Abstract
Observations by the Mars Reconnaissance Orbiter/Compact Reconnaissance Imaging Spectrometer for Mars in the Mawrth Vallis region show several phyllosilicate species, indicating a wide range of past aqueous activity. Iron/magnesium (Fe/Mg)–smectite is observed in light-toned outcrops that probably formed via aqueous alteration of basalt of the ancient cratered terrain.
This unit is overlain by rocks rich in hydrated silica, montmorillonite, and kaolinite that may have formed via subsequent leaching of Fe and Mg through extended aqueous events or a change in aqueous chemistry. A spectral feature attributed to an Fe2+ phase is present in many locations in the Mawrth Vallis region at the transition from Fe/Mg-smectite to aluminum/silicon (Al/Si)–rich units. Fe2+-bearing materials in terrestrial sediments are typically associated with microorganisms or changes in pH or cations and could be explained here by hydrothermal activity. The stratigraphy of Fe/Mg-smectite overlain by a ferrous phase, hydrated silica, and then Al-phyllosilicates implies a complex aqueous history.
The Mawrth Vallis outflow channel (~25°N, 20°W) cuts through the ancient cratered Noachian highland terrain near the border with the lowlands. A variety of rocks are exposed that reveal the early aqueous history of the planet. Indurated light-toned units with complex spatial and stratigraphic relations are overlain by a darker, more heavily cratered material. Erosion in many locations exposes phyllosilicate-bearing outcrop within the light-toned rocks (1). Here we describe the specific clay minerals identified.
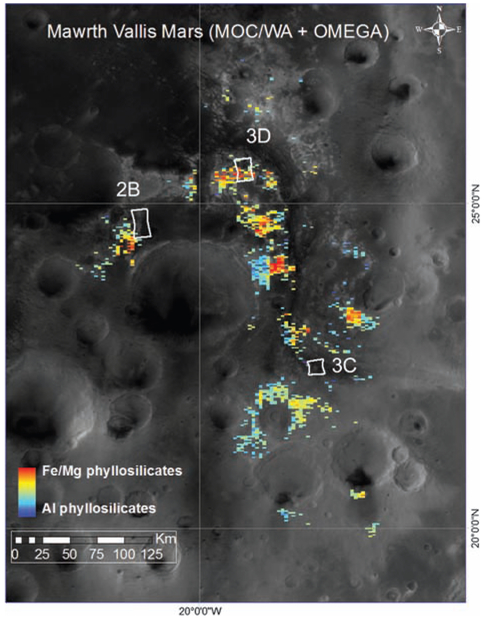
Hyperspectral images acquired by the Compact Reconnaissance Imaging Spectrometer for Mars (CRISM) (2,3) exhibit a variety of signatures in the visible/near-infrared (VNIR: ~0.4 to 4 μm) that are attributed to phyllosilicates and are consistent with observations of this area made by the Observatoire pour la Minéralogie, l’Eau, les Glaces et l’Activité (OMEGA) instrument on Mars Express (3-5). OMEGA data revealed the presence of montmorillonite and nontronite based on Al-OH and Fe-OH absorption bands near 2.2 and 2.3 μm, respectively (4, 6). OMEGA data (Fig. 1) show Fe/Mg-phyllosilicates near the outflow channel and in a few other large outcrops observable at a resolution of hundreds of meters per pixel. Al-phyllosilicates and/or hydrated silica (not distinguished in Fig. 1) are associated with many Fe/Mg-phyllosilicate outcrops. We used the increased spatial and spectral resolution of CRISM data to more precisely identify the types of phyllosilicates present, to map them on a finer scale, and to determine their stratigraphic relationships (7).
Fig. 1.
Mars Orbiter Camera wide-angle (MOC/WA) image overlain with OMEGA phyllosilicate detections and locations of the CRISM images presented. Fe/Mg-smectite was mapped with the band at 2.3 μm (orange), and a combination of Al-phyllosilicate (montmorillonite/kaolinite) and hydrated silica was mapped with the 2.2-μm band (blue). The ~10-km-wide footprints are shown for three targeted CRISM images.
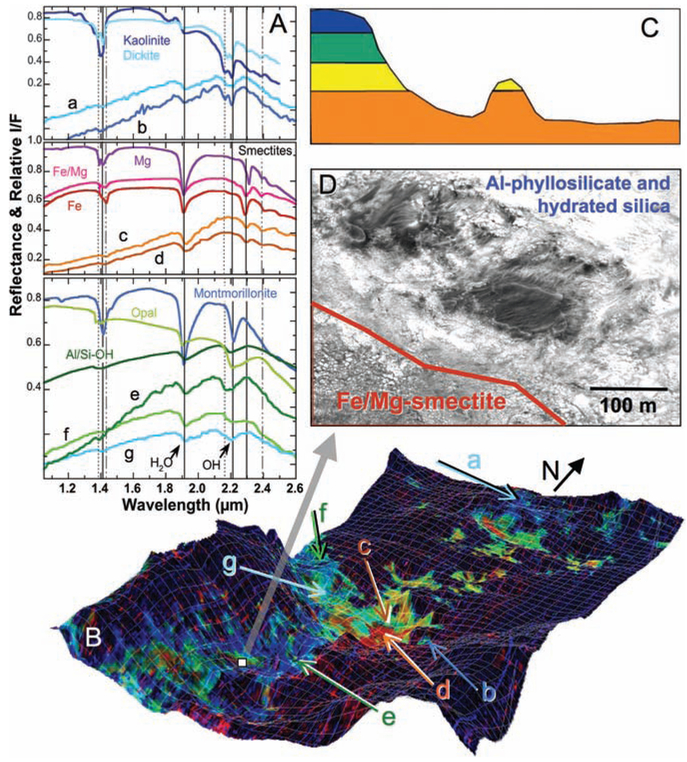
CRISM spectra (Fig. 2) in the western part of Mawrth Vallis, where an areally extensive (>10 km) exposure of phyllosilicate-bearing units is observed, exhibit bands at ~1.4,1.92,2.30, and 2.39 μm (Fig. 2A, spectra c and d; Fig. 3B, spectra e, g, and h). The 2.30- and 2.39-μm bands lie in between the band centers observed for the Fe-smectite nontronite and the Mg-smectite hectorite (3, 8) and are thus attributed to smectite having both Fe and Mg in octahedral sites such as Mg-bearing nontronite. An upward slope from ~1 to 1.5 μm is abundant in rocks surrounding the main exposure of the Fe/Mg-smectite (Fig. 2A, spectrum e; Fig. 3B, spectrum f) and is consistent with an Fe2+-bearing phase such as a ferrous clay. Other spectra at higher elevations around the Fe/Mg-smectite exhibit bands near 1.4, 1.92, and 2.21 μm (Fig. 2A, spectrum g; Fig. 3A, spectrum a) that are consistent with the Al-smectite montmorillonite (9). Similar spectra having broad features near 2.18 to 2.26 μm (Fig. 2A, spectra e and f; Fig. 3A, spectra c and d) are attributed to Al/Si-OH or Si-OH (hydrated silica) (10). The Al-phyllosilicate and hydrated silica appear to be intermixed in most places because the sharp 1.41-, 1.91-, and 2.21-μm bands characteristic of montmorillonite are rare, whereas broad bands centered near 1.38,1.93, and 2.21 μm (observed for opal) or 1.39, 1.92, and 2.19 μm (observed for Al/Si-OH species) are more common.
Fig. 2.
Phyllosilicate-bearing deposits in western Mawrth Vallis. (A)) CRISM spectra from image HRL000043EC (and the overlapping image FRT0000863E) compared to lab spectra of phyllosilicates and hydrated silica-bearing materials (15, 16). (B) Mineral indicator map draped over MOLA terrain with ×20 vertical enhancement showing Fe/Mg-smectite (orange/red), Al-phyllosilicate and hydrated silica (blue), and Fe2+-bearing phases (yellow/green). (C) Sample stratigraphy of phyllosilicates consistent with observations throughout the region, using the colors from (B). (D) Portion of HiRISE image PSP_005819_2050_RED, showing the transition from the polygonal Fe/Mg-smectite terrain in the lower left to the scaly Al-phyllosilicate and smooth hydrated silica in the upper right with two dark patches of mafic material.
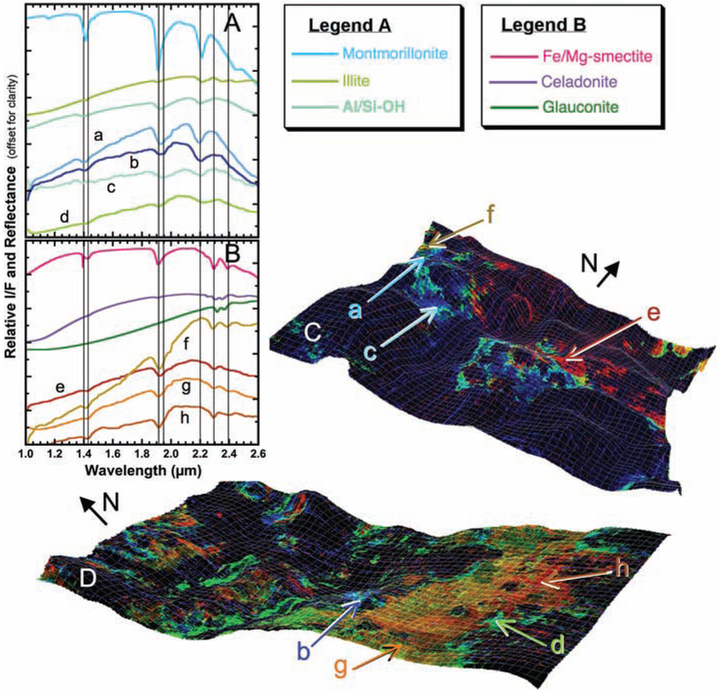
Fig. 3.
Phyllosilicate-bearing deposits along the Mawrth Vallis channel. (A and B) CRISM spectra compared to lab spectra of phyllosilicates and other hydrated phases. (C and D) Mineral indicator maps of CRISM images FRT00003BFB (C) and HRL0000285A (D) draped over MOLA terrain with ×10 vertical enhancement showing Fe/Mg-smectites (orange/red), Al-phyllosilicates and hydrated silica (blue), and Fe2+-bearing phases (yellow/green).
Observations from the Thermal Emission Spectrometer (with a spatial resolution of 3 to 6 km per pixel) show that the light-toned units are dominated by feldspar and a silica-rich phase (11). This silica detection is consistent with the Si-OH and Al/Si-OH bands identified in CRISM spectra here. Feldspar is not directly observable in the VNIR spectral region and could be present as well in the phyllosilicate-bearing rocks.
Additional spectra contain a band at ~1.4 μm and a doublet near 2.2 μm (Fig. 2A, spectra a and b), consistent with the kaolinite family. Many of these spectra also include a weak band near 1.93 μm attributed to bound water in a hydrated component (such as hydrated silica, montmorillonite, or ferrihydrite). A crisp doublet is observed at 2.16 and 2.20 μm (Fig. 2A, spectrum b) in a few cases that resembles kaolinite; in most spectra, a less resolved doublet is present at ~2.18 and 2.20 μm (Fig. 2A, spectrum a) that is more consistent with dickite.
Analyses of spectra collected at Mawrth Vallis in relation to Mars Orbiter Laser Altimeter (MOLA) topography (Figs. 2B and 3, C and D, and movie S1) reveal that Fe/Mg-smectite is prevalent in valley tributaries and other topographic lows (the southern part of the CRISM image in Fig. 3D and the central part of the image in Fig. 3C), whereas the raised knobs (Fig. 3C) and higher-elevation regions (Fig. 3D) contain Al-phyllosilicates. The Fe/Mg-smectite exposures appear to be thicker and more resistant to erosion, and in most areas they are the lowest unit visible. CRISM-MOLA analyses indicate that the Fe/Mg-smectite–rich rocks are typically 200 to 300 m lower in elevation than neighboring Al-phyllosilicate–rich and hydrated silica-rich layers and that the Al-phyllosilicates are probably covering the Fe/Mg-smectites (Fig. 2C). This is consistent with independent analyses of High Resolution Imaging Science Experiment (HiRISE) data (12). Many regions bordering the Fe/Mg-smectite–rich rocks (Fig. 3D) yield spectra with features similar to that of hydrated silica, plus a NIR slope that could indicate a ferrous mica such as celadonite or glauconite. However, laboratory spectra of these phyllosilicates also have weak bands near 2.2 to 2.35 μm that are not resolved in the CRISM spectra. A side view of a fissure through the channel wall (Fig. 3C) shows that Al-phyllosilicate–rich rocks lie stratigraphically above the Fe/Mg-smectite unit, which itself covers nonhydrated rocks below.
Units containing Fe/Mg-smectite, montmorillonite/hydrated silica, and kaolinite/hydrated silica exhibit distinct textures in the HiRISE images of this region (12). As demonstrated in Fig. 2D, the Fe/Mg-smectite unit exhibits a fractured polygonal surface consistent with the contraction of smectites exposed to dehydration. In contrast, the units containing hydrated silica possess a smooth texture, and those with montmorillonite are characterized by a polygonally fractured texture on a smaller scale than that observed for the Fe/Mg smectites. Kaolinite is observed in alteration regions as small as 50 m across, but in a few cases much larger, and appears to exhibit a smooth texture.
Phyllosilicates have been detected in a number of outcrops of Mars’ ancient crust surrounding Mawrth Vallis (13), suggesting that the processes forming phyllosilicates here may have been widespread and that only a fraction of the phyllosilicate-rich rocks has been exposed. The initial Fe/Mg-smectite probably formed via aqueous alteration of basalt, the dominant lithology of the Martian highlands (3, 14). This smectite unit appears to drape the topography and exhibits layered textures suggesting that ash was the basaltic precursor. Alteration could have occurred during direct deposition of ash onto an open body of water or by groundwater. Alternative sources include basaltic sediments and impact ejecta. The layering is complex, indicating perhaps multiple depositional events over a prolonged time period (1). The specific processes forming the additional clay minerals and stratigraphic relations remain unclear. Subsequent aqueous alteration and leaching of Fe and Mg from the smectite or ash could have produced hydrated silica, montmorillonite, mica, and kaolinite, as could a change in aqueous chemistry (for example, through hydrothermal activity) or alteration of a later Si-rich volcanic ash or sediment. Another possible scenario is the alteration of interbedded source rocks with different chemistries, resulting in changing alteration profiles.
Possible terrestrial analogs for the hydrated silica phases are the opal formed in hydrated ash on the southern rim of the Kilauea caldera (15) and hydrated amorphous Al/Si-OH phases formed in altered volcanic material at Haleakala crater in Hawaii (16). Acidic conditions and/or hot and humid climates support active hydrolysis and ion leaching that can result in formation of kaolinite and hydrated silica (17, 18). The NIR slope interpreted as an Fe2+ phase is unusual because iron typically precipitates out of solution as Fe3+ oxides/oxyhydroxides or sulfates and because the Fe2+ unit occurs on top of the Fe3+ nontronite-like unit. On Earth, Fe3+ is normally converted to Fe2+ in aqueous environments by acidophilic bacteria (19-21). If an Fe2+ phase does exist, then a reducing element must have been present at Mawrth Vallis in order to convert the Fe3+ to Fe2+, or some form of activity [such as hydrothermal processes (22-24)] could have reduced the nontronite or released abundant Fe2+ in solution that precipitated out before being converted to Fe3+. Thus, the complex and potentially multi-event formation conditions of phyllosilicates at Mawrth Vallis present a fascinating window into past aqueous activity on Mars.
Supplementary Material
Footnotes
Supporting Online Material
References and Notes
- 1.Michalski JR, Noe Dobrea EZ, Geology 35, 951 (2007). [Google Scholar]
- 2.Murchie S et al. , J. Geophys. Res. 112, 10.1029/2006JE002682 (2007). [DOI] [Google Scholar]
- 3.See supporting material on Science Online.
- 4.Poulet F et al. , Nature 438, 623 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Bibring J-P et al. , Science 307, 1576 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Loizeau D et al. , J. Geophys. Res. 112, 10.1029/2006JE002877 (2007). [DOI] [Google Scholar]
- 7.Mustard JF et al. , Nature, 10.1038/nature07097 (2008). [DOI] [Google Scholar]
- 8.Bishop JL, Murad E, Dyar MD, ClayMiner. 37, 617 (2002). [Google Scholar]
- 9.Bishop JL, Madejova J, Komadel P, Fröschl H, Clay Miner. 37, 607 (2002). [Google Scholar]
- 10.Milliken RE et al. , Geology, in press. [Google Scholar]
- 11.Michalski JR et al. , paper presented at the 7th International Mars Conference, Pasadena, CA, 2007. [Google Scholar]
- 12.Wray JJ, Ehlmann BL, Squyres S, Mustard JF, Kirk RL, Geophys. Res. Lett, 10.1029/2008GL034385 (2008). [DOI] [Google Scholar]
- 13.Noe Dobrea EZ et al. , paper presented at the Lunar and Planetary Science Conference, Lunar and Planetary Institute, Houston, TX, 2008. [Google Scholar]
- 14.Bandfield JL, Hamilton VE, Christensen PR, Science 287, 1626 (2000). [Google Scholar]
- 15.Bishop JL, Schiffman P, Lane MD, Dyar MD, paper presented at the Lunar and Planetary Science Conference, Lunar and Planetary Institute, Houston, TX, 2005. [Google Scholar]
- 16.Bishop JL et al. , Clays Clay Miner. 55, 1 (2007). [Google Scholar]
- 17.Chamley H, Clay Sedimentology (Springer-Verlag, New York, 1989). [Google Scholar]
- 18.Jackson ML, Clays Clay Miner. 6, 133 (1957). [Google Scholar]
- 19.Lowenstam HA, Science 211, 1126 (1981). [DOI] [PubMed] [Google Scholar]
- 20.Nealson KH, Annu. Rev. EarthPlanet. Sci. 25, 403 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Brock TD, Gustafson J, Appl. Environ. Microbiol. 32, 567 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson MJ, Newsom HE, Draper DS, Geochim. Cosmochim. Acta 69, 2701 (2005). [Google Scholar]
- 23.Stucki JW, Tessier D, Clays Clay Miner. 39, 137 (1991). [Google Scholar]
- 24.Browne PRL, Annu. Rev. Earh Planet. Sci 6, 229 (1978). [Google Scholar]
- 25.The authors thank E. Cloutis and an anonymous reviewer for helpful editorial comments and the CRISM Science Team for acquisition of the images. Supported by NASA’s Mars Reconnaissance Orbiter project, Mars Data Analysis program, Mars Fundamental Research program, and the NASA Astrobiology Institute.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.