Abstract
Aim:
To determine epilepsy risk factors after pediatric stroke.
Method:
A cohort of children with arterial ischemic stroke (from birth- age 18 years) was enrolled at 21 centers and followed for 1 year. Acute seizures (≤7 days post-stroke) and active epilepsy (≥ 1 unprovoked remote seizure + maintenance anti-convulsant at 1 year) were identified. Predictors were determined using logistic regression.
Results:
Among 114 patients (28 neonates and 86 children) enrolled, 26 neonates (93%) and 32 children (37%) had an acute seizure. Acute seizures lasted > 5 minutes in 23 (40%) and were frequently recurrent: 33 (57%) had 2–10 and 11 (19%) had >10 seizures. Among 109 patients with 1-year follow-up, 11 (10%) had active epilepsy. For each year younger, active epilepsy was 20% (OR 0.8, CI 0.5–1.0, p=0.04) more likely. Prolonged or recurrent acute seizures also increased epilepsy risk. Each additional 10-minutes of the longest acute seizure increased epilepsy risk five-fold (OR 4.7, 95% CI 1.7–13). Patients with >10 acute seizures had a 30-fold increased epilepsy risk (OR 30, CI 2.9–305).
Interpretation:
Pediatric stroke survivors, especially younger children, have a high risk of epilepsy one year post-stroke. Prolonged or recurrent acute seizures increase epilepsy risk in a dose-dependent manner.
Arterial ischemic stroke (AIS) is a leading cause of childhood brain injury. Many survivors suffer from permanent disability and epilepsy, although a subset recovers without significant deficits. One of the critical differences of pediatric stroke compared to stroke in adults is that children frequently experience acute symptomatic seizures. Only 2–4% of adults with stroke have an acute seizure.1 In contrast, an estimated 75–90% of neonates and 20–30% of children with AIS have acute seizures.2–7 Convulsions at stroke presentation are more likely in young children.8, 9 Although determinants of epilepsy after pediatric AIS are poorly understood, emerging data suggest early seizures may be associated.6, 10, 11 Therefore, the high incidence of acute seizures after pediatric AIS is worrisome. However, we know little about the characteristics of children who have acute seizures, or the mechanisms by which acute seizures are associated with epilepsy.
Large, prospective studies of neonates and children are critical to understand which patients are at greatest risk of epilepsy, and how acute seizures or other factors might influence that risk. While there are currently no known interventions to prevent epilepsy, it is conceivable that aggressive anti-convulsant treatment to reduce acute seizures could decrease later epilepsy. Moreover, accurate prediction of epilepsy risk will allow providers to appropriately counsel patients and families and guide future research. We aimed to prospectively determine incidence rates and risk factors of acute seizures and epilepsy after pediatric AIS. We hypothesized that age at the time of a stroke is an important determinant of both acute symptomatic seizures and later development of post-stroke epilepsy. Further, we hypothesized that the duration and frequency of acute seizures during the first week after stroke modifies epilepsy risk.
Method:
Seizures in Pediatric Stroke (SIPS) investigators at 21 sites (Appendix) enrolled newborns ≥ 37 weeks gestation and children ≤ 18 years within 14 days of stroke onset from March 2011- August 2012 using the infrastructure of the International Pediatric Stroke Study, a multicenter pediatric stroke consortium. All sites obtained approval from their local institutional review board and written informed consent from guardians. For all participants, a child neurologist (GD or CF) adjudicated brain imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) to confirm AIS with supplemental neuroradiologist review as needed. Inclusion criteria: (1) acute focal neurologic deficit or seizure consistent with stroke, and (2) arterial infarct on CT or MRI consistent with clinical symptom location and timing.
Local site investigators determined acute seizures on a clinical basis or by electroencephalography (EEG) performed for clinical indications, documented the number of acute seizures as an ordinal categorical variable (single, 2–10 seizures or > 10 seizures); and the duration of the longest acute seizure in minutes. At hospital discharge, guardians were given seizure diaries with instructions to document remote seizures. Longitudinal follow-up data including anticonvulsant use and presence and characteristics of seizures following hospital discharge were obtained by from review of health records, standardized telephone or in-person parental questionnaires at 3 months and in-person investigator visit 1 year post-stroke. All data were abstracted onto standardized case record forms.
Definitions:
Neonatal stroke was defined as a stroke presenting from birth - 28 days old; childhood stroke was defined as a stroke presenting at age 29 days – 18 years old. Acute seizures were defined as a seizure at stroke onset or within the subsequent seven days.12 Recurrent acute seizures were defined as more than one seizure during the seven days after stroke. Remote seizure was defined as a seizure more than 7 days post-stroke and excluded seizures provoked by fever, TIA or stroke recurrence.12 Active epilepsy was defined as treatment with a maintenance anti-convulsant at the one-year follow-up in a child who had at least one 1 remote seizure.13
Statistical analyses were performed using Stata 13 (College Station, Texas). Summary statistics were used to describe the cohort and acute seizures characteristics in neonates and children. We described the onset time of acute seizures in children but not in neonates, because the exact time of stroke onset for neonates is generally not known. Predictors of acute seizures and active epilepsy were investigated using logistic regression. Acute seizure analyses: We first estimated the probability of acute seizure by age in a linear model. Because the association of acute seizures and age had evidence of nonlinearity, we then depicted the association graphically as a cubic spline to allow variation across age. We considered variables with marginal outcome associations significant at the p<0.1 for inclusion in multivariable analysis of acute seizure predictors. In the final model, we included age in years but not neonatal stroke to avoid overfitting. Analyses of active epilepsy: We performed a test for trend to determine if acute seizures frequency (categorized as none, single, 2–10 or > 10 seizures) was associated with higher epilepsy risk. We did not report a multivariable model because of the limited number of epilepsy outcomes. Survival analysis methods were used to estimate remote seizure incidence rates. Time at risk began 7 days post-stroke; in neonates, stroke onset was assumed to be on the day of birth. Patients were right-censored at the last observed seizure-free date. We presented Kaplan Meier plots stratified by acute seizure (yes/no) and acute seizure frequency. Sensitivity analyses: We repeated predictor testing applying the assumption that patients with an acute seizure but incomplete data regarding duration or frequency had only brief (30 seconds) or a single acute seizure to provide conservative estimates.
Results:
We enrolled 114 patients (28 neonates and 86 children) with confirmed AIS (Figure 1). Median age of neonates at the time of AIS symptom onset was day of life 0 (range 0–20 days). Median age for children was 6.1 years (interquartile range [IQR] 1.4, 12). The presumed stroke risk factors differed between neonates and children (Table 1). Neonates were also more likely to have infarcts with cortical involvement and infarcts in the middle cerebral artery (MCA) territory.
Figure 1. Flow diagram of pediatric stroke cohort demonstrating seizures and epilepsy during the first year post-stroke.
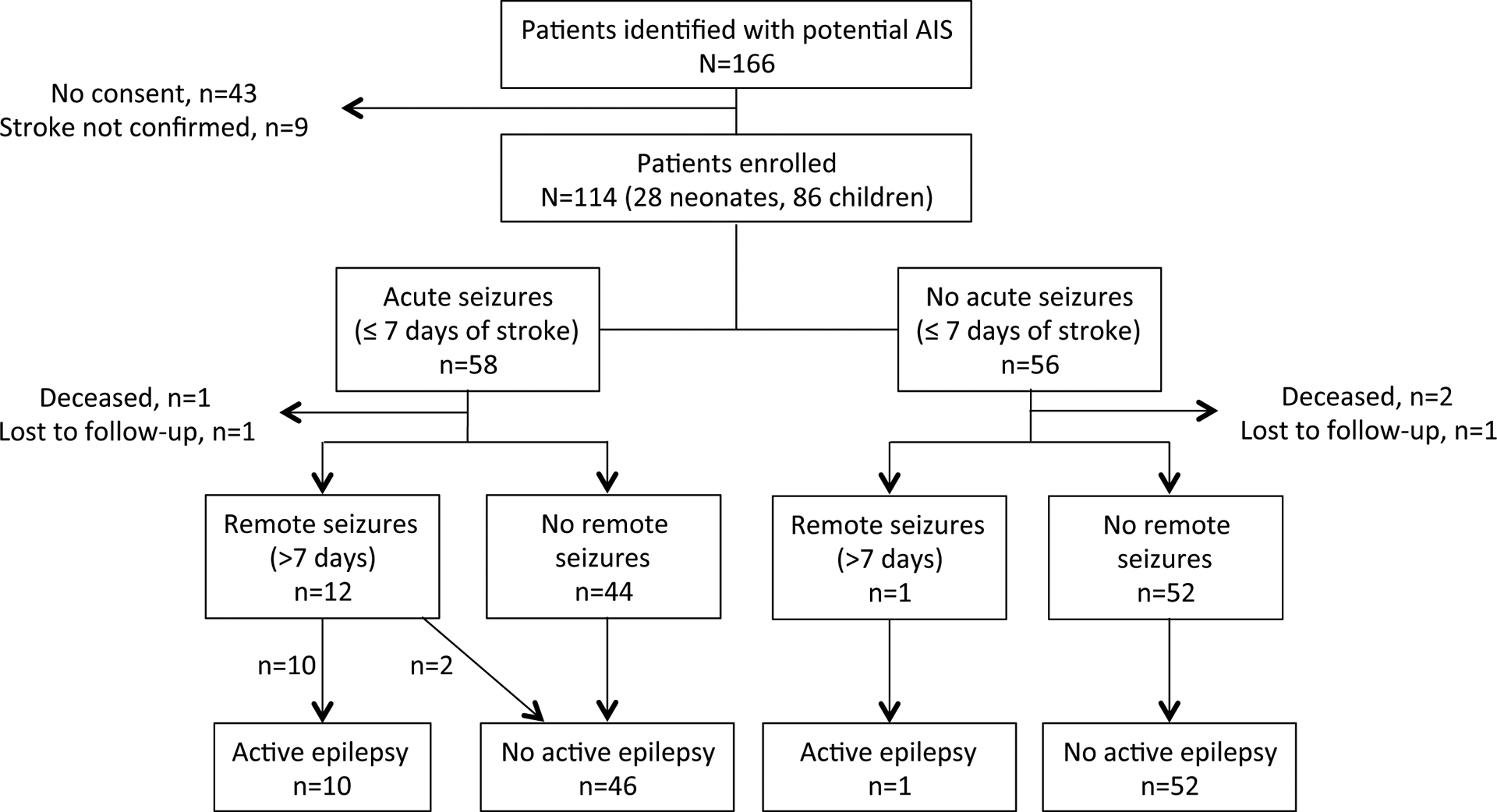
The final cohort included 114 participants. Among these, 109 completed one-year follow-up for a measurement of active epilepsy.
Table 1.
Demographics and clinical characteristics. Except for seizure frequency, categories were not mutually exclusive, so do not sum to 100%.
| Neonatal (N=28) n (%) | Childhood (N=86) n (%) | |
|---|---|---|
| Demographics | ||
| Male | 15 (54) | 51 (59) |
| North American | 20 (71) | 60 (70) |
| Hispanic parent | 6 (21) | 15 (17) |
| Stroke risk factors | ||
| Arteriopathy | 1 (4) | 33 (38) |
| Cardiac disease | 7 (26) | 22 (26) |
| Acute illness | 6 (22) | 21 (24) |
| Underlying chronic disease | 2 (7) | 24 (28) |
| Head trauma at time of stroke | 0 (0) | 7 (8) |
| No risk factor identified | 14 (50) | 15 (17) |
| Clinical characteristics | ||
| Prolonged acute seizure (≥5 minutes) | 8 (29) | 11 (13) |
| History of seizure prior to stroke | 0 (0) | 5 (6) |
| Family history of epilepsy | 1 (4) | 0 (0) |
| Discharged home | 24 (86) | 70 (81) |
| Infarct Description | ||
| MCA territory stroke | 23 (82) | 53 (62) |
| ACA territory stroke | 2 (7) | 12 (14) |
| PCA territory stroke | 3 (11) | 18 (21) |
| Cortical stroke location | 10 (36) | 15 (17) |
| Multifocal infarcts | 8 (29) | 29 (34) |
| Any hemorrhage | 4 (14) | 3 (3) |
| EEG done | 27 (96) | 35 (41) |
| Routine EEG (<30 min) | 10 (36) | 14 (16) |
| Prolongd EEG (30 min-2 hours) | 8 (29) | 13 (15) |
| Continuous EEG (>2 hours) | 9 (32) | 7 (8) |
| Acute Seizure | 26 (93) | 32 (37) |
| Clinical seizure | 19 (69) | 26 (30) |
| Electrographic seizure | 8 (29) | 3 (3) |
| Electroclinical seizure | 10 (36) | 4 (5) |
| Seizure frequency ‡ | ||
| None | 2 (7) | 54 (63) |
| single seizure | 3 (11) | 7 (8) |
| 2–10 seizures | 18 (64) | 15 (17) |
| > 10 seizures | 5 (18) | 6 (7) |
| Unknown frequency | 0 (0) | 4 (5) |
P ≤ 0.05.
P-value is for differences between acute seizure frequency groups, Fisher’s exact test. Abbreviations: MCA = middle cerebral artery, ACA = anterior cerebral artery, PCA = posterior cerebral artery.
An acute seizure was identified in 26 (93%) neonates and 32 (37%) children. Neonates were more likely to have recurrent acute seizures compared to older children (88% versus 66% of those with an acute seizure, p=0.04), but recurrent acute seizures were common overall. Acute seizures were often prolonged: (Figure 2, panel A). Among the 32 children with an acute seizure, 12 (38%) were seizing upon arrival to the emergency department. Onset of the first seizure was delayed by at least 8 hours after stroke presentation in another 12 (38%). Seizures were classified as focal motor in 21 (81%) neonates, with the remainder classified as generalized. Among children, the seizures were classified as focal motor (n=23; 72%), complex partial (n=5; 16%), simple partial (n=3; 9%), and bilateral convulsive (n=2; 6%). At the end of the stroke hospitalization, 23 neonates (82% of neonates in the cohort) and 25 children (29% of all children in the cohort) were discharged on an anti-convulsant medication, including 2 neonates and 3 children who did not have an acute seizure.
Figure 2. Acute and remote seizure characteristics and predictors after pediatric arterial ischemic stroke.
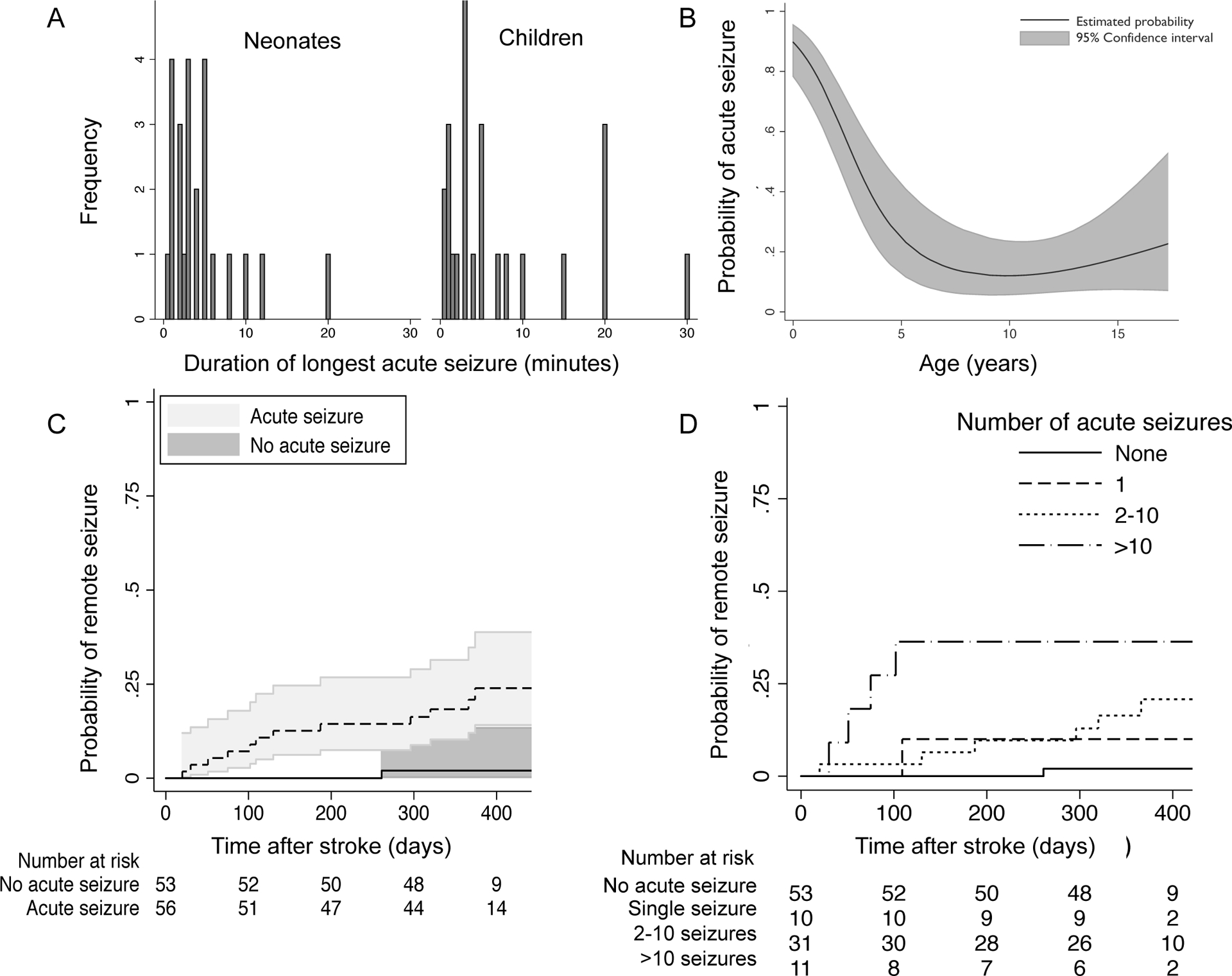
(A) Histogram demonstrating durations of the longest acute seizure (≤ 7 days post-stroke) after arterial ischemic stroke. (B) Younger age predicted acute seizures (black line = estimated probability of acute seizure by age, shading = 95% confidence intervals); model includes neonates and children (all participants birth to 18 years at the time of stroke). (C) Children with an acute post-stroke seizure had a 2% (95% CI 1.1%–3.4%) average monthly incidence rate of a first remote seizure and 18% (CI 10–31%) cumulative incidence at one-year post-stroke. (D) Recurrent acute seizures were associated with increasing remote seizure risk. Reference group: patients with no acute seizures; Single acute seizure: HR 5 (95% CI 0.3–84, p=0.2); 2–10 acute seizures: HR 10 (95% CI 1.2–85, p=0.03); > 10 acute seizures: HR 26 (95% CO 2.9–230, p=0.004).
On univariate analyses, younger age and stroke involving the middle cerebral artery territory predicted acute seizures (Table 2). Overall, acute seizures were 20% more likely (Odds Ratio [OR] 0.8, 95% confidence interval [CI] I 0.7 – 0.9, P < 0.001) for each year of age below age 18 at the time of AIS. However, the risk was non-linear, with greater probability of acute seizures under 4 years of age (shown in Figure 2, panel B). The magnitude of risk and shape of the curve was similar when neonates were excluded from the model, except that CI were wider at the youngest ages (online supplemental figure). Cerebrovascular arteriopathy decreased acute seizure risk overall, but not after age-adjustment (data not shown). We did not find an association between acute seizures and hemorrhagic conversion or cortical infarct location. In a multivariable model including age in years, the presence of an arteriopathy and MCA territory infarct, estimates of association were: younger age (OR 0.8, CI 0.7–0.9, p<0.001), arteriopathy (OR 0.3, 95% CI 0.1–0.8, p=0.02) and MCA territory infarct (OR 3.3, CI 1.2–9, p=0.02).
Table 2.
After pediatric arterial ischemic stroke (N=114), unadjusted odds ratios and 95% confidence intervals for clinical factors associated with acute seizures (≤7 days post-stroke) and epilepsy at one-year (N=109 with one year follow-up).
| Acute Seizures (N=58) | Epilepsy (N=11) | |||
|---|---|---|---|---|
| OR | P-value | OR | P-value | |
| Demographics | ||||
| Male | 0.8 (0.4–1.7) | 0.5 | 1.4 (0.4–5.2) | 0.6 |
| North American | 0.6 (0.3–1.4) | 0.3 | 1.1 (0.3–4.5) | 0.9 |
| Hispanic parent | 0.9 (0.3–2.2) | 0.7 | 1.0 (0.2–5.0) | 1 |
| Neonatal stroke | 22 (4.9–99) | <0.001* | 1.2 (0.3–4.7) | 0.8 |
| Years of age at stroke, median (IQR)† | 0.8 (0.7–0.9) | <0.001* | 0.7 (0.5–1.0) | 0.03* |
| Stroke Risk Factors | ||||
| Cardiac disease | 1.6 (0.7–3.7) | 0.3 | 0.6 (0.1–3.2) | 0.6 |
| Arteriopathy | 0.2 (0.1–0.6) | 0.001* | 0.9 (0.2–3.6) | 0.9 |
| Acute illness | 1.1 (0.5–2.3) | 0.9 | 2.8 (0.8–10) | 0.1 |
| Underlying chronic disease | 0.7 (0.3–1.6) | 0.3 | 0.7 (0.1–3.5) | 0.7 |
| Head trauma | 0.4 (0.1–2.0) | 0.2 | n/a | 1 |
| Infarct Description | ||||
| MCA territory | 2.8 (1.2–6.3) | 0.01* | 1.4 (0.3–5.4) | 0.7 |
| ACA territory | 0.7 (0.2–2.1) | 0.5 | 1.6 (0.3–8.3) | 0.6 |
| PCA territory | 0.7 (0.3–1.8) | 0.4 | 0.9 (0.2–4.6) | 0.9 |
| Cortical location | 1.3 (0.5–3.2) | 0.6 | 2.3 (0.6–9.0) | 0.2 |
| Multifocal | 1.2 (0.6–2.6) | 0.6 | 2.1 (0.6–7.4) | 0.3 |
| Hemorrhage | 1.3 (0.3–6.1) | 0.7 | 1.9 (0.2–18) | 0.6 |
| History of seizure before stroke | 4.1 (0.4–38) | 0.2 | n/a | 0.4 |
| Acute seizure (any) | 11 (1.4–92) | 0.02* | ||
| No acute seizure | Ref | 0.005‡ | ||
| Single acute seizure | 6 (0.3–101) | 0.2 | ||
| 2–10 acute seizures | 7.7 (0.8–72) | 0.07 | ||
| > 10 acute seizures | 31 (2.9–305) | 0.004* | ||
P ≤ 0.05.
Non-neonates.
P-value, test for trend across ordinal catgories. Abbreviations: OR = Odds ratio, CI= 95% confidence interval, IQR = interquartile range, MCA = middle cerebral artery, ACA = anterior cerebral artery, PCA = posterior cerebral artery
Follow-up was available for 109 children (three were deceased, two were lost to follow-up) at a median of 12.3 months (IQR 11.6 – 13) after stroke. All 13 of the children with a remote seizure had been discharged on an anti-convulsant after their stroke hospitalization. Children with an acute seizure had a 2% (95% CI 1.1%–3.4%) average monthly incidence rate of a first remote seizure with 18% (CI 10–31%) cumulative incidence at one-year post-stroke (Figure 2, panel C). At one year, 11 patients (10%, CI 4–16%) had active epilepsy, including 3 with neonatal stroke (11%) and 8 with childhood stroke (10%; p=0.8 for difference between neonates and children). Epilepsy severity varied. Over the year, 7 of the children with epilepsy had more than one remote seizure and 3 reported ≥100 seizures. Eight of the children had been treated with multiple anti-convulsants, and 4 of the children had ongoing treatment with concurrent anti-convulsants (Online supplemental table).
For each year younger than 18 at the time of stroke, active epilepsy risk increased 20% (OR 0.8, CI 0.6–1.0, p=0.04); this relationship was similar when neonatal strokes were excluded (OR 0.7 (95% CI 0.5–1.0, p=0.03). Acute seizures were also associated with active epilepsy (Table 2). Greater duration or frequency of acute seizures elevated epilepsy risk in a dose-dependent manner. Each additional 10-minute duration of the longest acute seizure increased active epilepsy risk five-fold (OR 4.7, CI 1.7–13). Children with a higher number of recurrent acute seizures had greater probability of a first remote seizure (Figure 2, panel D). Patients with >10 acute seizures had 30-fold increased risk of active epilepsy at one year compared to patients without an acute seizure (Table 2). Sensitivity analyses: Acute seizure duration was unknown in 10/109 (8%); four patients had at least one acute seizure with an unknown number of additional seizures. Assuming that patients with unknown acute seizure duration had brief (30 second) seizures, each additional 10-minutes in longest acute seizure duration still increased active epilepsy risk (OR 3.5, CI 1.4–9, p=0.01). Assuming the patients with an acute seizure but unknown frequency had only a single seizure did not change the estimate or magnitude of the association of seizure frequency and active epilepsy (OR 30, CI 2.9–305, p=0.004 for children with >10 acute seizures compared to those with no acute seizures). EEG characteristics were only available for the subset of children in which EEG was clinically performed (n=51). In this subset, abnormal slowing on the acute EEG was associated with ten-fold increased risk of active epilepsy (OR 9.7, CI 1.1–84, p=0.04) at one-year, but we did not find an association with the presence of epileptiform discharges (OR 2.9, CI 0.7–13, p=0.2).
Discussion:
In this prospective study, we demonstrated that a significant proportion of pediatric stroke survivors later develop epilepsy. Among children who had an acute symptomatic seizure, the incidence rate of a first remote seizure is about 2% each month during the year after stroke. By one-year poststroke, 10% of all neonates and children were on a maintenance medication for active epilepsy. The incidence rate of epilepsy in our study is higher than that reported in the population-based Kaiser Pediatric Stroke Study, in which 3.6% of children developed active epilepsy annually, with a cumulative incidence of 13% at 5 years.6 Our higher incidence rates may be explained by age differences between the stroke cohorts; our cohort included neonates with stroke. However, the Kaiser Pediatric Stroke Study was also retrospective and dependent on electronic database searches to identify patients with epilepsy, and may therefore have underestimated epilepsy incidence rates. Importantly, we did not find a leveling off of the remote seizure incidence rate by one year, and speculate that the risk likely persists well beyond the first year, resulting in higher incidence of post-stroke epilepsy over time.
We found younger age and MCA territory strokes predict acute seizures. The incidence of acute seizures after neonatal AIS should be considered with detection bias in mind because very young infants may not show other clinical signs of stroke. Yet, the magnitude and shape of the curve of acute seizure probabilities does not change for children when neonates were excluded, suggesting that this potential bias does not account for the observed association with young age. Younger age also predicts epilepsy at one year post-stroke; this was unchanged when neonates were excluded.
Few prior studies have directly measured epilepsy after pediatric AIS across age groups. Younger children may have had different infarct characteristics than older children, which could influence epileptogenesis and the age-related epilepsy risk. Alternatively, characteristics of the immature brain that favor excitatory neurotransmitter systems could account for both a vulnerability to acute seizures and the transformation of a normal neuronal network into a hyper-excitable after an ischemic infarct.14, 15 A third possibility is that acute seizures in the setting of ischemia have an epileptogenic effect.16 Acute post-stroke seizures were associated with epilepsy in our study and the Kaiser Pediatric Stroke Study.6 Building on this association, we found that epilepsy risk appears to increase in a dose-related manner with an increasing number and duration of acute seizures. Children with more than ten acute seizures have a thirty-fold increased epilepsy risk compared to children who did not have an acute seizure.
Could acute seizures, observed more frequently in younger children, explain the age-related epilepsy risk? A high burden of acute seizures could potentially contribute to epileptogenesis directly by inducing neurobiological changes or indirectly by increasing metabolic demand in poorly perfused brain and worsening secondary injury. Current pediatric stroke guidelines do not recommend continuous EEG monitoring nor routine prophylactic administration of anti-epileptic medications after AIS prior to acute seizures.17 Although acute seizures after pediatric AIS might be preventable, in practice, anti-convulsants are typically only started after a seizure occurs. When to start anti-convulsant treatment and how long to continue it after acutely provoked seizures subside, especially in neonates and younger children, remain open questions. However, given the possibility that prolonged or recurrent acute seizures are detrimental, our data suggest an important theoretical opportunity for intervention. A significant proportion of children did not seize at stroke onset, suggesting a window of time to start an early anti-convulsant. Further, most patients with an acute seizure in our cohort went on to have multiple seizures during the first week. Perhaps post-stroke epilepsy risk could be modified through more aggressive acute seizure management or early detection with continuous EEG monitoring, but further research is required to determine if this is possible.
Our study had limitations. First, the timing of onset, frequency and duration of acute seizures were classified at the judgment of the site investigator using all available clinical and EEG data. Some patients may have had clinical or subclinical seizures that were not identified, resulting in an underestimation of acute seizure frequency or duration. In neonates with AIS, an estimated 80% of seizures identified on continuous EEG may be clinically unsuspected.18 Systematic continuous EEG monitoring in all patients would be a better way to identify and quantify all seizures but was beyond the scope of our study. Misclassification of exposure is generally thought to result in bias towards the null, so the strength of the association of epilepsy risk with acute seizure presence, frequency and duration may also be underestimated. Second, remote seizures could have been related to factors other than stroke in our cohort, such as head trauma or underlying neurodevelopmental disorders. However, only 6% of the patients had head trauma at the time of the AIS and none of these patients had active epilepsy at one year post-stroke. While 5% of our patients did have a history of seizure prior to stroke, none of these children met our definition of active epilepsy at one year. Third, nearly half of the children in our cohort had been discharged from the stroke hospitalization on maintenance anticonvulsants. Ongoing anti-convulsant treatment during the first year post-stroke may have prevented a first remote seizure, resulting in an underestimation of the natural history of epilepsy development. Finally, the cohort was not population-based or a consecutively enrolled sample. Our estimates of acute seizure probability could have been skewed if investigators preferentially enrolled patients either with or without acute seizures. The cohort, enrolled at pediatric stroke centers that are generally regional tertiary care facilities, may have preferentially included children more severe strokes or greater medical illness who were then more likely to go on to develop epilepsy.
Our study highlights that children have an increased and age-related propensity for stroke-related seizures compared to adults. Approximately one in five school age children has an acute seizure related to AIS, and this proportion is even higher in younger children. Pediatric stroke survivors have a significant risk of developing epilepsy, particularly if they experienced prolonged or multiple acute seizures. Young children and those with MCA territory infarcts are important at-risk groups who could be targeted for closer EEG monitoring and rapid seizure management. Because acute seizures post-stroke are a potentially modifiable risk factor, future studies should evaluate whether prevention of recurrent seizures with anticonvulsants can reduce the risk of later epilepsy in this vulnerable population.
Supplementary Material
Supplemental Figure.Predicted probabilities of an acute seizure (≤7 days) after childhood arterial ischemic stroke in a model excluding patients who were neonates (birth to 28 days) at the time of the stroke.
What this paper adds:
Younger children are at higher risk of acute seizures and epilepsy after stroke.
One year after pediatric stroke, 10% of survivors are treated for epilepsy.
Each ten-minute increase in acute seizure duration increases epilepsy risk five-fold.
Children with >10 acute seizures after stroke have a 30-fold increased epilepsy risk.
Acknowledgements:
We thank Jessica Peattie, Julie Paterson, Samantha Chait and the SIPS research staff.
Sources of Funding: Pediatric Epilepsy Research Foundation (Grant # 112010-007); the Auxilium Foundation, NIH (2K12NS001692-11 and KL2TR000143, Dr. Fox), (U54GM104942 to the WVCTSI IDeA CTR, Dr. Pergami). The funders of the study were not involved in the study design, data collection, data analysis, manuscript preparation or publication decisions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors have stated that they had no interests that might be perceived as posing conflict or bias.
Appendix: SIPS sites and investigators.
Alberta Children’s Hospital, Canada – Adam Kirton
Akron Children’s Hospital, United States – Abdalla Abdalla
Aristotle University of Thessaloniki, Greece – Dimitrios Zafeiriou
Cleveland Clinic Children’s Hospital, United States – Neil Friedman
Children’s Central Hospital, Tbilisi, Georgia – Nana Tatishvili
Children’s Clinic of Tartu University Hospital, Estonia – Anneli Kolk
Children’s Hospital Colorado, United States – Jennifer Armstrong-Wells
Children’s Hospital of Philadelphia, United States – Rebecca Ichord
Seattle Children’s Hospital, United States – Catherine Amlie-Lefond
The Hospital for Sick Children, Canada – Gabrielle deVeber
Mother and Child Health Care Institute, Serbia – Gordana Kovacevic
Pontificia Universidad Catolica de Chile, Chile – Marta Hernandez Chavez
Royal Children’s Hospital Melbourne, Australia – Belinda Stojanovski
Hôpital Robert Debré, France – Luigi Titomanlio
St. Louis Children’s Hospital, United States– Kristin Guilliams
Stanford University, United States – Jorina Elbers
University of California San Francisco, United States – Christine Fox, Heather Fullerton
Primary Children’s Medical Center Utah, United States – Susan Benedict
University of Texas Southwestern Medical Center, United States –Patricia Plumb
Vanderbilt University Medical Center, United States – Lori Jordan
West Virginia University, United States – Paola Pergami
References:
- 1.Reith J, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Seizures in acute stroke: predictors and prognostic significance. The Copenhagen Stroke Study. Stroke; a journal of cerebral circulation. 1997;28(8):1585–9. [DOI] [PubMed] [Google Scholar]
- 2.Kirton A, Armstrong-Wells J, Chang T, et al. Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. Pediatrics. 2011;128(6):e1402–10. [DOI] [PubMed] [Google Scholar]
- 3.Lee JC, Lin KL, Wang HS, et al. Seizures in childhood ischemic stroke in Taiwan. Brain Dev. 2008. [DOI] [PubMed] [Google Scholar]
- 4.Golomb MR, Garg BP, Carvalho KS, Johnson CS, Williams LS. Perinatal stroke and the risk of developing childhood epilepsy. J Pediatr. 2007;151(4):409–13, 13 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wusthoff CJ, Kessler SK, Vossough A, et al. Risk of later seizure after perinatal arterial ischemic stroke: a prospective cohort study. Pediatrics.127(6):e1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox CK, Glass HC, Sidney S, Lowenstein DH, Fullerton HJ. Acute seizures predict epilepsy after childhood stroke. Annals of neurology. 2013;74(2):249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackay MT, Wiznitzer M, Benedict SL, Lee KJ, Deveber GA, Ganesan V. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol. 2011;69(1):130–40. [DOI] [PubMed] [Google Scholar]
- 8.Zimmer JA, Garg BP, Williams LS, Golomb MR. Age-related variation in presenting signs of childhood arterial ischemic stroke. Pediatric neurology. 2007;37(3):171–5. [DOI] [PubMed] [Google Scholar]
- 9.Abend NS, Beslow LA, Smith SE, et al. Seizures as a presenting symptom of acute arterial ischemic stroke in childhood. The Journal of pediatrics. 2011;159(3):479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh RK, Zecavati N, Singh J, et al. Seizures in acute childhood stroke. J Pediatr.160(2):291–6. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald KC, Williams LS, Garg BP, Golomb MR. Epilepsy in children with delayed presentation of perinatal stroke. J Child Neurol. 2007;22(11):1274–80. [DOI] [PubMed] [Google Scholar]
- 12.Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):671–5. [DOI] [PubMed] [Google Scholar]
- 13.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52 Suppl 7:2–26. [DOI] [PubMed] [Google Scholar]
- 14.Bender RA, Baram TZ. Epileptogenesis in the developing brain: what can we learn from animal models? Epilepsia. 2007;48 Suppl 5:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks-Kayal AR. Rearranging receptors. Epilepsia. 2005;46 Suppl 7:29–38. [DOI] [PubMed] [Google Scholar]
- 16.Loscher W, Hirsch LJ, Schmidt D. The enigma of the latent period in the development of symptomatic acquired epilepsy - Traditional view versus new concepts. Epilepsy Behav. 2015;52(Pt A):78–92. [DOI] [PubMed] [Google Scholar]
- 17.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke; a journal of cerebral circulation. 2008;39(9):2644–91. [DOI] [PubMed] [Google Scholar]
- 18.Low E, Mathieson SR, Stevenson NJ, et al. Early postnatal EEG features of perinatal arterial ischaemic stroke with seizures. PloS one. 2014;9(7):e100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure.Predicted probabilities of an acute seizure (≤7 days) after childhood arterial ischemic stroke in a model excluding patients who were neonates (birth to 28 days) at the time of the stroke.


