Abstract
Introduction
Hesperetin-5,7,3ʹ-O-trimethylether (HTME), a synthetic liposoluble hesperetin, has been reported to be a dual phosphodiesterase (PDE)3/4 inhibitor. We investigated its inhibitory effects on methacholine (MCh)-induced airway hyperresponsiveness (AHR) and its potential for treating atypical asthma and COPD.
Methods
FlexiVent system was used to determine AHR in ovalbumin (OVA) sensitized and challenged mice. Determination of cytokines was performed by using mouse T helper (Th)1/Th2 cytokine CBA kits, and of total immunoglobulin (Ig)E and OVA-specific IgE using ELISA kits. The number of inflammatory cells was counted using a hemocytometer. Xylazine/ketamine-induced anesthesia was to assess nausea, vomiting, and gastric hypersecretion in these mice.
Results
HTME dually and competitively inhibited PDE3/4 activities in the Lineweaver–Burk analysis. HTME (30 and 100 μmol/kg) dose-dependently and significantly decreased the airway resistance (RL) and increased lung dynamic compliance (Cdyn) values induced by MCh. It significantly suppressed numbers of total inflammatory cells and neutrophils, and levels of cytokines in bronchoalveolar lavage fluid (BALF). HTME dose-dependently and significantly inhibited total and OVA-specific IgE levels in the BALF and serum. However, HTME did not influence xylazine/ketamine-induced anesthesia.
Conclusion
HTME exerted anti-inflammatory and bronchodilator effects and may be useful in treating chronic obstructive pulmonary disease and allergic atypical asthma with no gastrointestinal side effects.
Keywords: allergic asthma, chronic obstructive pulmonary disease, therapeutic PDE4H/PDE4L ratio, ovalbumin, cytokines, bronchoalveolar lavage fluid, inflammation
Introduction
Phosphodiesterases (PDEs) comprise 11 distinct enzyme families. PDE3 plays a role in airway dilatation and up-regulates in airway smooth muscle from patients with asthma.1 PDE3 has two genes identified, known as PDE3A and PDE3B. PDE4 has four genes (A–D) identified, and has high (PDE4H) and low (PDE4L) affinities for rolipram. The therapeutic (PDE4H/PDE4L) ratio of selective PDE4 and dual PDE3/4 inhibitors for treating asthma and chronic obstructive pulmonary disease (COPD) has been established.2,3 However, the real therapeutic ratio of dual PDE3/4 inhibitors should be greater than that reported,3 because they are reported to have additive or synergistic effects compared to PDE3 or PDE4 inhibitors alone.4
Synthetic hesperetin-5,7,3ʹ-O-trimethylether (HTME) dually inhibited PDE3/4 with a therapeutic (PDE4H/PDE4L) ratio of 18.33 which is greater than that (3) of roflumilast, an orally administered selective PDE4 inhibitor.5 Roflumilast was approved by the European Commission6 and the US Food and Drug Administration3 as a bronchodilator for severe COPD and recently reported to reverse xylazine/ketamine-induced anesthesia in mice at orally effective dose, suggesting that roflumilast may have gastrointestinal (GI) side effects.7 Thus, selective PDE4 inhibitors were limited their development and wider use.4 We investigated the inhibiting effects of HTME on methacholine (MCh)-induced airway hyperresponsiveness (AHR), and clarified its potential for treating atypical asthma and COPD with few or no GI side effects.
Materials and Methods
Reagents and Animals
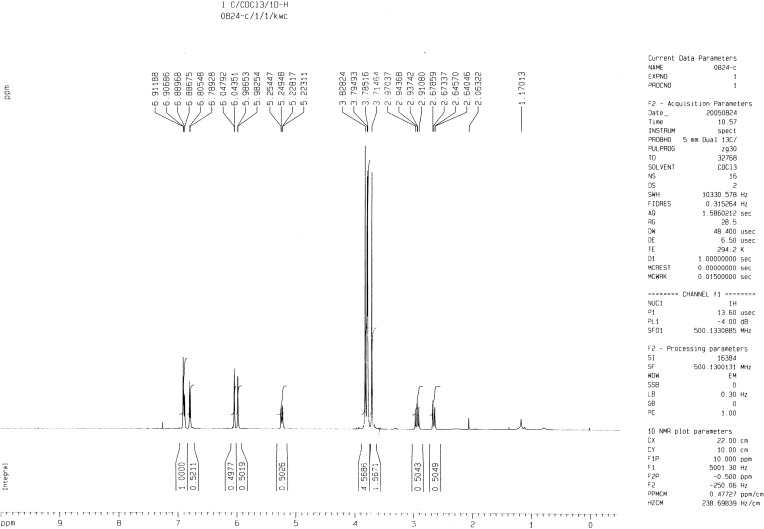
HTME (M.W., 344.28) was synthesized according to the previous method.8 The purity of HTME was 98% and the structure was determined by spectral methods [m.p. 158–160°C which was consistent with literature, IR: 1669 cm−1 (C=O),1H NMR δ (Figure 1): 2.65 (1H, dd, J=17.2 Hz, H-3), 2.94 (1H, dd, J=17.2, H-3), 3.71 (3H, s, -OCH3), 3.78–3.82 (9H, s, 3×-OCH3), 5.23 (1H, dd, J=2.5, H-2), 5.98 (1H, dd, H-6), 6.04 (1H, dd, H-8), 6.79 (1H, d, J=8.3, H-5ʹ), 6.88 (1H, dd, J=8.3, H-6ʹ), 6.90 (1H, d, J=2.51, H-2ʹ)].3 Aluminum sulfate, MCh, ovalbumin (OVA), ketamine, and xylazine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Milrinone and Ro 20–1724 were purchased from Biomol (Plymouth Meeting, PA, USA). Freund’s adjuvant (Mycobacterium butyricum) was purchased from Pierce Biotechnology (Rockford, IL, USA). Mouse T helper (Th)1/Th2 cytokine CBA kits, and mouse immunoglobulin (Ig)E enzyme-linked immunosorbent assay (ELISA) sets were purchased from Pharmingen (San Diego, CA, USA). Ethyl alcohol and polyethylene glycol (PEG) 400 were purchased from Merck (Darmstadt, Germany). [3H]cAMP was purchased from Amersham Pharmacia Biotech (Buckinghamshire, UK). HTME and Ro 20–1724 were dissolved in a mixture of ethyl alcohol and DMSO (1:1). Other reagents were dissolved in distilled water.
Figure 1.
The NMR spectrum of hesperetin-5,7,3ʹ-O-trimethylether (HTME).
Female BABL/c mice at 8–12 weeks’ old were purchased from Animal Center of the Ministry of Science and Technology (Taipei, Taiwan), and housed in ordinary cages at 22 ± 1°C with a humidity of 50%–60% under a constant 12/12-h light/dark cycle and provided with food and water ad libitum.
Ethical Statement
According to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Taipei Medical University, the following in vivo experiments were performed.
Competitive Inhibition of PDE3 and PDE4 Activities by HTME
According to the previous method,9 the Lineweaver–Burk analyses for HTME, milrinone,10 and Ro 20–172411 at various concentrations including its vehicle (0 μM, control) were performed. The total protein was assayed according to the previous method.12 PDE activities are reported as nmol/mg/min.
AHR in vivo
In accordance with the previous method and schedule,13 the AHR of female BABL/c mice was assessed by measuring changes in the airway resistance (RL, cmH2O/mL/sec) and lung dynamic compliance (Cdyn, mL/cmH2O) after challenge with aerosolized methacholine (MCh, 0.78~25 mg/mL) using the FlexiVent system (SCIREQ, Montreal, Quebec, Canada). Anesthetized (urethane 600 mg/kg and chloralose 120 mg/kg, i.p.), tracheostomized (stainless-steel cannula, 18 G) mice were mechanically ventilated (at 150 breaths/min, with a tidal volume of 10 mL/kg, positive end-expiratory pressure of 3 cmH2O).
Unrestrained mice were placed into the main chamber of a whole-body plethysmograph and nebulized with phosphate-buffered saline (PBS). Subsequently, nebulized with MCh 6.25–50 mg/mL for 3 min at each increasing concentration. Twenty-four hours later, bronchoalveolar lavage fluid (BALF) and blood of mice under anesthesia (pentobarbital 50 mg/kg, i.p.) were collected. Cytokines in the BALF were determined using mouse T helper (Th)1/Th2 cytokine CBA kits (Pharmingen, San Diego, CA, USA).13 Total and OVA-specific immunoglobulin (Ig)E in the BALF and serum were determined using ELISA kits (Pharmingen, San Diego, CA, USA).13,14 The number of inflammatory cells in the BALF and differentiation were performed as the previous method.13 All undetectable data (<1 pg/mL) of cytokines were taken as 0.5 pg/mL.
Xylazine/Ketamine-Induced Anesthesia
According to the previous methods,13,15 the ability of reversing xylazine/ketamine-induced anesthesia, a surrogate for determining GI side effects, by HTME, Ro 20–1724, or their vehicle was, respectively, determined in normal female BALB/c mice.
Statistical Analysis
All values are given as the median (min, max) and n is the number of experiments. The difference between two values was determined by using the Wilcoxon Rank-Sum Test (Mann–Whitney U). Differences among values were calculated by Kruskal–Wallis one-way analysis of variance on rank and then determined by Dunnett’s test by using SigmaPlot 10 (St. Louis, MO, USA). Significance was accepted when P< 0.05.
Results
Competitive Inhibition of PDE3 and PDE4 Activities by HTME
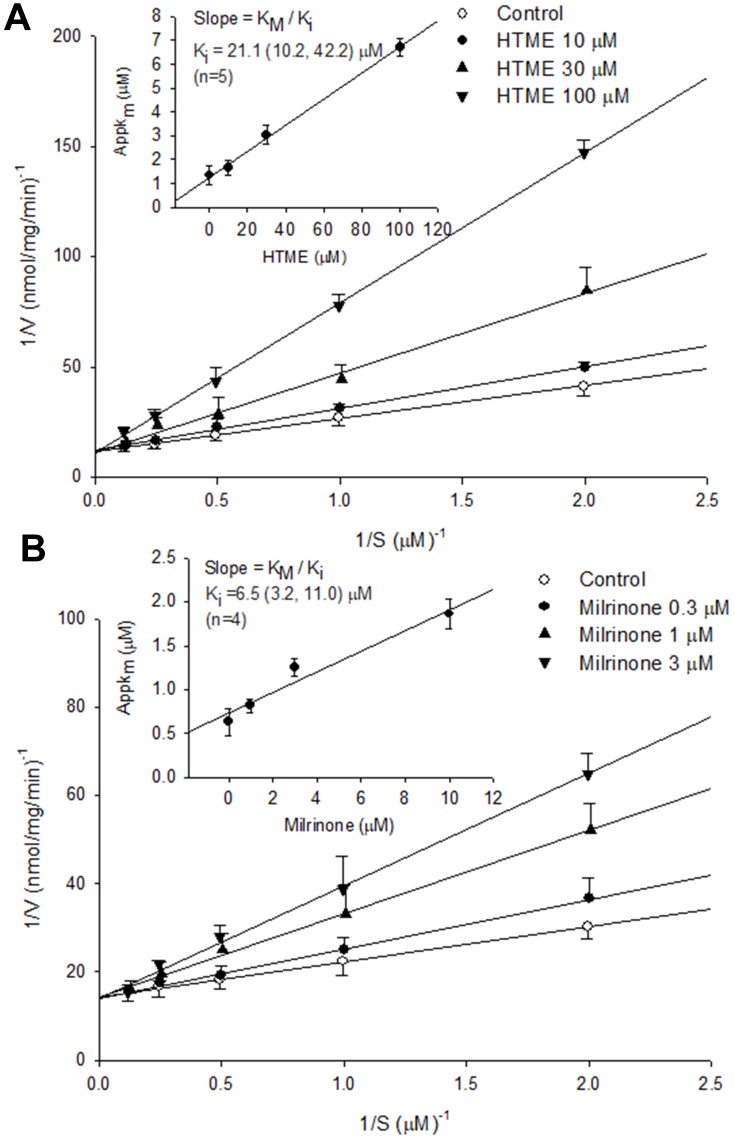
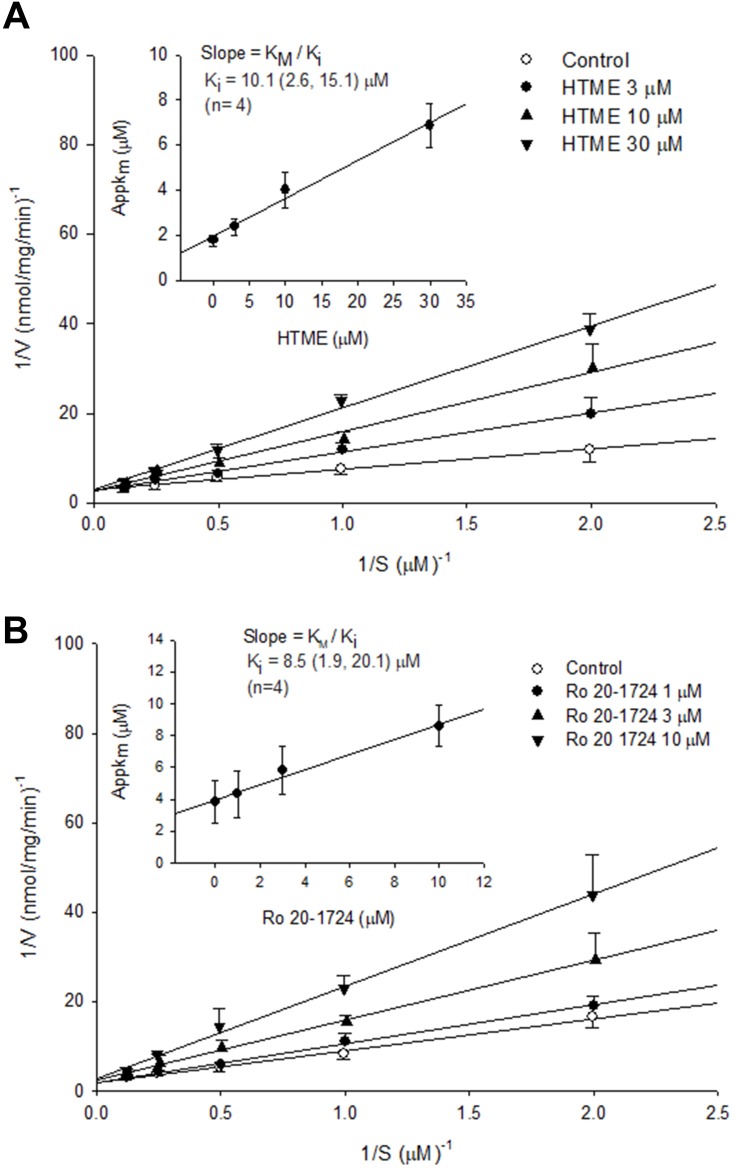
HTME (10–100 μM) and milrinone (0.3–3 μM) competitively inhibited PDE3 activity (Figure 2), as 1/Vmax values were not significantly influenced by various concentrations of HTME (a) or milrinone (b) in the Lineweaver–Burk analysis. Their dissociation constants for inhibitory binding (Ki) values were, respectively, calculated to be 21.1 (10.2, 42.2) (n=5) and 6.5 (3.2, 11.0) (n=4) μM (Figure 2 inset). Similarly, HTME (3–30 μM) and Ro 20–1724 (1–10 μM) competitively inhibited PDE4 activity (Figure 3A and B), with calculated Ki values of 10.1 (2.6, 15.1) (n=4) and 8.5 (1.9, 20.1) (n=4) μM, respectively (Figure 3 inset). The Ki value of HTME for PDE4 inhibition did not significantly differ from that for PDE3 inhibition, suggesting that the affinities of HTME for PDE3 and PDE4 were similar.
Figure 2.
Inhibition of PDE3-induced cAMP hydrolysis by HTME (A) and milrinone (B). Activities of PDE3 in the presence of various concentrations of HTME or milrinone, and the substrate (cAMP) were plotted according to a Lineweaver–Burk analysis. Ki was determined from the equation of the apparent Km as a function of the inhibitor concentration (inset).
Figure 3.
Inhibition of PDE4 induced cAMP hydrolysis by HTME (A) and Ro 20–1724 (B). Activities of PDE4 in the presence of various concentrations of HTME or Ro 20–1724, and the substrate (cAMP) were plotted according to a Lineweaver–Burk analysis. Ki was determined from the equation of the apparent Km as a function of the inhibitor concentration (inset).
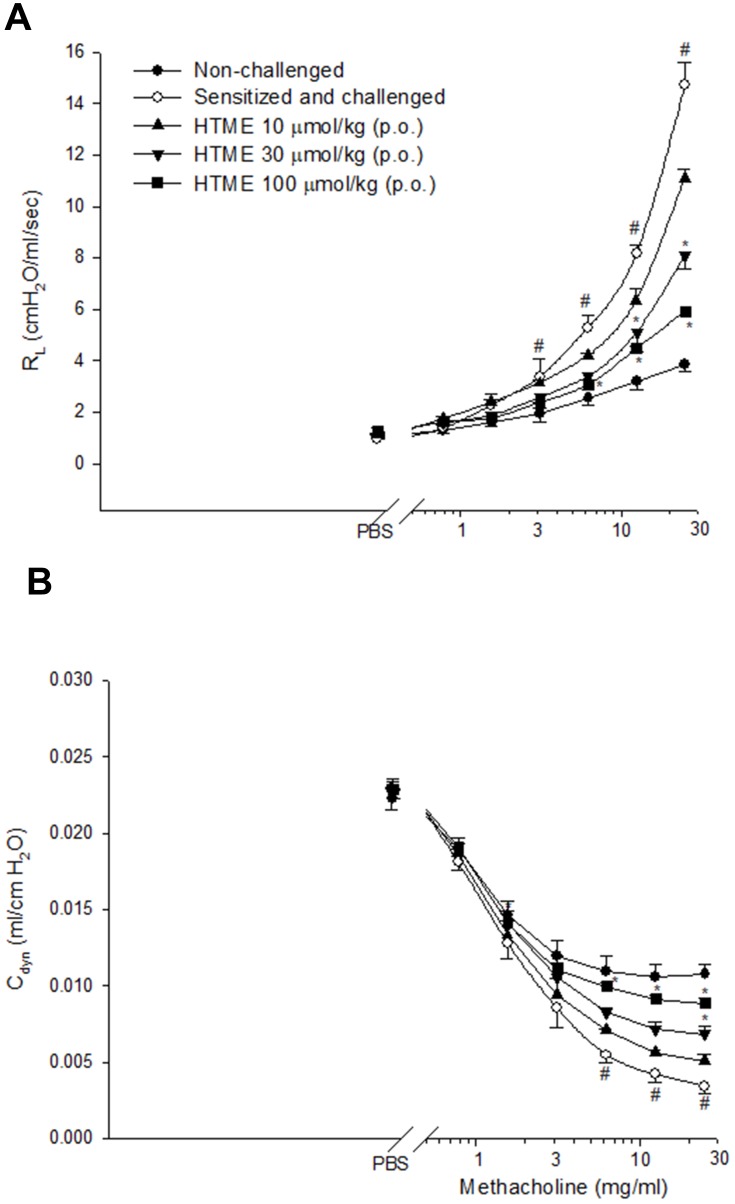
Suppression of AHR in vivo
Methacholine (6.25–25 mg/mL) concentration-dependently and significantly enhanced airway resistance (RL) values (Figure 4A), and reduced lung dynamic compliance (Cdyn) values (Figure 4B) in the sensitized and challenged mice compared to the non-challenged mice. HTME (100 μmol/kg, p.o.) significantly reversed these changes (Figure 4). Moreover, HTME (30 μmol/kg, p.o.) significantly suppressed airway resistance (RL) values at MCh 12.5 and 25 mg/mL (Figure 4A), and enhanced lung dynamic compliance (Cdyn) values at MCh 25 mg/mL (Figure 4B).
Figure 4.
Effects of HTME (10–100 µmol/kg, p.o.) on airway resistance (RL, A) and lung dynamic compliance (Cdyn, B) in sensitized and challenged mice which received aerosolized methacholine (6.25–50 mg/mL) 2 days after allergen challenge. # P< 0.05 compared to the non-challenged group. * P< 0.05 compared to the sensitized and challenged group administered (p.o.) vehicle alone. The number of mice in each group was 10. PBS, phosphate-buffered saline.
Suppression of Inflammatory Cells in BALF
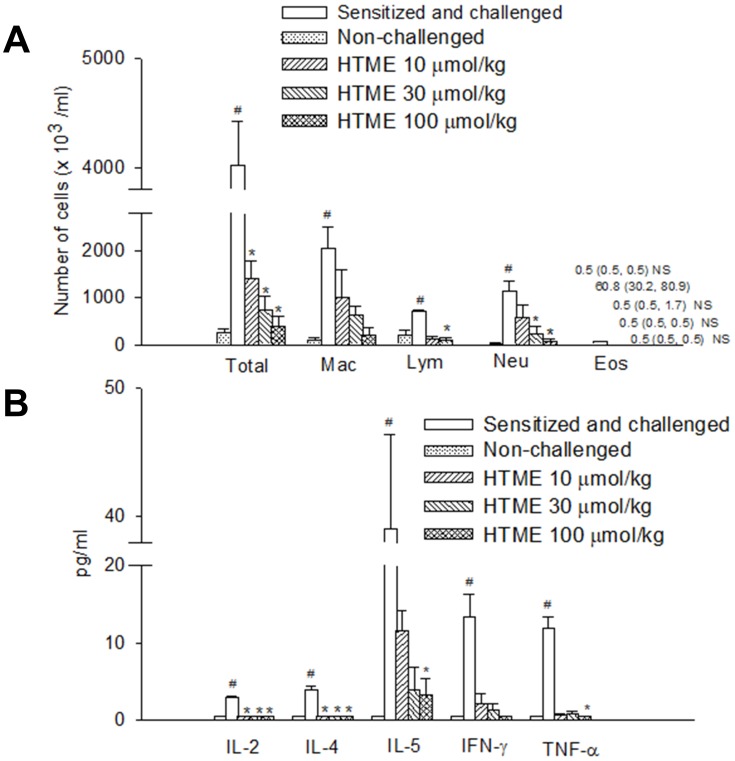
The number of total inflammatory cells from the BALF of sensitized and challenged mice significantly enhanced compared to that of non-challenged mice (Figure 5A). HTME (10, 30, and 100 μmol/kg, p.o.) also significantly attenuated the enhancement in the number of total inflammatory cells. HTME (30 and 100 μmol/kg, p.o.) also significantly attenuated the enhancements of neutrophils, but not macrophages, lymphocytes, and eosinophils, with an exception of the highest dose on lymphocytes (Figure 5A).
Figure 5.
Effects of HTME (10–100 µmol/kg, p.o.) on the inflammatory cells (A), and cytokines (B) in sensitized and challenged mice which received aerosolized methacholine (6.25–50 mg/mL) 2 days after allergen challenge. # P< 0.05 compared to the non-challenged group. * P< 0.05 compared to the sensitized and challenged group administered (p.o.) vehicle alone. The number of mice in each group was 10. Total, total cells; Mac, macrophages; Lym, lymphocytes; Neu, neutrophils; Eos, eosinophils; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor. NS: Not significantly different from those of sensitized and challenged group.
Suppression of Cytokines in BALF
Compared with those in non-challenged mice, levels of cytokines, such as interleukin (IL)-2, IL-4, IL-5, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ, in the BALF of sensitized and challenged mice significantly enhanced (Figure 5B). HTME (10~100 μmol/kg, p.o.) also significantly suppressed the enhancement in levels of IL-2 and IL-4, but not IFN-γ. However, HTME at the highest dose did those of IL-5 and TNF-α (Figure 5B).
IgE Levels Suppressed in the Serum and BALF
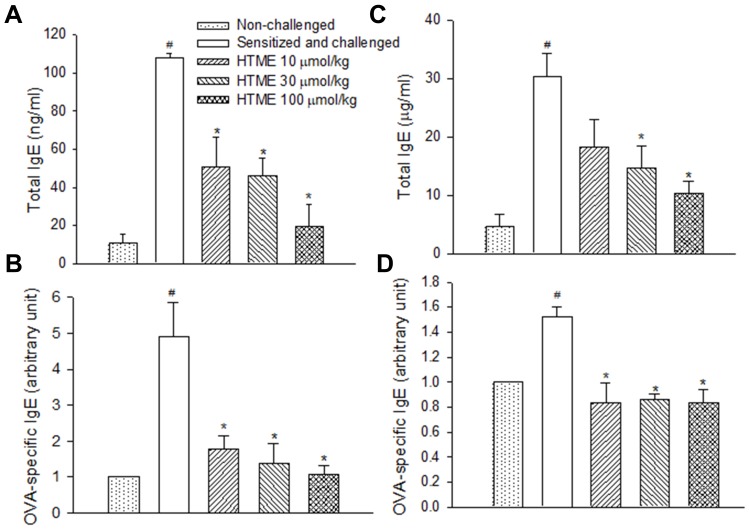
Total and OVA-specific IgE levels in the BALF (Figure 6A and B) and serum (Figure 6C and D) of sensitized and challenged mice were significantly increased compared to those of non-challenged mice (Figure 6). HTME (10–100 μmol/kg, p.o.) dose-dependently and significantly attenuated these enhancements with the exception that HTME at 10 µmol/kg did not significantly decrease the total IgE level in the serum (Figure 6C).
Figure 6.
Effects of HTME (10–100 μmol/kg, p.o.) on total IgE (A, C) and ovalbumin (OVA)-specific IgE (B, D) levels in bronchial alveolar lavage fluid (A, B) and serum (C, D) of sensitized and challenged mice which received aerosolized methacholine (6.25–50 mg/mL) 2 days after allergen challenge. # P< 0.05 compared to the non-challenged group. * P< 0.05 compared to the sensitized and challenged group administered (p.o.) vehicle alone. The number of mice in each group was 10.
No or Few GI Side Effects
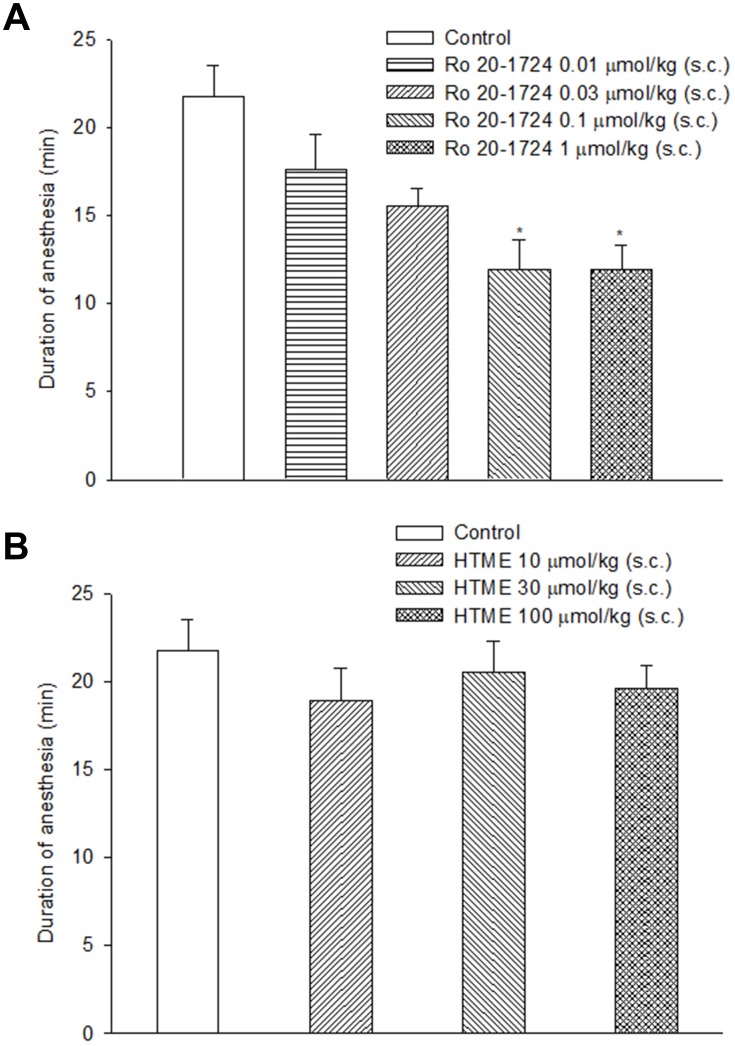
The durations of xylazine/ketamine-induced anesthesia in vehicle (control) of Ro 20-1724- and HTME-treated mice were 18.5 (14.3, 36.7) and 18.5 (12.1, 36.7) min, respectively, which do not differ from each other. Ro 20–1724 at 0.1 and 1 μmol/kg (s.c.) significantly reversed the duration (Figure 7A). On the opposite, the duration was not significantly affected by HTME (10~100 μmol/kg, s.c.) (Figure 7B).
Figure 7.
Effects of subcutaneously administered Ro 20–1724 (A) and HTME (B) on the duration of xylazine (10 mg/kg, i.p.)/ketamine (70 mg/kg, i.p.)-induced anesthesia in mice. Ro 20–1724 was administered 0.25 hr and HTME 1 hr before anesthesia. * P< 0.05 compared to the vehicle (control). The number of mice in each group was 10.
Discussion
This animal model was suggested for studying drug effects on the allergic atypical asthma and COPD.13 Neutrophilic inflammation is a hallmark of atypical asthma and COPD which is characterized by airway obstruction and worsening lung function, associated with enhanced airway inflammation. In the present results, HTME (30 and 100 μmol/kg, p.o.) significantly decreased RL (Figure 4A) and increased Cdyn (Figure 4B) at 6.25–25 mg/mL methacholine with some exceptions at lower dose. Also, the numbers of total inflammatory cells and neutrophils detected were almost reduced in the BALF of HTME-treated mice. These suggest that HTME may have beneficial effects for treating COPD and allergic atypical asthma. Cytokines released from Th2 cells are IL-4 and IL-5, and those from Th1 cells are IL-2, IFN-γ, and TNF-α.16 HTME (10–100 μmol/kg, p.o.) significantly inhibited the levels of IL-2 and IL-4, but not IFN-γ. However, HTME at the highest dose also did those of IL-5 and TNF-α (Figure 5B). Thus, HTME inhibits both Th1 and Th2 cells which have been implicated in autoimmune and atopic diseases, respectively.17 HTME 10–100 μmol/kg (p.o.) dose-dependently and significantly inhibited total and OVA-specific IgE levels in the BALF and serum of sensitized and challenged mice although with an exception of HTME at the lowest dose (Figure 6C), suggesting that HTME has immunoregulatory and anti-allergic asthmatic effects.
HTME dually and competitively inhibited PDE3/4 activities, although HTME was also reported to inhibit PDE1 activity.3 However, the IC50 value for PDE1 inhibition was significantly greater than that for PDE4 inhibition.3 Combined inhibition of PDE3 and PDE4 can have synergistic anti-inflammatory effects in CD4+ and CD8+ human T-lymphocytes18 and have a synergistic inhibitory effect on vascular cell adhesion molecule (VCAM)-1 expression and eosinophil adhesion to activated human lung microvascular endothelial cells.19 Furthermore, combined inhibition of PDE3 and PDE4 has synergistic inhibitory effects on allergen or leukotriene (LT) C4-induced contraction of human airway smooth muscle, but PDE3 or PDE4 inhibition alone has no effect on these contractions.20 Recently RPL 554, a dual PDE3/4 inhibitor, has been reported to effectively relax human bronchial smooth muscle precontracted by a number of contractile agents.4 Combined inhibition of PDE3 and PDE4 also stimulates mucociliary clearance in patients with COPD.4 Accordingly, the inhaled route of administration would be a clever strategy to take with PDE3/4 inhibitors to maximize the therapeutic ratio and reduce the side effects.4 However, inhaled administration of zardaverine, a PDE3/4 inhibitor, has been reported to elicit GI side effects, suggesting that the inhaled route is not sufficient alone to reduce these effects.4 RPL 554 should be carefully monitored these GI side effects in patients with asthma and COPD in clinical trials.
In the present results, HTME (10–100 µmol/kg, s.c.) did not affect xylazine/ketamine-induced anesthesia suggesting that HTME may have few or no GI adverse effects. In contrast, Ro 20–1724, a selective PDE4 inhibitor, reversed the anesthesia. The reversing effect has been suggested to occur through presynaptic α2-adrenoceptor inhibition,21 because MK-912, an α2-adrenoceptor antagonist, was reported to reverse xylazine/ketamine-induced anesthesia in rats22 and trigger vomiting in ferrets.21 In contrast, clonidine, an α2-adrenoceptor agonist, prevented emesis induced by PDE4 inhibitors in ferrets.21 The availability of an inhaled dual PDE3/4 inhibitor, RPL 554, is rapidly cleared from systemic circulation may provide a novel approach to treat COPD and other inflammatory airway disease.4 RPL 554 is in Phase 2 clinical trials with no significant cardiovascular side effects reported up today.23 It needs to further investigate whether HTME has cardiovascular side effects and how to competitively inhibit PDE3/4 activities.
Conclusions
HTME showed anti-inflammatory and bronchodilator actions in this murine model, including suppression of AHR, and attenuation of inflammatory cells and cytokines. Moreover, HTME did not reverse xylazine/ketamine-induced anesthesia, suggesting HTME has few or no GI adverse effects. Thus, HTME may be useful in treating COPD and allergic atypical asthma.
Acknowledgment
This work was supported by a grant (NSC 96-2320-B-038-020) from the Ministry of Science and Technology, Taipei, Taiwan.
Abbreviations
AHR, airway hyperresponsiveness; BALF, bronchoalveolar lavage fluid; Cdyn, lung dynamic compliance; COPD, chronic obstructive pulmonary disease; ELISA, enzyme-linked immunosorbent assay; GI, gastrointestinal; [3H]cAMP, [3H]adenosine 3ʹ,5ʹ cyclic monophosphate; HTME, hesperetin-5,7,3ʹ-O-trimethylether; IFN-γ, interferon-γ; IL, interleukin; Ig, immunoglobulin; LT, leukotriene; MCh, methacholine; OVA, ovalbumin; PDE, phosphodiesterase; RL, airway resistance; TNF-α, tumor necrosis factor-α.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yick CY, Zwinderman AH, Kunst PW, et al. Transcriptome sequencing (RNA-Seq) of human endobronchial biopsies: asthma versus controls. Eur Respir J. 2013;42(3):662–670. doi: 10.1183/09031936.00115412 [DOI] [PubMed] [Google Scholar]
- 2.Giembycz MA. Phosphodiesterase 4 inhibitors and the treatment of asthma: where are we now and where do we go from here? Drugs. 2000;59(2):193–212. doi: 10.2165/00003495-200059020-00004 [DOI] [PubMed] [Google Scholar]
- 3.Hsu HT, Wang WH, Han CY, et al. Inhibitory effects of hesperetin derivatives on guinea pig phosphodiesterases and their ratios between high- and low-affinity rolipram binding. J Pharm Sci. 2013;102(7):2120–2127. doi: 10.1002/jps.2359110.1002/jps.23591 [DOI] [PubMed] [Google Scholar]
- 4.Abbott-Banner KH, Page CP. Dual PDE3/4 and PDE4 inhibitors: novel treatments for COPD and other inflammatory airway diseases. Basic Clin Pharmacol Toxicol. 2014;114(5):365–376. doi: 10.1111/bcpt.12209 [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Zhang HT, O’Donnell JM. Inhibitor binding to type 4 phosphodiesterase (PDE4) assessed using [3H]piclamilast and [3H]rolipram. J Pharmacol Exp Ther. 2003;305(2):565–572. doi: 10.1124/jpet.102.047407 [DOI] [PubMed] [Google Scholar]
- 6.Giembycz MA, Field SK. Roflumilast: first phosphodiesterase 4 inhibitor approved for treatment of COPD. Drug Des Devel Ther. 2010;4:147–158. doi: 10.2147/dddt.s7667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YL, Chen CL, Chen CM, et al. Hesperetin-5,7,3ʹ-O-triacetate suppresses airway hyperresponsiveness in ovalbumin-sensitized and challenged mice without reversing xylazine/ketamine-induced anesthesia in normal mice. BMC Pharmacol Toxicol. 2017;18(1):39. doi: 10.1186/s40360-017-0146-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kupchan SM, Bauerschmidt E. Cytotoxic flavonols from Baccharis sarothroides. Phytochemistry. 1971;10:664–666. doi: 10.1016/S0031-9422(00)94716-2 [DOI] [Google Scholar]
- 9.Ko WC, Chen MC, Wang SH, et al. 3-O-methylquercetin more selectively inhibits phosphodiesterase subtype 3. Planta Med. 2003;69(4):310–315. doi: 10.1055/s-2003-38874 [DOI] [PubMed] [Google Scholar]
- 10.Carceles MD, Fuentes T, Aroca V, et al. Effects of milrinone on contractility and cyclic adenosine monophosphate production induced by β1- and β2-adrenergic receptor activation in human myocardium. Clin Ther. 2007;29(8):1718–1724. doi: 10.1016/j.clinthera.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 11.Peng S, Yang X, Liu GJ, et al. The phosphodiesterase-4 inhibitor Ro 20-1724 reverses learning and memory impairments, and downregulation of CREB in the hippocampus and cortex induced by ketamine anesthesia in immature rats. J Neurosurg Sci. 2014;58(4):231–237. doi:R38Y9999N00A140003 [pii] [PubMed] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 13.Yang YL, Hsu HT, Wang KH, et al. Hesperetin-7,3ʹ-O-dimethylether selectively inhibits phosphodiesterase 4 and effectively suppresses ovalbumin-induced airway hyperresponsiveness with a high therapeutic ratio. J Biomed Sci. 2011;18(1):84–95. doi: 10.1186/1423-0127-18-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melgert BN, Postma DS, Geerlings M, et al. Short-term smoke exposure attenuates ovalbumin-induced airway inflammation in allergic mice. Am J Respir Cell Mol Biol. 2004;30(6):880–885. doi: 10.1165/rcmb.2003-0178OC [DOI] [PubMed] [Google Scholar]
- 15.Robichaud A, Stamatiou PB, Jin SL, et al. Deletion of phosphodiesterase 4D in mice shortens α2-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110(7):1045–1052. doi: 10.1172/JCI0215506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revets H, Pynaert G, Grooten J, et al. Lipoprotein I, a TLR2/4 ligand modulates Th2-driven allergic immune responses. J Immunol. 2005;174(2):1097–1103. doi: 10.4049/jimmunol.174.2.1097 [DOI] [PubMed] [Google Scholar]
- 17.Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987;138(11):3688–3694. [PubMed] [Google Scholar]
- 18.Giembycz MA, Corrigan CJ, Seybold J, et al. Identification of cyclic AMP phosphodiesterases 3, 4 and 7 in human CD4+ and CD8+ T-lymphocytes: role in regulating proliferation and the biosynthesis of interleukin-2. Br J Pharmacol. 1996;118(8):1945–1958. doi: 10.1111/j.1476-5381.1996.tb15629.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blease K, Burke-Gaffney A, Hellewell PG. Modulation of cell adhesion molecule expression and function on human lung microvascular endothelial cells by inhibition of phosphodiesterases 3 and 4. Br J Pharmacol. 1998;124(1):229–237. doi: 10.1038/sj.bjp.0701833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt DT, Watson N, Dent G, et al. The effect of selective and non-selective phosphodiesterase inhibitors on allergen- and leukotriene C4-induced contractions in passively sensitized human airways. Br J Pharmacol. 2000;131(8):1607–1618. doi: 10.1038/sj.bjp.0703725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robichaud A, Savoie C, Stamatiou PB, et al. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway. Neuropharmacology. 2001;40(2):262–269. doi: 10.1016/S0028-3908(00)00142-8 [DOI] [PubMed] [Google Scholar]
- 22.Robichaud A, Savoie C, Stamatiou PB, et al. Assessing the emetic potential of PDE4 inhibitors in rats. Br J Pharmacol. 2002;135(1):113–118. doi: 10.1038/sj.bjp.0704457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franciosi LG, Diamant Z, Banner KH, et al. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: findings from four clinical trials. Lancet Respir Med. 2013;1(9):714–727. doi: 10.1016/S2213-2600(13)70187-510.1016/S2213-2600(13)70187-5 [DOI] [PubMed] [Google Scholar]