Abstract
Purpose
Antiretroviral monotherapy and treatment interruption are potential strategies for perinatally HIV-infected adolescents (PHIVA) who face challenges maintaining effective combination antiretroviral therapy (ART). We assessed the use and outcomes for adolescents receiving monotherapy or undergoing treatment interruption in a regional Asian cohort.
Methods
Regional Asian data (2001–2016) were analyzed to describe PHIVA who experienced ≥2 weeks of lamivudine or emtricitabine monotherapy or treatment interruption and trends in CD4 count and HIV viral load during and after episodes. Survival analyses were used for World Health Organization (WHO) stage III/IV clinical and immunologic event-free survival during monotherapy or treatment interruption, and a Poisson regression to determine factors associated with monotherapy or treatment interruption.
Results
Of 3,448 PHIVA, 84 (2.4%) experienced 94 monotherapy episodes, and 147 (4.3%) experienced 174 treatment interruptions. Monotherapy was associated with older age, HIV RNA >400 copies/mL, younger age at ART initiation, and exposure to ≥2 combination ART regimens. Treatment interruption was associated with CD4 count <350 cells/μL, HIV RNA ≥1,000 copies/mL, ART adverse event, and commencing ART age ≥10 years compared with age <3 years. WHO clinical stage III/IV 1-year event-free survival was 96% and 85% for monotherapy and treatment interruption cohorts, respectively. WHO immunologic stage III/IV 1-year event-free survival was 52% for both cohorts. Those who experienced monotherapy or treatment interruption for more than 6 months had worse immunologic and virologic outcomes.
Conclusions
Until challenges of treatment adherence, engagement in care, and combination ART durability/tolerability are met, monotherapy and treatment interruption will lead to poor long-term outcomes.
Keywords: HIV, Adolescent, Antiretroviral therapy, Monotherapy, Treatment interruption
Antiretroviral therapy (ART) in perinatally HIV-infected adolescents (PHIVA) is complicated by the impact of chronic disease on physical and psychosocial health, contributing to morbidity, treatment fatigue, and adherence vulnerability [1]. This not only has implications for the viability of ART during adolescence but also for future regimens through the development of antiretroviral resistance. To limit disease progression and/or the development of antiretroviral resistance during periods of inadequate adherence to combination ART or for those failing combination ART awaiting access to effective regimens, alternate treatment strategies such as antiretroviral monotherapy and treatment interruption may be considered [2,3]. Rationale for lamivudine or emtricitabine monotherapy has been derived from studies demonstrating the evolution of the M184V mutation in the setting of lamivudine monotherapy, which hinders viral fitness and impairs viral replication [4,5]. In addition, the M184V mutation in isolation does not confer cross-resistance and increases susceptibility to other nucleoside reverse transcriptase inhibitors [4]. Treatment interruptions have been considered a more viable option in children compared with adults because of concern over greater cumulative lifetime ART exposure, greater potential for long-term ART toxicity, and less available ART formulations; as well as their greater potential for immune reconstitution on recommencing ART [6].
Data regarding the viability of monotherapy or treatment interruption as alternate treatment strategies for PHIVA are mixed, with prior studies incorporating adolescents into broader cohorts with young adults [7] or children [2,3,8–13]. Lamivudine or emtricitabine monotherapy has been shown to result in worse immunologic outcomes than continuing nonsuppressive combination ART in a cohort of 33 adolescents and young adults from the International Maternal Pediatric Adolescent AIDS Clinical Trials network [7] while lamivudine monotherapy resulted in better immunologic and clinical outcomes when compared with completely stopping lamivudine-containing combination ART in an Italian cohort of 58 adults [14]. In the Paediatric European Network for the Treatment of AIDS 11 study, 109 children and adolescents across nine countries who underwent a planned treatment interruption encountered no serious clinical events during their treatment interruption of up to 48 weeks [6], with 101 participants observed up to 2 years after reinitiation of combination ART who encountered no serious adverse clinical, immunologic, or virologic consequences [3]. Whereas the Strategies for Management of Antiretroviral Therapy study demonstrated an increased risk of opportunistic disease and death associated with treatment interruption in an international cohort of 5,472 adults [15].
Given the challenges of maintaining PHIVA on long-term effective combination ART [1], a better understanding of the impact of monotherapy and treatment interruptions on PHIVA is required to better inform HIV healthcare providers, and their patients, on the viability of such alternate treatment strategies. This study aims to assess the use and impact of lamivudine or emtricitabine monotherapy and incident treatment interruption in an Asian regional cohort of PHIVA aged 10–19 years by describing characteristics of PHIVA who received monotherapy or had treatment interruptions, describing trends in CD4 count and HIV viral load during and after monotherapy and treatment interruption episodes, evaluating World Health Organization (WHO) stage III/IV event-free survival during episodes of monotherapy and treatment interruptions, and determining factors associated with monotherapy and treatment interruptions.
Methods
Study design and population
The Therapeutics Research, Education, and AIDS Training Asia pediatric HIV Observational Database (TApHOD) of International epidemiology Databases to Evaluate AIDS Asia-Pacific involves 16 sites across six countries (Cambodia, India, Indonesia, Malaysia, Thailand, and Vietnam) in Asia that collect data during HIV care, which are transferred to the Kirby Institute (University of New South Wales, Sydney, Australia) for management and analysis. Details of the TApHOD cohort have been previously reported [16]. For this study, adolescents aged 10–19 years with perinatal HIV infection who received care within TApHOD up to December 2016 were included. Ethics approval was obtained through the human research ethics committees at participant sites, Kirby Institute, and Therapeutics Research, Education, and AIDS Training Asia/amfAR (The Foundation for AIDS Research) coordinating center (Bangkok, Thailand).
Definitions
Adolescence was defined as 10–19 years of age. ART was defined as treatment with any antiretroviral agent, and combination ART defined as treatment with at least three antiretroviral agents. First combination ART regimen was defined as the initial combination ART regimen. Switch to a second combination ART regimen consisted of either a change from one antiretroviral class to another, addition of a new antiretroviral class, or a change in at least two antiretroviral agents. Monotherapy was defined as treatment with lamivudine or emtricitabine for at least 2 weeks, and treatment interruption defined as a minimum of 2 weeks without ART between two periods of any ART use (planned or unplanned). Only monotherapy or treatment interruption episodes that occurred during adolescence were included.
Viral suppression was defined as an HIV viral load of <400 copies/mL, and immunodeficiency as a CD4 count of <500 cells/μL [17]. Clinical and immunologic staging was in accordance with the WHO; with advanced HIV disease as WHO clinical stage III or a CD4 count 200–349 cells/μL, and severe HIV disease as WHO clinical stage IV or a CD4 count <200 cells/μL [17]. Baseline was defined as either a participants’ tenth birthday or their first clinic visit if it occurred after their 10th birthday. Loss to follow-up (LTFU) while on monotherapy was determined by the participant site reporting LTFU or the absence of individual-level data for >12 months from last data transfer if the participant remained on monotherapy at last clinic visit. LTFU could not be determined for patients with treatment interruptions, as they required reinitiation of ART to meet the criteria for treatment interruption, which required engagement in care.
Statistical analysis
Descriptive analyses were used to report demographic, immunologic, virologic, and clinical characteristics of PHIVA who received monotherapy, had a treatment interruption, and those who did not have monotherapy or a treatment interruption. For WHO clinical events and ART adverse events at baseline and initiation of monotherapy or treatment interruption, the most recent event within the preceding 6 months was recorded for each participant. For baseline CD4 count and HIV viral load, the closest measurement within a 6-month window period (i.e., 6 months before or after) was taken. Crude incidence rates with 95% confidence intervals (CIs) were calculated for monotherapy and treatment interruption episodes, with person-years calculated from baseline to death, LTFU, last reported clinic visit (if occurred at <20 years of age) or the day before their 20th birthday (if remained in care at ≥20 years of age). Trends in median CD4 count and HIV viral load categories (<400 copies/mL, 400–999 copies/mL, 1,000–9,999 copies/mL, and ≥10,000 copies/mL) during and after episodes of monotherapy or treatment interruption were reported at 6 monthly time points. The closest measurement to each time point within a 3-month window period (i.e., 3 months before or after) was taken. If a participant had a subsequent episode of monotherapy or treatment interruption, they were censored from contributing further data to their most recent monotherapy or treatment interruption episode and began contributing data to their current monotherapy or treatment interruption episode.
Kaplan–Meier survival analyses were used to determine event-free survival during monotherapy or treatment interruption for WHO clinical stage III/IV events and WHO advanced/severe HIV-associated immunodeficiency. The period of observation commenced at the start of either the monotherapy or treatment interruption episode and stopped when either the participant died, was LTFU, ceased monotherapy or treatment interruption, or at their last clinic visit if remained on monotherapy. Only participants who commenced monotherapy or treatment interruption with a CD4 count >350 cell/μL were included in the survival analyses for WHO advanced/severe HIV-associated immunodeficiency.
A Poisson regression analysis was used to determine incidence rate ratios and adjusted incidence rate ratios (aIRR) to determine factors associated with monotherapy or treatment interruption. Covariates included age, sex, CD4 count, HIV viral load, preceding (within 6 months) WHO clinical stage, preceding (within 6 months) ART adverse event, age at ART initiation, prior combination ART regimen (first or at least second), and prior monotherapy or treatment interruption. Age, CD4 count, and HIV viral load were analyzed as time-dependent variables. Unknown or missing data were included in the analyses as a separate category. Covariates with a p value of <.1 on univariate analysis were included in a multivariate analysis, which was conducted in a step-wise fashion maintaining covariate that retained a p value of <.05. Statistical analyses were performed using Stata, version 14.2 (StataCorp LP, College Station, TX).
Results
Study population
There were 3,448 PHIVA who ever received care within TApHOD up to December 2016. The period of adolescent data analyzed ranged from 2001 to 2016. The total period of observation was 16,400 person-years, with a median adolescent follow-up of 4.7 (interquartile range [IQR] 2.3, 7.1) years. Contribution of person-years by country was 9,939 person-years from Thailand, 2,234 person-years from Vietnam, 1,738 person-years from Cambodia, 1,142 person-years from Malaysia, 948 person-years from India, and 309 person-years from Indonesia. There were 84 PHIVA (2.4%) who experienced 94 monotherapy episodes over 16,071 person-years (crude incidence rate .6 [95% CI .5, .7] per 100 person-years), and 147 PHIVA (4.3%) who had 174 treatment interruption episodes over 16,067 person-years (crude incidence rate 1.1 [95% CI .9, 1.3] per 100 person-years). The median duration of monotherapy was 198 (IQR 117, 365) days, and the median duration of treatment interruption was 182 (IQR 65, 343) days. Table 1 summarizes the characteristics of PHIVA who received monotherapy, had a treatment interruption, and who did not have monotherapy or a treatment interruption.
Table 1:
Characteristics of study population
| Monotherapy, N = 94 | Treatment interruption, N = 174 | Without monotherapy or treatment interruption | |
|---|---|---|---|
| Number of patients | 84 | 147 | 3,233 |
| Median age, y (IQR) | 15.0 (12.9, 16.5) | 14.5 (12.5, 16.4) | 14.9 (12.4, 17.6)a |
| Median duration, d (IQR) | 198 (117, 365) | 182 (65, 343) | |
| Sex | |||
| Female | 59 (62.8) | 101 (58.1) | 1,644 (50.9) |
| Country | |||
| Cambodia | 1 (1.1) | 1 (.6) | 412 (12.8) |
| India | 1 (1.1) | 15 (8.6) | 208 (6.4) |
| Indonesia | 0 | 0 | 122 (3.8) |
| Malaysia | 14 (14.9) | 11 (6.3) | 241 (7.5) |
| Thailand | 77 (81.9) | 144 (82.8) | 1,463 (45.3) |
| Vietnam | 1 (1.1) | 3 (1.7) | 787 (24.3) |
| Age at ART initiation, years | |||
| <3 | 23 (24.5) | 10 (5.8) | 531 (16.4) |
| 3–4 | 12 (12.8) | 14 (8.1) | 519 (16.1) |
| 5–9 | 35 (37.2) | 73 (42.0) | 1,390 (43.0) |
| ≥10 | 24 (25.5) | 77 (44.3) | 665 (20.6) |
| Naïve | 0 | 0 | 128 (4.0) |
| Preceding CD4 count, cells/μLb | |||
| Median (IQR) | 403 (193, 697) | 349 (132, 673) | 675 (385, 947) |
| ≥500 | 38 (40.4) | 64 (36.8) | 1,978 (61.2) |
| 350–499 | 12 (12.8) | 19 (10.9) | 321 (9.9) |
| 200–349 | 14 (14.9) | 32 (18.4) | 217 (6.7) |
| <200 | 23 (24.5) | 52 (29.9) | 463 (14.3) |
| Unknown/missing | 7 (7.5) | 7 (4.0) | 254 (7.9) |
| Preceding HIV viral load, copies/mLb | |||
| <400 | 11 (11.7) | 57 (32.8) | 1,258 (38.9) |
| 400—999 | 4 (4.3) | 3 (1.7) | 59 (1.8) |
| 1,000—9,999 | 21 (22.3) | 23 (13.2) | 94 (2.9) |
| ≥10,000 | 49 (52.1) | 54 (31.0) | 315 (9.7) |
| Unknown/missing | 9 (9.6) | 37 (21.3) | 1,507 (46.6) |
| Preceding WHO clinical stageb | |||
| I/II | 93 (98.9) | 160 (92.0) | 2,870 (88.8) |
| III/IV | 1 (1.1) | 14 (8.0) | 363 (11.2) |
| Preceding ART adverse eventb | |||
| No | 89 (94.7) | 153 (87.9) | 3,190 (98.7) |
| Yes | 5 (5.3) | 21 (12.1) | 43 (1.3) |
| Combination ART regimens | |||
| 1 | 31 (33.0) | 101 (58.1) | 2,053 (63.5) |
| ≥2 | 56 (59.6) | 72 (41.4) | 1,046 (32.4) |
| Naïve | 7 (7.5) | 1 (.6) | 134 (4.1) |
Values n (%) unless otherwise specified.
ART = antiretroviral therapy; IQR = interquartile range; WHO = World Health Organization.
Age at last clinic visit.
Baseline data for population without monotherapy or treatment interruption.
For the 94 monotherapy episodes, preceding therapy was combination ART in 80 (85.1%), dual agent ART in 10 (10.6%), and no therapy in 4 (4.3%). One PHIVA died, and four were LTFU while on monotherapy, and 13 had continued monotherapy to the end of the follow-up period. The remaining 76 monotherapy episodes were followed by combination ART in 70 (92.1%) and treatment interruption in six (7.9%). For the 174 treatment interruption episodes, preceding therapy was combination ART in 147 (85.0%), dual agent ART in 17 (9.8%), and single-agent ART in nine (5.2%), whereas subsequent therapy was combination ART in 160 (93.6%), dual agent ART in four (2.3%), and single-agent ART in seven (4.1%).
CD4 count and HIV viral load trends
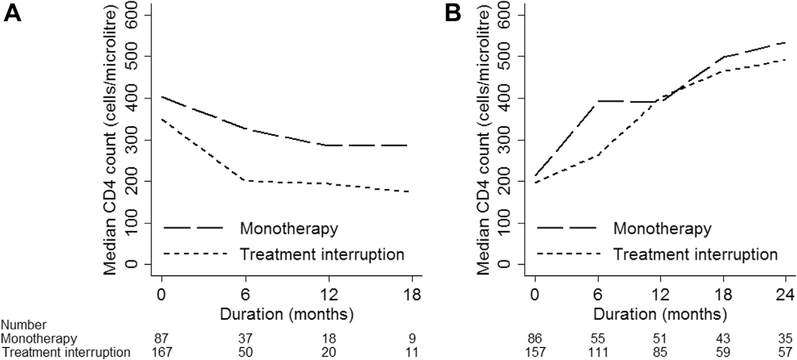
The monotherapy group experienced a decline in median CD4 count from 403 (IQR 193, 697) cells/μL to 285 (IQR 128, 512) cells/μL over 12 months, whereas the treatment interruption group demonstrated a decline in median CD4 count from 349 (IQR 132, 673) cell/μL to 200 (IQR 72, 397) cells/μL over 6 months (Figure 1A). The median CD4 count at the end of monotherapy and treatment interruption episodes was 213 (IQR 93, 435) cells/μL and 197 (IQR 48, 362) cells/μL, respectively. Figure 1B shows the trend in median CD4 count after recommencement of at least two agent ART for the monotherapy and treatment interruption groups. When stratified by the duration of monotherapy (≤6 months or >6 months), the groups demonstrated a similar median CD4 count at the end of their monotherapy episodes; however, those who had a monotherapy duration of ≤6 months displayed a better median CD4 count at 2 years after monotherapy compared with those who had a monotherapy episode of more than 6 months (Supplemental Figure 1, which shows CD4 count trends). For the treatment interruption group, those who had a treatment interruption episode of ≤6 months had a higher median CD4 count from the end of their treatment interruption episode through to 2 years after (Supplemental Figure 1, which shows CD4 count trends).
Figure 1.
Median CD4 trend (A) during monotherapy or treatment interruption and (B) with subsequent antiretroviral therapy after monotherapy or treatment interruption.
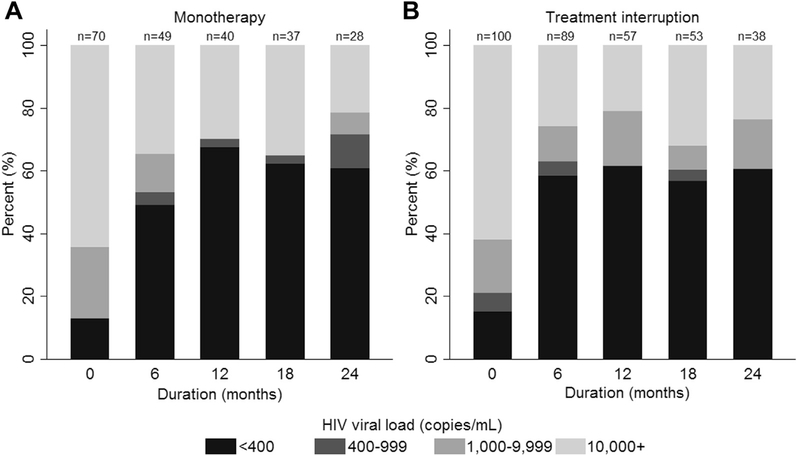
Viral suppression was achieved in around 60% of those who had viral load testing up to 2 years after subsequent management with at least two agent ART after monotherapy or treatment interruption (Figure 2). When stratified by duration of monotherapy or treatment interruption (≤6 months or >6 months), there was a higher proportion of those achieving viral suppression for both the monotherapy and treatment interruption groups who had a duration of ≤6 months (Supplemental Figure 2, which shows HIV viral load distribution for the monotherapy cohort; and Supplemental Figure 3, which shows HIV viral load distributions for the treatment interruption cohort).
Figure 2.
HIV viral load distribution with subsequent antiretroviral therapy following (A) monotherapy or (B) treatment interruption.
WHO advanced/severe HIV disease-free survival
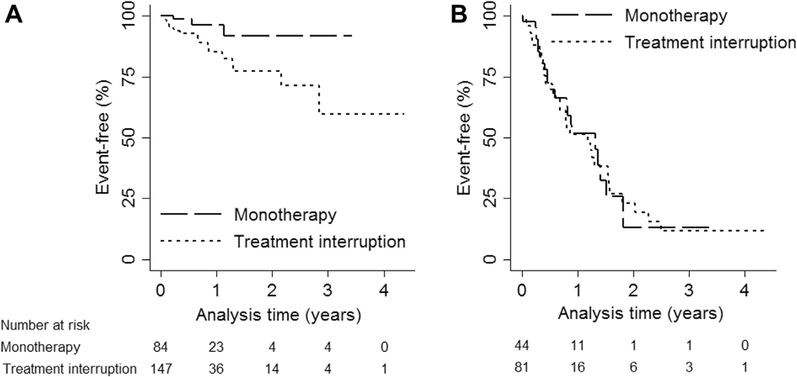
For the monotherapy group, there were 3/84 (3.5%) who had a WHO clinical stage III/IV event, with an event-free survival of 96.4% (95% CI 85.9, 99.1) at 1 year and 91.9% (95% CI 73.7, 97.7) at 3 years (Figure 3A). For the treatment interruption group, there were 18/147 (12.2%) who had a WHO clinical stage III/IV event, with an event-free survival of 85.2% (95% CI 74.7, 91.6) at 1 year and 59.8% (95% CI 31.6, 79.5) at 3 years (Figure 3A). For PHIVA who started monotherapy or treatment interruption with a CD4 count >350 cell/μL, 22/44 (50%) who received monotherapy and 37/81 (45.7%) who had a treatment interruption had a WHO immunologic stage III/IV event. Figure 3B shows the WHO immunologic stage III/IV event-free survival during episodes of monotherapy and treatment interruption, with an event-free survival of 51.9% (95% CI 33.6, 67.4) at 1 year and 13.0% (95% CI 1.2, 39.1) at 3 years for the monotherapy group, and an event-free survival of 52.2% (95% CI 37.4, 65.1) at 1 year and 11.7% (95% CI 3.1, 26.4) at 3 years for the treatment interruption group.
Figure 3.
(A) WHO clinical stage III/IV event-free survival and (B) WHO advanced/severe immunologic event-free survivala during monotherapy or treatment interruption. aOnly patients who commenced monotherapy or a treatment interruption with a CD4 count >350 cell/μL included in analysis. WHO = World Health Organization.
Factors associated with monotherapy or treatment interruption
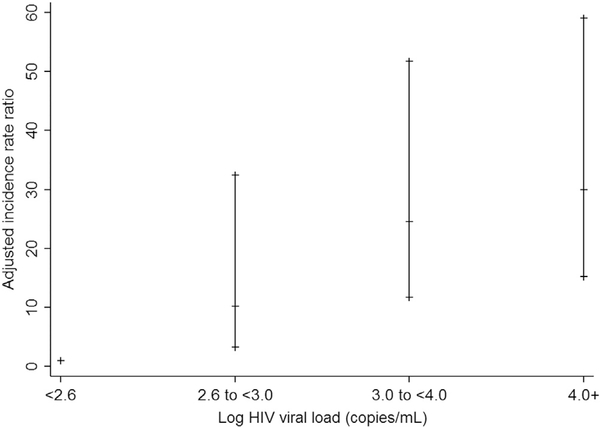
On multivariate regression analysis, the following factors were found to be associated with a higher rate of monotherapy: age 15–19 years compared with age 10–14 years (aIRR 2.0 [95% CI 1.3, 3.1]); HIV viral load ≥400 copies/mL compared to <400 copies/mL (400–999 copies/mL aIRR 10.2 [95% CI 3.2, 32.5]; 1,000–9,999 copies/mL aIRR 24.6 [11.7, 51.7]; ≥10,000 copies/mL aIRR 30.0 [95% CI 15.2, 59.1]; Figure 4); and exposure to at least two combination ART regimens compared with one combination ART regimen (aIRR 1.8 [95% CI 1.1, 3.0]; Table 2). Age ≥5 years at ART initiation was shown to be associated with a lower rate of monotherapy use compared with those commencing ARTat age <3 years (age 5–9 years aIRR .3 [95% CI .2, .6]; age ≥10 years aIRR .3 [.2, .6]).
Figure 4.
Adjusted incidence rate ratio (with 95% confidence intervals) of monotherapy stratified by log HIV viral load. Adjusted for significant variables on multivariate regression analysis (current age, age at antiretroviral initiation, and number of prior combination antiretroviral regimens).
Table 2.
Factors associated with monotherapy and treatment interruption
| Monotherapy |
Treatment interruption |
|||||
|---|---|---|---|---|---|---|
| Events/person-years | Multivariate analysis |
Events/person-years | Multivariate analysis |
|||
| aIRR (95% CI) | p | aIRR (95% CI) | p | |||
| Age category | ||||||
| 10–14 | 47/11,262 | 1.0 | 101/11,256 | 1.0 | ||
| 15–19 | 47/4,810 | 2.0 (1.3, 3.1) | .003 | 73,811 | 1.3 (.9, 1.9) | .11 |
| Sex | ||||||
| Male | 35/7,544 | 1.0 | 73/7,545 | 1.0 | ||
| Female CD4 count, cells/μL |
59/8,527 | 1.3 (.8, 2.0) | .32 | 101/8,522 | 1.1 (.8, 1.6) | .44 |
| ≥500 | 38/11,278 | 1.0 | 64/11,300 | 1.0 | ||
| 350–499 | 12/2,270 | .8 (.4, 1.5) | .42 | 19/2,252 | 1.0 (.6, 1.8) | .89 |
| 200–349 | 14/1,250 | 1.0 (.5, 1.9) | .94 | 32/1,254 | 2.3 (1.5, 3.8) | <.001 |
| <200 | 23/1,197 | .9 (.5, 1.6) | .67 | 52/1,183 | 2.9 (1.8, 4.5) | <.001 |
| Unknown/missing HIV viral load, copies/mL |
7/76 | 29.5 (11.8, 73.7) | <.001 | 7/79 | 24.9 (10.6, 58.7) | <.001 |
| <400 | 11/10,503 | 1.0 | 57/10,537 | 1.0 | ||
| 400–999 | 4/323 | 10.2 (3.2, 32.5) | <.001 | 3/324 | 1.1 (.3, 3.6) | .87 |
| 1,000–9,999 | 21/750 | 24.6(11.7,51.7) | <.001 | 23/739 | 3.8 (2.2, 6.5) | <.001 |
| ≥10,000 | 49/1,477 | 30.0(15.2, 59.1) | <.001 | 54/1,445 | 3.4 (2.1, 5.3) | <.001 |
| Unknown/missing WHO clinical stage |
9/3,018 | 6.1 (2.5, 15.3) | <.001 | 37/3,022 | 3.2 (2.0, 5.0) | <.001 |
| I/II | 93/15,807 | 1.0 | 160/15,804 | 1.0 | ||
| III/IV ART adverse event |
1/265 | .3 (.04, 1.9) | .19 | 14/263 | 1.5 (.8, 2.6) | .21 |
| No | 89/15,589 | 1.0 | 153/15,577 | 1.0 | ||
| Yes | 5/482 | 1.3 (.5, 3.3) | .56 | 21/490 | 2.9 (1.8, 4.7) | <.001 |
| Age at ART initiation, years | ||||||
| <3 | 23/1,973 | 1.0 | 10/1,973 | 1.0 | ||
| 3–4 | 12/1,994 | .5 (.2,1.1) | .09 | 14/1,994 | 1.4 (.6, 3.4) | .40 |
| 5–9 | 35/7,814 | .3 (.2, .6) | <.001 | 73/7,810 | 1.5 (.8, 3.1) | .24 |
| ≥10 | 24/4,012 | .3 (.2, .6) | .001 | 77/4,012 | 3.2 (1.5, 6.6) | .002 |
| Prior combination ART regimens | ||||||
| 1 | 31/10,578 | 1.0 | 101/10,569 | 1.0 | ||
| ≥2 | 56/4,532 | 1.8 (1.1, 3.0) | .02 | 72/4,536 | 1.4 (1.0, 2.0) | .06 |
| Naive Prior monotherapy |
7/962 | 1.2 (.5, 3.0) | .65 | 1/961 | .04 (.005, .3) | .001 |
| No | 84/15,843 | 1.0 | — | — | — | |
| Yes | 10/228 | 1.2 (.4, 3.9) | .79 | — | — | — |
| Prior treatment interruption | ||||||
| No | — | — | — | 147/15,620 | 1.0 | |
| Yes | — | — | — | 27/447 | .7 (.2, 2.2) | .56 |
aIRR = adjusted incidence rate ratio; ART = antiretroviral therapy; CI = confidence interval; WHO = World Health Organization.
A higher rate of treatment interruption was associated with the following factors on multivariate regression analysis: CD4 count <350 cells/μL compared with ≥500 cells/μL (200–349 cells/μL aIRR 2.3 [95% CI 1.5, 3.8]; <200 cells/μL aIRR 2.9 [95% CI 1.8, 4.5]); HIV viral load ≥1,000 copies/mL compared with <400 copies/mL (1,000–9,999 copies/mL aIRR 3.8 [95% CI 2.2, 6.5]; ≥10,000 copies/mL aIRR 3.4 [95% CI 2.1, 5.4]); ART adverse event (aIRR 2.9 [95% CI 1.8, 4.7]); and age ≥10 years at ART initiation compared with age <3 years (aIRR 3.2 [95% CI 1.5, 6.6]; Table 2). Those who were combination ART naïve (i.e., exposure to only single or dual agent ART) had a lower rate of treatment interruption compared with those who had received their first combination ART regimen (aIRR .1 [95% CI .01, .7]).
Discussion
This study provides insights into the use and impact of monotherapy and treatment interruption in a regional Asian PHIVA cohort. The combination of factors associated with monotherapy in our cohort, including older age, unsuppressed HIV viral loads, having had at least two prior combination ART regimens, and earlier age at ART initiation, suggests monotherapy more often occurred in treatment-experienced PHIVA at risk of treatment fatigue and in whom options for effective combination ART are limited. Monotherapy in these circumstances is in keeping with experiences in other regions [2,8,18], emphasizing common challenges in delivering suppressive combination ART to adolescents in low- and middle-income countries. The higher proportion of Thai PHIVA in the monotherapy and treatment interruption groups in part reflects the longer period of follow-up for Thai sites.
Those receiving monotherapy in our cohort demonstrated clinical stability, with 92% remaining free of a WHO clinical stage III/IV event at 3 years. However, they were vulnerable to immunologic deterioration, with a WHO immunologic stage III/IV event-free survival of 52% at 1 year and only 13% at 3 years. The clinical stability but immunologic vulnerability during episodes of monotherapy in our cohort is consistent with previous studies involving children and adolescents. In an observational study from South Africa, there were six WHO clinical stage III/IV events among 71 children and adolescents who received monotherapy over a mean duration of 2 years and a 50% probability of developing an “immunological event” (new WHO stage III/IV event, CD4 drop of ≥25%, and/or absolute drop to<200 cells/μL) at1 year [2]. In the International Maternal Pediatric Adolescent AIDS Clinical Trials P1094 study comparing monotherapy with nonsuppressive combination ART, there were no Centers for Disease Control and Prevention (CDC) category C clinical events recorded for 17 participants randomized to monotherapy, but their probability of experiencing “immunologic failure” (CD4 decline of ≥30% or CDC category C event) was 41% at 28 weeks [7].
Not surprisingly, those having treatment interruptions frequently had preceding low CD4 counts and high HIV viral loads, suggestive of poor treatment adherence and engagement in care. Interestingly, factors suggestive of treatment fatigue, such as earlier age at ART initiation or exposure to multiple combination ART regimens, were not found to be associated with treatment interruption, which may in part be because of the high proportion in our cohort commencing ART after age 5 years. The clinical course of the treatment interruption group showed a steady decline, with a WHO clinical stage III/IV event-free survival of 85% at 1 year and 60% at 3 years. However, as with our monotherapy group, there was considerable immunologic deterioration, with a WHO immunologic stage III/IV event-free survival of 52% at 1 year and 12% at 3 years. In the Pediatric European Network for the Treatment of AIDS 11 trial of planned treatment interruptions, there were no new CDC category C events during the periods of combination ART [6]. The higher proportion of clinical events in our cohort is likely because of unplanned and unstructured treatment interruptions being captured in our observational study among adolescents with preceding poor immunologic control.
In addition to the clinical and immunologic outcomes expected during monotherapy or treatment interruption, the expected responses following subsequent management with combination ART need to be considered. For our monotherapy and treatment interruption groups, of whom more than 90% were subsequently managed with combination ART, their virologic and immunologic responses were poor. Despite median CD4 counts returning to pre-monotherapy or pre-treatment interruption levels within several months of restarting treatment, the monotherapy group did not achieve a median CD4 count of 500 cells/μL until 18 months after monotherapy, and the treatment interruption group had not reached a median CD4 count of 500 cells/μL by 24 months after treatment interruption. This may reflect a limited capacity for immune reconstitution in PHIVA [19,20]. Among those with viral load testing, about 60% of both the monotherapy and treatment interruption groups achieved viral suppression up to 2 years after restarting treatment. These outcomes may be related to poor treatment adherence, emergence of drug resistance, or an insufficiently robust ART regimen. Those who had monotherapy or a treatment interruption for more than 6 months had poorer immunologic and virologic outcomes compared with those who had monotherapy or a treatment interruption episode of ≤6 months, further highlighting the limited viability of either treatment strategy.
The poor outcomes demonstrated during and after periods of monotherapy or treatment interruption further underscore the call for adolescent-specific approaches to overcome challenges related to treatment fatigue and nonadherence and engagement in care. Adolescent-friendly clinics, incorporating management aspects such as structured adolescent-focused adherence counseling, scheduled group activities promoting peer interactions, and individualized psychosocial support delivered by staff trained to meet the specific needs of HIV-infected adolescents have been shown to improve engagement in care, retention on ART, and viral suppression [21–23]. The evolution of HIV services to meet the needs of PHIVA are ever more important, given the expanding adolescent demographic within the HIV-infected population who are particularly vulnerable for poor outcomes [1,24,25].
The main limitation of this study is its observational nature including a heterogenous PHIVA population with varying ART exposures and subsequent ART regimens. There was insufficient data to evaluate treatment adherence, pill burden, or engagement in care as factors associated with monotherapy or treatment interruption or with outcomes after restarting treatment. We were unable to ascertain the reasons for monotherapy or treatment interruption and whether treatment interruptions were intentional or incidental. There was potential not to capture all treatment interruptions by excluding participants that had stopped but not recommenced ART by the end of the study period, which could lead to an underappreciation of the impact of engagement in care on treatment interruptions. Our criteria for a minimum 2-week duration of monotherapy and treatment interruption is less than some prior observational studies. These limitations hinder the generalizability of our results to all PHIVA who receive monotherapy or experience a treatment interruption. However, given the large majority had prior experience with combination ART and were subsequently managed with combination ART, this study population is a relatively representative cohort of adolescents from low- and middle-income countries in an era of combination ART.
In conclusion, we observed monotherapy in clinically stable PHIVA with extensive ART exposure and poor virologic control, while treatment interruptions were encountered in PHIVA with poor immunologic and virologic control suggestive of issues with treatment adherence and engagement in care. Both alternate treatment patterns render PHIVA immunologically vulnerable, and their virologic and immunologic responses after subsequent management with combination ART are poor. Enhanced treatment adherence support, the lifting of barriers to youth’s engagement in care, and increasing access to and durability/tolerability of suppressive ART regimens are all of paramount importance to avoid the poor long-term outcomes associated with the ineffective strategies of monotherapy and treatment interruption.
Supplementary Material
IMPLICATIONS AND CONTRIBUTION.
This analysis demonstrates that treatment fatigue and poor engagement in care are the main reasons perinatally HIV-infected adolescents are receiving antiretroviral monotherapy or undergoing treatment interruptions. These management strategies leave adolescents immunologically vulnerable during such periods and lead to poor immunologic and virologic outcomes with subsequent antiretroviral therapy.
Acknowledgments
The TREAT Asia Pediatric HIV Network: P.S. Ly*, and V. Khol, National Centre for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia; J. Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia; N. Kumarasamy*, and C. Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), VHS-Infectious Diseases Medical Centre, VHS, Chennai, India; A. Kinikar*, V. Mave, and S. Nimkar, B.J. Medical College and Sassoon General Hospitals, Maharashta, India; D.K. Wati*, D. Vedaswari, and I.B. Ramajaya, Sanglah Hospital, Udayana University, Bali, Indonesia; N. Kurniati*, and D. Muktiarti, Cipto Mangunkusumo–Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; S.M. Fong*, M. Lim, and F. Daut, Hospital Likas, Kota Kinabalu, Malaysia; N.K. Nik Yusoff*, and P. Mohamad, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia; T.J. Mohamed*‡, and M.R. Drawis, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; R. Nallusamy*, and K.C. Chan, Penang Hospital, Penang, Malaysia; T. Sudjaritruk*, V. Sirisanthana, and L. Aurpibul, Department of Pediatrics, Faculty of Medicine, and Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand; R. Hansudewechakul*, P. Ounchanum, S. Denjanta, and A. Kongphonoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; P. Lumbiganon*, P. Kosalaraksa, P. Tharnprisan, and T. Udomphanit, Division of Infectious Diseases, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; G. Jourdain, PHPT-IRD UMI 174 (Institut de recherche pour le développement and Chiang Mai University), Chiang Mai, Thailand; T. Puthanakit*, S. Anugulruengkit, W. Jantarabenjakul and R. Nadsasarn, Department of Pediatrics, Faculty of Medicine and Research Unit in Pediatric and Infectious Diseases, Chulalongkorn University, Bangkok, Thailand; K. Chokephaibulkit*‡, K. Lapphra, W. Phongsamart, and S. Sricharoenchai, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; K.H. Truong*, Q.T. Du, and C.H. Nguyen, Children’s Hospital 1, Ho Chi Minh City, Vietnam; V.C. Do*, T.M. Ha, and V.T. An, Children’s Hospital 2, Ho Chi Minh City, Vietnam; L.V. Nguyen*, D.T.K. Khu, A.N. Pham, and L.T. Nguyen, National Hospital of Pediatrics, Hanoi, Vietnam; O.N. Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam; A.H. Sohn*, J.L. Ross, and T. Suwanlerk, TREAT Asia/amfAR–The Foundation for AIDS Research, Bangkok, Thailand; M.G. Law* and A. Kariminia, The Kirby Institute, UNSW Australia, Sydney, Australia; (*Steering Committee members; † Current Steering Committee Chair; ‡ co-Chair).
Funding Sources
The TREAT Asia Pediatric HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute, National Institute of Mental Health, and National Institute on Drug Abuse as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing and is affiliated with the Faculty of Medicine, UNSW Australia. A.W.B. received support from an Australian Government Research Training Program Scholarship.
Conflicts of interest: A.W.B. receives grant support to his institution from Gilead Australia. A.H.S. has received travel and grant support to her institution from ViiV Healthcare. Other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Disclaimer: The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jadohealth.2019.05.025.
References
- [1].Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. J Int AIDS Soc 2013;16: 18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Linder V, Goldswain C, Adler H, et al. Lamivudine monotherapy: Experience of medium-term outcomes in HIV-infected children unable to adhere to triple therapy. Pediatr Infect Dis J 2016;35:e199–205. [DOI] [PubMed] [Google Scholar]
- [3].Bunupuradah T, Duong T, Compagnucci A, et al. Outcomes after reinitiating antiretroviral therapy in children randomized to planned treatment interruptions. AIDS 2013;27:579–89. [DOI] [PubMed] [Google Scholar]
- [4].Wainberg MA. The impact of the M184V substitution on drug resistance and viral fitness. Expert Rev Anti Infect Ther 2004;2:147–51. [DOI] [PubMed] [Google Scholar]
- [5].Wei X, Liang C, Gotte M, Wainberg MA. The M184V mutation in HIV-1 reverse transcriptase reduces the restoration of wild-type replication by attenuated viruses. AIDS 2002;16:2391–8. [DOI] [PubMed] [Google Scholar]
- [6].Paediatric European Network for Treatment of AIDS. Response to planned treatment interruptions in HIV infection varies across childhood. AIDS 2010;24:231–41. [DOI] [PubMed] [Google Scholar]
- [7].Agwu AL, Warshaw MG, McFarland EJ, et al. Decline in CD4 T lymphocytes with monotherapy bridging strategy for non-adherent adolescents living with HIV infection: Results of the IMPAACT P1094 randomized trial. PLoS One 2017;12:e0178075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lazarus EM, Otwombe K, Fairlie L, et al. Lamivudine monotherapy as a holding strategy in HIV-infected children in South Africa. J AIDS Clin Res 2013;4. [Google Scholar]
- [9].Saitoh A, Foca M, Viani RM, et al. Clinical outcomes after an unstructured treatment interruption in children and adolescents with perinatally acquired HIV infection. Pediatrics 2008;121:e513–21. [DOI] [PubMed] [Google Scholar]
- [10].Siberry GK, Patel K, Van Dyke RB, et al. CD4+ lymphocyte-based immunologic outcomes of perinatally HIV-infected children during antiretroviral therapy interruption. J Acquir Immune Defic Syndr 2011;57:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Klein N, Sefe D, Mosconi I, et al. The immunological and virological consequences of planned treatment interruptions in children with HIV infection. PLoS One 2013;8:e76582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gibb DM, Duong T, Leclezio VA, et al. Immunologic changes during unplanned treatment interruptions of highly active antiretroviral therapy in children with human immunodeficiency virus type 1 infection. Pediatr Infect Dis J 2004;23:446–50. [DOI] [PubMed] [Google Scholar]
- [13].Aupiais C,Faye A,LeChenadec J,et al. InterruptionofcARTinclinicalpracticeis associated with an increase in the long-term risk of subsequent immunosuppressioninHIV-1-infected children. Pediatr Infect Dis J 2014;33:1237–45. [DOI] [PubMed] [Google Scholar]
- [14].Castagna A, Danise A, Menzo S, et al. Lamivudine monotherapy in HIV-1infected patients harbouring a lamivudine-resistant virus: A randomized pilot study (E-184V study). AIDS 2006;20:795–803. [DOI] [PubMed] [Google Scholar]
- [15].Strategies for Management of Antiretroviral Therapy Study Group; El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–96. [DOI] [PubMed] [Google Scholar]
- [16].Kariminia A, Chokephaibulkit K, Pang J, et al. Cohort profile: The TREAT Asia pediatric HIV observational database. Int J Epidemiol 2011;40:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].World Health Organization. WHO case definitions of HIV surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Available at: http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf. Accessed March 7, 2018.
- [18].Fairlie L, Bernheimer J, Sipambo N, et al. Lamivudine monotherapy in children and adolescents: The devil is in the detail. S Afr Med J 2017;107: 1055–7. [DOI] [PubMed] [Google Scholar]
- [19].Bamford A, Turkova A, Lyall H, et al. Paediatric European Network for Treatment of AIDS (PENTA) guidelines for treatment of paediatric HIV-1 infection 2015: Optimizing health in preparation for adult life. HIV Med 2018;19:e1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Krogstad P, Patel K, Karalius B, et al. Incomplete immune reconstitution despite virologic suppression in HIV-1 infected children and adolescents. AIDS 2015;29:683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zanoni BC, Sibaya T, Cairns C, et al. Higher retention and viral suppression with adolescent-focused HIV clinic in South Africa. PLoS One 2017;12: e0190260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].MacKenzie RK, van Lettow M, Gondwe C, et al. Greater retention in care among adolescents on antiretroviral treatment accessing “Teen Club” an adolescent-centred differentiated care model compared with standard of care: A nested case-control study at a tertiary referral hospital in Malawi. J Int AIDS Soc 2017;20:e25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Izudi J, Mugenyi J, Mugabekazi M, et al. Retention of HIV-positive adolescents in care: A quality improvement intervention in Mid-Western Uganda. Biomed Res Int 2018;2018:1524016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: Exaggerated health disparities. AIDS Patient Care STDS 2014;28:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zanoni BC, Archary M, Buchan S, et al. Systematic review and meta-analysis of the adolescent HIV continuum of care in South Africa: The Cresting Wave. BMJ Glob Health 2016;1:e000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.