Abstract
Background:
Primary cutaneous lymphoma (PCL) represents a heterogeneous collection of non-Hodgkin lymphomas originating in the skin. Our study describes the clinical and histological findings of cutaneous lymphoma within Botswana to expand the paucity of data on this rare disease in sub-Saharan Africa.
Methods:
We conducted a retrospective review from the dermatology clinic at Princess Marina Hospital (Gaborone, Botswana) of patients evaluated by skin biopsy for cutaneous lymphoma between 2008–2017. Patients with initial diagnostic suspicion for cutaneous lymphoma had biopsies re-reviewed by experienced dermatopathologists and were given a final diagnosis of either 1) cutaneous lymphoma, 2) atypical lymphocytic infiltrate (ALI), or 3) a reactive cutaneous process.
Results:
Thirty-eight cases were identified with a mean age of 50.0 years and a M:F ratio of 13:6. Final diagnoses included: 27 cases of cutaneous lymphoma, 8 cases of ALI, and 3 cases of reactive cutaneous processes. Subtypes of cutaneous lymphoma diagnosed included: mycosis fungoides (81.5%), plasmablastic lymphoma (7.4%), Epstein-Barr virus-positive T-cell lymphoma (3.7%), subcutaneous panniculitis-like T-cell lymphoma (3.7%), and peripheral T-cell lymphoma, NOS (3.7%). The most common immunohistochemical staining profile in mycosis fungoides cases was CD8 predominance over CD4.
Conclusions:
PCL causes significant morbidity and mortality globally. Given the limited resources in sub-Saharan Africa, it is essential to educate providers on the manifestations and histology of PCL. This study is an important step towards understanding the demographics, clinical presentation, histologic features, and mortality of patients diagnosed with PCL in Botswana and similar low-resource settings.
Introduction
Primary cutaneous lymphoma (PCL) represents a heterogeneous collection of non-Hodgkin lymphomas originating in the skin, distinguished in part by distinct clinical and histologic presentations and the composition of immunologic markers found on these abnormal cells. The majority (70–80%) of PCLs are cutaneous T-cell lymphomas (CTCLs), with mycosis fungoides (MF) being the most common subtype within this category, comprising over 50% of cases.1–3 Clinical course is variable, ranging from indolent disease with an overall good prognosis to an aggressive life-threatening course with a poor prognosis.
Only a limited number of case reports and case series have described cutaneous lymphoma in sub-Saharan Africa, however, there have been no systematic reviews of the clinical and histological findings of cutaneous lymphoma within this population.4–11 Several issues, including HIV co-infection, lack of access to specialty providers, and a relatively large dispersed rural population, may contribute to burden and late presentation of disease that can be seen in this setting. The literature available suggests that cutaneous lymphoma is likely under recognized and under diagnosed in sub-Saharan Africa.6, 11, 12
In order to further evaluate this disease in sub-Saharan Africa, our study reviewed the clinical and histological findings of patients diagnosed with cutaneous lymphoma at Princess Marina Hospital (PMH) in Botswana from 2008–2017.
Materials and Methods
Setting: Princess Marina Hospital Dermatology Clinic
PMH serves as the largest referral hospital in the public healthcare sector of Botswana, and this system provides free healthcare to all citizens. PMH’s referral base spans the entire country, and it serves as the primary access point for specialized medicine, including dermatologic care. Given the limited availability of practitioners with expertise in complex medical dermatology and/or dermatopathology in Botswana, the dermatology clinic at PMH provides the majority of diagnoses of cutaneous lymphoma in the country.
Data collection
A retrospective chart review from the dermatology clinic at PMH was conducted in order to identify patients who were evaluated by skin biopsy for cutaneous lymphoma between 2008 and 2017. Patients with both clinical and histopathologic features concerning for cutaneous lymphoma were included in this study. Extracted patient data included age, sex, gender, HIV status, clinical history, clinical photos, lesion morphology, lesion distribution, histopathology results, histopathology photos, treatment, treatment response, length of follow up, and vital status.
Histopathology
All patients included in this study for original suspicion of cutaneous lymphoma were re-evaluated in an attempt to confirm a final diagnosis. Histologic descriptions and histology photos, when available, along with clinical data were reviewed by experienced dermatopathologists at Penn Cutaneous Pathology Services at the University of Pennsylvania to confirm their original diagnoses of cutaneous lymphoma. Final patient diagnoses fit into the following categories: 1) cutaneous lymphoma, 2) atypical lymphocytic infiltrate (ALI), or 3) a reactive cutaneous process (i.e. psoriasiform, eczematous, etc.). ALI met the following histologic criteria: 1) rare to scattered abnormal, hyperchromatic and irregularly contoured lymphocytes in a pattern suspicious but not diagnostic for MF and/or 2) an atypical immunophenotype (if available) with minimal cytologic atypia or other diagnostic findings. Reactive cutaneous processes were characterized as having either a spongiotic or psoriasiform pattern with lack of cytologic atypia and reactive appearing immunophenotypic markers (if available).
Immunohistochemistry
Immunohistochemistry results were available in a select number of cases, and included antibodies such as CD3, CD4, CD7, CD8, CD20, CD30, CD56, and Epstein-Barr encoded RNAs (EBER) in situ hybridization. Immunohistochemical stains, flow cytometry and clonal T-cell receptor gene rearrangement (TCR) analyses were not available in Botswana at the time of this study, and thus not performed in all cases due to the limited resources available for sending out specimens to a referral laboratory in South Africa for analysis. Available tissue blocks were obtained from the National Health Lab in Gaborone, Botswana to allow for additional immunohistochemical stains to be performed on 6 cases at Penn Cutaneous Pathology Services for this study.
Results
Clinical features
A total of 38 patients with clinical and histologic suspicion for cutaneous lymphoma were identified. The mean age at diagnosis for this cohort was 50.0 years with a M:F ratio of 2.2:1. After re-evaluation, a final diagnosis of cutaneous lymphoma was rendered for 27 of 38 cases (71.1%), ALI for 8 of 38 cases (21.1%), and reactive cutaneous processes for 3 of 38 cases (7.9%). Of the 27 cases of cutaneous lymphoma identified, the majority were classified as MF (22/27, 81.5%). Other subtypes diagnosed included plasmablastic lymphoma (2/27, 7.4%), Epstein-Barr virus (EBV)-positive T-cell lymphoma (1/27, 3.7%), subcutaneous panniculitis-like T cell lymphoma (1/27, 3.7%), and peripheral T-cell lymphoma, NOS (1/27, 3.7%). HIV data was available for 13 of 22 MF patients, of which 4 (18.2%) patients were positive. All patients diagnosed with non-MF subtypes of cutaneous lymphoma were found to be HIV positive. Table I summarizes the complete demographic profiles and clinical characteristics of the cohort.
Table I.
Summary of demographics and clinical features.
| Diagnosis (Total Cases) | Average age (years) | Sex (M:F) | HIV status (% positive) | Morphology | Lesion Distribution |
|---|---|---|---|---|---|
| MF (22) | 52.5 | 13:9 | 4/22 (18.2%) | -Macules/patches only (6) -Plaques only (3) -Patches, plaques, tumors (3) -Erythroderma (8) -Erythroderma, plaques (1) -Erythroderma, tumors (1) |
-Gen (10) -T predominant (3) -T, E (8) -E predominant (1) |
| PTCL NOS* (1) | 32 | 1:0 | 1/1 (100%) | -Nodules and tumors | -Arm, T, H/N in a sporotrichoid-like pattern |
| Plasmablastic lymphoma (2) | 37 | 2:0 | 2/2 (100%) | -Nodules and tumors | -Genitals (1) -T, LE (1) |
| EBV(+) CTCL (1) | 32 | 1:0 | 1/1 (100%) | -Necrotic plaque with perforation | -H/N, hard palate |
| SPTCL (1) | 39 | 0:1 | 1/1 (100%) | -Subcutaneous nodules | -T, UE |
| ALI (8) | 49 | 3:1 | 2/8 (25%) | -Plaques (5) -Erythroderma (3) |
-Gen (4) -T, E (4) |
| Reactive processes (3) | 56 | 3:0 | 1/3 (33%) | -Erythroderma (2) -Atrophic plaques (1) |
-Gen (2) -T, LE (1) |
ALI, atypical lymphocytic infiltrate; EBV, Epstein–Barr virus; MF, mycosis fungoides; PTCL NOS, peripheral T-cell lymphoma not otherwise specified; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; Gen, generalized; T, trunk; LE, lower extremities; E, extremities; H/N, head and neck; UE, upper extremities
Mycosis fungoides
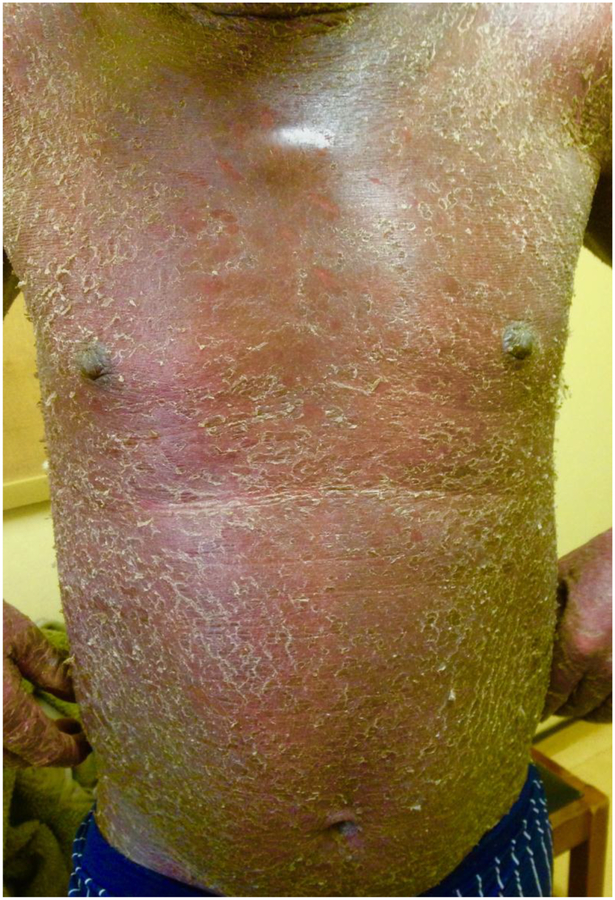
Of the 22 MF patients, a majority were male (59.1%) and the mean age at diagnosis was 52.5 years. The most common presentations of MF were exfoliative erythroderma (8/22, 36.4%) (Figure 1) and macules and/or patches (6/22, 27.3%). Five of 22 patients (22.7%) presented with a combination of more than one morphology which included tumors in some cases. The most common distribution was generalized (10/22, 45.5%). Flow cytometry for work up of blood involvement was available for only two patients, and results showed populations of <30% T lymphocytes with abnormal phenotypes. Clonality was not able to be assessed to aid in definitively ruling out Sezary Syndrome.1
Figure 1.
A patient presents with generalized exfoliative erythroderma.
Plasmablastic lymphoma
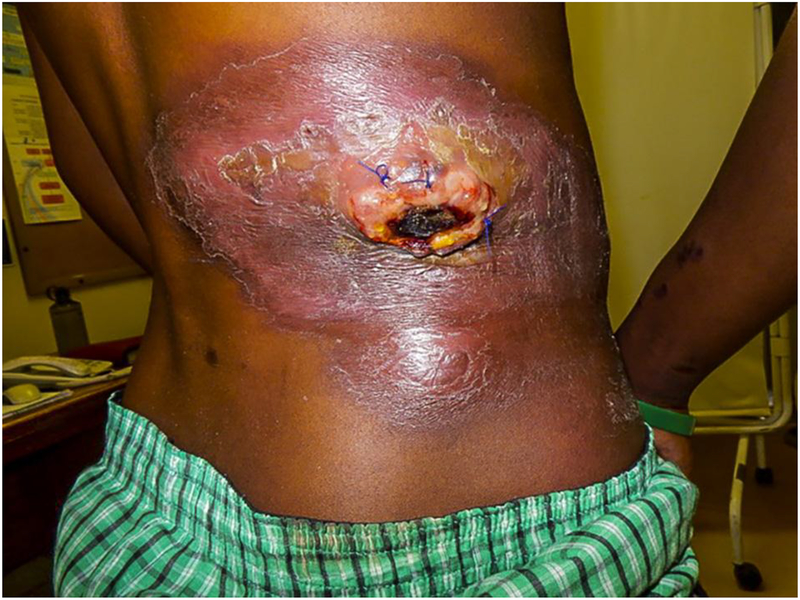
Two cases of plasmablastic lymphoma were included in this series. A 42-year-old HIV+ male presented with painful penile and perineal nodules. In addition, a 32-year-old HIV+ male presented with a large, exophytic, ulcerated, necrotic tumor on his right flank surrounded by an indurated plaque with multiple satellite tumors and an additional crusted plaque on his right thigh (Figure 2). He had cervical and inguinal lymphadenopathy.
Figure 2.
Large erythematous boggy plaque with a central necrotic, purulent mass and a rim of scale on the right lower back.
Epstein-Barr virus-positive T-cell lymphoma
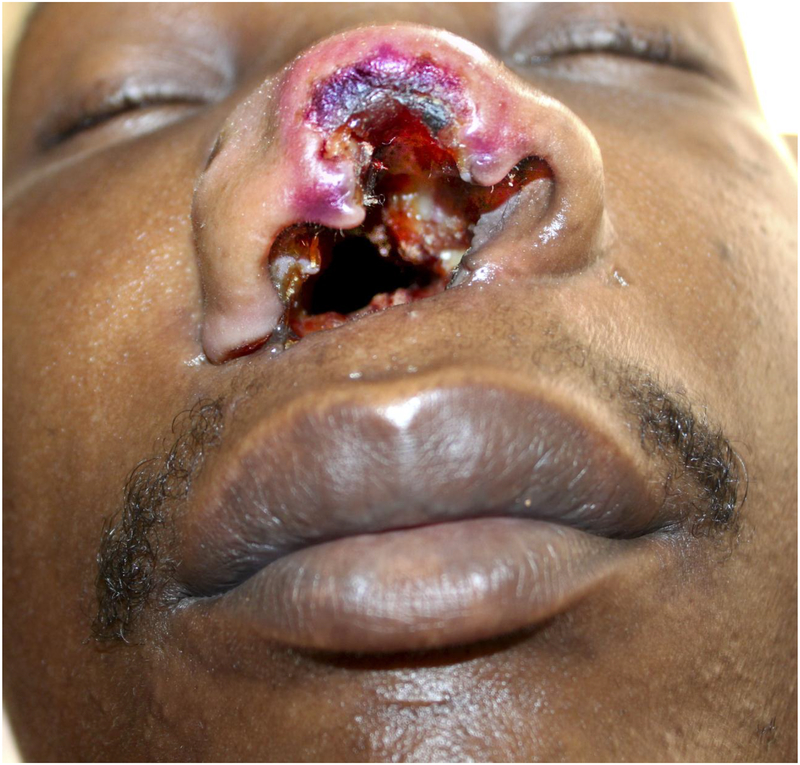
One case of Epstein-Barr virus-positive T-cell lymphoma was identified in a 32-year-old HIV+ male presenting with a hard palate ulceration which progressed to fistulation and necrosis of the nasal septum over a period of 4 months (Figure 3). The patient had significant anterior cervical and submandibular lymphadenopathy.
Figure 3.
Complete ulceration of the nasal septum fistulating to the hard palate (not shown) with deformation of the nasal tip and ala, necrosis of the nasal mucosa and surrounding erythema.
Peripheral T-cell lymphoma, NOS
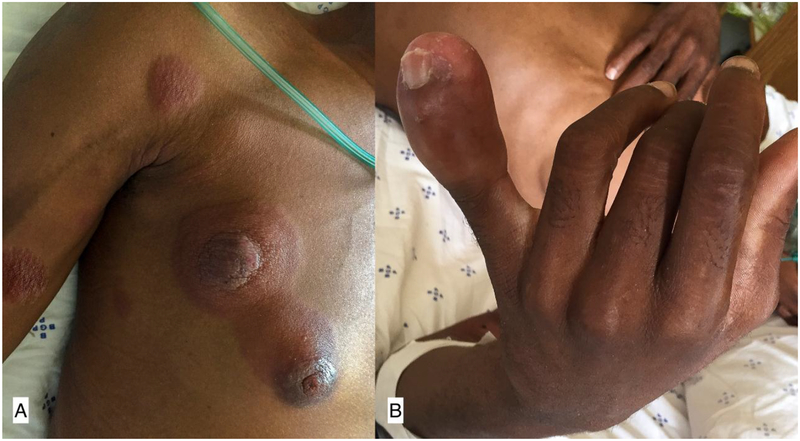
One case of peripheral T-cell lymphoma, NOS (PTCL-NOS) was diagnosed in a 32-year-old HIV positive male. He developed edema of the left 5th digit which evolved over weeks to a large nodule with associated erythematous plaques that spread cranially mimicking a sporotrichoid lymphocutaneous infection. (Figure 4). He also had cervical and axillary lymphadenopathy and left-sided parotid gland swelling.
Figure 4.
Erythematous indurated plaques and nodules extending linearly up right arm, chest (A) and face (not pictured) with associated erythema, edema and induration of the distal left fifth digit (B).
Subcutaneous panniculitis-like T-cell lymphoma
One case of subcutaneous panniculitis-like T-cell lymphoma (SPTCL) was diagnosed in a 39-year-old HIV+ female with firm subcutaneous nodules of the left deltoid, right proximal upper extremity and lower back.
Histopathologic and immunohistochemical characteristics
A full summary of the histopathologic and immunohistochemical details of all cases reviewed is provided in Table II. Sixteen of 22 MF specimens had slides available for review and epidermotropism was the most common histologic feature (13/16, 81.3%). CD8 predominance over CD4 was the most common immunohistochemical staining profile. TCR gene rearrangement analysis was available for a single case and was negative.
Table II.
Summary of histopathology and immunohistochemistry data.
| Diagnosis (±) | Epidermo-tropism | Adnexo-tropism | Large cells | CD4 vs. CD8 predominance | Comments |
|---|---|---|---|---|---|
| MF (16) | 13/16 (81%) | 6/16 (38%) | 3/16 (19%) | CD8 (8), CD4 (6), null* (2) | -Significant CD30+ T cells (4) -Rare CD30+ T cells, EBV- (1) -CD5+, EBV-, CD56- (1) -CD8+ (1) |
| PTCL NOS (1) | 0/1 (0%) | 0/1 (0%) | 1/1 (100%) | NA | CD5, EBV+, HHV8-, BCL6-, CD10-, PD1-, TIA1- |
| Plasmablastic lymphoma (1) | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | NA | CD138+, Ki67+, MUM1+, BCL2-, BCL6-, CD10-, CD15-, CD79a-, Tdt- |
| EBV(+) CTCL (1) | 0/1 (0%) | 0/1 (0%) | 1/1 (100%) | NA | CD3+, CD5+, CD8+, alpha/beta+, EBER+, CD56- |
| SPTCL (1) | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | CD4 = CD8 | Atypical lymphocytes rimming adipocytes, CD3+, CD8+, CD56, predominantly TIA1+ |
| ALI (4) | 3/4 (75%) | 0/4 (0%) | 0/4 (0%) | CD8(3), WNL** | |
| Reactive processes (1) | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | WNL |
includes only cases with histology available for review
null: CD4, CD8 negative
WNL: within normal limits
NA: not applicable as in CD4 and/or CD8 not tested
ALI, atypical lymphocytic infiltrate; EBV, Epstein–Barr virus; MF, mycosis fungoides; PTCL NOS, peripheral T-cell lymphoma not otherwise specified; SPTCL, subcutaneous panniculitis-like T-cell lymphoma
Plasmablastic lymphoma cases showed the characteristic histologic findings of this disease, including medium to large plasmablastic lymphoid cells and scattered macrophages creating a ‘starry sky’ pattern. Histology of the EBV+ T cell lymphoma showed large, atypical cells with diffuse EBER positivity. This patient’s clinical presentation resembled extranodal natural killer (NK)/T-cell lymphoma (NK/T-cell) but the immunophenotype (CD3+, CD5+, CD8+, alpha/beta+, EBER+, CD56-) was inconsistent (due to the CD3+, CD56-), though known cases of CD56- NK/TCL do occur. Histology of the PTCL-NOS case revealed highly atypical lymphocytes with an unusual skin immunophenotype (CD5+, EBV+, BCL6-, CD10-, HHV-8-, PD-1-, TIA-1-), leading to a determination that the patient’s disease was best classified as a PTCL-NOS. Histology of the SPTCL case showed atypical lymphocytes rimming adipocytes that were CD3+, CD8+, CD56- and predominantly TIA1+.
Treatment
Of the 22 MF patients, treatment data for 15 patients (68.2%) was available for analysis. Thirteen of these 15 (86.7%) MF patients received topical steroids, 4 (26.7%) were advised to use natural sunlight phototherapy, and 5 (33.3%) were referred to oncology. Two erythrodermic MF patients received oral steroids, and one of these patients was also treated with cyclosporine and methotrexate. Treatment response information was only available for two patients with MF who had partial response to topical steroids and sunlight.
The first case of plasmablastic lymphoma localized to the genitals was lost to follow up without any documented treatment. The second case of plasmablastic lymphoma (Figure 2) was successfully treated with 7 cycles of cyclophosphamide, hydroxydaunorubicin, oncovin, etoposide, and prednisone (CHOEP) with complete clinical response. The patient with EBV+ T cell lymphoma (Figure 3) was successfully treated with 8 cycles of CHOEP with a complete clinical response. The patient with PTCL-NOS (Figure 4) passed away before appropriate treatment could be started. The case of SPTCL was treated with topical and oral corticosteroids with a partial clinical response.
Follow-up and mortality
Follow-up data to assess vital status was available for 17 of 38 cases (44.7%) with a mean duration of follow-up of 256.8 days (standard deviation 479.6 days, median 81 days, range 12–1932 days). At the time of this publication, 5 patients from this series had a documented vital status of deceased. Deaths occurred in patients with advanced presentations of MF including erythroderma, plaques and tumors (3), and the patient with rapidly progressive PTCL, NOS.
Comments
Only a few reports have been published on the occurrence of cutaneous lymphoma in sub-Saharan Africa, and our study adds a significant number of cases from which additional knowledge can be gained.6; 9; 11; 12 Given the comorbidities and access issues faced by populations in low-resource settings, as well as the variability in clinical presentation, the diagnosis and treatment of cutaneous lymphomas can be clinically challenging. Through our systematic analysis of these cases, we aim to improve the ability of clinicians in resource-limited settings to recognize, accurately diagnose, and appropriately manage patients presenting with these diseases.
MF is the most common subtype of PCL, and typically has an indolent course with slow progression over years, from patches to infiltrated plaques and eventually to tumor-stage.1 The prognosis of patients with MF is dependent on the type and extent of skin lesions and the presence of extracutaneous disease. Patients with stage IA disease (<10% body surface area) generally do not experience a change in overall survival,13 whereas patients with stage IV disease have a median survival of 2.47 years.14 In our series, erythroderma was the most common presentation of both MF and reactive processes, highlighting the diverse etiologies of erythroderma and the need for appropriate work up to identify the correct underlying diagnosis. A common limitation is the difficulty in assessing peripheral blood to rule out Sezary Syndrome (SS) in MF patients presenting with erythroderma. In our setting, a peripheral blood smear analysis and fine needle aspiration for enlarged lymph nodes are available for work up, but accuracy of results is highly dependent on the skill and experience of the performing physician. Peripheral blood flow cytometry with T cell receptor rearrangement analysis is only rarely available for definitive diagnosis. Thus, we group erythrodermic patients into the MF category, and as a result, there may be unconfirmed cases of SS contributing to the higher rates of erythroderma seen in our MF cohort compared to what is reported in the literature.1
Any evaluating clinician can initiate treatment with skin directed therapies such as topical steroids or natural sunlight therapy which are first line options for treatment of both early stage MF and many reactive processes.15 However, not all cutaneous lymphomas will be responsive to topical agents and lack of response should prompt clinicians to consider further work up with a skin biopsy for unconfirmed diagnoses and/or referral to a dermatologist. For patients with advanced stage MF and non-MF subtypes of PCL, treatment often needs to be escalated to systemic immunomodulators and chemotherapeutic agents, in which case coordination with oncology is essential.
Non-MF cutaneous lymphomas are rare but important to be aware of because they have a worse prognosis compared to MF and are similarly challenging to diagnose due to variable presentations and clinical behaviors.1 Non-MF subtypes of cutaneous lymphomas can overlap with the morphologies and histologic phenotypes of non-malignant processes, such as eczema, psoriasis, pityriasis rubra pilaris, drug eruptions and other inflammatory or infectious cutaneous processes. Our case of PTCL-NOS presented unusually with a sporotrichoid pattern most commonly seen with deep fungal infections, illustrating the importance of considering lymphoma in the differential for immunosuppressed patients presenting with skin plaques/nodules and systemic symptoms. Rapid skin biopsy for confirmation of diagnosis is key in these atypical cases, as non-MF cutaneous lymphomas can be aggressive, as evidenced by this patient passing away before treatment could be initiated. Similarly, our case of SPTCL presented with non-specific subcutaneous nodules, which required biopsy, immunohistochemistry and close clinical correlation to distinguish from a much more common diagnosis of lupus profundus.
Twenty-nine percent of patients in our cohort who initially had clinical and histopathologic features concerning for cutaneous lymphoma were determined to fit best into other diagnostic categories after expert re-evaluation, highlighting the common challenges faced in cutaneous lymphoma diagnosis. We demonstrate that clinical and histopathologic overlap with a reactive process, ALI, or other inflammatory dermatoses is common. However, we caution clinicians against ruling out cutaneous lymphoma with just one biopsy. Skin lesions of cutaneous lymphoma, particularly the MF subtype, can take years to evolve and develop enough diagnostic features to allow confirmation of a malignant lymphoma. Therefore, for patients with an atypical or recalcitrant dermatitis that does not fit well into another diagnostic category, it is critical to maintain a low threshold for biopsy and be open to doing multiple biopsies over months to years to make the most accurate final diagnosis, and avoid missing a diagnosis of cutaneous lymphoma.
The importance of immunohistochemistry and the complexity of characterizing subtypes of cutaneous lymphoma is again highlighted by our case of EBV+ T cell lymphoma. This patient displayed aggressive destruction of the nasal cavity, clinically resembling extranodal NK/T cell lymphoma, nasal type. However, extranodal NK/T cell lymphoma are typically EBV positive, CD56 positive and CD3 negative, and in this case, CD3 was positive and CD56 was negative on immunohistochemistry. Although this case did not have the prototypical extranodal NK/T cell lymphoma, nasal type staining pattern, aberrant immunophenotypic cases have been described, and it is important to remain vigilant as these variants can also display aggressive behavior. Identification of the subtype and immunohistochemical profile of cutaneous lymphomas can help predict the disease course, prognosis, and strongly influences treatment decisions, thus access to histopathology with immunostaining is crucial for proper management of cutaneous lymphomas.
The majority of the patients in our study presented to dermatology with skin disease suggestive of advanced stage III//IV disease, indicating that referral to dermatology may have occurred at a late stage in their disease course. These cases highlight the important role primary care providers can play as the key initiators of successful cutaneous lymphoma management. Clinicians must consider the diagnosis of cutaneous lymphoma early when presented with a persistent chronic dermatitis resistant to initial treatments or featuring concerning morphologies such as erythroderma, nodules, tumors, and lymphadenopathy. Prompt referral to a facility with dermatology and pathology specialists is essential for appropriate diagnostic work-up. If not available locally, tissue can be sent out to neighboring countries with immunohistochemistry capability for dermatopathology consultation.
Dermatologists and even primary care physicians supported by remote dermatology specialists can manage early patch or plaque stage MF, which comprise the majority of patients with cutaneous lymphoma. However, oncology should be involved when tumors, lymphadenopathy, erythroderma or signs of internal involvement are present, for patients with non-MF cutaneous lymphoma subtypes or for any rapidly progressive disease. When specialized care is not available, telemedicine can be a vital resource, particularly for dermatopathology which is a scarce skill in low and middle-income countries. Teledermatology and telepathology resources were successfully utilized in a number of cases in this series to aid in diagnosis and management. For advanced cases or critically ill patients, transfer to neighboring countries with more dermatology and oncology resources should also be considered.
Limitations
There are several limitations to this study. First, due to the retrospective nature of this series, the data reviewed was incomplete for some cases, especially given that our government institution does not employ an electronic medical record in the outpatient setting and not all biopsy samples were able to be recovered for further analysis. In addition, rotating dermatology physicians were working in the clinic for short periods of time, leading to less continuity, and lapses in the detail of clinical records. Limitations in the resources needed to perform full staging in the public health system of Botswana, made it difficult to differentiate erythrodermic MF from SS and to confirm that lymphoma cases originated from a primary cutaneous site.
Conclusion
PCL causes significant morbidity and mortality globally. Given the paucity of published epidemiological data from sub-Saharan Africa, this study begins to address a significant gap in our understanding of this disease, but further investigations are needed, particularly to prospectively investigate treatments response and outcomes. In our study population, the majority of patients presented to dermatology with advanced disease manifestations including erythroderma, nodules and tumors. MF was the most common subtype diagnosed, however it is important for clinicians to be aware of the non-MF subtypes, which can present atypically and be associated with more aggressive disease. We highlight the importance of educating primary healthcare providers in limited resource settings to identify skin lesions suspicious for cutaneous lymphoma early and understand when patients need to be referred for specialized care to dermatology and oncology to allow for appropriate diagnosis and treatment intervention.
Although this is only a small snapshot of the impact of PCL on the population of Botswana, it is an important step in our understanding of the demographics, clinical presentation, histologic features, and mortality of patients diagnosed with cutaneous lymphoma in Botswana and similar low-resource settings.
Funding/Support
The project described was supported in part by the National Heart, Lung and Blood Institute, National Institutes of Health, through grant R25- HL084665 (OR).
Footnotes
Conflicts of Interest: None
References
- 1.Willemze R, Jaffe ES, Burg G et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005; 105: 3768–3785. [DOI] [PubMed] [Google Scholar]
- 2.Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood 2009; 113: 5064–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samimi S, Morrissey K, Anshelevich S et al. Romidepsin and interferon gamma: a novel combination for refractory cutaneous T-cell lymphoma. J Am Acad Dermatol 2013; 68: e5–6. [DOI] [PubMed] [Google Scholar]
- 4.Malviya N, Rainwater YB, Vandergriff T, Mauskar MM. Cutaneous extranodal NK/T-cell lymphoma mimicking cellulitis an HIV positive patient without lymphopenia. J Cutan Pathol 2017; 44: 296–299. [DOI] [PubMed] [Google Scholar]
- 5.Gahongayire F. Mycosis fungoides and Sezary syndrome against a human immunodeficiency virus-positive background: case report. Int J Dermatol 2007; 46 Suppl 1: 32–35. [DOI] [PubMed] [Google Scholar]
- 6.Ulrickson M, Okuku F, Walusansa V et al. Cutaneous T-cell lymphoma in sub-Saharan Africa. J Natl Compr Canc Netw 2013; 11: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toutoukpo Y, Kone M, Tea D, Abissey A. [Sezary’s syndrome. 4 cases observed at the University Hospital Center of Treichville (Abidjan, Ivory Coast)]. Med Trop (Mars) 1991; 51: 451–454. [PubMed] [Google Scholar]
- 8.Perret JL, Moussavou-Kombila JB, Delaporte E, Coniquet S, Nguemby-Mbina C, Normand P. [Mycosis fungoides in a Gabonese patient infected with HTLV-I]. Med Trop (Mars) 1996; 56: 66–68. [PubMed] [Google Scholar]
- 9.Campbell OB, George AO, Shokunbi WA, Akang EE, Aghadiuno PU. Problems in the management of mycosis fungoides in Nigeria. Trop Geogr Med 1991; 43: 317–322. [PubMed] [Google Scholar]
- 10.Akinbami AA, Osikomaiya BI, John-Olabode SO et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep 2014; 7: 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouchard N, Mahe A, Huerre M et al. Cutaneous T cell lymphomas: mycosis fungoides, Sezary syndrome and HTLV-I-associated adult T cell leukemia (ATL) in Mali, West Africa: a clinical, pathological and immunovirological study of 14 cases and a review of the African ATL cases. Leukemia 1998; 12: 578–585. [DOI] [PubMed] [Google Scholar]
- 12.Grijsen ML, Mtayangulwa RG, Naafs B et al. The clinical spectrum of mycosis fungoides in Tanzania, East Africa. Br J Dermatol 2017; 176: 1653–1656. [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Jensen RA, Watanabe GL, Varghese A, Hoppe RT. Clinical stage IA (limited patch and plaque) mycosis fungoides. A long-term outcome analysis. Arch Dermatol 1996; 132: 1309–1313 [PubMed] [Google Scholar]
- 14.Alberti-Violetti S, Talpur R, Schlichte M, Sui D, Duvic M. Advanced-stage mycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk 2015; 15: e105–112 [DOI] [PubMed] [Google Scholar]
- 15.Rodd AL, Ververis K, Karagiannis TC. Current and Emerging Therapeutics for Cutaneous T-Cell Lymphoma: Histone Deacetylase Inhibitors. Lymphoma 2012; 2012: 10 [Google Scholar]