Abstract
Isolated 17,20-lyase deficiency may be caused by mutations in the CYP17A1 (coding for cytochrome P450c17), POR (coding for cytochrome P450 oxidoreductase) and CYB5A (coding for microsomal cytochrome b5) genes. Of these, mutations in the CYB5A gene have thus far only been described in genetic males who presented with methemoglobinemia and 46,XY disorders of sex development (DSD) due to 17,20-lyase deficiency.
A 24-year-old Chinese woman presented to the hematology outpatient clinic with purplish discoloration of fingers, toes, and lips since childhood. Investigations confirmed methemoglobinemia. A homozygous c.105C>G (p.Tyr35Ter) nonsense mutation was detected in the CYB5A gene. Hormonal studies showed isolated 17,20-lyase deficiency. Interestingly, she had a completely normal female phenotype with no DSD, normal pubertal development, and spontaneous pregnancy giving birth uneventfully to a healthy female infant.
The sex hormone-related features of genetic females with 17,20-lyase deficiency due to cytochrome b5 gene mutation appear to differ from that of females with 17,20-lyase deficiency caused by other genetic defects who presented with hypergonadotropic hypogonadism and infertility and differ from genetic males with the same mutation.
Keywords: CYB5A; 17,20-lyase deficiency; methemoglobinemia
1. Introduction
Isolated 17,20-lyase deficiency is a rare condition that results in sex steroid deficiency. Clinically, 46,XY patients present with disorders of sex development (DSD), while 46,XX patients have absent or disturbed pubertal development, depending on the severity of enzyme defect.
Mutations in genes encoding cytochrome P450c17 (CYP17A1), P450 oxidoreductase (POR), and microsomal cytochrome b5 (CYB5A) had been reported to account for 17,20-lyase deficiency [1]. The cytochrome P450c17 microsomal enzyme is expressed in steroidogenic tissues including adrenal cortexes, testes, and ovaries. This single enzyme catalyzes both 17α-hydroxylase and 17,20-lyase activities to produce glucocorticoid and sex steroids, respectively. The 17,20-lyase activity is dependent on the availability of POR, the obligatory electron transfer protein required by all microsomal P450 enzymes. Cytochrome b5 acts as an allosteric cofactor in facilitating interaction between POR and P450c17, enhancing electron flux and favoring 17,20-lyase reaction. On the other hand, 17α-hydroxylase activity is not modulated by cytochrome b5 [1].
Thus far, all reported cases of mutations in the cytochrome b5 gene (CYB5A) were identified in genetic males [2–5]. To our knowledge, this is the first report of a female case in the literature.
We report the clinical and biochemical findings of a 24-year-old Chinese woman who had a homozygous mutation c.105C>G (p.Tyr35Ter) in the CYB5A gene. She presented with methemoglobinemia. Biochemical findings suggested deficiency of 17,20-lyase activity, but she had normal female phenotype and was able to conceive spontaneously and go through an uneventful pregnancy.
1.1. Case Description
The patient first presented to the accident and emergency department with dizziness and malaise at the age of 24 years and was found to have purplish coloration over fingers, toes, and lips. She recalled having bluish fingers and lips since childhood. Exercise tolerance was good all along. There was no family history of methemoglobinemia. Oxygen saturation was 94% in room air. Hemoglobin level was 14.7 g/dL. Test for glucose-6-phosphate dehydrogenase enzyme activity was normal. Methemoglobin level was 13.4% (reference interval [RI]: <1.5%). There was no contact with dye, and she took no medication. She was referred to the hematology clinic for further management. Hemoglobinopathy was excluded by hemoglobin pattern testing. The patient was keen for genetic testing for methemoglobinemia as she was then pregnant and wanted to know if the methemoglobinemia might be transmitted to her offspring.
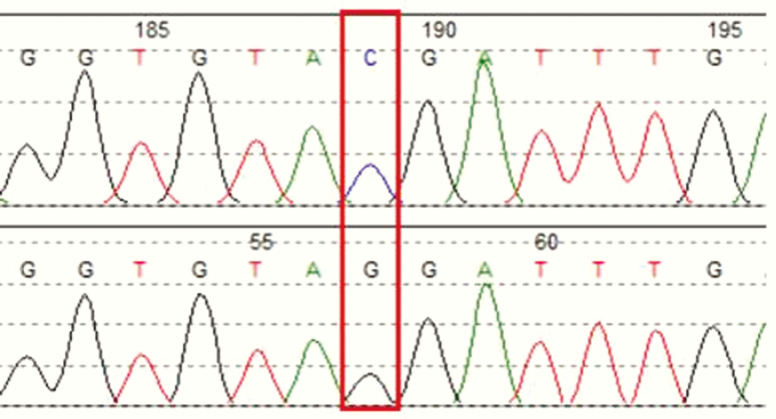
Molecular genetic analysis was performed after obtaining informed and written consent from the patient. Genomic deoxyribonucleic acid was extracted from peripheral leukocytes and subjected to polymerase chain reaction amplification. Primers were first designed to amplify exons 1 to 9 of the CYB5R3 gene, which codes for cytochrome b5 reductase. Sanger sequencing of all the coding regions of this gene did not reveal any pathogenic variants, hence we proceeded with the molecular analysis of the CYB5A gene located on chromosome 18, which codes for the microsomal enzyme cytochrome b5. A homozygous nonsense variant c.105C>G was identified in the CYB5A gene. (Fig. 1) This is a reported disease-causing variant located at the first of the 5 exons in the CYB5A gene, changing codon 35 from TAC to TAG, which is a termination codon (p.Tyr35Ter). The substituted stop codon leads to premature termination of peptide translation, and the truncated protein is expected to be nonfunctioning or have significantly defective cytochrome b5 activity, causing congenital methemoglobinemia type IV.
Figure 1.
Electrophoregram of exon 1 of the CYB5A gene of the patient.
Homozygous NM_148923.3:c.105C>G p.(Tyr35Ter) variant was detected. Keys: Upper, reference; lower, patient.
As cytochrome b5 is also an allosteric effector that interacts with the P450c17 oxidoreductase complex to stimulate 17,20-lyase activity, and other patients with CYB5A mutations had been reported to have 17,20-lyase deficiency, the patient was referred to the endocrinology clinic for further evaluation and hormonal analysis.
Physical examination revealed normal secondary sexual characteristics, with normal adult female breasts and normal axillary and pubic hair. Blood pressure was normal. Menarche was at 11 years of age, with regular monthly cycles all along.
Plasma electrolytes and renal function tests were normal. Blood tests in the follicular phase showed mildly elevated 17-hydroxyprogesterone (17-OHP) at 11 nmol/L (RI: <4 nmol/L). Progesterone was 4.8 nmol/L (RI: <1.0 nmol/L), estradiol 163 pmol/L (RI: 77–921 pmol/L), luteinizing hormone 24.6 IU/L (RI: 1.8–11.8 IU/L), follicle-stimulating hormone 7.7 IU/L (RI: 3.0–8.1 IU/L), androstenedione < 0.5 nmol/L (RI: 1.1–6.5 nmol/L), testosterone < 0.2 nmol/L (RI: <1.7 nmol/L), dehydroepiandrosterone (DHEA)-sulphate 1.0 umol/L (RI: 3.6–11.1 umol/L). Cortisol was 545 nmol/L (RI: 101–536 nmol/L), renin 2.78 ng/ml/hr (RI: 0.98–4.18 ng/ml/hr), aldosterone 251 pmol/L (RI: <777 pmol/L). A 250-microgram adrenocorticotropin hormone (ACTH) stimulation test resulted in a normal cortisol response of 545, 675, and 787 nmol/L at 0, 30, and 60 minutes, respectively, while 17-OHP response was absent with 11, 11, and 11 nmol/L at 0, 30, and 60 minutes, respectively. The normal basal and stimulated cortisol results confirmed a normal 17-hydroxylase activity, whereas the lack of response in 17-OHP and low androstenedione, testosterone, and DHEA-sulphate levels suggested a selective impairment in 17,20-lyase activity (Table 1) [6–7]. Urinary steroid profiling revealed very low androgen metabolites and elevation of metabolites of 17-OH pregnenolone, 17-OHP, and pregnenolone, also compatible with 17,20-lyase deficiency (Fig. 2). To complete the evaluation, karyotype was performed that confirmed 46,XX.
Table 1.
Hormonal Tests and 250 Microgram ACTH Stimulation Test Results
| Basal | 30 Min | 60 Min | Reference Interval | |
|---|---|---|---|---|
| Cortisol | 545 | 675 | 787 | 101–536 nmol/L |
| 17-hydroxyprogesterone | 11 | 11 | 11 | basal < 4 nmol/L; 30 min post synacthen 2–8 nmol/L |
| Androstenedione | <0.5 | 0.6 | 0.7 | 1.1–6.5 nmol/L |
| 21-deoxycortisol | <2.5 | <2.5 | <2.5 | ≤2.5 nmol/L |
| 11-deoxycortisol | 1.8 | 3.5 | 4.4 | ≤4.3 nmol/L |
| Testosterone | 1.0 | 0.9 | 1.0 | <1.7 nmol/L |
| DHEA-sulphate | <0.2 | <0.2 | 0.2 | 2.6–13.9 umol/L |
| LH | 24.6 | 1.8–11.8 IU/L | ||
| FSH | 7.7 | 3.0–18.1 IU/L | ||
| Progesterone | 4.8 | <1.0 nmol/L | ||
| Estradiol | 163 | 77–921 pmol/L | ||
| Renin, erect | 2.78 | 0.98–4.18 ng/ml/hr | ||
| Aldosterone, erect | 251 | ≤777 pmol/L |
Abbreviations: DHEA, FSH, follicle-stimulating hormone; LH, luteinizing hormone; dehydroepiandrosterone; min, minutes.
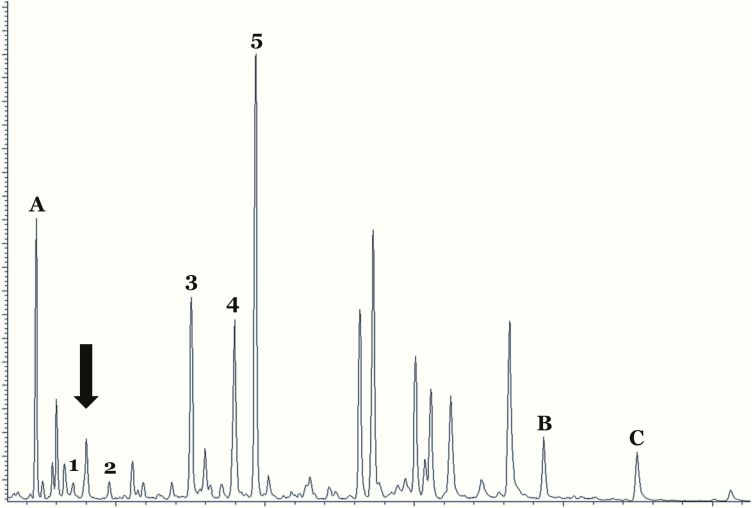
Figure 2.
Gas chromatogram of urinary steroid profiling of the patient. Androgen metabolites were low (large arrow). 17-hydroxypregnanolone (metabolite of 17-hydroxyprogesterone), pregnanediol (metabolite of progesterone), and pregnanetriol (metabolite of 17-hydroxyprogesterone) were elevated (3–5), compatible with 17,20-lyase deficiency.Keys: A, B and C are internal standards. 1, androsterone; 2, DHEA; 3, 17-hydroxypregnanolone; 4, pregnanediol; 5, pregnanetriol.
Abbreviation: DHEA, dehydroepiandrosterone.
The patient’s pregnancy was closely monitored by obstetricians and endocrinologists. It was uneventful, and she gave birth to a healthy female infant. The blood methemoglobin levels varied from 8.2% to 14.3% at follow-up.
2. Discussion
To date, 4 mutations in the CYP17A1 gene (p.Arg347His, p.Arg347Cys, p.Arg358Gln, and p.Glu305Gly), 1 mutation in the POR gene (p.Gly539Arg), and 4 mutations in the CYB5A gene have been identified as the causes for isolated 17,20-lyase deficiency [3–5, 8–9].
Essentially, all patients with CYP17A1 or POR mutations had variable impairment of glucocorticoid production, with insufficient cortisol responses to ACTH stimulation as well as a DSD due to 17,20-lyase activity, with the latter dominating the clinical picture. They may require glucocorticoid supplementation during stress [10].
In contrast, patients with CYB5A mutations all had normal glucocorticoid production. The first case of CYB5A mutation was reported by Hegesh et al in 1986 [2]. A female infant, later found to be a male pseudohermaphrodite, presented with methemoglobinemia on day 7 of life. She had a 16-bp deletion in the messenger ribonucleic acid, resulting from a mutation c.130-2A>G in the 3' splicing junction of intron 1 [8]. A second case was reported by Kok et al in 2010 [3]. The infant boy presented with ambiguous genitalia. He carried a homozygous nonsense mutation c.81G>A (p.Trp27Ter) in exon 1 of the CYB5A gene causing a premature stop codon. The absence of residues E48 and E49 and the C-terminal part of cytochrome b5 affected 17,20-lyase activity, resulting in deficient androgen productions and 46,XY DSD. The third CYB5A mutation, a missense mutation c.131A>T (p.His44Leu), was reported by Idkowiak et al in 2012 in 3 children in a family from Pakistan [4]. All of them had 46,XY DSD due to isolated 17,20-lyase deficiency and methemoglobinemia. In 2014, another novel nonsense mutation in the CYB5A gene, c.105C>G (p.Tyr35Ter), was reported locally in a patient with methemoglobinemia [5]. She also had 46,XY DSD and isolated 17,20-lyase deficiency. At birth, there was ambiguous genitalia with palpable gonads at the vulva. A diagnosis of testicular feminization was made initially, and the infant was reared as a female. She had breast development at 12 years of age and pubic hair growth at age 13 years. Methemoglobin level was 7.7 % (RI <0.5%) when assessed at the age of 29 years.
To date, all reported cases of CYB5A mutation were in genetic XY patients. They all had severe DSD (Table 2). To our knowledge, this is the first reported case of a genetic female with a CYB5A mutation who has the same mutation as the XY patient reported locally. Like her XY counterpart, she also had methemoglobinemia. Biochemical findings of elevated 17-OHP with blunted response to ACTH stimulation, low androstenedione, testosterone, and DHEA-sulphate levels together with findings from urine steroid profiling suggestive of blockage in the conversion of 17-hydroxypregnenolone to DHEA and 17-OHP to androstenedione were consistent with the diagnosis of isolated 17,20-lyase deficiency. It has been well established that cytochrome b5 enhances 17,20-lyase, probably by affecting the activity of POR as an allosteric cofactor. Absent or aberrant action of cytochrome b5 leads to reduced, though not absent, 17,20-lyase activity [3, 11]. Intriguingly, the patient did not have any clinical features of DSD and was able to spontaneously conceive and deliver a healthy infant. This component of 17,20-lyase deficiency thus appears to follow sex-limited recessive genetics. As reported in other cases, serum cortisol and cortisol response in the ACTH stimulation test were normal.
Table 2.
Summary of CYB5A Cases
| Hegesh et al, 1986 [2] | Kok et al, 2010 [3] | Idkowiak et al, 2012 [4] | Idkowiak et al, (Sib 1) [4] | Idkowiak et al, (Sib 2) [4] | Yeung et al, 2014 [5] | Current Case | |
|---|---|---|---|---|---|---|---|
| Age of presentation | 7 days | 0 day | 0 day | 13 years | 0 day | 0 day | 13 years |
| Ethnicity | Jewish | Netherlander | Pakistani | Pakistani | Pakistani | Chinese | Chinese |
| Symptoms | Cyanosis, pseudohermaphrodite | Ambiguous genitalia | Ambiguous genitalia | Absent puberty, cliteromegaly | Ambiguous genitalia | Ambiguous genitalia | Cyanosis |
| Rearing gender | Female | Male | Male | Female | Male | Female | Female |
| Karyotype | 46 XY | 46 XY | 46 XY | 46 XY | 46 XY | 46 XY | Not done |
| Biochemical findings | Not mentioned | Isolated 17,20-lyase deficiency | Isolated 17,20-lyase deficiency | Isolated 17,20-lyase deficiency | Isolated 17,20-lyase deficiency | Isolated 17,20-lyase deficiency | Isolated 17,20-lyase deficiency |
| Genetic analysis | Homozygous splicing 16-bp deletion | Homozygous nonsense p.W27X mutation | Homozygous missense p.H44L mutation | Homozygous missense p.H44L mutation | Homozygous missense p.H44L mutation | Homozygous nonsense p.Y35X mutation | Homozygous nonsense p.Y35X mutation |
| Methemoglobin level | 19% | 0.061 mmol/mol Hb (<0.015) | 0.063 mmol/mol Hb (<0.015) | 0.061 mmol/mol Hb (<0.015) | 0.085 mmol/mol Hb (<0.015) | 7.7% (<0.5%) | 13.4% (<1.5%) |
Abbreviation: Hb, hemoglobin.
Genetic females with 17,20-lyase deficiency due to CYP17A1 or POR mutations presented with hypergonadotropic hypogonadism and infertility [9]. Spontaneous sexual development and menarche had been reported, but not spontaneous pregnancy. There was only 1 report of pregnancy after transfer of cryopreserved embryos. [9, 12] Anovulation, uterine hypoplasia, and high progesterone and low estrogen levels were postulated to contribute to infertility.
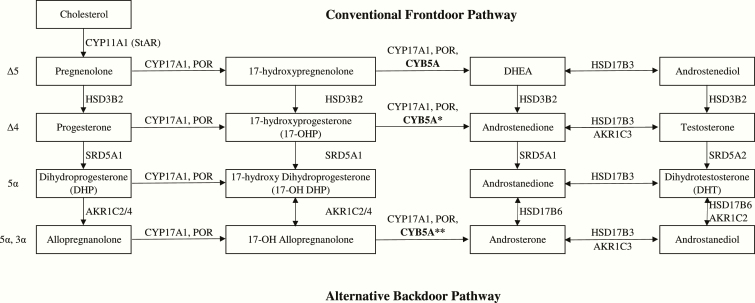
The ability of an affected female to go through normal puberty and pregnancy and an affected male to have sufficient estrogen for breast development and androgen for pubic hair growth in cases with cytochrome b5 mutation had prompted interest in an alternative pathway in sex steroid production. While the classical (“frontdoor”) pathway produces dihydrotestosterone (DHT) by converting 17-hydroxypregnenolone to DHEA, and then androstenediol and testosterone, there is now increasing evidence of a “backdoor” pathway for sex steroid production [13].
This backdoor pathway converts 17OHP, through 17-hydroxy-dihydroprogesterone, 17-hydroxy-allopregnanolone, androsterone, and androstanediol to DHT (Fig. 3). The pathway was first discovered in the testes of the tammar wallaby pouch young [14]. Subsequent studies of urine steroid metabolites in patients with cytochrome P450 oxidoreductase (POR) deficiency found significantly increased 17-OH allopregnanolone levels and androsterone to etiocholanolone ratios during early infancy, demonstrating the presence of a backdoor pathway in humans [15]. However, the activity of the backdoor pathway is usually only detected during infancy when the activity of 5α-reductase, which converts 17-OHP to 17-hydroxy-allopregnanolone, is high. After infancy, 5α-reductase activity decreases with age, leading to less efficient 5α-reduction of 17-OHP to 17-hydroxy-allopregnanolone. In parallel with an increasing 17,20-lyase activity, 17-OHP would be directed into the classical pathway instead of the backdoor pathway for androgen production [16].
Figure 3.
The conventional front door pathway (Δ5 and Δ4 routes via testosterone) and the alternative backdoor pathway (5α and 5α, 3α routes bypassing testosterone). The 17,20-lyase activity of CYP17A1 is low for 17-OHP in the frontdoor pathway (*) and high for 17-OH allopregnanolone in the backdoor pathway (**).Abbreviations: AKR1C2/4, 3α-hydroxysteroid dehydrogenase type 3/1; AKR1C3, 3α-hydroxy- steroid dehydrogenase type 2; CYB5A, cytochrome b5; CYP11A1, cholesterol side chain cleavage; CYP17A1, 17α-hydroxylase, 17/20-lyase; HSD3B2, 3β-hydroxysteroid dehydrogenase; HSD17B3, 17β-hydroxysteroid dehydrogenase type 3; HSD17B6, 17β-hydroxysteroid dehydrogenase type 6; POR, cytochrome P450 oxidoreductase; SRD5A1, 5α-reductase type 1; SRD5A2, 5α-reductase type 2.
Expression of genes coding for enzymes of the backdoor pathway, including aldoketo reductases (AKR1C1-4), 5α-reductases (SRD5A1-2), and retinol dehydrogenase (RoDH) had been found in the human ovary, with higher expression found in ovaries in polycystic ovarian syndrome, suggesting a possible role of human ovary in the backdoor pathway for DHT synthesis [17].
Discovery of mutations in AKR1C2, a key enzyme that participates in the backdoor pathway in 46,XY individuals with DSD, further illustrated the importance of a backdoor pathway in normal human sexual differentiation and fetal androgen biosynthesis in the testes [17]. Retrospective analysis of urine steroid profiles of patients with 21α-hydroxylase deficiency suggested that the backdoor pathway contributed to androgen biosynthesis in the human adrenal cortex [18], as the accumulated 17-OHP was found to be a good substrate for 5α-reductase.
This pathway has therefore been implicated in the variable virilization of genetic females with both 21α-hydroxylase deficiency and POR deficiency as well as androgen excess in some patients with polycystic ovarian syndrome [13, 15–19]. In the backdoor pathway, 17,20-lyase activity is minimally dependent on cytochrome b5, in contrast to the conventional front door pathway. In patients with cytochrome b5 mutation, it is conceivable that the backdoor pathway may be less impaired than the classical pathway, producing the androgens for the normal development of ovarian follicles and reproduction in our female patient as well as supplying androgen and, through aromatization, estrogen for pubic hair and breast development in an affected male.
Androsterone and etiocholanolone are both androgen metabolites derived from androgen precursors and active androgens. While etiocholanolone is produced exclusively along the front door pathway, androsterone can be derived from both androstenedione in the classical Δ 4 pathway and DHT but also from the backdoor pathway via 5α-pregnane-3α,17α-diol-20-one.
A high androsterone/etiocholanolone ratio in the urine steroid profile can therefore indicate activity in the backdoor pathway [16,18]. In our patient, however, the estimation of androsterone to etiocholanolone ratio could not be considered as accurate with the very low androgen levels.
In summary, we report the first case of a CYB5A mutation coding for cytochrome b5 in a genetic female. She presented with methemoglobinemia alone and with normal pubertal development, menarche, secondary sexual characteristics, and pregnancy despite a biochemical picture of isolated 17,20-lyase deficiency with elevation of 17-hydroxyprogesterone, progesterone, and reduced DHEA-sulphate. Glucocorticoid production was normal. Further studies are required to look into the mechanisms of sex hormone production in patients with CYB5A mutations.
Glossary
Abbreviations
- 17-OH
17-hydroxy
- 17-OHP
17-hydroxyprogesterone
- ACTH
adrenocorticotropin hormone
- AKR1
aldoketo reductases
- CYB5
cytochrome b5
- CYP17A1
cytochrome P450c17
- DHEA
dehydroepiandrosterone
- DHT
dihydrotestosterone
- DSD
disorders of sex development
- POR
P450 oxidoreductase
- RI
reference interval
Acknowledgments
Financial Support: This work received no financial support.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
References and Notes
- 1. Miller WL. The syndrome of 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hegesh E, Hegesh J, Kaftory A. Congenital methemoglobinemia with a deficiency of cytochrome b5. N Engl J Med. 1986;314(12):757–761. [DOI] [PubMed] [Google Scholar]
- 3. Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, de Jong FH. Isolated 17,20-lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab. 2010;95(3):994–999. [DOI] [PubMed] [Google Scholar]
- 4. Idkowiak J, Randell T, Dhir V, et al. A missense mutation in the human cytochrome b5 gene causes 46,XY disorder of sex development due to true isolated 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97(3):E465–E475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeung TWY, Chan AoK, Li RHW, Shek CC, Ho PC, Ng EHY. The diagnosis and management of an adult 46xy female with isolated 17, 20-lyase deficiency due to a novel mutation P. Y35xi cytochrome B5a gene. Austin J Obstet Gynecol. 2014;1(1):4. [Google Scholar]
- 6. Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem. 2003;278(49):48563–48569. [DOI] [PubMed] [Google Scholar]
- 7. Simsek E, Ozdemir I, Lin L, Achermann JC. Isolated 17,20-lyase (desmolase) deficiency in a 46,XX female presenting with delayed puberty. Fertil Steril. 2005;83(5):1548.e23-1548.e26. [DOI] [PubMed] [Google Scholar]
- 8. Giordano SJ, Kaftory A, Steggles AW. A splicing mutation in the cytochrome b5 gene from a patient with congenital methemoglobinemia and pseudohermaphrodism. Hum Genet. 1994;93(5):568–570. [DOI] [PubMed] [Google Scholar]
- 9. Marsh CA, Auchus RJ. Fertility in patients with genetic deficiencies of cytochrome P450c17 (CYP17A1): combined 17-hydroxylase/17,20-lyase deficiency and isolated 17,20-lyase deficiency. Fertil Steril. 2014;101(2):317–322. [DOI] [PubMed] [Google Scholar]
- 10. Van Den Akker EL, Koper JW, Boehmer AL, et al. Differential inhibition of 17alpha-hydroxylase and 17,20-lyase activities by three novel missense CYP17 mutations identified in patients with P450c17 deficiency. J Clin Endocrinol Metab. 2002;87(12):5714–5721. [DOI] [PubMed] [Google Scholar]
- 11. Geller DH, Auchus RJ, Miller WL. P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5. Mol Endocrinol. 1999;13(1):167–175. [DOI] [PubMed] [Google Scholar]
- 12. Levran D, Ben-Shlomo I, Pariente C, Dor J, Mashiach S, Weissman A. Familial partial 17,20-desmolase and 17alpha-hydroxylase deficiency presenting as infertility. J Assist Reprod Genet. 2003;20(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukami M, Homma K, Hasegawa T, Ogata T. Backdoor pathway for dihydrotestosterone biosynthesis: implications for normal and abnormal human sex development. Dev Dyn. 2013;242(4):320–329. [DOI] [PubMed] [Google Scholar]
- 14. Wilson JD, Auchus RJ, Leihy MW, et al. 5alpha-androstane-3alpha,17beta-diol is formed in tammar wallaby pouch young testes by a pathway involving 5alpha-pregnane-3alpha,17alpha-diol-20-one as a key intermediate. Endocrinology. 2003;144(2):575–580. [DOI] [PubMed] [Google Scholar]
- 15. Homma K, Hasegawa T, Nagai T, et al. Urine steroid hormone profile analysis in cytochrome P450 oxidoreductase deficiency: implication for the backdoor pathway to dihydrotestosterone. J Clin Endocrinol Metab. 2006;91(7):2643–2649. [DOI] [PubMed] [Google Scholar]
- 16. Kamrath C, Hartmann MF, Remer T, Wudy SA. The activities of 5α-reductase and 17,20-lyase determine the direction through androgen synthesis pathways in patients with 21-hydroxylase deficiency. Steroids. 2012;77(13):1391–1397. [DOI] [PubMed] [Google Scholar]
- 17. Biason-Lauber A, Miller WL, Pandey AV, Flück CE. Of marsupials and men: “Backdoor” dihydrotestosterone synthesis in male sexual differentiation. Mol Cell Endocrinol. 2013;371(1-2):124–132. [DOI] [PubMed] [Google Scholar]
- 18. Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J Clin Endocrinol Metab. 2012;97(3):E367–E375. [DOI] [PubMed] [Google Scholar]
- 19. Marti N, Galván JA, Pandey AV, et al. Genes and proteins of the alternative steroid backdoor pathway for dihydrotestosterone synthesis are expressed in the human ovary and seem enhanced in the polycystic ovary syndrome. Mol Cell Endocrinol. 2017;441:116–123. [DOI] [PubMed] [Google Scholar]