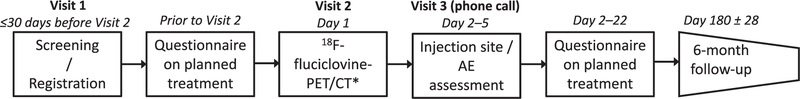
Baseline screening was performed during the first visit of each patient (fig. 1). The physician completed a questionnaire to document the patient treatment plan before and again 2 to 22 days after 18F-fluciclovine PET/CT to document changes resulting from the scan (supplementary fig. 1, http://jurology.com/). A change of management involving a new treatment modality (eg salvage radiotherapy to systemic therapy) was classified as major while any change in a modality (eg modified radiotherapy fields) was classified as other. At 26 ± 4 weeks after PET/CT the actual treatment was recorded to document changes from the revised plan.
Figure 1.
LOCATE study design. AE, adverse event. Asterisk indicates that biopsy may be done after scan when clinically indicated.
18F-Fluciclovine Positron Emission Tomography/ Computerized Tomography
18F-fluciclovine was manufactured by automated radiosynthesis. Patients were prepared according to standardized procedures and 18F-fluciclovine was administered by bolus intravenous injection (mean ± SD 370 ± 20% MBq).18
Readers were trained to interpret 18F-fluciclovine PET/ CT images according to 2014 consensus guidelines (supplementary Appendix, http://jurology.com/).19 Anatomical locations were categorized as positive or negative for uptake when determining the positivity rate at the subject level for the prostate/prostate bed and for extraprostatic regions (lymph nodes, bone or soft tissue).
Statistics
The sample size calculation was based on binding the width of the 95% CI within 0.20 using the Clopper-Pearson exact method in PASS, version 12 (NCSS, Kaysville, Utah), the worldwide leading software to determine sample size. We assumed that approximately 30% of positive scans would change the intended management plan.20 Thus, 89 patients with positive 18F-fluciclovine PET/CT findings were needed to assess the primary end point. The prevalence of positive 18F-fluciclovine PET/CT was assumed to be about 40% and, thus, we expected to enroll 223 patients. Because of the random nature of the number of patients meeting clinical criteria in prospective studies, we applied a sample size correction.21 By adding 25 extra patients we could be 90% certain to yield the required number of patients who met the criteria. Assuming a 15% potential data loss, a total sample of 292 patients was estimated.
RESULTS
Patients
Because of a higher positivity rate and a lower data loss than anticipated, recruitment was terminated following the enrollment of 221 patients. Two patients subsequently withdrew consent and screening failed in 6. Thus, 213 evaluable patients were scanned between June 2016 and May 2017 (supplementary fig. 2, http://jurology.com/). Patients were a median 54 months after the initial diagnosis, median age was 67.0 years and median prescan PSA was 1.00 ng/ml (see table).
Patient characteristics
| No. pts | 213 | |
| Mean ± SD age/median (range) | 66.4 ± 7.75/67.0 | (46–90) |
| No. race (%): | ||
| Black/African American | 17 | (8.0) |
| American Indian or Alaska native | 1 | (0.5) |
| Native Hawaiian or other Pacific islander | 2 | (0.9) |
| White | 188 | (88) |
| Other | 3 | (1.4) |
| Missing | 2 | (0.9) |
| Mean ± SD mos since initial diagnosis/median (range) | 70.0 ± 55.4/54.3 | (4.2–270.9) |
| Time since adjuvant treatment: | ||
| No. pts | 51 | |
| Mean ± SD mos/median (range) | 38.9 ± 32.8/30.0 | (3.9–171.5) |
| No. primary prostatectomy (%):* | 164 | (77) |
| + Radiotherapy | 43 | (26) |
| No radiotherapy | 121 | (74) |
| No. no primary prostatectomy (%):† | 49 | (23) |
| No. Radiotherapy alone (%): | 24 | (49) |
| External beam radiotherapy only | 21 | (43) |
| Brachytherapy only | 1 | (2.0) |
| External beam radiotherapy + brachytherapy | 2 | (4.1) |
| No. primary radiotherapy ± other treatments (%):† | 22 | (45) |
| External beam radiotherapy + ADT | 17 | (35) |
| External beam radiotherapy + cryotherapy | 2 | (4.1) |
| Brachytherapy + ADT | 1 | (2.0) |
| External beam radiotherapy, brachytherapy + ADT | 2 | (4.1) |
| No. other primary treatment (%):† | 3 | (6.1) |
| Cryotherapy | 1 | (2.0) |
| High intensity focal ultrasound | 1 | (2.0) |
| High dose rate brachytherapy | 1 | (2.0) |
| Mean ± SD ng/ml PSA/median (range) | 4.24 ± 10.22/1.00 | (0.2–93.5) |
| No. Gleason score (%):‡ | ||
| 6 or Less | 27 | (13) |
| 7 | 134 | (63) |
| 8 or Greater | 50 | (23) |
| Missing | 2 | (0.9) |
Denominator was number of patients with prostatectomy.
Denominator was number of patients without prostatectomy.
Data from biopsy in 183 patients and surgery in 150.
Imaging
Figure 2 shows representative images. Conventional imaging revealed at least 1 equivocal finding in 34 patients (16%) while the remainder had negative results. The most recent result in each patient occurred a median of 30 days (range 2 to 124) before scanning. Within the preceding 60 days bone scan, CT or MRI was negative/equivocal in 175, 133 and 38 patients, respectively. Of the negative/equivocal bone scans the subsequent 18F-fluciclovine PET/CT detected bone lesions in 9.7%. 18F-fluciclovine PET/CT detected prostate/bed or pelvic lesions in 52% and 47% of those with negative/equivocal pelvic CT and MRI, respectively.
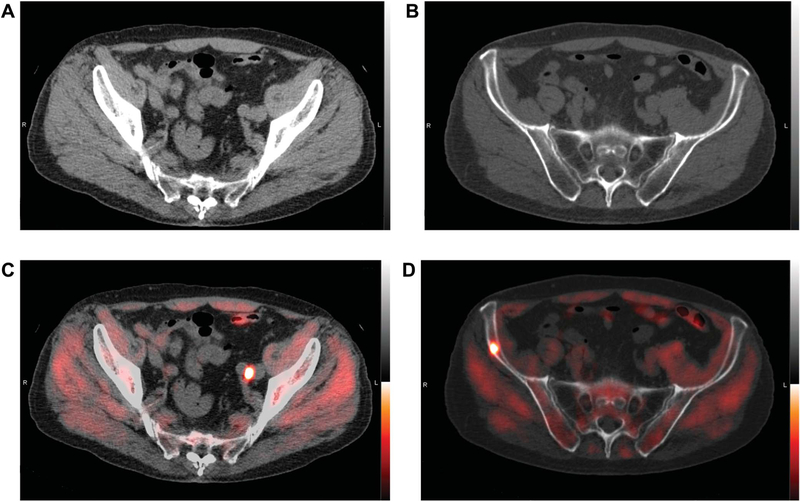
Figure2.
Representative images of 77-year-old man with BCR after radical prostatectomy (Gleason 3 + 4 T2cN0Mx) in 2006.PSA was 1.38 ng/ml. In October 2016 abdominopelvic CT and bone scintigraphy were negative. 18F-fluciclovine PET/CT reveals positive, nonenlarged left common iliac node (A and C) and focus in right ilium without CT correlate, most consistent with bone metastasis (B and D). Prescan management plan was whole pelvis salvage radiotherapy. After PET/CT plan was revised to ADT and prostate bed salvage radiotherapy. Posttreatment PSA was 0.05 ng/ml in March 2017.
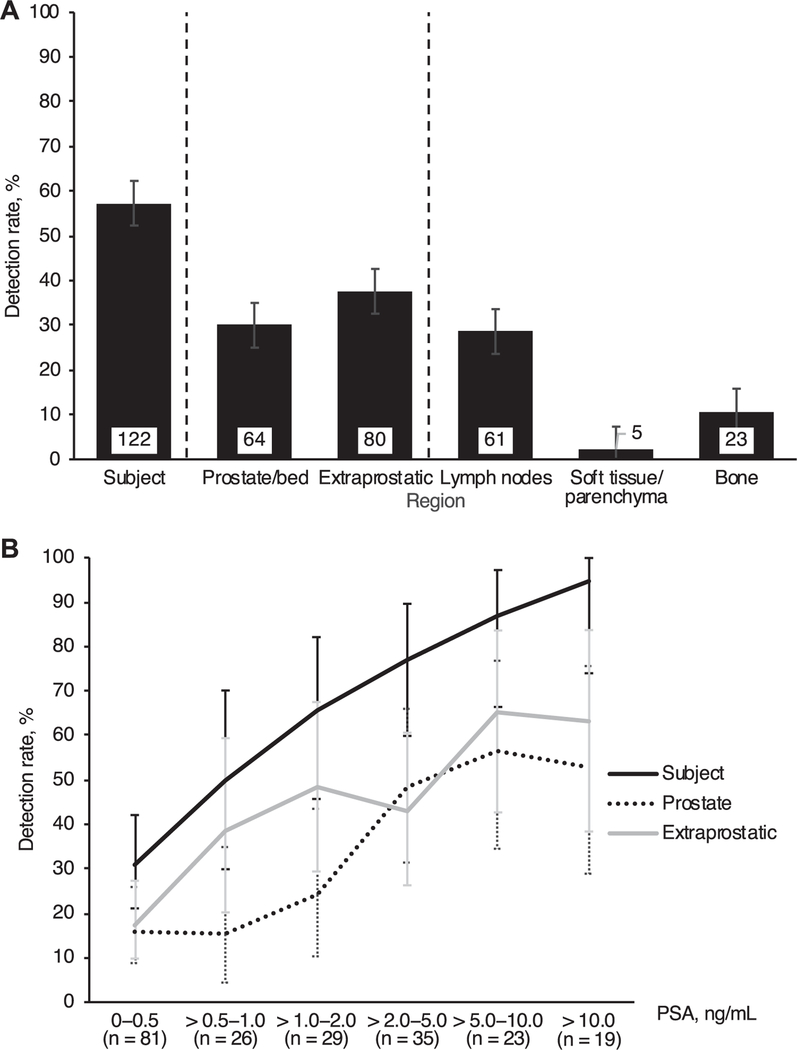
Overall 18F-fluciclovine avid lesions were detected in 122 of 213 patients (57%) (fig. 3, A). The detection rate was 30% in the prostate/prostate bed and 38% outside the prostate, including 29% in lymph nodes, 2.3% in soft tissue and 11% in bone.
Figure 3.
18F-fluciclovine imaging detection rate by region (A) and PSA (B). Extraprostatic region consisted of lymph nodes, soft tissues/ parenchyma and bone. Lymph nodes consisted of pelvic and extrapelvic (retroperitoneal and other) nodes with overall 24% and 12% detection rate, respectively. Soft tissue/parenchyma positivity was identified in bowel and lung. Error bars indicate 95% CI.
Detection Rate vs Prostate Specific Antigen
The 18F-fluciclovine PET/CT detection rate was broadly proportional to prescan PSA (fig. 3, B). Lesions were detected in 84 of 106 patients (79%) with PSA greater than 1.0 ng/ml and in 65 of 77 (84%) with PSA greater than 2.0 ng/ml.
Among those with the lowest PSA (0 to 0.5 ng/ml) the patient level detection rate was 31%, including 16% for the prostate/prostate bed and 17% for extraprostatic sites. In patients with PSA greater than 0.5 to 1.0 ng/ml positivity was 50%, which rose to 66% in those with PSA greater than 1.0 to 2.0 ng/ ml. For extrapelvic lymph nodes (retroperitoneal and other) positivity ranged from 3.7% at PSA 0.5 ng/ml or less to 42% at PSA greater than 10 ng/ml. Skeletal positivity was 7.4% at PSA 0.5 ng/ml or less and 14% at PSA greater than 2 ng/ml.
Therapeutic Management Plans
Intended.
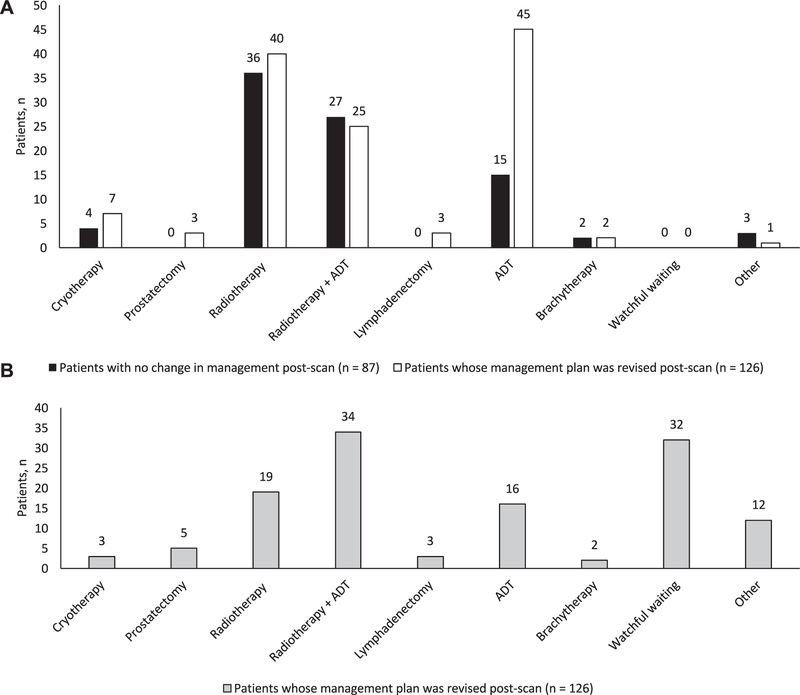
The most common prescan management plan was radiotherapy performed in 128 cases (60%). In 52 of these patients (41%) adjuvant ADT was also planned and in a further 60 (28%) ADT monotherapy was planned (fig. 4, A).
Figure 4.
Intended treatment before (A) and after (B) 18F-fluciclovine PET/CT. Other than when indicated in men with no treatment plan change, adjuvant ADT was planned in1 of 2 whose intended plan was brachytherapy. Of patients with post-scan change in plan adjuvant ADT was planned in 3 of 7 scheduled for cryotherapy and in 1 of 3 scheduled for lymphadenectomy.
Revised.
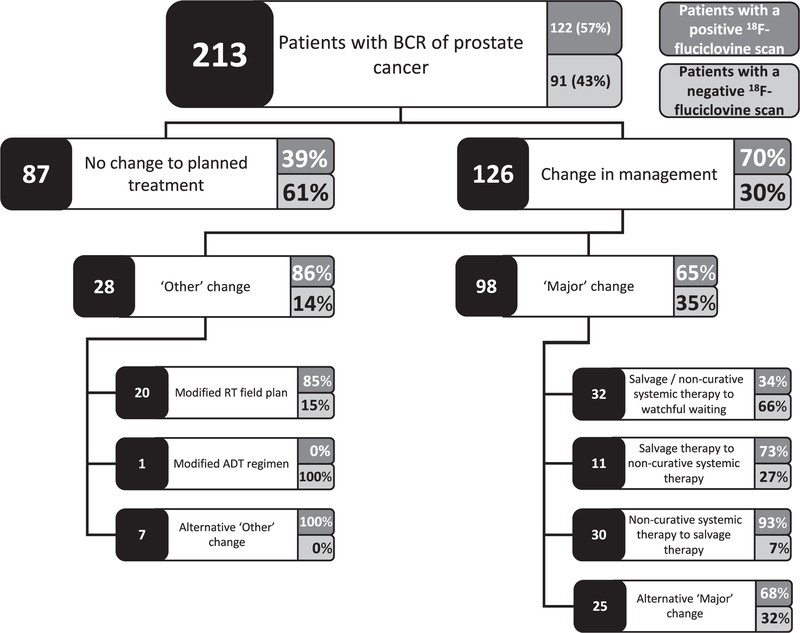
Following 18F-fluciclovine PET/CT planned management was revised in 126 of 213 patients (59%, 95% CI 52–66, figs. 4 and 5). Revisions were associated with positive 18F-fluciclovine PET/CT in 88 of 126 cases (70%). They were attributable to findings consistent with prostate cancer in 122 of 126 cases (97%) and to incidental findings in 4 (3%). Major management changes occurred in 98 of 126 cases (78%).
Figure 5.
Change in management decisions and associated 18F-fluciclovine PET/CT positivity. RT, radiotherapy.
Following 18F-fluciclovine PET/CT treatment plans were typically revised in 1 of 4 main ways. 1) The most frequent change was to withhold planned salvage or noncurative systemic therapy in favor of watchful waiting. This applied to 15% of patients and accounted for 32 of all 126 management changes (25%), including 11 from intended ADT, 16 from radiotherapy (4 with adjuvant ADT), 2 from cryotherapy (1 with adjuvant ADT), 2 from prostatectomy and 1 from lymphadenectomy. The decision to revise plans to watchful waiting was informed by negative 18F-fluciclovine PET/CT in 21 patients (66%).
2) At a comparable frequency planned ADT was revised to curative intent salvage therapy (radiotherapy, brachytherapy or cryotherapy) in 30 of 126 patients (24%). PET/CT was positive in 28 of these patients (93%). Of 60 patients whose original plan was ADT monotherapy 45 (75%) had treatment changed after PET/CT. There were major changes in 41 of the 45 cases (91%), most commonly a change to salvage radiotherapy in 15, including 9 with adjuvant ADT.
3) Of all 126 changes 11 (9%) were from salvage therapy with or without ADT to systemic monotherapy with ADT. Eight of these patients (73%) had positive 18F-fluciclovine PET/CT, often with distant metastases, eg to retroperitoneal lymph nodes (5 patients) or distant soft tissue and parenchymal metastasis (bowel in 1 patient). Of the 128 men whose intended plan was salvage radiotherapy with or without ADT 65 (51%) had the treatment changed after the scan and 44 were major changes, including 19 changes to ADT (ADT plus radiotherapy in 13 and ADT monotherapy in 6).
4) The majority of management changes classified as other were modifications to radiotherapy fields. These modifications accounted for 20 of all 126 management changes (16%). They were informed by positive PET/CT in 85% of cases, most commonly when a positive finding was identified that was not targeted by the original field.
Actual.
Six-month followup data documenting the actual treatment that patients received were available on 211 of the 213 patients (99%). While treatment in 132 patients (63%) was concurrent with the post-PET/CT plans, a clinically important difference was found in 79 (37%). 18F-fluciclovine PET/CT was negative in 42% of these patients.
The documented changes comprised a delay to planned treatment in 3.8% of patients, revision following further imaging, tests or specialist opinion in 15%, an altered regimen of planned treatment in 5.1%, addition of a new therapy in 13%, receipt of only 1 of 2 planned elements given to date in 7.6%, a change to watchful waiting based on patient preference in 44% or another change in 11%. Of the 35 patients who elected watchful waiting 57% had a negative 18F-fluciclovine PET/CT.
Importantly only 4 of the 79 modifications (5.1%) from the post-PET plan restored treatment back to the prePET plan. Of the 79 patients whose actual treatment changed 46% had no prePET to post-PET change.
DISCUSSION
To determine whether disease localization with 18F-fluciclovine PET/CT would influence patient treatment we enrolled 213 patients at a total of 15 centers in the United States. Lesions were detected in 57% of patients and in 59% the management plan was changed after the scan. Approximately three-quarters of the changes were major changes from one modality to another.
Our findings are similar to those recently presented from the smaller United Kingdom based FALCON (Fluciclovine [18F] PET/CT in Biochemical Recurrence of Prostate Cancer) trial.22 FALCON data showed that 61% of 85 men with BCR had management plans changed following 18F-fluciclovine PET/CT and approximately 60% of the changes were major.
Standard imaging for BCR has limited accuracy, particularly in men with low PSA.5,6,16 The current findings confirm and extend previous histologically confirmed findings showing 18F-fluciclovine detected disease not seen on standard imaging.15,23
The usefulness of alternative PET radiotracers to inform disease management decisions24,25 is in the same range as we report for 18F-fluciclovine. A study evaluating the impact of choline PET/CT on the treatment of 33 patients with BCR (median 2 ng/ml PSA, range 0.16 to 79) showed choline uptake in the prostate, prostate fossa and pelvic lymph nodes in 17 and remote disease in 9.24 However, the cause of BCR was not identified on 7 scans, together leading to modification of treatment plans for 18 patients (55%). 68Ga-PSMA-11 PET/CT informed major changes to initial management plans in 53% of 126 patients with BCR and minor changes in 6.4%.25
Patients who initially undergo gland sparing treatments are typically offered salvage focal therapy such as brachytherapy or cryotherapy (with or without ADT).5,6,16 However, the majority of men with BCR who are considered for salvage therapy after curative intent prostatectomy will undergo salvage prostate bed irradiation without prior identification of the actual recurrence site(s). Indeed, in the 77% of men in our cohort who underwent prostatectomy as initial therapy more than half were scheduled to undergo salvage radiotherapy before the results of 18F-fluciclovine PET/CT were available.
Because of the poor performance of conventional imaging, salvage therapy decisions have traditionally been based on clinical judgment, patient and physician preference, the probability of disease progression and the risk-to-benefit profile of each treatment.7 The current study demonstrates a role for 18F-fluciclovine PET/CT in disease staging and management planning. However, additional studies are needed to assess the impact on patient outcomes as a result of refining management plans to increase the chance of durable progression-free survival or the avoidance of therapy that will ultimately be futile.
We frequently observed the modification of salvage or systemic therapy to watchful waiting in patients with negative PET/CT, suggesting that the patient and the physician were reassured by the absence of visible disease and decided not to intervene but instead to monitor closely. Positive 18F-fluciclovine PET/CT also led to treatment modification, notably when distant metastases were identified by PET/CT, eg in retroperitoneal lymph nodes or bowel soft tissue, prompting the decision to abandon intended salvage radiotherapy to the prostate bed and/or pelvis. Consequently these men were spared the morbidity of salvage radiotherapy.26,27 Similarly, approximately a sixth of patients were spared ADT and its associated morbidity8,9 after 18F-fluciclovine PET/CT showed anatomically localized positive lesions which could be better targeted by a salvage modality.
The ability of 18F-fluciclovine PET/CT to assist radiotherapy planning was reported previously.20 We frequently observed lesions outside the prescan treatment field in patients scheduled to undergo salvage radiotherapy. In a randomized, prospective trial in 87 subjects with post-prostatectomy BCR initial radiotherapy plans were compiled based on conventional imaging.20 Of the 87 patients 42 were randomized to undergo additional 18F-fluciclovine PET/CT. Of the men 81% had positive PET/CT results, leading to revised radiotherapy plans in 41%. In a sub analysis of a cohort of the first enrolled patients in the study 18F-fluciclovine PET/CT led to the augmentation of planned radiotherapy target volumes for the prostate bed and the lymph nodes in 30 of 41 patients (73%).28,29
As a change in management does not necessarily confer improved outcomes, longer term followup ideally via a patient registry is needed to confirm how incorporating 18F-fluciclovine PET/CT into therapy planning would affect outcomes. Approximately a third of patients at the 6-month followup had a clinically significant modification from the post-18F-fluciclovine PET/CT treatment plan. Reassuringly the rate of change was similar among those whose initial treatment plan was changed by 18F-fluciclovine PET/CT vs those whose initial treatment plan was not changed. Moreover, only 5% of the changes recorded at 6 months restored management to that intended before 18F-fluciclovine PET/CT.
While in the current study we did not routinely confirm imaging results with histological findings, prior histologically confirmed data demonstrate good diagnostic performance of 18F-fluciclovine PET/CT.15 However, higher specificity is noted in extraprostatic lesions compared with the treated prostate, perhaps because of overlap between malignancy, benign hyperplasia and prostatitis.15
CONCLUSIONS
Localization of recurrent prostate cancer with 18F-fluciclovine PET/CT had considerable impact on patient treatment with the majority of patients receiving a treatment modality that differed from the prescan management plan. 18F-fluciclovine PET/CT has the potential to improve management but further investigation is warranted.
APPENDIX
Additional LOCATE study group members: Lee P. Adler, Laurence H. Belkoff, Daniel Burzon, Paul Dato, Michael Farwell, Stephen Fogelson, Peter Gardiner, Lucy Hanna, John M. Hoffman, Charles Intenzo, David Josephson, Jed Kaminetsky, Michael Kipper, Borys Krynyckyi, Karen E. Linder, Helga Marques, John Melnick, Matthew P. Miller, William Oh, Shaile Philips, Judith Rose, Bital Savir-Baruch, Daniel J. Stevens, Ashutosh Tewari, Przemyslaw Twardowski, Penelope Ward, Martha Wasserman, Sharon Weick and Jian Q. (Michael) Yu.
Supplementary Material
ACKNOWLEDGMENT
Imaging assistance was provided at the ACR® (American College of Radiology) Core Laboratory, Philadelphia, Pennsylvania. Catriona Turnbull, Correlate Medical, provided writing support.
Supported by the NCI (National Cancer Institute), NIDDK (National Institutes of Diabetes and Digestive and Kidney Diseases), the PCF (Prostate Cancer Foundation), the Peter Michael Foundation, the St. Louis Men’s Group Against Cancer and the Barnes-Jewish Hospital Foundation (GLA).
Abbreviations and Acronyms
- ADT
androgen deprivation therapy
- BCR
biochemical recurrence
- CT
computerized tomography
- LOCATE
18F Fluciclovine (FACBC) PET/CT in Patients with Rising PSA after Initial Prostate Cancer Treatment
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- PSA
prostate specific antigen
- PSMA
prostate specific membrane antigen
Footnotes
Financial interest and/or other relationship with 3D Biopsy, Augmenix, Blue Earth Diagnostics, Medivation and Progenics.
Financial interest and/or other relationship with Blue Earth Diagnostics.
No direct or indirect commercial incentive associated with publishing this article.
Contributor Information
Gerald L. Andriole, Division of Urologic Surgery, Department of Surgery, Washington University School of Medicine, St. Louis, Missouri; Alvin J. Siteman Cancer Center, Washington University School of Medicine, St. Louis, Missouri.
Lale Kostakoglu, Division of Nuclear Medicine and Molecular Imaging, Icahn School of Medicine, Mount Sinai, New York, New York.
Albert Chau, Blue Earth Diagnostics, Oxford, United Kingdom.
Fenghai Duan, Department of Biostatistics and Center for Statistical Sciences, Brown University School of Public Health, Providence, Rhode Island.
Umar Mahmood, Department of Radiology, Massachusetts General Hospital, Boston, Massachusetts.
David A. Mankoff, Department of Radiology, University of Pennsylvania, Philadelphia, Pennsylvania.
David M. Schuster, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology and Imaging Sciences, Emory University, Atlanta, Georgia.
Barry A. Siegel, Division of Nuclear Medicine, Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri; Alvin J. Siteman Cancer Center, Washington University School of Medicine, St. Louis, Missouri.
REFERENCES
- 1.Kim MM, Hoffman KE, Levy LB et al. : Improvement in prostate cancer survival over time: a 20-year analysis. Cancer J 2012; 18: 1. [DOI] [PubMed] [Google Scholar]
- 2.Bruce JY, Lang JM, McNeel DG et al. : Current controversies in the management of biochemical failure in prostate cancer. Clin Adv Hematol Oncol 2012; 10: 716. [PubMed] [Google Scholar]
- 3.Roehl KA, Han M, Ramos CG et al. : Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol 2004; 172: 910. [DOI] [PubMed] [Google Scholar]
- 4.Simmons MN, Stephenson AJ and Klein EA:Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol 2007; 51: 1175. [DOI] [PubMed] [Google Scholar]
- 5.Mottet N, Bellmunt J, Bolla M et al. : EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71: 618. [DOI] [PubMed] [Google Scholar]
- 6.Cornford P, Bellmunt J, Bolla M et al. : EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 2017; 71: 630. [DOI] [PubMed] [Google Scholar]
- 7.Artibani W, Porcaro AB, De Marco V et al. : Management of biochemical recurrence after primary curative treatment for prostate cancer: a review. Urol Int 2018; 100: 251. [DOI] [PubMed] [Google Scholar]
- 8.Tsai HK, D’Amico AV, Sadetsky N et al. : Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst 2007; 99: 1516. [DOI] [PubMed] [Google Scholar]
- 9.Saigal CS, Gore JL, Krupski TL et al. : Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer 2007; 110: 1493. [DOI] [PubMed] [Google Scholar]
- 10.Giovacchini G, Giovannini E, Leoncini R et al. : PET and PET/CT with radiolabeled choline in prostate cancer: a critical reappraisal of 20 years of clinical studies. Eur J Nucl Med Mol Imaging 2017; 44: 1751. [DOI] [PubMed] [Google Scholar]
- 11.Schiavina R and Martorana G: The promise of choline-PET/CT in the detection of recurrent prostate cancer: what are the limits of our investigation? Eur Urol 2013; 63: 797. [DOI] [PubMed] [Google Scholar]
- 12.Picchio M, Spinapolice EG, Fallanca F et al. : [11C] choline PET/CT detection of bone metastases in patients with PSA progression after primary treatment for prostate cancer: comparison with bone scintigraphy. Eur J Nucl Med Mol Imaging 2012; 39: 13. [DOI] [PubMed] [Google Scholar]
- 13.Giovacchini G, Picchio M, Coradeschi E et al. : Predictive factors of [11C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging 2010; 37: 301. [DOI] [PubMed] [Google Scholar]
- 14.Perera M, Papa N, Christidis D et al. : Sensitivity, specificity, and predictors of positive 68Gaprostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol 2016; 70: 926. [DOI] [PubMed] [Google Scholar]
- 15.Bach-Gansmo T, Nanni C, Nieh PT et al. : Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine (18F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol 2017; 197: 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network: NCCN Guidelines® and Clinical Resources. Clinical Practice Guidelines in Oncology: Prostate Cancer, Version 2.2018. Available at https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed August 17, 2018.
- 17.Roach M, Hanks G, Thames H et al. : Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006; 65: 965. [DOI] [PubMed] [Google Scholar]
- 18.Blue Earth Diagnostics: Axumin® Prescribing Information (August 2016). Available at http://www.axumin.com/pdf/Axumin_PI_08_2016_Clean.pdf. Accessed August 17, 2018.
- 19.Miller MP, Kostakoglu L, Pryma D et al. : Reader training for the restaging of biochemically recurrent prostate cancer using 18F-fluciclovine PET/CT. J Nucl Med 2017; 58: 1596. [DOI] [PubMed] [Google Scholar]
- 20.Akin-Akintayo OO, Jani AB, Odewole O et al. : Change in salvage radiotherapy management based on guidance with FACBC (fluciclovine) PET/CT in postprostatectomy recurrent prostate cancer. Clin Nucl Med 2017; 42: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepe M: The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford, United Kingdom: Oxford University Press 2003. [Google Scholar]
- 22.Teoh EJ, Bottomley DM, Scarsbrook A et al. : The FALCON trial: impact of 18F-fluciclovine PET/CT on clinical management choices for men with biochemically recurrent prostate cancer. J Clin Oncol 2018; 36: 165. [Google Scholar]
- 23.Zanoni L, Nanni C, Bach-Gansmo T et al. : Multisite experience of fluciclovine (18F) PET/CT imaging in biochemically recurrent prostate cancer: impact of clinical factors and intersite variation. J Clin Oncol 2017; 35: 163. [Google Scholar]
- 24.Goldstein J, Even-Sapir E, Ben-Haim S et al. : Does choline PET/CT change the management of prostate cancer patients with biochemical failure? Am J Clin Oncol 2017; 40: 256. [DOI] [PubMed] [Google Scholar]
- 25.Hope TA, Aggarwal R, Chee B et al. : Impact of 68Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med 2017; 58: 1956. [DOI] [PubMed] [Google Scholar]
- 26.van Stam MA, Aaronson NK, Pos FJ et al. : The effect of salvage radiotherapy and its timing on the health-related quality of life of prostate cancer patients. Eur Urol 2016; 70: 751. [DOI] [PubMed] [Google Scholar]
- 27.Spratt DE: Salvage radiotherapy after prostatectomy: two sides of the coin. Eur Urol 2016; 70: 758. [DOI] [PubMed] [Google Scholar]
- 28.Schreibmann E, Schuster D, Rossi P et al. : Image guided planning for prostate carcinomas with incorporation of anti-3-[18F]FACBC (fluciclovine) positron emission tomography: workflow and initial findings from a randomized trial. Int J Radiat Oncol Biol Phys 2016; 96: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jani AB, Schreibmann E, Rossi PJ et al. : Impact of 18F-fluciclovine PET on target volume definition for postprostatectomy salvage radiotherapy: initial findings from a randomized trial. J Nucl Med 2017; 58: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.