Abstract
Purpose:
To investigate the dimensions of the tensor veli palatini (TVP) muscle using high image resolution 3D MRI of the soft palate among children with normal velopharyngeal and craniofacial anatomy and to compare values to individuals with a diagnosis of 22q11.2 deletion syndrome (22q11DS). We also sought to determine if there is a relationship between hypoplasia of the TVP and severity of middle ear dysfunction and hearing loss.
Methods:
3D MRI were used to collect and analyze data obtained across 53 children between 4 and 12 years of age, including 40 children with normal velopharyngeal and craniofacial anatomy and 13 children with a diagnosis of 22q11.2 DS. TVP muscle length, thickness, and volume as well as bihamular distance were compared among participant groups.
Results:
A Welch’s T-test revealed that the TVP in participants with 22q11DS is significantly shorter (p = .005, 17.3 mm vs. 19.0 mm), thinner (p = < .001, 1.1 mm vs. 1.8 mm), and less voluminous (p = < .001. 457.5 mm3 vs. 667.3 mm3) than participants without 22q11DS. Participants with 22q11DS also had a greater (p = .006, 27.7 mm vs. 24.7 mm) bihamular distance than participants without 22q11DS. There was an inverse relationship between TVP abnormalities noted above and the severity of audiologic and otologic histories.
Conclusion:
The TVP muscle is substantially reduced in volume, length, and thickness in children with 22q11DS. These findings serve as preliminary support for the association of patient hearing and otologic severity and TVP dysmorphology.
Keywords: tensor veli palatini, morphology, magnetic resonance imaging, three-dimensional reconstruction, 22q11.2 deletion syndrome, Eustachian tube dysfunction
Introduction
22q11.2 deletion syndrome (22q11DS) is a genetic disorder affecting 1 in 4,000 live births (Ryan et al., 1997; Devriendt et al., 1998). Verheij et al. (2017) conducted a systematic review including 21 studies, which demonstrated a substantial increase in abnormal hearing and otologic findings among patients with 22q11DS compared to controls. Although rates vary across studies, prevalence of recurrent or chronic otitis media (OME) has been reported to be as high as 89.8% in patients with 22q11DS (Dyce et al., 2002), which is substantially higher than rates reported (7.8%) among the general populations (Niskar et al., 1998; Digilio et al., 1999; Reyes, LeBlanc and Bassila, 1999; Ford, Sulprizio, Rasgon, 2000; Dyce et al., 2002; Robin and Shprintzen, 2005; Kobrynski and Sullivan, 2007; Macintyre et al., 2010; Bassett et al., 2011; Feder et al., 2017).
The etiology of conductive hearing loss among children with 22q11DS is largely due to chronic OME and its sequelae (e.g., perforations of the tympanic membrane, impairment of ossicular sound transfer, ossicular remodeling). OME is presumably caused by Eustachian tube (ET) dysfunction (Verheij et al., 2017), such as variations in the angle of the ET, patency, and obstruction of the orifice of the ET in the nasopharynx, and may be exacerbated by immune deficiency in 22q11DS. Because 22q11DS is commonly associated (75%) with palatal and pharyngeal anomalies affecting velopharyngeal function (Ryan et al., 1997; Bassett et al., 2011), it is likely that ET dysfunction may have a similar pathophysiology in causing OME in patients with a history of cleft palate (Sheahan et al., 2003; Heidsieck et al., 2016). Loos et al. (2016) also observed anatomic malformation of middle and inner ear structures, which is postulated to contribute to the presence of hearing loss among children with 22q11DS.
Tensor veli palatini (TVP) abnormalities have also been reported among adults with palatal anomalies and may be a contributing factor to the high incidence of OME reported among this population (George et al., 2018). Specifically, the TVP has been observed to be significantly smaller in volume and present with an overall shorter muscle length in adults with repaired cleft palate compared to adults without cleft palate (George et al., 2018). However, no studies have examined if the TVP abnormalities observed in individuals with cleft palate are also found in individuals with 22q11DS. In addition, the report by George et al. (2018) did not include detailed audiologic or otologic histories of adult participants. As a result, it is unclear whether abnormalities of the TVP directly relate to middle ear health.
Untreated and/or pervasive hearing loss during childhood has negative effects on the development of speech and language (Heidsieck et al., 2016). Such delays are often further associated with the development of negative behaviors and/or delays in language, literacy, and learning. This can have a compounding effect for patients with 22q11DS given the high incidence of speech and/or language disorders, even in the absence of hearing loss (Solot et al., 2000; Persson et al., 2003). Given this strong predisposition toward speech and language issues, early diagnosis and treatment of hearing loss and/or recurrent episodes of OME is particularly important to optimize speech and learning outcomes (Digilio et al., 1999; Rosenfeld et al., 2004; Bluestone and Klein, 2007; Verheij et al., 2017).
The purpose of this study is to investigate the dimensions of the TVP muscle using high image resolution three-dimensional (3D) magnetic resonance imaging (MRI) of the soft palate among children with normal velopharyngeal and craniofacial anatomy and to compare these values to individuals with a diagnosis of 22q11DS. Specifically, we examined if the length, diameter, and volume of the TVP muscle differ significantly between groups. Additionally, we compared differences between groups regarding the hamular process width. Because hypoplasia of the TVP muscle has been observed in adults with repaired cleft palate (George et al., 2018), it was hypothesized that hypoplasia in the TVP muscle would also be observed among this clinical population. We expected TVP muscle hypoplasia to be accompanied by a smaller total muscle volume. Lastly, we expected to find a spectrum of severity of hearing and middle ear abnormalities: a greater degree of dysmorphology of the TVP among children with more severe hearing loss and otologic medical histories compared to patients who presented with minimal loss to normal hearing.
The overarching aim of this study is to expand our understanding of structural abnormalities common to individuals born with 22q11DS. Ultimately, insights of this line of research are aimed to improve our understanding of the pathophysiology behind hearing loss and OME common among this population to improve treatment and patient outcomes.
Methods
Participants
In accordance with the local Institutional Review Boards, 53 children between 4 and 12 years of age were recruited for this study. Participants included 40 children with normal velopharyngeal and craniofacial anatomy (control participants; 21 males and 19 females) with a mean age of 8.4 years (SD = 0.59 years), and 13 children (5 males and 8 females) with a diagnosis of 22q11DS with a mean age of 8.3 years (SD = 2.8 years). None of the participants with 22q11DS had overt cleft palate or displayed overt signs of submucous cleft palate. Inspection of MRI data showed three children with 22q11DS presented with complete diastasis of the levator veli palatini (levator) muscle. An additional four of the 13 child participants with 22q11DS showed irregularity of the levator muscle through the velar midline. More specifically, in these cases the levator muscle was cohesive through a portion of the sling with diastasis apparent in the region closest to the posterior hard palate.
Children with 22q11DS were recruited through use of flyers that were disseminated and posted on social media sites to make available to all patients who met the following inclusion criteria: 1) diagnosis of 22q11.2 deletion as confirmed by fluorescence in situ hybridization analysis or microarray; 2) between 4 and 12 years of age; 3) have a negative history of overt cleft or other genetic disorders; and 4) were no less than six months post adenoidectomy or tonsillectomy. Individuals with normal velopharyngeal and craniofacial anatomy were selected from a larger dataset (Perry et al., 2016) and were chosen because they represented individuals who were similar in age to those in the 22q11DS group. Parents of the participants in the control group indicated no history of hearing loss, chronic ear infections, ear infections at the time of the study, or prior surgeries. Parents further reported their child to be free of syndromes, swallowing disorders, neurologic disorders, or musculoskeletal disorders and were identified to be otherwise healthy normal controls.
Magnetic Resonance Imaging
All participants were imaged in the supine position using a Siemens 3 Tesla Trio (Erlangen, Germany) MRI scanner and a 12-channel Siemens Trio head coil. A high resolution T2-weighted 3D turbo spin echo (TSE) anatomical scan called Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE) sequence was acquired with .8 mm isotropic spatial resolution in 4:52 minutes covering the anatomy of interest with a sagittal acquisition (25.6 X 19.2 X 15.4 cm). Parameters include a repetition time of 2,500 ms, echo time of 268 ms, and an echo-train length of 171. Parallel imaging acceleration with a factor of two in both phase-encode and slice directions was used across the images. Children were not sedated nor intubated for this study and the MRI procedures were not done in coordination with other procedures (e.g., MRI for assessing carotid arteries). Rather, each MRI study was done solely for the purposes of this study.
Image analyses and Data Collection
The MRI data were imported into Thermo Scientific™ Amira™ Software (Thermo Fisher Scientific, Waltham, MA, US). This software has a native Digital Imaging and Communication in Medicine (DICOM) support program which allows for the preservation of geometric structures present in the data sets. The TVP muscle was visualized by slicing the 3D data along an oblique coronal plane at an angle that allowed for the muscle bellies to be displayed fully from the origin to the insertion. Sequential slices were used to create a 3D volumetric reconstruction of the muscle, as described by Perry et al. (2013), Kotlarek et al. (2017), and George et al. (2018). A free-handed paintbrush tool in Amira segmentation editor was used to identify TVP muscle fibers, thereby creating a voxel set. Threshold analyses were used to ensure the selected voxels represented the muscle fibers, as determined by the voxel threshold obtained at the midline of the TVP muscle. The voxel set was subsequently used to calculate volumetric values of the TVP muscle.
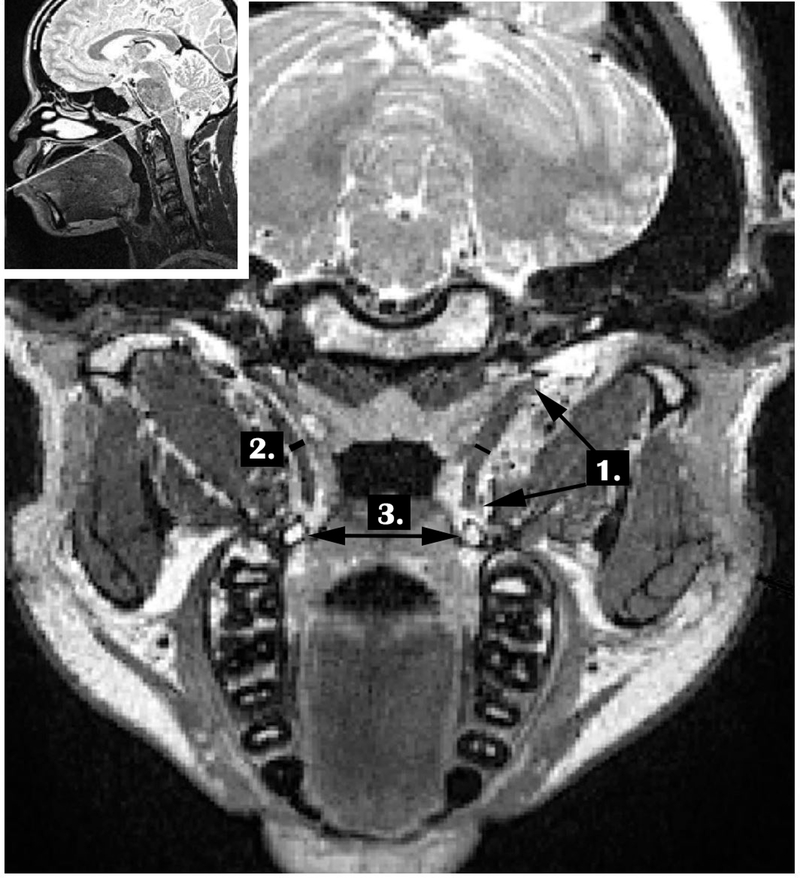
Figure 1 shows the measures that were carried out for each participant. Similar to Terzi et al. (2016) and George et al. (2018), the variables of the TVP used for comparison in the present study included the length of the TVP, thickness of the TVP, the bihamular distance, and the volume of the TVP. The TVP muscle length was obtained using the image with the longest portion of the muscle bilaterally. The length was measured from the origin of the muscle along the lateral lamina of the Eustachian tube until the muscle descended to wrap around the hamulus. TVP muscle thickness was measured at the muscle belly with the greatest diameter. To obtain the TVP muscle length and thickness, the values of the right and left muscle bundles were averaged. The total volume of the TVP muscle was determined by manually segmenting the muscle through sequential images and included both muscle bundles bilaterally.
Figure 1.
Demonstration of the measures including 1. TVP length, 2. TVP thickness, and 3. Bihamular distance. TVP volume is extracted using sequential images and manually segmenting the TVP length.
Audiologic and otologic histories were obtained for each of the participants with 22q11 DS via retrospective medical records review. Results from audiologic and otologic evaluations in closest proximity to scan date were recorded as normal or abnormal for each ear. For audiologic data, results of hearing evaluations were reviewed and considered abnormal for any type of hearing loss (air conduction thresholds > 20 dB HL at any frequency) documented via behavioral audiometric testing or abnormal results on tympanometric testing. For otologic data, results from physician or nurse practitioner otoscopic examination were recorded as abnormal for any report of middle ear effusion, tympanic membrane perforation, active otorrhea with or without the presence of tympanostomy tubes, or reduced movement on pneumatic otoscopy.
Data from additional interim evaluations were also recorded whenever available to establish whether audiologic and otologic abnormalities were persistent. The total number of audiologic exams completed per participant ranged from 0 to 8 while the total number of otologic exams ranged from 0 to 23. An exam was recorded as abnormal regardless of whether the abnormal findings were reported in one ear or both ears. The total number of abnormal exams for each participant was documented, and a resulting proportion of abnormal exams was devised for audiologic and otologic exams, respectively. For children with no history of audiologic or otologic evaluation, proportions were recorded as not applicable (NA). All but one participant (participant ID 09, Table 1) had at least one audiologic or otologic evaluation to record. This participant had parent report that indicated no chronic history or concerns regarding hearing or middle ear health that warranted such evaluations. Therefore, this participant was coded as having a severity index of “0.0” given the absent medical history. Ultimately, the audiologic and otologic proportions were averaged to create one severity score for each participant. For participants who only had either audiologic or otologic exams completed, their severity score was recorded as whichever score was available. Severity scores ranged from 0.0 to 1.0, with scores below 0.50 judged to be minimal-to-no significant history, 0.50 to 0.69 was considered to be moderate history, and 0.70 to 1.00 reflecting severe history of ear and hearing issues. See Table 1 for individual participant information, including laterality of hearing/ear issues.
Table 1.
Audiologic and otologic history data used to inform the overall severity index score used for participants with 22q11DS and laterality of hearing/ear issues for each participant. Children whose laterality of hearing/ear issues changed over time were labeled as “fluctuant”.
| Participant ID |
Proportion Abnormal ENT Exams |
Proportion Abnormal Audiology Exams |
Severity Index Score |
Hearing/Ear Laterality |
|---|---|---|---|---|
| 01 | 1.00 | 1.00 | 1.00 | Fluctuant |
| 02 | 0.00 | 1.00 | 0.50 | Bilateral |
| 03 | 0.75 | 0.50 | 0.63 | Unilateral- Left |
| 04 | 1.00 | 1.00 | 1.00 | Unilateral- Left |
| 05 | 0.00 | 0.00 | 0.00 | NA |
| 06 | 0.87 | 0.71 | 0.79 | Bilateral |
| 07 | 0.78 | 0.50 | 0.64 | Unilateral- Right |
| 08 | NA | 0.00 | 0.00 | NA |
| 09 | NA | NA | 0.00 | NA |
| 10 | 0.50 | 0.50 | 0.50 | Bilateral |
| 11 | 0.78 | 0.88 | 0.83 | Fluctuant |
| 12 | 0.00 | NA | 0.00 | NA |
| 13 | 0.00 | NA | 0.00 | NA |
NA=not applicable, indicating the parent report did not warrant concerns with patient hearing to warrant such testing.
Statistical Analyses
The assumption of homogeneity of variances was violated for TVP length, as assessed by Levene’s test for equality of variance (p < .001). Due to the unbalanced design (i.e., unequal group sizes) and inhomogeneity of variance as assessed by Levene’s test (p > .05), the Welch t-test was used to compare between the two groups (22q11 DS and control participants) across the TVP variables of interest. We used a Pearson correlation and frequency analysis to examine if there appeared to be a relationship between an abnormal TVP and the ear and hearing severity index, which was related to the participant’s hearing and otologic medical history as outlined above.
Inter- and intra-rater reliability measures were obtained using an intra-class correlation coefficient (ICC) performed across a random sample of 30% of participants. ICC estimates and their 95% confident intervals were calculated using Statistical Package for Social Sciences version 23 (SPSS; IBM Corp. Armonk, NY) based on a 2-way mixed-effects model. Intra-rater agreement was between .87 and .97 and inter-rater between .81 to .95. This indicates good to excellent reliability across measures.
Results
Descriptive statistics are presented in Table 2. Welch t-tests were completed to determine if there were differences in the TVP variables between study groups. Results demonstrated a significantly shorter TVP muscle length in individuals with 22q11DS (mean = 17.3±1.8 mm) compared to controls (mean = 19.0±.6), t(12.829) = 3.384, p = .005. Individuals with 22q11DS also presented with a significantly thinner TVP muscle (mean = 1.1±.2 mm) compared to control participants (mean = 1.8±.2 mm), t(20.622) = 10.238, p < .001. Overall, the average TVP volume was 210 mm3 smaller in the 22q11DS group, representing a significant difference between groups, t(16.326) = 5.289, p < .001. Bihamular distance was significantly wider in those with 22q11DS (mean = 27.7±3.2 mm) compared to controls (mean = 24.7±2.2 mm), t(15.749) = −3.136, p = .006).
Table 2.
Descriptive statistics and test statistics comparing control participants (n = 40) to participants with 22q11DS (n = 13) across TVP variables. Volume measures are in mm3 while all other measures are in mm.
| Descriptive Statistics | Welch’s T-Test | ||||
|---|---|---|---|---|---|
| Variable | Control | 22q11DS | t | df | p value |
| TVP Volume | 667.3±96.0 | 457.5±132.1 | 5.289 | 16.326 | < .001 |
| TVP Length | 19.0±.6 | 17.3±1.8 | 3.384 | 12.829 | .005 |
| TVP Thickness | 1.8±.2 | 1.1±.2 | 10.238 | 20.622 | < .001 |
| Bihamular Distance | 24.7±2.2 | 27.7±3.2 | −3.136 | 15.749 | .006 |
A significant negative (inverse) correlation (p < .001) was found between hearing severity index score and TVP volume, TVP length, and TVP thickness. As noted in Table 1, 4 children presented with hearing severity index scores that were considered severe (ranging from 0.7 to 1.0), 4 showed a moderate severity index score (ranging from 0.5 to 0.69), and 5 presented with minimal-to-no history of hearing or otologic abnormalities (score under 0.5). Also seen in Table 1, the mean TVP length and volume decreases as hearing severity index score increases. For participants with 22q11DS who had minimal-to-no audiologic and otologic histories, TVP volume (mean = 457.3 mm3) was similar to those in the control group (mean = 667.3 mm3). However, using an independent samples t-test, both TVP thickness (p = .001) and volume (p = .028) were still significantly different between the control participants and 22q11DS participants who presented with minimal-to-no audiologic and otologic history. Similarly, participants with a moderate severity rating index and a severe severity rating index all had significantly smaller (p < .05) TVP thickness and volume measures when compared by severity index group to the control participants separately using independent samples t-tests.
Discussion
This is one of the first reports of TVP muscle abnormalities in children with 22q11DS. We hypothesized that TVP abnormalities would be observed in this clinical population given the known palatal and pharyngeal anomalies, and such TVP differences have been reported in individuals born with cleft palate. George et al. (2018) provided a preliminary comparison of normal adult measures of TVP in vivo to that of 6 adult participants with repaired cleft palate. Significant differences in the TVP volume and length were observed between groups. Research has indicated that the TVP not only appears hypoplastic in those with cleft palate, but may be absent (Schonmeyr and Sadhu, 2014). Shibahara and Sando (1988) using histology of specimens with cleft palate observed significantly more acute angles between the axis of the TVP and the superior portion of the ET lumen and the lateral lamina. This in turn causes a less efficient pull when attempting to dilate the ET, leading to less effective draining and ventilation of the middle ear (Heidsieck et al., 2016). Fara and Dvorak (1970) completed a study on 18 infants with unrepaired cleft palate and 4 without cleft palate. The TVP muscle was observed to be significantly thinner in the infants with cleft palate compared to the infants without cleft palate (Fara and Dvorak, 1970). Future studies should compare TVP measures in children with 22q11DS to those of age-matched children with repaired cleft palate.
We aimed to examine if observations of TVP muscle dysmorphology was common in individuals with 22q11DS. Our results demonstrate a significantly reduced TVP volume, length, and thickness among participants with 2211DS. George et al. (2018) attributed the TVP volume differences in adults with cleft palate to be primarily related to an abnormally shortened TVP muscle. In contrast, among the participant group with 22q11DS in the present study, we observed the TVP to be significantly reduced in all dimensions. This indicates an overall hypoplastic muscle. It is unclear whether this reflects age-related differences or a syndrome-specific finding regarding the TVP.
There was a negative or inverse relationship between TVP abnormalities and audiologic and otologic histories. That is, as the index of hearing and otologic severity increased, TVP volume, length, and thickness decreased. This was an expected finding. However, caution should be taken given the small sample size of patients with 22q11DS. Further research using a larger sample size with more consistent audiologic and otologic follow-up is needed to understand if there are specific aspects related to the audiologic and otologic histories that are more likely related to the TVP abnormalities observed in this study. Given the relatively small sample size in the present study, we combined multiple aspects of each patient’s medical history into a single severity score to represent the severity index. A limitation of this study is that the audiologic and otologic histories were obtained retrospectively. Therefore, testing was only conducted when warranted, such as due to patient or parent report or clinical observations on team visits. Future studies should prospectively collect such data and ensure data is collected even in the absence of negative history reported by parents, for example. Additionally, while our severity index score did yield discrete groups showing a graded TVP to hearing severity index relationship, it is possible only certain aspects of this score (e.g., audiometric findings versus otologic findings or vice versa) are driving these results. Future studies may also consider the influence of non-anatomic factors such as immunologic profiles, which may also influence the frequency or severity of middle ear disease in 22q11DS.
A difference between findings in the present study among children with 22q11DS compared to past reports of individuals with repaired cleft palate (George et al., 2018) was the difference in the bihamular width. George et al. (2018) reported no significant differences in bihamular width between adults with and without cleft palate. In the present study, participants with 22q11DS demonstrated a significantly greater bihamular distance, on average 3 mm greater than that of control participants. The bodily structures primarily affected in 22q11DS are derivatives of the embryonic pharyngeal arches and pouches, and therefore, 22q11DS has been described as a developmental field defect of the pharyngeal apparatus (Yamagishi, 2002). Based on studies involving the Pax3 and endothelin-I genes in animals, it is believed that that 22q11DS results in part from abnormal development of the neural crest cells within the pharyngeal arches (Kirby et al., 1983; Kirby & Waldo, 1995; Kurihara et al., 1995; Conway et al., 1997). The pterygoid hamulus, part of the basi-pre-sphenoid that originates from the neural crest cells of the first pharyngeal arch, is likely impacted during embryonic development in 22q11DS (Catala, 2003). In contrast, nonsyndromic cleft palate, such as that examined by George et al. (2018), most commonly results from a failure of the palatal shelves to fuse at midline and has not been related to neural crest cell deficiency or the development of the pharyngeal arches (Yu et al., 2009). This may explain why differences in bihamular distance are present in the participants with 22q11DS but not in participants with nonsyndromic repaired cleft palate. It may also explain the greater degree of TVP dysmorphology observed in participants with 22q11DS compared to nonsyndromic cleft palate. Future research is needed to make comparisons between these two populations using age-matched groups. Because our relatively small sample size, we were not able to compare growth differences between our study groups. Future research is should consider if these observed differences in the TVP are observed across the age span or if differences emerge at particular timepoints during growth.
The review of the literature provided by Verheij et al. (2017) supports a likely multifactorial cause to hearing loss among patients with 22q11DS. This study contributes to the literature by providing evidence of TVP hypoplasia in patients with 22q11DS and preliminary support for the association of patient hearing and otologic severity and TVP dysmorphology. However, even with a negative hearing history, participants with 22q11DS still demonstrated hypoplasia of the TVP. Hypoplasia of the levator muscle has also been reported in the literature as an associated feature of patients with 22q11DS (Kollara et al., 2017). Fuchs et al. (2015) observed a relationship between OME and a hypoplastic levator muscle using two mice models of 22q11DS (Df1/+ and Tbx1+/−). Of particular interest, in the mice with unilateral OME, a significantly smaller levator muscle was observed on the same side as the affected ear when compared to the levator muscle on the side of the unaffected ear. However, Finkelstein et al. (1990) did not observe contribution of the levator P to ET function among children. Differences in observations between studies (Finkelstein et al., 1990 and Fuchs et al., 2015) may be related to differences in animal and human studies. Nonetheless, this may demonstrate a possible association of palatal muscle dysmorphology and OME and subsequent hearing loss commonly seen in children with 22q11DS. Although the TVP and levator have different muscle functions, the two muscles course parallel, are both classified as palatal muscles, and arise from the pharyngeal arches. As such, it is possible there is a positive correlation between TVP and levator muscle morphology. Such findings may indicate a strong correlation between hearing and ear health, and velopharyngeal function for speech (i.e., resonance issues). Future studies are needed to explore these relationships and to examine the clinical significance of such findings.
Conclusion
The current study provides new insights into the morphology of the TVP in individuals with and without 22q11DS using MRI. A comparison of 40 control participants to 13 participants with 22q11DS found that the TVP is significantly shorter, thinner, and less voluminous than in participants without 22q11DS. Findings serve as preliminary support for the association of patient hearing and otologic severity and TVP dysmorphology. Results also showed an increased bihamular distance in the group with 22q11DS. These findings suggest that known differences in embryologic development in individuals with 22q11DS may alter the form of the TVP. Future longitudinal research is needed to understand the impact of the TVP on ET function and middle ear health over time.
Table 3.
Comparison of means and standard deviations (noted in parentheses) for control participants to participants with 22q11DS subdivided into audiologic and otologic severity scores as showing either a minimum, moderate, or severe audiologic and otologic hearing history.
| Variable | Control participants n = 40 |
22q11DS Minimal-to-no history (score of < 0.5) n = 5 |
22q11DS Moderate history (score of 0.5–.69) n = 4 |
22q11DS Severe history (score of 0.7–1.0) n = 4 |
|---|---|---|---|---|
| TVP volume | 667.3 (96) | 650.3 (16) | 429.6 (70) | 414.3 (80) |
| TVP length | 19.0 (.6) | 18.9 (.1) | 17.0 (.9) | 16.8 (.9) |
| TVP thick | 1.8 (.2) | 1.7 (.05) | 1.2 (.1) | 1.1 (.1) |
| Bihamular distance | 24.7 (2.2) | 25.2 (.4) | 27.4 (.9) | 26.1 (1.4) |
References
- Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, Marino B, Oskarsdottir S, Philip N, Sullivan K, Swillen A. Practical guidelines for managing patients with 22q11. 2 deletion syndrome. J pediatr. 2011;159(2):332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis AL, Watson PJ, Moller KT. Structural and functional causes of hypernasality in velocardiofacial syndrome. Folia Phoniatrica et Logopaedica. 2009;61(2):93–6. [DOI] [PubMed] [Google Scholar]
- Bluestone CD, Klein JO. Otitis media in infants and children. PMPH-USA; 2007. [Google Scholar]
- Conway SJ, Henderson DJ, Copp AJ: Pax3 is required for cardiac neural crest migration in the mouse: evidence from the splotch (Sp2H) mutant. Development. 1997;124:505–514. [DOI] [PubMed] [Google Scholar]
- Catala M Embryology of the sphenoid bone. J Neuroradiol. 2003;30(4):196–200. [PubMed] [Google Scholar]
- Devriendt K, Fryns JP, Mortier G, van Thienen MN, Keymolen K The annual incidence of DiGeorge/velocardiofacial syndrome. J. Med. Genet. 1998;35: 789–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digilio MC, Angioni A, De Santis M, Lombardo A, Giannotti A, Dallapiccola B, Marino B. Spectrum of clinical variability in familial deletion 22q11. 2: from full manifestation to extremely mild clinical anomalies. Clin genet. 2003;63(4):308–13. [DOI] [PubMed] [Google Scholar]
- Digilio MC, Pacifico C, Tieri L, Marino B, Giannotti A, Dallapiccola B. Audiological findings in patients with microdeletion 22qll (Di George/velocardiofacial syndrome). Br. J. Audiol. 1999;33(5):329–33. [DOI] [PubMed] [Google Scholar]
- Doyle WJ, Cantekin EI, Bluestone CD. Eustachian tube function in cleft palate children. Ann Otol Rhinol Laryngol. 1980;89(3_suppl):34–40. [DOI] [PubMed] [Google Scholar]
- Dyce O, McDonald-McGinn D, Kirschner RE, Zackai E, Young K, Jacobs IN. Otolaryngologic manifestations of the 22q11. 2 deletion syndrome. Arch Otolaryngol Head Neck Surg. 2002;128(12):1408–12. [DOI] [PubMed] [Google Scholar]
- Fára M, Dvorák J. Abnormal anatomy of the muscles of palatopharyngeal closure in cleft palates: Anatomical and surgical considerations based on the autopsies of 18 unoperated cleft palates. Plast Reconst Surg. 1970;46(5): 488. [PubMed] [Google Scholar]
- Feder KP, Michaud D, McNamee J, Fitzpatrick E, Ramage-Morin P, Beauregard Y. Prevalence of hearing loss among a representative sample of Canadian children and adolescents, 3 to 19 years of age. Ear Hear. 2017;38(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford LC, Sulprizio SL, Rasgon BM. Otolaryngological manifestations of velocardiofacial syndrome: a retrospective review of 35 patients. Laryngoscope. 2000;110:362–367. [DOI] [PubMed] [Google Scholar]
- Fuchs JC, Linden JF, Baldini A, Tucker AS. A defect in early myogenesis causes Otitis media in two mouse models of 22q11.2 Deletion Syndrome. Hum Mol Genet. 2015;24(7):1869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TN, Kotlarek KJ, Kuehn DP, Sutton BP, Perry JL. Differences in the Tensor Veli Palatini Between Adults With and Without Cleft Palate Using High-Resolution 3-Dimensional Magnetic Resonance Imaging. Cleft Palate Craniofac J. 2018;55(5):697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyanwali B, Li H, Xie L, Zhu M, Wu Z, He G, Tang A. The role of tensor veli platini muscle (TVP) and levetor veli platini muscle (LVP) in the opening and closing of pharyngeal orifice of Eustachian tube. Acta oto-laryngologica. 2016;136(3):249–55. [DOI] [PubMed] [Google Scholar]
- Heidsieck DSP, Smarius BJA, Oomen KPQ, & Breugem CC. The role of the tensor veli palatini muscle in the development of cleft palate associated middle ear problems. Clin Oral Investig. 2016;20:1389–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE: Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–1061 [DOI] [PubMed] [Google Scholar]
- Kirby ML, Waldo KL: Neural crest and cardiovascular patterning. Circ Res. 1995; 77: 211–215 [DOI] [PubMed] [Google Scholar]
- Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet. 2007;370:1443–1452. [DOI] [PubMed] [Google Scholar]
- Kollara L, Schenck GC, Jaskolka M, Perry JL. Examining a new method to studying velopharyngeal structures in a child with 22q11.2 DS. J Speech Lang Hear Res. 2017;60:892–896. [DOI] [PubMed] [Google Scholar]
- Kotlarek KJ, Perry JL, Fang X. Morphology of the Levator Veli Palatini Muscle in Adults with Repaired Cleft Palate. J Craniofac Surg. 2017; 28(3): 833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, Oda H, Maemura K, Nagai R, Ishikawa T, Yazaki Y: Aortic arch malformations and ventricular septal defect in mice deficient in endothelin-1. J Clin Invest. 1995;96:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos E, Verhaert N, Willaert A, Devriendt K, Swillen A, Hermans R, Op de Beeck K, Hens G. Malformations of the middle and inner ear on CT imaging in 22q11 deletion syndrome. Am J Med Genet A. 2016;170(11):2975–83. [DOI] [PubMed] [Google Scholar]
- Macintyre EA, Karr CJ, Koehoorn M, Demers P, Tamburic L, Lencar C, Brauer M. Otitis media incidence and risk factors in a population-based birth cohort. Paediatr Child Health. 2010;15:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan DS, Pandian SS, Murugesan S, Kumar R. The incidence of secretory otitis media in cases of cleft palate. J Clin Diagn Res. 2013;7(7):1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niskar AS, Kieszak SM, Holmes A, Esteban E, Rubin C, Brody DJ. Prevalence of hearing loss among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey. JAMA. 1998;279(14):1071–5. [DOI] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP. Morphology of the levator veli palatini muscle using magnetic resonance imaging. Cleft Palate Craniofac J. 2013;50(1):64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C, Lohmander A, Jönsson R, Óskarsdóttir S, Söderpalm E. A prospective cross-sectional study of speech in patients with the 22q11 deletion syndrome. J Comm Disord. 2003;36(1):13–47. [DOI] [PubMed] [Google Scholar]
- Reyes MRT, LeBlanc EM, Bassila MK. Hearing loss and otitis media in velo-cardio-facial syndrome. Int J Pediatr Otorhinolaryngol. 1999;47:227–233. [DOI] [PubMed] [Google Scholar]
- Robin NH, Shprintzen RJ. Defining the clinical spectrum of deletion 22q11.2. J Pediatr. 2005;147:90–96. [DOI] [PubMed] [Google Scholar]
- Rosenfeld RM, Culpepper L, Doyle KJ, Grundfast KM, Hoberman A, Kenna MA, Lieberthal AS, Mahoney M, Wahl RA, Woods CR Jr, Yawn B. Clinical practice guideline: otitis media with effusion. Otolaryngol Head Neck Surg. 2004;130(5):S95–118. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, Schuffenhauer S, Oechsler H, Belohradsky B, Prieur M, Aurias A. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet. 1997;34(10):798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönmeyr B, Sadhu P. A review of the tensor veli palatine function and its relevance to palatoplasty. J Plast Surg Hand Surg. 2014;48(1):5–9. [DOI] [PubMed] [Google Scholar]
- Sheahan P, Miller I, Sheahan JN, Earley MJ, Blayney AW. Incidence and outcome of middle ear disease in cleft lip and/or cleft palate. Int J Pediatr Otorhinolaryngo. 2003;67(7):785–93. [DOI] [PubMed] [Google Scholar]
- Shibahara Y, Sando I. Histopathologic study of Eustachian tube in cleft palate patients. Ann Otol Rhinol Laryngol. 1988;97(4Pt 1):403–408. [DOI] [PubMed] [Google Scholar]
- Solot CB, Knightly C, Handler SD, Gerdes M, Mcdonald-Mcginn DM, Moss E, Wang P, Cohen M, Randall P, Larossa D, Driscoll DA. Communication disorders in the 22Q11.2 microdeletion syndrome. J Commun Disord. 2000; 33:187–203. [DOI] [PubMed] [Google Scholar]
- Terzi S, Beyazal Çeliker F, Özgür A, Çeliker M, Beyazal M, Demirci M, Dursun E. The evaluation of eustachian tube paratubal structures using magnetic resonance imaging in patients with chronic suppurative otitis media. Acta oto-laryngologica. 2012;136(7):673–6. [DOI] [PubMed] [Google Scholar]
- Verheij E, Kist AL, van der Molen AM, Stegeman I, van Zanten GA, Grolman W, Thomeer HG. Otologic and audiologic findings in 22q11. 2 deletion syndrome. Eur Arch Otorhinolaryngol. 2017;274(2):765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H The 22q11.2 deletion syndrome. Keio J Med. 2002; 51(2): 77–88. [DOI] [PubMed] [Google Scholar]
- Yu W, Serrano M, San Miguel S, Ruest LB, Svoboda KKH. Cleft lip and palate genetics and application in early embryological development. Indian J Plast Surg. 2009;42(Suppl):S35–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]