Abstract
Introduction
Encapsulated Streptococcus pneumoniae strains cause high morbidity and mortality, mainly in countries with no pneumococcal conjugate vaccines (PCVs) immunization program. This study investigated the epidemiological changes of S. pneumoniae isolates including serotype distribution and antimicrobial susceptibility in Tehran, Iran.
Methods
A total of 80 S. pneumoniae samples were collected from patients admitted to Shariati hospital over two periods. Half of the isolates were collected from February to September 2017 and the other half from July 2018 to March 2019. The antimicrobial susceptibility testing and PCV-13 serotype coverage of S. pneumoniae isolates were evaluated among patients with invasive and non-invasive infections.
Results
The most common serotypes were 23F (17.5%), 14 (16.3%), 3 (16.3%) 19F (12.5%), and 19A (12.5%) in the present study. The vaccine coverage rates of PCV-7, PCV-10 and PCV-13 were 52.6%, 52.6%, and 83.7%, respectively. S. pneumoniae isolates with the serotype of the PCV-13 showed an increasing trend during the study. Nearly half of the S. pneumoniae strains were MDR, while MDR serotype 19A increased (40%) during the study periods. A small minority of isolates (16%) belonged to non-vaccine serotypes, 65% of which were assigned to MDR. In general, the frequency of penicillin resistant and MDR strains were estimated about 27.5% and 51%, respectively. An increase was observed in resistance to erythromycin and co-trimoxazole.
Conclusion
The results showed that majority of the circulating serotypes in our study are related to PCV-13 serotypes. The use of conjugate vaccine in the immunization program and surveillance of antimicrobial resistance can be effective in reducing the pneumococcal clinical burden.
Keywords: Streptococcus pneumoniae, serotype, pneumococcal conjugate vaccine, PCV, antibiotic susceptibility, multi-drug resistance, MDR
Introduction
Streptococcus pneumoniae is one of the main respiratory pathogens may cause severe threats to human health, such as pneumonia, septicemia, and meningitis1,2 especially in children and the elderly with about 500.000 deaths in the children aging below five, annually.3 Despite the serotype variety, only certain types lead to invasive pneumococcal diseases worldwide.4 The complications of infections and increasing rate of antibiotic resistance, mainly to β-lactams, demonstrate the importance of disease prevention. After the introduction of 7-valent pneumococcal conjugate vaccine (PCV-7) in 2000 in the United States, significant reduction occurred in cases of invasive pneumococcal disease (IPD) due to the vaccine serotypes (VT). As a result of the “replacement phenomenon” over the years, non-vaccine serotypes (NVT) became the major cause of the remaining pneumococcal diseases.5 The distribution of S. pneumoniae serotypes, clones, and antimicrobial resistance patterns differ based on age and geographical area. Therefore, the type of vaccines should be selected with regard to the circulating serotypes in each country.6 Quellung test is the gold standard technique for pneumococcal serotyping which is costly, time-consuming and requires experts for its interpretation. Thus, PCR-based assays such as multiplex PCR can be used instead of the conventional methods. The sequential multiplex PCR assay developed by CDC is easy to perform and cost-effective.7 High carriage rate and genome remodeling capability, expedite the spread of antibiotic resistance and evasion of vaccine-induced immunity.8 The increase of multi-drug resistant (MDR) S. pneumoniae has been reported in the Middle Eastern countries, which is becoming a serious problem around the world.9 High antibiotic resistances have been reported in some serotypes, such as 23F, 19A, 19F, and 14.10 Usually, pneumococcal vaccines target several serotypes belonging to antimicrobial-non-susceptible clones i.e. 19A – clonal complex 320, thus attention to antibiotic resistance patterns is an essential note in vaccination policies.11 Pneumococcal vaccines are not included in Iran national immunization program, while recommended for a limited number of high-risk people. Limited data of pneumococcal serotypes from Iran is available for the last decade.12,13 In addition, applying PCV-13 in the national immunization schedule reduces the incidence of pneumococcal diseases in both vaccinated and unvaccinated individuals of all ages.14
The present study aims to analyze the serotype distribution of S. pneumoniae isolates to determine the PCV-13 serotype coverage. In addition, the serotype fluctuation and antibiotic susceptibility changes were evaluated before the introduction of pneumococcal vaccines, during the study periods.
Materials and Methods
Ethics Statement
The present study was approved by the Medical Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.SPH.REC.1397.178). Further, the written informed consent was obtained from all patients. Furthermore, the present study is based on the principles of the Declaration of Helsinki.
Bacterial Isolates
A total of 80 S. pneumoniae clinical isolates were collected from outpatients and inpatients of pneumococcal infections, admitted to Shariati hospital, Tehran, Iran, during two periods of February to September 2015, and July 2018 to March 2019, each period collecting 40 isolates. The present study has been conducted on patients of 1 month or above. None of the patients had received pneumococcal vaccines. Conventional microbiology tests described by CDC standards and lytA, i.e. species-specific primer amplification were performed to confirm the isolates as S. pneumoniae.15,16 All strains were stored at 80°C in trypticase soy broth Merck, Germany, containing 20% glycerol Merck, Germany and 10% horse serum.
Antimicrobial Susceptibility Testing
Antibiotic susceptibility testing was performed based on the Clinical Laboratory and Standards Institute (CLSI) guidelines.17 The susceptibility of all isolates to erythromycin (15 μg), clindamycin (2 μg), tetracycline (30 μg), trimethoprim/sulfamethoxazole (25 μg), chloramphenicol (30 μg), and vancomycin (30 μg) (MAST, UK), was determined by the Kirby–Bauer disk diffusion method. Minimum inhibitory concentrations (MICs) of penicillin and ceftriaxone were determined by MIC test strip (MTS, Liofilchem, Italy) for all oxacillin resistant isolates in cases of oxacillin zone <20 mm. The meningitis MIC breakpoints for penicillin were as follows: susceptible, MIC ≤ 0.06 μg/mL; resistant, MIC ≥ 0.12 μg/mL. Non-meningitis MIC breakpoints for penicillin were as follows: susceptible, MIC ≤ 2 μg/mL; resistant, MIC ≥ 8 μg/mL. For ceftriaxone, meningitis criteria considered as follows: susceptible, MIC ≤ 0.5 μg/mL; resistant, MIC ≥ 2 μg/mL. Non-meningitis criteria for ceftriaxone considered as follows: susceptible, MIC ≤ 1 μg/mL; resistant, MIC ≥ 4 μg/mL. MDR was defined as nonsusceptibility to at least one agent in three or more antimicrobial categories. S. pneumoniae ATCC 49619 was used as the standard strain for quality control.
DNA Extraction
Genomic DNA was extracted from the purified bacterial cultures by the High Pure PCR Template Preparation Kit (Roche Co., Germany) according to the manufacturer’s instructions. The extracted DNA was stored at −20°C.
Molecular Capsular Typing by Multiplex PCR
The PCV13 serotype distribution was determined by the multiplex PCR assay, based on coskun-Ari study.18 The primers were selected based on CDC recommendation.19 Each reaction comprised four primer pairs, targeting serotype-specific genes, and an internal control, the cpsA gene as a conserved region located at the cps loci. Serotypes with positive control band for cpsA that cannot be identified by the PCR method were classified as non-PCV13 types.
The final volume of reaction was 25 µL HotstarTaq master mix kit (Sinaclon, Iran), containing: 12.5 μL 2x HotstarTaq master mix (3 mM MgCl2, 0.4 mM of each dNTP, 0.2 U/µL Taq DNA polymerase), 2 μL DNA, 10 pmol of each primer and 6.5 µL ddH2O. The PCR steps were optimized as follows: 4 mins incubation at 94°C, 35 cycles consisting of 45-s denaturation at 94°C, 30-s annealing at 58°C and 1 min elongation at 72°C, and 10 mins final extension at 72°C. The PCR products were analyzed by 2% agarose gel (Biotium Inc, USA), in 0.5x TBE buffer at 80 V for 60 mins. The size of the amplicons was determined by the standard (100 bp ladder; Sinaclon, Iran).
Statistical Analysis
Statistical analyses were done in IBM SPSS Statics, Version 19.0 (IBM Crop. Released 2010. IBM SPSS Statics for Windows, Version 19.0. Armonk, NY: IBM Crop). Comparisons were performed with Chi-square test or Fisher’s exact test. A cutoff P value of ≤0.05 (two tailed) was considered statistically significant.
Results
Totally 80 isolates were collected during the study periods, 48.7% (n=38) of which belonged to invasive infections. Females accounted for 43.8% (n=35) of the cases. Age associated with isolates ranged from 1 month to 72 years old with a mean of 18 years. The clinical sources of isolates were blood 32.5% (n=26), CSF 7.5% (n=6), ascites fluid 1.3% (n=1), pleural fluid 5% (n=4), synovial fluid 1.3% (n=1), neck lymph node aspiration 1.3% (n=1), ear discharge 2.5% (n=2) and eye discharge 5% (n=4). Respiratory specimens were taken, including trachea 23.8% (n=19), sputum 10% (n=8), broncho alveolar lavage 3.8% (n=3) and nasal discharge 6.3% (n=5).
Approximately 42% (n=16) of all IPD cases were observed in children ≤5 years, and the prevalence of invasive infections in period II increased during the first 5 years of life, compared to period I (P=0.02).
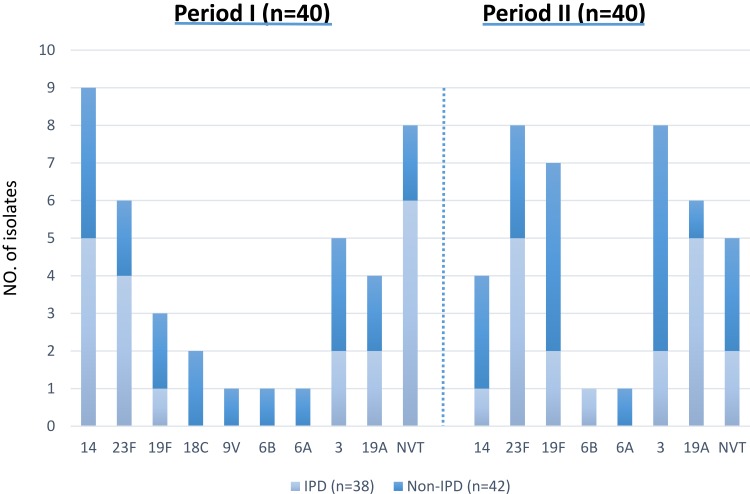
Pneumococcal serotypes of 67 isolates were determined by the multiplex PCR method. About 84% of the isolates were associated with serotypes of 23F, 14, 3, 19F, 19A, 6A, 6B, 9V, and 18C. The most common serotypes were 23F, 17.5% (n=14), 14, 16.3% (n=13), 3, 16.3% (n=13), 19F, 12.5% (n=10) and 19A, 12.5% (n=10). The frequency of serotypes according to the type of infection over the study periods is shown in Figure 1. Among invasive isolates the major serotypes were 23F, 23.6% (n=9), 19A, 18.4% (n=7), and 14, 15.7% (n=6), while serotype 3, 26.1% (n=11), 19F, 21.4% (n=9), and 23F, 11.9% (n=5) were the prominent serotypes among the non-invasive cases.
Figure 1.
Distribution of S. pneumoniae serotypes isolated from IPD & non-IPD.
Abbreviations: IPD, invasive pneumococcal disease; Non-IPD, non-invasive pneumococcal disease; NVT, non-vaccine type.
The potential serotype coverage for PCV-7, PCV-10 and PCV-13 were 55%, 55%, and 80%, respectively, during the study period I, and 50%, 50%, and 87.5%, respectively, during the study period II.
Serotypes 19A, 21% (n=6), 3, 23F, and 14, each 14.2% (n=4) were the most common serotypes in children ≤5 years. Also, serotype 19A was observed in 35.2% (n=6) of IPD cases (P=0.04), and serotypes 3, 23% (n=3), 23F and 14, each 15.3% (n=2) were the most non-IPD serotypes in this age group. In elderly patients (≥64 years), serotype 23F, 50% (n=2) showed the highest frequency. However, no significant difference was found between serotype and age. The serotype coverage rates of PCV-7 and PCV-10 decreased from 54.5% to 35.2% among children ≤5 years, which is the target vaccine population, while the vaccine coverage of PCV-13 showed similar rates in the same case over the two study periods (period I: 81.8%, period II: 82.3%).
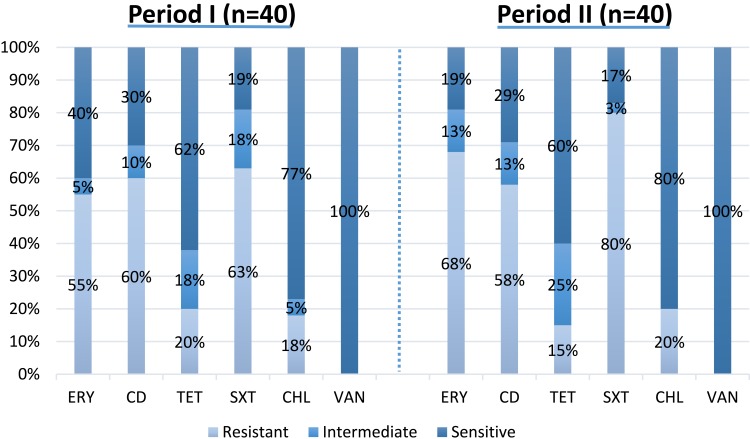
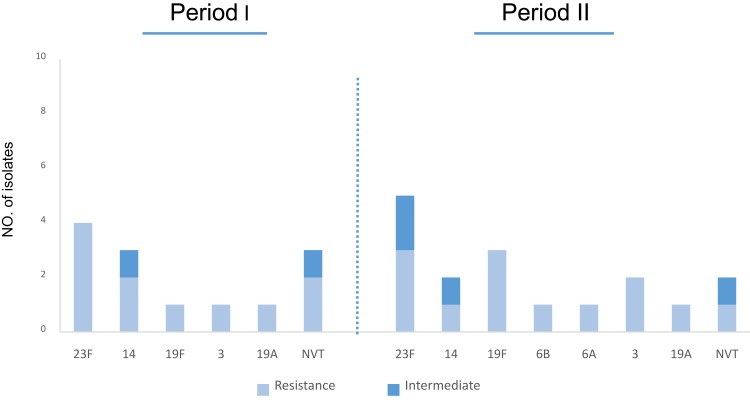
Antibiotic susceptibility profile of S. pneumoniae isolates, collected during two periods is shown in Table 1 and Figure 2. Totally 8.7% (n=7) and 27.5% (n=22) of isolates were penicillin-non-susceptible and resistant pneumococci, among which serotype 23F was the most common one (Figure 3). In addition, non-susceptibility and resistance rates to ceftriaxone were 6.3% (n=5) and 16.2% (n=13), respectively. The resistance rates to erythromycin, co-trimoxazole increased to 68% (n=27) and 80% (n=32) in period II, respectively. The number of isolates resistant to clindamycin, tetracycline, and chloramphenicol, indicated no statistically significant fluctuations during the study periods.
Table 1.
Minimum Inhibitory Concentrations of S. pneumoniae Isolates
| Penicillin | Ceftriaxone | ||||
|---|---|---|---|---|---|
| I% | R% | I% | R% | ||
| MIC break point (µg/mL) |
Meningitis | — | ≥ 0.12 | 1 | ≥ 2 |
| Non-meningitis | 4 | ≥ 8 | 2 | ≥ 4 | |
| Period I | Meningitis (n=4) | 25 (1) | 25 (1) | 0 (0) | 50 (2) |
| Non-meningitis (n=36) | 2.7 (1) | 27.7 (10) | 2.7 (1) | 5.5 (4) | |
| Period II | Meningitis (n=2) | 0 (0) | 50 (1) | 0 (0) | 0 (0) |
| Non-meningitis (n=38) | 13.1 (5) | 26.3 (10) | 10.5 (4) | 18.4 (7) | |
Abbreviations: I, intermediate; R, resistant.
Figure 2.
Antibiotic susceptibility of S. pneumoniae isolates.
Figure 3.
Serotype distribution of S. pneumoniae isolates according to penicillin susceptibility.
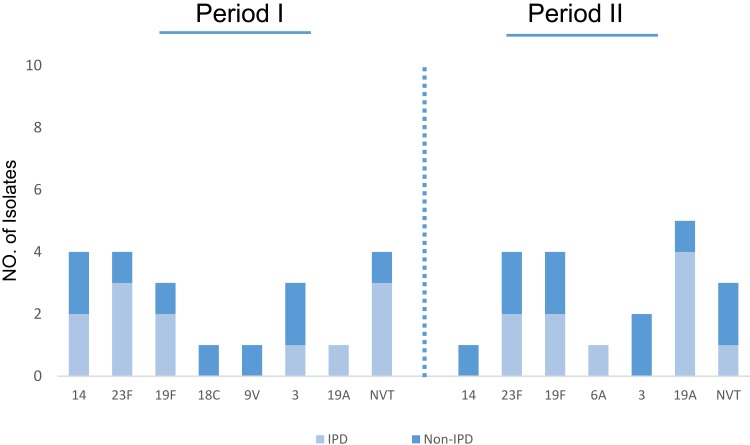
Among 6–18 year cases, the penicillin resistance rates were higher than those in the other age groups 46.2% (n=37). Serotypes 23F, 11.2% (n=9) and 14, 6.2% (n=5), were the most common serotypes showing resistance to penicillin (Figure 3). The frequency of MDR isolates was 51% (n=41), including 52% (n=21) in period I and 50% (n=20) in period II. Serotype 23F, 19.5% (n=8) was the most common serotype among MDR strains (Figure 4). The percentage of MDR isolates among invasive 53% (n=17) and non-invasive 50% (n=24) pneumococci was almost equal.
Figure 4.
Serotype distribution of MDR S. pneumoniae isolates.
Abbreviations: IPD, invasive pneumococcal disease; Non-IPD, non-invasive pneumococcal disease.
Discussion
Vaccination programs lead to a high reduction of pneumococcal diseases worldwide.20 Pneumococcal vaccines are not still included in Iran national immunization program, merely recommended for high-risk groups. Determining PCV-13 vaccine coverage is critical for decision-making on PCV utilization in Iran. The present study investigated pneumococcal serotype distribution and antimicrobial resistance during two periods in a teaching hospital in Iran. The most common serotypes in the order of frequency were 14, 3, 23F, and 19F in our study, which is consistent with the predominant serotypes reported in previous studies in Asia.21,22 In addition, the common serotypes accounted for half of the IPD cases in the present study, similar to findings of pneumococcal global serotype project.23 The circulating pneumococcal serotypes vary during different periods in the same country. Fluctuations in the frequency of serotypes can happen naturally or by vaccine selective pressure.24 The diversity of serotypes in the present study may be affected by limited vaccination in children and high-risk individuals during the two study periods. However, serotype replacement was reported even before introducing the first pneumococcal vaccine.25 The vaccines PCV7, PCV10, and PCV13 covered 52.6%, 52.6%, and 83.7% of the isolates, respectively. The serotypes 19A, 3, and 23F increased during the second period, compared to the first one. Serotype 19A was estimated as the particular emerging serotype among IPD cases (19%), similar to many other countries before the PCV introduction.26 However, the main reasons are not completely understood, the significant rise of serotype 19A MDR isolates may be due to antibiotic selective pressure or migration. Serotype 3 has received global attention due to high virulence and mortality rate.27 However, in line with other studies, we have found serotypes 3 to be a frequent cause of non-IPD.28,29
Serotypes 3 and 19A are not recently covered by PCV-10, thus using these vaccines may not be sufficient. The data showed low serotype coverage of PCV-7 (50%) in period II which is in concordance with data from other Asian countries such as Nepal.30 However, 68% of serotypes causing pneumococcal infection in India were covered by PCV-7.31 On the other hand, the rising incidence of NVT pneumococcal infections indicate a need to use extended-valency PCVs or new generation vaccines. At present, PCVs are only recommended to a limited number of high-risk people in Iran. The results suggest that PVC-13 with 82.8% coverage can be included in Iran immunization schedule. Clinical impacts of pneumococcal antibiotic resistance is a global concern. The resistant pneumococci have a high risk of causing invasive disease, so high frequency of resistant isolates can be expected among invasive strains.32 However, the proportion of MDR isolates was almost equal among invasive and non-invasive pneumococci, in this study. The results confirmed high β-lactams and macrolides pneumococcal resistance. In addition, high resistances to penicillin, erythromycin, and co-trimoxazole were found during the two study periods. There were no significant differences in resistance rates to tetracycline and clindamycin among the two study periods. Penicillin non-susceptibility was observed in about one third of S. pneumoniae isolates (27.5% resistant and 8.7% intermediate). The resistance rate to penicillin has increased over the study periods, 1.5 times higher than other recent study in Iran.33 Serotype 23F was the most common serotype showing resistance to penicillin, while many countries have reported increasing prevalence of resistant NVTs following PCV-13, such as 23A, 15B, and 15C.34 Furthermore, non-susceptibility rate to ceftriaxone has increased slightly, compared to the previous study in the same area in 2017.12 Increasing trend of resistance to ceftriaxone is related to selective pressure by antibiotic overuse, while ceftriaxone is still a good choice for empirical treatment. Similar to several other studies, the rate of erythromycin-resistance was high (61.25%) in the present study, mainly due to its highly overuse for the treatment of respiratory tract infections.35,36 Seventy-six percent of pediatric patients were affected by IPD, half of whose invasive isolates were MDR. In concordance, other studies, serotypes 23F, 19F, and 19A were predominant serotypes in MDR isolates.37 Among NVT strains, 53.8% of isolates were identified as MDR, indicating the emergence of high resistant strains not included in PCVs. Finally, however, the number of collected isolates in the present study was low, the data can help to monitor the vaccine serotype changes and provide clinical guidance for administering the appropriate antimicrobial therapies.
Acknowledgments
This work was supported by Tehran University of Medical Sciences, Tehran, Iran and performed as a part of M.Sc. thesis [grant no. 39112].
Abbreviations
IPD, invasive pneumococcal disease; NVT, none-vaccine type; ERY, erythromycin; CD, clindamycin; TET, tetracycline; SXT, trimethoprim/sulfamethoxazole; CHL, chloramphenicol; VAN, vancomycin.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16(6):355–367. doi: 10.1038/s41579-018-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniel P, Rodrigo C, Bewick T, et al. 13-Valent vaccine serotype pneumococcal community acquired pneumonia in adults in high clinical risk groups. Vaccine. 2018;36(12):1614–1620. doi: 10.1016/j.vaccine.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 3.Wahl B, O’Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–2015. Lancet Glob Health. 2018;6(7):e744–e757. doi: 10.1016/S2214-109X(18)30247-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jauneikaite E, Tocheva AS, Jefferies JM, et al. Current methods for capsular typing of Streptococcus pneumoniae. J Microbiol Methods. 2015;113:41–49. doi: 10.1016/j.mimet.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 5.Warren JL, Pingali SC, Weinberger DM. Spatial variability in the persistence of pneumococcal conjugate vaccine-targeted pneumococcal serotypes among adults. Epidemiology. 2017;28(1):119–126. doi: 10.1097/EDE.0000000000000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi W, Zhou K, Yuan L, et al. Serotype distribution, antibiotic resistance patterns and molecular characteristics of serogroup 6 Streptococcus pneumoniae isolates collected from Chinese children before the introduction of PCV13. J Glob Antimicrob Resist. 2018;14:23–28. doi: 10.1016/j.jgar.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 7.Mauffrey F, Fournier E, Demczuk W, et al. Comparison of sequential multiplex PCR, sequetyping and whole genome sequencing for serotyping of Streptococcus pneumoniae. PLoS One. 2017;12(12):e0189163. doi: 10.1371/journal.pone.0189163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Moujaber G, Osman M, Rafei R, Dabboussi F, Hamze M. Molecular mechanisms and epidemiology of resistance in Streptococcus pneumoniae in the Middle East region. J Med Microbiol. 2017;66(7):847–858. doi: 10.1099/jmm.0.000503 [DOI] [PubMed] [Google Scholar]
- 9.Talebi M, Azadegan A, Sadeghi J, et al. Determination of characteristics of erythromycin resistant Streptococcus pneumoniae with preferred PCV usage in Iran. PLoS One. 2016;11(12):e0167803. doi: 10.1371/journal.pone.0167803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). Drug resistance; 2017. Available from: www.cdc.gov/pneumococcal/drug-resistance.html. Accessed March19, 2019.
- 11.Setchanova L, Alexandrova A, Pencheva D, et al. Rise of multidrug-resistant Streptococcus pneumoniae clones expressing non-vaccine serotypes among children following introduction of the 10-valent pneumococcal conjugate vaccine in Bulgaria. J Glob Antimicrob Resist. 2018;15:6–11. doi: 10.1016/j.jgar.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 12.Houri H, Tabatabaei SR, Saee Y, Fallah F, Rahbar M, Karimi A. Distribution of capsular types and drug resistance patterns of invasive pediatric Streptococcus pneumoniae isolates in Tehran, Iran. Nt J Infect Dis. 2017;57:21–26. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadi A, Yaghoubi S, Irajian G. Molecular analysis of PBP1A in Streptococcus pneumoniae isolated from clinical and normal flora samples in Tehran, Iran: a multicenter study. Microb Drug Resist. 2019;25(1):39–46. doi: 10.1089/mdr.2017.0326 [DOI] [PubMed] [Google Scholar]
- 14.Esposito S, Principi N. Impacts of the 13-valent pneumococcal conjugate vaccine in children. J Immunol Res. 2015;2015:591580. doi: 10.1155/2015/591580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC). Identification and characterization of Streptococcus pneumoniae; 2017. Available from: www.cdc.gov/meningitis/lab-manual/chpt08-id-characterizationstreppneumo.html Accessed March12, 2019.
- 16.Azarsa M, Salami SA, Pourmand MR, Forushani AR, Kazemian H. Evaluation of lytB gene for detection of Streptococcus pneumoniae in isolates and clinical specimens by real-Time PCR. Jundishapur J Microbiol. 2017;10(6):e14378. doi: 10.5812/jjm [DOI] [Google Scholar]
- 17.Wayne PA Clinical and Laboratory Standards Institute: performance standards for antimicrobial susceptibility testing: 26th informational supplement. CLSI document M100-S20. 2015 January1.
- 18.Coskun-Ari FF, Guldemir D, Durmaz R. One-step multiplex PCR assay for detecting Streptococcus pneumoniae serogroups/types covered by 13-valent pneumococcal conjugate vaccine (PCV13). PLoS One. 2012;7(12):e50406. doi: 10.1371/journal.pone.0050406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). Multiplex conventional PCR schemes for pneumococcal serotype deduction; 2018. Available from https://www.cdc.gov/streplab/pneumococcus/resources.html. Accessed January24, 2020.
- 20.Centers for Disease Control and Prevention (CDC). Global pneumococcal disease and vaccine; 2017. Available from: https://www.cdc.gov/pneumococcal/global.html. Accessed March12, 2019..
- 21.Nagaraj S, Kalal BS, Manoharan A, Shet A. Streptococcus pneumoniae serotype prevalence and antibiotic resistance among young children with invasive pneumococcal disease: experience from a tertiary care center in South India. Germs. 2017;7(2):78–85. doi: 10.18683/germs.2017.1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai SS. Streptococcus pneumoniae serotype distribution and pneumococcal conjugate vaccine serotype coverage among pediatric patients in East and Southeast Asia, 2000–2014: a pooled data analysis. Vaccines. 2016;4:1. doi: 10.3390/vaccines4010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson HL, Deloria-Knoll M, Levine OS, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7:10. doi: 10.1371/journal.pmed.1000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medeiros MIC, Almeida SCG, Guerra M, da Silva P, Carneiro AMM, de Andrade D. Distribution of Streptococcus pneumoniae serotypes in the northeast macro-region of Sao Paulo state/Brazil after the introduction of conjugate vaccine. BMC Infect Dis. 2017;17(1):590. doi: 10.1186/s12879-017-2696-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwambana-Adams B, Hanson B, Worwui A, et al. Rapid replacement by non-vaccine pneumococcal serotypes may mitigate the impact of the pneumococcal conjugate vaccine on nasopharyngeal bacterial ecology. Sci Rep. 2017;7(1):8127. doi: 10.1038/s41598-017-08717-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi EH, Kim SH, Eun BW, et al. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis. 2008;14(2):275–281. doi: 10.3201/eid1402.070807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Bae IK, Park D, et al. Serotype distribution and antimicrobial resistance of streptococcus pneumoniae isolates causing invasive and noninvasive pneumococcal diseases in Korea from 2008 to 2014. Biomed Res Int. 2016;2016:6950482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugimoto N, Yamagishi Y, Hirai J, et al. Invasive pneumococcal disease caused by mucoid serotype 3 Streptococcus pneumoniae: a case report and literature review. BMC Res Notes. 2017;10(1):21. doi: 10.1186/s13104-016-2353-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maraki S, Mavromanolaki VE, Stafylaki D, Hamilos G, Samonis G. The evolving epidemiology of serotype distribution and antimicrobial resistance of streptococcus pneumoniae strains isolated from adults in Crete, Greece, 2009–2016. Infect Chemother. 2018;50(4):328–339. doi: 10.3947/ic.2018.50.4.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah AS, Knoll MD, Sharma PR, et al. Invasive pneumococcal disease in Kanti Children’s Hospital, Nepal, as observed by the South Asian Pneumococcal Alliance network. Clin Infect Dis. 2009;48(Suppl 2):S123–S128. doi: 10.1086/596490 [DOI] [PubMed] [Google Scholar]
- 31.Manoharan A, Manchanda V, Balasubramanian S, et al. Invasive pneumococcal disease in children aged younger than 5 years in India: a surveillance study. Lancet Infect Dis. 2017;17(3):305–312. doi: 10.1016/S1473-3099(16)30466-2 [DOI] [PubMed] [Google Scholar]
- 32.Wang CY, Chen YH, Fang C, et al. Antibiotic resistance profiles and multidrug resistance patterns of Streptococcus pneumoniae in pediatrics: a multicenter retrospective study in mainland China. Medicine. 2019;98(24):e15942. doi: 10.1097/MD.0000000000015942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadeghi J, Ahamadi A, Douraghi M, Pourshafie MR, Talebi M. Molecular analysis of pbp2b in Streptococcus pneumonia isolated from clinical and normal flora samples. Curr Microbiol. 2015;70(2):206–211. doi: 10.1007/s00284-014-0704-7 [DOI] [PubMed] [Google Scholar]
- 34.Choe YJ, Lee HJ, Lee H, et al. Emergence of antibiotic-resistant non-vaccine serotype pneumococci in nasopharyngeal carriage in children after the use of extended-valency pneumococcal conjugate vaccines in Korea. Vaccine. 2016;34(40):4771–4776. doi: 10.1016/j.vaccine.2016.08.030 [DOI] [PubMed] [Google Scholar]
- 35.Kim SH, Song JH, Chung DR, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56(3):1418–1426. doi: 10.1128/AAC.05658-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azadegan A, Ahmadi A, Lari AR, Talebi M. Detection of the efflux-mediated erythromycin resistance transposon in Streptococcus pneumoniae. Ann Lab Med. 2015;35(1):57–61. doi: 10.3343/alm.2015.35.1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassiolato AP, Almeida SCG, Andrade AL, Minamisava R, Brandileone MCC. Expansion of the multidrug-resistant clonal complex 320 among invasive Streptococcus pneumoniae serotype 19A after the introduction of a ten-valent pneumococcal conjugate vaccine in Brazil. PLoS One. 2018;13(11):e0208211. doi: 10.1371/journal.pone.0208211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Centers for Disease Control and Prevention (CDC). Drug resistance; 2017. Available from: www.cdc.gov/pneumococcal/drug-resistance.html. Accessed March19, 2019.